Abstract
Background
In HER2-positive breast cancer, time elapsed between completion of (neo)adjuvant trastuzumab and diagnosis of metastatic disease (‘trastuzumab-free interval’, TFI) is crucial to choose the optimal first-line treatment. Nevertheless, there is no clear evidence to support its possible prognostic role.
Methods
In the Adjuvant Lapatinib and/or Trastuzumab Treatment Optimisation (ALTTO) trial, patients with HER2-positive early breast cancer were randomised to 1 year of either trastuzumab alone, lapatinib alone, their sequence or their combination. This exploratory analysis included only patients in the trastuzumab alone or trastuzumab plus lapatinib arms who developed a distant disease-free survival (DDFS) event. Overall survival (OS) was defined as time between date of DDFS event and death; age at diagnosis, tumour size and hormone receptor status were the variables included in the multivariate models.
Results
Out of 8381 patients included in ALTTO, 404 patients in the trastuzumab alone and trastuzumab plus lapatinib arms developed a DDFS event, of which 201 occurred <12 months (group A) and 203 >12 months (group B) after completion of adjuvant trastuzumab. No significant difference in location of first DDFS event was observed (p=0.073); a numerically higher number of patients in group A than in group B developed brain metastasis (26% vs 15%). Choice of first-line therapy differed between the two groups (p=0.022): in group A, more patients received lapatinib (25% vs 11%) and less pertuzumab (8% vs 17%). Median OS was 29.3 and 18.4 months in groups B and A, respectively (adjusted HR 0.69; 95% CI 0.54–0.89; p=0.004). The longer OS for patients in group B was observed across the analysed subgroups without interaction according to hormone receptor status (p=0.814) nor type of administered adjuvant anti-HER2 treatment (p=0.233).
Conclusions
TFI has prognostic value in patients with HER2-positive early breast cancer treated with adjuvant trastuzumab-based therapy. TFI is a valid tool to better individualise clinical recommendations and to design future first-line treatment trials for metastatic patients.
Keywords: breast cancer, HER2-positive, adjuvant therapy, trastuzumab, prognosis
Key questions.
What is already known about this subject?
Despite its crucial role in guiding first-line treatment choices, there is currently lack of clear evidence to support the possible prognostic value of the time elapsed between completion of adjuvant trastuzumab and diagnosis of metastatic disease (‘trastuzumab-free interval’, TFI). A more profound understanding of how this interval might influence outcomes is crucial and may contribute to further individualise clinical recommendations and design future trials.
What does this study add?
This exploratory analysis of the Adjuvant Lapatinib and/or Trastuzumab Treatment Optimisation (ALTTO) trial aimed to investigate the prognostic role of TFI in patients with HER2-positive early breast cancer that developed a distant disease-free survival event after exposure to adjuvant trastuzumab-based therapy. We observed that TFI has a strong prognostic value with a significantly better overall survival for patients relapsing >12 months following completion of adjuvant trastuzumab-based therapy. This result was observed irrespective of hormone receptor status and type of administered adjuvant anti-HER2 therapy. These findings may indirectly support the current use of the 12-month cut-off to differently manage patients with HER2-positive breast cancer relapsing after prior exposure to anti-HER2 therapy in the early setting.
Key questions.
How might this impact on clinical practice?
These results may help physicians in counselling patients with HER2-positive disease relapsing after exposure to anti-HER2 therapy in the early setting. They confirm that TFI is a valid tool to further individualise clinical recommendations and to design future trials in the metastatic setting. Additional research efforts are awaited to further explore the performance of first-line anti-HER2 targeted therapy in patients relapsing following exposure to these agents in the early setting.
Introduction
Being historically considered an indicator of resistance to previously administered treatments, the time elapsed between the end of (neo)adjuvant therapy and diagnosis of metastatic breast cancer is among the crucial factors in the choice of the optimal first-line treatment following disease recurrence.1 This concept was first demonstrated in pivotal chemotherapy trials.2 3 Similarly, for patients with hormone receptor-positive breast cancer, the time since the end of adjuvant endocrine therapy is currently used by international guidelines to define resistance to endocrine therapy to guide not only treatment decision-making but also the eligibility for first-line endocrine treatment trials.1
Disease-free interval has also a crucial role in patients with HER2-positive breast cancer to the extent that it should be considered as a key factor in the choice of the optimal first-line anti-HER2 therapy. Based on the inclusion criteria and results of the CLEOPATRA and EMILIA trials,4 5 respectively, the recommended first-line treatment is the combination of a taxane plus dual anti-HER2 blockade with trastuzumab and pertuzumab in patients recurring more than 12 months after the completion of (neo)adjuvant trastuzumab while T-DM1 should be used in those developing disease recurrence while on (neo)adjuvant trastuzumab or within 6 months from its completion.6 In addition, the indication for T-DM1 has been extrapolated for patients relapsing within 12 months of (neo)adjuvant trastuzumab completion although no data are available to guide the choice of the best first-line therapy when the time elapsed since completion of adjuvant trastuzumab is between 6 and 12 months.6 Notably, limited data are available on the performance of first-line therapies in patients relapsing after prior exposure to anti-HER2 targeted agents in the early setting.7
Despite its crucial role in guiding first-line treatment choices, there is currently lack of clear evidence to support the possible prognostic impact of the time elapsed between completion of adjuvant trastuzumab and diagnosis of metastatic disease.8 Therefore, a more profound understanding of how this interval might influence outcomes is crucial and may contribute to further individualise clinical recommendations and design future trials. To address this important issue, we performed an exploratory analysis of the Adjuvant Lapatinib and/or Trastuzumab Treatment Optimisation (ALTTO) trial, the largest adjuvant randomised study conducted in patients with early-stage HER2-positive disease.9 In ALTTO, type and timing of first site of disease recurrence, as well as information on choice of first-line treatments received by patients who relapsed have been prospectively collected. Hence, this was a unique opportunity to investigate the impact of the time elapsed between completion of adjuvant trastuzumab and diagnosis of a distant relapse (‘trastuzumab-free interval’, TFI) on patients’ prognosis.
Methods
Study design and patients
Details on the study design of the ALTTO trial were previously reported.9 Briefly, ALTTO (Breast International Group (BIG) 2-06/EGF106708 and North Central Cancer Treatment Group (Alliance) N063D) was an international, intergroup, open-label, randomised phase III study assessing the use of trastuzumab and/or lapatinib as adjuvant anti-HER2 targeted therapy in patients with HER2-positive early breast cancer.
To be eligible for the trial, the following inclusion criteria were required: completely excised and histologically confirmed invasive HER2-positive early breast cancer, node-positive disease or pathological tumour size of ≥1 cm in the case of no nodal involvement. Primary tumour samples from all patients were centrally tested to assess HER2 and hormone receptor status. HER2-positivity was defined according to the 2007 American Society of Clinical Oncology/College of American Pathologists guidelines.10 Expression of oestrogen and/or progesterone receptors in ≥1% tumour cells was the criteria used to define hormone receptor positivity.
Study procedures
Stratified permuted blocks with a 1:1:1:1 allocation ratio were used to prepare randomisation lists. Randomisation was performed with an interactive voice response system to one of the four anti-HER2 treatment arms: trastuzumab alone, lapatinib alone, sequential treatment with trastuzumab for 12 weeks followed by a 6-week washout period before other 34 weeks of lapatinib, and dual anti-HER2 blockade with trastuzumab plus lapatinib. In all treatment arms, adjuvant anti-HER2 therapy was administered for 1 year. Following the first interim analysis in 2011, the lapatinib arm was closed and adjuvant commercial trastuzumab was offered; a total of 1087 (51.8%) out of 2100 patients received at least one dose of trastuzumab.
For the purpose of the present analysis, only patients randomised to the 1-year duration trastuzumab arms (trastuzumab alone and trastuzumab plus lapatinib) were included.
Anti-HER2 treatment could be administered as per physician’s choice following chemotherapy completion (design 1), or concomitantly with a taxane after anthracycline-based chemotherapy (design 2) or with 6 cycles of docetaxel and carboplatin in an anthracycline-free regimen (design 2B). Adjuvant endocrine therapy had to be administered unless contraindicated according to local guidelines to all patients with hormone receptor-positive disease for at least 5 years. Radiotherapy was mandatory following breast-conserving surgery and was performed after mastectomy based on the guidelines of each participating institution. Endocrine therapy and radiotherapy were administered after chemotherapy completion and concomitantly with anti-HER2 therapy.
As per study protocol, type and timing of first site of disease recurrence, as well as information on choice of first-line treatments that were administered to patients who developed disease recurrence had to be prospectively reported. For the purpose of the present analysis, only patients randomised in the trastuzumab alone and trastuzumab plus lapatinib arms who developed a distant recurrence were included. Patients who had other invasive disease-free survival (DFS) events (local and regional relapses, contralateral invasive breast cancers, second non-breast malignancies and deaths without event) with no distant recurrence were excluded from the present analysis.
The 12-month cut-off between the completion of adjuvant trastuzumab and the development of the first distant DFS (DDFS) event was used as the criteria to distinguish between two groups of patients relapsing with a TFI of ≤12 months (group A) and >12 months (group B). The choice of the 12-month cut-off was based considering the current recommendation for the choice of the first-line treatment,6 as well as a prior similar BIG research project conducted within the HERA trial.8
Objectives and endpoints
The aim of the current analysis was to investigate the prognostic role of TFI in patients with HER2-positive early breast cancer in terms of overall survival (OS) following the development of a DDFS event.
Baseline patients’ characteristics, patterns of relapse and choice of first-line treatment for metastatic disease were also assessed.
Statistical methods
Statistical assumptions and sample size calculations of the ALTTO trial were previously reported.9 The present analysis should be regarded as exploratory considering that it was not preplanned in the study protocol and the power of the statistical analyses performed was not prespecified. The updated ALTTO Database was used for all the analyses with a cut-off date of 14th of December 2016.11
Baseline characteristics, patterns of relapse and the choice of first-line treatment were compared between group A and group B according to TFI. Categorical variables were summarised with proportions and differences tested using Χ2 test; continuous variables were reported using medians and IQR and differences were tested using Wilcoxon rank-sum test.
OS was defined as the time between the date of DDFS event and death. The impact of TFI on OS from distant recurrence was assessed in both univariate and multivariate Cox proportional hazards models. Age at diagnosis, tumour size and hormone receptor status were the variables included in the final multivariate models. Likelihood ratio test considered whether there was evidence of an interaction between TFI and hormone receptor status as well as between TFI and type of administered adjuvant anti-HER2 treatment. Results were presented using the Kaplan-Meier survival plots.
Considering the significant OS benefit associated with the use of pertuzumab-based first-line therapy and the observed different distribution of its use between groups A and B, an exploratory OS analysis was conducted by excluding patients exposed to this anti-HER2 agent.
Statistical analyses were two-sided; p values of <0.05 were considered statistically significant. Statistical analyses were performed by DAT using SAS V.9.4.
Results
A total of 8381 patients with HER2-positive early breast cancer were randomised in ALTTO between June 2007 and July 2011. Among patients enrolled in the trastuzumab and trastuzumab plus lapatinib arms (N=4190), 404 (9.6%) developed a DDFS event of which 201 occurred <12 months (group A) and 203 >12 months (group B) following completion of adjuvant trastuzumab (online supplemental figure S1).
esmoopen-2020-000979supp001.pdf (312.8KB, pdf)
Baseline clinicopathological characteristics are summarised in table 1. As compared with patients in group B, those in group A were older (p=0.013), had larger tumours (p=0.004) and had more often hormone receptor-negative disease (p<0.001). No difference in nodal status, nor in type of administered systemic therapy was observed between the two groups.
Table 1.
Baseline clinicopathological characteristics
| Group A (≤12 months) n=201 No. (%) |
Group B (>12 months) n=203 No. (%) |
P value | |
| Age at breast cancer diagnosis (median) (years) | 50.1 (41.8–59.7) | 47.3 (39.7–56.8) | 0.124 |
| Age at diagnosis | 0.013 | ||
| ≤40 years | 36 (18) | 53 (26) | |
| 41–64 years | 140 (70) | 139 (68) | |
| ≥65 years | 25 (12) | 11 (5) | |
| Menopausal status at diagnosis | 0.320 | ||
| Pre-menopausal | 97 (48) | 108 (53) | |
| Post-menopausal | 104 (52) | 95 (47) | |
| BMI (kg/m2) | 0.801 | ||
| Underweight (BMI <18.5) | 8 (4) | 5 (2) | |
| Normal (BMI=18.5–24.9) | 82 (41) | 89 (44) | |
| Overweight (BMI=25–29.9) | 62 (31) | 60 (30) | |
| Obese (BMI ≥30) | 49 (24) | 49 (24) | |
| Surgery | |||
| Breast-conserving surgery | 57 (28) | 59 (29) | 0.875 |
| Mastectomy | 144 (72) | 144 (71) | |
| Histology | 0.583 | ||
| Ductal carcinoma | 184 (92) | 182 (90) | |
| Lobular carcinoma | 7 (3) | 6 (3) | |
| Others/missing | 10 (5) | 15 (7) | |
| Tumour size | 0.004 | ||
| pT1 | 32 (16) | 63 (31) | |
| pT2 | 100 (50) | 92 (45) | |
| pT3–4 | 26 (13) | 19 (9) | |
| Not applicable (NACT) | 41 (20) | 29 (14) | |
| Missing | 2 (<1) | 0 (0) | |
| Nodal status | 0.233 | ||
| pN0 | 38 (19) | 32 (16) | |
| pN1 | 41 (20) | 44 (22) | |
| pN2–3 | 81 (40) | 98 (48) | |
| Not applicable (NACT) | 41 (20) | 29 (14) | |
| Tumour grade | 0.620 | ||
| G1 | 5 (2) | 3 (1) | |
| G2 | 65 (32) | 77 (38) | |
| G3 | 122 (61) | 114 (56) | |
| Missing | 9 (4) | 9 (4) | |
| Type of expression of hormone receptors | <0.001 | ||
| ER-positive and/or PR-positive | 94 (47) | 130 (64) | |
| ER-negative and PR-negative | 107 (53) | 73 (36) | |
| Treatment arm | 0.270 | ||
| Trastuzumab | 113 (56) | 103 (51) | |
| Trastuzumab plus lapatinib | 88 (44) | 100 (49) | |
| Type of chemotherapy | 0.223 | ||
| Anthracycline-based and taxane-based regimens | 139 (69) | 124 (61) | |
| Anthracycline-based regimens | 56 (28) | 70 (34) | |
| Taxane-based regimens | 6 (3) | 9 (4) | |
| Timing of chemotherapy | 0.509 | ||
| Sequential (design 1) | 135 (67) | 130 (64) | |
| Concurrent (design 2 and design 2B) | 66 (33) | 73 (36) | |
| Adjuvant endocrine therapy* | 0.053 | ||
| Administered | 79 (84) | 120 (92) | |
| Not administered | 15 (16) | 10 (8) | |
| Type of adjuvant endocrine therapy | 0.164 | ||
| SERM | 47 (59) | 82 (68) | |
| SERM and AI | 5 (6) | 10 (8) | |
| AI | 25 (32) | 28 (23) | |
| LHRHa alone | 2 (3) | 0 (0) |
*Calculated on the total number of patients with hormone receptor-positive breast cancer (94 in group A and 130 in group B).
AI, aromatase inhibitors; BMI, body mass index; ER, oestrogen receptor; G, grade; LHRHa, luteinising hormone-releasing hormone agonist; NACT, neoadjuvant chemotherapy; PR, progesterone receptors; SERM, selective oestrogen receptor modulator.
The majority of patients (267, 66%) developed visceral metastasis as first DDFS event with no difference between the two groups (table 2). No significant difference in location of first DDFS event was observed (p=0.073); however, a numerically higher number of patients in group A developed brain metastasis (26% vs 15%).
Table 2.
Location of first disease relapse at a distant site
| Group A (≤12 months) n=201 No. (%) |
Group B (>12 months) n=203 No. (%) |
P value | |
| Type of metastatic presentation (distant relapse)* | 0.195 | ||
| Visceral | 139 (69) | 128 (63) | |
| Non-visceral | 62 (31) | 75 (37) | |
| Metastatic site (distant relapse)† | 0.073 | ||
| Brain | 52 (26) | 31 (15) | |
| Liver | 46 (23) | 60 (30) | |
| Lung | 37 (18) | 33 (16) | |
| Bone | 43 (21) | 51 (25) | |
| Others | 23 (11) | 28 (14) | |
| Type of metastatic presentation (distant relapse)* | 0.289 | ||
| Visceral | 139 (77) | 128 (72) | |
| Bone (without visceral) | 42 (23) | 50 (28) | |
*Patients with bone, skin, lymph node and soft tissue were considered as non-visceral; all the others were considered as visceral.
†For patients who developed relapse in more than one organ, the first site of distant metastasis was defined by prespecified importance in the following order: brain, liver, lung, bone and others. The category ‘others’ included: skin, lymph node, soft tissue, pleura and other rarer sites of relapse.
After the development of DDFS events, no significant differences between the two groups were observed in terms of local treatments, use and type of chemotherapy (online supplemental table 1). First-line chemotherapy was administered to 251 (62%) patients. Taxane-based regimens were the most frequently used (57%). Among patients with hormone receptor-positive breast cancer, more patients in group A than group B received endocrine therapy as part of their first-line therapy (46% vs 29%; p=0.011). Anti-HER2 targeted therapy was part of the first-line treatment in 231 (57%) of the patients. The type of anti-HER2 treatment was differently distributed in the two groups (p=0.022): more patients in group A received lapatinib (25% vs 11%) and less pertuzumab (8% vs 17%).
esmoopen-2020-000979supp002.pdf (89.1KB, pdf)
Median follow-up after development of DDFS events was 17.5 months (IQR, 6.3–31.6 months), with no difference (p=0.092) between group A (15.2 months, IQR, 5.7–29.1 months) and group B (19.2 months, IQR, 7.1–33.6 months).
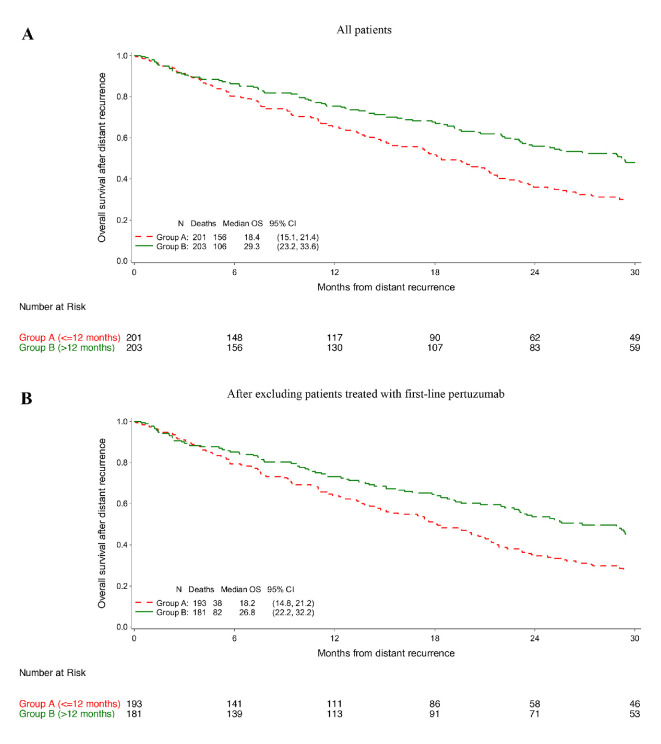
Patients in group B had a significantly longer OS as compared with those in group A. Median OS was 29.3 and 18.4 months in groups B and A, respectively (adjusted HR 0.69; 95% CI 0.54–0.89; p=0.004; figure 1A and online supplemental table 2). Similar results were observed when patients treated with first-line pertuzumab-based therapy (n=29) were excluded with a median OS of 26.8 and 18.2 months in groups B and A, respectively (adjusted HR 0.66; 95% CI 0.51–0.86; p=0.002; figure 1B and online supplemental table 2).
Figure 1.
Overall survival (OS) in the whole cohort of patients (A) and after excluding those who received first-line pertuzumab-based therapy (B).
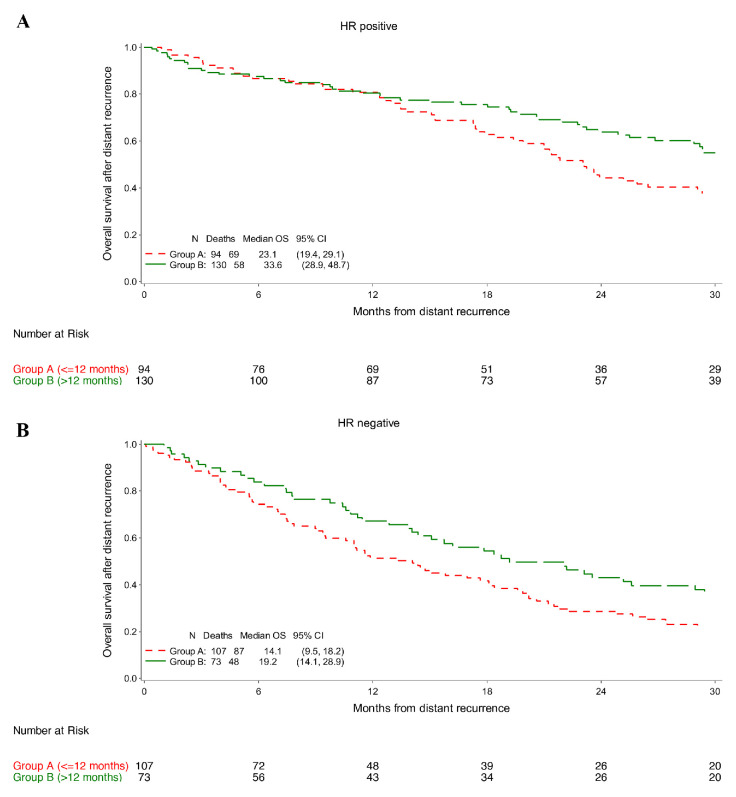
The longer OS for patients in group B as compared with those in group A was observed across the analysed subgroups with no interaction according to hormone receptor status (p=0.814) nor type of administered adjuvant anti-HER2 treatment (p=0.233).
In groups B and A, respectively, median OS was 33.6 and 23.1 months among patients with hormone receptor-positive disease (adjusted HR 0.69; 95% CI 0.48–0.99; figure 2A and online supplemental table 2), and 19.2 and 14.1 months among those with hormone receptor-negative disease (adjusted HR 0.68; 95% CI 0.48–0.98; figure 2B and online supplemental table 2).
Figure 2.
Overall survival (OS) in patients with hormone receptor (HR)-positive breast cancer (A) and in patients with HR-negative breast cancer (B).
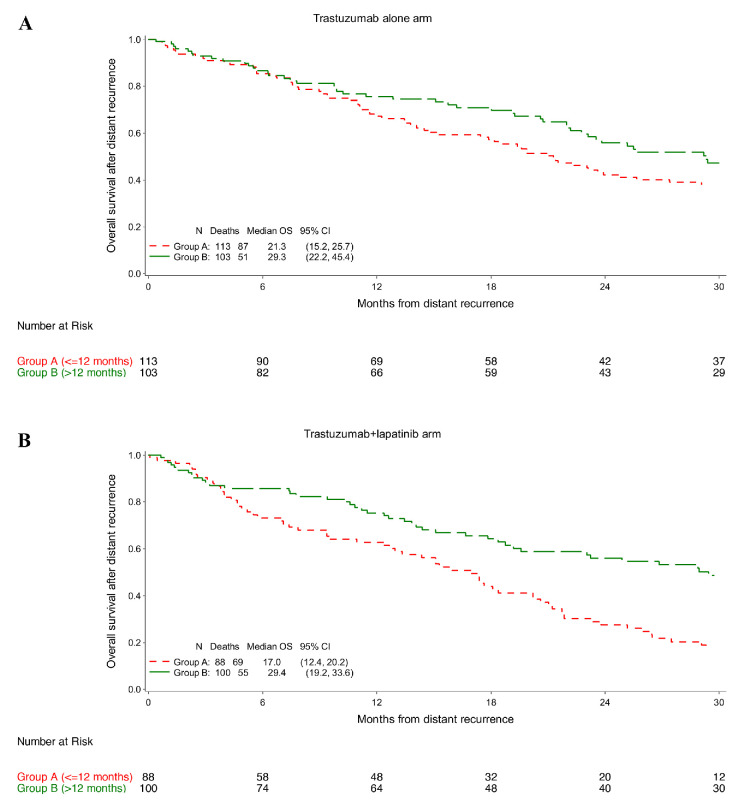
In groups B and A, respectively, median OS was 29.3 and 21.3 months among patients who received trastuzumab alone (adjusted HR 0.80; 95% CI 0.55–1.17; figure 3A and online supplemental table 2), and 29.4 and 17.0 months among those who received trastuzumab plus lapatinib (adjusted HR 0.55; 95% CI 0.38–0.80; figure 3B and online supplemental table 2) as adjuvant anti-HER2 treatment.
Figure 3.
Overall survival (OS) in patients who received adjuvant trastuzumab (A) and in patients who received adjuvant trastuzumab plus lapatinib (B).
Discussion
To our knowledge, this is the largest analysis aiming to investigate the prognostic impact of TFI in patients with HER2-positive early breast cancer that developed a DDFS event after exposure to adjuvant trastuzumab-based therapy. We observed that TFI has a strong prognostic role with a significantly better OS for patients relapsing >12 months following the end of adjuvant trastuzumab-based therapy. This result was observed irrespective of hormone receptor status and type of administered adjuvant anti-HER2 therapy. These findings may indirectly support the current use of the 12-month cut-off to differently manage patients with HER2-positive breast cancer relapsing after prior exposure to anti-HER2 therapy.
Whereas the 12-month cut-off is commonly used in clinical practice, limited evidence is available so far on its role in influencing the prognosis of patients. A prior exploratory analysis of the HERA trial including 187 patients developing a DDFS events following exposure to adjuvant trastuzumab investigated the potential prognostic role of TFI.8 No statistically significant relationship between TFI and OS was observed (adjusted HR 0.98; p=0.27). However, median OS from the development of a DDFS event was numerically longer among patients with a TFI >12 months after the end of adjuvant trastuzumab (23.7 vs 17.8 months; p=0.47).8 Similarly, an Italian retrospective study including 101 patients showed that the 6-month cut-off for the TFI appeared to have prognostic value but the result did not reach statistical significance.12 Median OS was 48.3 and 29.5 months for patients with a TFI of more and less than 6 months, respectively (HR 0.73; 95% CI 0.39–1.37; p=0.331).12 With a larger sample size, our analysis shows the important prognostic role of TFI with an almost 11-month absolute median OS advantage (18.4 vs 29.3 months) for patients relapsing >12 months following completion of adjuvant trastuzumab (adjusted HR 0.69; 95% CI 0.54–0.89; p=0.004).
In addition, our results provide further evidence on the different behaviour of HER2-positive tumours according to hormone receptor status.13–18 As compared to patients with hormone receptor-positive disease, those with hormone receptor-negative tumours had higher likelihood to relapse within 12 months following completion of adjuvant trastuzumab and had poorer OS irrespective of TFI. These data further highlight the need to pursue in research efforts aiming to improve the outcomes of patients with metastatic HER2-positive disease with different approaches according to hormone receptor status. These efforts would be even more relevant for patients relapsing during or soon after completion of anti-HER2 therapy who are characterised by particularly poor outcomes.
The different recommendations on the use of first-line therapy in patients relapsing after prior exposure to anti-HER2 therapy are currently mostly based on the inclusion criteria of major randomised trials conducted in the metastatic setting.4 5 However, a small number of patients included in these trials were enrolled after failure of (neo)adjuvant anti-HER2 therapy.4 5 Limited evidence, derived mostly from small retrospective studies, is currently available on the performance of first-line anti-HER2 therapy in patients relapsing after prior exposure to targeted agents in the early setting.8 12 19–25 Based on these studies, it cannot be excluded that patients exposed to anti-HER2 targeted treatment in the early setting may experience a smaller benefit with first-line therapy. In ALTTO, type of treatment received after experiencing disease relapse was collected, but no proper data are available on the duration of first-line systemic therapy with a specific focus on anti-HER2 agents. Therefore, the performance of first-line anti-HER2 treatments in patients with relapse after adjuvant trastuzumab-based therapy could not be assessed. In the ongoing adjuvant trials, collecting information on duration of exposure to subsequent lines of therapy should be considered a research priority to improve the counselling of patients relapsing following exposure to anti-HER2 targeted treatment in the early setting. In addition, real-world data from the currently ongoing registries in the advanced setting should also be considered an important source of data to explore this unmet medical need.26–29 With the current availability of several effective targeted agents as (neo)adjuvant and post-neoadjuvant treatment for patients with HER2-positive breast cancer,30–34 defining the optimal performance of first-line anti-HER2 therapies in those relapsing after prior exposure in the early setting is becoming a clinically relevant research area.
The present analysis has some limitations that should be acknowledged. This is an unplanned exploratory analysis and with a relatively small sample size. Some potential important prognostic factors (like performance status and disease volume) were not available and so they could not be included in the multivariate model. Notably, 66% of the patients included in this analysis received trastuzumab sequentially to chemotherapy (design 1). In addition, only 57% of the patients received first-line anti-HER2 therapy and few of them received the more recently approved targeted therapies including pertuzumab and T-DM1. These circumstances may explain the poorer OS observed in our study when compared with current expectations in this setting.35 However, major strengths are that these results come from a large phase III trial, and that the data used for the analyses were prospectively collected during trial conduction as requested and detailed in the study protocol. Only patients receiving adjuvant trastuzumab-based treatment for 1 year were included to mirror current clinical standard.
In conclusion, our exploratory analysis conducted within the ALTTO trial suggests that the 12-month cut-off for the TFI has a strong prognostic role. Patients experiencing a DDFS event ≤12 months following the completion of adjuvant trastuzumab had a significantly shorter OS (defined as time between the development of DDFS event and death) than those relapsing >12 months. These findings may help physicians in counselling patients with HER2-positive disease relapsing after exposure to anti-HER2 therapy in the early setting and confirm that TFI is a valid tool to further individualise clinical recommendations and to design future trials in the metastatic setting. Additional research efforts are awaited to further explore the performance of first-line anti-HER2 targeted therapy in patients relapsing following exposure to these agents in the early setting.
Acknowledgments
ML acknowledges the support from the European Society for Medical Oncology (ESMO) for a Translational Research Fellowship at the Institut Jules Bordet in Brussels (Belgium) during the conduction of this study. We also acknowledge the ALTTO staff of the BrEAST Data Centre at Institut Jules Bordet in Brussels (Belgium) for clinical record online management, and Sebastien Guillaume of the BrEAST Data Centre at Institut Jules Bordet in Brussels (Belgium) for administrative support.
Footnotes
Twitter: @matteolambe, @E_de_Azambuja
Contributors: ML, MJP and EdA—study conception and study design. ML, OM-F, NFP, FP, FSH, LAK, SC, OW, LDM, RC, VM, AM-A, MJP and EdA—data acquisition. ML, DA-T, OM-F and NFP—data analysis and interpretation. ML—paper preparation. ML, DA-T and EdA—paper editing. All authors—paper review.
Funding: The ALTTO trial received financial support from GlaxoSmithKline (until January 2015), Novartis Pharma AG (as of January 2015) and the National Cancer Institute of the National Institutes of Health (NCI-NIH; Grant No. U10CA180821 and U10CA180882 to the Alliance for Clinical Trials in Oncology and Grant No. CA025224 to the legacy North Central Cancer Treatment Group). The funders and sponsors had no role in the design or conduct of the study, in the collection, analysis, or interpretation of the data, neither in the preparation, review or approval of the present manuscript.
Disclaimer: The present analysis did not receive additional funding.
Competing interests: ML acted as a consultant for Roche and Novartis, and received speaker honoraria from Theramex, Roche, Takeda, Novartis, Pfizer and Lilly outside the submitted work. NFP acted as a consultant for Lilly; received speaker honoraria from AstraZeneca, Novartis, Lilly and Roche-Genentech, travel support from Novartis and research grants from Daiichi Sankyo, MSD and BMS outside the submitted work. OW reports employment at Novartis. LDM acted as a consultant for Roche, Novartis, MSD, Pfizer, Ipsen, AstraZeneca, Genomic Health, Lilly, Seattle Genetics, Eisai, Pierre Fabre, Daiichi Sankyo; received speaker honoraria from Roche, Novartis, Lilly and MSD, and travel grants from Roche, Pfizer and Celgene outside the submitted work. RC received speaker fees from Boehringer-Ingelheim, AstraZeneca and Janssen, and travel support from AstraZeneca and Pfizer, outside the submitted work. AM-A received research grants from GSK/Novartis (to the institution) outside the submitted work. MJP served as board member of Oncolytics; received honoraria from AstraZeneca, Camel-IDS, Crescendo Biologics, Debiopharm, G1 Therapeutics, Roche-Genentech, Huya, Immunomedics, Lilly, Menarini, MSD, Novartis, Odone, Periphagen, Pfizer, Roche, Seattle Genetics, research grants from AstraZeneca, Lilly, MEDSD, Novartis, Pfizer, Radius, Roche-Genentech, Servier and Synthon (to the institution) outside the submitted work. EdA received honoraria and/or advisory board from Roche/GNE, Novartis, Seattle Genetics and Zodiac, travel grants from Roche/GNE and GSK/Novartis, research grants to his institution from Roche/GNE, Astra-Zeneca, GSK/Novartis and Servier outside the submitted work; his institution has received research grants for the conduct of ALTTO.
Ethics approval: The ALTTO trial was approved by the Ethics Committees/Independent Review Boards of participating institutions and all patients provided written informed consent before inclusion in the study. The study was performed in accordance with the Declaration of Helsinki. The current exploratory analysis received approval by the ALTTO Executive and Steering Committees.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon reasonable request. Data and results are available at the BrEAST Data Centre at Institut Jules Bordet in Brussels (Belgium) and can be made available upon approval of a research proposal.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
References
- 1. Cardoso F, Senkus E, Costa A, et al. 4th ESO–ESMO International Consensus guidelines for advanced breast cancer (ABC 4). Ann Oncol 2018;29:1634–57. 10.1093/annonc/mdy192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Buzdar AU, Legha SS, Hortobagyi GN, et al. Management of breast cancer patients failing adjuvant chemotherapy with adriamycin-containing regimens. Cancer 1981;47:2798–802. [DOI] [PubMed] [Google Scholar]
- 3. Valagussa P, Tancini G, Bonadonna G. Salvage treatment of patients suffering relapse after adjuvant CMF chemotherapy. Cancer 1986;58:1411–7. [DOI] [PubMed] [Google Scholar]
- 4. Swain SM, Miles D, Kim S-B, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): end-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol 2020;21:519–30. 10.1016/S1470-2045(19)30863-0 [DOI] [PubMed] [Google Scholar]
- 5. Diéras V, Miles D, Verma S, et al. Trastuzumab emtansine versus capecitabine plus lapatinib in patients with previously treated HER2-positive advanced breast cancer (EMILIA): a descriptive analysis of final overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol 2017;18:732–42. 10.1016/S1470-2045(17)30312-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Giordano SH, Temin S, Chandarlapaty S, et al. Systemic therapy for patients with advanced human epidermal growth factor receptor 2–Positive breast cancer: ASCO clinical practice guideline update. J Clin Oncol 2018;36:2736–40. 10.1200/JCO.2018.79.2697 [DOI] [PubMed] [Google Scholar]
- 7. Lambertini M, Vaz-Luis I. Is HER2-positive metastatic breast cancer still an incurable disease? Lancet Oncol 2020;21:471–2. 10.1016/S1470-2045(20)30058-9 [DOI] [PubMed] [Google Scholar]
- 8. Metzger-Filho O, de Azambuja E, Procter M, et al. Trastuzumab re-treatment following adjuvant trastuzumab and the importance of distant disease-free interval: the HERA trial experience. Breast Cancer Res Treat 2016;155:127–32. 10.1007/s10549-015-3656-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Piccart-Gebhart M, Holmes E, Baselga J, et al. Adjuvant lapatinib and trastuzumab for early human epidermal growth factor receptor 2–Positive breast cancer: results from the randomized phase III adjuvant lapatinib and/or trastuzumab treatment optimization trial. J Clin Oncol 2016;34:1034–42. 10.1200/JCO.2015.62.1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wolff AC, Hammond MEH, Schwartz JN, et al. American Society of clinical Oncology/College of American pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol 2007;26:118–45. 10.1200/JCO.2006.09.2775 [DOI] [PubMed] [Google Scholar]
- 11. Moreno-Aspitia A, Holmes EM, Jackisch C, et al. Updated results from the phase III ALTTO trial (big 2-06; NCCTG (Alliance) N063D) comparing one year of anti-HER2 therapy with lapatinib alone (L), trastuzumab alone (T), their sequence (T→L) or their combination (L+T) in the adjuvant treatment of HER2-positive early breast cancer. J Clin Oncol 2017;35(suppl; abstr 502. [Google Scholar]
- 12. Lambertini M, Ferreira AR, Poggio F, et al. Patterns of care and clinical outcomes of first‐line trastuzumab‐based therapy in HER2‐positive metastatic breast cancer patients relapsing after (Neo)adjuvant trastuzumab: an Italian multicenter retrospective cohort study. Oncologist 2015;20:880–9. 10.1634/theoncologist.2015-0020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vaz-Luis I, Winer EP, Lin NU. Human epidermal growth factor receptor-2-positive breast cancer: does estrogen receptor status define two distinct subtypes? Ann Oncol 2013;24:283–91. 10.1093/annonc/mds286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vaz-Luis I, Seah D, Olson EM, et al. Clinicopathological features among patients with advanced human epidermal growth Factor–2-Positive breast cancer with prolonged clinical benefit to first-line trastuzumab-based therapy: a retrospective cohort study. Clin Breast Cancer 2013;13:254–63. 10.1016/j.clbc.2013.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yardley DA, Tripathy D, Brufsky AM, et al. Long-term survivor characteristics in HER2-positive metastatic breast cancer from registHER. Br J Cancer 2014;110:2756–64. 10.1038/bjc.2014.174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yang H-Y, Ma D, Liu Y-R, et al. Impact of hormone receptor status and distant recurrence-free interval on survival benefits from trastuzumab in HER2-positive metastatic breast cancer. Sci Rep 2017;7:1134 10.1038/s41598-017-00663-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lambertini M, Campbell C, Gelber RD, et al. Dissecting the effect of hormone receptor status in patients with HER2-positive early breast cancer: exploratory analysis from the ALTTO (big 2-06) randomized clinical trial. Breast Cancer Res Treat 2019;177:103–14. 10.1007/s10549-019-05284-y [DOI] [PubMed] [Google Scholar]
- 18. Cobleigh M, Yardley DA, Brufsky AM, et al. Baseline characteristics, treatment patterns, and outcomes in patients with HER2-positive metastatic breast cancer by hormone receptor status from SystHERs. Clin Cancer Res 2020;26:1105–13. 10.1158/1078-0432.CCR-19-2350 [DOI] [PubMed] [Google Scholar]
- 19. Krell J, James CR, Shah D, et al. Human epidermal growth factor receptor 2–Positive breast cancer relapsing Post-Adjuvant trastuzumab: pattern of recurrence, treatment and outcome. Clin Breast Cancer 2011;11:153–60. 10.1016/j.clbc.2011.03.012 [DOI] [PubMed] [Google Scholar]
- 20. Láng I, Bell R, Feng FY, et al. Trastuzumab retreatment after relapse on adjuvant trastuzumab therapy for human epidermal growth factor receptor 2-positive breast cancer: final results of the retreatment after Herceptin adjuvant trial. Clin Oncol 2014;26:81–9. 10.1016/j.clon.2013.08.011 [DOI] [PubMed] [Google Scholar]
- 21. Murthy RK, Varma A, Mishra P, et al. Effect of adjuvant/neoadjuvant trastuzumab on clinical outcomes in patients with HER2-positive metastatic breast cancer. Cancer 2014;120:1932–8. 10.1002/cncr.28689 [DOI] [PubMed] [Google Scholar]
- 22. Negri E, Zambelli A, Franchi M, et al. Effectiveness of trastuzumab in First‐Line HER2+ metastatic breast cancer after failure in adjuvant setting: a controlled cohort study. Oncologist 2014;19:1209–15. 10.1634/theoncologist.2014-0227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Blondeaux E, Ferreira AR, Poggio F, et al. Clinical outcomes of patients with breast cancer relapsing after (neo)adjuvant trastuzumab and receiving trastuzumab rechallenge or lapatinib-based therapy: a multicentre retrospective cohort study. ESMO Open 2020;5:e000719. 10.1136/esmoopen-2020-000719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yamashiro H, Sawaki M, Masuda N, et al. Survival outcomes of retreatment with trastuzumab and cytotoxic chemotherapy for HER2-positive recurrent patients with breast cancer who had been treated with Neo/adjuvant trastuzumab plus multidrug chemotherapy: a Japanese multicenter observational study. Breast Cancer 2018;12:117822341878624 10.1177/1178223418786243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Daniels B, Kiely BE, Houssami N, et al. Survival outcomes for Australian women receiving trastuzumab for HER2-positive metastatic breast cancer following (neo)adjuvant trastuzumab: a national population-based observational study (2006-2014). Br J Cancer 2018;118:441–7. 10.1038/bjc.2017.405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tripathy D, Rugo HS, Kaufman PA, et al. The SystHERs registry: an observational cohort study of treatment patterns and outcomes in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. BMC Cancer 2014;14:307. 10.1186/1471-2407-14-307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hurvitz SA, O'Shaughnessy J, Mason G, et al. Central nervous system metastasis in patients with HER2-positive metastatic breast cancer: patient characteristics, treatment, and survival from SystHERs. Clin Cancer Res 2019;25:2433–41. 10.1158/1078-0432.CCR-18-2366 [DOI] [PubMed] [Google Scholar]
- 28. Tripathy D, Brufsky A, Cobleigh M, et al. De novo versus recurrent HER2-positive metastatic breast cancer: patient characteristics, treatment, and survival from the SystHERs registry. Oncologist 2020;25:e214–22. 10.1634/theoncologist.2019-0446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Conte B, Fabi A, Poggio F, et al. T-DM1 efficacy in patients with HER2-positive metastatic breast cancer progressing after a taxane plus pertuzumab and trastuzumab: an Italian multicenter observational study. Clin Breast Cancer 2020;20:e181–7. 10.1016/j.clbc.2019.09.001 [DOI] [PubMed] [Google Scholar]
- 30. Kourie HR, El Rassy E, Clatot F, et al. Emerging treatments for HER2-positive early-stage breast cancer: focus on neratinib. Onco Targets Ther 2017;10:3363–72. 10.2147/OTT.S122397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Brandão M, Pondé NF, Poggio F, et al. Combination therapies for the treatment of HER2-positive breast cancer: current and future prospects. Expert Rev Anticancer Ther 2018;18:629–49. 10.1080/14737140.2018.1477596 [DOI] [PubMed] [Google Scholar]
- 32. Eiger D, Pondé NF, de Azambuja E. Pertuzumab in HER2-positive early breast cancer: current use and perspectives. Future Oncol 2019;15:1823–43. 10.2217/fon-2018-0896 [DOI] [PubMed] [Google Scholar]
- 33. Pusztai L, Foldi J, Dhawan A, et al. Changing frameworks in treatment sequencing of triple-negative and HER2-positive, early-stage breast cancers. Lancet Oncol 2019;20:e390–6. 10.1016/S1470-2045(19)30158-5 [DOI] [PubMed] [Google Scholar]
- 34. Arecco L, Blondeaux E, Damassi A, et al. Moving trastuzumab emtansine (T-DM1) to the early setting of breast cancer treatment. Ann Palliat Med 2020;9:512–6. 10.21037/apm.2020.01.09 [DOI] [PubMed] [Google Scholar]
- 35. Gobbini E, Ezzalfani M, Dieras V, et al. Time trends of overall survival among metastatic breast cancer patients in the real-life ESME cohort. Eur J Cancer 2018;96:17–24. 10.1016/j.ejca.2018.03.015 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
esmoopen-2020-000979supp001.pdf (312.8KB, pdf)
esmoopen-2020-000979supp002.pdf (89.1KB, pdf)