Abstract
Background
Bacterial biofilms have become a major threat to human health. The objective of this study was to isolate amylase-producing bacteria from soil to determine the overall inhibition of certain pathogenic bacterial biofilms.
Methods
We used serial dilution and the streaking method to obtain a total of 75 positive amylase isolates. The starch-agar plate method was used to screen the amylolytic activities of these isolates, and we used morphological and biochemical methods to characterize the isolates. Optimal conditions for amylase production and purification using Sephadex G-200 and SDS-PAGE were monitored. We screened these isolates’ antagonistic activities and the purified amylase against pathogenic and multi-drug-resistant human bacteria using the agar disk diffusion method. Some standard antibiotics were controlled according to their degree of sensitivity. Finally, we used spectrophotometric methods to screen the antibiofilm 24 and 48 h after application of filtering and purifying enzymes in order to determine its efficacy at human pathogenic bacteria.
Results
The isolated Bacillus species were Bacillus megaterium (26.7%), Bacillus subtilis (16%), Bacillus cereus (13.3%), Bacillus thuringiesis (10.7%), Bacillus lentus (10.7%), Bacillus mycoides (5.3%), Bacillus alvei (5.3%), Bacillus polymyxa (4%), Bacillus circulans (4%), and Micrococcus roseus (4%). Interestingly, all isolates showed a high antagonism to target pathogens. B. alevi had the highest recorded activity (48 mm) and B. polymyxa had the lowest recorded activity (12 mm) against Staphylococcus aureus (MRSA) and Escherichia coli, respectively. On the other hand, we detected no antibacterial activity for purified amylase. The supernatant of the isolated amylase-producing bacteria and its purified amylase showed significant inhibition for biofilm: 93.7% and 78.8%, respectively. This suggests that supernatant and purified amylase may be effective for clinical and environmental biofilm control.
Discussion
Our results showed that soil bacterial isolates such as Bacillus sp. supernatant and its purified amylase are good antibiofilm tools that can inhibit multidrug-resistant former strains. They could be beneficial for pharmaceutical use. While purified amylase was effective as an antibiofilm, the isolated supernatant showed better results.
Keywords: Amylase, Bacillus, Soil bacteria, Antibiofilm, Pathogenic bacteria, SDS-PAGE profile
Introduction
Bacterial biofilms have increasingly become a serious threat to human health (Hall-Stoodley, Costerton & Stoodley, 2004; Saber et al., 2017). These substances have a high level of antibiotic resistance and are hosts to immune response stimulants (Rodrigues et al., 2016; Sharma, Misba & Khan, 2019). They also play an essential role in the pathogenicity of several chronic human infections (Parsek & Singh, 2003). Biofilm removal is a particularly difficult task. The principal method for preventing biofilm formation is applying chemicals or antimicrobials, such as chemical biocides, detergents, and surfactants. Biofilm destruction and prevention are effective methods, as are mechanical removal techniques such as shredding, sonication, freezing, and thawing (De Carvalho, 2007; Kalpana, Aarthy & Pandian, 2012; Elamary, Albarakaty & Salem, 2020). However, because the exopolysaccharide biofilm cells are protected (Kalpana, Aarthy & Pandian, 2012), it is difficult to completely remove biofilms using these methods. Using enzymes is also a good strategy for biofilm removal because enzymes are rabidly biodegradable and environmentally harmless (Xavier et al., 2005). Amylase is a member of the glycosidic hydrolases, which are digestive enzymes that hydrolyze starch glycosidic bonds (Kaur et al., 2012). This family also includes maltotriotic glucose, dextrin, and maltose. Amylase has exhibited excellent antibiotic activity against Pseudomonas aeruginosa and Staphylococcus aureus marine-derived biofilm-forming bacteria (Vaikundamoorthya et al., 2018). Soil is the main part of the terrestrial environment, which is compared with aquatic environments with a large association of microorganisms. Among terrestrial bacteria, Bacillus sp. is the best source of amylase producers, including Bacillus subtilis, Bacillus cereus, and Bacillus polymyxa (El-Fallal et al., 2012; Dash, Rahman & Sarker, 2015). Bacillus amylase is thermostable, and retains a high pH, osmolarity, and high pressure, which are important for manufacturing (Islam et al., 2017). Antibiotics produced by Bacillus sp. such as bacitracin, gramicidin S, polymyxin, and tyrotricidin have exhibited great efficacy against gram-positive and gram-negative bacteria (Perez, Suarez & Castro, 1992; Perez, Suarez & Castro, 1993; Yilmaza, Sorana & Beyatlib, 2006). In this study, we identified and isolated Bacillus spp. from soil using morphological and biochemical assays. We compared the antimicrobial activity of these isolates against five human pathogenic strains. We optimized and purified the amylase after determining the optimal temperature, pH, incubation period, and starch levels needed for the greatest purification. Finally, we monitored the antibiofilm activity of the filtrate and purified amylase from these isolates.
Materials & Methods
Soil sample collection
We collected 100 soil samples during January 2019 from different sites across the Luxor governorate (Monshaat Al Amari, 25°41′14″N32°41′40″E, 16.2 km), Egypt. Samples were collected in sterile plastic bags under aseptic conditions and were transported to the laboratory (Reed & Rigney, 1947). We added 1 gram of soil to 5 ml of tryptic soy broth (Oxoid, Hampshire, United Kingdom), which we modified with 1% starch to make enrichment broth. Samples were incubated at 37 °C for 24 h. The landowner, Mohamed El sanousy, approved field sampling.
Screening and isolation of amylase-producing bacteria
Serial dilution techniques are one of the most precise methods for isolating bacteria from soil (Jamil et al., 2007; Rasooli et al., 2008). We performed serial dilutions up to 10−7. We aseptically transferred 100 µl from each dilution, which we spread into tryptic soy agar media fortified with 1% starch. The plates were incubated at 37 °C for 24 h to determine the colony-forming unit (CFU)/ml. The plates were then flooded with iodine that turns blue when it reacts to unhydrolyzed starch. If the starch was hydrolyzed, a clear halo zone would appear against a dark blue background around the colonies that produce amylase (Gupta et al., 2003; Abd-Elhahlem et al., 2015). We further subcultured bacterial isolates to obtain a pure culture and identified isolates using standard morphological techniques based on colony shape, Gram’s staining, spore formation, and biochemical characterization (Cruickshank et al., 1975; Collins & Lyne, 1984; Koneman et al., 1992). Isolates were then maintained in a 70% sterilized glycerol stock at −70 °C for further use.
Selecting isolates for amylase purification
We selected isolates for amylase extraction and purification, as well as for comparing the purified amylase’s antibiofilm activity against some human pathogenic bacteria, according to the starch hydrolysis ratio (SHR) that we calculated using the following equation (Pranay et al., 2019):
Isolates were subcultured on starch agar plates, which were incubated for 24 h at 37 °C. After incubation, the plates were flooded with iodine. Finally, we calculated SHR using the equation above.
Optimization of amylase production
Effect of temperature and incubation periods
The starch nutrient medium was prepared and the pH was adjusted to 7.5. We then inoculated the medium with the tested isolates. The culture was allowed to grow on a rotatory shaker (250 revs/min) at temperatures ranging from 15 to 65 °C over 48 h. We took 20 ml from each culture at all temperatures and time intervals (18, 24, 48, 72, 96, and 120 h) and centrifuged them to remove the bacterial cells. Finally, the supernatant was collected to assay the amylase activity (Nimisha, Moksha & Gangawane, 2019).
Effect of pH
We prepared the starch nutrient medium and adjusted the pH to different values (5, 6, 7, 8, 9, and 10). Each isolate was inoculated into a portion of this medium and were grown at 50 °C for 24 h. We then collected 20 ml from each isolate and applied the same treatment as above to determine amylase activity (Nimisha, Moksha & Gangawane, 2019).
Effect of starch concentration
All Bacillus isolates were grown on nutrient broth medium with a pH of 9, except B. subtilis which was grown at a pH of 7. Different soluble starch quantities were added to fresh medium to give final concentrations of 0.1, 0.5, 1, 1.5, 2, 2.5, and 3%. We inoculated each isolate in this medium at 50 °C for 24 h to determine their amylase activity (Nimisha, Moksha & Gangawane, 2019).
Determining amylase activity under optimum conditions
The assay mixture contained 2 ml of a solution made up of 1% starch in 50 mM sodium phosphate buffer (pH 7) and 0.1 ml of enzyme solution. After 10 min. of incubation at 40 °C, we stopped the reaction by adding 2 ml of 3,5 dinitrosalicylic acid (DNS) reagent, and heated the tubes at 100 °C for 5 min. The absorbance was measured spectrophotometrically at 540 nm using a blank containing buffer instead of the culture supernatant. We calculated the amount of reduced sugars from a maltose standard curve (Meyer, Fisher & Bernfeld, 1951). Protein was determined using Bradford’s (1976) method.
Enzyme purification
Ammonium sulfate precipitation
The crude amylase enzyme was brought to 45% saturation with ammonium sulfate and was kept overnight in a cold room at 4 °C. We removed the precipitate, brought the supernatant to 85% saturation with ammonium sulfate, and centrifuged it at 8,000 rpm for 10 min at 4 °C. After collecting the precipitate during this step, we stored it at 4 °C (Shinde & Soni, 2014).
Dialysis
This step was conducted to exclude the ammonium sulfate remains and to concentrate the enzyme. We used the dialysis tubes, which were previously soaked in 0.1 M phosphate buffer (pH 6.2), for precipitate dialysis. The precipitate was dissolved in 0.1 M phosphate buffer and was dialyzed against the same buffer (Roe, 2001).
DEAE Sephadex G-200
The crude enzyme preparations of the six culture filtrates were applied separately to a column of DEAE-Sephadex G-200. The enzyme was eluted with a linear gradient of sodium chloride (0 –0.4 M) in 200 ml of sodium phosphate buffer (0.05 M and pH 7), the flow rate was adjusted to 1 ml per 1 min., and 200 ml of eluents were collected into 40 tubes (1 × 7 cm) using an automatic circular fraction collector. We determined enzyme activity and protein concentration in each fraction using the described assay method. Fractions of the highest specific activity were pooled together and kept for further study.
SDS-PAGE
We carried out polyacrylamide gel electrophoresis according to Laemmli’s (1970) method using 10% polyacrylamide gel. Purified B. alvei and B. cereus amylase was loaded into wells parallel to the standard protein markers. The protein bands were stained with Coomassie brilliant blue (Sigma, St. Louis, MO, USA). We estimated the enzyme’s relative molecular weight by comparing it to molecular mass standard markers (Fermentas, Vilnius, Lithuania).
Antibacterial activities
Antagonistic efficacy of the isolated bacteria
We compared the antagonistic efficacy of all isolates against five human pathogenic strains (Escherichia coli, P. aeruginosa, S. aureus (MRSA), Klebsiella pneumoniae, and Acinetobacter baumanii). Strains were kindly provided by the International Luxor Hospital in Luxor Governorate, Egypt. We performed screening using the disc diffusion method. All bacteria were cultured on TSB modified with 1% starch, adjusted to OD595 = 0.01, and incubated at 37 °C at 24 h. The isolated bacterial cultures were centrifuged to exclude the cell debris (6,000 rm for 15 min., Biofuge). We then modified 20 ml of TSA with 1% starch, and poured it in a sterile Petri plate (100 mm diameter). We streaked 100 µl of the five tested pathogens on the plates and punched 6-mm wells in the plates using a sterile borer. The wells were then filled with 100 µl of the isolated bacteria filtrate, and the plates were incubated at 37 °C for 24 h. The inhibition zone was measured using a ruler (Reinheimer, Demkov & Condioti, 1990). Standard antibiotics were used as the controls according to the Kirby Bauer disk diffusion method (Bauer, Sherris & Turk, 1966). The antibiotics were chloramphenicol (C; 30 µg, Oxoid), oxacillin (OX; 1 mcg, Bioanalyse®), vancomycin (VA; 30 mcg, Bioanalyse®), ampicillin/sulbactam (SAM; 10/10 mcg, Bioanalyse®), penicillin G (P; 10 U; Bioanalyse®), erythromycin (E; 15 mcg, Bioanalyse®), sulfamethoxazole/trimethoprim (SXT; 23.75/1.25 µg, BBL™), cefotaxime (CTX; 30 mcg, Bioanalyse®), gentamycin (GM; 10 µg, Bioanalyse®), meropenem (MEM; 10µg, Bioanalyse®), piperacillin (PIP; 100 µg, Bioanalyse®), and piperacillin-tazobactam (PTZ; 100/10 µg, Bioanalyse®). We interpreted the results using the Clinical Laboratory Standard Institute guidelines (CLSI, 2017) to determine whether the tested pathogens were resistant, intermediate, or sensitive against the antibiotics.
Antibacterial activity of purified amylase enzyme from the isolated Bacillus
We placed 100 µl of purified amylase from the selected isolates according to their SHR in the wells of the agar plates inoculated with the target strains. The plates were incubated at 37 °C for 24 h. The halo zone was measured using a ruler.
Biofilm formation assay
We determined the biofilm formation ability of the tested pathogens (E. coli, P. aeruginosa, S. aureus (MRSA), K. pneumoniae, and A. baumanii) using 96-well polystyrene plates (Seper et al., 2011) and the methods described by Salem et al. (2015a) and Salem et al. (2015b): isolates were subcultured on tryptic soy agar for 24 h at 37 °C, suspended in tryptic soy broth, and adjusted to an OD595 of 0.02. We placed 130 µl of each adjusted isolate culture in the microtitre plate (U bottom, Sterilin) for 24 and 48 h at 37 °C. After incubation, the wells were washed with distilled water (six times) and were stained with 0.1% crystal violet for 10 min. The wells were then washed again with distilled water (four times) to remove excess stain. Finally, the wells were destained using 210 µl of ethanol 96%, and the OD595 was read using an Infinite® F50 Robotic (Ostrich) Microplate Plate to quantify the amount of biofilm.
Antibiofilm activity of the isolated Bacillus sp. filtrate and its purified amylase enzyme
We compared the antibiofilm effects of the isolated bacteria filtrate and the purified amylase from the selected isolates against the five human biofilm pathogenic bacteria using the following spectrophotometric methods: a fresh isolate culture was prepared and adjusted to 0.5 McFarland (106 CFU/ml), and 30 µl (this volume was selected according to a preliminary experiment) of these cultures and purified amylase enzyme were added to 130 µl of the tested pathogens at an OD595 of 0.02 after 24 h of incubation at 37 °C to allow biofilm formation. The plates were then incubated for 24 and 48 h and stained with crystal violet. Wells without isolated cultures or amylase served as controls.
Statistical analysis
The variability degree of the results was expressed in the form of mean ± standard deviation (mean ±SD) based on three independent determinations (n = 3). We statistically analyzed the data by one-way ANOVA analysis and compared the control and treatment groups using the least significant difference (LSD) test at 1% (*) levels (Snedeco & Cochran, 1980).
Results and Discussion
Screening and isolating amylase-producing bacteria
Microorganisms that produce amylase are generally isolated from soil and other sources (Fossi, Taveaand & Ndjonenkeu, 2005). Our study explored the isolation of amylase-producing bacteria from soil using the serial dilution spread plate technique. Singh & Kumari (2016) used a similar method by diluting soil samples on starch agar plates and flooding the plates with an iodine solution. The presence of a halo zone around certain colonies indicated amylase production, and a total of 75 bacterial isolates showed a zone of clearance with a starch agar medium. Bacterial isolates were selected according to their amylolytic activity (Table 1). A similar method was also employed by Magalhaes (2010). We further characterized isolates using morphological and biochemical tests shown in Tables 1 and 2. Our results showed that the 75 isolates were comprised of 19 B. megaterium, 12 B. subtilis, 10 B. cereus, eight B. thuringiesis, eight B. lentus, four B. mycoides, four B. alvei, three B. polymyxa, three B. circulans, and three Micrococcus roseus. B. megaterium had the highest recorded prevalence (26.7%) and B. circulans and Micrococcus roseus had the lowest (4%). The CFU of the amylase-producing bacteria in our 100 soil samples ranged from 115 ×103–198 ×105 CFU/ml (Table 1).
Table 1. Prevalence of Bacillus species isolated from soil.
| Isolatesa/Parameters | No. of isolatesb | Percentagec (%) | CFUml−1d | |
|---|---|---|---|---|
| Bacillus megaterium | 20 | 26.7 | 115 ×103 –198 ×105 | |
| Bacillus subtilis | 12 | 16 | ||
| Bacillus cereus | 10 | 13.3 | ||
| Bacillus thuringiesis | 8 | 10.7 | ||
| Bacillus lentus | 8 | 10.7 | ||
| Bacillus mycoides | 4 | 5.3 | ||
| Bacillus alvei | 4 | 5.3 | ||
| Bacillus polymyxa | 3 | 4 | ||
| Bacillus circulans | 3 | 4 | ||
| Micrococcus roseus | 3 | 4 | ||
| Total | 10 | 75 | 100 | |
Notes.
The amylase-producing bacteria isolated from soil.
Number of each isolated type from the total number of the positive isolated sample.
Percentage of each isolate.
Average colony-forming unit of amylase-producing bacteria per ml of 100 g soil samples (highest value to lowest value).
Table 2. Biochemical activities of amylase-producing bacterial isolates and their starch hydrolysis rates.
| Isolate/Testsa | Gram reaction | Motility | Catalase | Egg yolk lecithinase | Nitrate reduction | Vogas proskauer | Citrate utilization | Gelatin hydrolysis | Starch hydrolysis | Indole production |
|---|---|---|---|---|---|---|---|---|---|---|
| Bacillus megaterium | + | + | + | – | – | – | + | + | + | – |
| Bacillus subtilis | + | + | + | – | + | + | + | + | + | – |
| Bacillus cereus | + | + | + | + | + | + | + | – | + | – |
| Bacillus thuringiesis | + | + | + | + | – | + | + | – | + | – |
| Bacillus lentus | + | + | + | – | – | – | – | + | + | – |
| Bacillus mycoides | + | – | + | + | – | + | + | – | + | + |
| Bacillus alvei | + | + | + | – | – | + | – | + | + | + |
| Bacillus polymyxa | + | + | + | – | + | + | – | + | + | – |
| Bacillus circulans | + | + | + | – | + | – | – | + | + | – |
| Micrococcus roseus | + | + | + | – | + | – | + | – | + | – |
Notes.
Morphological and biochemical tests used for identifying isolated bacteria.
Positive.
Negative.
Starch hydrolysis rate.
Optimizing amylase production
Using the starch hydrolysis rates shown in Table 2, we selected the six isolates with the highest hydrolysis rates, namely B. alvei, B. thuringiesis, B. megaterium, B. subtilis, B. cerus, and B. lentus with SHRs of 6.0, 5.67, 5.33, 5.0, 4.0, and 3.5 mm, respectively, for amylase purification.
Effect of temperature and time intervals
All isolates showed maximum amylase production after 24 h. Similar results were obtained by Singh & Kumari (2016), who observed that the highest amylase activity of some Bacillus sp. occurred at 24 h of incubation and that the activity began to decrease after 48 and 72 h of incubation time (Fig. 1B). B. megaterium, B. subtilis, and B. cereus showed maximum amylase production at 45 °C, while other isolates showed maximum amylase production at 55 °C. Mohamed, Malki & Kumosani (2009) similarly reported that some amylase were stable at 40 °C and some at 50 °C (Fig. 1A).
Figure 1. Optimization and purification conditions of amylase enzyme from selected Bacillus sp.
(A) The effect of temperature. B. megaterium, B. subtilis, and B. cereus showed maximum amylase production at 45 °C, while other isolates showed maximum amylase production at 55 °C. (B) The effect of incubation time. All isolates showed maximum amylase production after 24 h incubation. (C) The effect of pH. All Bacillus isolates showed maximum amylase production at a pH of 8.0 except B. subtilis, which maximally produced amylase at a pH of 7.0. (D) The effect of starch concentration. B. subtilis and B. cereus had maximum amylase production at 1.5% soluble starch concentration. The remaining isolates had maximum amylase production at 2.0% soluble starch concentration.
Effect of pH
All Bacillus isolates showed maximum amylase production at a pH of 8, except for B. subtilis which maximally produced amylase at a pH of 7. A previous study by Behal et al. (2006) found that the optimum pH for amylase production was 8. Another study by Singh & Kumari (2016) reported that while amylase activity was recorded at different pH levels from 5 to 10, maximum activity was observed at pH 7 (Fig. 1C).
Effect of substrate concentration
Our results showed that B. subtilis and B. cereus had maximum amylase production at 1.5% soluble starch concentration. The remaining isolates showed maximum amylase production at 2.0% soluble starch concetration (Fig. 1D). Mishra & Behera (2008) reported that Bacillus strains produced the maximum yield of amylase at a starch concentration of 2%.
Enzyme activity
We purified extracellular amylase from the Bacillus isolated from soil to homogeneity using 45–85% ammonium sulfate precipitation and Sephadex G-200 (Fig. 2). As shown in Table 3, the highest amylase activity was found in B. alvei (96.02 U/ml), followed by B. thuringiesis (88.64 U/ml). B. megaterium, B. subtilis, and B. cereus showed amylase activities of 80.03, 76.0, and 55.9 U/ ml, respectively. B. lentus showed the lowest amylase activity of 45.69 U/ml.
Figure 2. Elution profile of isolated Bacillus sp. on Sephadex G-200.
The figure shows the total protein concentrations (mg/ml) along with the enzyme activity (U/ml) for (A) B. megaterium; (B) B. subtilis; (C) B. cereus; (D) B. thuringiesis; (E) B. lentus; and (F) B. alvei.
Table 3. Purification profile of amylase produced from different Bacillus sp. isolates.
| Isolatesa Purification stepb | Bacillus megaterium | Bacillus subtilis | Bacillus cereus | Bacillus thuringiesis | Bacillus lentus | Bacillus alvei | |
|---|---|---|---|---|---|---|---|
| Crude | TP(mg/ml) | 0.66 | 0.96 | 0.81 | 0.54 | 0.99 | 0.86 |
| TA(U) | 6630 | 5000 | 3932 | 7890 | 2780 | 10040 | |
| EA(U/ml) | 33.15 | 25.0 | 19.66 | 39.45 | 13.9 | 50.2 | |
| Ammonium sulfate | TP(mg/ml) | 0.58 | 0.88 | 0.75 | 0.39 | 0.79 | 0.77 |
| TA(U) | 701.0 | 640 | 461.0 | 820 | 308 | 1029 | |
| EA(U/ml) | 35.06 | 32.0 | 23.08 | 41.0 | 15.4 | 51.47 | |
| Dialysis | TP(mg/ml) | 0.50 | 0.82 | 0.71 | 0.37 | 0.60 | 0.70 |
| TA(U) | 718 | 700 | 502 | 805 | 365 | 591 | |
| EA(U/ml) | 35.9 | 35.0 | 25.12 | 40.26 | 18.26 | 29.56 | |
| Sephadex G-200 | TP(mg/ml) | 0.45 | 0.76 | 0.64 | 0.29 | 0.48 | 0.59 |
| TA(U) | 800 | 760 | 559 | 886 | 456.9 | 960 | |
| EA(U/ml) | 80.03 | 76.0 | 55.9 | 88.64 | 45.69 | 96.02 | |
Notes.
Isolated Bacillus sp. selected for amylase purification according to SHR.
Different purification steps of amylase purification.
- TP
- total protein
- TA
- total activity
- EA
- enzyme activity
SDS-PAGE
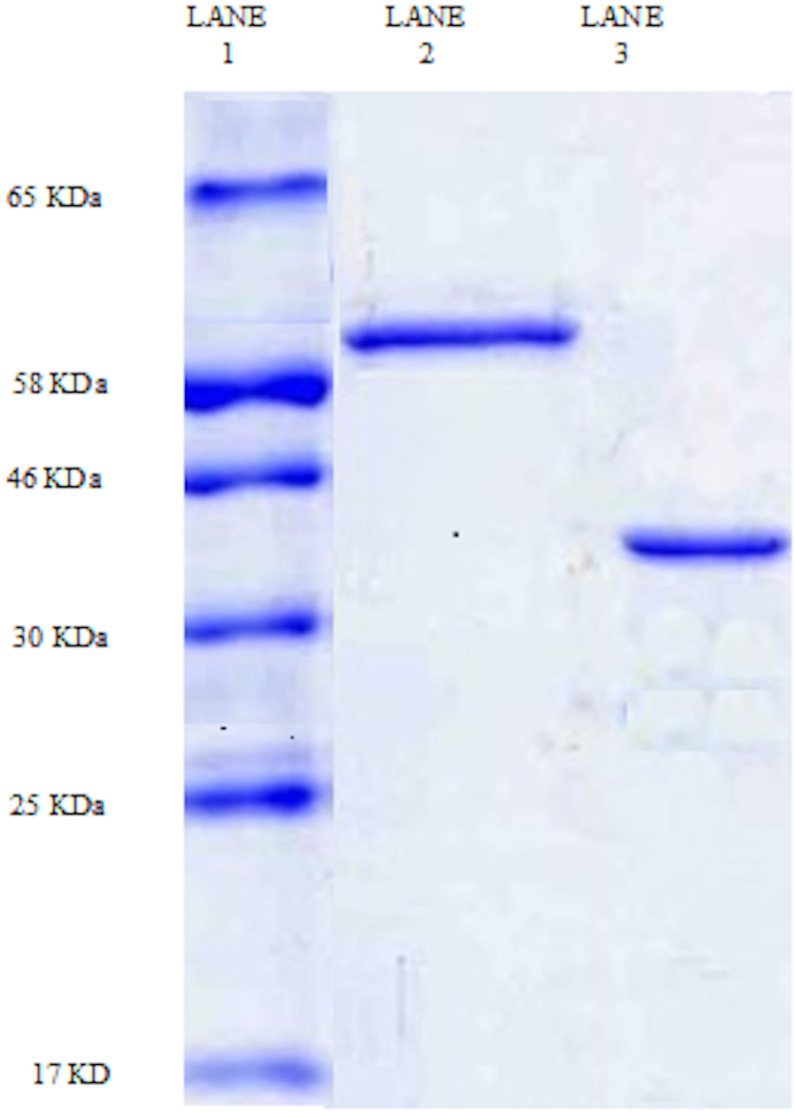
After purification, the SDS- PAGE profile showed a single protein band of amylase for each bacteria, confirming that the enzyme has been purified to homogeneity. The molecular weights of B. alvei and B. cereus were 60 KDa and 43 KDa, respectively (Fig. 3). The molecular weight of B. alvei was similar to that of the amylase isolated from B. subtilis (56 KDa and 55 KDa, respectively) (Bano et al., 2011; Takkinen et al., 1983). The molecular weight of B. cereus was equal to the molecular weight of amylase from B. cereus and B. subtilis (42 KDa) (Annamalai et al., 2011; Das, Doley & Mukherjee, 2004). Previous studies have reported different molecular weights for amylase isolated from Bacillus sp. Lin, Chyau & Hsu (1998) found that Bacillus sp. can produce five different forms of amylase.
Figure 3. SDS-PAGE amylase profile for B. alvei and B. cereus.
Lane 1: molecular weight marker. Lane 2: amylase purified from B. alvei; Lane 3: amylase purified from B. cereus.
Antibacterial activity
Antagonistic efficacy of the isolated bacteria and purified amylase enzyme from selected isolates
In this study, we compared the antimicrobial activity of Bacillus supernatant and purified amylase with standard antibiotics against human pathogens (Table 4). The standard antibiotics served as the control group since pathogenic bacteria can become extremely resistant to widely-used antibiotics. The pharmaceutical industry is in need of new and natural antimicrobials that can overcome the problem of multidrug-resistant strains (Schmidt, 2004; Salem et al., 2015a; Salem et al., 2015b; Salem et al., 2017). Several soil organisms can produce antibiotics using a survival mechanism that can eliminate their competition (Talaro & Talaro, 1996; Jensen & Wright, 1997). The Bacillus genus is a terrestrial strain that can produce inhibitory compounds from peptide-derivative and lipopolypeptide antibiotics (Mannanov & Sattarova, 2001; Tamehiro et al., 2002; Stein, 2005). Oscariz, Lasa & Pisabarro (1999) and Yilmaza, Sorana & Beyatlib (2006) found that isolated bacteriocin-producing strains such as Bacillus sp. were active against gram-negative and gram-positive bacteria. We compared the antimicrobial activity of the isolated amylase-producing bacteria and purified amylase against five human pathogenic bacteria (Table 4). We found that E. coli was resistant to sulfamethoxazole-trimethoprim (23.75/1.25 mcg), gentamycin (10 µg), cefotaxime (30 µg), piperacillin (100 µg), and piperacillin-tazobactam (100/10 µg); showed intermediate sensitivity to ampicillin-sulbactam (10/10 mcg); and was sensitive to chloramphenicol (30 µg) and meropenem (10 µg). Notably, we observed that all isolated bacteria showed high antimicrobial activity in response to E. coli, with B. polymyxa showing the most activity (36 mm) and B. subtilis and B. cereus showing the least (12 mm). B. mycoides and M. roseus showed no antimicrobial activity in response to E. coli. Our results were consistent with the results of Moshafi et al. (2011), who observed that one soil bacterial isolate, identified as Bacillus sp., was found to inhibit six pathogenic bacteria, namely E. coli, K. pneumoniae, S. typhi, P. aeruginosa, S. aureus, and S. epidermidis. When examining the antimicrobial activity in response to K. pneumoniae, we found that although K. pneumoniae was resistant to all tested antibiotics, it showed intermediate sensitivity to ampicillin-sulbactam (10/10 mcg). It is worth mentioning that all isolates had great antimicrobial effects, with B. megaterium showing the highest inhibition (26 mm) and B. polymyxa showing the lowest (17 mm) (Table 4). In contrast, B. mycoides and M. roseus were resistant to K. pneumoniae. In a recent study, (Reed & Rigney, 1947) reported that B. subtilis metabolites inhibited K. pneumoniae, P. aeruginosa, S. aureus, E. coli, P. mirabilis, and other bacteria. A. baumanii was resistant to all tested antibiotics and was sensitive only to chloramphenicol (30 µg). However, all isolated bacteria showed improved antibacterial effects against the tested pathogens, with B. alvei and B. cirulans showing the greatest inhibitory effects (39 mm), and B. subtilis and B. thuringiesis showing the lowest (21 mm) (Table 4). We found that B. mycoides and Micrococcus roseus displayed no inhibitory effects against the tested pathogens. Ramachandran et al. (2014) reported that B. subtilis showed antimicrobial activity against A. baumanii, E. coli, K. pneumoniae, P. aeruginosa, and S. aureus. The susceptibility level of P. aeruginosa indicated that it was resistant to sulfamethoxazole-trimethoprim (23.75/1.25 µg), cefotaxime (30 mcg), gentamycin (10 µg), meropenem (10 µg), and piperacillin (100 µg). It had intermediate sensitivity to chloramphenicol (30 µg) and ampicillin-sulbactam (10/10 mcg), and was sensitive to piperacillin-tazobactam (100/10 µg). We noted that all isolates showed great antibacterial effects against the tested pathogens, with B. lentus and B. cirulans having the greatest effects (32 mm) and B. subtilis having the least (15 mm). Salem et al. (2015a) and Salem et al. (2015b) similarly reported that Bacillus strains exhibited antimicrobial activity against P. aeruginosa, E. coli, and S. typhi. Perez, Suarez & Castro (1992) and Aslim, Saglam & Beyatli (2002) found that B. subtilis, B. thuringiesis, and B. megaterium showed antibacterial activity against E. coli and P. aeruginosa. We found that S. aureus (MRSA) was resistant to oxacillin (1 mcg), vancomycin (30 mcg), penicillin G (10 U), cefotaxime (30 mcg), and gentamycin (10 µg), and showed intermediate sensitivity to chloramphenicol (30 µg), erythromycin (15 mcg), and sulfamethoxazole-trimethoprim (23.75/1.25 µg). The isolated amylase-producing bacteria showed better antibacterial effects on the tested pathogens, with the greatest effect shown by B. alvei (48 mm) and the least effect shown by B. cereus (14 mm) (Table 4). However, B. mycoides and M. roseus did not affect S. aureus. Similar results were obtained by Moshafi et al. (2011) and Ramachandran et al. (2014). In contrast to the high antimicrobial activity observed in the isolated soil bacteria, the purified amylase from the selected isolates had very little effect on E. coli and K. pneumoniae (the highest inhibition diameter was 7.5 mm), and no recorded effect in response to the other tested pathogens (Table 4). This result is similar to that of Kalpana, Aarthy & Pandian (2012), who confirmed that amylase enzyme has no antibacterial effect.
Table 4. Comparing the antibacterial activity of Bacillus sp. and purified amylase enzyme to different standard antibiotics.
| Tested pathogens/Antibiotica/Isolates | Escherichia coli | Klebsiella pneumoniae | Acinetobacter baumanii | Pseudomonas aeuroginosa | Staphylococcus aureus (MRSA) | ||
|---|---|---|---|---|---|---|---|
| Chloramphenicol (30 µg) | S | R | S | I | I | ||
| Oxacillin (1 mcg) | NA | NA | NA | NA | R | ||
| Vancomycin (30 mcg) | NA | NA | NA | NA | R | ||
| Ampicillin- sulbactam (10/10 mcg) | I | I | R | I | NA | ||
| Pencillin G (10 U) | NA | NA | NA | NA | R | ||
| Erythromycin (15 mcg) | NA | NA | NA | NA | I | ||
| Sulfameth.-trimethoprim (23.75/1.25 µg) | R | R | R | R | I | ||
| Cefotaxime (30 mcg) | R | R | R | R | R | ||
| Gentamycin (10 µg) | R | R | R | R | R | ||
| Meropenem (10 µg) | S | R | R | R | NA | ||
| Piperacillin (100 µg) | R | R | R | R | NA | ||
| Piperacillin-tazobactam (100/10 µg) | R | R | R | S | NA | ||
Notes.
Comparing the antimicrobial susceptibility of a group of standard antibiotics (CLSI, 2017) against five human pathogenic strains (control).
Antimicrobial activity of the isolated Bacillus sp.
- R
- Resistant
- S
- sensitive
- I
- intermediate
- NA
- Not applicable (for antibiotics that were not specific to the bacterial strains)
- NI
- No inhibition
- C
- antimicrobial activity of purified amylase from some isolated Bacillus sp.
- ABM
- amylase purified from Bacillus megaterium
- ABS
- amylase purified from Bacillus subtilis
- ABC
- amylase purified from purified Bacillus cereus
- ABT
- amylase purified from Bacillus thuringiesis
- ABL
- amylase purified from Bacillus lentus
- ABA
- amylase purified from Bacillus alvei
Values expressed as mean ± SD.
Biofilm formation assay
We quantitatively determined the amount of biofilm (OD595) in the tested pathogens and designated the 24 and 48 h treatments as the control groups (Figs. 4, 5, 6, and 7). Using the OD595 nm mean values, we defined the pathogens as low, moderate, or high bacterial biofilm formers when the OD595 nm was > 1, 1 - 2.9, and < 2.9, respectively. A. baumanii and Klebsiella pneumoniae were high biofilm formers while E. coli, P. aeruginosa, and S. aureus (MRSA) were low biofilm formers.
Figure 4. Comparing the antibiofilm activity of Bacillus-producing amylase-filtrate against some pathogenic bacteria after 24 h treatment.
The figure shows Bacillus sp. filtrate, T1: B. megaterium, T2: B. subtilis, T3: B. cereus, T4: B. thuringiesis, T5: B. lentus, T6: B. alvei, T7: B. polymyxa, T8: B. circulans. The tested pathogenic bacteria are (A) E. coli, (B) P. aeruginosa, (C) S. aureus (MRSA), (D) K. pneumoniae, and (E) A. baumanii. The figure shows the averages from at least three independent measurements. The error bars indicate the standard deviations using the least significant difference (LSD). The 1% LSD for E. coli, P. aeruginosa, S. aureus (MRSA), K. pneumoniae, and A. baumanii was 0.016, 0.013, 0.014, 0.04, and 0.17, respectively. Significant differences between controls and treated samples are marked by asterisks. P < 0.05; Krustal-Wallis test and post hoc Bonferroni post-tests. Scales are different for A–C versus D and E.
Figure 5. Comparing the antibiofilm activity of Bacillus-producing amylase-filtrate against some pathogenic bacteria after 48 h treatment.
The figure shows Bacillus sp. filtrate, T1: B. megaterium, T2: B. subtilis, T3: B. cereus, T4: B. thuringiesis, T5: B. lentus, T6: B. alvei, T7: B. polymyxa, T8: B. circulans. The tested pathogenic bacteria are (A) E. coli, (B) P. aeruginosa, (C) S. aureus (MRSA), (D) K. pneumoniae, and (E) A. baumanii. The figure shows the averages from at least three independent measurements. The error bars indicate the standard deviations using the least significant difference (LSD). An LSD of 1% for E. coli, P. aeruginosa, S. aureus (MRSA), K. pneumoniae, and A. baumanii was 0.027, 0.014, 0.013, 0.08 and 0.19, respectively. Significant differences between controls and treated samples are marked by asterisks. P < 0.05; Krustal-Wallis test and post hoc Bonferroni post-tests. Scales are different for A-C versus D and E.
Figure 6. Comparing the antibiofilm activity of Bacillus- purified amylase enzyme against some pathogenic bacteria after 24 h treatment.
The figure shows Bacillus sp. filtrate, T1: B. megaterium, T2: B. subtilis, T3: B. cereus, T4: B. thuringiesis, T5: B. lentus, T6: B. alvei. The tested pathogenic bacteria are (A) E. coli, (B) P. aeruginosa, (C) S. aureus (MRSA), (D) K. pneumoniae, and (E) A. baumanii. The figure shows the averages from at least three independent measurements. The error bars indicate the standard deviations using the least significant difference (LSD). An LSD of 1% for E. coli, P. aeruginosa, S. aureus (MRSA), K. pneumoniae, and A. baumanii was 0.11, 0.01, 0.03, 0.01, and 0.02, respectively. Significant differences between controls and treated samples are marked by asterisks. P < 0.05; Krustal-Wallis test and post hoc Bonferroni post-tests. Scales are different for A–C versus D and E.
Figure 7. Comparing the antibiofilm activity of Bacillus- purified amylase enzyme against some pathogenic bacteria after 48 h treatment.
The figure shows Bacillus sp. filtrate, T1: B. megaterium, T2: B. subtilis, T3: B. cereus, T4: B. thuringiesis, T5: B. lentus, T6: B. alvei. The tested pathogenic bacteria are (A) E. coli, (B) P. aeruginosa, (C) S. aureus (MRSA), (D) K. pneumoniae, and (E) A. baumanii. The figure shows the averages from at least three independent measurements. The error bars indicate the standard deviations using the least significant difference (LSD). An LSD of 1% for E. coli, P. aeruginosa, S. aureus (MRSA), K. pneumoniae, and A. baumanii was 0.01, 0.009, 0.007, 0.018, and 0.35, respectively. Significant differences between controls and treated samples are marked by asterisks. P < 0.05; Krustal-Wallis test and post hoc Bonferroni post-tests. Scales are different for A-C versus D and E.
Antibiofilm activity of isolated bacterial filtrate and purified amylase enzyme from selected Bacillus isolates
In a natural ecosystem, bacteria can be exist in two forms: planktonic cells, which are susceptible to antibiotics and other antimicrobial agents, and biofilm, which are resistant to antibiotics and disinfectants (Limoli, Jones & Wozniak, 2015). A biofilm is a complex community of bacteria attached to a surface or interface enclosed in an exopolysaccharide matrix, protected from unfavorable antibiotics, host defenses, or oxidative stresses (Shakibaie, 2018). Microbial biofilms have created huge problems in the treatment of both community and hospital infections. Most antimicrobial agents are unable to penetrate biofilm due to its extracellular polymeric substances (EPS), which act as a barrier protecting the bacterial cells within the biofilm. Therefore, we must use compounds that have the potential to degrade the biofilm’s EPS. Enzymes have proven to be effective in EPS degradation (Kalpana, Aarthy & Pandian, 2012; Lequette et al., 2010). In our study, we compared the antibiofilm activity of the Bacillus sp. that we isolated from soil (supernatant) and the purified amylase from these isolates against five human pathogenic biofilm former strains. Our study has reported that Bacillus supernatant and amylase enzyme can inhibit the biofilm formation in various pathogens. We confirmed the ability of pathogenic bacterial strains to form biofilm formation using spectrophotometric methods before applying the antibiofilm treatments of bacterial filtrate and purified amylase enzyme (Figs. 4, 5, 6, and 7). The antibiofilm activity was screened using a spectrophotometric method with crystal violet staining. Our results showed that the bacteria isolated from soil exhibited significant antibiolfilm effects against the tested pathogenic strains after 24 h of treatment. The percentage of inhibition significantly increased after 48 h of treatment. The highest percentage of inhibition was recorded for B. circulans against K. pneumonia: 93.7% after 48 h of treatment (Fig. 5D, T8). We also monitored the efficacy of the purified amylase enzyme as an antibiofilm against the same tested pathogens. Our results revealed that the purified amylase showed significant antibiofilm effects after 24 h of treatment. The percentages of inhibition significantly increased after 48 h of treatment. We observed the highest percentage for B. alvei against K. pneumonia: 78.8% after 48 h of treatment (Fig. 7D, T6). Our results also showed the greatest enzyme activity from B. alvei (96.02 U/ ml), followed by B. thuringiesis (88.64 U/ ml), B. megaterium (80.03 U/ ml), and B. subtilis (76.0 U/ml). The lowest antibiofilm efficacy was recorded in B. cereus, with an enzyme activity of 55.9 U/ml, and B. lentus, with an enzyme activity of 45.69 U/ml. Kalpana, Aarthy & Pandian (2012) first reported that purified amylase enzyme from B. subtilis was a good antibiofilm agent against biofilm-forming clinical pathogens. The purified enzyme caused 68.33%, 64.84%, 61.81%, and 59.2% of inhibition in V. cholerae (VC5, VC26), MRSA (102), and P. aeruginosa ATCC10145, respectively. Another study by Vaikundamoorthya et al. (2018) confirmed the antibiofilm efficacy of the thermostable amylase enzyme from B. cereus.
It is worth noting that the isolated bacteria filtrate showed great antibiofilm activity compared to the purified amylase enzyme from the selected isolates. This may be due to the accumulation of some extracellular and intracellular metabolites in the medium, which is further explained by the metabolic overflow theory (Pinu, Villas-Boas & Aggio, 2017; Pinu et al., 2018; Horak et al., 2019). Bacillus also showed great efficacy in the production of carbohydrate-active enzymes and bioactive compounds, as well as the secretion of a variety of extracellular metabolites and lytic enzymes (Abdel-Aziz, 2013). Additionally, Bacillus species are the most efficient at producing peptide antibiotic compounds such as polymyxin, colistin, and circulin (Katz & Demain, 1997; Atanasova-Pancevska et al., 2016). Our results also indicated a great inhibition of biofilm from the amylase enzymes of B. alvei (96.02 U/ml), followed by B. thuringiesis (88.64 U/ml), B. megaterium (80.03 U/ml), B. subtilis (76.0 U/ml), B. cereus (55.9 U/ml), and B. lentus (45.69 U/ml) (Table 3). This may be due to the increased enzyme activity in each species.
Conclusion
Our results indicated that the ability of Bacillus sp. to produce extracellular and intracellular metabolites, lytic enzymes, and some peptide antibiotics directly affects the antimicrobial functions of various Bacillus sp. (amylase producers) in the soil. We observed the highest inhibition rate (93.7%) when comparing the species’ antibiofilm effects against five human pathogenic strains. We observed an inhibition rate of 78.8% when comparing the antibiotic biofilm activity of purified amylase against the strains. Our study showed that Bacillus filtrate is an effective clinical antibiofilm. Futher studies are being conducted to determine the exact composition of the filtrate and its active agents.
Supplemental Information
Values of fractions, enzyme activities and protein concentrations for Fig 2; Values of OD595 for the bacterial biofilm of Bacillus-producing amylase-filtrate and/or purified amylase enzyme against some pathogenic bacteria after 24 or 48h for Figs 4- 7.
Funding Statement
The authors received no funding for this work.
Contributor Information
Rokaia Elamary, Email: roka.elamary_88@yahoo.com.
Wesam M. Salem, Email: wesam.salem@svu.edu.eg.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Rokaia Elamary conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Wesam M. Salem conceived and designed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Field Study Permissions
The following information was supplied relating to field study approvals (i.e., approving body and any reference numbers):
The landowner, Mohamed El sanousy, approved field sampling.
Data Availability
The following information was supplied regarding data availability:
Raw data are available as Supplemental Files.
References
- Abd-Elhahlem et al. (2015).Abd-Elhahlem BT, El-Saway M, Gamal RF, Abou-Taleb KA. Production of amylases from Bacillus amyloliquefaciens under submerged fermentation using some agro-industrial by-products. Annals of Agricultural Sciences. 2015;60(2):193–202. doi: 10.1016/j.aoas.2015.06.001. [DOI] [Google Scholar]
- Abdel-Aziz (2013).Abdel-Aziz SM. Extracellular metabolites produced by a novel strain, Bacillus alvei NRC-14: 5. multiple plant-growth promoting properties. Journal of Basic and Applied Scientific Research. 2013;3(1):670–682. [Google Scholar]
- Annamalai et al. (2011).Annamalai N, Thavasi R, Vijayalakshmi S, Balasubramanian T. Extraction, Purification and characterization of thermostable, alkaline tolerant a-amylase from Bacillus cereus. Indian Journal of Microbiology. 2011;51(4):424–429. doi: 10.1007/s12088-011-0160-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslim, Saglam & Beyatli (2002).Aslim B, Saglam N, Beyatli Y. Determination of some properties of Bacillus isolated from soil. Turkish Journal Of Biology. 2002;26:41–48. [Google Scholar]
- Atanasova-Pancevska et al. (2016).Atanasova-Pancevska N, Popovska I, Davalieva K, Kungulovski D. Screening for antimicrobial activity of Bacillus subtilis and Paenibacillus Alvei isolated from rotten apples compost. Acta Microbiologica Bulgarica. 2016;32(1):56–64. [Google Scholar]
- Bano et al. (2011).Bano S, Qader SAUl, Aman A, Syed MN, Azhar A. Purification and characterization of novel α-Amylase from Bacillus subtilis KIBGE HAS. An Official Journal of the American Association of Pharmaceutical Scientists. 2011;12(1):255–261. doi: 10.1208/s12249-011-9586-1. [DOI] [Google Scholar]
- Bauer, Sherris & Turk (1966).Bauer KA, Sherris J, Turk M. Antibiotic susceptibility testing by standardized single disc method. American Journal of Clinical Pathology. 1966;45:493–496. doi: 10.1093/ajcp/45.4_ts.493. [DOI] [PubMed] [Google Scholar]
- Behal et al. (2006).Behal A, Singh J, Sharma MK, Puri P, Batra N. Studied thermostable amylase producing Bacillus sp. International Journal of Agriculture and Biology. 2006;8:80–83. [Google Scholar]
- Bradford (1976).Bradford MM. A rapid and sensitive method for the quantition of microgram quantities of protein -dye binding. Analytical Biochemistry. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- CLSI (2017).Clinical Laboratory Standards Institute(CLSI) Performance standards for susceptibility testing. 27th edition Document M100- S27 Wayne: Clin Lab Stand Inst; 2017. [Google Scholar]
- Collins & Lyne (1984).Collins C, Lyne P. Microbiological methods 5th microbiology laboratory manual. British Liberary. Butter Worth Inc; United Kingdom: 1984. [Google Scholar]
- Cruickshank et al. (1975).Cruickshank R, Duguid JP, Marmion BP, Swain RHA. Medical microbiology. 12th edition. Volume Two. Edinburg, London and New York: The practice of Medical Microbiology; 1975. [Google Scholar]
- Das, Doley & Mukherjee (2004).Das K, Doley R, Mukherjee AK. Purification and biochemical characterization of a thermostable, alkaliphilic, extracellular a-amylase from Bacillus subtilis DM-03, a strain isolated from the traditional fermented food of India. Biotechnology and Applied Biochemistry. 2004;40:291–298. doi: 10.1042/BA20040034. [DOI] [PubMed] [Google Scholar]
- Dash, Rahman & Sarker (2015).Dash BK, Rahman MM, Sarker PK. Molecular identification of a newly isolated Bacillus subtilis BI19 and optimization of production conditions for enhanced production of extracellular amylase. BioMed Research International. 2015;2015:859805. doi: 10.1155/2015/859805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Carvalho (2007).De Carvalho CCCCR. Biofilms: recent developments on an old battle. Recent Patents on Biotechnology. 2007;1:49–57. doi: 10.2174/187220807779813965. [DOI] [PubMed] [Google Scholar]
- El-Fallal et al. (2012).El-Fallal A, Dobara MA, El-Sayed A, Omar N. Starch and microbial á-amylases: from concepts to biotechnological applications. Carbohydrates—comprehensive Studies on Glycobiology and Glycotechnology. 2012. pp. 459–488. Epub ahead of print Nov 21 2012.
- Elamary, Albarakaty & Salem (2020).Elamary RB, Albarakaty FM, Salem WM. Efficacy of Acacia nilotica aqueous extract in treating biofilm-forming and multidrug resistant uropathogens isolated from patients with UTI syndrome. Scientific Reports. 2020;10(1):1–14. doi: 10.1038/s41598-019-56847-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossi, Taveaand & Ndjonenkeu (2005).Fossi BT, Taveaand F, Ndjonenkeu T. Production and partial characterization of a themostable amylase from ascomycetes yeast strain isolated from starchy soils. African Journal of Biotechnology. 2005;4:14–18. [Google Scholar]
- Gupta et al. (2003).Gupta R, Gigras P, Mohapatra H, Goswami VK, Chauhan B. Microbial-amylases: a biotechnological perspective. Process Biochemistry. 2003;38:1599–1616. doi: 10.1016/S0032-9592(03)00053-0. [DOI] [Google Scholar]
- Hall-Stoodley, Costerton & Stoodley (2004).Hall-Stoodley L, Costerton JW, Stoodley P. Bacterial biofilms: from the natural environment to infectious diseases. Nature Reviews Microbiology. 2004;2:95–108. doi: 10.1038/nrmicro821. [DOI] [PubMed] [Google Scholar]
- Horak et al. (2019).Horak G, Engelbrecht PJ, Rensburg JV, Claassens S. Microbial metabolomics: essential definitions and the importance of cultivation conditions for utilizing Bacillus species as bionematicides. Journal of Applied Microbiology. 2019;127:326–343. doi: 10.1111/jam.14218. [DOI] [PubMed] [Google Scholar]
- Islam et al. (2017).Islam T, Choudury N, Hossain MM, Khan TT. Isolation of amylase producing bacteria from soil, identification by 16S rRNA gene sequencing and characterization of amylase. Bangladesh Journal of Microbiology. 2017;34(1):01–06. [Google Scholar]
- Jamil et al. (2007).Jamil B, Hasan F, Hameed A, Ahmed S. Isolation of Bacillus subtilis MH-4 from soil and its potential of polypeptidic antibiotic production. Pakistan Journal of Pharmaceutical Sciences. 2007;20(1):26–31. [PubMed] [Google Scholar]
- Jensen & Wright (1997).Jensen MJ, Wright DN. Microbiology for the health sciences. Prentice Hall; New York: 1997. Chemotherapeutic agents; pp. 132–145. [Google Scholar]
- Kalpana, Aarthy & Pandian (2012).Kalpana BJ, Aarthy S, Pandian SK. Antibiofilm activity of α-amylase from Bacillus subtilis S8-18 against biofilm forming human bacterial pathogens. Applied Biochemistry and Biotechnology. 2012;167:1778–1794. doi: 10.1007/s12010-011-9526-2. [DOI] [PubMed] [Google Scholar]
- Katz & Demain (1997).Katz E, Demain AL. The peptide antibiotics of Bacillus, chemistry, biogenesis, and possible functions. Bacteriological reviews. 1997;41(2):449–474. doi: 10.1128/br.41.2.449-474.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur et al. (2012).Kaur A, Kaur M, Samyal ML, Ahmed Z. Isolation, characterization and identification of bacterial strain producing amylase. Journal of Microbiology and Biotechnology Research. 2012;2(4):573–579. [Google Scholar]
- Koneman et al. (1992).Koneman E, Allen S, Janda W, Schrenchen P, Winn W. Color atlas and text book of diagnostic microbiolog. 4th Edition L.B Lippincott Company; Philadelphia: 1992. [Google Scholar]
- Laemmli (1970).Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lequette et al. (2010).Lequette Y, Boelsb G, Clarissea M, Faille C. Using enzymes to remove biofilms of bacterial isolates sampled in the food-industry. Biofouling. 2010;26:421–431. doi: 10.1080/08927011003699535. [DOI] [PubMed] [Google Scholar]
- Limoli, Jones & Wozniak (2015).Limoli DH, Jones CJ, Wozniak DJ. Bacterial extracellular polysaccharides in biofilm formation and function. Microbial Biofilms. 2015:223–247. doi: 10.1128/microbiolspec.MB-0011-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, Chyau & Hsu (1998).Lin LL, Chyau CC, Hsu WH. Production and properties of a raw-starch-degrading amylase from the thermophilic and alkaliphilic Bacillus sp. TS-23. Biotechnology and Applied Biochemistry. 1998;28:61–68. [PubMed] [Google Scholar]
- Magalhaes (2010).Magalhaes Application of microbial alpha amylase in industry. Brazilian Journal of Microbiology. 2010;41(4):850–861. doi: 10.1590/S1517-83822010000400004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannanov & Sattarova (2001).Mannanov RN, Sattarova RK. Antibiotics produced by Bacillus bacteria. Chemistry of Natural Compounds. 2001;37:117–123. doi: 10.1023/A:1012314516354. [DOI] [Google Scholar]
- Meyer, Fisher & Bernfeld (1951).Meyer KH, Fisher EH, Bernfeld P. In: General biochemistry. 2nd edition Fritton SS, Simmods S, editors. New York: J. Wiley; 1951. pp. 434–450. [Google Scholar]
- Mishra & Behera (2008).Mishra S, Behera N. Amylase activity of a starch degrading bacteria isolated from soil receiving kitchen wastes. African journal of Biotechnology. 2008;7(18):3326–3331. [Google Scholar]
- Mohamed, Malki & Kumosani (2009).Mohamed SA, Malki AL, Kumosani TA. Studied effect of temperature on amylase production by Bacillus sp. Australian Journal of Basic and Applied Sciences. 2009;3:1740–1748. [Google Scholar]
- Moshafi et al. (2011).Moshafi MH, Forootanfar H, Ameri A, Shakibaie M, Dehghan-Noudeh G, Razavi M. Antimicrobial activity of Bacillus Sp. Strain Fas1 isolated from soil. Pakistan Journal of Pharmaceutical Sciences. 2011;24(3):269–275. [PubMed] [Google Scholar]
- Nimisha, Moksha & Gangawane (2019).Nimisha P, Moksha S, Gangawane AK. Amylase activity of starch degrading bacteria isolated from soil. International Journal of Current Microbiology and Applied Sciences. 2019;8(4):659–671. [Google Scholar]
- Oscariz, Lasa & Pisabarro (1999).Oscariz JC, Lasa I, Pisabarro AG. Detection and characterization of cerein 7, a new bacteriocin produced by Bacillus cereus with a broad spectrum of activity. FEMS Microbiology Letters. 1999;178:337–341. doi: 10.1111/j.1574-6968.1999.tb08696.x. [DOI] [PubMed] [Google Scholar]
- Parsek & Singh (2003).Parsek MR, Singh PK. Bacterial biofilms: an emerging link to disease pathogenesis. Annual Reviews in Microbiology. 2003;57(1):677–701. doi: 10.1146/annurev.micro.57.030502.090720. [DOI] [PubMed] [Google Scholar]
- Perez, Suarez & Castro (1992).Perez C, Suarez C, Castro GR. Production of antimicrobials by Bacillus subtilis MIR 15. Journal of Biotechnology. 1992;26:331–336. doi: 10.1016/0168-1656(92)90017-4. [DOI] [PubMed] [Google Scholar]
- Perez, Suarez & Castro (1993).Perez C, Suarez C, Castro GR. Antimicrobial activity determined in strains of Bacillus circulans cluster. Folia Microbiologica. 1993;38(1):25–28. doi: 10.1007/BF02814544. [DOI] [PubMed] [Google Scholar]
- Pinu et al. (2018).Pinu FR, Granucci N, Daniell J, Han TL, Carneiro S, Rocha I, Nielsen J, Villas-Boas SG. Metabolite secretion in microorganisms: the theory of metabolic overflow put to the test. Metabolomics. 2018;14(4):43. doi: 10.1007/s11306-018-1339-7. [DOI] [PubMed] [Google Scholar]
- Pinu, Villas-Boas & Aggio (2017).Pinu FR, Villas-Boas SG, Aggio R. Analysis of intracellular metabolites from microorganisms: quenching and extraction protocols. Metabolites. 2017;7(4):53. doi: 10.3390/metabo7040053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pranay et al. (2019).Pranay K, Padmadeo SR, Jha V, Prasad B. Screening and identification of amylase producing strains of Bacillus. Journal of Applied Biology & Biotechnology. 2019;7(04):57–62. doi: 10.7324/JABB.2019.70409. [DOI] [Google Scholar]
- Ramachandran et al. (2014).Ramachandran R, Chalasani AG, Lal R, Roy U. A broad-spectrum antimicrobial activity of Bacillus subtilis RLID 12.1. The Scientific World Journal. 2014;2014:968487. doi: 10.1155/2014/968487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasooli et al. (2008).Rasooli I, Astaneh SDA, Borna H, Barchini KAA. Thermostable a-amylase producing natural variant of bacillus s pp. isolated from soil in Iran. American Journal of Agricultural and Biological Sciences. 2008;3(3):591–596. doi: 10.3844/ajabssp.2008.591.596. [DOI] [Google Scholar]
- Reed & Rigney (1947).Reed JF, Rigney JA. Soil sampling from fields of uniform and nonuniform appearance and soil types. Journal of the American Society of Agronomy. 1947;39(1):26–40. doi: 10.2134/agronj1947.00021962003900010003x. [DOI] [Google Scholar]
- Reinheimer, Demkov & Condioti (1990).Reinheimer JA, Demkov MR, Condioti MC. Inhibition of coliform bacteria by lactic cultures. Australian Journal of Dairy Technology. 1990;45(1):5–9. [Google Scholar]
- Rodrigues et al. (2016).Rodrigues WF, Miguel CB, Nogueira APO, Ueira-Vieira C, Paulino TDP, Soares SDC, DeResende EAMR, Lazo-Chica JE, Araújo MC, Oliveira CJ. Antibiotic resistance of bacteria involved in urinary infections in Brazil: a cross-sectional and retrospective study. International Journal of Environmental Research and Public Health. 2016;13(9):918. doi: 10.3390/ijerph13090918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe (2001).Roe S. In: Purification and concentration by precipitation. Roe S, editor. Oxford University Press; New York: 2001. [Google Scholar]
- Saber et al. (2017).Saber H, Alwaleed EA, Ebnalwaled KA, Sayed A, Salem W. Efficacy of silver nanoparticles mediated by Jania rubens and Sargassum dentifolium macroalgae; characterization and biomedical applications. Egyptian Journal of Basic and Applied Sciences. 2017;4(4):249–255. [Google Scholar]
- Salem et al. (2015a).Salem SH, El-Sheikh HH, Naguib MM, Heikal YA. Potential antimicrobial activity of the antagonistic Bacillus strains to Saccharomyces cerevisiae. JIPBS. 2015a;2(4):632–645. [Google Scholar]
- Salem et al. (2017).Salem WM, El-Hamed DS, Sayed W, Elamary R. Alterations in virulence and antibiotic resistant genes of multidrug-resistant Salmonella serovars isolated from poultry: the bactericidal efficacy of Allium sativum. Microbial Pathogenesis. 2017;108:91–100. doi: 10.1016/j.micpath.2017.05.008. [DOI] [PubMed] [Google Scholar]
- Salem et al. (2015b).Salem WM, Schneditz G, Dornisch E, Schild S. In vitro effects on biofilm viability, cytotoxicity, and antibacterial activity of green Ag and ZnO nanoparticles against Nontypeable Haemophilus influenza strains. JEAAS. 2015b;1:369–383. [Google Scholar]
- Schmidt (2004).Schmidt FR. The challenge of multidrug resistance: actual strategies in the development of novel antibacterials. Applied microbiology and biotechnology. 2004;63(4):335–343. doi: 10.1007/s00253-003-1344-1. [DOI] [PubMed] [Google Scholar]
- Seper et al. (2011).Seper A, Fengler VH, Roier S, Wolinski H, Kohlwein SD, Bishop AL, Camilli A, Reidl J, Schild S. Extracellular nucleases and extracellular DNA play important roles in Vibrio cholerae biofilm formation. Molecular Microbiology. 2011;82:1015–1037. doi: 10.1111/j.1365-2958.2011.07867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakibaie (2018).Shakibaie MR. Bacterial biofilm and its clinical implications. Annals of Microbiology and Research. 2018;2(1):45–50. [Google Scholar]
- Sharma, Misba & Khan (2019).Sharma D, Misba L, Khan AU. Antibiotics versus biofilm: an emerging battleground in microbial communities. Antimicrobial Resistance and Infection Control. 2019;8(1):76. doi: 10.1186/s13756-019-0533-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinde & Soni (2014).Shinde S, Soni R. Production and partial purification of α-amylase from bacterial strains. International Journal of Genetic Engine Biotechnolo. 2014;974(1):57–62. [Google Scholar]
- Singh & Kumari (2016).Singh P, Kumari P. Isolation and characterization of amylase producing Bacillus spp. from selected soil sample. International Journal of Research in BioSciences. 2016;5(2):24–29. [Google Scholar]
- Snedeco & Cochran (1980).Snedeco GW, Cochran WG. Statistical methods. 7th Edition Ames: Iowa State University Press; 1980. [Google Scholar]
- Stein (2005).Stein T. Bacillus subtilis antibiotics: structures, syntheses and specific functions. Molecular Microbiology. 2005;56:845–857. doi: 10.1111/j.1365-2958.2005.04587.x. [DOI] [PubMed] [Google Scholar]
- Takkinen et al. (1983).Takkinen K, Pettersson RF, Kalkkinen N, Palva I, Söderlund H, Kääriäinen L. Amino acid sequence of alpha-amylase from Bacillus amyloliquefaciens deduced from the nucleotide sequence of the cloned gene. Journal of Biological Chemistry. 1983;258:1007–1013. [PubMed] [Google Scholar]
- Talaro & Talaro (1996).Talaro A, Talaro K. Drugs, microbes, host. The elements of chemotherapy in foundations in microbiology. W.M. Brown; New York: 1996. [Google Scholar]
- Tamehiro et al. (2002).Tamehiro N, Okamoto-Hosoya Y, Okamoto S, Ubukata M, Masa HM, Naganawa H, Ochi K. Bacilysocin, a novel phospholipid antibiotic produced by Bacillus subtilis 168. Antimicrobial Agents and Chemotherapy. 2002;46:315–320. doi: 10.1128/AAC.46.2.315-320.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaikundamoorthya et al. (2018).Vaikundamoorthya R, Rajendrana R, Selvaraju A, Moorthy K, Perumal S. Development of thermostable amylase enzyme from Bacillus cereus for potential antibiofilm activity. Bioorganic Chemistry. 2018;77:494–506. doi: 10.1016/j.bioorg.2018.02.014. [DOI] [PubMed] [Google Scholar]
- Xavier et al. (2005).Xavier JB, Picioreanu C, Rani SA, Van Loosdrecht MCM, Stewart PS. Biofilm control strategies based on enzymatic disruption of the extracellular polymeric substance matrix—a modeling study. Microbiology. 2005;51:3817–3832. doi: 10.1099/mic.0.28165-0. [DOI] [PubMed] [Google Scholar]
- Yilmaza, Sorana & Beyatlib (2006).Yilmaza M, Sorana H, Beyatlib Y. Antimicrobial activities of some Bacillus spp. strains isolated from the soil. Microbiological Research. 2006;161:127–131. doi: 10.1016/j.micres.2005.07.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Values of fractions, enzyme activities and protein concentrations for Fig 2; Values of OD595 for the bacterial biofilm of Bacillus-producing amylase-filtrate and/or purified amylase enzyme against some pathogenic bacteria after 24 or 48h for Figs 4- 7.
Data Availability Statement
The following information was supplied regarding data availability:
Raw data are available as Supplemental Files.