Abstract
Background
Chronic congestive heart failure is a common condition that, if untreated, markedly impairs the quality of life and is associated with a high risk of recurrent hospitalization and death.
Methods
This review is based on articles retrieved by a selective search in PubMed, as well as on relevant guidelines.
Results
Evidence-based treatment options are available only for congestive heart failure with a low ejection fraction. Pharmacotherapy is based on neurohumoral inhibition of the renin-angiotensin-aldosterone system and the adrenergic system. The prognosis of patients with this condition has been further improved recently through the introduction of combined angiotensin receptor antagonists and neprilysin inhibitors. Modern implantable devices are a further component of treatment. Implantable defibrillators and special pacemakers for cardiac resynchronization are well established; the utility of alternative devices (baroreflex modulation or cardiac contractility modulation) needs to be investigated in further studies. It was recently shown that the catheter-based treatment of secondary mitral regurgitation with a MitraClip improves the outcome of selected patients.
Conclusion
The treatment of chronic systolic heart failure as recommended in the relevant guidelines, with drugs and implanted devices if indicated, can significantly improve the clinical outcome.
Chronic heart failure is one of the most frequent causes of death and reasons for hospitalization in industrialized countries. If left untreated, patients have a poor prognosis (1). The introduction of new drugs and the rigorous implementation of evidence-based recommendations in the guidelines on heart failure has led to a reduction in recent years in mortality and frequency of hospitalizations in patients with heart failure and reduced ejection fraction (HFrEF) (2). In addition, established devices such as implantable defibrillators and resynchronization therapy have improved patients‘ symptoms and prognosis. Newer devices are currently being investigated in studies or have already shown early success in smaller studies. The aim of this article is to provide an overview of current drug therapy while taking into account new treatment approaches as well as to outline the possibilities presented by various device-based treatments.
Prevalence.
Heart failure is common: The prevalence of heart failure in the western world is approximately 1–2%.
Learning objectives
After reading this article, the reader should:
Be familiar with the problem of the rising prevalence and, if left untreated, poor prognosis of the syndrome of heart failure
Be able to name current drug therapies used to treat heart failure
Be familiar with the most important device-based treatments and their indications.
Method
Different types of heart failure.
A distinction needs to be made between three different types of heart failure depending on left ventricular ejection fraction.
A selective literature search was conducted in an international database (PubMed). The authors took into consideration the current guidelines of the European Society of Cardiology (ESC) and the German Cardiac Society (Deutsche Gesellschaft für Kardiologie, DGK), as well as the German national treatment guideline (Nationale Versorgungsleitlinie, NVL) on heart failure.
Epidemiology
The prevalence of heart failure in western industrialized nations is around 1–2% and increases steadily with advancing age—from below 1% in under 55-year-olds to approximately 10% in over 80-year-olds (3). Due to changes in age structure, a significant increase in the prevalence of heart failure is forecast in the coming years—accompanied by the anticipated economic consequences.
The prognosis of affected patients is poor: approximately 50% of patients diagnosed with heart failure die within 5 years (e1). European data from the ESC-HF pilot study show a 17% overall mortality rate and 44% rehospitalization rate in the first 12 months following hospital stay (4).
Pharmacological treatment approaches
Prognosis.
The prognosis of affected patients is poor: Approximately 50% of patients diagnosed with heart failure die within 5 years.
A distinction is made between three different types of heart failure depending on left ventricular ejection fraction (LVEF) (table 1) (2). All types of heart failure are associated with a reduction in stroke volume and cardiac output. There is differing evidence to support the treatment of the various types. Due to a lack of studies, the current ESC recommendations provide no clear recommendations on the treatment of patients with heart failure with mid-range ejection fraction (HFmrEF). There are analyses based only on post-hoc analyses from studies on HFrEF and/or HFpEF (heart failure with preserved ejection fraction, [diastolic heart failure]) using subgroup analyses of patients that are now classified as HFmrEF (5).
Table 1. Classification and frequency of the different types of heart failure according to the extent of left ventricular dysfunction*1.
| Abbreviation | Description | Frequency in the ESC Heart Failure Long Term Registry (e22) | Characteristics | Evidence-based therapy | ||
| Symptoms | LVEF | Other criteria | ||||
| HFrEF | HF with reduced ejection fraction | 59.8% | Symptoms ± signs | <40% | +*2 | |
| HFmrEF | HF with mid-range ejection fraction | 24.2% | Symptoms ± signs | 40–49% | 1. Elevated serum levels of natriuretic peptides 2. At least one additional criterion:
|
– |
| HFpEF | HF with preserved ejection fraction | 16% | Symptoms ± signs | ≥ 50% | 1. Elevated serum levels of natriuretic peptides 2. At least one additional criterion:
|
– |
Furthermore, no treatment strategy in HFpEF patients has shown a significant improvement in prognosis as yet. Other studies, particularly in relation to the latter, are currently underway and their results are eagerly awaited. In everyday routine, HFpEF patients are often prescribed the same drugs as patients with HFrEF, for which, however, there is no scientific basis, given that the evidence is neutral. Nevertheless, HFmrEF patients appear to benefit from beta-blockers and renin-angiotensin-aldosterone system (RAAS) blockade (5). There are clear recommendations on HFrEF treatment that have been demonstrated in numerous randomized studies and which are therefore evidence-based.
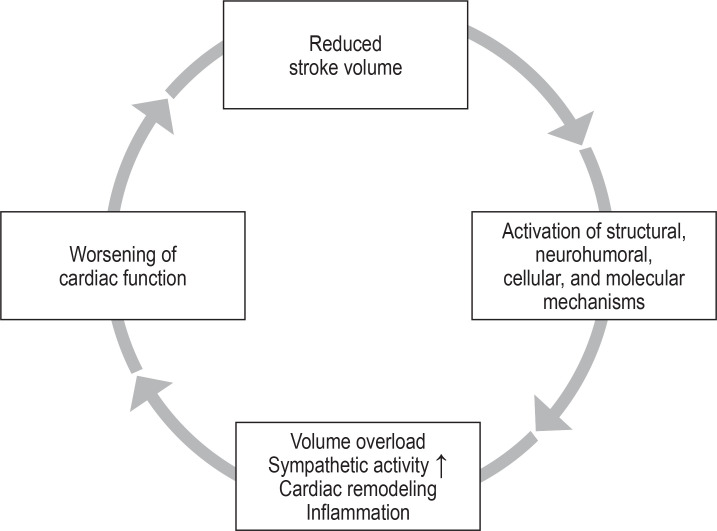
As a result of the reduced ejection fraction and reduced stroke volume, a “vicious circle” is set in motion (figure 1). The goal of pharmacological management of HFrEF, as well as that of some devices, is to interrupt these harmful maladaptive processes (e2).
Figure 1.
Simplified representation of the vicious circle in heart failure ultimately responsible for the disease‘s poor prognosis. The aim of drug therapy as well as device-based therapy is to stop or interrupt this downward spiral.
Evidence-based treatments.
Evidence-based treatments are available only for heart failure with reduced ejection fraction (HFrEF).
The basic principle here—besides treating the underlying cause (for example, by means of revascularization or heart valve surgery)—is neurohumoral inhibition by means of ACE inhibitors, angiotensin II receptor blockers (ARB), or angiotensin receptor neprilysin inhibitors (ARNI), as well as mineralocorticoid receptor antagonists (MRA) and beta-blockers.
Numerous randomized studies have demonstrated the efficacy of these treatment approaches (2).
The basis of drug therapy
Treatment with ACE inhibitors and beta-blockers has led to a significant improvement in the prognosis of heart failure patients.
What is important is to appropriately increase the dose to the respective target dose. A large European study (BIOSTAT-CHF) only recently demonstrated once again the prognostic relevance of appropriate dosing of ACE inhibitors and beta-blockers (6). ARB represent an alternative for patients unable to tolerate ACE inhibitors due to cough or angioedema. Table 2 provides an overview of the effects of heart failure treatment. The treatment is supported by diuretic therapy tailored to the patient‘s symptoms.
Table 2. Effects and typical side effects of the various heart failure drugs*1.
| Drugs | Overall mortality HR [95% CI] | NNT for mortality (standardized for 36 months) | Heart failure- related hospitalizations HR [95% CI] | Typical side effects | Typical active substances | Initial daily dose | Target daily dose |
| ACE inhibitors (e25, 38) | 0.84 [0.67; 1.01] |
26 | 0.52 [0.32; 0.76] |
Impaired renal function, hyperkalemia, hypotension, cough, angioedema |
Captopril | 3 × 6.25 mg | 3 × 50 mg |
| Enalapril | 2 × 2.5 mg | 2 × 10–20 mg | |||||
| Lisinopril | 1 × 2.5–5.0 | 1 × 20–35 mg | |||||
| Ramipril | 1 × 2.5 mg | 1 × 10 mg | |||||
| Trandolapril | 1 × 0.5 mg | 1 × 4 mg | |||||
| Angiotensin receptor blocker (e25, 38) | 0.89 [0.61; 1.27] |
0.53 [0.26; 1.03] |
Impaired renal function, hyperkalemia, hypotension |
Candesartan | 1 × 4–8 mg | 1 × 32 mg | |
| Losartan | 1 × 50 mg | 1 × 150 mg | |||||
| Valsartan | 2 × 40 mg | 2 × 160 mg | |||||
| Beta-blockers (e25, 38) | 0.58 [0.34; 0.95] |
9 | 0.45 [0.13; 1.39] |
Bradycardia, hypotension, impaired peripheral perfusion, bronchoconstriction |
Bisoprolol | 1 × 1.25 mg | 1 × 10 mg |
| Carvedilol | 2 × 3.125 mg | 2 × 25 mg | |||||
| Metoprolol succinate | 1 × 12.5–25 mg | 1 × 200 mg | |||||
| Nebivolol | 1 × 1.25 mg | 1 × 10 mg | |||||
| MRA (e25, 38) |
0.58 [0.36; 0.90]*1 |
6 | 0.36 [0.12; 0.96]*1 |
Hyperkalemia, impaired renal function, hypotension (primarily spironolactone); gynecomastia, impotence, menstrual disorders (spironolactone) |
Eplerenone | 1 × 25 mg | 1 × 50 mg |
| Spironolactone | 1 × 25 mg | 1 × 50 mg | |||||
| If channel blockers (e24) | 0.96 [0.87; 1.05] |
NA | 0.81 [0.73; 0.89] |
Symptomatic bradycardia, impaired vision (phosphenes, blurred vision), atrial fibrillation |
Ivabradine | 2 × 5 mg | 2 × 7.5 mg |
| ARNI (10, e26) | 0.84 [0.76; 0.93]*2 |
35*2 | 0.79 [0.71; 0.89]*2 |
Impaired renal function, hyperkalemia, hypotension, angioedema |
Sacubitril/ valsartan |
2 × 49/51 mg | 2 × 97/103 mg |
| SGLT2 inhibitors (21)*3 |
0.83 [0.71; 0.97] |
22 | 0.70 [0.59; 0.83] |
Genital infections, urinary tract infections, hypoglycemia (when combined with sulfonylureas or insulin), diabetic ketoacidosis, dysuria, polyuria, volume depletion |
Dapagliflozin | 1 × 10 mg | – |
| Empagliflozin | 1 ×10 mg (increasing if appropriate to 1 × 25 mg) |
*1 In combination with ACE inhibitors, *2vs ACE inhibitors, *3the mentioned side effects relate to the results of the DAPA-HF study (dapagliflozin vs. placebo in addition to an existing pharmacological heart failure treatment); modified from (10, 13, 21, e24– e26, 38)
ARNI, angiotensin receptor neprilysin inhibitors; CI, confidence interval; HR, hazard ratio; MRA, mineralocorticoid receptor antagonists, NNT, number needed to treat; SGLT2, sodium-glucose linked transporter 2
The basis of drug therapy.
Treatment with ACE inhibitors and beta-blockers remains the basis of heart failure therapy.
The prognostically beneficial effect of MRA is also established—not only in patients with severe symptoms using spironolactone (NYHA III–IV [7]), but also in those with less severe symptoms using eplerenone (NYHA II [8]). According to the current guidelines, all patients with an LVEF ≤ 35% that remain symptomatic under treatment with an ACE inhibitor as well as a beta-blocker should receive an MRA (2) (figure 2). Compared to eplerenone, spironolactone is a non-selective MRA that also activates progesterone and androgen receptors and can therefore lead to gynecomastia, impotence, and menstrual disorders (9). Furthermore, since the blood pressure-lowering effect of spironolactone is stronger than that of eplerenone, the latter can be preferentially used in the case of low blood pressure.
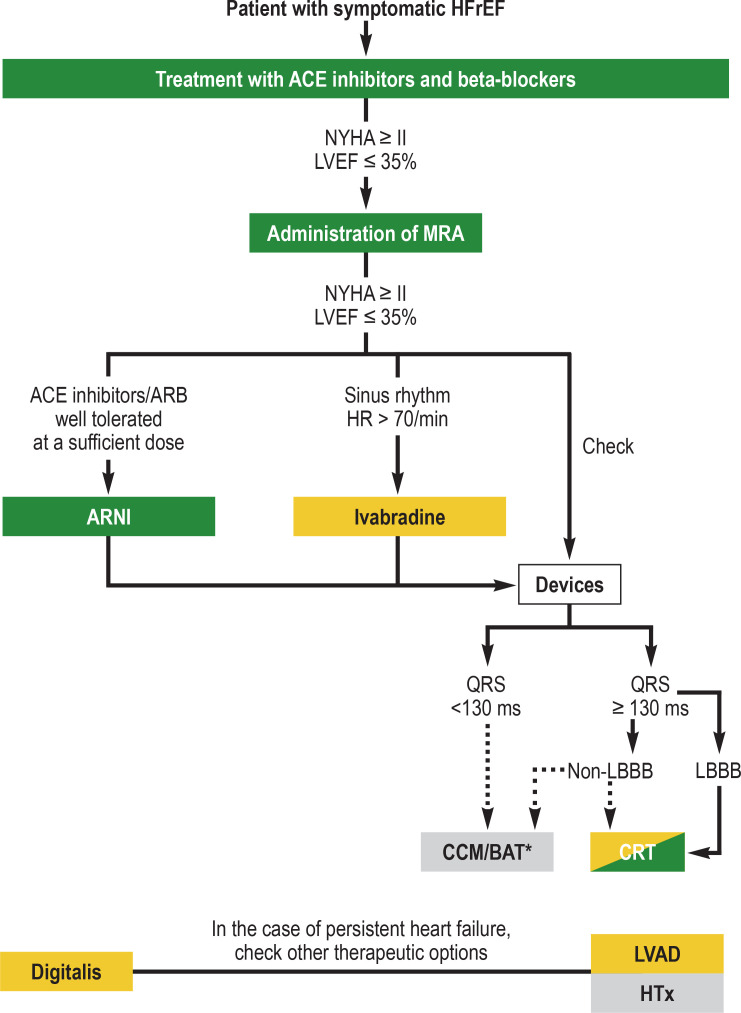
Figure 2.
Overview of drug therapy and possible device-based therapies for heart failure with reduced systolic left ventricular function (HFrEF) (modified from [2, e27]). To treat symptoms, diuretic therapy should be additionally used, as well as implantation of a cardioverter-defibrillator due to the risk of malignant cardiac arrhythmia in persistently reduced left ventricular function (LVEF <35%). In the case of intolerance due to cough, an ACE inhibitor should be swapped for an angiotensin receptor blocker. Color denotes the level of recommendation:
green, class I recommendation; yellow, class II recommendation;
gray, no clear level of recommendation in the 2016 ESC guidelines *Consider therapy
ARB, angiotensin II receptor blocker; ARNI, angiotensin receptor neprilysin inhibitor; BAT, baroreflex modulation therapy; CCM, cardiac contractility modulation; CRT, cardiac resynchronization therapy; HF, heart rate; HTx, heart transplantation; LBBB, left bundle branch block; LVAD, left ventricular assist device; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; non-LBBB, non-left bundle branch block; NYHA, New York Heart Association class
Treatment with the direct renin inhibitor aliskiren is not recommended in heart failure treatment, since it has not been demonstrated to be superior to ACE inhibitors (2, e3, e4).
Angiotensin receptor neprilysin inhibitors
Angiotensin receptor neprilysin inhibitors (ARNI) combine the established inhibition of the renin–angiotensin–aldosterone system (RAAS) with inhibition of the degradation of endogenously released natriuretic peptides.
Natriuretic peptides are released upon cardiomyocyte hypertrophy and cause an increase in intracellular cyclic guanosine monophosphate (cGMP), natriuresis, as well as a reduction in renal renin secretion and a weakening of the angiotensin II-induced hypertrophic signal transduction in cardiomyocytes (e5).
The only substance available in this drug group is the combination comprising the angiotensin II receptor blocker valsartan and the neprilysin inhibitor sacubitril. Neprilysin (synonym, neutral endopeptidase [NEP]) breaks down natriuretic peptides and various other vasoactive substances (for example, bradykinin, endothelin-1, and adrenomedullin).
The drug aliskiren.
Aliskiren is not recommended in the treatment of heart failure.
The PARADIGM-HF study on patients with symptomatic HFrEF (NYHA II–IV; LVEF ≤ 40%, modified during the course of the study to ≤ 35%) and elevated levels of natriuretic peptides, compared sacubitril/valsartan therapy with treatment using the ACE inhibitor enalapril (10). Sacubitril/valsartan therapy resulted in a significant reduction in the primary endpoint of cardiovascular mortality and hospitalization due to heart failure (21.8% versus 26.5%). In addition, cardiovascular mortality (13.3% versus 16.5%), overall mortality (17.0% versus 19.8%), and heart failure-related hospitalizations (12.8% versus 15.6%) were significantly improved (10).
Achieving target doses.
Achieving target doses of ACE inhibitors and beta-blockers is prognostically relevant.
Mineralocorticoid receptor antagonists.
In the case of persistent symptoms (NYHA ≥ II) and LVEF ≤35% despite ACE inhibitor and beta-blocker therapy, treatment should be complemented by a mineralocorticoid receptor antagonist.
Subanalyses of the study also show that sacubitril/valsartan reduced the frequency of heart-failure–related rehospitalizations and significantly improved quality of life (e6). In addition, the rate of ventricular arrhythmias was lower in an observational study (e7). More recently, another observational study, the PROVE-HF trial, demonstrated a positive effect on cardiac remodeling (e8). The current ESC guidelines recommend sacubitril/valsartan for all patients (class IB recommendation) that would have fulfilled the inclusion criteria and that remain symptomatic despite treatment with an ACE inhibitor or ARB, a beta-blocker, and an MRA (2) (Figure 2 and Box).
BOX. Treatment recommendations for routine practice.
Switch ACE inhibitor to angiotensin receptor neprilysin inhibitor sacubitril/valsartan
When switching an ACE inhibitor to the angiotensin receptor neprilysin inhibitor sacubitril/valsartan, ACE inhibitor use needs to be discontinued at least 36 h before the first use of sacubitril/valsartan. The background to this is that both substances—neprilysin and ACE—degrade bradykinin. Therefore, in principle, the simultaneous use of ACE inhibitors and sacubitril can lead to an accumulation of bradykinin and, thus, to angioedema. An angiotensin II receptor blocker can be directly swapped for sacubitril/valsartan.
Important to note with digoxin
Due to the narrow therapeutic range in patients with impaired renal function, the use of digitoxin should be preferred in this patient group, since digoxin is excreted primarily via the kidneys. Serum levels should be determined 4–6 weeks following initiation of treatment with cardiac glycosides (39). In general, doses should be lower than those commonly previously used (i.e., digoxin 0.1–0.2 mg/day, digitoxin 0.05–0.07 mg/day) (40).
| Target level: | Digoxin: 0.5–0.9 ng/mL |
| Digitoxin: 8–18 ng/mL |
Indication for cardiac resynchronization therapy
-
For the indication to cardiac resynchronization therapy, the current ESC guidelines on the treatment of heart failure (2) give a:
Class I recommendation for patients with a left bundle branch block (LBBB) and a QRS duration of ≥ 150 ms (IA) or 130–149 ms (IB)
Class II recommendation for patients with non-LBBB morphology
Class III recommendation (contraindication) for patients with a QRS duration of <130 ms
Sacubitril/valsartan compared to the ACE inhibitor enalapril.
In the PARADIGM-HF study, sacubitril/valsartan led to a significant reduction in mortality and hospitalization rates compared to the ACE inhibitor enalapril.
Typical side effects of sacubitril/valsartan therapy compared to the comparison substance in the PARADIGM-HF study, enalapril, include the onset of (symptomatic) hypotension, whereas an elevated serum potassium levels as well as increased retention values were more often found with enalapril (10).
Hyperkalemia as a relevant side effect—which, under RAAS inhibitors, often prevents the uptitration of heart failure medication in clinical routine—could be treated in future with potassium binders such as patiromer. However, further studies are required here in order to demonstrate that patiromer is associated with an improvement in prognosis in the treatment of heart failure.
Less is more: heart rate monitoring
As a result of the reduced cardiac output due to the reduced ejection fraction, the heart rate increases as a reflex. In heart failure patients, an elevated heart rate leads to less economical ventricular function and has been repeatedly associated with a poorer prognosis (e9).
Treatment with the If channel blocker ivabradine is able to achieve a rate reduction in patients in sinus rhythm without the blood pressure-lowering effect of beta-blockers. In the SHIFT study, treatment with ivabradine, in addition to the guideline-based heart failure therapy including beta-blockers, resulted in:
A significant reduction in heart failure-related hospitalizations and cardiovascular mortality (hazard ratio [HR]: 0.82; 95% confidence interval [0.75; 0.90]) (11)
An improved quality of life (e10)
An improvement in left ventricular function and a reduction in left ventricular volume (12).
The combined primary endpoint of the SHIFT study was largely driven by the reduction in hospitalizations (table 2).
The current ESC guidelines (national treatment guideline) recommend treatment with ivabradine for HFrEF patients (LVEF ≤ 35%) in sinus rhythm with a heart rate of ≥ 70 (≥ 75) beats/min that remain symptomatic despite therapy with an ACE inhibitor or angiotensin II receptor blocker, a beta-blocker, and a mineralocorticoid receptor antagonist (2, 13) (figure 2).
Still unclear: the value of cardiac glycosides
Heart rate monitoring.
An elevated heart rate is associated with a poorer prognosis.
Although cardiac glycosides have long been used in heart failure, their role is unclear and they are classified in the ESC guidelines as well as the German national treatment guideline on heart failure as a “back-up drug” in advanced symptomatic heart failure under existing optimal drug therapy (2, 13). The only large randomized study, the DIG trial (14), on digoxin in heart failure patients was deemed neutral, since the primary endpoint of overall mortality was not affected by the treatment; however, heart failure-related hospitalizations and mortality were significantly reduced. Subgroup analyses showed a mortality benefit for patients with low serum digoxin levels compared to patients with high levels (15). A meta-analysis on the studies available to date on digitalis in heart failure revealed that treatment with digitalis reduces hospitalizations and improves the symptoms of heart failure (16). In older, multimorbid patients with reduced renal function, digoxin poses the risk of accumulation and possible toxicity. The alternative cardiac glycoside, digitoxin, is less dependent on renal function and appears to be beneficial in patients with reduced renal function (box).
A large randomized study to investigate the role of digitoxin in heart failure patients in addition to a modern, up-to-date drug therapy is currently underway: the DIGIT-HF study (DIGitoxin to Improve ouTcomes in patients with advanced chronic Heart Failure, EudraCT-Nr.: 2013-005326-38) (17).
Treating comorbidities
The comorbidities of heart failure warrant particular attention. For example, iron deficiency reduces physical capacity and is associated with a poorer prognosis (18). In proven iron deficiency (ferritin <100 mg/L or ferritin 100–299 µg/L and transferrin saturation <20%), iron replacement therapy even in the absence of anemia led to improved quality of life and physical capacity (19, 20). The current guidelines recommend intravenous iron therapy in symptomatic patients with heart failure and confirmed iron deficiency (2). The FAIR-HF2-DZHK5 study is currently investigating the prognostic effect of iron therapy on mortality and hospitalizations.
The value of cardiac glycosides.
The value of cardiac glycosides in the treatment of heart failure has not been fully elucidated as yet; they are used especially in patients with atrial fibrillation and high ventricular rate.
Primary prevention defibrillator implantation.
In symptomatic patients with an LVEF ≤ 35% defibrillator implantation is recommended in order to prevent sudden cardiac death.
The SGLT2 inhibitors (sodium-glucose linked transporter 2) are a highly promising drug group in patients with heart failure with and without diabetes mellitus. The 2016 ESC guideline stated that empagliflozin should be considered in patients with type 2 diabetes in order to prevent or delay the onset of heart failure. This recommendation was recently expanded to include the alternative SGLT2 inhibitors canagliflozin and dapagliflozin (5). The results of the DAPA-HF study have also been presented, showing a significant reduction in mortality and heart failure-related hospitalizations in HFrEF patients under dapagliflozin treatment irrespective of the presence of diabetes (HR 0.74; [0.65; 0.85]; p <0.001) (table 2) (21).
Novel treatment approaches
Two new treatment approaches in chronic heart failure include vericiguat, a stimulator of soluble guanylatcyclase (sGC), and omecamtiv mecarbil, a myosine activator. In the recently published VICTORIA study, the primary composite endpoint of death from cardiovascular causes and heart failure-related hospitalization was significantly reduced in HFrEF patients under vericiguat treatment (HR 0.90; [0.82; 0,98]; p = 0.02) (e11). For omecamtiv mecarbil, further studies are still required to demonstrate its value in current modern heart failure therapy. The results of the GALACTIC-HF study on the relevance of omecamtiv mecarbil in HFrEF are expected in 2021.
Devices in the treatment of heart failure
Implantable cardioverter-defibrillators
Resynchronization therapy.
Resynchronization therapy in patients with left bundle branch block significantly reduces heart-failure–related hospitalizations as well as cardiovascular and overall mortality.
To avoid sudden cardiac death, primary prevention defibrillator therapy (implantable cardioverter-defibrillator [ICD]) is recommended in patients with LVEF ≤ 35% despite optimized drug therapy (2, 22). For optimally treated patients (including cardiac resynchronization therapy) with non-ischemic heart failure, the DANISH study showed no significant difference in relation to all-cause mortality (21.6% versus 23.4%; HR 0.87; [0.68; 1.12], p = 0.28) (23), whereas the onset of sudden cardiac death was significantly reduced (4.3% versus 8.2%; HR 0.50; [0.31; 0.82], p = 0.005). However, in a subgroup analysis, a significant survival benefit was demonstrated for patients ≤ 70 years also in terms of all-cause mortality (HR 0.70; [0.51; 0.96], p = 0.03) (24). The authors of the German national treatment guideline on heart failure do not infer from this “a specific recommendation for the use of implantable cardioverter-defibrillators in the primary prevention indication in patients with non-ischemic cardiomyopathy,” but instead recommend “establishing an individual indication by appropriately specialized cardiologists” (13). In patients with advanced heart failure (NYHA class IV) to whom therapeutic options such as resynchronization therapy, a left-ventricular assist device (LVAD), or transplantation are not available, implantation of a cardioverter-defibrillator is currently not recommended (22). This needs to be discussed critically with the patient and their relatives. As a bridging measure, i.e., as protection against malignant arrhythmias during the optimization phase of drug therapy, a wearable defibrillator can be prescribed in the first months (25).
Cardiac resynchronization therapy
In patients with heart failure, a left bundle branch block (LBBB) causes intraventricular (between the interventricular septum and the posterolateral left ventricular wall), as well as an interventricular (between the right and left ventricle) dyssynchrony. This worsens ventricular remodeling, cardiac output per minute, and existing functional mitral insufficiency.
Cardiac resynchronization therapy (CRT) using specialized pacemaker systems equipped with an additional left ventricular lead implanted in the coronary sinus makes it possible to resolve or reduce this dyssychrony. This achieved an improvement in heart failure symptoms and physical capacity, as well as having a positive effect on cardiac remodeling (e12, e13, >– 26) and positive effects on heart failure-related hospitalizations and mortality (HR 0.63; [0.51; 0.77]; p <0.001) in the CARE-HF study (CRT) compared to optimal drug therapy; (HR 0.66; [0.52; 0.84]; p <0.001) in the MADIT-CRT study (CRT+defibrillator compared to ICD) (27– 30). A high percentage (target: 98%) of LV pacing is crucial to treatment success (2). Mortality and morbidity increase with each percentage decline in left ventricular stimulation (31).
Patients with a broad QRS complex of >130 ms but non-LBBB morphology do not benefit from cardiac resynchronization therapy to the same extent in the large studies (28, e14, e15). However, a recently conducted registry analysis found that cardiac resynchronization may be beneficial in patients with a QRS duration of more than 180 ms irrespective of QRS morphology (e16). In the case of a narrow QRS complex (<130 ms) despite echocardiographically confirmed mechanical dyssynchrony, no prognostic improvement was conferred by cardiac resynchronization therapy—on the contrary, an excess mortality was seen in the cardiac resynchronization therapy arm (e17). This gives rise to the recommendations in the current ESC guidelines shown in the Box.
Devices in narrow QRS complex
Success of resynchronization therapy.
A high percentage of LV pacing is crucial to treatment success.
Only around 20% of patients have a QRS duration of >120 ms (e18), meaning that cardiac resynchronization therapy is not indicated in the majority of HFrEF patients. Since modulation of the autonomic nervous system by means of vagal nerve stimulation was unsuccessful (e19), baroreflex activation therapy (BAT) and cardiac contractility modulation (CCM) could represent potential alternative therapies in the future for patients with a narrow QRS complex. Both therapies are relatively new devices for heart failure and could be considered for HFrEF patients with a narrow QRS complex that remain symptomatic despite optimal guideline-compliant drug therapy. The Food and Drug Administration has already approved baroreflex activation therapy and cardiac contractility modulation in the USA to improve HFrEF symptoms. Hard data on improvement of prognosis (mortality) are currently still pending. The German heart failure treatment guideline (NVL) deems the available evidence on baroflex activation therapy and cardiac contractility modulation as hitherto insufficient for the purposes of making specific recommendations (13). Both devices currently play a secondary role in the clinical treatment of heart failure patients and are only used on the basis of individual assessments in specialized centers.
Secondary mitral regurgitation
Patients with HFrEF frequently develop secondary mitral regurgitation (MR); in patients with an LVEF ≤ 35%, mitral regurgitation of at least moderate severity was detected in 49% of cases (32). Typically, the valve itself is intact in secondary mitral regurgitation. The regurgitation is the result of an imbalance between the closing and tethering forces on the valve due to changes in left ventricular geometry (e20). The prognosis of patients with HFrEF worsens with increasing severity of mitral regurgitation (32, e21).
Treatment comprises optimal heart failure drug therapy, as well as cardiac resynchronization therapy where indicated (2). The value of isolated surgical treatment of secondary mitral regurgitation has not been elucidated as yet and is viewed with caution in the current guidelines (2). Alternatively, interventional procedures have been available for some years; MitraClip therapy in particular is an established treatment option.
Alternative device-based therapies.
Alternative device-based therapies in patients with a narrow QRS complex comprise baroreflex activation therapy and cardiac contractility modulation.
In 2018, two studies were published on the value of MitraClip therapy of severe secondary mitral regurgitation in HFrEF: The French MITRA-FR study (33) found no significant difference between treatment with MitraClip and optimal drug therapy on the combined endpoint of all-cause mortality and heart failure-related hospitalizations following MitraClip therapy. In the COAPT study, on the other hand, interventional mitral valve repair conferred an improvement in prognosis in selected patients with heart failure (LVEF 20–50%) and moderate-to-severe mitral regurgitation following previously optimized heart failure treatment, with a reduction in hospitalizations and death (34). Quality of life was also significantly improved (35).
Possible reasons (e22) for these differing results could lie on the one hand in the severity of mitral regurgitation, which was greater in the COAPT study. Another difference lay in the fact that the left ventricular end-diastolic volume was higher in the MITRA-FR study, i.e., patients with more advanced heart failure and a higher degree of LV dilatation were included. These two studies resulted in the coining of the term “proportionate” as compared to “disproportionate” functional mitral regurgitation compared to the size of the left ventricle (36). The COAPT study primarily included patients with the latter, meaning that these patients appear more likely to benefit from the MitraClip intervention (5).
The results of the COAPT study demonstrated, for the first time, a significant improvement in prognosis as a result of interventional therapy in patients with severe secondary mitral regurgitation. The number needed to treat for mortality in this study is six. Therefore, the possibility of MitraClip therapy should be assessed in patients with HFrEF, optimal heart failure therapy, and severe secondary mitral regurgitation in order to improve prognosis in this group.
Implementing treatment recommendations in the outpatient sector
Particularly in the outpatient sector, recommendations on the treatment of HFrEF are not sufficiently implemented in daily routine. Uptitration of heart failure drugs is often inadequate. Comorbidities (for example, COPD, depression, sleep apnea) hamper the diagnosis and treatment of heart failure, are often not taken into account, or are underestimated in terms of their prognostic effect. Therapy needs to be optimized by means of heart failure networks made up of specialized heart failure practices, clinics, and supraregional centers in order to guarantee the best possible treatment of heart failure patients. The German Cardiac Society (DGK) certifies appropriate facilities (37). Specialized heart failure nurses and medical assistants play an important role here, as do telemedicine approaches, which are able to indicate overhydration early on (for example, CardioMEMS, a pressure sensor that is implanted in the pulmonary artery), and could help to promptly identify decompensation and prevent hospitalizations in the future.
MitraClip therapy.
A positive effect on mortality and hospitalization rates was seen for MitraClip therapy in selected patients with secondary mitral regurgitation.
Conclusion
Further advances have been made in the treatment of HFrEF in recent years and the prognosis of these patients has significantly improved. In addition to the introduction of angiotensin receptor neprilysin inhibitors, new pharmacological approaches such as SGLT2 inhibitors and sGC activators, as well as novel devices, are showing promise.
Results of the COAPT study.
The results of the COAPT study demonstrated, for the first time, a significant improvement in prognosis as a result of interventional therapy in patients with severe secondary mitral regurgitation.
Further information on CME.
Participation in the CME certification program is only possible online: cme.aerzteblatt.de. The submission deadline is 21.5.2021. Submissions by letter, e-mail, or fax cannot be considered.
The completion time for all newly started CME units is 12 months. The results can be accessed 4 weeks following the start of the CME unit. Please note the respective submission deadline at: cme.aerzteblatt.de.
This article has been certified by the North Rhine Academy for Continuing Medical Education. CME points can be managed using the “uniform CME number” (einheitliche Fortbildungsnummer, EFN). The EFN must be stated during registration on www.aerzteblatt.de (“Mein DÄ”) or entered in “Meine Daten” and consent must be given for results to be communicated. The 15-digit EFN can be found on the CME card (8027XXXXXXXXXXX).
Participation is possible at cme.aerzteblatt.de. The submission deadline is 21 May 2021. Only one answer is possible per question. Please select the answer that is most appropriate.
Question 1
How high is the prevalence of heart failure in over 80-year-olds?
2%
4%
6%
8%
10%
Question 2
Which substance classes form the basis of treatment for heart failure with reduced ejection fraction?
ACE inhibitors and beta-blockers
Digoxin and ACE inhibitors
Angiotensin receptor neprilysin inhibitors and digitoxin
If channel blockers and beta-blockers
SGLT2 inhibitors (SGLT, sodium dependent glucose transporter) and angiotensin receptor neprilysin inhibitors
Question 3
By what percentage can the administration of beta-blockers reduce overall mortality in heart failure?
10%
22%
30%
42%
58%
Question 4
What is a typical side effect of mineralocorticoid receptor antagonists?
Angioedema
Bradycardia
Hyperkalemia
Impaired peripheral perfusion
Tachycardia
Question 5
Approximately how many heart failure patients require treatment with a mineralocorticoid receptor antagonist in order to prevent death within 3 years?
3
6
9
12
15
Question 6
What recommendation does the national treatment guideline make regarding the use of implantable cardioverter-defibrillators?
Indicated in individual cases of patients with an LVEF ≤ 35% with non-ischemic cardiomyopathy
As a flanking measure in the case of moderate response to beta-blockers and ACE inhibitors
As an alternative to the use of ivabradine if sinus rhythm is present
Mandatory in patients with an LVEF of 35–50%
For use in very physically active patients
Question 7
When can a wearable defibrillator be prescribed?
In the case of symptomatic heart failure and preserved pump function
In the case of advanced (NYHA IV) heart failure and a lack of treatment options
In patients with poor compliance
As a bridging measure until LVEF improves or until definitive treatment with an implantable cardioverter-defibrillator
If the patient does not tolerate amiodarone and other antiarrhythmic drugs
Question 8
Which treatment option has a class I recommendation if LVEF remains ≤ 35% in NYHA ≥ II after use of beta-blockers, ACE inhibitors, and a mineralocorticoid receptor antagonist?
Ivabradine
Vericiguat
Angiotensin receptor neprilysin inhibitor
Baroreflex modulation therapy
Cardiac contractility modulation
Question 9
What importance is attributed to the use of cardiac glycosides in advanced heart failure?
They form an integral part of first-line therapy.
They can replace mineralocorticoid receptor antagonists in the case of intolerance.
They should only be used in patients that have a cardioverter-defibrillator.
Digoxin should be used particularly in the case of impaired renal function.
They are considered a back-up drug in optimal pharmacotherapy.
Question 10
What is a typical side effect of SGLT2 inhibitors?
Angioedema
AV block
Bronchoconstriction
Gynecomastia
Volume depletion
►Participation is only possible online: cme.aerzteblatt.de
Acknowledgments
Translated from the original German by Christine Rye.
Footnotes
Conflict of interests
Dr. Berliner received consultancy fees from Novartis. He received funds for the preparation of scientific meetings from Orion Pharma, Abbott Vascular, and Novartis Pharma GmbH. He received funds to conduct clinical trials from Zoll Medical Corporation, CVRx, and Novartis.
Dr. Hänselmann was reimbursed for congress participation fees as well as travel and accommodation costs by Bayer and Böhringer Ingelheim. She received funds for the preparation of scientific meetings from Novartis.
Prof. Bauersachs received consultancy fees from Astra Zeneca, Bayer, BMS, Böhringer Ingelheim, Novartis, and Servier Vifor. He was reimbursed for travel and accommodation costs by Bayer, Böhringer Ingelheim, and Servier. He received funds for the preparation of scientific meetings from Abiomed, Astra Zeneca, Bayer, BMS, Böhringer Ingelheim, CVRX, Medtronic, MSD, and Novartis. He received funds for a research project of his own initiation as well as to conduct clinical trials from Abiomed, Bayer, Böhringer Ingelheim, CVRX, Medtronic, MSD, Vifor, and Zolll.
References
- 1.Lindenfeld J, Albert NM, Boehmer JP, et al. HFSA 2010 Comprehensive heart failure practice guideline. J Card Fail. 2010;16 e1-194. doi: 10.1016/j.cardfail.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 2.Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: The task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18:891–975. doi: 10.1002/ejhf.592. [DOI] [PubMed] [Google Scholar]
- 3.Mosterd A, Hoes AW. Clinical epidemiology of heart failure. Heart. 2007;93:1137–1146. doi: 10.1136/hrt.2003.025270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maggioni AP, Dahlstrom U, Filippatos G, et al. EURObservational Research Programme: the Heart Failure Pilot Survey (ESC-HF Pilot) Eur J Heart Fail. 2010;12:1076–1084. doi: 10.1093/eurjhf/hfq154. [DOI] [PubMed] [Google Scholar]
- 5.Seferovic PM, Ponikowski P, Anker SD, et al. Clinical practice update on heart failure 2019: pharmacotherapy, procedures, devices and patient management An expert consensus meeting report of The Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2019;21:1169–1186. doi: 10.1002/ejhf.1531. [DOI] [PubMed] [Google Scholar]
- 6.Ouwerkerk W, Voors AA, Anker SD, et al. Determinants and clinical outcome of uptitration of ACE-inhibitors and beta-blockers in patients with heart failure: a prospective European study. Eur Heart J. 2017;38:1883–1890. doi: 10.1093/eurheartj/ehx026. [DOI] [PubMed] [Google Scholar]
- 7.Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med. 1999;341:709–717. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 8.Zannad F, McMurray JJ, Krum H, et al. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364:11–21. doi: 10.1056/NEJMoa1009492. [DOI] [PubMed] [Google Scholar]
- 9.Zannad F, Gattis Stough W, Rossignol P, et al. Mineralocorticoid receptor antagonists for heart failure with reduced ejection fraction: integrating evidence into clinical practice. Eur Heart J. 2012;33:2782–2795. doi: 10.1093/eurheartj/ehs257. [DOI] [PubMed] [Google Scholar]
- 10.McMurray JJ, Packer M, Desai AS, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004. doi: 10.1056/NEJMoa1409077. [DOI] [PubMed] [Google Scholar]
- 11.Swedberg K, Komajda M, Böhm M, et al. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet. 2010;376:875–885. doi: 10.1016/S0140-6736(10)61198-1. [DOI] [PubMed] [Google Scholar]
- 12.Tardif JC, O‘Meara E, Komajda M, et al. Effects of selective heart rate reduction with ivabradine on left ventricular remodelling and function: results from the SHIFT echocardiography substudy. Eur Heart J. 2011;32:2507–2515. doi: 10.1093/eurheartj/ehr311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bundesärztekammer (BÄK), Kassenärztliche Bundesvereinigung (KBV), Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften (AWMF) Nationale Versorgungs-Leitlinie Chronische Herzinsuffizienz -Langfassung 3. Auflage. 2019 (last acessed on 31 October 2019) [Google Scholar]
- 14.Digitalis Investigation Group. The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med. 1997;336:525–533. doi: 10.1056/NEJM199702203360801. [DOI] [PubMed] [Google Scholar]
- 15.Adams KF, Butler J, Patterson JH, et al. Dose response characterization of the association of serum digoxin concentration with mortality outcomes in the Digitalis Investigation Group trial. Eur J Heart Fail. 2016;18:1072–1081. doi: 10.1002/ejhf.584. [DOI] [PubMed] [Google Scholar]
- 16.Hood JWB, Dans AL, Guyatt GH, Jaeschke R, McMurray JJV. Digitalis for treatment of heart failure in patients in sinus rhythm. Cochrane Database Syst Rev. 2014;4 doi: 10.1002/14651858.CD002901.pub3. CD002901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bavendiek U, Berliner D, Davila LA, et al. Rationale and design of the DIGIT-HF trial (DIGitoxin to Improve ouTcomes in patients with advanced chronic Heart Failure): a randomized, double-blind, placebo-controlled study. Eur J Heart Fail. 2019;21:676–684. doi: 10.1002/ejhf.1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jankowska EA, Rozentryt P, Witkowska A, et al. Iron deficiency: an ominous sign in patients with systolic chronic heart failure. Eur Heart J. 2010;31:1872–1880. doi: 10.1093/eurheartj/ehq158. [DOI] [PubMed] [Google Scholar]
- 19.Anker SD, Comin Colet J, Filippatos G, et al. Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med. 2009;361:2436–2448. doi: 10.1056/NEJMoa0908355. [DOI] [PubMed] [Google Scholar]
- 20.Ponikowski P, van Veldhuisen DJ, Comin-Colet J, et al. Beneficial effects of long-term intra-venous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiency. Eur Heart J. 2015;36:657–668. doi: 10.1093/eurheartj/ehu385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995–1908. doi: 10.1056/NEJMoa1911303. [DOI] [PubMed] [Google Scholar]
- 22.Priori SG, Blomström-Lundqvist C, Mazzanti A, et al. 2015 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The task force for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death of the European Society of Cardiology (ESC) endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC) Eur Heart J. 2015;36:2793–2867. doi: 10.1093/eurheartj/ehv316. [DOI] [PubMed] [Google Scholar]
- 23.Kober L, Thune JJ, Nielsen JC, et al. Defibrillator implantation in patients with nonischemic systolic heart failure. N Engl J Med. 2016;375:1221–1230. doi: 10.1056/NEJMoa1608029. [DOI] [PubMed] [Google Scholar]
- 24.Elming MB, Nielsen JC, Haarbo J, et al. Age and outcomes of primary prevention implantable cardioverter-defibrillators in patients with nonischemic systolic heart failure. Circulation. 2017;136:1772–1780. doi: 10.1161/CIRCULATIONAHA.117.028829. [DOI] [PubMed] [Google Scholar]
- 25.Duncker D, Veltmann C. Role of the wearable defibrillator in newly diagnosed heart failure. Curr Heart fail Rep. 2018;15:368–375. doi: 10.1007/s11897-018-0415-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abraham WT, Fisher WG, Smith AL, et al. Cardiac resynchronization in chronic heart failure. N Engl J Med. 2002;346:1845–1853. doi: 10.1056/NEJMoa013168. [DOI] [PubMed] [Google Scholar]
- 27.Moss AJ, Hall WJ, Cannom DS, et al. Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med. 2009;361:1329–1338. doi: 10.1056/NEJMoa0906431. [DOI] [PubMed] [Google Scholar]
- 28.Tang AS, Wells GA, Talajic M, et al. Cardiac-resynchronization therapy for mild-to-moderate heart failure. N Engl J Med. 2010;363:2385–2395. doi: 10.1056/NEJMoa1009540. [DOI] [PubMed] [Google Scholar]
- 29.Cleland JG, Daubert JC, Erdmann E, et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–1549. doi: 10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 30.Bristow MR, Saxon LA, Boehmer J, et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140–2150. doi: 10.1056/NEJMoa032423. [DOI] [PubMed] [Google Scholar]
- 31.Hayes DL, Boehmer JP, Day JD, et al. Cardiac resynchronization therapy and the relationship of percent biventricular pacing to symptoms and survival. Heart rhythm. 2011;8:1469–1475. doi: 10.1016/j.hrthm.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 32.Koelling TM, Aaronson KD, Cody RJ, Bach DS, Armstrong WF. Prognostic significance of mitral regurgitation and tricuspid regurgitation in patients with left ventricular systolic dysfunction. Am Heart J. 2002;144:524–529. doi: 10.1067/mhj.2002.123575. [DOI] [PubMed] [Google Scholar]
- 33.Obadia J-F, Messika-Zeitoun D, Leurent G, et al. Percutaneous repair or medical treatment for secondary mitral regurgitation. N Engl J Med. 2018;379:2297–2306. doi: 10.1056/NEJMoa1805374. [DOI] [PubMed] [Google Scholar]
- 34.Stone GW, Lindenfeld J, Abraham WT, et al. Transcatheter mitral-valve repair in patients with heart failure. N Engl J Med. 2018;379:2307–2318. doi: 10.1056/NEJMoa1806640. [DOI] [PubMed] [Google Scholar]
- 35.Arnold SV, Chinnakondepalli KM, Spertus JA, et al. Health status after transcatheter mitral-valve repair in heart failure and secondary mitral regurgitation. COAPT Trial. 2019;73:2123–2132. doi: 10.1016/j.jacc.2019.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grayburn PA, Sannino A, Packer M. Proportionate and disproportionate functional mitral regurgitation: a new conceptual framework that reconciles the results of the MITRA-FR and CO-APT trials. JACC Cardiovasc Imaging. 2019;12:353–362. doi: 10.1016/j.jcmg.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 37.Ertl G, Angermann CE, Bekeredjian R, et al. Aufbau und Organisation von Herzinsuffizienz-Netzwerken (HF NETs) und Herzinsuffizienz-Einheiten („Heart Failure Units“, HFUs) zur Optimierung der Behandlung der akuten und chronischen Herzinsuffizienz. Der Kardiologe. 2016;10:222–235. [Google Scholar]
- 38.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure A report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. 2013;62:e147–e239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 39.Bavendiek U, Aguirre Davila L, Koch A, Bauersachs J. Assumption versus evidence: the case of digoxin in atrial fibrillation and heart failure. Eur Heart J. 2017;38:2095–2099. doi: 10.1093/eurheartj/ehw577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bavendiek U, Aguirre Davila L, Schwab J, et al. P6168Digitoxin serum concentrations affecting patient safety and potential outcome in patients with HFrEF-analyses of the ongoing DIGIT-HF-trial. Eur Heart J. 2017;38 [Google Scholar]
- E1.Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart Disease and Stroke Statistics-2017 Update: A report from the American Heart Association. Circulation. 2017;135:e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E2.Ferrari R, Ceconi C, Tavazzi L, Ghio S, Boffa G, Fucili A. Heart failure: 150 questions & answers 2nd ed. Neuilly-sur-Seine Cedex: Servier. 2011 [Google Scholar]
- E3.Gheorghiade M, Bohm M, Greene SJ, et al. Effect of aliskiren on postdischarge mortality and heart failure readmissions among patients hospitalized for heart failure: the ASTRONAUT randomized trial. JAMA. 2013;309:1125–1135. doi: 10.1001/jama.2013.1954. [DOI] [PubMed] [Google Scholar]
- E4.McMurray JJ, Krum H, Abraham WT, et al. Aliskiren, Enalapril, or Aliskiren and Enalapril in heart failure. N Engl J Med. 2016;374:1521–1532. doi: 10.1056/NEJMoa1514859. [DOI] [PubMed] [Google Scholar]
- E5.D‘Elia E, Iacovoni A, Vaduganathan M, Lorini FL, Perlini S, Senni M. Neprilysin inhibition in heart failure: mechanisms and substrates beyond modulating natriuretic peptides. Eur J Heart Fail. 2017;19:710–717. doi: 10.1002/ejhf.799. [DOI] [PubMed] [Google Scholar]
- E6.Lewis EF, Claggett BL, McMurray JJV, et al. Health-related quality of life outcomes in PAR-ADIGM-HF. Circ Heart Fail. 2017;10 doi: 10.1161/CIRCHEARTFAILURE.116.003430. [DOI] [PubMed] [Google Scholar]
- E7.de Diego C, Gonzalez-Torres L, Nunez JM, et al. Effects of angiotensin-neprilysin inhibition compared to angiotensin inhibition on ventricular arrhythmias in reduced ejection fraction patients under continuous remote monitoring of implantable defibrillator devices. Heart rhythm. 2018;15:395–402. doi: 10.1016/j.hrthm.2017.11.012. [DOI] [PubMed] [Google Scholar]
- E8.Januzzi JL Jr, Prescott MF, Butler J, et al. Association of change in N-terminal pro-B-type natriuretic peptide following initiation of sacubitril-Valsartan treatment with cardiac structure and function in patients with heart failure with reduced ejection fraction. JAMA. 2019;322:1085–1095. doi: 10.1001/jama.2019.12821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E9.Böhm M, Swedberg K, Komajda M, et al. Heart rate as a risk factor in chronic heart failure (SHIFT): the association between heart rate and outcomes in a randomised placebo-controlled trial. Lancet. 2010;376:886–894. doi: 10.1016/S0140-6736(10)61259-7. [DOI] [PubMed] [Google Scholar]
- E10.Ekman I, Chassany O, Komajda M, et al. Heart rate reduction with ivabradine and health related quality of life in patients with chronic heart failure: results from the SHIFT study. Eur Heart J. 2011;32:2395–2404. doi: 10.1093/eurheartj/ehr343. [DOI] [PubMed] [Google Scholar]
- E11.Armstrong PW, Pieske B, Anstrom KJ, et al. Vericiguat in patients with heart failure and reduced ejection fraction. N Engl J Med. 2020 [epub ahead of print] doi: 10.1056/NEJMoa1915928. [DOI] [PubMed] [Google Scholar]
- E12.Cazeau S, Leclercq C, Lavergne T, et al. Effects of multisite biventricular pacing in patients with heart failure and intraventricular conduction delay. N Engl J Med. 2001;344:873–880. doi: 10.1056/NEJM200103223441202. [DOI] [PubMed] [Google Scholar]
- E13.Auricchio A, Stellbrink C, Sack S, et al. Long-term clinical effect of hemodynamically optimized cardiac resynchronization therapy in patients with heart failure and ventricular conduction delay. J Am Coll Cardiol. 2002;39:2026–2033. doi: 10.1016/s0735-1097(02)01895-8. [DOI] [PubMed] [Google Scholar]
- E14.Gervais R, Leclercq C, Shankar A, et al. Surface electrocardiogram to predict outcome in candidates for cardiac resynchronization therapy: a sub-analysis of the CARE-HF trial. Eur J Heart Fail. 2009;11:699–705. doi: 10.1093/eurjhf/hfp074. [DOI] [PubMed] [Google Scholar]
- E15.Zareba W, Klein H, Cygankiewicz I, et al. Effectiveness of cardiac resynchronization therapy by QRS morphology in the multicenter automatic defibrillator implantation trial-cardiac resynchronization therapy (MADIT-CRT) Circulation. 2011;123:1061–1072. doi: 10.1161/CIRCULATIONAHA.110.960898. [DOI] [PubMed] [Google Scholar]
- E16.Sundaram V, Sahadevan J, Waldo AL, et al. Implantable cardioverter-defibrillators with versus without resynchronization therapy in patients with a QRS duration > 180 ms. J Am Coll Cardiol. 2017;69:2026–2036. doi: 10.1016/j.jacc.2017.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E17.Ruschitzka F, Abraham WT, Singh JP, et al. Cardiac-resynchronization therapy in heart failure with a narrow QRS complex. N Engl J Med. 2013;369:1395–1405. doi: 10.1056/NEJMoa1306687. [DOI] [PubMed] [Google Scholar]
- E18.Shenkman HJ, Pampati V, Khandelwal AK, et al. Congestive heart failure and QRS duration: establishing prognosis study. CHEST. 2002;122:528–534. doi: 10.1378/chest.122.2.528. [DOI] [PubMed] [Google Scholar]
- E19.Gold MR, Van Veldhuisen DJ, Hauptman PJ, et al. Vagus nerve stimulation for the treatment of heart failure: the INOVATE-HF trial. J Am Coll Cardiol. 2016;68:149–158. doi: 10.1016/j.jacc.2016.03.525. [DOI] [PubMed] [Google Scholar]
- E20.Baumgartner H, Falk V, Bax JJ, et al. 2017 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. 2017;38:2739–2791. doi: 10.1093/eurheartj/ehx391. [DOI] [PubMed] [Google Scholar]
- E21.Bursi F, Barbieri A, Grigioni F, et al. Prognostic implications of functional mitral regurgitation according to the severity of the underlying chronic heart failure: a long-term outcome study. Eur J Heart Fail. 2010;12:382–388. doi: 10.1093/eurjhf/hfq014. [DOI] [PubMed] [Google Scholar]
- E22.Pfister R, Hausleiter J, Boekstegers P, et al. Role of percutaneous edge-to-edge repair in secondary mitral regurgitation after MITRA-FR and COAPT : a comment by the section of AV-valve treatment of the Working Group of Interventional Cardiology (AGIK) of the German Society of Cardiology (DGK) Clin Res Cardiol. 2019;108:969–973. doi: 10.1007/s00392-019-01457-3. [DOI] [PubMed] [Google Scholar]
- E23.Chioncel O, Lainscak M, Seferovic PM, et al. Epidemiology and one-year outcomes in patients with chronic heart failure and preserved, mid-range and reduced ejection fraction: an analysis of the ESC Heart Failure Long-Term Registry. Eur J Heart Fail. 2017;19:1574–1585. doi: 10.1002/ejhf.813. [DOI] [PubMed] [Google Scholar]
- E24.Fox K, Komajda M, Ford I, et al. Effect of ivabradine in patients with left-ventricular systolic dysfunction: a pooled analysis of individual patient data from the BEAUTIFUL and SHIFT trials. Eur Heart J. 2013;34:2263–2270. doi: 10.1093/eurheartj/eht101. [DOI] [PubMed] [Google Scholar]
- E25.Komajda M, Böhm M, Borer JS, et al. Incremental benefit of drug therapies for chronic heart failure with reduced ejection fraction: a network meta-analysis. Eur J Heart Fail. 2018;20:1315–1322. doi: 10.1002/ejhf.1234. [DOI] [PubMed] [Google Scholar]
- E26.Srivastava PK, Claggett BL, Solomon SD, et al. Estimated 5-year number needed to treat to prevent cardiovascular death or heart failure hospitalization with Angiotensin Receptor-Neprilysin inhibition vs standard therapy for patients with heart failure with reduced ejection fraction: An analysis of data from the PARADIGM-HF trial. JAMA Cardiology. 2018;3:1226–1231. doi: 10.1001/jamacardio.2018.3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E27.Duncker D, Veltmann C. Device therapy in heart failure with reduced ejection fraction—cardiac resynchronization therapy and more. Herz. 2018;43:415–422. doi: 10.1007/s00059-018-4710-6. [DOI] [PubMed] [Google Scholar]