Abstract
Introduction:
Dual human immunodeficiency virus/syphilis rapid diagnostic devices can play an important role in prevention efforts. The field performance of the INSTI Multiplex HIV-1/HIV-2/Syphilis Antibody Test (Multiplex) was evaluated.
Methods:
Fingerstick whole blood was tested using the rapid test. A 4th generation HIV laboratory assay and treponenal-specific laboratory assays were used as reference. Rapid plasma reagin (RPR) was used to stratify treponemal results. Sensitivity and specificity were calculated.
Results:
Overall, 274 patients participated. Sensitivity of the Multiplex for detection of HIV was 98.8% (95% CI, 93.4%−100%) and specificity was 100% (95% CI, 98.1%−100%). Sensitivity for detection of syphilis antibodies was 56.8% (95% CI, 44.7%−68.2%) and specificity was 98.5% (95% CI, 95.7%−99.7%). Sensitivity for treponemal antibodies improved with increasing RPR and was 100% (95% CI, 78.2%–100%) among samples with RPR titers≥1:8.
Conclusions:
The Multiplex showed excellent performance for detection of HIV antibodies and increasing sensitivity for detection of treponemal antibody with increasing RPR titer.
Keywords: diagnosis, Treponema pallidum, HIV, rapid test, dual test
1. Introduction
Despite a decrease in the number of new cases of human immunodeficiency virus (HIV) infection (Centers for Disease Control and Prevention), the rate of new syphilis cases in the United States is increasing (Centers for Disease Control and Prevention). Men who have sex with men account for the majority of new HIV and syphilis cases and the rate of HIV/syphilis co-infection is also very high (Centers for Disease Control and Prevention). Syphilis infection can facilitate transmission and acquisition of HIV (Zetola and Klausner 2007). Novel diagnostic strategies for timely identification and treatment are needed to reduce the burden of those infections and reduce transmission.
Dual rapid assays are point-of-care tests that simultaneously detect antibodies for HIV and Treponema pallidum (TP), the organism that causes syphilis. They offer a convenient way to screen for both infections using one specimen and one test. Dual testing requires a single finger prick, which reduces patient discomfort, and may have the potential to reduce costs(Bristow et al. 2016). This could have significant applications in resource-limited areas, populations with high prevalence of both infections, antenatal screening and population targeted screening. Despite obvious advantages of these dual tests, to date there is no FDA-approved dual device test for use in the United States.
The INSTI Multiplex HIV-1/HIV-2/Syphilis Antibody Test (BioLytical, Richmond, BC, Canada) is a new rapid point-of-care device for the detection of antibodies to HIV and Treponema pallidum and it has shown high performance in laboratory evaluations (Herbst de Cortina et al. 2016). The goal of this study was to evaluate the field performance of the INSTI Multiplex in a community clinical setting using laboratory-based reference tests, and to compare it to the performance of the currently used standard of care rapid screening assays for HIV and syphilis.
2. Methodology
2.1. Study population and setting:
Eligible participants were ≥18 years old who presented to four outpatient clinics of the AIDS Healthcare Foundation (AHF) in Los Angeles and in New York City between August 2016 and December 2017. The clinics provide healthcare to patients with HIV infection and offer free HIV and sexually transmitted diseases testing. Participants gave oral informed consent for participation. A $25 gift card was given to all participants for their time.
2.2. Under evaluation assays:
The INSTI Multiplex is a single use, rapid flow-through in vitro qualitative immunoassay for the detection of antibodies to HIV-1, HIV-2 and TP in human whole blood, fingerstick blood, serum or plasma (bioLytical Laboratories Inc.). The test detects IgG antibodies to gp41 antigen (HIV-1), gp36 antigen (HIV-2) and p17 and p47 domains of TP. The test cartridge has three dots for result interpretation; one for the internal control, one for HIV and another for TP antibodies. The kit result does not differentiate between HIV-1 and HIV-2 antibody detection. Test results can be read within one minute of inoculation with the specimen.
Two rapid tests are currently used at AHF clinics for HIV and syphilis screening, the INSTI® HIV-1/HIV-2 Rapid Antibody Test (BioLytical, Richmond, BC, Canada) and the Syphilis Health Check (Diagnostics Direct, LLC, Stone Harbor, NJ, USA), respectively. The INSTI HIV test follows the same procedures as the INSTI Multiplex and contains the same HIV antibody targets. The Syphilis Health Check yields results in 10 minutes and detects TP-specific antibodies for p15, p17 and p44 domains of the Treponema pallidum (Trinity Biotect Plc.). Figure 1 shows the technical characteristics from the test package inserts of all the rapid tests used in the study.
Figure 1.
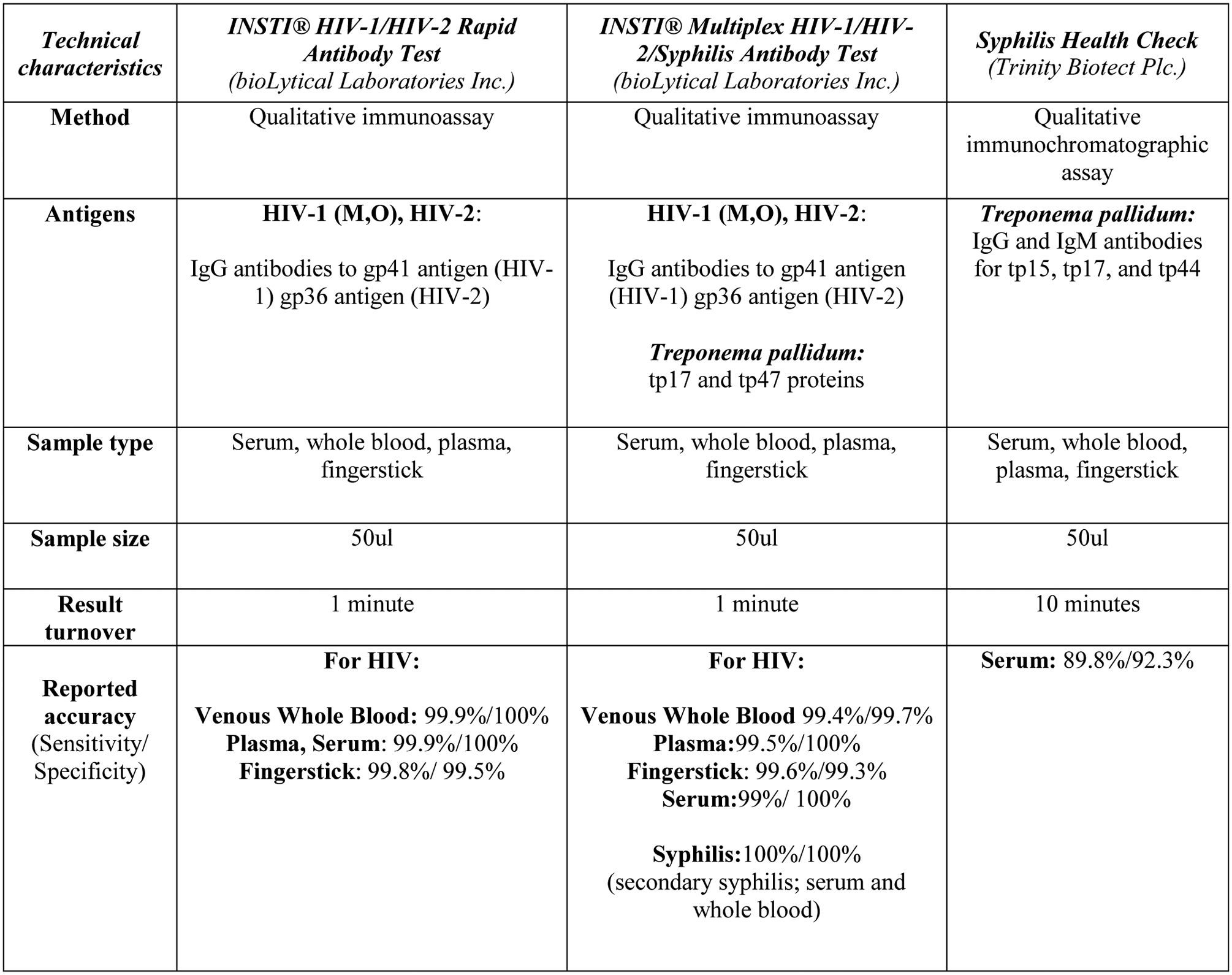
Manufacturer technical characteristics of the rapid test under evaluation (INSTI Multiplex) and the standard of care rapid tests used by the clinics.
Testing with each rapid test (INSTI Multiplex, INSTI HIV, and Syphilis Health Check) requires 50 μl of blood, which is equal to two drops of blood. Fingerstick whole blood was tested on each rapid test. Specimen collection and testing were performed by a trained counselor, according the manufacturer’s instructions.
A venipuncture specimen was also collected from each participant and serum was used to perform HIV and syphilis reference testing. Participant infection status for HIV was determined using a 4th generation assay. The Los Angeles sites used the Abbott ARCHITECT HIV Ag/Ab Combo (Abbott, Illinois, USA) and the New York sites the 4th generation ADVIA Centaur XP HIV-1/O/2 Antigen/Antibody (Siemens, USA). For syphilis, the infection status was determined using the TP particle agglutination (Serodia TPPA, Fujirebio Inc, PA) in Los Angeles sites and TP Enzyme Immunoassay (TPEIA; Bioplex 2200, Bio-Rad Industries, California, USA) in New York. Rapid plasma reagin (RPR; Gold Standard Diagnostics, California, USA) was performed on TP positive specimens.
2.3. Data Analysis:
We extracted demographic information (gender, age) from patient medical records. Data from all sites were combined before analysis. A sample was considered positive for HIV, if it tested positive in either 4th generation HIV assay. Similarly, a sample was considered positive for TP antibodies, if it tested positive in either TPPA or TPEIA.
The sensitivity and specificity of the three rapid devices for the detection of HIV and TP antibody detection were calculated; in addition, sensitivity for TP antibody detection was stratified by RPR titer (Non-Reactive, 1:1–1:2, 1:4, ≥1:8). Concordance between the experimental device and the reference laboratory tests was calculated using Cohen’s kappa statistic. The exact binomial method was used to determine 95% confidence intervals (CI). Data analysis was conducted using the IBM SPSS version 22. The two proportion z-test was used to assess difference in sensitivity in syphilis detection between the two rapid tests for TP detection. A result was considered significant, when the p value was less than 0.05.
2.4. Human Subjects Review approval
The study was approved by the UCLA Institutional Review Board with IRB# 16–000489.
3. Results
In total, 274 patients (244 men, 30 women) participated in the study, 98 patients from New York and 176 patients from Los Angeles sites. Among the participants, 82 patients had detectable HIV antibodies and 74 had antibodies for TP on reference tests. Among those positive for TP, 58 participants had reactive RPR (titer range = 1:1 – 1:512) with 15 with an RPR titer ≥1:8. No rapid tests gave invalid results.
3.1. Performance of the INSTI Multiplex
Sensitivity for HIV antibodies was 98.8% (95% CI 93.4 – 99.9%) and specificity was 100% (95% CI, 98.1 – 100%). Sensitivity for TPantibodies was 56.8% (95% CI, 44.7% – 68.2%) and specificity 98.5% (95% CI, 95.7 – 99.7%). Table 1 summarizes the performance of the INSTI Multiplex. Sensitivity among TP-reactive specimens varied based on the RPR titer, ranging from 25% (95% CI, 7.3 – 52.4%) in samples where RPR was non-reactive to 100% (95% CI, 78.2% - 100%) when the RPR titer was ≥1:8 [Table 2]. There were 8 patients positive for HIV infection with positive TP antibodies and RPR titer≥1/8. The agreement between the HIV rapid component and the reference laboratory assay was 100%(8/8). Similarly, for TP antibody detection.
Table 1.
Performance of the INSTI Multiplex HIV-1/HIV-2/Syphilis Antibody Test kit, the INSTI HIV-1/HIV-2 Antibody Test kit and Syphilis Health Check.
| HIV antibody detection* | |||||
| Positive (n=82) | Negative (n=192) | Sensitivity 95% CI | Specificity 95% CI | Cohen’s kappa statistic | |
| Multiplex HIV | 81 | 192 | 98.8% (93.40 – 100%) | 100% (98.1–100%) | 0.99 (0.97–1) |
| INSTI HIV | 81 | 192 | |||
| Treponema pallidum antibody detection** | |||||
| Positive (n=74) | Negative (n=200) | Sensitivity 95% CI | Specificity 95% CI | Cohen’s kappa statistic | |
| Multiplex TP | 42 | 197 | 56.8% (44.7– 68.2%) | 98.5% (95.7–99.7%) | 0.63 (0.52–0.74) |
| Syphilis Healthcheck | 54 | 194 | 73% (61.4–82.6%) | 97% (93.6–98.9%) | 0.75 (0.56–0.86) |
Reference tests: Abbott ARCHITECT HIV Ag/Ab Combo; 4th generation ADVIA Centaur XP HIV-1/O/2 Antigen/Antibody
Reference tests: Serodia TPPA, Fujirebio Inc, PA, USA; TPEIA Bioplex 2200, Bio-Rad Industries, CA, USA
Table 2.
Sensitivity of the INSTI Multiplex and the Syphilis Health Check in TPPA positive samples (n=74) stratified by RPR titer.
| INSTI Multiplex TP | Syphilis Health Check | |||
|---|---|---|---|---|
| RPR titer | Sensitivity (95% CI) | Samples detected | Sensitivity (95% CI) | Samples detected |
| Non-Reactive | 25% (7.3 – 52.4%) | 4/16 | 43.8% (19.7 – 70.1%) | 7/16 |
| 1/1 or 1/2 | 50% (7.3 −52.4%) | 17/34 | 73.5% (55.6 – 87.1%) | 25/34 |
| 1/4 | 66.6% (29.9 – 92.5%) | 6/9 | 77.8% (40 – 97.2%) | 7/9 |
| ≥1/8 | 100% (78.2 – 100%) | 15/15 | 100% (78.2 – 100%) | 15/15 |
3.2. Performance of the INSTI HIV
The performance of the INSTI HIV was identical to the HIV component of the INSTI Multiplex (Table 1). Sensitivity for HIV antibodies was 98.8% (95% CI, 93.4 – 99.9%) and specificity was 100% (95% CI, 98.1 – 100%). There was only one false negative result.
3.3. Performance of the Syphilis Health Check and comparison with the INSTI Multiplex
Sensitivity for TP- specific antibodies was 73% (95% CI: 61.4% - 82.6%) and specificity 97% (95% CI, 93.6 – 98.9%) [Table 1]. Among TPPA reactive specimens, sensitivity ranged from 43.8% (95% CI, 19.7 – 70.1%), when the RPR was non-reactive, to 100% (95% CI, 78.2 – 100%), when RPR titer was ≥1:8 [Table 2]. The syphilis health check demonstrated higher sensitivity than the INSTI Multiplex for treponemal antibody detection (73% versus 56.8%, p=0.04).
4. Discussion
We conducted a field evaluation of the INSTI Multiplex, a dual rapid test for detection of HIV and syphilis, in clinical settings in the United States. The INSTI Multiplex showed excellent performance in detecting HIV antibodies. Unsurprisingly, the INSTI HIV showed identical performance to the HIV detection of the INSTI Multiplex as both test kits use the same antigens for detection of HIV antibodies. Performance of both tests was similar to the sensitivity and specificity reported by the manufacturer(bioLytical Laboratories Inc.), as well as previous studies evaluating the INSTI HIV on fingerstick whole blood samples (Bergman et al. 2013),(Adams et al. 2017). This finding suggests that the addition of the TP component of the test does not negatively impact the detection of HIV antibodies.
The overall specificity for treponemal antibodies was very high (98.5%), but the sensitivity of the test is significant lower (56.8%) than the one reported by the manufacturer (100%) (bioLytical Laboratories Inc.). Specimen type used for the evaluation, as well as the setting of the evaluation may have affected the performance of the test (Gliddon et al. 2017). The manufacturer used contrived/spiked whole blood samples in the laboratory (bioLytical Laboratories Inc.), while in our study we used fresh fingerstick whole blood specimens in a real-world clinical setting.
Another important finding is that the sensitivity of treponemal antibody detection increased with increasing RPR titers. This observation has also been reported on another dual HIV/syphilis tests by Black et al. (2016)(Black et al. 2016). This finding is critical because those with higher RPR titers may be those with active or recent infection and therefore may be more likely to require treatment.
The performance of the INSTI Multiplex for both HIV and syphilis antibodies is similar to that of other dual devices. In a recent meta-analysis evaluating the performance of multiple dual tests, Gliddon et al. showed that the range of sensitivity in whole blood samples for HIV antibody detection ranged from 94% to 99% and for specificity ranged from 97% to 100%, while sensitivity for syphilis detection ranged between 47% - 96% and specificity ranged between 91% and 100% (Gliddon et al. 2017). In our study, we found that the performance of the INSTI Multiplex is within this range for both infections, indicating that the INSTI Multiplex performs as well as other dual rapid tests.
A recent performance evaluation of the Syphilis Health Check with fingerstick samples and TP-EIA as a reference standard showed similar performance to our results; sensitivity 71.4% (95% CI, 41.9 – 95.1%) and specificity 91.5% (95% CI, 87.5 – 95.5%)(Matthias et al. 2016). When compared to the syphilis component of the INSTI Multiplex rapid test, both tests showed high specificity. However, the FDA-cleared syphilis health check demonstrated higher sensitivity than the INSTI Multiplex for treponemal antibody detection. The INSTI Multiplex utilizes two TP-specific antigens (tp17, tp47)(bioLytical Laboratories Inc.). In comparison, the FDA-approved Syphilis Health Check detects three TP-specific antigens (tp15, tp17, tp44)(Trinity Biotect Plc.), which could explain some of the difference in their performance.
Our study is subject to some limitations. The moderate sample size of the study limits the precision of our estimates, especially the small size of RPR titer subgroups. The test under evaluation (INSTI Multiplex) was not evaluated for acute HIV infections or cases of primary syphilis infection, so we cannot make estimates on its performance in those groups of patients. Additionally, our study did not perform further analyses to detect the HIV strains of participants with HIV infection. Based on the geographic distribution of HIV strains (Buonaguro et al. 2007), HIV-1 subtype B is the dominant genetic subtype in North America, thus our study is likely to have evaluated the performance of the test kit on the particular strain. Further studies should be conducted to evaluate the performance in the various HIV strains and subtypes.
5. Conclusions
In conclusion, the INSTI Multiplex showed excellent performance in detecting HIV antibodies, while its sensitivity in detecting Treponema pallidum antibodies increased with increasing RPR titer. Furthermore, it is simple to use, with one-minute result turnover, could be performed by non-medical personnel. Considering the proven cost reduction dual rapid tests could yield and its performance, the under evaluation test could be used in high-prevalence areas or screening populations with history of syphilis or syphilis treatment. Further research is needed on how to best use those tests in clinical and outreach settings.
Highlights.
- Performance of INSTI Multiplex HIV-1/HIV-2/Syphilis Antibody Test
- HIV: Sensitivity=98.8%(93.4%−100%), Specificity=98.8%(95% CI, 93.4%−100%)
- Syphilis: Sensitivity=56.8%(44.7%−68.2%), specificity=98.5%(95.7%−99.7%)
- Sensitivity for detection of syphilis improved for RPR titer≥1:8
Performance of INSTI® HIV-1/HIV-2 Rapid: Sensitivity=98.8%(93.4%−100%), Specificity=98.8%(95% CI, 93.4%−100%)
Performance of Syphilis Healthcheck: Sensitivity=73%(61.4% - 82.6%), specificity=97%(93.6 – 98.9%)
Funding:
This work was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award numbers: T32AI007384 (to CCB), K01AI136725 (to CCB), the UCLA Center for AIDS Research (P30AI028697 to JDK) and National Institutes of Health UCLA Center for HIV Identification, Prevention and Treatment Services (P30MH058107 to JDK). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts of interest statement: INSTI Multiplex tests were donated by the manufacturer, BioLytical, Richmond, BC, Canada.
Data statement: data are available upon request by the corresponding author.
References:
- Adams S, Luo W, Wesolowski L, Cohen SE, Peters PJ, Owen SM, et al. Performance evaluation of the point-of-care INSTITM HIV-1/2 antibody test in early and established HIV infections. J Clin Virol [Internet]. 2017. June 1 [cited 2018 Mar 4];91:90–4. Available from: https://www.sciencedirect.com/science/article/pii/S1386653217300896?via%3Dihub [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman J, Gratrix J, Plitt S, Fenton J, Archibald C, Wong T, et al. Feasibility and Field Performance of a Simultaneous Syphilis and HIV Point-of-Care Test Based Screening Strategy in at Risk Populations in Edmonton, Canada. AIDS Res Treat [Internet]. 2013. [cited 2018 Mar 5];2013:819593 Available from: http://www.ncbi.nlm.nih.gov/pubmed/24527210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- bioLytical Laboratories Inc. INSTI® Multiplex HIV-1/HIV-2/ Syphilis Antibody Test Kit Package Insert [Internet]. [cited 2018 Mar 10]. Available from: http://biolytical.com/products/insti-multiplex/?country=CA
- Black V, Williams BG, Maseko V, Radebe F, Rees HV, Lewis DA. Field evaluation of Standard Diagnostics’ Bioline HIV/Syphilis Duo test among female sex workers in Johannesburg, South Africa. Sex Transm Infect. 2016;92(7):495–8. [DOI] [PubMed] [Google Scholar]
- Bristow CC, Larson E, Anderson LJ, Klausner JD. Cost-effectiveness of HIV and syphilis antenatal screening: a modelling study. Sex Transm Infect [Internet]. 2016. August [cited 2018 Feb 2];92(5):340–6. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26920867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonaguro L, Tornesello ML, Buonaguro FM. Human immunodeficiency virus type 1 subtype distribution in the worldwide epidemic: pathogenetic and therapeutic implications. J Virol [Internet]. 2007. October 1 [cited 2018 Aug 27];81(19):10209–19. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17634242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. HIV in the United States | Statistics Overview [Internet]. [cited 2018a Mar 10]. Available from: https://www.cdc.gov/hiv/statistics/overview/ataglance.html
- Centers for Disease Control and Prevention. Syphilis - 2016 STD Surveillance Report [Internet]. [cited 2018b Mar 10]. Available from: https://www.cdc.gov/std/stats16/Syphilis.htm
- Gliddon HD, Peeling RW, Kamb ML, Toskin I, Wi TE, Taylor MM. A systematic review and meta-analysis of studies evaluating the performance and operational characteristics of dual point-of-care tests for HIV and syphilis. Sex Transm Infect [Internet]. 2017. December 1 [cited 2018 Feb 2];93(S4):S3–15. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28747410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbst de Cortina S, Bristow CC, Vargas SK, Perez DG, Konda KA, Caceres CF, et al. Laboratory Evaluation of a Point-of-Care Downward-Flow Assay for Simultaneous Detection of Antibodies to Treponema pallidum and Human Immunodeficiency Virus. J Clin Microbiol [Internet]. 2016. July 1 [cited 2018 Feb 2];54(7):1922–4. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27147725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inc. bioLytical L. INSTI HIV-1/HIV-2 Antibody Test Kit Package Insert.
- Matthias J, Dwiggins P, Totten Y, Blackmore C, Wilson C, Peterman TA. Evaluation of the Sensitivity and Specificity of a Commercially Available Rapid Syphilis Test -- Escambia County, Florida, 2016. MMWR Morb Mortal Wkly Rep [Internet]. 2016. October 28 [cited 2016 Nov 1];65(42):1174–5. Available from: http://www.cdc.gov/mmwr/volumes/65/wr/mm6542a5.htm [DOI] [PubMed] [Google Scholar]
- Trinity Biotect Plc. Syphilis Health Check [Internet]. [cited 2017 Aug 22]. Available from: http://www.trinitybiotech.com/wp-content/uploads/2015/07/Syphilis-Health-Check-test_PI-rev-O-04-2015.pdf
- Zetola NM, Klausner JD. Syphilis and HIV infection: an update. Clin Infect Dis [Internet]. 2007. May 1 [cited 2016 Sep 1];44(9):1222–8. Available from: http://cid.oxfordjournals.org/lookup/doi/10.1086/513427 [DOI] [PubMed] [Google Scholar]


