Abstract
Background
Vulnerability to cannabis use (CU) initiation and problematic use have been shown to be affected by both genetic and environmental factors, with still inconclusive and uncertain evidence.
Objective
Aim of the present study was to investigate the possible interplay between gene polymorphisms and psychosocial conditions in CU susceptibility.
Methods
Ninety-two cannabis users and ninety-three controls have been included in the study. Exclusion criteria were serious mental health disorders and severe somatic disorders, use of other drugs and alcohol abuse; control subjects were not screened to remove Reward Deficiency Syndrome (RDS) behaviors. A candidate gene association study was performed, including variants related to dopaminergic and endocannabinoids pathways. Adverse childhood experiences and quality of parental care have been retrospectively explored utilizing ACES (Adverse Children Experience Scale), CECA-q (Child Experience of Care and Abuse Questionnaire), PBI (Parental Bonding Instrument).
Results
Our findings evidenced a significant association between rs1800497 Taq1A of ANKK1 gene and CU. Parental care was found to be protective factor, with emotional and physical neglect specifically influencing CU. Gender also played a role in CU, with males smoking more than females. However, when tested together genotypes and psychosocial variables, the significance of observed genetic differences disappeared.
Conclusions
Our results confirm a significant role of Taq1A polymorphism in CU vulnerability. A primary role of environmental factors in mediating genetic risk has been highlighted: parental care could be considered the main target to design early prevention programs and strategies.
Keywords: Cannabis, risk factors, Genetics, Biomarkers, Adverse childhood experiences, Parental care
Introduction
A variety of studies has investigated the influence of genetics on cannabis use disorders development. Family, adoption and twin studies have assessed the total variance in genetic risk of cannabis use (CU) estimated to be in the range between 30 and 80% (Agrawal and Lynskey, 2009). Adoption studies, investigating in general substance abuse vulnerability, have found that abuse or dependence of adoptees is more related to abuse or dependence of their biological parents than their adoptive parents (Cadoret et al., 1995), once again indicating a significant role for genetic factors. A twin study, including a large number of female twins samples (n=1934), suggested that genetic factors have a substantial impact on the liability of women to develop cannabis use, abuse and dependence (Kendler and Prescott, 1998).
From the Human Genome Project to date, several polymorphisms have been identified as attractive candidates for CU susceptibility. These variants have been reported to be associated to both endocannabinoid and dopaminergic functions. Cannabis rewarding effect due to its psychoactive component Δ9-tetrahydrocannabinol (THC), in fact, seems to be attributable to endocannabinoids receptors stimulation that in turn affects dopamine signals. Evidence of cross talk between the dopamine and endocannabinoid systems seems to suggest that cannabinoid receptors respond to THC by increasing dopamine release (Cheer et al., 2004; Tanda et al., 1997) from the nucleus accumbens and prefrontal cortex (Gessa et al., 1998).
Among the Single Nucleotide Polymorphisms (SNPs), rs1049353 and rs806380 in CNR1 gene, and rs324420 in FAAH gene have been found involved in endocannabinoid system regulation and linked significantly with cannabis related phenotypes (Bühler et al., 2015; Hartman et al., 2009; Tyndale et al., 2007).
As regards to dopaminergic pathway, genetic variants have been also proposed to increase the risk of cannabis use disorders, in particular TaqA1 allele (rs1800497, ANKK1) (Nacak et al., 2012), the exon 3 VNTR of the dopamine receptor 4 gene (DRD4) (McGeary, 2009), COMT Val158Met polymorphism rs4680, in the COMT enzyme involved in the catecholamines degradation pathway (Nieman et al., 2016).
The rs6277 variant of DRD2 gene could also have an important role in CU susceptibility, in light of its crucial role in affecting dopamine receptor 2 gene expression (Duan et al., 2003) and density in cortex and thalamus (Hirvonen et al., 2009). In contrast, the 9R/9R homozygous genotype of VNTR 3’UTR (DAT1/SLC6A3) has been suggested to confer a general protective effect against risky behaviours, included cannabis use (Guo et al., 2010).
Moreover, genome-wide association studies (GWAS) have identified novel variants related to cannabis use disorders, with loci located in or near the gene that could play a role in the neural conduction and synaptic transmission, as the ANKFN1 gene (Agrawal et al., 2011), RP11–206 M11.7, SLC35G1 and CSMD1 (Sherva et al., 2016).
In parallel with genetic risk factors, specific environmental predictors that may trigger CU have already been identified. Several studies provide evidence that childhood negative experiences maltreatment, childhood neglect, physical abuse, sexual abuse, lack of parental care, reduced bonding to family, exposure to community violence and other life traumatic events, as severe negative early life experiences, could influence cannabis consumption (Licanin and Redzić, 2005; Windle and Wiesner, 2004). In particular, subjects who developed posttraumatic stress disorder (PTSD) following a range of community and family-based traumas reported greater vulnerability for CU (Lipschitz et al., 2003). Family has a well-known key role in the development and progression of substance use disorder and many studies highlighted how the perception of parental care could also be determinant: childhood history of neglect and low perception of parental care in cocaine addicts were associated with specific neuroendocrine changes, less resiliency facing stressful events and greater risk to use crack (Schweitzer & Lawton 1989; Gerra et al., 2009; Pettenon et al., 2014).
Considering the risk conditions in a more integrated perspective, a growing body of evidence appears to underline the possible interaction of genes and environmental factors in the development of cannabis use and cannabis use disorders (Olivares et al., 2016; Kendler et al., 2008). More precisely, vulnerability to both cannabis use initiation and problematic use has been shown to be about 48-59 % genes, 15-25% shared environment and 21-29% unshared environment (Verweij et al., 2010). Genetic influences could vary considerably as a function of environmental conditions such as parenting, attachment, bonding to family and supervision in early childhood and adolescence (Harden et al., 2008; Chabrol et al., 2006).
In this complex scenario, the reciprocal influences of inherited and environmental factors remain not clearly defined and inconclusive. To this purpose, studies adopting polygenic techniques and integrating genetic variation with measures of environmental risk, such as childhood adversity, have been considered promising in exploring new leads (Bogdan et al., 2016).
For these reasons, we decided to investigate genetic and environmental risk factors in a sample of 92 cannabis users, compared to 93 controls. In particular, the present study aimed to investigate which risk factors can trigger or exacerbate CU vulnerability, through two main goals: (1) To verify the potential role of gene polymorphisms in the development of CU. Gene association studies were performed, analysing the allele frequencies and the genotype distributions of polymorphisms involved in dopaminergic and endocannabinoid function. (2) To investigate the role of environmental factors in the susceptibility of CU. Adverse childhood experiences and quality of parenting (ACES, CECA-q, PBI) have been investigated.
The hypothesis of the study was that gene variants involved in the function of the reward dopaminergic system and endogenous cannabinoids would underlie CU vulnerability, in particular when modulated by concurrent environmental factors and social risky conditions.
Materials and methods
Subjects
Ninety-two (92) unrelated Caucasian cannabis users (73% males, aged 18-60 years, mean age 29.5 ± 9.2 years), were included in the study. The study design was approved by the Local Ethics Committee of Parma, Italy (PROT.n. 33816) and written informed consent was obtained from all participants. The subjects were not paid for their participation and accepted to enter the study as volunteers.
Cannabis users were recruited according to the following criteria: regular adult Caucasian smokers of marijuana, daily or near daily cannabis users, who got in touch with Addiction Treatment Centres (Italy).
Most of them approached the services because of the legal provisions imposing to drug users to have at least a few weeks contact with treatment services in case of possession of controlled drugs for personal consumption. Other subjects were treatment seekers for behavioural or psychological problems induced by cannabis. Cannabis users who participated in the study provided positive urines for cannabis and negative urines for all other drugs metabolites at the beginning of the study.
Ninety-three (93) unrelated healthy individuals from the same geographical areas (36% males, aged 18-60 years, mean age 33.5 ± 7.7 years), who have never smoked marijuana used other drugs or abused alcohol, were selected as controls. They were recruited as volunteers (with no payment) from hospital and university staff workers, blood donors and university students. Control subjects were requested to provide negative urines for cannabis and all other drugs at the beginning of the study.
Exclusion criteria
Exclusion criteria included serious mental health disorders, clearly pre-existing to cannabis use, and severe somatic disorders (chronic liver or renal disorder, endocrinopathies, immunopathies and HIV disease), use of other drugs (cocaine amphetamines heroin benzodiazepines prescription drugs) and alcohol abuse. The subjects submitted to prescribe psychopharmacological long-term interventions were also excluded.
Demographic and psychometric measures
All the participants, subjects and controls, were submitted to an interview about demographic data and three psychometric tests: ACES (Adverse Children Experience Scale), CECA-q (Child Experience of Care and Abuse Questionnaire), PBI (Parental Bonding Instrument) (Felitti et al., 1998; Bifulco et al., 2005; Parker et al., 1978).
ACES was used to measure emotional and physical abuse, emotional and physical neglect, household dysfunction, parental separation, parental mental illness, sexual abuse. CECA-q measured parental antipathy, neglect, abuse, sexual abuse screen and severity. PBI investigated parental care and protection, evaluated as neglectful parenting, affectionless control, affectionate constrain, optimal parenting.
Sample collection
Samples collection and analyses were conducted following the workflow study shown in Figure 1. Blood with FTA classic cards (Whatman) has been collected by Addiction Treatment service as part of the routinary diagnosis process; the subjects volounteering as controls has been requested to provide saliva samples with buccal swab (Whatman) by our lab.
Figure 1.
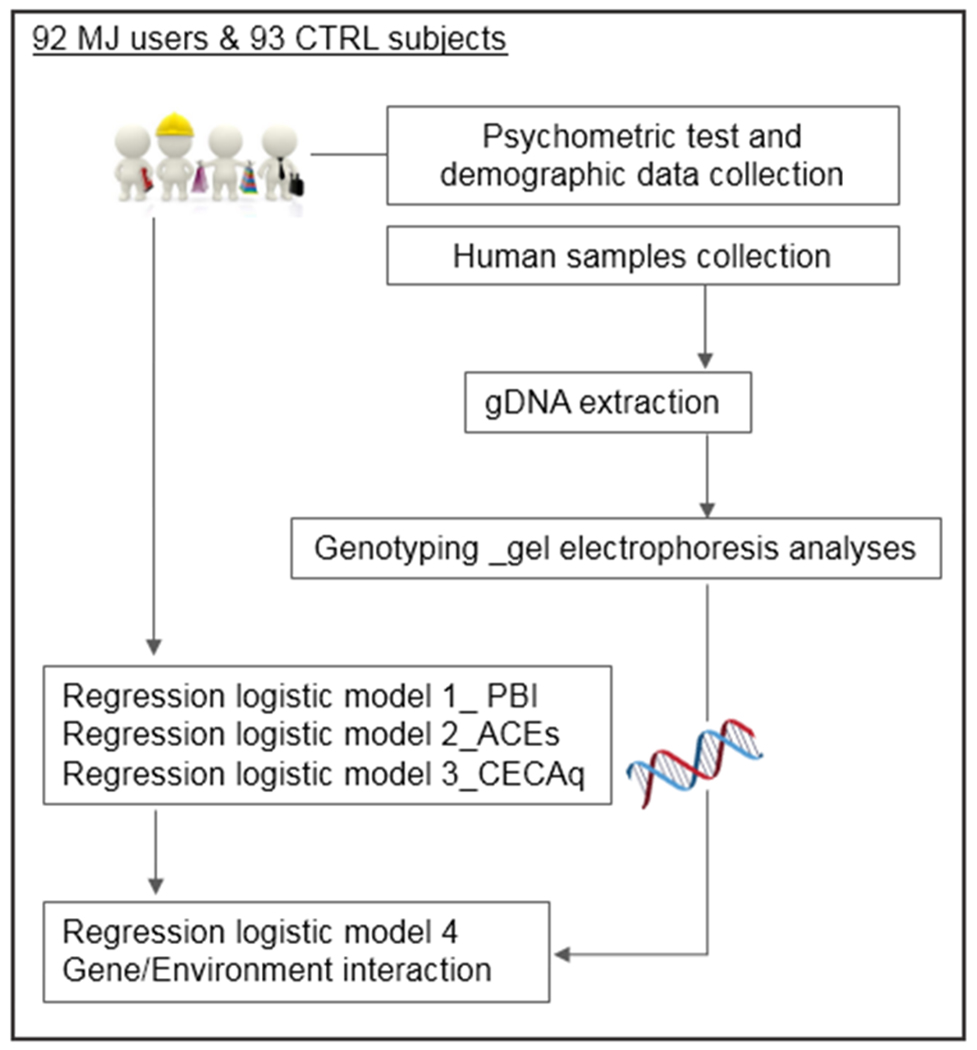
Workflow study.
Genotyping
The polymorphisms related to the genes listed in Table 1 have been genotyped in cannabis users and controls. The genotyping procedure was carried out in four main steps. (1) Biological sample collection. (2) Genomic DNA extraction/purification. The buccal swabs were immediately subjected to the DNA extraction using QIAamp® DNA Mini Kit. FTA classic cards were instead purified with FTA Classic Cards Purification Protocol. (3) Amplification of the polymorphic regions through PCR. For most of the genes a standard PCR protocol was applied, using human oligonucleotide primers previously selected (See Table 1). The master mix was assembled and incubated with the samples in a thermal cycler at 94°C for 2 minutes to completely denature the template. After 35 cycles of PCR amplification (denaturing 94°C for 30 s, annealing 55°C for 30 s, extension 72°C for 30 s) the samples were incubated for an additional 7 min at 72°C and maintained the reaction at 4°C. Further optimization of the standard protocol was required for DRD4 and CNR1 genes, since these regions are templates with high GC content and high secondary structure. In particular, to avoid nonspecific amplicons, touchdown PCR was performed in combination with additional denaturing agent (10% DMSO). The samples were then stored at −20°C until use. (4) Identification of allelic variants using agarose gel electrophoresis. In case of length polymorphism (VNTR), after PCR reaction, DNA amplicons were directly loaded on agarose gel electrophoresis. In case of SNPs, the PCR products were subjected to restriction digestion, using the enzymes listed in Table 1, before electrophoresis analysis.
Table 1.
List of candidate genes and relative variants analysed, DNA sequence variations and functional consequences. Forward (FW) and reverse (RV) primers used in the PCR reactions, restriction enzymes and references are reported for each variants.
| Gene, SNP/VNTR | DNA variation | Functional Consequence | Primer, FW and RV (5’-3’) | Restriction enzyme | Reference |
|---|---|---|---|---|---|
| CNR1, rs1049353 | A/G (REV) | synonymous codon: Thr ⇒ Thr | FW- GAAAGCTGCATCAAGAGCCC RV- TTTTCCTGTGCTGCCAGGG |
MspI | Gadzicki et al., 1999 |
| FAAH, rs324420 | A/C (FWD) | missense: Pro ⇒ Thr | FW-ATGTTGCTGGTTACCCCTCTCC RV- TCACAGGGACGCCATAGAGCTG |
EcoO109I | Morita et al., 2005 |
| COMT, rs4680 | A/G (FWD) | missense, upstream variant 2KB: Val ⇒ Met | FW-TCGTGGACGCCGTGATTCAGG RV- AGGTCTGACAACGGGTCAGGC |
NlaIII | Hong et al., 2003 |
| DRD2, rs6277 | C/T (REV)* | synonymous codon: Pro ⇒ Pro | FW-a) ACCACGGTCTCCACAGCACTCT b) ACCATGGTCTCCACAGCACTCT’ RV- ATGGCGAGCATCTGAGTGGCT |
TaqaI / BslI | Hirvonen et al., 2009 |
| ANKK1, rs1800497 | C/T (REV) | missense: Glu⇒ Lys | FW-CCGTCGACGGCTGGCCAAGTTGTCTA RV- CCGTCGACCCTTCCTGAGTGTCATCA |
TaqaI | Grandy et al., 1993 |
| DAT1, VNTR 3’UTR | 40bp, 3-11 repeats | 3’UTR | FW-TGTGGTGTAGGGAACGGCCG AG RV- CTTCCTGGAGGTCACGGCTCAAGG |
/ | Santtila et al., 2010 |
| DRD4, VNTR exon 3 | 48bp, 2-11 repeats | exon 3 | FW-AGGTGGCACGTCGCGCCAAGCTGCA RV- TCTGCGGTGGAGTCTGGGGTGGGAG |
/ | Mitsuyasu et al., 2001 |
rs6277 C957T SNP, has been studied in association with the mutation G1101A
Statistical analyses
Fisher’s exact test was applied to investigate the relationship between both allele frequencies and genotypic distribution on the use of cannabis. In the case of the SNP rs1049353, due to the lack of a reasonable number of homozygous A/A subjects, Fisher’s exact test was performed in two different ways, including and excluding A/A homozygous subjects. The chi-square (χ2) test was used to assess the deviations of genotype distribution from the Hardy-Weinberg equilibrium.
Logistic regression was used to assess first the association between CU and environmental factors. The first model (Logistic regression model 1 - explanatory variables: gender, PBI father, PBI mother; dependent variable: MJ use) evaluated the influence of gender and parenting on the risk of CU. Other two models (Logistic Regression model 2 - explanatory variables: gender, ACES variables; dependent variable: MJ use. Logistic Regression model 3 - explanatory variables: gender, CECAq variables; dependent variable: MJ use) introduced respectively ACES and CECAq data, with the aim of deepening the influence of different aspects of parental bonding on the risk of CU. Logistic regression was then used to evaluate genetics and environmental factors together on CU. This final model (Logistic Regression model 4) included only those variables previously resulted statistically significant. Parental bonding variables were not considered in this last model because of multi-collinearity of PBI and some of the variables of CECAq and ACES.
The PBI, ACES and CECA-Q scores were also considered in association with the presence/absence of the Taq1A allele, ANKK1 gene, through Fisher exact test.
Because of the different gender composition of the two groups, it was considered appropriate to insert gender in the logistic regression models in order to evaluate if gender is associated with adversity experiences and substance misuse, however the low number of marijuana user women did not allow to divide the sample in two gender subgroups and then all the other variables have been evaluated net of gender effect.
For all the statistical analyses, results were considered statistically significant for p ≤0.05.
Results
The genotypic distribution and allele frequencies of 93 controls and 92 cannabis users related to the five SNPs and two VNTRs analysed are reported in Table 2. The observed genotypes in the subjects did not differ significantly from those expected from the Hardy–Weinberg equilibrium (p>0.05).
Table 2.
Association of SNPs and VNTRs with cannabis use. Genotype and allele frequency analyses.
| SNP ID (gene) | Genotypes and Alleles | Subjects | Fisher’s exact test | SNP ID / VNTR (gene) | Genotypes and Alleles | Subjects | Fisher’s exact test | |||
| CTRLs | MJ users | CTRLs | MJ users | |||||||
| rs1049353 (CNR1) | GG | 61.8% | 52.17% | 0.051(*) | rs6277 (DRD2) | CC | 11.83% | 16% | 0.51 | |
| AA | 4.30% | 0.00% | TT | 29.03% | 33% | |||||
| GA | 38.20% | 47.83% | CT | 59.14% | 51% | |||||
| G allele | 80.90% | 76.09% | 0.3 | C allele | 41.40% | 41.85% | 1 | |||
| A allele | 19.10% | 23.91% | T allele | 58.60% | 58.15% | |||||
|
rs324420 (FAAH) |
CC | 62.37% | 68.48% | 0.52 |
rs1800497 (ANKK1) |
CC | 76.34% | 57.61% | 0.034 | |
| AA | 6.45% | 3.26% | TT | 2.15% | 4.35% | |||||
| CA | 31.18% | 28.26% | TC | 21.51% | 38.04% | |||||
| C allele | 77.96% | 82.61% | 0.29 | C allele | 87.10% | 76.63% | 0.032 | |||
| A allele | 22.04% | 17.39% | T allele | 12.90% | 23.37% | |||||
|
rs4680 (COMT) |
GG | 33.33% | 31.52% | 0.97 |
VNTR 3’UTR (DAT1) |
9R/9R | 10.75% | 7.61% | 0.81 | |
| AA | 16.13% | 16.30% | 10R/10R | 43.01% | 44.57% | |||||
| GA | 50.54% | 52.17% | 9R/10R | 43.01% | 43.48% | |||||
| G allele | 58.60% | 57.61% | 0.91 | 9R | 65.57% | 67.78% | 0.73 | |||
| A allele | 41.40% | 42.39% | 10R | 34.43% | 32.22% | |||||
| VNTR-48 bp (DRD4) | R<7 (S) | 84.41% | 88.04% | 0.36 (**) | ||||||
| R≥7 (L) | 15.59% | 11.96% | ||||||||
Due to the lack of a reasonable number of homozygous A/A subjects, for the SNP rs1049353, Fisher’s exact test was performed in two different ways, including and excluding A/A homozygous subjects (no significant differences were revealed excluding A/A subjects).
For DRD4 VNTR, since the high number of alleles, the number of observations does not allow the statistical analysis for the genotype distribution; in this case statistical analysis is reported only on alleles.
Our findings evidenced a statistically significant association between rs1800497 Taq1A of ANKK1 gene (p=0.03) and CU, and a tendency to significant association between rs1049353 of CNR1 gene (p=0.051) and CU. The prevalence of Taq1A allele of the SNP rs1800497, ANKK1 gene, was significantly higher in the cannabis group compared to controls (p=0.034). This result was also reflected in the genotypic distribution, where heterozygous T/C (thymine/cytosine; A1/A2) was more frequent in the cannabis users (p=0.032) whereas C (A2) allele and homozygous CC (A2/A2) genotype were most represented in the control group. As for the SNP rs1049353 (G1539A) of CNR1 gene, the first statistical analysis including the homozygous A/A subjects revealed a higher frequency of heterozygous G/A carriers among cannabis users than controls, although only with a tendency to significance (p=0.051). The second one, excluding A/A homozygous subjects, did not reveal any statistically significant difference.
The environmental data collected are reported in Table 3 and related statistical analyses are shown in Table 4. PBI mean scores (Table 4a), as expression of parent-child attachment and perception of parental care, were significantly lower in the CU group (p<0.000 paternal and p=0.002 maternal bonding). In particular, subjects reporting a good parenting were 85-90% less likely to be (85.7% father; 90% mother) cannabis users than those who reported affectionless control or affectionate constraint. In addition, males present about a six-time higher risk to develop cannabis use disorder compared to females (OR=5.74, p=0.001).
Table 3.
(A) Gender (B) PBI scores related to mother and father (C) ACES and CECA-q scores.
| A | Gender | B | PBI - father | PBI - mother | |||||||
| female | male | neglectful parenting | affectionless control | affectionate constrain | optimal parenting | neglectful parenting | affectionless control | affectionate constrain | optimal parenting | ||
| Control subjects | 64% | 36% | 0% | 4% | 1% | 95% | 0% | 4% | 0% | 96% | |
| Marijuana users | 27% | 73% | 2% | 38% | 9% | 51% | 0% | 33% | 2% | 64% | |
| C | ACEs | |||||||||||||||
| Emotional abuse | Physical abuse | Household dysfunction | Emotional neglect | Physical neglect | Parental separation | Parental mental illness | Sexual abuse | |||||||||
| no | yes | no | yes | no | yes | no | yes | no | yes | no | yes | no | yes | no | yes | |
| Control subjects | 93% | 7% | 87% | 13% | 94% | 6% | 99% | 1% | 99% | 1% | 91% | 9% | 81% | 19% | 97% | 3% |
| Marijuana users | 71% | 29% | 56% | 44% | 80% | 20% | 60% | 40% | 71% | 29% | 78% | 22% | 69% | 31% | 89% | 11% |
| CECA-q | ||||||||||||||||
| Antipathy mother | Antipathy father | Neglect mother | Neglect father | Physical abuse mother | Physical abuse father | Sexual abuse screen | Sexual abuse severity | |||||||||
| no | yes | no | yes | no | yes | no | yes | no | yes | no | yes | no | yes | no | yes | |
| Control subjects | 99% | 1% | 95% | 5% | 99% | 1% | 94% | 6% | 96% | 4% | 97% | 3% | 97% | 3% | 97% | 3% |
| Marijuana users | 84% | 16% | 73% | 27% | 96% | 4% | 76% | 24% | 76% | 24% | 80% | 20% | 87% | 13% | 87% | 13% |
Table 4.
Environmental influences on cannabis use (A) Logistic regression model 1- explanatory variables: gender, PBI father, PBI mother; dependent variable: MJ use. (B) Logistic Regression model 2 - explanatory variables: gender, ACES variables; dependent variable: MJ use. (C) Logistic Regression model 3 - explanatory variables: gender, CECAq variables; dependent variable: MJ use.
| A_ Variables in the Equation | |||||||
|---|---|---|---|---|---|---|---|
| B | S.E. | Wald | df | Sig. | Exp(B) | ||
| Gender (ref. female) | |||||||
| Male | 1.748 | .514 | 11.549 | 1 | .001 | 5.745 | |
| PBI_father (ref. optimal parenting) | |||||||
| neglectful parenting or | 2.655 | .629 | 17.838 | 1 | .000 | 14.223 | |
| affectionless control or | |||||||
| affectionate constrain | |||||||
| PBI_mother (ref. optimal parenting) | |||||||
| neglectful parenting or | 2.223 | .722 | 9.475 | 1 | .002 | 9.238 | |
| affectionless control or | |||||||
| affectionate constrain | |||||||
| Constant | −2.654 | .461 | 33.091 | 1 | .000 | .070 | |
| B_Variables in the Equation | |||||||
| B | S.E. | Wald | df | Sig. | Exp(B) | ||
| Gender (ref. female) | |||||||
| Male | 1.474 | .518 | 8.102 | 1 | .004 | 4.366 | |
| ACES | Emotional abuse(1) | −.393 | .863 | .207 | 1 | .649 | .675 |
| Physical abuse(1) | 1.282 | .690 | 3.451 | 1 | .063 | 3.602 | |
| Household disfunction(1) | −.554 | .902 | .377 | 1 | .539 | .575 | |
| Emotional neglect(1) | 3.129 | 1.160 | 7.270 | 1 | .007 | 22.841 | |
| Physical neglect(1) | 2.554 | 1.214 | 4.423 | 1 | .035 | 12.860 | |
| Parental separation(1) | −.132 | .831 | .025 | 1 | .874 | .876 | |
| Parental mentalillness(1) | .157 | .616 | .065 | 1 | .799 | 1.170 | |
| Sexual abuse(1) | −.553 | 1.310 | .178 | 1 | .673 | .575 | |
| Constant | −2.360 | .450 | 27.518 | 1 | .000 | .094 | |
| C_Variables in the Equation | |||||||
| B | S.E. | Wald | df | Sig. | Exp(B) | ||
| Gender (ref. female) | |||||||
| male | 1.497 | .460 | 10.596 | 1 | .001 | 4.470 | |
| CECA q | Antipathy mother(1) | 1.728 | 1.260 | 1.878 | 1 | .171 | 5.627 |
| Antipathy father(1) | 1.242 | .729 | 2.902 | 1 | .088 | 3.464 | |
| Neglect mother(1) | .147 | 1.471 | .010 | 1 | .921 | 1.158 | |
| Neglect father(1) | .626 | .642 | .950 | 1 | .330 | 1.870 | |
| Physical abuse mother(1) | .323 | .890 | .131 | 1 | .717 | 1.381 | |
| Physical abuse father(1) | .707 | 1.003 | .497 | 1 | .481 | 2.028 | |
| Sexual abuse screen(1) | 1.277 | .883 | 2.093 | 1 | .148 | 3.584 | |
| Constant | −2.161 | .388 | 30.985 | 1 | .000 | .115 | |
The following two logistic regression models, including respectively ACES and CECAq (Table 4 -B and -C) reflecting conditions of trauma, physical abuse, emotional neglect, adverse experiences in childhood and adolescence, revealed that emotional neglect, (p=0.007) physical neglect (p=0.035), and again gender, significantly associated with CU. In particular, individuals reporting emotional neglect show a 22.8 times higher risk to develop cannabis abuse as well as those reporting physical neglect are 12.8 times more likely to develop cannabis addiction compared to subjects who do not have the perception of these psychological and physical damages.
A final model (Table 5) tested simultaneously the influence of genetic and environmental risk factors. In this model, the statistical significance for gene variants disappeared, indicating the primary role of environmental factor in CU susceptibility. In particular, the psychometric variables that remained significantly associated with CU were emotional neglect and physical neglect (coefficients 25.417 and 13.341, respectively).
Table 5.
Environmental and genetic effect on cannabis use. Logistic Regression model 4 - explanatory variables: gender, physical/emotional neglect, presence/absence of T allele, SNP rs1800497 of ANKK1 gene; presence/absence of A allele, SNP rs1049353 of CNR1 gene; dependent variable: MJ use.
| Variables in the Equation | B | S.E. | Wald | Df | Sig. | Exp(B) |
|---|---|---|---|---|---|---|
| Gender (ref. female) male |
1.479 | .505 | 8.585 | 1 | .003 | 4.387 |
| rs1800497 (ref. C allele) T allele |
−.767 | .519 | 2.188 | 1 | .139 | .464 |
| rs1049353 (ref. G allele) A allele |
.259 | .502 | .265 | 1 | .606 | 1.295 |
| Emotional neglect(1) | 3.235 | 1.143 | 8.017 | 1 | .005 | 25.417 |
| Physical neglect(1) | 2.591 | 1.229 | 4.448 | 1 | .035 | 13.341 |
| Constant | −1.834 | .605 | 9.203 | 1 | .002 | .160 |
Males were confirmed to present a statistically significant higher risk of cannabis use compared to females (p=0.003).
In addition, the individuals carrying Taq1A allele (T allele, rs1800497, ANKK1 gene) had higher score in affectionless maternal control (p=0.015) (PBI) compared to subjects not carrying Taq1A allele. T allele frequency was also significantly associated with emotional neglect (p=0.027) and emotional abuse (p=0.019), being higher in subjects who reporting poor parental bonding. None of these associations were reported related to the rs1049353 CNR1 gene variant (data not shown).
Discussion
The findings of our genetic association study indicate Taq1A SNP of ANKK1 gene to be significant associated with cannabis use. However, the low number of minor allele homozygotes highlights the need to confirm the results increasing the number of observations. The ANKK1 gene has been reported to encode for a serine/threonine kinase highly expressed in the brain (Neville et al., 2004) with an ankyrin repeat domain involved in protein-protein interactions. In line with our results, TaqIA SNP, possibly influencing dopamine function in the motivational system, has been found associated with different kinds of substance use disorders (Ponce et al., 2009; Yang et al., 2008), especially with vulnerability to alcoholism (Blum et al., 1990; Noble et al., 1991) and cannabis dependence (Nacak et al., 2012).
At the molecular level, Taq1A allele (rs1800497) was reported in association with reduced dopamine D2 receptor density in the brain (Jönsson et al., 1999) and a lower D2 receptor binding potential in healthy carriers of the minor allele A1 (Lys713) (Gluskin and Mickey; 2016). The variation in Lys (K) residue caused by Taq1A polymorphism, located in the ankirin repeat domain, could profoundly alter protein-protein interactions and therefore subsequent signal transduction pathways (Meylan and Tschopp, 2005).
As regard to the SNP rs1049353, in CNR1 gene, directly involved in cannabis mechanism of action, was also found associated with CU in our study, although only with a tendency to significance. Heterozygous GA has shown a higher frequency in cannabis users. It should be noted, however, that the observed difference in this population did not survive to the statistical analysis excluding AA genotypes, leading to assume a possible role of A allele. The A allele of this SNP was previously found associated with vulnerability to alcohol withdrawal delirium (Schmidt et al., 2002) and with enhanced impulsivity (Buchmann et al., 2015). Our findings were not consistent with the nominal association evidenced between the G allele, SNP rs1049353, and cannabis dependence symptoms (Hartman et al., 2009), casting doubts concerning the strength of our results. The contradictory evidence available until now in this field suggest the need of further investigation, increasing the size of the samples and comparing more homogeneous methodologies and measures.
Interestingly, Isir and colleagues (2016) have recently shown that the interaction between the 1359 G/A polymorphisms of CNR1 gene and the Taq1A polymorphism plays a decisive role in CB1 and D2 receptors interaction, promoting CU development or reducing CU risk (Isir et al., 2016).
The genotyping data of our study confirm that CU is influenced by genetic factors.
As previously reported by other authors concerning the role of environmental risk factors in substance use disorders susceptibility (Olivares et al., 2016; Kendler et al., 2008; Verweij et al., 2010), our data highlighted the importance of poor parenting and early stressful conditions, such as neglect and abuse, in the risk for CU. Psychosocial factors may represent more than a simple association, but the factors that mainly contributed to the risk condition, mediating gene variant effects and enabling the expression of behavioural and personality traits phenotypes.
To this purpose, logistic regression models revealed four parameters, gender, parental bonding, emotional neglect and physical neglect, as crucial concurrent conditions to cannabis use development. Subjects who reported emotional and physical neglect showed a risk respectively about 22.8 and 12.8 times higher to develop CU than subjects who did not have the perception of these psychosocial problems. In addition, subjects who reported an optimal parenting has approximately a risk 85–90% lower to be cannabis users.
When genetic and environmental risk factors were considered all together with the regression model analysis, the significance of gene variants association with CU decreases until to disappear, indicating the primary role of environmental factor in CU susceptibility.
In addition, the higher scores concerning affectionless mother control (PBI), and emotional neglect and abuse (CECA-Q) among subjects carrying Taq1A polymorphism, respect to those not carrying this gene variant, may suggest a more complex interpretation. Dopamine-related gene variant would have contributed to CU susceptibility not only directly, influencing behavioral attitude, personality traits and positive response to cannabis in adolescence or adulthood, but also modulating temperamental traits in early childhood, in turn undermining child-parent attachment and the quality of care in the family (Balleyguier, 1991; Mayseless and Scher, 2000; Mäntymaa et al., 2006).
Our results are consistent with previous studies, where early life events (Perkonigg et al., 2008), experience of stress attributed to family instability, family disruption (Flewelling and Bauman, 1990; Butters, 2002) and early childhood maltreatment (Oshri et al., 2011) were already suggested as possible predictor factors for cannabis initiation and cannabis use disorders (Volkow et al., 2016). Male subjects presented a higher risk to develop cannabis use disorders, compared to female, confirming the importance of gender in this area of research and possible gender-related resilience factors (Agrawal and Lynskey, 2007, Perkonigg et al., 2008, Farmer et al., 2015).
Finally, it should be noted that the present study has certain limitations: the sample size could be increased for genotyping analysis to obtain more reliable results. In addition, genome-wide association study is known one of the best approach to identify markers across the complete sets genomes, and it could be used to further investigation of the observed association of risk polymorphisms with cannabis use. Retrospective perception measures of the quality of parenting reported by our subjects should also be considered with caution, being themselves influenced by personality traits in adulthood and complex cultural conditions.
Overall, our results suggest a possible role in cannabis use for genes encoding proteins involved in the dopamine function and probably in the endocannabinoid system. Parental care seems to play the role of a strong protective factor, being able to mitigate or strongly reduce the risk related to genetic variants. For this reasons, parental care should be consider as a primary target to design early prevention programs and strategies for substance use disorders in adolescence and later in life.
Acknowledgements
We thank all subjects who participated to the project. This work was supported by the GENERISK project (D91J09000480001), Presidency of the Council of the Ministers, Italy.
Footnotes
Disclosure of interest
The authors report no conflict of interest.
References
- 1.Agrawal A, Lynskey MT (2009) Candidate genes for cannabis use disorders: findings, challenges and directions. Addiction 104 (4):518–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agrawal A, Lynskey MT, Hinrichs A et al. (2011) A genome-wide association study of DSM-IV cannabis dependence. Addict Biol 16(3):514–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agrawal A, Lynskey MT (2007) Does gender contribute to heterogeneity in criteria for cannabis abuse and dependence? Results from the national epidemiological survey on alcohol and related conditions. Drug Alcohol Depend 88 (2-3):300–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balleyguier G (1991) The development of attachment according to the temperament of the newborn. Psychiatr Enfant 34(2):641–57. [PubMed] [Google Scholar]
- 5.Bifulco A, Bernazzani O, Moran PM et al. (2005) The childhood experience of care and abuse questionnaire (CECA.Q): validation in a community series. Br J Clin Psychol 44(Pt 4):563–81. [DOI] [PubMed] [Google Scholar]
- 6.Blum K, Noble EP, Sheridan PJ, et al. (1990) Allelic association of human dopamine D2 receptor gene in alcoholism. JAMA 263(15):2055–60. [PubMed] [Google Scholar]
- 7.Bogdan R, Winstone JM, Agrawal A (2016) Genetic and Environmental Factors Associated with Cannabis Involvement. Curr Addict Rep 3(2):199–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buchmann AF, Hohm E, Witt SH, et al. (2015) Role of CNR1 polymorphisms in moderating the effects of psychosocial adversity on impulsivity in adolescents. J Neural Transm 122(3):455–63. [DOI] [PubMed] [Google Scholar]
- 9.Bühler KM, Giné E, Echeverry-Alzate V, et al. (2015) Common single nucleotide variants underlying drug addiction: more than a decade of research. Addict Biol 20(5):845–71. [DOI] [PubMed] [Google Scholar]
- 10.Butters JE (2002) Family stressors and adolescent cannabis use: a pathway to problem use. J Adolesc 25(6):645–54. [DOI] [PubMed] [Google Scholar]
- 11.Cadoret RJ, Yates WR, Troughton E, et al. (1995) Adoption study demonstrating two genetic pathways to drug abuse. Arch Gen Psychiatry 52(1):42–52. [DOI] [PubMed] [Google Scholar]
- 12.Chabrol H, Chauchard E, Mabila JD, et al. (2006) Contributions of social influences and expectations of use to cannabis use in high-school students. Addict Behav 31(11):2116–9. [DOI] [PubMed] [Google Scholar]
- 13.Cheer JF, Wassum KM, Heien ML, et al. (2004) Cannabinoids enhance subsecond dopamine release in the nucleus accumbens of awake rats. J Neurosci 24(18):4393–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dick DM, Viken R, Purcell S, et al. (2007) Parental monitoring moderates the importance of genetic and environmental influences on adolescent smoking. J Abnorm Psychol 116(1):213–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duan J, Wainwright MS, Comeron JM, et al. (2003) Synonymous mutations in the human dopamine receptor D2 (DRD2) affect mRNA stability and synthesis of the receptor. Hum Mol Genet 12(3):205–16. [DOI] [PubMed] [Google Scholar]
- 16.Farmer RF, Seeley JR, Kosty DB, et al. (2015) Internalizing and externalizing psychopathology as predictors of cannabis use disorder onset during adolescence and early adulthood. Psychol Addict Behav 29 (3): 541–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Felitti VJ, Anda RF, Nordenberg D, et al. (1998) Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med 14(4):245–58. [DOI] [PubMed] [Google Scholar]
- 18.Flewelling RL, Bauman KE (1990) Family structure as a predictor of initial substance use and sexual intercourse in early adolescence. J Marriage Fam 52: 171–81 [Google Scholar]
- 19.Gerra G, Leonardi C, Cortese E, et al. (2009) Childhood neglect and parental care perception in cocaine addicts: relation with psychiatric symptoms and biological correlates. Neurosci Biobehav Rev 33(4):601–10. [DOI] [PubMed] [Google Scholar]
- 20.Gessa GL, Melis M, Muntoni AL, et al. (1998) Cannabinoids activate mesolimbic dopamine neurons by an action on cannabinoid CB1 receptors. Eur J Pharmacol 2;341(1):39–44. [DOI] [PubMed] [Google Scholar]
- 21.Gluskin BS, Mickey BJ. Genetic variation and dopamine D2 receptor availability: a systematic review and meta-analysis of human in vivo molecular imaging studies (2016) Transl Psychiatry 6:e747. doi: 10.1038/tp.2016.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo G, Cai T, Guo R, et al. (2010) The dopamine transporter gene, a spectrum of most common risky behaviors, and the legal status of the behaviors. PLoS One 5(2):e9352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harden KP, Hill JE, Turkheimer E, et al. (2008) Gene-environment correlation and interaction in peer effects on adolescent alcohol and tobacco use. Behav Genet 38(4):339–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hartman CA, Hopfer CJ, Haberstick B, et al. (2009) The association between cannabinoid receptor 1 gene (CNR1) and cannabis dependence symptoms in adolescents and young adults. Drug Alcohol Depend 104 (1-2):11–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hirvonen MM, Lumme V, Hirvonen J, et al. (2009) C957T polymorphism of the human dopamine D2 receptor gene predicts extrastriatal dopamine receptor availability in vivo. Prog Neuropsychopharmacol Biol Psychiatry 33(4):630–6. [DOI] [PubMed] [Google Scholar]
- 26.Isir AB, Baransel C, Nacak M (2016) An Information Theoretical Study of the Epistasis Between the CNR1 1359 G/A Polymorphism and the Taq1A and Taq1B DRD2 Polymorphisms: Assessing the Susceptibility to Cannabis Addiction in a Turkish Population. J Mol Neurosci 58(4):456–60. [DOI] [PubMed] [Google Scholar]
- 27.Jönsson EG, Nöthen MM, Grünhage F, et al. (1999) Polymorphisms in the dopamine D2 receptor gene and their relationships to striatal dopamine receptor density of healthy volunteers. Mol Psychiatry 4(3):290–6. [DOI] [PubMed] [Google Scholar]
- 28.Kendler KS, Prescott CA (1998) Cannabis use, abuse, and dependence in a population-based sample of female twins. Am J Psychiatry 155(8):1016–22. [DOI] [PubMed] [Google Scholar]
- 29.Kendler KS, Schmitt E, Aggen SH, et al. (2008) Genetic and environmental influences on alcohol, caffeine, cannabis, and nicotine use from early adolescence to middle adulthood. Arch Gen Psychiatry 65(6):674–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Licanin I, Redzić A (2005) Psycho-social characteristics of cannabis abusing youth. Bosn J Basic Med Sci 5(1):72–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lipschitz DS, Rasmusson AM, Anyan W, et al. (2003) Posttraumatic stress disorder and substance use in inner-city adolescent girls. J Nerv Ment Dis 191(11):714–21. [DOI] [PubMed] [Google Scholar]
- 32.Mäntymaa M, Puura K, Luoma I, et al. (2006) Mother’s early perception of her infant’s difficult temperament, parenting stress and early mother-infant interaction. Nord J Psychiatry 60(5):379–86. [DOI] [PubMed] [Google Scholar]
- 33.Mayseless O, Scher A (2000) Mother’s attachment concerns regarding spouse and infant’s temperament as modulators of maternal separation anxiety. J Child Psychol Psychiatry 41(7):917–25. [PubMed] [Google Scholar]
- 34.McGeary J (2009) The DRD4 exon 3 VNTR polymorphism and addiction-related phenotypes: a review. Pharmacol Biochem Behav 93(3):222–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meylan E, Tschopp J (2005) The RIP kinases: crucial integrators of cellular stress. Trends Biochem Sci 30(3):151–9. [DOI] [PubMed] [Google Scholar]
- 36.Nacak M, Isir AB, Balci SO, et al. (2012) Analysis of dopamine D2 receptor (DRD2) gene polymorphisms in cannabinoid addicts. J Forensic Sci 57(6):1621–4. [DOI] [PubMed] [Google Scholar]
- 37.Neville MJ, Johnstone EC, Walton RT (2004) Identification and characterization of ANKK1: a novel kinase gene closely linked to DRD2 on chromosome band 11q23.1. Hum Mutat 23(6):540–5. [DOI] [PubMed] [Google Scholar]
- 38.Noble EP, Blum K, Ritchie T, et al. (1991) Allelic association of the D2 dopamine receptor gene with receptor-binding characteristics in alcoholism. Arch Gen Psychiatry; 48(7):648–54. [DOI] [PubMed] [Google Scholar]
- 39.Nieman DH, Dragt S, van Duin ED, et al. (2016) COMT Val(158)Met genotype and cannabis use in people with an At Risk Mental State for psychosis: Exploring Gene x Environment interactions. Schizophr Res 174 (1-3):24–8. [DOI] [PubMed] [Google Scholar]
- 40.Olivares EL, Kendler KS, Neale MC, et al. (2016) The Genetic and Environmental Association Between Parental Monitoring and Risk of Cannabis, Stimulants, and Cocaine Initiation in a Sample of Male Twins: Does Parenting Matter? Twin Res Hum Genet 19(4):297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oshri A, Rogosch FA, Burnette ML, et al. (2011) Developmental pathways to adolescent cannabis abuse and dependence: child maltreatment, emerging personality, and internalizing versus externalizing psychopathology. Psychol Addict Behav 25(4):634–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parker G, Tupling H, Brown LB (1978) A Parental Bonding Instrument. British Journal of Medical Psychology. [Google Scholar]
- 43.Perkonigg A, Goodwin RD, Fiedler A, et al. (2008) The natural course of cannabis use, abuse and dependence during the first decades of life. Addiction 103(3):439–49. [DOI] [PubMed] [Google Scholar]
- 44.Pettenon M, Kessler FH, Guimarães LS et al. (2014) Perceptions of parental bonding in freebase cocaine users versus non-illicit drug users. Indian J Med Res 139(6):835–40. [PMC free article] [PubMed] [Google Scholar]
- 45.Ponce G, Pérez-González R, Aragüés M, et al. (2009) The ANKK1 kinase gene and psychiatric disorders. Neurotox Res 16(1):50–9. [DOI] [PubMed] [Google Scholar]
- 46.Schmidt LG, Samochowiec J, Finckh U, et al. (2002) Association of a CB1 cannabinoid receptor gene (CNR1) polymorphism with severe alcohol dependence. Drug Alcohol Depend 65(3):221–4. [DOI] [PubMed] [Google Scholar]
- 47.Schweitzer RD, Lawton PA (1989). Drug abusers’ perceptions of their parents. Br J Addict 84(3):309–14. [DOI] [PubMed] [Google Scholar]
- 48.Sherva R, Wang Q, Kranzler H, et al. (2016) Genome-wide Association Study of Cannabis Dependence Severity, Novel Risk Variants, and Shared Genetic Risks. JAMA Psychiatry 73(5):472–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stringer S, Minică CC, Verweij KJ, et al. (2016) Genome-wide association study of lifetime cannabis use based on a large meta-analytic sample of 32 330 subjects from the International Cannabis Consortium. Transl Psychiatry 6:e769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tanda G, Pontieri FE, Di Chiara G (1997) Cannabinoid and heroin activation of mesolimbic dopamine transmission by a common mu1 opioid receptor mechanism. Science 276(5321):2048–50. [DOI] [PubMed] [Google Scholar]
- 51.Tyndale RF, Payne JI, Gerber AL, et al. (2007) The fatty acid amide hydrolase C385A (P129T) missense variant in cannabis users: studies of drug use and dependence in Caucasians. Am J Med Genet B Neuropsychiatr Genet 144B (5):660–6. [DOI] [PubMed] [Google Scholar]
- 52.Verweij KJ, Zietsch BP, Lynskey MT, et al. (2010) Genetic and environmental influences on cannabis use initiation and problematic use: a meta-analysis of twin studies. Addiction 105(3):417–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Volkow ND, Koob GF, McLellan AT (2016) Neurobiologic Advances from the Brain Disease Model of Addiction. N Engl J Med 374(4):363–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Windle M, Wiesner M (2004) Trajectories of marijuana use from adolescence to young adulthood: predictors and outcomes. Dev Psychopathol 16(4):1007–27. [DOI] [PubMed] [Google Scholar]
- 55.Yang BZ, Kranzler HR, Zhao H, et al. (2008) Haplotypic variants in DRD2, ANKK1, TTC12, and NCAM1 are associated with comorbid alcohol and drug dependence Alcohol Clin Exp Res 32(12):2117–27. [DOI] [PMC free article] [PubMed] [Google Scholar]


