Abstract
Background
Static changes in local brain activity in patients suffering from amyotrophic lateral sclerosis (ALS) have been studied. However, the dynamic characteristics of local brain activity are poorly understood. Whether dynamic alterations could differentiate patients with ALS from healthy controls (HCs) remains unclear.
Methods
A total of 54 patients with ALS (mean age = 48.71 years, male/female = 36/18) and 54 (mean age = 48.30 years, male/female = 36/18) HCs underwent magnetic resonance imaging scans. To depict static alterations in cortical activity, amplitude of low-frequency fluctuations (ALFF) which measures the total power of regional activity was computed. Dynamic ALFF (d-ALFF) from all subjects was calculated using a sliding-window approach. Statistical differences in ALFF and d-ALFF between both groups were used as features to explore whether they could differentiate ALS from HC through support vector machine method.
Results
In contrast with HCs, patients with ALS displayed increased ALFF in the right inferior temporal gyrus and bilateral frontal gyrus and decreased ALFF in the left middle occipital gyrus and left precentral gyrus. Furthermore, patients with ALS demonstrated lower d-ALFF in widespread regions, including the right lingual gyrus, left superior temporal gyrus, bilateral precentral gyrus, and left paracentral lobule by comparison with HCs. In addition, the ALFF in the left superior orbitofrontal gyrus had a tendency of correlation with ALSFRS-R score and disease progression rate. The classification performance in distinguishing ALS was higher with both features of ALFF and d-ALFF than that with a single approach.
Conclusions
Decreased dynamic brain activity in the precentral gyrus, paracentral gyrus, lingual gyrus, and temporal regions was found in the ALS group. The combined ALFF and d-ALFF could distinguish ALS from HCs with a higher accuracy than ALFF and d-ALFF alone. These findings may provide important evidence for understanding the neuropathology underlying ALS.
Keywords: Amyotrophic lateral sclerosis, Amplitude of low-frequency fluctuations, Dynamic, Resting state, Static
Introduction
Amyotrophic lateral sclerosis (ALS) is a devastating disease which involves dysfunctions in movement and cognition (Hardiman et al., 2017; Van Es et al., 2017). Patients with ALS usually died within 3–5 years after symptoms appear (Roth & Shacka, 2009). At present, the therapeutic options for ALS are limited. Nevertheless, increasing lines of evidence demonstrate that early diagnosis is important for selecting available pharmacologic therapy and that appropriate palliative care has an active influence on patients’ living quality and survival (Bourke et al., 2006; Volanti et al., 2011). Now the diagnosis of ALS is still clinical, and a pronounced delay exists between the onset of symptoms and diagnosis, possibly beyond the therapeutic window (Turner et al., 2009). Timely and accurate diagnosis of ALS is urgently needed to date, and imaging biomarkers should be developed.
Recent studies combining functional and structural data depicted that functional alterations at resting state may precede structural changes in patients with cognitive impairments (Kawagoe, Onoda & Yamaguchi, 2019; Sun et al., 2016) and ALS (Abidi et al., 2020; Chipika et al., 2019). Neuroimaging approaches provide convenience for studying local brain activities and may facilitate expanding our understanding of early diagnosis of ALS (Huynh et al., 2016; Verstraete & Foerster, 2015). Resting-state functional magnetic resonance imaging (rs-fMRI) is an ideal instrument used to probe into cortical activities based on blood oxygenation level-dependent signals without performing variable tasks (Biswal et al., 1995). As an effective index to measure local brain activity, amplitude of low frequency fluctuation (ALFF) (Guo et al., 2012; Liu et al., 2013; Yu-Feng et al., 2007) has been extensively employed in ALS research. Using this approach, scholars have discovered that patients with ALS showed aberrant activation in the precentral gyrus, frontal gyrus, and occipital regions; this finding suggests that ALS is a disease involving many system with brain impairment spreading beyond the motor cortex (Bueno et al., 2019; Ma et al., 2016; Shen et al., 2018). In addition, the increased ALFF in the frontal lobe could be a candidate biomarker in ALS (Luo et al., 2012). However, these studies are on the strength of the hypothesis that the signal of rs-fMRI is static during scanning, ignoring the dynamic behavior of activities of people’s brains (Allen et al., 2014; Fu et al., 2017; Liu et al., 2017).
Dynamic amplitude of low-frequency fluctuation (d-ALFF), an indicator of the variance of ALFF, is an effective tool to explore brain dynamics in healthy people (Liao et al., 2019; Zou et al., 2009) and patients with neuropsychiatric disorders, including schizophrenia (Yang et al., 2019), generalized anxiety disorder (GAD) (Cui et al., 2019), and Parkinson’s disease (Zhang et al., 2019). In addition, Li et al. (2018a) discovered that in contrast to static ALFF abnormalities, d-ALFF abnormalities could predict the severity of suicidal ideation in major depressive disorders. d-ALFF may contribute more than ALFF in differentiating between patients diagnosed with GAD and normal controls (Cui et al., 2019). However, as far as we know, the dynamic signatures of ALFF have been rarely elucidated in ALS; furthermore, the performance of d-ALFF compared with ALFF in recognizing ALS patients from healthy controls (HCs) at an individual level remains poorly documented.
Motivated by previous studies, we utilized d-ALFF to detect changes of dynamic patterns of brain activity in ALS. We assumed that patients with ALS would exhibit altered d-ALFF patterns contrast to HCs, and that such changes could be used as features to distinguish them. We also hypothesized that the classification performance with altered d-ALFF as features would be comparable with that using altered static ALFF.
Materials & Methods
Subjects
In western countries, the rates of ALS is probably between 1 and 3 per 100,000 per year per person-years (Robberecht & Philips, 2013), while the exact prevalence in China remains unclear (Chen et al., 2015). ALS is more widespread in men than in women in different countries (Chen et al., 2015; Oskarsson, Gendron & Staff, 2018). Patients were employed from January 1, 2009 to December 31, 2013 in this work. Fifty-four patients diagnosed with ALS and 54 HCs matched in gender and age were enrolled from Southwest Hospital. The inclusion criteria were as follows: patients can lie down flat in the scanner for at least 40 min and receive none therapeutic interventions before participating in this study; and patients with ALS diagnosed on the basis of the revised El Escorial criteria of the World Federation of Neurology (Brooks et al., 2000). The exclusion criteria were as follows: patients diagnosed with frontotemporal dementia or other mental and neurological disorders; patients with major systemic diseases; patients with family trait of motor neuron diseases and other neurodegenerative disorders; and patients with cognitive impairment. The clinical status based on ALS Functional Rating Scale-Revised (ALSFRS-R) was obtained for each patient. Disease duration was computed from symptom onset to examination date. By using the equation: (48-ALSFRS-R score)/Disease duration (Ellis et al., 1999), rate of disease progression was achieved. Demographic and clinical information of subjects are displayed in Table 1.
Table 1. Demographic and clinical characteristics of the ALS patients and HCs.
| Variables | ALS (n = 54) | HC (n = 54) | p value |
|---|---|---|---|
| Gender (female/male) | 18/36 | 18/36 | 1a |
| Age (years) | 48.71 ± 10.21 | 48.30 ± 8.74 | 0.82b |
| Gray matter | 649.56 ± 2842.17 | 668.23 ± 3251.82 | 0.08b |
| Disease duration (months) | 20.93 ± 21.56 | – | – |
| ALSFRS-R | 32.56 ± 6.83 | – | – |
| Disease progression rate | 1.28 ± 1.15 | – | – |
Notes.
Values are mean ± variance.
- ALS
- Amyotrophic Lateral Sclerosis
- HC
- Healthy Control
- ALSFRS-R
- ALS Functional Rating Scale-revised
Disease progression rate, (48-ALSFRS-R score)/time from symptom onset.
The p value was obtained by Chi-square t-test.
The p value was obtained by two-sample t-test.
The measurements of the Edinburgh Handedness Inventory indicated that all the subjects were right-handed. The medical research ethics committee of Southwest Hospital (the First Affiliated Hospital of the Third Military Medical University of the Chinese People’s Liberation Army) authorized this study to proceed. Informed consent from each participant was collected.
Data acquisition
Data were collected as described in our former research (Ma et al., 2015). The following parameters were used in collecting functional data: echo time (TE) = 30 ms, repetition time (TR) = 2,000 ms, flip angle (FA) = 90°, 36 slices, 1 mm gap, field of view (FOV) = 192 mm × 192 mm, thickness = 3 mm, matrix size = 64 × 64 and voxel size = 3 mm × 3 mm × 3 mm. Two hundred and forty volumes were collected for each subject. T1-weighted structural data was gathered using the following settings: TE = 2.52 ms, TR = 1,900 ms, FA = 9°, slice thickness = 1 mm, 176 slices, 0 mm gap, FOV = 256 mm × 256 mm, matrix size = 256 × 256, and voxel size = 1 mm × 1 mm × 1 mm.
Data analysis
Preprocessing
fMRI data preprocessing was performed with the Data Processing Assistant for Resting-state fMRI (DPARSF) (Yan & Zang, 2010). To ensure the reliabilty of functional data, we abandoned the first 10 volumes of images. Slice timing and head motion have been done in the remaining 230 volumes of images. No subject had a head movement bigger than 1.5 mm or rotation larger than 1.5°. The images were then normalized to the standard echo planar imaging (EPI) template (resampled voxel size: 3 mm × 3 mm × 3 mm). The following images were smoothed (full-width at half-maximum Gaussian kernel: 4 mm). After normalization, the time series was linearly detrended. Except global signal, 24 parameters of head motion (Friston et al., 1996), signals of white matter, and signals of cerebrospinal fluid were all removed. ALFF/d-ALFF was based on the frequency spectrum of rs-fMRI signals.
Total gray matter (GM) was obtained with the VBM8 toolbox as elaborated in the earlier work (Ma et al., 2016; Ma et al., 2015).
Static ALFF computation
ALFF was calculated using DPARSF toolkit as used in prior research (Cheng et al., 2019; Luo et al., 2012). With the aid of fast Fourier transform, the time series was transformed from the time domain to a frequency domain, from which the power spectrum was achieved. With the power spectrum of each voxel from all subjects, the square root was collected at each frequency and then averaged in the region of 0.01–0.08 Hz (Guo et al., 2013). The square root obtained was known as the ALFF at the given voxel. We divided the ALFF by the global mean ALFF for standardization.
d-ALFF computation
d-ALFF was processed with Temporal Dynamic Analysis (TDA) toolkit which was dependent on DPABI (Yan et al., 2016). According to a former report (Sakoglu et al., 2010), the window length was supposed to be sufficiently short to capture transient signals and long enough to detect slow changing signals. A sliding window with moderate size of 32 TR and a moving step length of 1 TR were selected in this study (Chen et al., 2019b). The 230 time points were divided into 199 windows. ALFF value was computed within each moving window for all participants. Then, the standard deviation (SD) of all ALFF maps from moving windows was computed to evaluate the variability of ALFF. Here, SD was used as d-ALFF.
Statistical analysis
Statistical analyses were processed with SPM12 toolkit. To compare differences in ALFF and d-ALFF of two groups, we employed two-sample t-tests method. The factors such as age, total GM volume, and gender were regressed. Gaussian Random Field (GRF) approach was adopted to perform multiple comparisons. The voxel level and the cluster level was set p < 0.01 and p < 0.05 respectively (the minimum cluster size in ALFF and d-ALFF analyses was 78 voxels) in the GRF correction.
Correlation analysis
Based on region of interest (ROI), Pearson’s correlation was analyzed to probe the relation of alterations in ALFF/d-ALFF to the clinical data of ALS. The mean ALFF/d-ALFF value of each significant clusters (ROIs) was used. A residual term was employed to correlate with clinical data. Meanwhile, the total GM volume, age, and gender were regressed. Bonferroni correction was introduced (significant level: p < 0.05/N) in the present study. Here, N = 15/12 represented the amount of comparisons using ALFF and d-ALFF.
Classification analysis
Support vector machine (SVM) method was utilized to compare classification ability among static ALFF, d-ALFF, and their combination for patients/HCs. The mean ALFF and d-ALFF of each static ALFF’s ROIs and d-ALFF’s ROIs were used as classification features. Liblinear toolbox with default parameter was utilized. Given that we aimed to compare the classification ability among ALFF, d-ALFF, and their combination, a leave-one-out cross validation (LOOCV) was accepted. LOOCV could obtain stable performance and prevent the possibility of overfitting (Chen et al., 2019a; Liu et al., 2015). There were m (m = 108) LOOCV loops. In each loop, we choose one participants’ information to test the categorization model and the m − 1 participants’ information was selected for model training. Finally, specificity, sensitivity and accuracy were collected to evaluate classifier performance.
Validation analysis
In order to confirm the main findings of d-ALFF, d-ALFF data with window lengths of 40 TRs and 50 TRs was recollected.
Results
Differences in static ALFF
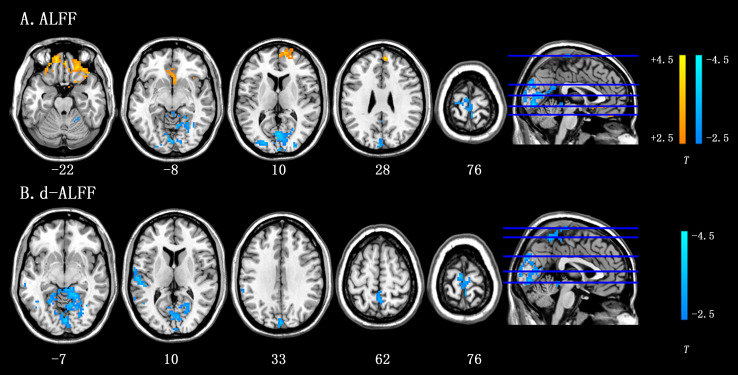
The ALFF in the ALS group increased in the right inferior temporal gyrus, right medial superior frontal gyrus, and right medial superior frontal gyrus and reduced in the left middle occipital gyrus and left precentral gyrus. The details were available in Table 2 and Fig. 1A.
Table 2. Differences in ALFF between ALS and HC groups.
| Clusters | Brain regions | Cluster size (voxels) | MNI (X, Y, Z) | T value |
|---|---|---|---|---|
| ALS >HC | ||||
| Cluster 1 | Right inferior temporal gyrus | 128 | 45, −6, −45 | 4.14 |
| Cluster 2 | Left superior orbitofrontal gyrus | 442 | −15, 18, −15 | 4.45 |
| Cluster 3 | Right medial superior frontal gyrus | 93 | 6, 54, 27 | 4.35 |
| ALS <HC | ||||
| Cluster 1 | Left middle occipital gyrus | 895 | −27, 90, −3 | −4.56 |
| Cluster 2 | Left precentral gyrus | 108 | −27, −21, 72 | −3.81 |
Notes.
MNI, Montreal Neurological Institute. X, Y, Z, coordinates of primary peak locations in the MNI space.
- ALS
- Amyotrophic Lateral Sclerosis
- HC
- Healthy Control
T value denotes the statistic value of two-sample t-test by contrasting the ALS patients to the controls (p < 0.01, GRF-corrected at a cluster level of p < 0.05).
Figure 1. Results of ALFF and d-ALFF analyses by two-sample t-tests between ALS group and HC group.
(A) Brain regions with significant difference in static ALFF between the ALS group and HC group. (B) Brain regions with significant difference in d-ALFF between the ALS group and HC group. The voxel level was set at p < 0.01, and the cluster level was set at p < 0.05 with GRF corrected. The color bar represents the T value of the between-group analysis. Hot colors represent higher ALFF/d-ALFF in the ALS group than in the healthy control group, and cool colors represent the lower ALFF/d-ALFF in the ALS group than the healthy control group.
Differences in d-ALFF
As shown in Table 3 and Fig. 1B, d-ALFF did not increase in ALS group. Decreased d-ALFF was seen in the right lingual gyrus, left superior temporal gyrus, bilateral precentral gyrus, and left paracentral lobule.
Table 3. Differences in d-ALFF between ALS and HC groups.
| Clusters | Brain regions | Cluster size (voxels) | MNI (X, Y, Z) | T value |
|---|---|---|---|---|
| ALS >HC | ||||
| None | ||||
| ALS <HC | ||||
| Cluster 1 | Right lingual gyrus | 988 | 24,−45, −9 | −4.35 |
| Cluster 2 | Left superior temporal gyrus | 176 | −48,−27, 12 | −3.82 |
| Cluster 3 | Bilateral precentral gyrus | 90 | 51,−9, 24 | −3.45 |
| Cluster 4 | Left paracentral lobule | 125 | −6, −15, 75 | −3.70 |
Notes.
MNI, Montreal Neurological Institute. X, Y, Z, coordinates of primary peak locations in the MNI space.
- ALS
- Amyotrophic Lateral Sclerosis
- HC
- Healthy Control
T value denotes the statistic value of two-sample t-test by contrasting the ALS patients to the controls (p < 0.01, GRF-corrected at a cluster level of p < 0.05).
Correlation analysis
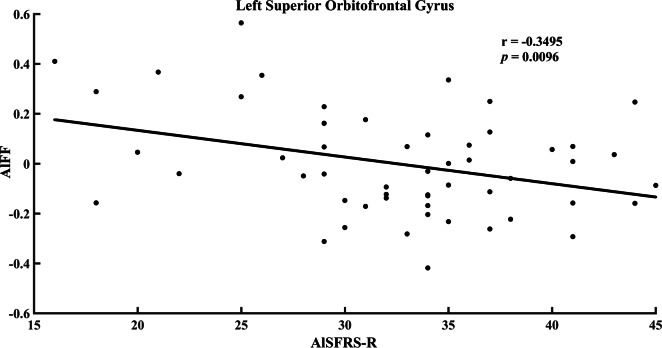
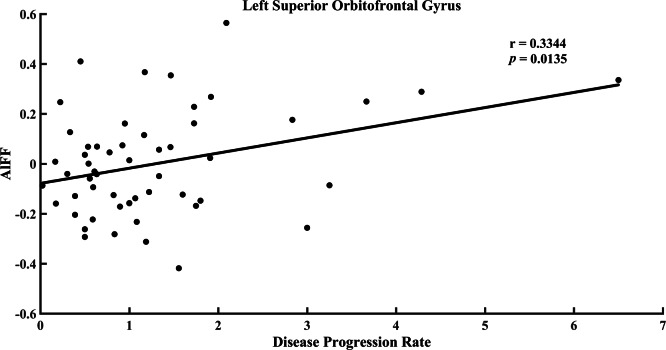
No correlation was detected between ALFF and clinical data in ALS. However, as shown in Fig. 2, the ALFF in the left superior orbitofrontal gyrus had a negative correlation with ALSFRS-R score at a trend level (p = 0.0096, r = − 0.3495, uncorrected). The ALFF in the left superior orbitofrontal gyrus demonstrated a tendency of positive correlation with disease progression rate (Fig. 3, p = 0.0135, r = 0.3344, uncorrected). In addition, no significant difference between d-ALFF and clinical data was found.
Figure 2. Correlation between ALFF value in the left superior orbitofrontal gyrus and ALSFRS-R score in the ALS group.
Figure 3. Correlation between ALFF value in the left superior orbitofrontal gyrus and disease progression rate in the ALS group.
Performance of classification
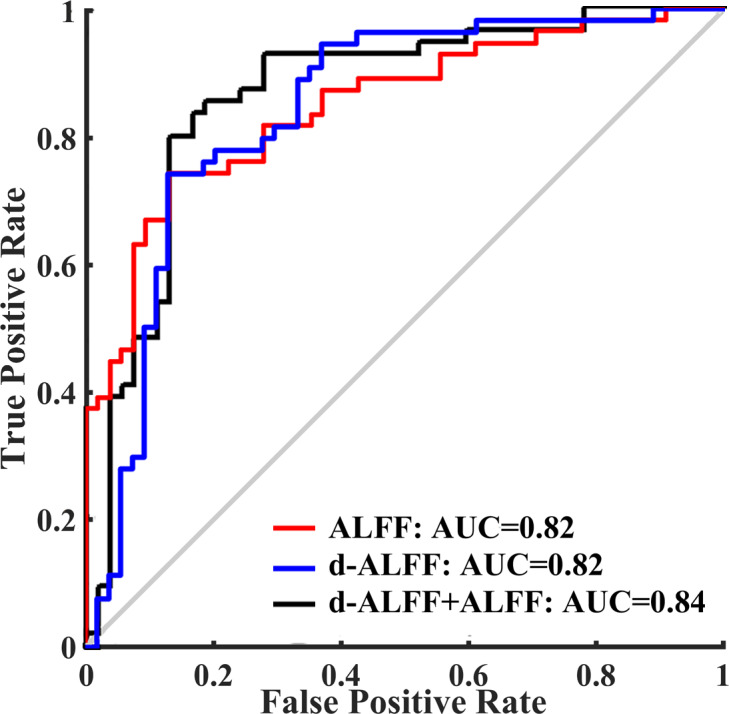
Figure 4 shows the receiver operating characteristic curve (ROC) with ALFF, d-ALFF, and their combination. The numerical data of the area under the curve (AUC) with ALFF, d-ALFF, and their combination were 0.82, 0.82 and 0.84 respectively.
Figure 4. Receiver operating characteristic curve of the classifier with ALFF, d-ALFF, and their combination.
As shown in Table 4, the ALFF method showed a classification accuracy of 76.85%, a specificity of 72.22%, and a sensitivity of 81.48%. The d-ALFF index exhibited a classification accuracy of 76.85%, a specificity of 62.96%, and a sensitivity of 90.74%. The accuracy, specificity, and sensitivity of the combined ALFF and d-ALFF were 79.63%, 72.22%, and 87.04%, respectively.
Table 4. Performance evaluation of classifier using ALFF, d-ALFF and combined ALFF and d-ALFF.
| Sensitivity (%) | Specificity (%) | Accuracy (%) | |
|---|---|---|---|
| ALFF | 81.48 | 72.22 | 76.85 |
| d-ALFF | 90.74 | 62.96 | 76.85 |
| Combined ALFF and d-ALFF | 87.04 | 72.22 | 79.63 |
Validation results
The results of d-ALFF using window sizes of 40 TRs and 50 TRs were very similar to the major results of 32 TRs. Validation results were available in Supplementary Materials (Figs. S1A and S1B).
Discussion
The present research studied the dynamic brain activity in ALS by using d-ALFF for the first time. We found that: (1) patients with ALS showed decreased d-ALFF in the right lingual gyrus, left superior temporal gyrus, bilateral precentral gyrus, and left paracentral lobule at resting state, and (2) the combined ALFF and d-ALFF distinguished ALS from HCs with higher accuracy than ALFF or d-ALFF alone.
Alterations in static ALFF
The brain areas with static ALFF differences in patients with ALS are consistent with previous reports (Luo et al., 2012; Ma et al., 2016), except that the right inferior temporal gyrus had increased ALFF. The temporal lobe with aberrant activation and connection in patients with ALS was discovered in preceding rs-fMRI articles (Li et al., 2018b; Loewe et al., 2017; Zhou et al., 2016). Besides, the thinning of cerebral cortex in the right inferior temporal gyrus is related to rapid clinical progression in ALS (Verstraete et al., 2012). The right inferior temporal gyrus is generally thought to be associated with social information processing for objects, places, and faces (Grill-Spector, 2003; Hall, Fussell & Summerfield, 2005). Of note, the cognitive impairment of ALS includes deficits in social cognition and executive functions (Beeldman et al., 2016). Moreover, deficits in recognition of facial expressions of emotion in ALS have been documented (Zimmerman et al., 2007). However, the change in the ALFF value was not found in previous research on ALS (Luo et al., 2012). The finding was probably caused by the situation that patients from the two studies were at different stages of the disease. The previous study recruited patients with an earlier stage compared with that in the present study, where the amplitude feature in this area may not altered. However, more longitudinal research is need to make the result clear. Therefore, this functional alteration may be the imaging evidence for understanding the impaired recognition of emotional stimuli in ALS at a certain stage. Elevated ALFF in the left superior orbitofrontal gyrus was relevant to rate of disease progression and ALSFRS-R score at a trend level. Hence, increased ALFF in this area might be useful to understand the progress of ALS.
Alterations in d-ALFF
Compared with HCs, the d-ALFF in the right lingual gyrus was lower in ALS group. Dysfunction of the right lingual gyrus in ALS was documented, including metabolic difference (Verma et al., 2013) and functional connectivity (Li et al., 2018b). The lingual gyrus is an important area in the visual system (Yang et al., 2015), and 24.13% of Chinese ALS population are considered with visuospatial disability (Wei et al., 2015). Thus, we concluded that impairment in the right lingual gyrus over time might underlie the phenomenon of visual dysfunction in ALS.
This study also found reduced d-ALFF in the left superior temporal gyrus in ALS group. The abnormality in left superior temporal gyrus was same with previous fMRI studies in regional functional connectivity density (Li et al., 2018b) and with anatomical MRI studies in gray matter volume (Buhour et al., 2017; Kim et al., 2017; Sheng et al., 2015). Electroencephalography study (McMackin et al., 2019) shows decreased power in the left superior temporal gyrus when patients with ALS underwent auditory frequency-mismatch oddball paradigm. The left superior temporal gyrus was considered to be related to the function of auditory working memory (Leff et al., 2009). The quieter activity in the left superior temporal gyrus over time at resting state in ALS can be explained as the reason of memory decline in ALS.
We also observed decreased d-ALFF in the bilateral precentral gyrus and left paracentral lobule in ALS. These motor regions are hallmark areas for patients with ALS who had structural (Cosottini et al., 2012; Schmidt et al., 2014; Thorns et al., 2013) and functional (Ma et al., 2016; Zhang et al., 2017; Zhou et al., 2014) abnormalities. These motor regions were detected with static ALFF and d-ALFF indices in the current research, indicating the vital role of these regions in studying ALS.
Relationship between static ALFF and d-ALFF changes
d-ALFF and ALFF detected decreased activity in the precentral gyrus in patients suffering from ALS. These findings provide a helpful perspective for our understanding the motor neuron dysfunction of this disease. In addition, d-ALFF could provide other different changes compared with traditional ALFF method, showing that dynamic brain activity may be an important neuroimaging feature to track pathological changes in ALS.
Altered d-ALFF could identify patients with ALS from HCs, and the classification performance is similar to that of ALFF. However, when both static and dynamic ALFF features were combined, the classification performance achieved the highest overall accuracy rate. These results consolidated that ALFF and d-ALFF were different approaches used to characterize brain activity from different perspectives. In contrast to ALFF, d-ALFF could provide complementary information to understand ALS better. The findings also provided a novel way to help distinguish patients with ALS from the healthy population.
Limitations and further considerations
Several limitations should be noted in this work. First, the features in classification were based on prior knowledge, which may increase the overall accuracy rate. The combined ALFF and d-ALFF approach would enhance accuracy with a single feature. More subjects and further sub-group analysis should be considered to obtain stable and more precise results.
Conclusions
ALFF and d-ALFF patterns were altered in patients with ALS. The alterations in the two features could identify ALS at the individual level with nearly the same performance. However, when the two features were combined, the classification performance achieved the highest overall accuracy rate. These results provide evidence for applying dynamic spontaneous neural activity (d-ALFF) to uncover the neuropathology of ALS.
Supplemental Information
Data imagings (T map), subject information and software for img format (xjview).
a. Results of the sliding-window length of 40 TR. b. Results of the sliding-window length of 50 TR.
Funding Statement
The work was supported by the Cangzhou Science and Technology Research and Development Project (No. 162302137) and the China Postdoctoral Science Foundation Grant (No. 2019M653383). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Contributor Information
Guiran Yang, Email: yangguiran2006@126.com.
Jiuquan Zhang, Email: zhangjq_radiol@foxmail.com.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Xujing Ma and Fengmei Lu analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Heng Chen, Caihong Hu, Jiao Wang, Sheng Zhang, Shuqin Zhang and Guiran Yang analyzed the data, authored or reviewed drafts of the paper, and approved the final draft.
Jiuquan Zhang conceived and designed the experiments, performed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Human Ethics
The following information was supplied relating to ethical approvals (i.e., approving body and any reference numbers):
This study was approved by the medical research ethics committee of Southwest Hospital (The First Affiliated Hospital of the Third Military Medical University of the Chinese People’s Liberation Army).
Data Availability
The following information was supplied regarding data availability:
The raw data are available in the Supplemental Files.
References
- Abidi et al. (2020).Abidi M, De Marco G, Couillandre A, Feron M, Mseddi E, Termoz N, Querin G, Pradat PF, Bede P. Adaptive functional reorganization in amyotrophic lateral sclerosis: coexisting degenerative and compensatory changes. European Journal of Neurology. 2020;27:121–128. doi: 10.1111/ene.14042. [DOI] [PubMed] [Google Scholar]
- Allen et al. (2014).Allen EA, Damaraju E, Plis SM, Erhardt EB, Eichele T, Calhoun VD. Tracking whole-brain connectivity dynamics in the resting state. Cereb Cortex. 2014;24:663–676. doi: 10.1093/cercor/bhs352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeldman et al. (2016).Beeldman E, Raaphorst J, Klein Twennaar M, De Visser M, Schmand BA, De Haan RJ. The cognitive profile of ALS: a systematic review and meta-analysis update. Journal of Neurology, Neurosurgery and Psychiatry. 2016;87:611–619. doi: 10.1136/jnnp-2015-310734. [DOI] [PubMed] [Google Scholar]
- Biswal et al. (1995).Biswal B, Yetkin FZerrin, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magnetic Resonance in Medicine. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Bourke et al. (2006).Bourke SC, Tomlinson M, Williams TL, Bullock RE, Shaw PJ, Gibson GJ. Effects of non-invasive ventilation on survival and quality of life in patients with amyotrophic lateral sclerosis: a randomised controlled trial. The Lancet Neurology. 2006;5:140–147. doi: 10.1016/S1474-4422(05)70326-4. [DOI] [PubMed] [Google Scholar]
- Brooks et al. (2000).Brooks B, Miller RG, Swash M, Munsat TL. World federation of neurology research group on motor neuron diseases. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. 2000 doi: 10.1080/146608200300079536. [DOI] [PubMed]
- Bueno et al. (2019).Bueno APA, Pinaya WHL, Rebello K, De Souza LC, Hornberger M, Sato JR. Regional dynamics of the resting brain in amyotrophic lateral sclerosis using fractional amplitude of low-frequency fluctuations and regional homogeneity analyses. Brain Connectivity. 2019;9:356–364. doi: 10.1089/brain.2019.0663. [DOI] [PubMed] [Google Scholar]
- Buhour et al. (2017).Buhour M-S, Doidy F, Laisney M, Pitel AL, De La Sayette V, Viader F, Eustache F, Desgranges B. Pathophysiology of the behavioral variant of frontotemporal lobar degeneration: a study combining MRI and FDG-PET. Brain Imaging and Behavior. 2017;11:240–252. doi: 10.1007/s11682-016-9521-x. [DOI] [PubMed] [Google Scholar]
- Chen et al. (2019b).Chen J, Sun D, Shi Y, Jin W, Wang Y, Xi Q, Ren C. Dynamic alterations in spontaneous neural activity in multiple brain networks in subacute stroke patients: a Resting-State fMRI Study. Frontiers in Neuroscience. 2019b;12:994–994. doi: 10.3389/fnins.2018.00994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen et al. (2019a).Chen H, Uddin LQ, Guo X, Wang J, Wang R, Wang X, Duan X, Chen H. Parsing brain structural heterogeneity in males with autism spectrum disorder reveals distinct clinical subtypes. Hum Brain Mapp. 2019a;40:628–637. doi: 10.1002/hbm.24400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen et al. (2015).Chen L, Zhang B, Chen R, Tang L, Liu R, Yang Y, Yang Y, Liu X, Ye S, Zhan S, Fan D. Natural history and clinical features of sporadic amyotrophic lateral sclerosis in China. Journal of Neurology, Neurosurgery, and Psychiatry. 2015;86(10):1075–1081. doi: 10.1136/jnnp-2015-310471. [DOI] [PubMed] [Google Scholar]
- Cheng et al. (2019).Cheng C, Dong D, Jiang Y, Ming Q, Zhong X, Sun X, Xiong G, Gao Y, Yao S. State-related alterations of spontaneous neural activity in current and remitted depression revealed by resting-state fMRI. Frontiers in Psychology. 2019;10 doi: 10.3389/fpsyg.2019.00245. Article 245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chipika et al. (2019).Chipika RH, Finegan E, Shing SLH, Hardiman O, Bede P. Tracking a fast-moving disease: longitudinal markers, monitoring, and clinical trial endpoints in ALS. Frontiers in Neurology. 2019;10 doi: 10.3389/fneur.2019.00229. Article 229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosottini et al. (2012).Cosottini M, Pesaresi I, Piazza S, Diciotti S, Cecchi P, Fabbri S, Carlesi C, Mascalchi M, Siciliano G. Structural and functional evaluation of cortical motor areas in amyotrophic lateral sclerosis. Experimental Neurology. 2012;234:169–180. doi: 10.1016/j.expneurol.2011.12.024. [DOI] [PubMed] [Google Scholar]
- Cui et al. (2019).Cui Q, Sheng W, Chen Y, Pang Y, Lu F, Tang Q, Han S, Shen Q, Wang Y, Xie A. Dynamic changes of amplitude of low-frequency fluctuations in patients with generalized anxiety disorder. Human Brain Mapping. 2019;41(6):1667–1676. doi: 10.1002/hbm.24902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis et al. (1999).Ellis C, Simmons A, Jones D, Bland J, Dawson J, Horsfield M, Williams S, Leigh P. Diffusion tensor MRI assesses corticospinal tract damages in ALS. Neurology. 1999;53(5):1051–1058. doi: 10.1212/WNL.53.5.1051. [DOI] [PubMed] [Google Scholar]
- Friston et al. (1996).Friston KJ, Williams S, Howard R, Frackowiak RS, Turner R. Movement-related effects in fMRI time-series. Magnetic Resonance in Medicine. 1996;35:346–355. doi: 10.1002/mrm.1910350312. [DOI] [PubMed] [Google Scholar]
- Fu et al. (2017).Fu Z, Tu Y, Di X, Biswal BB, Calhoun VD, Zhang Z. Associations between functional connectivity dynamics and BOLD dynamics are heterogeneous across brain networks. Frontiers in Human Neuroscience. 2017;11 doi: 10.3389/fnhum.2017.00593. Article 593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill-Spector (2003).Grill-Spector K. The neural basis of object perception. Current Opinion in Neurobiology. 2003;13:159–166. doi: 10.1016/S0959-4388(03)00040-0. [DOI] [PubMed] [Google Scholar]
- Guo et al. (2013).Guo W, Liu F, Dai Y, Jiang M, Zhang J, Yu L, Long L, Chen H, Gao Q, Xiao C. Decreased interhemispheric resting-state functional connectivity in first-episode, drug-naive major depressive disorder. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2013;41:24–29. doi: 10.1016/j.pnpbp.2012.11.003. [DOI] [PubMed] [Google Scholar]
- Guo et al. (2012).Guo W-B, Liu F, Xue Z-M, Xu X-J, Wu R-R, Ma C-Q, Wooderson SC, Tan C-L, Sun X-L, Chen J-D. Alterations of the amplitude of low-frequency fluctuations in treatment-resistant and treatment-response depression: a resting-state fMRI study. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2012;37:153–160. doi: 10.1016/j.pnpbp.2012.01.011. [DOI] [PubMed] [Google Scholar]
- Hall, Fussell & Summerfield (2005).Hall DA, Fussell C, Summerfield AQ. Reading fluent speech from talking faces: typical brain networks and individual differences. Journal of Cognitive Neuroscience. 2005;17:939–953. doi: 10.1162/0898929054021175. [DOI] [PubMed] [Google Scholar]
- Hardiman et al. (2017).Hardiman O, Al-Chalabi A, Chio A, Corr EM, Logroscino G, Robberecht W, Shaw PJ, Simmons Z, Van den Berg LH. Amyotrophic lateral sclerosis. Nature Reviews Disease Primers. 2017;3 doi: 10.1038/nrdp.2017.71. Article 17071. [DOI] [PubMed] [Google Scholar]
- Huynh et al. (2016).Huynh W, Simon NG, Grosskreutz J, Turner MR, Vucic S, Kiernan MC. Assessment of the upper motor neuron in amyotrophic lateral sclerosis. Clinical Neurophysiology. 2016;127:2643–2660. doi: 10.1016/j.clinph.2016.04.025. [DOI] [PubMed] [Google Scholar]
- Kawagoe, Onoda & Yamaguchi (2019).Kawagoe T, Onoda K, Yamaguchi S. Subjective memory complaints are associated with altered resting-state functional connectivity but not structural atrophy. NeuroImage: Clinical. 2019;21 doi: 10.1016/j.nicl.2019.101675. Article 101675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim et al. (2017).Kim H-J, De Leon M, Wang X, Kim HY, Lee Y-J, Kim Y-H, Kim SH. Relationship between clinical parameters and brain structure in sporadic amyotrophic lateral sclerosis patients according to onset type: a voxel-based morphometric study. PLOS ONE. 2017;12:e0168424. doi: 10.1371/journal.pone.0168424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leff et al. (2009).Leff AP, Price CJ, Seghier ML, Schofield TM, Grogan A, Crinion JT, Green DW. The left superior temporal gyrus is a shared substrate for auditory short-term memory and speech comprehension: evidence from 210 patients with stroke. Brain. 2009;132:3401–3410. doi: 10.1093/brain/awp273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li et al. (2018a).Li J, Duan X, Cui Q, Chen H, Liao W. More than just statics: temporal dynamics of intrinsic brain activity predicts the suicidal ideation in depressed patients. Psychological Medicine. 2018a;49:852–860. doi: 10.1017/S0033291718001502. [DOI] [PubMed] [Google Scholar]
- Li et al. (2018b).Li W, Zhang J, Zhou C, Hou W, Hu J, Feng H, Zheng X. Abnormal functional connectivity density in amyotrophic lateral sclerosis. Frontiers in Aging Neuroscience. 2018b;10:215. doi: 10.3389/fnagi.2018.00215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao et al. (2019).Liao W, Li J, Ji G-J, Wu G-R, Long Z, Xu Q, Duan X, Cui Q, Biswal BB, Chen H. Endless fluctuations: temporal dynamics of the amplitude of low frequency fluctuations. IEEE Transactions on Medical Imaging. 2019;38:2523–2532. doi: 10.1109/TMI.2019.2904555. [DOI] [PubMed] [Google Scholar]
- Liu et al. (2015).Liu F, Guo W, Fouche J-P, Wang Y, Wang W, Ding J, Zeng L, Qiu C, Gong Q, Zhang W, Chen H. Multivariate classification of social anxiety disorder using whole brain functional connectivity. Brain Structure and Function. 2015;220:101–115. doi: 10.1007/s00429-013-0641-4. [DOI] [PubMed] [Google Scholar]
- Liu et al. (2013).Liu F, Guo W, Liu L, Long Z, Ma C, Xue Z, Wang Y, Li J, Hu M, Zhang J. Abnormal amplitude low-frequency oscillations in medication-naive, first-episode patients with major depressive disorder: a resting-state fMRI study. Journal of Affective Disorders. 2013;146:401–406. doi: 10.1016/j.jad.2012.10.001. [DOI] [PubMed] [Google Scholar]
- Liu et al. (2017).Liu F, Wang Y, Li M, Wang W, Li R, Zhang Z, Lu G, Chen H. Dynamic functional network connectivity in idiopathic generalized epilepsy with generalized tonic–clonic seizure. Human Brain Mapping. 2017;38:957–973. doi: 10.1002/hbm.23430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewe et al. (2017).Loewe K, Machts J, Kaufmann J, Petri S, Heinze HJ, Borgelt C, Harris JA, Vielhaber S, Schoenfeld MA. Widespread temporo-occipital lobe dysfunction in amyotrophic lateral sclerosis. Scientific Reports. 2017;7:40252. doi: 10.1038/srep40252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo et al. (2012).Luo C, Chen Q, Huang R, Chen X, Chen K, Huang X, Tang H, Gong Q, Shang H. Patterns of spontaneous brain activity in amyotrophic lateral sclerosis: a resting-state fMRI study. PLOS ONE. 2012;7(9):e45470. doi: 10.1371/journal.pone.0045470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma et al. (2016).Ma X, Zhang J, Zhang Y, Chen H, Li R, Long Z, Zheng J, Wang J, Chen H. Frequency-specific alterations in the fractional amplitude of low-frequency fluctuations in amyotrophic lateral sclerosis. Neurological Sciences. 2016;37:1283–1291. doi: 10.1007/s10072-016-2583-0. [DOI] [PubMed] [Google Scholar]
- Ma et al. (2015).Ma X, Zhang J, Zhang Y, Chen H, Li R, Wang J, Chen H. Altered cortical hubs in functional brain networks in amyotrophic lateral sclerosis. Neurological Sciences. 2015;36:2097–2104. doi: 10.1007/s10072-015-2319-6. [DOI] [PubMed] [Google Scholar]
- McMackin et al. (2019).McMackin R, Dukic S, Broderick M, Iyer PM, Pinto-Grau M, Mohr K, Chipika R, Coffey A, Buxo T, Schuster C, Gavin B, Heverin M, Bede P, Pender N, Lalor EC, Muthuraman M, Hardiman O, Nasseroleslami B. Dysfunction of attention switching networks in amyotrophic lateral sclerosis. NeuroImage: Clinical. 2019;22 doi: 10.1016/j.nicl.2019.101707. Article 101707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oskarsson, Gendron & Staff (2018).Oskarsson B, Gendron TF, Staff NP. Amyotrophic lateral sclerosis: an update for 2018. Mayo Clinic Proceedings. 2018;93:1617–1628. doi: 10.1016/j.mayocp.2018.04.007. [DOI] [PubMed] [Google Scholar]
- Robberecht & Philips (2013).Robberecht W, Philips T. The changing scene of amyotrophic lateral sclerosis. Nature Reviews Neuroscience. 2013;14:248–264. doi: 10.1038/nrn3430. [DOI] [PubMed] [Google Scholar]
- Roth & Shacka (2009).Roth KA, Shacka JJ. Apoptosis in neurodegenerative disease. In: Squire LR, editor. Encyclopedia of neuroscience. Academic Press; Oxford: 2009. pp. 531–537. [Google Scholar]
- Sakoglu et al. (2010).Sakoglu U, Pearlson GD, Kiehl KA, Wang YM, Michael AM, Calhoun VD. A method for evaluating dynamic functional network connectivity and task-modulation: application to schizophrenia. MAGMA. 2010;23:351–366. doi: 10.1007/s10334-010-0197-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt et al. (2014).Schmidt R, Verstraete E, De Reus MA, Veldink JH, Van den Berg LH, Van den Heuvel MP. Correlation between structural and functional connectivity impairment in amyotrophic lateral sclerosis. Human Brain Mapping. 2014;35:4386–4395. doi: 10.1002/hbm.22481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen et al. (2018).Shen DC, Hou B, Cui B, Li XL, Peng P, Tai HF, Zhang K, Liu SW, Fu HH, Liu MS, Feng F, Cui LY. Resting-state functional MRI studies of amyotrophic lateral sclerosis patients with various levels of cognitive impairment. Zhonghua Yi Xue Za Zhi. 2018;98:2002–2006. doi: 10.3760/cma.j.issn.0376-2491.2018.25.007. [DOI] [PubMed] [Google Scholar]
- Sheng et al. (2015).Sheng L, Ma H, Zhong J, Shang H, Shi H, Pan P. Motor and extra-motor gray matter atrophy in amyotrophic lateral sclerosis: quantitative meta-analyses of voxel-based morphometry studies. Neurobiology of Aging. 2015;36:3288–3299. doi: 10.1016/j.neurobiolaging.2015.08.018. [DOI] [PubMed] [Google Scholar]
- Sun et al. (2016).Sun Y, Dai Z, Li Y, Sheng C, Li H, Wang X, Chen X, He Y, Han Y. Subjective cognitive decline: mapping functional and structural brain changes—a combined resting-state functional and structural MR imaging study. Radiology. 2016;281:185–192. doi: 10.1148/radiol.2016151771. [DOI] [PubMed] [Google Scholar]
- Thorns et al. (2013).Thorns J, Jansma H, Peschel T, Grosskreutz J, Mohammadi B, Dengler R, Münte TF. Extent of cortical involvement in amyotrophic lateral sclerosis—an analysis based on cortical thickness. BMC Neurology. 2013;13:148. doi: 10.1186/1471-2377-13-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner et al. (2009).Turner M, Kiernan MC, Leigh PN, Talbot K. Biomarkers in amyotrophic lateral sclerosis. Lancet Neurology. 2009;8:94–109. doi: 10.1016/S1474-4422(08)70293-X. [DOI] [PubMed] [Google Scholar]
- Van Es et al. (2017).Van Es MA, Hardiman O, Chio A, Al-Chalabi A, Pasterkamp RJ, Veldink JH, Van den Berg LH. Amyotrophic lateral sclerosis. Lancet. 2017;390:2084–2098. doi: 10.1016/S0140-6736(17)31287-4. [DOI] [PubMed] [Google Scholar]
- Verma et al. (2013).Verma G, Woo JH, Chawla S, Wang S, Sheriff S, Elman LB, McCluskey LF, Grossman M, Melhem ER, Maudsley AA, Poptani H. Whole-brain analysis of amyotrophic lateral sclerosis by using echo-planar spectroscopic imaging. Radiology. 2013;267:851–857. doi: 10.1148/radiol.13121148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstraete & Foerster (2015).Verstraete E, Foerster BR. Neuroimaging as a new diagnostic modality in amyotrophic lateral sclerosis. Neurotherapeutics. 2015;12:403–416. doi: 10.1007/s13311-015-0347-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstraete et al. (2012).Verstraete E, Veldink JH, Hendrikse J, Schelhaas HJ, Van den Heuvel MP, Van den Berg LH. Structural MRI reveals cortical thinning in amyotrophic lateral sclerosis. Journal of Neurology, Neurosurgery & Psychiatry. 2012;83:383–388. doi: 10.1136/jnnp-2011-300909. [DOI] [PubMed] [Google Scholar]
- Volanti et al. (2011).Volanti P, Cibella F, Sarvà M, De Cicco D, Spanevello A, Mora G, La Bella V. Predictors of non-invasive ventilation tolerance in amyotrophic lateral sclerosis. Journal of the Neurological Sciences. 2011;303:114–118. doi: 10.1016/j.jns.2010.12.021. [DOI] [PubMed] [Google Scholar]
- Wei et al. (2015).Wei Q, Chen X, Zheng Z, Huang R, Guo X, Cao B, Bak TH, Shang H. Screening for cognitive impairment in a Chinese ALS population. Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration. 2015;16:40–45. doi: 10.3109/21678421.2014.966311. [DOI] [PubMed] [Google Scholar]
- Yan et al. (2016).Yan CG, Wang XD, Zuo XN, Zang YF. DPABI: data processing & analysis for (resting-state) brain imaging. Neuroinformatics. 2016;14:339–351. doi: 10.1007/s12021-016-9299-4. [DOI] [PubMed] [Google Scholar]
- Yan & Zang (2010).Yan C-G, Zang Y-F. DPARSF: a MatLab toolbox for pipeline data analysis of resting-state fMRI. Frontiers in Systems Neuroscience. 2010;4 doi: 10.3389/fnsys.2010.00013. Article 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang et al. (2015).Yang Y-L, Deng H-X, Xing G-Y, Xia X-L, Li H-F. Brain functional network connectivity based on a visual task: visual information processing-related brain regions are significantly activated in the task state. Neural Regeneration Research. 2015;10:298–307. doi: 10.4103/1673-5374.152386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang et al. (2019).Yang S, Meng Y, Li J, Fan Y-S, Du L, Chen H, Liao W. Temporal dynamic changes of intrinsic brain activity in schizophrenia with cigarette smoking. Schizophrenia Research. 2019;210:66–72. doi: 10.1016/j.schres.2019.06.012. [DOI] [PubMed] [Google Scholar]
- Yu-Feng et al. (2007).Yu-Feng Z, Yong H, Chao-Zhe Z, Qing-Jiu C, Man-Qiu S, Meng L, Li-Xia T, Tian-Zi J, Yu-Feng W. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain and Development. 2007;29:83–91. doi: 10.1016/j.braindev.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Zhang et al. (2019).Zhang C, Dou B, Wang J, Xu K, Zhang H, Sami MU, Hu C, Tao R, Chen N, Li K. Dynamic alterations of spontaneous neural activity in parkinson’s disease: a resting-state fMRI study. Frontiers in Neurology. 2019;10 doi: 10.3389/fneur.2019.01052. Article 1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang et al. (2017).Zhang J, Ji B, Hu J, Zhou C, Li L, Li Z, Huang X, Hu X. Aberrant interhemispheric homotopic functional and structural connectivity in amyotrophic lateral sclerosis. Journal of Neurology, Neurosurgery and Psychiatry. 2017;88:369–370. doi: 10.1136/jnnp-2016-314567. [DOI] [PubMed] [Google Scholar]
- Zhou et al. (2016).Zhou C, Hu X, Hu J, Liang M, Yin X, Chen L, Zhang J, Wang J. Altered brain network in amyotrophic lateral sclerosis: a resting graph theory-based network study at voxel-wise level. Frontiers in Neuroscience. 2016;10:204–204. doi: 10.3389/fnins.2016.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou et al. (2014).Zhou F, Xu R, Dowd E, Zang Y, Gong H, Wang Z. Alterations in regional functional coherence within the sensory-motor network in amyotrophic lateral sclerosis. Neuroscience Letters. 2014;558:192–196. doi: 10.1016/j.neulet.2013.11.022. [DOI] [PubMed] [Google Scholar]
- Zimmerman et al. (2007).Zimmerman EK, Eslinger PJ, Simmons Z, Barrett AM. Emotional perception deficits in amyotrophic lateral sclerosis. Cognitive and Behavioral Neurology. 2007;20:79–82. doi: 10.1097/WNN.0b013e31804c700b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou et al. (2009).Zou Q, Wu CW, Stein EA, Zang Y, Yang Y. Static and dynamic characteristics of cerebral blood flow during the resting state. NeuroImage. 2009;48:515–524. doi: 10.1016/j.neuroimage.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data imagings (T map), subject information and software for img format (xjview).
a. Results of the sliding-window length of 40 TR. b. Results of the sliding-window length of 50 TR.
Data Availability Statement
The following information was supplied regarding data availability:
The raw data are available in the Supplemental Files.