Abstract
Background
Low-dose persistent organic pollutants (POPs), especially organochlorine pesticides (OCPs), have emerged as a new risk factor of many chronic diseases. As serum concentrations of POPs in humans are mainly determined by both their release from adipose tissue to circulation and their elimination from circulation, management of these internal pathways may be important in controlling the serum concentrations of POPs. As habitual physical activity can increase the elimination of POPs from circulation, we evaluated whether chronic physical activity is related to low serum POP concentrations.
Methods
A cross-sectional study of 1,850 healthy adults (age ≥20 years) without cardio-metabolic diseases who participated in the U.S. National Health and Nutrition Examination Survey 1999 to 2004 was conducted. Information on moderate or vigorous leisure-time physical activity was obtained based on questionnaires. Serum concentrations of OCPs and polychlorinated biphenyls were investigated as typical POPs.
Results
Serum concentrations of OCPs among physically active subjects were significantly lower than those among physically inactive subjects (312.8 ng/g lipid vs. 538.0 ng/g lipid, P<0.001). This difference was maintained after adjustment for potential confounders. When analyses were restricted to physically active subjects, there were small decreases in the serum concentrations of OCPs with increasing duration of physical activity, showing a curvilinear relationship over the whole range of physical activity (Pquadratic <0.001). In analyses stratified by age, sex, body mass index, and smoking status, a strong inverse association was similarly observed among all subgroups.
Conclusion
Physical activity may assist in decreasing serum concentrations of lipophilic chemical mixtures such as OCPs.
Keywords: Adipose tissue, Complex mixtures, Environmental exposure, Environmental pollutants, Exercise, Organic chemicals, Pesticides, Polychlorinated biphenyls
INTRODUCTION
Serum concentration of persistent organic pollutants (POPs) has recently emerged as a risk factor for common chronic diseases, such as diabetes mellitus, dyslipidemia, cancer, and dementia [1,2,3,4,5]. POPs include various strong lipophilic organic compounds that are resistant to biodegradation and highly persistent in the environment. Typical examples of POPs are organochlorine pesticides (OCPs) and polychlorinated biphenyls (PCBs). Although most OCPs and PCBs were banned several decades ago, current general populations are still exposed to these persistent chemicals because they have widely contaminated the food chain, especially fatty animal products such as meat, dairy, and fish [1].
In general, serum concentrations of environmental chemicals reflect recent exposure levels to these chemicals from the environment. However, POPs are different. When POPs enter the body from external exposure sources, they are primarily stored in adipose tissue and then slowly released into circulation through lipolysis [6]. Therefore, serum concentrations of POPs are mainly determined by the rate of release from adipose tissue and the rate of elimination from circulation. Consequently, mechanisms to mitigate these internal pathways may play a key role in decreasing serum concentrations of POPs [7]. Serum concentrations of POPs can be elevated in persons with increased lipolysis of adipocytes [8] or decreased bile secretion because bile is the main route for the elimination of these chemicals [9,10].
Habitual physical activity can assist in decreasing serum concentrations of POPs through various mechanisms. First, as physical activity increases insulin sensitivity [11,12], physical activity without weight loss can decrease insulin resistance-related uncontrolled release of POPs from adipose tissue to the circulatory system [8]. Second, physical activity can increase the metabolism and elimination of chemical mixtures by increasing biotransformation through liver enzyme activity [13]. Third, physical activity increases the excretion of endogenous and exogenous chemicals from the circulatory system by increasing biliary clearance, as demonstrated in an animal experimental study [14]. Fourth, POPs can be eliminated through sweat during physical activity [15,16].
To our knowledge, only one human study on this topic has been published. This small-scale cross-sectional study showed lower serum concentrations of POPs among endurance athletes compared to obese persons [17]. However, the association between habitual physical activity and serum concentrations of POPs has never been investigated among the general population.
A randomized controlled trial is generally considered the most appropriate study design to evaluate any beneficial effect of physical activity. However, a cross-sectional study among appropriate study subjects has the merit of evaluating the long-term effect of physical activity on serum concentrations of POPs because serum concentrations of POPs are continuously affected by the dynamic equilibrium with their concentrations in adipose tissue. Therefore, the effects of physical activity on POPs would be difficult to discern in a short-term randomized trial. In addition, weight loss that accompanies physical activity during clinical trials can distort results because adipocyte lipolysis during weight loss leads to a temporary increase in serum POP concentrations [18,19]. Even a prospective study with repeated measurement of POPs makes it difficult to test the effect of physical activity on POPs, because contemporary humans frequently experience weight cycling which directly affects serum concentrations of POPs [5].
Therefore, this cross-sectional study was performed to investigate whether habitual physical activity was related to serum concentrations of OCPs or PCBs in the United States general population. As physical activity is commonly recommended in patients with metabolic disturbances such as type 2 diabetes mellitus, dyslipidemia, or hypertension and these diseases are reported to be associated with increased serum concentrations of POPs [1,2,5], we excluded patients with cardio-metabolic diseases and focused on healthy persons.
METHODS
Study participants
We used the data from the National Health and Nutrition Examination Survey (NHANES) conducted by the Centers for Disease Control and Prevention (CDC) in the United States (CDC and National Center for Health Statistics). The NHANES is an ongoing program designed to assess the health and nutritional status of the civilian non-institutionalized United States population. Detailed information on the sampling design, survey procedure, data collection, and consent to participate in NHANES are reported elsewhere (www.cdc.gov/nchs/nhanes/about_nhanes.htm). The study protocol was reviewed and approved by the Institutional Review Board of the CDC in the United States (Protocol #98-12). Written informed consent was obtained from all participants.
Concentrations of OCPs and PCBs in serum were measured in one-third of the participants aged ≥12 years who met the subsample requirements of the 1999 to 2004 NHANES. Participants in the subsample were randomly pre-assigned into subgroups which were selected to be representative of the survey [20]. The number of participants with serum OCP measurements was 7,158 and with serum PCB measurements was 7,106. Among them, we included study participants aged at least 20 years who had information on the serum concentrations of either OCPs or PCBs. We excluded patients who had physician-diagnosed hypertension, diabetes mellitus, dyslipidemia, coronary heart disease, stroke, or cancer. The final sample size was 1,850 for analyses of OCPs and 1,702 for analyses of PCBs. About two-thirds of the analysis sample had measurements for both OCPs and PCBs.
Data collection and measurements
Information on demographic and socioeconomic characteristics, dietary habits, health behaviors, and health status was collected by interview. Venous blood samples were collected and frozen at−20℃. Individual congeners of OCPs were measured by high-resolution gas chromatography/high-resolution mass spectrometry using isotope dilution. The instruments used were as follows: Thermo Finnigan MAT95 XP Mass Spectrometer (5 kV) with X-caliber data systems (Thermo Finnigan, San Jose, CA, USA), Agilent Technologies 6890 Gas Chromatograph (Agilent Technologies, Palo Alto, CA, USA), and GC-Pal autosampler (Leap Technologies, Carrboro, NC, USA) [21]. Total serum cholesterol and triglyceride levels were measured enzymatically (Hitachi 704 analyzer; Roche Diagnostics, Indianapolis, IN, USA).
Leisure-time physical activity was calculated using the formula provided in the NHANES physical activity and cardiovascular fitness data tutorial. The data tutorial and details on SAS syntax are available on the Internet [22]. The study participants were asked whether they had performed moderate and/or vigorous leisure-time activities at intervals of 10 minutes or more over the last 30 days. Moderate activities cause light sweating or a slight-to-moderate increase in breathing or heart rates (for example, brisk walking, cycling for pleasure, golf, or dancing). Vigorous activities cause heavy sweating or large increases in breathing or heart rates (for example, running, lap swimming, aerobics classes, or fast cycling). The study participants reported multiple physical activities, which have predetermined metabolic equivalent of task (MET) scores according to the level of activity (moderate or vigorous). The daily duration of leisure-time physical activity (min/day) was calculated by summing the frequency and duration for each physical activity. Daily MET-minutes of leisure-time physical activity (MET-min/day) were calculated by summing the products of frequency, duration, and predetermined MET score for each physical activity.
We selected six OCPs (β-hexachlorocyclohexane, p,p′-DDE, p,p′-DDT, oxychlordane, trans-nonachlor, and heptachlor epoxide) and 12 PCBs (PCB74, PCB99, PCB118, PCB138, PCB146, PCB153, PCB156, PCB170, PCB180, PCB187, 3,3′,4,4′,5-pentachlorobiphenyl, and 3,3′,4,4′,5,5′-hexachlorobiphenyl) that are frequently detected among the general population. The lipid-standardized concentration was calculated by dividing the wet weight concentrations by total lipids: total lipids (mg/dL)=2.27×total cholesterol+triglycerides+62.3 [23]. We reported the results for both lipid-adjusted concentrations and wet-weight concentrations.
Statistical analysis
The duration of moderate or vigorous physical activity was categorized as 0, 1 to <10, 10 to <30, 30 to <60, and ≥60 min/day. MET-minutes were categorized as <10, 10 to <50, 50 to <100, 100 to <200, 200 to <500, and ≥500 MET-min/day, after consideration of the sample sizes in each category. The main dependent variables were the summary measures of OCPs (ΣOCPs) or PCBs (ΣPCBs), calculated by summing the absolute concentration of the individual OCP or PCB compounds. Crude and adjusted geometric means of ΣOCPs or ΣPCBs stratified by the level of physical activity were estimated using generalized linear models. For OCPs, we also presented the results for individual OCP compounds. In addition, analyses stratified by age (<40, 40 to 59, and ≥60), sex (men and women), body mass index (BMI, <30 and ≥30 kg/m2), and smoking status (non-current smokers and current smokers) were conducted to evaluate the associations of ΣOCPs or ΣPCBs with physical activity in the various subgroups.
Demographic-, obesity-, and diet-related variables were considered as covariates because the serum concentrations of OCPs or PCBs could be affected by these variables [1]. Specifically, we adjusted for age, sex, race-ethnicity (non-Hispanic White and others, including multi-racial), smoking status (non-current smokers and current smokers), BMI (kg/m2), weight change over the past year (current body weight minus body weight 1 year ago, kg), total polyunsaturated fatty acid intake (g), total monounsaturated fatty acid intake (g), total saturated fatty acid intake (g), and total energy intake (kcal).
Missing covariates values for individual participants were substituted by the median values of the specified variables. The numbers of participants with missing data for each parameter were 70 for weight change and 132 for each fatty acid intake variable among those with information on OCPs and 63 for weight change and 136 for each fatty acid intake variable among those with information on PCBs. Although the exclusion of participants with missing data for covariates did not significantly impact the results, we included these participants to ensure sufficient sample sizes in the stratified analyses.
Estimates of the main results were calculated, accounting for the NHANES stratification and clustering [24]; they were adjusted for age, sex, and race-ethnicity instead of using sample weights; this adjustment has been regarded as a good compromise between efficiency and bias [25]. All statistical analyses were performed using SAS 9.4 software (SAS Institute, Cary, NC, USA) in 2018.
RESULTS
General characteristics of the study participants
Table 1 shows the general characteristics of the study participants; the mean age was 39 years and 47% were male. Physically active participants tended to be younger, men, of white race, non-obese, non-current smokers, and with higher total energy and fatty acid intakes; however, these trends in the dietary variables were not linear.
Table 1.
General characteristics of study participants with information on organochlorine pesticides
| Characteristic | Total (n=1,850) | Daily duration of leisure-time moderate to vigorous physical activity, min/day |
Ptrenda | ||||
|---|---|---|---|---|---|---|---|
| 0 (n=750) | 1 to <10 (n=286) | 10 to <30 (n=328) | 30 to <60 (n=256) | 60≤ (n=230) | |||
| Age, yr | 39.4±15.9 | 42.2±17.2 | 36.2±13.9 | 37.9±14.5 | 38.5±14.9 | 37.0±15.4 | <0.001 |
| BMI, kg/m2 | 27.3±5.7 | 27.7±5.7 | 27.0±5.6 | 27.7±6.3 | 26.0±5.2 | 27.0±5.6 | 0.006 |
| Weight change for recent 1 year, kg | 1.8±8.3 | 1.8±8.6 | 2.1±7.5 | 2.7±9.0 | 1.5±7.4 | 0.3±7.9 | 0.102 |
| Total energy, kcal | 2,264.7±1,043.1 | 2,103.2±972.0 | 2,390.3±1,056.4 | 2,248.6±1,033.7 | 2,335.2±919.5 | 2,579.2±1,276.1 | <0.001 |
| Total monounsaturated fatty acids, g | 31.4±18.8 | 28.6±17.9 | 33.2±18.9 | 31.2±17.9 | 33.1±18.0 | 36.8±22.1 | <0.001 |
| Total polyunsaturated fatty acids, g | 17.0±11.5 | 15.5±10.7 | 18.4±12.2 | 17.4±10.9 | 17.3±11.4 | 19.3±13.3 | <0.001 |
| Total saturated fatty acids, g | 27.6±17.1 | 25.0±15.7 | 29.9±17.4 | 27.7±17.4 | 28.7±16.5 | 32.2±20.1 | <0.001 |
| Male sex | 877 (47.4) | 333 (44.4) | 138 (48.3) | 138 (42.1) | 123 (48.1) | 145(63.0) | <0.001 |
| Non-Hispanic white | 834 (45.1) | 258 (34.4) | 151 (52.8) | 163 (49.7) | 147 (57.4) | 115 (50.0) | <0.001 |
| BMI ≥30 kg/m2 | 473 (25.6) | 218 (29.1) | 66 (23.1) | 91 (27.7) | 44 (17.2) | 54 (23.5) | 0.005 |
| Current smokers | 476 (25.7) | 216 (28.8) | 84 (29.4) | 67 (20.4) | 55 (21.5) | 54 (23.5) | 0.004 |
Values are presented as mean±standard deviation or number (%).
BMI, body mass index.
A generalized linear model was used for continuous variables and a Cochran-Armitage trend analysis was used for categorical variables.
Physical activity and serum concentrations of OCPs/PCBs
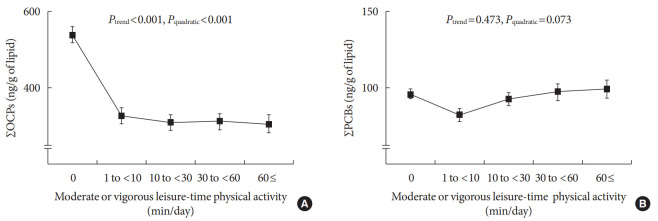
Fig. 1 shows the distribution of serum concentrations for the summary measures of OCPs by the level of moderate or vigorous leisure-time physical activity. The largest difference was observed when physically inactive persons were compared with physically active persons. The geometric mean values of serum concentrations of OCPs among physically active persons (312.8 ng/g of lipid) were significantly lower than those among physically inactive persons (538.0 ng/g of lipid) (P<0.001) (Fig. 1A).
Fig. 1. Distribution of serum concentrations of persistent organic pollutants based on the level of moderate or vigorous leisure-time physical activity. Geometric mean±standard error. (A) ΣOCPs, sum of six OCPs (β-hexachlorocyclohexane, p,p′-DDE, p,p′-DDT, oxychlordane, trans-nonachlor, and heptachlor epoxide). (B) ΣPCBs, sum of 12 PCBs (PCB74, PCB99, PCB118, PCB138, PCB146, PCB153, PCB156, PCB170, PCB180, PCB187, 3,3′4,4′,5-pentachlorobiphenyl, and 3,3′,4,4′,5,5′-hexachlorobiphenyl).
Among physically active persons, decreased serum concentrations of OCPs were observed among persons who reported moderate or vigorous leisure-time physical activity of up to 30 min/day. When the duration exceeded 30 minutes, serum concentrations of OCPs did not decrease further, conforming to a curvilinear dose–response relationship over the whole range of leisure-time physical activity (Pquadratic<0.001). Conversely, the serum concentrations of PCBs were not clearly associated with physical activity.
Adjustment for possible confounders did not materially change the unadjusted results, which are presented in Fig. 1 (Table 2). Adjusted serum concentrations of the summary measures for OCPs were 426.9, 374.2, 323.3, 334.3, and 334.3 ng/g of lipids according to the five categories of duration of leisure time physical activity (Ptrend<0.001, Pquadratic=0.013). In the adjusted models, as the generally negative slope seemed to be more important than non-linearity, we focused on Ptrend.
Table 2.
Lipid-adjusted serum concentrations of persistent organic pollutants according to the duration of leisure-time physical activity
| Variable | Modela | Daily duration of leisure-time moderate to vigorous physical activity, min/day |
Ptrend | Pquadratic | |||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 to <10 | 10 to <30 | 30 to <60 | 60≤ | |||||
| No. of subjects | 750 | 286 | 328 | 256 | 230 | ||||
| ∑OCPs | 1 | 429.5±14.7 | 371.0±20.0 | 321.7±16.2 | 338.3±19.2 | 335.2±20.2 | <0.001 | 0.009 | |
| 2 | 426.9±14.8 | 374.2±20.1 | 323.3±16.3 | 334.3±19.2 | 334.3±20.2 | <0.001 | 0.013 | ||
| β-Hexachlorocyclohexane | 1 | 9.5±0.3 | 7.6±0.4 | 7.4±0.4 | 7.4±0.4 | 7.3±0.5 | <0.001 | 0.016 | |
| 2 | 9.4±0.3 | 7.7±0.4 | 7.4±0.4 | 7.5±0.5 | 7.3±0.5 | <0.001 | 0.022 | ||
| p,p'-DDE | 1 | 350.4±13.1 | 307.2±18.0 | 261.2±14.3 | 278.7±17.3 | 274.3±18.0 | <0.001 | 0.018 | |
| 2 | 348.4±13.2 | 309.8±18.2 | 262.4±14.5 | 274.7±17.2 | 273.2±18.0 | <0.001 | 0.026 | ||
| p,p'-DDT | 1 | 8.7±0.3 | 7.4±0.4 | 6.7±0.3 | 6.8±0.4 | 6.7±0.4 | <0.001 | 0.016 | |
| 2 | 8.5±0.3 | 7.4±0.4 | 6.6±0.3 | 6.6±0.4 | 6.6±0.4 | <0.001 | 0.024 | ||
| Oxychlordane | 1 | 9.7±0.2 | 9.2±0.4 | 9.0±0.3 | 8.8±0.4 | 9.8±0.4 | 0.390 | 0.018 | |
| 2 | 9.8±0.2 | 9.3±0.4 | 9.3±0.3 | 9.0±0.4 | 10.0±0.4 | 0.607 | 0.045 | ||
| Trans-nonachlor | 1 | 14.4±0.4 | 14.2±0.6 | 14.0±0.6 | 13.7±0.6 | 14.8±0.7 | 0.897 | 0.251 | |
| 2 | 14.6±0.4 | 14.5±0.6 | 14.4±0.6 | 14.0±0.6 | 15.1±0.7 | 0.918 | 0.382 | ||
| Heptachlor epoxide | 1 | 5.3±0.1 | 5.1±0.2 | 5.2±0.2 | 4.9±0.2 | 4.6±0.2 | 0.006 | 0.383 | |
| 2 | 5.3±0.1 | 5.1±0.2 | 5.3±0.2 | 5.0±0.2 | 4.6±0.2 | 0.016 | 0.205 | ||
| No. of subjects | 671 | 252 | 321 | 234 | 224 | ||||
| ∑PCBs | 1 | 90.5±2.2 | 89.8±3.4 | 97.3±3.3 | 99.1±4.0 | 104.9±4.3 | 0.001 | 0.592 | |
| 2 | 90.9±2.2 | 90.2±3.4 | 98.4±3.4 | 99.1±3.9 | 104.6±4.3 | <0.001 | 0.734 | ||
Values are presented as geometric mean±standard error (ng/g of lipid).
ΣOCPs, sum of six OCPs (β-hexachlorocyclohexane, p,p'-DDE, p,p'-DDT, oxychlordane, trans-nonachlor, and heptachlor epoxide); ΣPCBs, sum of 12 PCBs (PCB74, PCB99, PCB118, PCB138, PCB146, PCB153, PCB156, PCB170, PCB180, PCB187, 3,3',4,4',5-pentachlorobiphenyl, and 3,3',4,4',5,5'-hexachlorobiphenyl).
Model 1: adjusted for age, sex, and race/ethnicity; Model 2: further adjustment for smoking status, body mass index, changes in weight over the past year, total calorie intake, dietary intake of total monounsaturated fatty acids, dietary intake of total polyunsaturated fatty acids, and dietary intake of total saturated fatty acids.
When six individual OCPs were separately analyzed, the serum concentrations of β-hexachlorocyclohexane, p,p′-DDE, p,p′-DDT, and heptachlor epoxide were found to be significantly lower with increasing duration of physical activity (Ptrend<0.001, <0.001, <0.001, and 0.016, respectively); however, oxychlordane showed a significant U-shaped association with physical activity (Pquadratic=0.045).
Wet weight concentrations of POPs had a similar association with the duration of leisure-time physical activity (Supplementary Table 1). Results based on the MET-minutes of leisure-time physical activity were similar to those for the duration of leisure-time physical activity (Supplementary Tables 2 and 3). Unlike OCPs, the adjusted serum concentrations of PCBs showed an increasing trend with the duration of leisure-time physical activity. The results after excluding subjects with missing values were similar to those obtained by replacing the missing values with the median (Supplementary Tables 4 and 5).
Subgroup analysis: physical activity and serum OCP concentrations
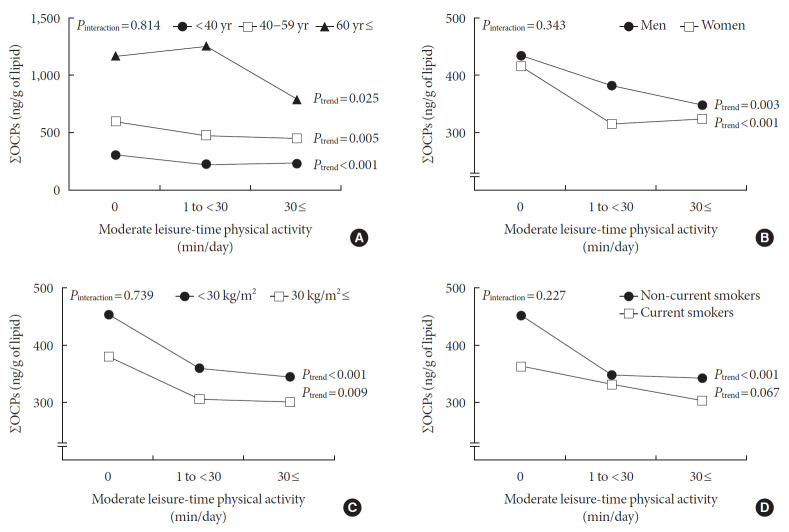
Fig. 2 shows the analyses for the relationship between the duration of leisure-time physical activity and OCP concentrations stratified by age, sex, BMI, and smoking status. When stratified by age, all three groups showed inverse associations, even though the associations were stronger among persons aged less than 60 years than among those aged ≥60 years (Ptrend <0.001 and 0.005 for participants aged less than 40 years and those aged 40 to 59 years; Ptrend=0.025 for participants aged 60 years or older) (Fig. 2A). Men and women had similar inverse associations between the duration of leisure-time physical activity and serum OCP concentrations (Ptrend=0.003 for men, Ptrend <0.001 for women) (Fig. 2B). When stratified by BMI or smoking status (Fig. 2C and D), the inverse associations were clearly observed among participants with BMI <30 kg/m2 (Ptrend <0.001) and non-current smokers (Ptrend <0.001), whereas this inverse association was only weakly observed among obese participants with BMI ≥30 kg/m2 (Ptrend=0.009) and current smokers (Ptrend=0.067).
Fig. 2. Stratified analyses for the associations between duration of leisure-time physical activity and the concentrations of organochlorine pesticides (OCPs). Geometric mean±standard error. (A) Stratified by age (sample sizes: 1,087 for age <40; 518 for age 40–59; 245 for age ≥60). (B) Stratified by sex (sample sizes: 877 for men; 973 for women). (C) Stratified by body mass index (sample sizes: 1,377 for body mass index [BMI] <30 kg/m2; 473 for BMI ≥30 kg/m2). (D) Stratified by smoking status (sample sizes: 476 for current smokers; 1,374 for non-current smoker). All of these analyses were adjusted for age, sex, race/ethnicity, smoking status, body mass index, changes in weight over the past year, dietary intake of total monounsaturated fatty acids, total polyunsaturated fatty acids, total saturated fatty acids, and total energy intake. Among all covariates mentioned above, the variable used for stratification was excluded from adjustment in each analysis. ΣOCPs, sum of six OCPs (β-hexachlorocyclohexane, p,p′-DDE, p,p′-DDT, oxychlordane, trans-nonachlor, and heptachlor epoxide).
The results of subgroup analyses on serum concentrations of PCBs differed from those of subgroup analyses on serum concentrations of OCPs (Supplementary Fig. 1). The positive association between exercise and PCBs disappeared in the subgroup analysis stratified by age; however, the positive trend was maintained in both sexes and in non-obese persons.
DISCUSSION
In this study, we show that serum OCP concentrations in physically active persons are considerably lower than those in physically inactive persons among the healthy United States population without any cardio-metabolic disease. Exclusion of patients with cardio-metabolic diseases was very important for the current study purpose because physical activity is commonly recommended to patients with cardio-metabolic conditions and higher serum concentrations of POPs are linked to such diseases [1,2]. Mechanistically, the release of POPs from adipocytes through uncontrolled lipolysis, which is common in patients with cardio-metabolic diseases [8], can distort the relationship between physical activity and serum concentrations of POPs.
Compared to the sharp contrast observed between physically active and inactive persons, however, the inverse association between different durations of physical activity and serum concentrations of POPs was not very clear, although there was a decreasing linear trend for the serum concentrations of OCPs among some sub-groups (i.e., men or non-obese persons) with a longer duration of physical activity.
On the other hand, PCBs did not show an inverse association with physical activity. In fact, lipid adjusted PCBs showed a positive association with physical activity even after adjustment for potential confounders. However, the positive association disappeared in the stratified analysis by age, suggesting the possibility of residual confounders related to age. Considering that all OCPs and PCBs are strongly lipophilic chemicals, the different patterns between them seemed strange. However, the previous small-scale cross-sectional study also reported similar findings [17]. When the plasma concentrations of POPs for eight endurance athletes who practiced sports intensively for 30 years on average were compared with those of lean sedentary and obese persons, the athletes had the lowest plasma concentrations of POPs, in particular OCPs. In fact, toxicokinetics of OCPs may be different from those of PCBs because the elimination patterns of OCPs and PCBs through sweat and urine differed during exercise [15,16].
Another possibility is that serum concentrations of OCPs better reflect release from adipose tissue than do serum concentrations of PCBs. This speculation is supported by different cross-sectional associations of OCPs or PCBs with obesity in epidemiological studies [26,27]; serum concentrations of OCPs tend to be positively associated with indices of obesity, whereas PCBs were inversely associated with them. Even though adipose tissue plays a role as a storage organ of POPs, obesity with dysfunctional adipocytes and/or insulin resistance is toxicokinetically related to increased release of lipophilic chemicals from adipose tissue through uncontrolled lipolysis [8]. Therefore, if serum concentrations of PCBs are primarily determined by the release of PCBs from adipose tissue like OCPs, there should be positive associations between serum concentration of PCBs and obesity. Consequently, serum concentrations of PCBs may have a different meaning from those of OCPs from the viewpoint of toxicokinetics.
Furthermore, epidemiological studies on the associations between serum concentrations of POPs and clinical outcomes, including studies using the same NHANES database, have reported that OCPs have different patterns from PCBs [28,29,30]. Compared with PCBs, OCPs demonstrated more consistent and stronger results. Therefore, we interpret that the clear inverse association between physical activity and serum concentrations of OCPs has significant implications for humans.
In our study, decreased serum concentrations of OCPs on the basis of physical activity levels were more apparent among younger persons than in the elderly. As a decrease in the physiological ability to metabolize and excrete xenobiotics in elderly people is expected [31], the weak association among elderly persons can be biologically justified. However, it might also be related to the variation in the accuracy of information regarding physical activity, which was assessed using a questionnaire. A previous study found that measurement of self-reported physical activity is more difficult in elderly persons than in younger persons [32]. Further, moderate or vigorous physical activity may not be appropriate to assess the levels of physical activity among the elderly because they tend to engage more in light physical activity [33].
The decrease in OCP concentrations on the basis of the physical activity level was clearer among non-obese persons than among obese persons. The inverse associations between physical activity and serum OCP concentrations may be weakened among obese persons because uncontrolled lipolysis is more common among obese persons than lean persons [34]; thus, the effect of physical activity on serum OCP concentrations could be partially masked among obese persons. However, it is important to note that more adiposity may actually be helpful in decreasing serum OCP concentrations by safely sequestering them into adipocytes as long as the adipose tissue is healthy [8].
The 2008 Physical Activity Guidelines for Americans recommended that adults should engage in at least 150 min/week of moderate-intensity aerobic physical activity, 75 min/week of vigorous-intensity aerobic physical activity, or an equivalent combination of moderate- and vigorous-intensity aerobic activity [35]. Additionally, the guidelines emphasize that inactivity be avoided; our study is in support of these guidelines. The lowest serum OCP concentration was observed among subjects who engaged in moderate or vigorous physical activity for 10 to 30 min/day. Additionally, the largest difference in the serum OCP concentrations was observed between physically inactive and active persons.
The absence of further decrease in the serum concentration of OCPs among persons who reported moderate or vigorous physical activity for more than 30 min/day may be related to increased lipolysis among these persons. Athletes (i.e., trained subjects or experienced marathon runners) showed an increase in adipose tissue lipolysis compared with lean sedentary controls [36]. Even though we adjusted for weight change over the past year, the information on weight loss would be crude in epidemiological studies considering the dynamics of weight change in humans, and it would be almost impossible to adjust for this effect without a residual confounding effect. Highly physically active persons may experience frequent, but subtle, weight loss, which can contribute to increased serum concentrations of OCPs.
This study has several limitations. First, as a cross-sectional study, this study was not able to establish temporality. However, the possibility of reverse causality was unlikely because it is difficult to believe that serum OCP concentrations, which were unknown to participants, would affect physical activity among healthy persons without any cardio-metabolic diseases. Additionally, similar associations among most subgroups stratified by age, sex, and BMI suggest that the current findings may be valid. In fact, a cross-sectional design has advantages in evaluation of the effects of habitual physical activity on serum concentrations of OCPs rather than a short-term experimental study, which is greatly affected by the dynamic nature of the serum concentrations of OCPs. The value of a cohort study is also limited unless all factors affecting weight change and serum concentrations of OCPs are continuously monitored. Therefore, if there is biological plausibility, the findings from a cross-sectional study could be causal.
Second, physical activity was measured on the basis of a self-reported questionnaire. Although physical activity questionnaires are considered to have acceptable reliability and validity [37], there are substantial discrepancies between self-reported and objectively measured physical activity. Thus, motion sensors, such as pedometers or accelerometers, are increasingly used as an additional measure to determine physical activity [38]. However, there are several issues associated with the validity of motion sensors [39]. In the current study, the assessment of physical activity using self-reported questionnaires might have led to under-estimation of the association because measurement error would not differ on the basis of serum OCP concentrations. Third, there could be residual confounders even though we extensively adjusted for covariates, including dietary fat intake and weight changes. However, the consistent associations observed in most subgroups cannot easily be explained by residual confounders.
In conclusion, the current study suggests that moderate to vigorous habitual physical activity can be helpful in decreasing serum OCP concentrations. Notably, serum concentrations of these chemicals need to be considered as a general marker of chemical mixtures that are released from adipose tissue into the circulatory system; they include OCPs and other lipophilic chemicals coexisting with OCPs. Avoidance of exposure to low dose lipophilic chemical mixtures such as OCPs is impossible because of the wide contamination of human adipose tissues [7]. Accordingly, physical activity should be evaluated as a practical method to decrease serum concentrations of lipophilic chemical mixtures by controlling their toxicokinetics in the human body.
ACKNOWLEDGMENTS
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST) (No. 2019R1af2-dmj-2019-0158C1008958). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Conception or design: D.H.L.
Acquisition, analysis, or interpretation of data: Y.M.L., S.A.K.
Drafting the work or revising: Y.M.L., J.Y.S., D.R.J., D.H.L.
Final approval of the manuscript: Y.M.L., J.Y.S., S.A.K., D.R.J., D.H.L.
SUPPLEMENTARY MATERIALS
Supplementary materials related to this article can be found online at https://doi.org/10.4093/dmj.2019.0158.
Wet serum concentrations of persistent organic pollutants according to the duration of leisure-time physical activity
Lipid-adjusted serum concentrations of persistent organic pollutants according to the metabolic equivalent (MET)-minutes of leisure-time physical activity
Wet serum concentrations of persistent organic pollutants according to the metabolic equivalent (MET)-minutes of leisure-time physical activity
Lipid-adjusted serum concentrations of persistent organic pollutants according to the duration of leisure-time physical activity after excluding subjects with the missing values
Lipid-adjusted serum concentrations of persistent organic pollutants according to the metabolic equivalent (MET)-minutes of leisure-time physical activity after excluding subjects with the missing values
Stratified analyses for the associations between duration of leisure-time physical activity and the concentrations of polychlorinated biphenyls (PCBs). Geometric mean±standard error. (A) Stratified by age (sample sizes: 1,001 for age <40; 461 for age 40 to 59; 240 for age ≥60). (B) Stratified by sex (sample sizes: 777 for men; 925 for women). (C) Stratified by body mass index (BMI) (sample sizes: 1,298 for BMI <30 kg/m2; 404 for BMI ≥30 kg/m2). (D) Stratified by smoking status (sample sizes: 421 for current smokers; 1,281 for non-current smoker). All of these analyses were adjusted for age, sex, race/ethnicity, smoking status, BMI, changes in weight over the past year, dietary intake of total monounsaturated fatty acids, total polyunsaturated fatty acids, total saturated fatty acids, and total energy intake. Among all covariates mentioned above, the variable used for stratification was excluded from adjustment in each analysis. ΣPCBs, sum of 12 PCBs (PCB74, PCB99, PCB118, PCB138, PCB146, PCB153, PCB156, PCB170, PCB180, PCB187, 3,3′,4,4′,5-pentachlorobiphenyl, and 3,3′,4,4′,5,5′-hexachlorobiphenyl).
References
- 1.Lee DH, Porta M, Jacobs DR, Jr, Vandenberg LN. Chlorinated persistent organic pollutants, obesity, and type 2 diabetes. Endocr Rev. 2014;35:557–601. doi: 10.1210/er.2013-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ruzzin J, Lee DH, Carpenter DO, Jacobs DR., Jr Reconsidering metabolic diseases: the impacts of persistent organic pollutants. Atherosclerosis. 2012;224:1–3. doi: 10.1016/j.atherosclerosis.2012.02.039. [DOI] [PubMed] [Google Scholar]
- 3.Lee DH, Jacobs DR, Jr, Park HY, Carpenter DO. A role of low dose chemical mixtures in adipose tissue in carcinogenesis. Environ Int. 2017;108:170–175. doi: 10.1016/j.envint.2017.08.015. [DOI] [PubMed] [Google Scholar]
- 4.Lee DH, Porta M, Lind L, Lind PM, Jacobs DR., Jr Neurotoxic chemicals in adipose tissue: a role in puzzling findings on obesity and dementia. Neurology. 2018;90:176–182. doi: 10.1212/WNL.0000000000004851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee YM, Jacobs DR, Jr, Lee DH. Persistent organic pollutants and type 2 diabetes: a critical review of review articles. Front Endocrinol (Lausanne) 2018;9:712. doi: 10.3389/fendo.2018.00712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Needham LL, Burse VW, Head SL, Korver MP, McClure PC, Andrews JS, Jr, Rowley DL, Sung J, Kahn SE. Adipose tissue/serum partitioning of chlorinated hydrocarbon pesticides in humans. Chemosphere. 1990;20:975–980. [Google Scholar]
- 7.Lee DH, Jacobs DR., Jr New approaches to cope with possible harms of low-dose environmental chemicals. J Epidemiol Community Health. 2019;73:193–197. doi: 10.1136/jech-2018-210920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee YM, Kim KS, Jacobs DR, Jr, Lee DH. Persistent organic pollutants in adipose tissue should be considered in obesity research. Obes Rev. 2017;18:129–139. doi: 10.1111/obr.12481. [DOI] [PubMed] [Google Scholar]
- 9.Birnbaum LS. The role of structure in the disposition of halogenated aromatic xenobiotics. Environ Health Perspect. 1985;61:11–20. doi: 10.1289/ehp.856111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Macdonald TL. Chemical mechanisms of halocarbon metabolism. Crit Rev Toxicol. 1983;11:85–120. doi: 10.3109/10408448309089849. [DOI] [PubMed] [Google Scholar]
- 11.Balkau B, Mhamdi L, Oppert JM, Nolan J, Golay A, Porcellati F, Laakso M, Ferrannini E EGIR-RISC Study Group. Physical activity and insulin sensitivity: the RISC study. Diabetes. 2008;57:2613–2618. doi: 10.2337/db07-1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mayer-Davis EJ, D'Agostino R, Jr, Karter AJ, Haffner SM, Rewers MJ, Saad M, Bergman RN. Intensity and amount of physical activity in relation to insulin sensitivity: the Insulin Resistance Atherosclerosis Study. JAMA. 1998;279:669–674. doi: 10.1001/jama.279.9.669. [DOI] [PubMed] [Google Scholar]
- 13.Yiamouyiannis CA, Sanders RA, Watkins JB, 3rd, Martin BJ. Chronic physical activity: hepatic hypertrophy and increased total biotransformation enzyme activity. Biochem Pharmacol. 1992;44:121–127. doi: 10.1016/0006-2952(92)90045-k. [DOI] [PubMed] [Google Scholar]
- 14.Watkins JB, 3rd, Crawford ST, Sanders RA. Chronic voluntary exercise may alter hepatobiliary clearance of endogenous and exogenous chemicals in rats. Drug Metab Dispos. 1994;22:537–543. [PubMed] [Google Scholar]
- 15.Genuis SJ, Beesoon S, Birkholz D. Biomonitoring and elimination of perfluorinated compounds and polychlorinated biphenyls through perspiration: blood, urine, and sweat study. ISRN Toxicol. 2013;2013:483832. doi: 10.1155/2013/483832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Genuis SJ, Lane K, Birkholz D. Human elimination of organochlorine pesticides: blood, urine, and sweat study. Biomed Res Int. 2016;2016:1624643. doi: 10.1155/2016/1624643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pelletier C, Despres JP, Tremblay A. Plasma organochlorine concentrations in endurance athletes and obese individuals. Med Sci Sports Exerc. 2002;34:1971–1975. doi: 10.1249/01.MSS.0000040820.48707.99. [DOI] [PubMed] [Google Scholar]
- 18.Imbeault P, Chevrier J, Dewailly E, Ayotte P, Despres JP, Mauriege P, Tremblay A. Increase in plasma pollutant levels in response to weight loss is associated with the reduction of fasting insulin levels in men but not in women. Metabolism. 2002;51:482–486. doi: 10.1053/meta.2002.31338. [DOI] [PubMed] [Google Scholar]
- 19.Jansen A, Lyche JL, Polder A, Aaseth J, Skaug MA. Increased blood levels of persistent organic pollutants (POP) in obese individuals after weight loss: a review. J Toxicol Environ Health B Crit Rev. 2017;20:22–37. doi: 10.1080/10937404.2016.1246391. [DOI] [PubMed] [Google Scholar]
- 20.Curtin LR, Mohadjer LK, Dohrmann SM, Montaquila JM, Kruszan-Moran D, Mirel LB, Carroll MD, Hirsch R, Schober S, Johnson CL. The National Health and Nutrition Examination Survey: sample design, 1999-2006. Vital Health Stat 2. 2012;(155):1–39. [PubMed] [Google Scholar]
- 21.Centers for Disease Control Prevention/National Center for Health Statistics. Laboratory procedure manual, PCBs and persistent pesticides (lab protocol for NHANES 1999-2000 data) [cited 2020 Feb 21]. Available from: https://www.cdc.gov/nchs/data/nhanes/nhanes_03_04/l28_c_met_%20PCBs_and_Persistent_Pesticides.pdf.
- 22.Centers for Disease Control Prevention. How to create new variables to describe leisure-time physical activity. [cited 2020 Feb 21]. Available from: https://www.cdc.gov/nchs/tutorials/PhysicalActivity/Preparing/PAQ/Task4_Step2c.htm.
- 23.Phillips DL, Pirkle JL, Burse VW, Bernert JT, Jr, Henderson LO, Needham LL. Chlorinated hydrocarbon levels in human serum: effects of fasting and feeding. Arch Environ Contam Toxicol. 1989;18:495–500. doi: 10.1007/BF01055015. [DOI] [PubMed] [Google Scholar]
- 24.Korn EL, Graubard BI. Epidemiologic studies utilizing surveys: accounting for the sampling design. Am J Public Health. 1991;81:1166–1173. doi: 10.2105/ajph.81.9.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Graubard BI, Korn EL. Analyzing health surveys for cancer-related objectives. J Natl Cancer Inst. 1999;91:1005–1016. doi: 10.1093/jnci/91.12.1005. [DOI] [PubMed] [Google Scholar]
- 26.Lee DH, Lee IK, Song K, Steffes M, Toscano W, Baker BA, Jacobs DR., Jr A strong dose-response relation between serum concentrations of persistent organic pollutants and diabetes: results from the National Health and Examination Survey 1999-2002. Diabetes Care. 2006;29:1638–1644. doi: 10.2337/dc06-0543. [DOI] [PubMed] [Google Scholar]
- 27.Lee DH, Lind L, Jacobs DR, Jr, Salihovic S, van Bavel B, Lind PM. Associations of persistent organic pollutants with abdominal obesity in the elderly: the Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS) study. Environ Int. 2012;40:170–178. doi: 10.1016/j.envint.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 28.Kim HW, Kim JH, Lee DW, Cho SH, Jung JH, Kim KS, Lee DH. Different associations of albuminuria with total and cardiovascular mortality by concentrations of persistent organic pollutants in the elderly. Environ Res. 2017;155:175–181. doi: 10.1016/j.envres.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 29.Kim SA, Kim KS, Lee YM, Jacobs DR, Lee DH. Associations of organochlorine pesticides and polychlorinated biphenyls with total, cardiovascular, and cancer mortality in elders with differing fat mass. Environ Res. 2015;138:1–7. doi: 10.1016/j.envres.2015.01.021. [DOI] [PubMed] [Google Scholar]
- 30.Lee YM, Ha CM, Kim SA, Thoudam T, Yoon YR, Kim DJ, Kim HC, Moon HB, Park S, Lee IK, Lee DH. Low-dose persistent organic pollutants impair insulin secretory function of pancreatic β-cells: human and in vitro evidence. Diabetes. 2017;66:2669–2680. doi: 10.2337/db17-0188. [DOI] [PubMed] [Google Scholar]
- 31.McLachlan AJ, Pont LG. Drug metabolism in older people: a key consideration in achieving optimal outcomes with medicines. J Gerontol A Biol Sci Med Sci. 2012;67:175–180. doi: 10.1093/gerona/glr118. [DOI] [PubMed] [Google Scholar]
- 32.Washburn RA, Jette AM, Janney CA. Using age-neutral physical activity questionnaires in research with the elderly. J Aging Health. 1990;2:341–356. [Google Scholar]
- 33.Takagi D, Nishida Y, Fujita D. Age-associated changes in the level of physical activity in elderly adults. J Phys Ther Sci. 2015;27:3685–3687. doi: 10.1589/jpts.27.3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saponaro C, Gaggini M, Carli F, Gastaldelli A. The subtle balance between lipolysis and lipogenesis: a critical point in metabolic homeostasis. Nutrients. 2015;7:9453–9474. doi: 10.3390/nu7115475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.U.S. Department of Health and Human Services. 2008 Physical activity guidelines for Americans: be active, healthy, and happy! [cited 2020 Feb 21]. Available from: https://health.gov/our-work/physical-activity/previous-guidelines/2008-physical-activity-guidelines.
- 36.Despres JP, Bouchard C, Savard R, Tremblay A, Marcotte M, Theriault G. Level of physical fitness and adipocyte lipolysis in humans. J Appl Physiol Respir Environ Exerc Physiol. 1984;56:1157–1161. doi: 10.1152/jappl.1984.56.5.1157. [DOI] [PubMed] [Google Scholar]
- 37.Helmerhorst HJ, Brage S, Warren J, Besson H, Ekelund U. A systematic review of reliability and objective criterion-related validity of physical activity questionnaires. Int J Behav Nutr Phys Act. 2012;9:103. doi: 10.1186/1479-5868-9-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Skender S, Ose J, Chang-Claude J, Paskow M, Bruhmann B, Siegel EM, Steindorf K, Ulrich CM. Accelerometry and physical activity questionnaires: a systematic review. BMC Public Health. 2016;16:515. doi: 10.1186/s12889-016-3172-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pedisic Z, Bauman A. Accelerometer-based measures in physical activity surveillance: current practices and issues. Br J Sports Med. 2015;49:219–223. doi: 10.1136/bjsports-2013-093407. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Wet serum concentrations of persistent organic pollutants according to the duration of leisure-time physical activity
Lipid-adjusted serum concentrations of persistent organic pollutants according to the metabolic equivalent (MET)-minutes of leisure-time physical activity
Wet serum concentrations of persistent organic pollutants according to the metabolic equivalent (MET)-minutes of leisure-time physical activity
Lipid-adjusted serum concentrations of persistent organic pollutants according to the duration of leisure-time physical activity after excluding subjects with the missing values
Lipid-adjusted serum concentrations of persistent organic pollutants according to the metabolic equivalent (MET)-minutes of leisure-time physical activity after excluding subjects with the missing values
Stratified analyses for the associations between duration of leisure-time physical activity and the concentrations of polychlorinated biphenyls (PCBs). Geometric mean±standard error. (A) Stratified by age (sample sizes: 1,001 for age <40; 461 for age 40 to 59; 240 for age ≥60). (B) Stratified by sex (sample sizes: 777 for men; 925 for women). (C) Stratified by body mass index (BMI) (sample sizes: 1,298 for BMI <30 kg/m2; 404 for BMI ≥30 kg/m2). (D) Stratified by smoking status (sample sizes: 421 for current smokers; 1,281 for non-current smoker). All of these analyses were adjusted for age, sex, race/ethnicity, smoking status, BMI, changes in weight over the past year, dietary intake of total monounsaturated fatty acids, total polyunsaturated fatty acids, total saturated fatty acids, and total energy intake. Among all covariates mentioned above, the variable used for stratification was excluded from adjustment in each analysis. ΣPCBs, sum of 12 PCBs (PCB74, PCB99, PCB118, PCB138, PCB146, PCB153, PCB156, PCB170, PCB180, PCB187, 3,3′,4,4′,5-pentachlorobiphenyl, and 3,3′,4,4′,5,5′-hexachlorobiphenyl).