Abstract
The most common and malignant primary brain tumor in adults is glioblastoma (GBM). In vitro 3D brain models are needed to better understand the pathological processes underlying GBM and ultimately develop more efficient antineoplastic agents. Here, we describe the bioprinting methods that have been used to fabricate volumetric GBM models. We explain several factors that should be considered for 3D bioprinting, including bioinks, cells and construct designs, in relation to GBM modeling. Although 3D-bioprinted brain models are still to be improved, they have the potential to become a powerful tool for drug screening.
Keywords: : 3D bioprinting, bioinks, drug screening, glioblastoma, neurological disease models
Background
The most common and malignant primary brain tumor in adults is glioblastoma (GBM), also classified by the WHO as grade IV astrocytoma [1]. The incidence among adults aged greater than or equal to 65 is as high as 10.6 per 100,000, while the overall incidence in adults is about 3.2 in 100,000 [2]. The response to therapeutic agents and treatment outcomes of patients with GBM is still challenging, even with recent improvements in the field in research [3], surgery [4,5], radiation [6] and chemotherapy [5,6]. The prognosis of GBM is very poor; with treatment (resection, radiotherapy and chemotherapy), the median survival is only 15 months [1,7]. Therefore, improved tools are needed to study GBM and its intrinsic resistance to radio- and chemotherapeutics for a better management of patients [8]. Different cell responses between 2D and 3D cancer models have been shown in protein and gene expressions, cell signaling, migration, morphology, proliferation, viability and drug responses [9]. This might be the reason why most anticancer drugs are effective in vitro but exhibit low therapeutic efficacies in clinical trials [10]. This fact was recently illustrated by Lee et al. when they compared responses to a novel molecular treatment between their 3D-bioprinted constructs [11] and their 2D culture model [12]. The treatment consisted of a cocktail of 10 μM of forskolin, 10 μM of ISX9, 3 μM of CHIR99021, 2 μM of IBET 151 and 5 μM of DAPT (FICBD). Their previous 2D culture model showed that the FICBD cocktail could reprogram GBM cells to early neurons at a high rate [12]. However, in their recently published 3D model, cells treated with this same cocktail did not show elongated neurites as previously reported in 2D culture, neither were they impaired in their ability to form spheroids with cancer stem cells. Their previously published 2D culture model also reported that FICBD-treated cells had a significant increase in the expression of early neuronal markers such as TUJ1 and DCX when compared with control cells [12]; in contrast, the 3D-bioprinted model exhibited no significant differences in TUJ1 or DCX expressions when compared with untreated samples [11]. These results suggested that drugs in development could benefit substantially from optimizations in 3D-bioprinted GBM models before moving onto animal or clinical trials.
To this end, 3D bioprinting is an enabling method to generate volumetric, biomimetic microenvironments emulating the central nervous system [13]. 3D bioprinting offers relatively fast fabrication of living tissue constructs of diverse 3D topologies and rapid freedom prototyping capabilities [14] and exceptional versatility in cell positioning [15]. These features of 3D bioprinting can help achieve intercellular communication, as well as proper transport of bioactive molecules and therapeutic agents [16]. As such, the neuronal microenvironments may be controlled more precisely with the 3D bioprinting methods [17] than the traditional 2D cultures [18] by formulating bioinks specific for neuronal tissues and spatially patterning relevant cell types and structural properties, to better reproduce the GBM characteristics in vitro.
Disease processes can be better understood and new therapies may be developed using 3D models of the brain tumor. Individual patients will also benefit through the identification of therapeutic targets that confer maximum efficacy based on systemic and genetic signatures specific to an individual's cancer.
This article will present the in vivo molecular and phenotypical characteristics of the GBM microenvironment sought to be replicated and the bioprinting techniques that are currently used in producing 3D GBM models. It continues to describe some of the most important 3D-bioprinted GBM models that show potential to study angiogenesis, GBM biology and identify suitable chemotherapeutics to treat GBM tumors resistant to standard first-line therapy. This article also emphasizes a number of considerations ought to be taken into account when constructing 3D-bioprinted GBM models; such as choosing the right bioinks, cellular components and mechanical properties. We finalize this article by providing several ideas for future endeavors regarding 3D-bioprinted GBM models that will contribute to our understanding of not only tumor etiology, but also progression and resistance to therapy.
Glioblastoma
GBM usually appears in two clinical situations: the most frequent as a newly occurring disease in elderly adults (primary GBM) and less frequently in young patients as a lesser degree of astrocytoma progression (secondary GBM) [1]. The Cancer Genome Atlas Network has identified patterns of molecular alteration in GBM that allow these tumors to be classified into four molecular subtypes: classical, proneural, neural and mesenchymal [19].
The classical subtype represents the majority of primary GBM and is characterized by mutations of the suppressor gene of PTEN tumors, deletions of chromosome 10 and amplification of the EGFR oncogene [1,19]. Also, frequent are the focal deletions of chromosome 9p21, which determine a deletion of the tumor suppressor gene CDKN2A that encodes two tumor suppressors, p16/INK4a and p14ARF, which increase the activity of RB and p53. The proneural type, which is the most frequent type in secondary GBM, is characterized by TP53 mutations and point mutations of the isocitrate dehydrogenase genes (IDH1 and IDH2) [1,19]. Proneural GBM commonly also shows overexpression of the platelet-derived growth factor alpha receptor (PDGFRA). The neural type is characterized by higher levels of expression of the neural markers, such as NEFL, GABRA1, SYT1 and SLC12A5 [1,19]. The mesenchymal type is characterized by deletions of the NF1 gene on chromosome 17 and a lower expression of the NF1 protein [1,19]. The genes involved in the TNF and NF-κB pathway are highly expressed in mesenchymal GBM.
There are three key components of the native GBM microenvironment. The first is a primarily biochemical component, the brain extracellular matrix (ECM)-like microenvironment [20]. The second and third are two biophysical components, the cancerous mass with its anatomical regions surrounded by the vascularized stroma and the oxygen gradient [20]. GBM is anatomically divided in the core, intermediate and peripheral anatomical regions [20]. In the core region, a necrotizing zone is formed with pseudopalisading cells due to severe hypoxia. In the intermediate region, there is excessive proliferation of anaplastic cells that secrete cytokines to survive. In the peripheral region is where invasion and hyperplasia of microvessels occur.
The 3D microenvironment achieved through 3D bioprinting likely allows cells to maintain their intrinsic characteristics and functions, such as cell–cell and cell–ECM interactions, spatiotemporal signaling, metabolic gradients and mechanical restriction [21]. Also of utmost importance is the capability of 3D-bioprinted models to reflect the in vivo malignant progression, for example, hypoxia or necrosis, stem cell niche properties, slow proliferation and drug resistance of cancer cells [22]. Furthermore, essential differences between 2D and 3D cancer models have been observed such as protein and gene expressions, cell signaling, migration, morphology, proliferation, viability, organization and drug response [9]. This could explain the effective in vitro, but low therapeutically efficacy reported in clinical trials [10]. Bioprinting can especially be used to create geometrically sophisticated volumetric tissues containing viable and relevant cells and it is also a method with relatively low cost, higher throughput and precise reproducibility [9]. 3D bioprinting has offered a broad range of applications in disease modeling, pharmaceutical research and cancer research [9,23].
Bioprinting techniques currently used in producing 3D GBM models
The bioprinting strategies currently used to produce 3D GBM models have almost solely relied on extrusion bioprinting. This bioprinting technique applies pneumatic, piston- or screw-based mechanisms to achieve a continuous deposition of the bioink in defined patterns in space [9]. The primary issue with this method is the shear stress exerted on the cells during extrusion, because this system applies pressure to deposit the bioink through a nozzle [24], although such a limitation might be mitigated through the adoption of shear-thinning bioinks [25]. The adoption of additional bioprinting approaches, such as those based on inkjet, light and others [13,26,27], remains to be explored.
GBM modeling through 3D bioprinting
To ensure that therapeutic responses are correctly identified for individual patients, researchers are aiming to develop patient-specific cancer models that reflect their original identity along with its multifactorial complexity [28]. Yi et al. developed an extrusion-based 3D-bioprinted GBM model consisting of patient-derived tumor cells, vascular endothelial cells and decellularized ECM from the brain tissue in a compartmentalized cancer-stroma concentric ring structure that sustains a radial oxygen gradient (Figure 1) [20]. This bioprinted GBM-on-a-chip reflected biochemical and biophysical properties of the tumor microenvironment (TME): the heterogeneous composition of the ECM, the oxygen gradient leading to central hypoxia and the mass of cancerous tissue surrounded by dysfunctional microvessels. A bioink solution was developed to bioprint the GBM-on-a-chip and it was composed of patient-specific GBM-15, -26, -278, -211, -28 and -51 cells obtained during brain tumor removal surgeries before receiving chemoradiation for them to be compared with GBM-37 and -103 cells obtained from the tumors after chemoradiation; in addition, there were human umbilical vein endothelial cells embedded in brain decellularized ECM and collagen hydrogels. This model reproduced patient-specific resistances to treatment with radiation and temozolomide (TMZ). The results demonstrated that the model could be potentially used to determine drug combinations associated with more efficient tumor ablation and it might help identify superior treatments for GBM patients resistant to standard first-line treatment.
Figure 1. . 3D-bioprinted GBM-on-a-chip reproduced patient responses to chemoradiotherapy.

(A) Pathological features of the GBM. (B) The illustration showing the process to fabricate the GBM-on-a-chip with GBM cells and endothelial cells in the BdECM-based bioinks. (C) GBM cells (red) and endothelial cells (green) in the GBM-on-a-chip; scale bars: 500 μm. (D) Recapitulating patient pathological features in the GBM-on-a-chip by bioprinting GBM-211 cells with the SP+GR+ conditions (SP+ indicates compartmentalized cells, GR+ indicates presence of an oxygen gradient). (E) Treating patient-specific GBM-on-a-chips with CCRT using different drug combinations.
BdECM: Brain decellularized extracellular matrix; CCRT: Concurrent chemoradiation; GBM: Glioblastoma.
Reproduced with permission from [20] © Springer Nature Customer Service Centre GmbH (2019).
The fact that glioma-associated macrophages (GAMs) support tumor progression, invasion and angiogenesis [29] has been of great interest in GBM treatment. Preclinical models have shown that the inhibition between cancer cells and macrophages can delay GBM progression. Through extrusion 3D bioprinting, we constructed a miniaturized brain model comprising macrophages and incorporating a well-defined mass of GBM cells [30]. The goal was to study phenotypic alterations in macrophages and cancer cells resulting from this interaction (Figure 2) [30]. This model was constructed using a two-step bioprinting process. First, the mini-brain was bioprinted using a bioink encapsulating mouse macrophages (RAW264.7). During the second step, the cavity was filled with mouse GBM cells (GL261) embedded into the bioink, where the construct was subsequently photocrosslinked. In both cases, the bioink consisted of a mixture of gelatin methacryloyl (GelMA) and gelatin. This model can decouple the specific interactions between macrophages and GBM cells, because it excludes the influence from other TME components. Indeed, the interactions between macrophages and GBM cells resulted in overexpression of GAM-specific markers and markers for matrix remodeling. It is also important to note that GBM cells attained a migratory phenotype. The correlations of markers that are linked to poor patient survival demonstrated that the expressions of genes in this model were of profound clinical relevance. Finally, carbomustine (BCNU), AS1517499 and BLZ945 were used to demonstrate the suitability of this model for drug screening. This bioprinted model can be used to investigate the effects of immunomodulatory and chemotherapeutic agents as well as facilitate treatment strategies.
Figure 2. . 3D-bioprinted mini-brain consisting of macrophages and GBM cells to study their interactions and evaluate therapeutics.
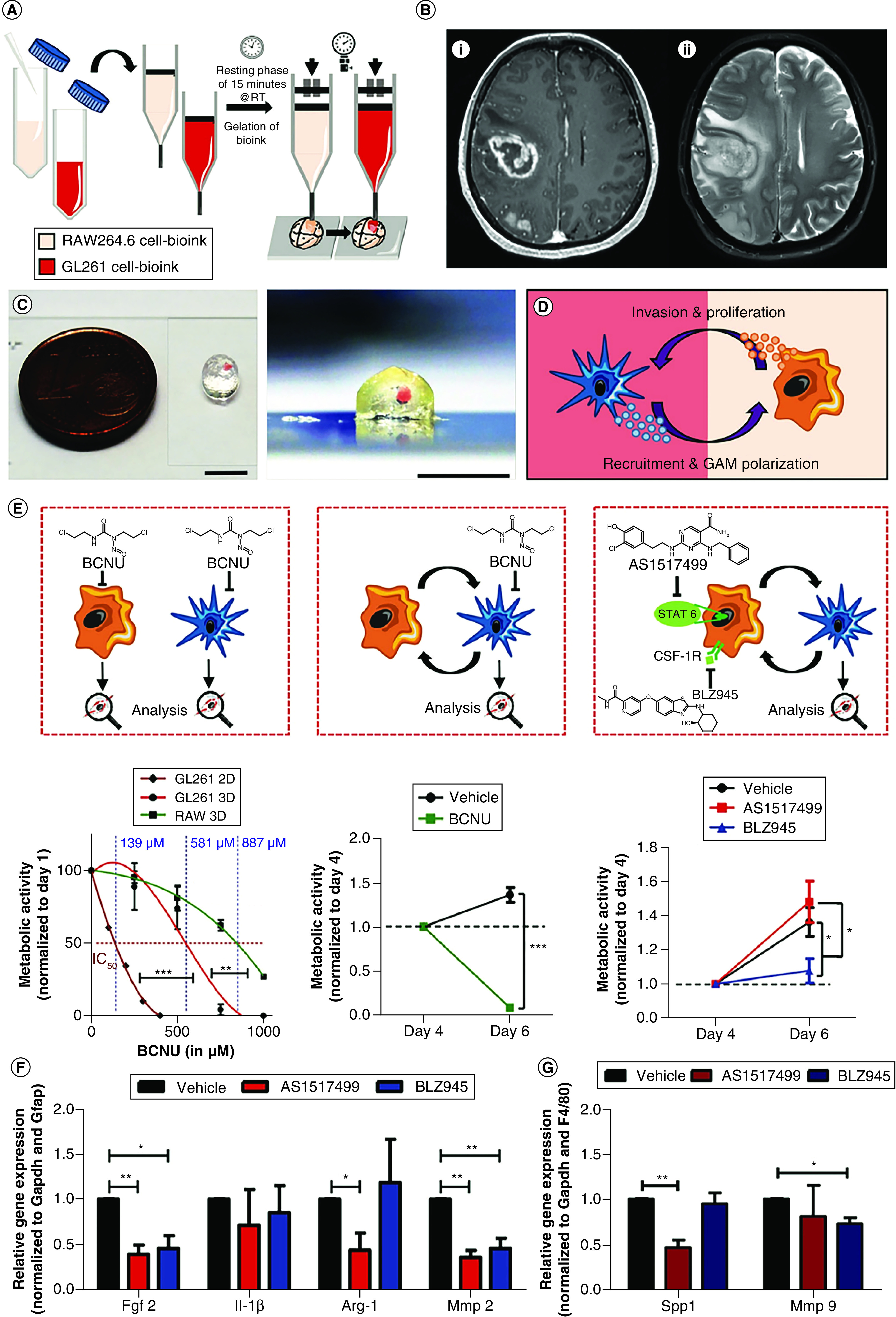
(A) The schematic showing the two-steps bioprinting process of two types of GelMA-gelatin bioinks. (B) A GBM is shown in (i) this axial T1-weighted post contrast right-side frontal mass measuring 4.1 × 3.6 × 3.3 cm3 and a second bilobed lesion in the right parietal lobe, found in surgery to be multifocal GBM multiforme; (ii) axial T2-weighted-fluid-attenuated inversion recovery shows extensive vasogenic edema surrounding the lesions. (C) Photos of bioprinted mini-brains in top and side views; scale bar: 5 mm. (D) The scheme of the interaction between GBM cells and GAMs. (E) Treatment effects of immuno- and chemo-therapeutic drugs in the bioprinted mini-brain. (F–G) Elected gene expressions in (F) GAMs or (G) GBM cells after treating with different drugs in the bioprinted models (A, C–G).
GAM: Glioma-associated macrophage; GBM: Glioblastoma; GelMA: Gelatin methacryloyl.
Reproduced with permission from [30] © John Wiley & Sons (2019); permission conveyed through Copyright Clearance Center, Inc.
The intrinsic resistance of glioma stem cell biology to chemotherapeutics has arisen the demand for models that will help achieve better management of the patients. Dai et al. constructed an extrusion-based 3D-bioprinted glioma stem cell model using a bioink encapsulating a glioma stem cell (GSC) line (SU3) or glioma cells (U87), transglutaminase, gelatin, sodium alginate and fibrinogen [10]. This model showed that the bioprinted GSCs maintain their inherent characteristics (nestin as a marker) but showed differentiation and vascularization potential (glial fibrillary acidic protein and β-tubulin III as markers). Tumor angiogenesis biomarker and VEGF were detected by immunohistochemistry to verify the vascularization potential of GSCs, where there was an increase in expression from week 1 to 3. Drug screening tests revealed that the 3D-bioprinted model was more resistant to TMZ than the 2D monolayer model. In comparison to 2D-cultured cells, 3D-bioprinted GSCs proliferated slower at the first several days, but the growth rate increased gradually, which lasted for 3 weeks in vitro [10]. This result demonstrates that 3D bioprinting might facilitate long-term in vitro cell culture with high cell viability. This model could provide an alternative to study angiogenesis, GSC biology, drug resistance and anticancer drug susceptibility in vitro.
An in vitro model to study the mechanism of GBM invasion is also necessary to develop more efficient treatments for patients with GBM. To this end, Ozturk et al. used sacrificial extrusion bioprinting to create two fluidic vascular channels; a GBM spheroid was placed in between these channels [31]. Next, human umbilical vein endothelial cells (HUVECs) in suspension were injected into the channels to create cell lining on the inner channel surface [31]. Collagen layers were also bioprinted at the top and bottom of these structures. After this, tumor cell responses to drug treatment were compared in 2D monolayer culture (Figure 3A–B) [31] versus 3D spheroid in suspension culture (Figure 3C & D) [31]. It was found that the overgrowth of cells was a mechanism of resistance to the long-term TMZ treatment. The 2D-cultured GBM cells formed a densely populated cell monolayer, while the 3D spheroids irregularly created satellite cell clumps. Furthermore, decreased metabolic activity of GBM cells was observed under both cell culture conditions with higher TMZ dose over time. The suspended 3D spheroids (Figure 3C) [31] showed greater decrease in metabolic activity than the 2D monolayers after 21 days of long-term TMZ treatment (Figure 3A), while also exposing a shrinkage of the tumor mass (Figure 3D) [31]. The regrowth of GBM cells was also shown in the 3D-bioprinted model (Figure 3E). Invasion from the embedded GBM 3D spheroids took place within the first week after fabrication (Figure 3E, day 6) [31]. During the first 3–5 weeks, the GBM cells travelled 1–2 mm from the spheroids, invading the surrounding matrix (Figure 3E, day 26) [31]. On day 26, drug treatment started with perfusion of 100 μM of TMZ. A slight decrease in the fluorescent intensity and the regression of individual tumor cells in the invasion area was observed during the first 3 weeks of subsequent drug treatment (Figure 3E, 14 days after drug treatment) [31]. Despite this, some GBM cells survived the treatment, and they resumed matrix invasion and proliferation even with continuing drug administration (Figure 3E, 31 days after drug treatment) [31].
Figure 3. . Drug response seen in GBM cells cultured in different settings.

(A) Graphics show AlamarBlue test results for metabolic activities of tumor cells in the 2D monolayer culture. (B) Overlay of phase-contract and fluorescence images of GBM cells cultured in 2D monolayers. (C) Graphics show AlamarBlue results for metabolic activities of tumor cells in the 3D spheroid culture. (D) Overlay of phase-contrast and fluorescence images of GBM cells cultured in 3D spheroids. (E) Invasion behavior of the GBM cells. On day 26, cells in the embedded spheroids aggressively invaded into the surrounding matrix and the vasculature and drug treatment was started. Then, 14 days after drug treatment was started, the cells regressed and shrinkage of the tumor core was observed. However, 31 days after drug treatment, it was observed that some GBM cells survived the treatment and resumed active invasion even with ongoing treatment.
EC: Endothelial cell; GBM: Glioblastoma; TMZ: Temozolomide.
Reproduced with permission from [31] © the American Association for the Advancement of Science on an Attribution 4.0 International (CC-BY 4.0) license (2020).
Considerations in 3D bioprinting for modeling glioblastoma
Choosing the bioinks
Choosing the right bioink is important because it makes up a significant proportion of the model, which also impacts printability as well as subsequent cell growth and functions [32]. The main properties of a bioink include viscosity, gelation (structural stabilization) and bioactivity [33]. There are two major classes of hydrogels: synthetic and natural. Natural hydrogels have excellent biocompatibility, while synthetic hydrogels possess defined properties that can easily be manipulated. A rational combination of both biomaterials is perhaps the best to simultaneously recapitulate biological behaviors as well as functionality. Furthermore, the creation of printable bioinks with proteoglycans from the lectican family and their binding partners hyaluronan, link proteins and tenascins [34] – found in the ECM within the CNS – is also an emerging alternative to improve the biomimetic properties of the bioprinted models. Some of the bioinks used to bioprint 3D brain tumor models are shown in Table 1. Even if 3D bioprinting is proven effective to replicate the complex neural microenvironments through concurrent manipulation of multiple materials and cell types, there is still significant room for the development of GBM-specific bioinks, which is oftentimes also patient-specific.
Table 1. . Bioinks and cells used for 3D bioprinting of glioblastoma models.
| Bioink | Cell | Method | Ref. |
|---|---|---|---|
| GelMA and gelatin | Macrophages cell line (RAW264.7), mouse GBM cells (GL261) | Extrusion | [30] |
| BdECM | Human GBM cells (-15, -26, -278, -211, -28, -51, -37 and -103), HUVECs, | Extrusion | [20] |
| Gelatin, TG, sodium alginate and fibrinogen | GSCs cell line (SU3), human GBM cell line (U87) | Extrusion | [10] |
| Gelatin, collagen type I | HUVECs, human GBM (isocitrate dehydrogenase-wild type) cells | Extrusion | [31] |
BdECM: Brain decellularized extracellular matrix; GBM: Glioblastoma; GelMA: Gelatin methacryloyl; GSC: Glioma stem cell; HUVEC: Human umbilical vein endothelial cell; TG: Transglutaminase.
Cellular components
Brain functions rely heavily on spatial arrangements of its cells and their interactions [35]. It is important to respect spatial distribution and consider cell co-cultures when fabricating neuronal tissues. Within the GBM microenvironment, interactions between cancer cells and other cells give place to tumor growth, migration and metastasis [29]. For instance, macrophages are recruited and polarized by cancer cells toward GAMs, which contribute to tumor progression, invasion and angiogenesis [36]. Another key feature of GBM is its high degree of vascularization [37]. The uncontrolled vascular growth observed in GBM is essential for the invasion and progression of the tumor [38]. A subpopulation of GSCs can trans-differentiate into endothelial cells to promote tumor angiogenesis through the secretion of VEGF [39]. The biological behaviors of cancer cells are in addition, influenced by the ECM that surrounds them [36]. One of the major benefits obtained through bioprinting include the precisely controlled distribution of biomaterials, cells and cytokines in 3D structures to maintain the physiologically and pathologically relevant bioactivity of cells [40]. Through the patterned positioning of these cells we can continue to expand our understanding of 3D neuronal network formation. A few different 3D models explored the interactions between immunological, vascular and glioma cells to discern their roles in GBM (Table 1).
Mechanical properties
Cell behaviors are greatly affected by the mechanical properties of the matrices; thus, the rheological properties, swelling characteristics and shape retention of potential bioinks must be taken into consideration altogether [41]. It has been shown that the stiffness of the matrix influences cell signaling, growth, survival and motility [41]. Soft hydrogels with low interfacial tension would ideally mimic the stiffness of the brain tissue, which is approximately of 0.5 kPa [42]. The desired stiffness can be achieved by combining natural and synthetic materials, tuning the extent of light exposure in photocrosslinkable bioinks and by controlling the amount of crosslinking agent in forming the hydrogels. For instance, Wang et al. observed that changes in matrix stiffness play a role in GBM cell differentiation, spreading and gene expression [43]. There was decreased GBM cell proliferation within stiffer hydrogels in 3D. This could be explained by the fact that cells present an increased retractive force in a stiffer matrix environment with a higher crosslinking density per volume [43]. Cancer cells in a soft environment have fewer crosslinks to cleave with matrix metalloproteinases (MMPs), which are important enzymes involved in tumor growth, migration and ECM remodeling. Their data showed that there was an enhanced MMP-9 expression in soft hydrogels (1 kPa), whereas increased matrix stiffness (26 kPa) led to upregulation of MMP-1 [43]. Hydrogel stiffness also had effects on gene expression; hyaluronic acid synthase 1 was upregulated in stiff hydrogels, whereas an increase in hyaluronic acid synthase 2 gene expression was present in soft hydrogels [43]. This suggested that varying the matrix stiffness could lead to differential ECM deposition and remodeling by employing different hyaluronic acid synthases or MMPs. In addition, there was an upregulation of proteins from mechanosensing pathways, such as RhoA and ROCK1, in stiff hydrogels, which might explain the spindle-like morphology with longer actin protrusions observed in cells, in contrast to cell morphology in soft hydrogels, which displayed fewer and shorter protrusions [43]. However, when culturing cells on 2D hydrogels, Ulrich et al. demonstrated enhanced GBM cell proliferation with increased hydrogel stiffness [44]. Cell-niche interactions might explain the difference in cell response when cultured in 2D versus 3D and therefore dimensionality is an important factor to consider in models. Engineered GBM models offer the opportunity to precisely dissect mechanisms of GBM progression, accelerate clinical testing and provide a platform for precision medicine. However, validation strategies need to be standardized to ensure that in vitro discoveries are predictive and of clinical relevance. Compositional and mechanical parameters of a model need to match those of the brain to make the model predictive of in vivo behaviors [45]. Additionally, cell migration, morphologies, relative gene expressions and chemosensitivity should be similar to the in vivo phenotype; as well as verification that the tumor progression has similar underlying biochemical mechanisms that govern drug resistance [45].
Hydrogels can be designed to degrade over the course of days or weeks and consequently most of the time these matrices are intended to serve a temporary purpose. It has been demonstrated that tensile strength and stiffness change over the incubation period in hydrogels with no cells, whereas these changes can be expedited, reduced or eliminated in scaffolds with cells [46]. It is important not to think of mechanical properties as a static property, since it is a function of culture time as the cells remodel their surroundings.
Future perspective
3D-bioprinted models provide the environmental cues that drive the pathological progression of cancer. Therefore, they are capable of reproducing the treatment responses of patients while at the same time indicating the optimal treatment regimens specific to an individual [20]. These abilities provided by the 3D bioprinting technologies allow for the creation of refined models of patient-specific cancer ecology, more specifically through the incorporation of robust heterogeneities in the cells, ECM components and the anatomically distinct regions associated with GBM [47].
All types of breakthroughs are anticipated in studying tumorigenesis, tumor heterogeneity, invasion and metastasis mechanisms, as well as identification of sensitivity to specific antineoplastic drugs, due to the precise patterning achieved through 3D bioprinting of human-based volumetric models, which can most accurately mimic the in vivo TME compared with 2D models, or more easily decouple these components compared with animal models [9,23]. 3D cancer models can also provide a tool to study multiple unknown regulatory feedback mechanisms between tumor and stromal cells [48]. For brain tumors, chemotherapy drug resistance is a great challenge; this resistance is primarily associated with GSCs [49]. The key solution to drug resistance and tumor recurrence is research on brain tumor stem cell biology. Ultimately, tumors in patients can perhaps be effectively replicated in vitro and individualized tumor can be tested for pretreatments of antineoplastic drugs. The final objective is to realize individualized accurate treatment of cancer [50]. Given the fact that 3D bioprinting allows the establishment of a relatively large number of models in a reasonable timeframe, it would be feasible to conduct point-of-care testing in a clinical setting in the future [20]. The extremely high lethality of GBM requires urgent and appropriate treatment, thus requiring fast production of GBM models that would offer a remarkable advantage. It is hoped that future cancer models will eventually help guide clinical decisions [20].
Neural tissue engineering will continue to advance with future developments in the 3D bioprinting technologies well-beyond those based on extrusion bioprinting, but also various other methods such as inkjet and light-based bioprinting strategies [13]. For example, stereolithography is amenable to vascular integration of 3D GBM models; high-resolution vascular patterns could be bioprinted to model the disordered tumor vascularization with potential multi-material capacity [51].
One can easily envision a future where cells are collected from patients with neurological diseases including GBM and then bioprinted as in vitro tissue models, which replicate the structure of their in vivo counterparts and the genetics of the corresponding patients [52]. Clinicians and researchers could study the mechanism of disease progression, design and test therapies in a higher throughput and efficient way through these human-based volumetric in vitro platforms.
Each anatomical area of the GBM tumor has different tissue stiffness, cellular composition and microenvironment [20]. Therefore, we foresee the importance of developing primarily three different models; each of them focusing on one anatomical area to understand tumor etiology, progression and resistance to therapy.
The first model consists of a bioprinted mini-brain with cancer cells, blood vessels and white matter tracts [20]. Currently, the effects of available anti-angiogenic therapies like VEGF inhibition have a transient clinical response due to acquired resistance [53]. Therefore, it is important to integrate proteoglycans, glycosaminoglycans, endothelial cells, astrocytes, immune cells, stem-like cells, cytokines and growth factors in the in vitro GBM models to emulate the immune-vascular, cell-matrix interactions and other clinically relevant hallmarks of the in vivo GBM microenvironment [54]. For instance, increased apoptotic resistance, proliferation and migration has been associated to ECM binding to GBM cell integrins [55]. It has been well-demonstrated that integrin αvβ3 can regulate angiogenesis and GBM progression [56]. Another mechanism by which GBM creates a proangiogenic microenvironment is through the secretion of VEGF and TGF-β1, which also suppress cytotoxic T-cell proliferation and function to hinder anti-angiogenic therapy and immunotherapy [57]. Therefore, Cui et al. engineered a 3D GBM angiogenesis model that recapitulated the tumor-associated macrophage-mediated proangiogenic and immunosuppressive niche. They discovered that the dual blockade of TGFβ-R1 and αvβ3 integrin significantly suppressed angiogenic and endothelial sprouting in their 3D GBM model, compared with the effects of current single-target anti-angiogenic GBM therapies, such as anti-VEGF (Cediranib) [58]. This 3D GBM angiogenesis model may instigate the development and screening of new therapeutic strategies that combine chemotherapy with biologic therapy such as immunotherapy and anti-angiogenic therapy. It also shows that 3D in vitro angiogenesis models are more physiologically relevant to in vivo GBM tumors than 2D in vitro capillary network formation models. Differences in pro- and anti-inflammatory signaling have been observed in 2D and 3D environments [59], which indicates a regulatory role for pro-angiogenic factor secretion [60]. Another clinical consideration is that anti-angiogenic therapeutics are delivered through the vasculature before they diffuse into the GBM tumor site, which can only be replicated in models featuring 3D vascularized TME [58]. Consequently, we can expect to find more therapeutic targets as we continue to understand the implications of each molecular pathway recapitulated through 3D models.
The second model would focus on the penumbra or the microenvironment at the periphery of the tumor, which is rich in migration and potentially the site where some of the most dangerous GSCs are present [20]. Hubert et al. found that the vast majority of proliferative activity, determined by Ki-67 staining, was localized in the peripheral rim of their GBM model [61]. This makes sense since this region has the highest levels of growth factors, oxygen and nutrients needed for proliferative activity [20]. They also investigated the relationship between SOX2 and expression of another key GSC gene, OLIG2. It was found that the overlap expression between these two genes was particularly prominent in the proliferative rim of the GBM model, while cancer stem cells in the core were more heterogenous in their expression of stem cell markers [61]. This might indicate that there are different molecular subpopulations within the GBM stem cell hierarchy and their distribution may be influenced by their microenvironment. Darmanis et al. discovered that despite the genomic heterogeneity of neoplastic cells from each individual patient, infiltrating cells (originating from the peripheral tissue) share common characteristics regardless of the patient of origin [62]. The homogenous gene signature of infiltrating cells presents a potential novel therapeutic avenue. The second model that we propose could help determine the molecular profiles of these cancer stem cell subtypes along with their influence upon each other and their non-stem GBM cell neighbors. Accordingly, combining novel therapies capable of targeting critical axes within the distinct phenotypes in future clinical trials may give place to improved outcomes in comparison to the ‘nontargeted’ therapeutic plans applied today [63].
The third model would replicate the core portion of the GBM, a hostile microenvironment that will show us how cancer cells survive and proliferate under high stress conditions [20]. Hubert et al. also found a rare population of SOX2+ cells in the hypoxic core and staining for cleaved caspase 3 revealed high apoptotic rates in the model's periphery compared with the core, indicating that the proliferative edge region had a higher cell turnover surrounded by a more stable core [61]. The recognition of a long-lived population of SOX2+ cells in the core that has retained the ability to divide but does so very infrequently was exposed by this 3D GBM model due to the fact that it could maintain greater complexity of heterogenous tumor cell population compared with other culture techniques. This breakthrough has major clinical implications because cytotoxic cancer therapies may be less effective in nonproliferative cancer cells [64]. Indeed, they demonstrated radiosensitive nonstem cells in the model's periphery and radioresistant cancer stem cells that were also growing within the model’s core [61]. All of this underscores the potential utility for developing 3D GBM models, such as the one we propose, which maintain hypoxic gradients and heterogeneity within areas of the tumor for screening assays, for the development of therapies and especially for identifying therapeutic resistance.
Due to the fact that each model will focus on a different anatomical area of the GBM, we expect each model to have different matrix stiffness and different interplay with the immune system, among others. Ultimately, these models will provide a helpful tool to uncover the genetic and phenotypic characteristics seen in recurrent GBM after being treated with chemotherapy and radiotherapy, while at the same time helping us understand what makes them different from primary GBM.
Conclusion
3D bioprinting has the potential to allow for better replication of molecular and phenotypical characteristics of GBM microenvironment. Several 3D-bioprinted GBM models were described, each of them showing a unique potential to advance our understanding of the mechanisms involved in GBM pathophysiology and resistance to specific chemotherapeutics. Furthermore, we explained how a number of variables play a pivotal role in 3D-bioprinted GBM models. The right integration of the bioink, cellular components and mechanical properties will allow to faithfully replicate GBM in vitro characteristics as seen in vivo. In the future, we envision three different GBM models; each replicating different microenvironment characteristics found in GBM to allow us to attain a better understanding of GBM growth and response to therapy.
Executive summary.
Background
Glioblastoma (GBM) is the most common primary brain tumor in adults, with the median survival only 15 months.
GBM has been classified into four molecular subtypes: classical, proneural, neural and mesenchymal
The classification for molecular subtypes might be important to determine GBM therapeutic susceptibility.
GBM is anatomically divided in core, intermediate and peripheral regions.
Each anatomical region has a different microenvironment, stiffness and interplay with the immune system.
Glioblastoma
Bioprinting techniques currently used in producing 3D glioblastoma models
The production of 3D GBM models has solely relied on extrusion bioprinting.
Other bioprinting modalities should be explored upon for creating 3D GBM models.
GBM modeling through 3D bioprinting
Several 3D-bioprinted GBM models have shown potential to study angiogenesis, glioma stem cell biology and identify superior chemotherapeutics to treat GBM tumors resistant to standard first-line therapies.
Considerations in 3D bioprinting for modeling GBM
Choosing the bioink
It is important to consider the viscosity, gelation kinetics and bioactivity of the bioink used for 3D bioprinting of GBM models.
Cellular components
Interactions between glioma stem cells, immune cells and vascular cells permit tumor growth and metastasis.
It is crucial to integrate various relevant cell types into the model.
Mechanical properties
Matrix stiffness will influence tumor cell signaling, growth, migration and survival.
The ideal matrix stiffness can possibly be achieved through the rational combination of natural and synthetic materials.
Future Perspectives
We envision the development of three different models: one focused on the migration of cells in the normal brain, a second one reproducing the microenvironment at the peripheral anatomical region of GBM and a third one modeling the core anatomical region of GBM.
We foresee that these models will shed some light on tumor etiology, progression and most importantly GBM’s resistance to therapy leading to development of better therapeutic outcomes.
Acknowledgments
We further thank D Garcia and G De Biase for providing the MRI images of the glioblastoma used in Figure 2B.
Footnotes
Financial & competing interests disclosure
YS Zhang gratefully acknowledges funding from the National Institutes of Health (K99CA201603, R00CA201603, R21EB025270, R21EB026175, R01EB028143, R01GM134036) and the Brigham Research Institute. A Quiñones-Hinojosa was supported by the Mayo Clinic Professorship, the Mayo Clinic Clinician Investigator award, the Florida Department of Health Cancer Research Chair Fund, as well as the National Institutes of Health (R43CA221490, R01CA200399, R01CA195503, R01CA216855). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Kumar V, Abbas AK, Fausto N, Aster JC. Robbins and Cotran Pathologic Basis of Disease, Professional Edition E-Book. Elsevier Health Sciences, PA, USA: (2014). [Google Scholar]
- 2.Hazzard WR, Halter JB. Hazzard's Geriatric Medicine and Gerontology (6th Edition). McGraw-Hill Education/Medical, NY, USA: (2009). [Google Scholar]
- 3.Jiang S, Eberhart CG, Lim M. et al. Identifying recurrent malignant glioma after treatment using amide proton transfer-weighted MR imaging: a validation study with image-guided stereotactic biopsy. Clin. Cancer Res. 25(2), 552–561 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mampre D, Ehresman J, Pinilla-Monsalve G. et al. Extending the resection beyond the contrast-enhancement for glioblastoma: feasibility, efficacy and outcomes. Br. J. Neurosug. 32(5), 528–535 (2018). [DOI] [PubMed] [Google Scholar]
- 5.Lu VM, Goyal A, Graffeo CS. et al. Survival benefit of maximal resection for glioblastoma reoperation in the temozolomide era: a meta-analysis. World Neurosurg. 127, 31–37 (2019). [DOI] [PubMed] [Google Scholar]
- 6.Lu VM, Kerezoudis P, Brown DA, Burns TC, Quinones-Hinojosa A, Chaichana KL. Hypofractionated versus standard radiation therapy in combination with temozolomide for glioblastoma in the elderly: a meta-analysis. J. Neurooncol. 143(2), 177–185 (2019). [DOI] [PubMed] [Google Scholar]
- 7.Cantrell JN, Waddle MR, Rotman M. et al. Progress toward long-term survivors of glioblastoma. Mayo Clin. Proc. 94(7), 1278–1286 (2019). [DOI] [PubMed] [Google Scholar]
- 8.Persano L, Rampazzo E, Basso G, Viola G. Glioblastoma cancer stem cells: role of the microenvironment and therapeutic targeting. Biochem. Pharmacol. 85(5), 612–622 (2013). [DOI] [PubMed] [Google Scholar]
- 9.Knowlton S, Onal S, Yu CH, Zhao JJ, Tasoglu S. Bioprinting for cancer research. Trends Biotechnol. 33(9), 504–513 (2015). [DOI] [PubMed] [Google Scholar]
- 10.Dai X, Ma C, Lan Q, Xu T. 3D bioprinted glioma stem cells for brain tumor model and applications of drug susceptibility. Biofabrication 8(4), 045005 (2016). [DOI] [PubMed] [Google Scholar]
- 11.Lee C, Abelseth E, de la Vega L, Willerth S. Bioprinting a novel glioblastoma tumor model using a fibrin-based bioink for drug screening. Materials Today Chemistry 12, 78–84 (2019). [Google Scholar]
- 12.Lee C, Robinson M, Willerth SM. Direct reprogramming of glioblastoma cells into neurons using small molecules. ACS Chem. Neurosci. 9(12), 3175–3185 (2018). [DOI] [PubMed] [Google Scholar]
- 13.Heinrich MA, Liu W, Jimenez A. et al. 3D bioprinting: from benches to translational applications. Small 15(23), 1805510 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang S, Leong KF, Du Z, Chua CK. The design of scaffolds for use in tissue engineering. Part II. Rapid prototyping techniques. Tissue Eng. 8(1), 1–11 (2002). [DOI] [PubMed] [Google Scholar]
- 15.Knowlton S, Cho Y, Li XJ, Khademhosseini A, Tasoglu S. Utilizing stem cells for three-dimensional neural tissue engineering. Biomater. Sci. 4(5), 768–784 (2016). [DOI] [PubMed] [Google Scholar]
- 16.Melissinaki V, Gill AA, Ortega I. et al. Direct laser writing of 3D scaffolds for neural tissue engineering applications. Biofabrication 3(4), 045005 (2011). [DOI] [PubMed] [Google Scholar]
- 17.Placone AL, McGuiggan PM, Bergles DE, Guerrero-Cazares H, Quiñones-Hinojosa A, Searson PC. Human astrocytes develop physiological morphology and remain quiescent in a novel 3D matrix. Biomaterials 42, 134–143 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levy AF, Zayats M, Guerrero-Cazares H, Quinones-Hinojosa A, Searson PC. Influence of basement membrane proteins and endothelial cell-derived factors on the morphology of human fetal-derived astrocytes in 2D. PloS One 9(3), (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Verhaak RG, Hoadley KA, Purdom E. et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR and NF1. Cancer Cell 17(1), 98–110 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yi HG, Jeong YH, Kim Y. et al. A bioprinted human-glioblastoma-on-a-chip for the identification of patient-specific responses to chemoradiotherapy. Nat. Biomed. Eng. 3(7), 509–519 (2019). [DOI] [PubMed] [Google Scholar]; •• Bioprinted glioblastoma-on-a-chip that reflected biophysicochemical properties of the tumor microenvironment and patient responses.
- 21.Hirschhaeuser F, Menne H, Dittfeld C, West J, Mueller-Klieser W, Kunz-Schughart LA. Multicellular tumor spheroids: an underestimated tool is catching up again. J. Biotechnol. 148(1), 3–15 (2010). [DOI] [PubMed] [Google Scholar]
- 22.Yang MY, Chiao MT, Lee HT. et al. An innovative three-dimensional gelatin foam culture system for improved study of glioblastoma stem cell behavior. J. Biomed. Mater. Res. B Appl. Biomater. 103(3), 618–628 (2015). [DOI] [PubMed] [Google Scholar]
- 23.Ozbolat IT, Peng W, Ozbolat V. Application areas of 3D bioprinting. Drug Discov. Today 21(8), 1257–1271 (2016). [DOI] [PubMed] [Google Scholar]
- 24.Nair K, Gandhi M, Khalil S. et al. Characterization of cell viability during bioprinting processes. Biotechnol. J. 4(8), 1168–1177 (2009). [DOI] [PubMed] [Google Scholar]
- 25.Liu W, Heinrich MA, Zhou Y. et al. Extrusion bioprinting of shear-thinning gelatin methacryloyl bioinks. Adv. Healthc. Mater. 6(12), 1601451 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moroni L, Burdick JA, Highley C. et al. Biofabrication strategies for 3D in vitro models and regenerative medicine. Nat. Rev. Mat. 3(5), 21–37 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li W, Mille LS, Robledo JA, Uribe T, Huerta V, Zhang YS. Recent Advances in Formulating and Processing Biomaterial Inks for Vat Polymerization-Based 3D Printing. Adv. Healthcare Mater. 9(15), e2000156 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schreiber SL, Shamji AF, Clemons PA. et al. Towards patient-based cancer therapeutics. Nat. Biotechnol. 28(9), 904–906 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roesch S, Rapp C, Dettling S, Herold-Mende C. When immune cells turn bad-tumor-associated microglia/macrophages in glioma. Int. J. Mol. Sci. 19(2), 436 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heinrich MA, Bansal R, Lammers T, Zhang YS, Michel Schiffelers R, Prakash J. 3D-bioprinted mini-brain: a glioblastoma model to study cellular interactions and therapeutics. Adv. Mater. 31(14), e1806590 (2019). [DOI] [PubMed] [Google Scholar]; • Miniaturized brain model to study phenotypic alterations in macrophages and cancer cells that drive glioblastoma progression.
- 31.Ozturk MS, Lee VK, Zou H, Friedel RH, Intes X, Dai G. High-resolution tomographic analysis of in vitro 3D glioblastoma tumor model under long-term drug treatment. Sci. Adv. 6(10), eaay7513 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Evaluation of the biological behaviors and drug responses of 3D glioblastoma spheroids compared with 2D glioblastoma monolayer cell culture.
- 32.Jungst T, Smolan W, Schacht K, Scheibel T, Groll J. Strategies and molecular design criteria for 3D printable hydrogels. Chem. Rev. 116(3), 1496–1539 (2016). [DOI] [PubMed] [Google Scholar]
- 33.Malda J, Visser J, Melchels FP. et al. 25th anniversary article: engineering hydrogels for biofabrication. Adv. Mater. 25(36), 5011–5028 (2013). [DOI] [PubMed] [Google Scholar]
- 34.Zimmermann DR, Dours-Zimmermann MT. Extracellular matrix of the central nervous system: from neglect to challenge. Histochem. Cell Biol. 130(4), 635–653 (2008). [DOI] [PubMed] [Google Scholar]
- 35.Nicholls J, Martin A, Wallace B, Fuchs P. From Neuron to Brain. Sinauer Associates. Inc., MA, USA: (2001). [Google Scholar]
- 36.Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nat. Rev. Clin. Oncol. 14(7), 399 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kleihues P, Louis DN, Scheithauer BW. et al. The WHO classification of tumors of the nervous system. J. Neuropathol. Exp. Neurol. 61(3), 215–225 (2002). [DOI] [PubMed] [Google Scholar]
- 38.Hardee ME, Zagzag D. Mechanisms of glioma-associated neovascularization. Am. J. Pathol. 181(4), 1126–1141 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ricci-Vitiani L, Pallini R, Biffoni M. et al. Tumour vascularization via endothelial differentiation of glioblastoma stem-like cells. Nature 468(7325), 824–828 (2010). [DOI] [PubMed] [Google Scholar]
- 40.Ozbolat IT, Hospodiuk M. Current advances and future perspectives in extrusion-based bioprinting. Biomaterials 76, 321–343 (2016). [DOI] [PubMed] [Google Scholar]
- 41.Wells RG. The role of matrix stiffness in regulating cell behavior. Hepatology 47(4), 1394–1400 (2008). [DOI] [PubMed] [Google Scholar]
- 42.Crompton KE, Goud JD, Bellamkonda RV. et al. Polylysine-functionalised thermoresponsive chitosan hydrogel for neural tissue engineering. Biomaterials 28(3), 441–449 (2007). [DOI] [PubMed] [Google Scholar]
- 43.Wang C, Tong X, Yang F. Bioengineered 3D brain tumor model to elucidate the effects of matrix stiffness on glioblastoma cell behavior using PEG-based hydrogels. Mol. Pharm. 11(7), 2115–2125 (2014). [DOI] [PubMed] [Google Scholar]
- 44.Ulrich TA, de Juan Pardo EM, Kumar S. The mechanical rigidity of the extracellular matrix regulates the structure, motility and proliferation of glioma cells. Cancer Res. 69(10), 4167–4174 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wolf KJ, Chen J, Coombes JD, Aghi MK, Kumar S. Dissecting and rebuilding the glioblastoma microenvironment with engineered materials. Nat. Rev. Mater. 4(10), 651–668 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Duan B, Hockaday LA, Kang KH, Butcher JT. 3D bioprinting of heterogeneous aortic valve conduits with alginate/gelatin hydrogels. J. Biomed. Mater. Res. A 101(5), 1255–1264 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Choi YJ, Yi HG, Kim SW, Cho DW. 3D cell printed tissue analogues: a new platform for theranostics. Theranostics 7(12), 3118 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu F, Celli J, Rizvi I, Moon S, Hasan T, Demirci U. A three-dimensional in vitro ovarian cancer coculture model using a high-throughput cell patterning platform. Biotechnol. J. 6(2), 204–212 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sørensen MD, Fosmark S, Hellwege S, Beier D, Kristensen BW, Beier CP. Chemoresistance and chemotherapy targeting stem-like cells in malignant glioma. In: stem cell biology in neoplasms of the central nervous system. Adv. Exp. Med. Biol. 853, 111–138 (2015). [DOI] [PubMed] [Google Scholar]
- 50.Türeci Ö, Vormehr M, Diken M, Kreiter S, Huber C, Sahin U. Targeting the heterogeneity of cancer with individualized neoepitope vaccines. Clin. Cancer Res. 22(8), 1885–1896 (2016). [DOI] [PubMed] [Google Scholar]; •• A more general discussion on the adoption of three-dimensional bioprinting strategies for the construction of in vitro tumor tissue models.
- 51.Liu T, Delavaux C, Zhang YS. 3D bioprinting for oncology applications. J. 3D Print. Med. 3(2), 55–58 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Knowlton S, Anand S, Shah T, Tasoglu S. Bioprinting for neural tissue engineering. Trends Neurosci. 41(1), 31–46 (2018). [DOI] [PubMed] [Google Scholar]
- 53.Gerstner ER, Batchelor TT. Antiangiogenic therapy for glioblastoma. Cancer J. 18(1), 45 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Calabrese C, Poppleton H, Kocak M. et al. A perivascular niche for brain tumor stem cells. Cancer Cell 11(1), 69–82 (2007). [DOI] [PubMed] [Google Scholar]
- 55.Guo W, Giancotti FG. Integrin signalling during tumour progression. Nature Rev. Mol. Cell. Biol. 5(10), 816–826 (2004). [DOI] [PubMed] [Google Scholar]
- 56.Choi H-J, Zhang H, Park H. et al. Yes-associated protein regulates endothelial cell contact-mediated expression of angiopoietin-2. Nat. Commun. 6(1), 1–14 (2015). [DOI] [PubMed] [Google Scholar]
- 57.Hambardzumyan D, Bergers G. Glioblastoma: defining tumor niches. Trends Cancer 1(4), 252–265 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cui X, Morales R-TT, Qian W. et al. Hacking macrophage-associated immunosuppression for regulating glioblastoma angiogenesis. Biomaterials 161, 164–178 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.DelNero P, Lane M, Verbridge SS. et al. 3D culture broadly regulates tumor cell hypoxia response and angiogenesis via pro-inflammatory pathways. Biomaterials 55, 110–118 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sun J-l, Jiao K, Niu L-n. et al. Intrafibrillar silicified collagen scaffold modulates monocyte to promote cell homing, angiogenesis and bone regeneration. Biomaterials 113, 203–216 (2017). [DOI] [PubMed] [Google Scholar]
- 61.Hubert CG, Rivera M, Spangler LC. et al. A three-dimensional organoid culture system derived from human glioblastomas recapitulates the hypoxic gradients and cancer stem cell heterogeneity of tumors found in vivo. Cancer Res. 76(8), 2465–2477 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• The findings indicate that there might be different molecular subpopulations within the glioma stem cell hierarchy and their distribution may be influenced by their microenvironment.
- 62.Darmanis S, Sloan SA, Croote D. et al. Single-cell RNA-seq analysis of infiltrating neoplastic cells at the migrating front of human glioblastoma. Cell Rep. 21(5), 1399–1410 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bernstock JD, Mooney JH, Ilyas A. et al. Molecular and cellular intratumoral heterogeneity in primary glioblastoma: clinical and translational implications. J. Neurosurg. (2019) (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 64.Das S, Srikanth M, Kessler JA. Cancer stem cells and glioma. Nat. Clin. Pract. Neurol. 4(8), 427–435 (2008). [DOI] [PubMed] [Google Scholar]


