Abstract
Leishmaniasis is a global health problem that affects more than 2 billion people worldwide. Recent advances in research have demonstrated critical roles for cytoplasmic sensors and inflammasomes during Leishmania spp. infection and pathogenesis. Specifically, several studies have focused on the role of nod-like receptor family, pyrin domain-containing protein 3 (NLRP3) inflammasome and inflammasome-associated cytokines IL-1β and IL-18 in leishmaniasis. Despite these studies, our understanding of the priming and activation events that lead to NLRP3 inflammasome activation during Leishmania spp. infection is limited. Furthermore, whether NLRP3 plays a protective or pathogenic role during Leishmania spp. infection is far from resolved, with some studies showing a protective role and others showing a pathogenic role. In this review, we performed a critical review of the literature to provide a current update on priming and activating signals required for NLRP3 inflammasome activation during Leishmania spp. infection. Finally, we provide a thorough review of the literature to reconcile differences in the observed protective vs pathogenic roles of the NLRP3 inflammasome during Leishmania spp. infection.
Keywords: caspase-1, IL-18, IL-1β, inflammasome, Leishmania, NLRP3
1 |. LEISHMANIASIS
Leishmania spp. are protozoan parasites and the causative agents of leishmaniasis, a neglected tropical disease with two predominant forms: visceral or kala-azar (VL) and cutaneous/mucocutaneous (CL).1 VL, the deadliest leishmaniasis, has an estimated incidence of 0.2–0.4 million new cases each year and is caused by L donovani and L infantum/L chagasi. CL, the most common form of the disease, has an incidence of 0.7–1.2 million new occurrences annually and is caused by infection from L major, L amazonensis, and L braziliensis.2 The life cycle of Leishmania spp. is well characterized.3–6 Infectious metacyclic promastigotes are transferred to mammalian hosts via sandfly (Phlebotomus spp.) bite, where they are phagocytosed by macrophages.7 Once inside the macrophage, promastigotes transform into amastigotes and multiply, eventually escaping to infect other cells and establish infection. Subsequently, uninfected sandflies consume amastigote-laden macrophages from infected hosts, which proceed to transform into promastigotes and multiply in the sandfly midgut. These promastigotes migrate to the sandfly proboscis, allowing the cycle to repeat when the sandfly takes its next blood meal.
Investigation of immunological response to Leishmania spp. infection continues to be an area of research interest. Both innate and adaptive immune cells play a central role in controlling the outcome of Leishmania spp. infection. Neutrophils and macrophages are the primary innate immune cells which have been investigated for their role in controlling Leishmania spp. infection. Neutrophils depletion studies have shown that they can have both protective 8–10 or pathogenic roles.11,12 This could potentially depend on the genetic background of the host, as neutrophils provide a protective role by promoting clearance of L major in C57BL/6 mice, but promote persistence of L major infection in BALB/c mice.13 Macrophages are the sentinel cell for Leishmania spp. infection and survival.14 Thus, macrophages play a dual role during Leishmania spp. infection; that is, they are not only important for clearance but also provide a cellular niche for these parasites. Thus, the outcome of a Leishmania spp. infection depends on the quality of the macrophage response that is generated in the infected host. Leishmania spp. infection can promote either an M1 and M2 macrophage phenotype.15 As reviewed extensively by Tomiotto-Pellissier et al16, in general, M1 macrophages promote clearance and resolve Leishmania spp. infection while M2 macrophages promote parasite survival and disease. With regard to adaptive immunity, helper T-cell responses have been shown to play critical roles in deciding the fate of the disease during Leishmania spp. infection. In an infected host, T helper (Th)1 response hallmarked by interferon (IFN)-γ production promotes parasite clearance and disease resolution, while a Th2 response dominated by interleukin (IL)-4 cytokines fails to clear the parasite and promote Leishmania spp.-mediated pathology.17–21 Specifically, C57BL/6 mice, which resolve Leishmania spp. infection and are resistant to re-infection, produce a Th1 dominant response. In contrast, BALB/c mice, which fail to resolve infection, produce a Th2-dominant response. It is now well established that innate immune sensors play an important role in modulating Th1/Th2 responses bias in the host. In this review, we will critically analyze the literature involving the nod-like receptor family, pyrin domain-containing protein 3 (NLRP3) inflammasome and Leishmania spp. infection and provide perspectives to reconcile the differences in studies reporting protective vs pathogenic roles for the NLRP3 inflammasome in leishmaniasis.
2 |. THE NLRP3 INFLAMMASOME
Inflammasomes are multimeric protein complexes that promote maturation of pro-inflammatory cytokines IL-1β and IL-18, and induction of pyroptotic cell death.22–26 The NLRP3 inflammasome is the most well-studied inflammasome and is assembled through a two-step process referred to here as priming and activation (Figure 1). Priming requires activation of nuclear factor kappa B (NF-κB) and extracellular signal-related kinase (ERK) signaling pathways (likely via stimulation of a surface toll-like receptor [TLRs]), which leads to transcription of NLRP3, pro-IL1β, and pro-IL18. To assemble the inflammasome, various pattern and damage-associated molecular patterns (PAMP and DAMP) provide the activation signal, prompting K+ efflux, NLRP3 activation and oligomerization. It should be noted, however, that K+ efflux is not universally required for activation of the NLRP3 inflammasome. Indeed, a recent study showed that small molecules such as imiquimod and CL097, which target mitochondria, can directly activate NLRP3 inflammasome in a reactive oxygen species (ROS)-dependent but K+ efflux-independent manner.27 A separate study showed that another small molecule, GB111-NH2, disrupted glycolysis to promote NLRP3 inflammasome activation in macrophages.28 Notably, GB111-NH2-induced NLRP3 inflammasome activation was K+ efflux-independent. NLRP3 oligomerization promotes association with adaptor protein ASC (apoptosis-associated speck like protein containing CARD) and pro-caspase-1 via Pyrin-Pyrin and CARD-CARD homotypic interactions, respectively. This multimeric protein complex of NLRP3, ASC, and pro-caspase-1 is known as the NLRP3 inflammasome. Alternatively, activation of caspase-11 has been shown to indirectly activate the NLRP3 inflammasome (also known as the non-canonical NLRP3 inflammasome).29–31 Mechanistic studies have demonstrated that Gram-negative bacteria-associated lipopolysaccharides (LPS) that access the cytosol are directly sensed by caspase-11, which subsequently activates the non-canonical NLRP3 inflammasome.32–35
FIGURE 1.
The NLRP3 inflammasome. Activation of the NLRP3 inflammasome requires two critical steps: (a) Priming: the first step of NLRP3 activation, referred to as priming, is mainly provided by engagement of toll-like receptors (TLR) by TLR ligands that include both pathogen-associated molecular pattern (PAMP) and damage-associated molecular pattern (DAMP). Engagement of TLR promotes activation of NFκB and MAPK signaling pathway, which ultimately promotes upregulation of NLRP3 and pro-IL-1β. (b) Activation: the second signal, known as the activation step, includes PAMP and DAMP which promote NLRP3 oligomerization and complex formation. PAMP and DAMP such as nigericin (via Pannexin1), ATP (via P2X7 receptor) and particulate crystals (lysosomal rupture), the known activating signals, ultimately promote potassium efflux. Change in the potassium concentration initiates oligomerization of NLRP3 and formation of the NLRP3 inflammasome complex consisting of NLRP3, ASC, and caspase-1. Within the NLRP3 inflammasome, caspase-1 is activated, which cleaves pro-IL-1β and pro-IL-18 to generate mature bioactive IL-1β and IL-18. In addition, active capase-1 also cleaves GSDMD to release N-terminal fragment (N-GSDMD), which forms pores in the cell membrane to induce pyroptosis
Within the NLRP3 inflammasome complex, caspase-1 is activated, which has been proposed to be mediated by proximity-induced caspase-1 autoproteolysis. A recent study by Boucher et al36 suggests that a full-length caspase-1 p46 dimer and a heterodimer of caspase-1 p33-p10 subunits are the active caspase-1 subunits, which mediate downstream cellular signaling events. Active caspase-1 promotes two major functions: (a) cleavage of pro-IL-1β and pro-IL-18 into their bioactive mature forms and (b) cleavage of gasdermin D (GSDMD), releasing the N-terminal fragment (ie, N-GSDMD) which then assembles pores in the cell membrane to promote pyroptotic cell death.37,38 This review will discuss the current cellular and molecular mechanisms that have been proposed to drive NLRP3 inflammasome-dependent protective and pathogenic responses during Leishmania spp. infection.
3 |. ACTIVATION OF THE NLRP3 INFLAMMASOME DURING LEISHMANIA SPP. INFECTION
As discussed above (Figure 1), activation of the NLRP3 inflammasome requires both priming and activation signals. However, how Leishmania spp. activates the NLRP3 inflammasome remains relatively unknown. A priming signal results in activation of NFκB and/or mitogen-activated protein kinase (MAPK) signaling, which then promotes upregulation of NLRP3 and pro-IL-1β; both essential components of the NLRP3 inflammasome.39 In addition, several studies have shown that a priming event promotes transcriptional-independent NLRP3 deubiquitylation, which conditions NLRP3 for subsequent activation.40–43 In this section, we review the literature on the current state of our understanding on the priming and activating signals required for activation of the NLRP3 inflammasome during Leishmania spp. infection.
3.1 |. Priming events that promote NLRP3 inflammasome activation during Leishmania spp. infection
In general, activation of TLRs serve as the priming signal for activation of the NLRP3 inflammasome.39 In concurrence, several TLRs have been shown to induce signaling in response to Leishmania spp. infection. As reviewed previously,44 Leishmania spp. lipophosphoglycan (LPG) can directly engage TLR2 to activate MAPK signaling and production of pro-inflammatory cytokine tumor necrosis factor (TNF),45–47 L donovani glycoproteins and glycosphingophoshpolipids engage TLR4,48,49 and Leishmania spp. DNA engage TLR9 to promote IL-12.50,51 Collectively, these reports highlight the ability of Leishmania spp.-associated antigens to engage and activate TLRs; however, the role of TLRs in priming of the NLRP3 inflammasome during Leishmania spp. infection still remains obscure.
A study by Gupta et al52 showed that skin biopsies from L braziliensis-infected human localized cutaneous leishmaniasis (LCL) patients have increased mRNA transcript levels of NLRP3, IL-1β, and other inflammasome-associated markers including caspase-1, AIM2, and NLRP1. Importantly, immunohistochemistry staining for NLRP3 and IL-1β shows increased levels of these proteins in LCL biopsies compared to normal control skin biopsies.52 In addition, several studies in mice have shown that Leishmania spp. infection promotes IL-1β production in vivo.12,19,53 While these studies suggest that Leishmania spp. can provide priming signals for NLRP3 inflammasome activation in vivo, the specific priming signals that lead to upregulation of NLRP3 and IL-1β remain undefined. Ives et al54 demonstrated that Leishmania spp. can harbor dsRNA leishmania RNA virus (LRV) which can directly engage TLR3 in bone marrow-derived macrophages (BMDM) in vitro to activate pro-inflammatory signaling that include chemokines and cytokines such as C-C motif chemokine ligand 5 (CCL5), C-X-C motif chemokine ligand 10 (CXCL10), IL-6, and TNF. Thus, LRV can be a potential ligand that can directly activate TLR3 and provide a necessary priming signal for activation of the NLRP3 inflammasome. A recent study from Dey et al55 show that sandfly midgut bacteria provide priming signals to activate the NLRP3 inflammasomes during natural Leishmania donovani infection. In this study, infected sandflies were used to transmit L donovani in mice, and it was determined that the sandfly bite also transmits sandfly gut microbes. Indeed, sandfly gut microbes are sufficient to induce induction of pro-Il1b mRNA even in the absence of L donovani.55 Interestingly, the upregulation of NLRP3 and pro-IL1β at the protein level was only observed when the sandfly transmits both L donovani and microbes together. In concurrence, as early as 6 hours after a sandfly bite, mature IL-1β is detected at significantly higher levels in the ears of mice that are bitten by L donovani-harboring sandflies compared to IL-1β levels in the ears of mice bitten by uninfected sandflies.55 To further demonstrate the role for sandfly microbes in the activation of the NLRP3 inflammasome, L donovani-harboring sandflies were left untreated or treated with antibiotics. As expected, infection of mice with L donovanii-harboring sandflies on antibiotics induce dramatically reduced levels of IL-1β and NLRP3 inflammasome activation.55 Together, these data demonstrate that the sandfly gut microbes work synergistically with L donovani to provide the necessary priming signals for activation of the NLRP3 inflammasome. We postulate that the sandfly microbes are engaging TLR to provide priming signals necessary for upregulating NLRP3 and pro-IL-1β; however, it is possible that non-TLR pathogen recognition receptors are also involved in the priming process. In support of the microbial co-infection hypothesis put forth by Dey et al,55 most in vitro studies with Leishmania spp. infection of macrophages require TLR priming (mostly LPS) to activate the NLRP3 inflammasome and IL-1β production.12,19,56 This seminal study by Dey et al55 also invokes an important question that we think needs to be discussed, which is, how does an in vivo infection with Leishmania spp. induce NLRP3 inflammasome activation as shown by several different studies? We propose two possible explanations. First, most studies that have shown IL-1β production and inflammasome activation following sterile infection with Leishmania spp. (syringe injection) at chronic time points in the cutaneous lesions. Fecal exposure of these cutaneous lesions in the cage could provide the additional bacterial/TLR signal for inflammasome activation. Second, at chronic time points, significant tissue damage is evident in the cutaneous lesions, which could result in the release of several DAMP such as high mobility group box 1 (HMGB1)57 and nucleic acids,58–63 providing the necessary signals for inflammasome activation.
Thus, in regard to priming requirements for NLRP3 inflammasome activation during Leishmania spp. infection, stimulation of TLR via LRV or microbial co-infection (in the form of sandfly microbes or direct exposure of lesions to environmental microbes) may be important, although it is still possible that direct TLR stimulation via Leishmania spp. antigens also play important roles (Figure 2). Interestingly, a recent study from Carvahlo et al64 suggests that macrophage priming may actually be dispensable for NLRP3 inflammasome activation; nonetheless, priming with TLR agonists was still required for pro-IL-1β induction, suggesting that priming accounts for IL-1β generated during Leishmania spp. infection.
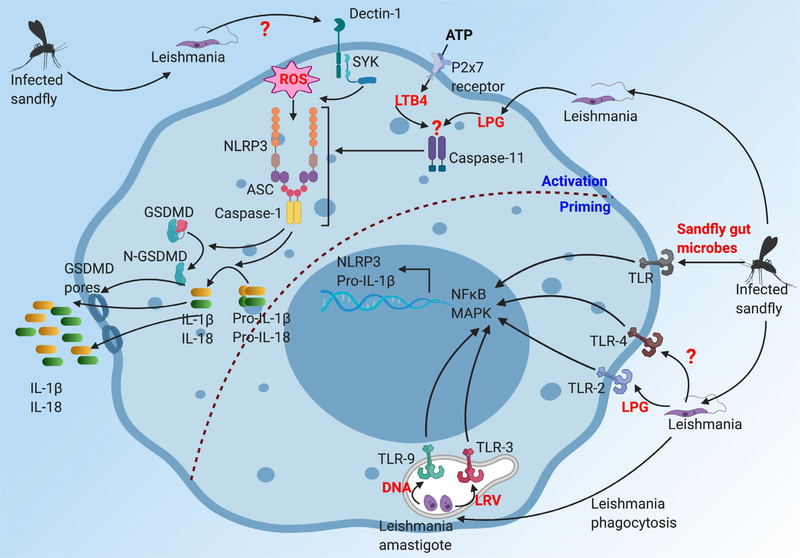
FIGURE 2.
Leishmania spp.-induced activation of the NLRP3 inflammasome. Priming: Several TLR-dependent mechanisms have been proposed for activating the NFκB and MAPK signaling to upregulate expression of NLRP3 and pro-IL-1β during Leishmania spp. infection. Sandfly microbiota, which are released with Leishmania spp. during sandfly bites, promote pro-IL-1β and NLRP3 upregulation, suggesting a role for TLR, although specific TLR have not been examined. Leishmania spp. lipophosphoglycan (LPG) can directly bind TLR2 to induce MAPK signaling. Leishmania spp.-associated LRV (dsDNA leishmania RNA virus) can activate TLR3 signaling, although whether it directly impacts NLRP3 and pro-IL-1β production is not clear. Leishmania spp. associated glycoproteins and glycosphingophospholipids can activate TLR4 signaling. Finally, Leishmania spp. DNA can activate TLR9 to promote IL-12 production. All these data provide evidence that sandfly microbiota and different leishmania antigens can activate TLR signaling pathway to promote NFκB and MAPK signaling, which can then promote upregulation of NLRP3 and pro-IL-1β. Activation: Several different mechanisms have been proposed on upstream events that lead to assembly of the NLRP3 inflammasome. Leishmania spp. engages Dectin-1/SYK signaling to promote ROS production, which activates the NLRP3 inflammasome. The leishmania antigen recognized by Dectin-1 to initiate this signaling cascade is currently unknown. Leishmania spp. LPG, once inside a cell, can indirectly activate caspase-11. Activated caspase-11 can then directly activate the NLRP3 inflammasome, which is also known as non-canonical NLRP3 inflammasome. Addition of ATP during Leishmania spp. infection has been shown to induce LTB4 (leukotriene B4) to activate caspase-11-dependent non-canonical NLRP3 inflammasome. Altogether, several different mechanisms have been proposed for providing activation signals for assembly of the NLRP3 inflammasome. Importantly, active caspase-11 and caspase-1 promote IL-1β, IL-18, and GSDMD-dependent pyroptotic cell death which play important roles in regulating Leishmania spp.-associated pathologies
3.2 |. Activating signals that promote NLRP3 inflammasome activation during Leishmania spp. infection
Following the priming events, assembly of the NLRP3 inflammasome complex requires additional activating PAMP and DAMP signals such as ATP, nigericin, particulate matters (uric acid crystals, alum, silica, asbestos, cholesterol crystals), mitochondrial damage, calcium mobilization, and ROS.37 Given that a wide array of signals promote NLRP3 oligomerization, it is now understood that NLRP3 does not directly sense all of these different activating signals; rather, most of these activating signals lead to potassium ion efflux37 that can then engage/activate molecules such as NEK765–67 and DDX3X68 to promote NLRP3 oligomerization and activation of the NLRP3 inflammasome.
Reactive oxygen species produced in response to PAMPs (candida, influenza virus)69,70 and DAMPs (ATP, silica, asbestos, monosodium urate)71,72 have been shown to be important for the activation of the NLRP3 inflammasome.73 Leishmania spp. infection induces ROS production from both neutrophils and macrophages, and importantly, ROS is critical for limiting Leishmania spp. infection.74 To examine whether ROS is involved in inflammasome activation, Lefevre et al75 treated peritoneal macrophages with N-acetylcysteine during L infantum infection, which resulted in 60%−80% inhibition of IL-1β release, suggesting a role for ROS in inflammasome activation. Lima-Junior et al76 further showed that L amazonenesis induced robust ROS production by BMDM. A previous study by Lima-Junior et al56 show that L amazonensis infection of BMDM activates the NLRP3 inflammasome. To investigate whether ROS is required for activation of the NLRP3 inflammasome, Lima-Junior et al76 treated L amazonensis-infected BMDM with apocynin or DPI to inhibit NADPH oxidase activity. In concurrence with previous studies, inhibiting NADPH oxidase activity abrogates L amazonensis-induced caspase-1 activation and IL-1β production in vitro.76 These studies altogether demonstrate that Leishmania spp.-induced ROS is one of the activating signals required for NLRP3 inflammasome activation.
So, what are the upstream events that promote ROS production during Leishmania spp. infection? During fungal infection, the Dectin-1/SYK signaling pathway induces ROS production, which then activates the NLRP3 inflammasome.69,77 Indeed, L infantum infection of peritoneal macrophages leads to increased mRNA expression of C-type lectin receptors including Clec7a which encodes for Dectin-1.75 Dectin-1 sensing of L infantum is critical for optimal ROS production as demonstrated by reduced levels of ROS produced by Dectin-1-deficient macrophages when compared to wildtype (WT) macrophages.75 In addition, treatment of WT macrophages with SYK inhibitor Bay 61–3606 (Bay) completely inhibit L infantum-induced ROS production demonstrating the role for SYK in ROS production as well.75 Similarly, L amazonensis-induced ROS production is abrogated in Dectin-1-deficient BMDM, further highlighting the role for Dectin-1 sensing in ROS production.76 These data altogether suggest that Dectin-1 and SYK are important for Leishmania spp.-induced ROS generation; but are they required for inflammasome activation? To answer this question, Lefevre et al showed that L infantum-induced IL-1β production, and caspase-1 activation as observed by western blotting for active caspase-1 p20 fragments, are reduced in Dectin-1-deficient macrophages.75 In concurrence with the study by Lefevre et al,75 Lima-Junior et al further showed that L amazonensis-induced caspase-1 activation (as observed by active caspase-1 staining by flow cytometry using FAM-YVAD staining) and subsequent IL-1β production is also dependent on Dectin-1 signaling.76 L amazonensis-induced phosphorylation of SYK in WT BMDM is completely abrogated in Dectin-1-deficient BMDM, demonstrating Dectin-1 sensing as the upstream event that promotes SYK activation. Importantly, similar to Dectin-1 deficiency, pharmacological inhibition of WT BMDM with three different SYK inhibitors (20 μmol/L Piceatannol, 20 μmol/L 3,4-methyl-enedioxy- β-nitrostyrene or 20 μmol/L Syk inhibitor III) lead to significant inhibition of L amazonensis-induced caspase-1 activation and IL-1β production.76 Altogether, these studies demonstrate that during Leishmania spp. infection, Dectin-1 and SYK signaling pathway are involved in ROS generation, which ultimately activate the NLRP3 inflammasome. While these studies place Dectin-1, SYK and ROS directly upstream of the NLRP3 inflammasome during Leishmania spp. infection, much remains unknown, including the Leishmania spp. ligand sensed by Dectin-1 (Figure 2).
In addition to the canonical activation of the NLRP3 inflammasome, direct activation of caspase-11 by intracellular LPS can promote non-canonical NLRP3 inflammasome activation.30,33–35,78,79 de Carvalho et al64 observed that caspase-11 is activated in BMDM infected with L major, L braziliensis, and L amazonensis, and importantly, Leishmania spp.-induced IL-1β and caspase-1 activation are reduced in Casp11−/− mice. Given that LPG is the most abundant glycoconjugate on the surface of Leishmania spp.80 and can directly engage TLR2,45,47 de Carvalho proposed that LPG may activate caspase-11. Indeed, transfection of L major, L braziliensis, or L amazonensis LPG into Pam3CSK4 (TLR2 agonist)-primed or TNF-primed BMDM induce robust caspase-1 activation and IL-1β production.64 Importantly, LPG-induced caspase-1 activation is abrogated in Casp11−/− and Nlrp3−/− BMDM, demonstrating the role of intracellular LPG in activating caspase-11-mediated non-canonical NLRP3 inflammasome.64 In support of LPG as the activator of the non-canonical NLRP3 inflammasome, LPG-deficient L major do not activate caspase-1 or induce IL-1β production in WT BMDM in vitro.64 However, unlike LPS, which directly binds to caspase-11,35 LPG fails to bind to caspase-11. ROS production has also been suggested to regulate the activation of caspase-11 and non-canonical NLRP3 inflammasome.81 Thus, it is possible that ROS is the intermediate molecule that links LPG to caspase-11 activation, which requires further investigation.
More recently, ATP-induced leukotriene B4 (LTB4) has been suggested to activate caspase-11-mediated non-canonical NLRP3 inflammasome during L amazonensis infection 82,83 (Figure 2). It should be noted, however, that Chaves et al used exogenous ATP or LTB4 during L amazonensis infection to activate the non-canonical NLRP3 inflammasome.82 Mechanistically, ATP signals via P2X7 receptor to induce LTB4 production in L amazonensis-infected peritoneal macrophages in vitro, demonstrating the importance of ATP-P2X7 receptor signaling in the production of LTB4.83 LTB4 then engages caspase-11 and promotes activation of the non-canonical NLRP3 inflammasome via a yet-unknown mechanism.82 Given that these studies used exogenous addition of ATP or LTB4 to engage caspase-11 pathway, whether endogenous ATP and/or LTB4 are generated during L amazonensis infection and how they might contribute to caspase-11 activation and non-canonical NLRP3 inflammasome is not clear.
To summarize, two major activation signals have been suggested to activate NLRP3 inflammasome during Leishmania spp. infection: (a) Dectin-1/SYK/ROS signaling activates the canonical NLRP3 inflammasome, and (b) the LPG-caspase-11 pathway activates the non-canonical NLRP3 inflammasome (Figure 2).
4 |. CONSEQUENCES OF NLRP3 INFLAMMASOME ACTIVATION IN LEISHMANIASIS
Activation of the NLRP3 inflammasome has both positive and negative effects on the health of the host. NLRP3 inflammasome activation during bacterial, viral, and fungal infection is essential for mounting an appropriate immune response and providing protection from lethal disease.84 Conversely, harnessing aberrant NLRP3 inflammasome activation has been on the forefront of several autoinflammatory syndromes85 including Parkinson’s86 and inflammatory bowel disease.87 While there is not a plethora of studies on the role of NLRP3 during Leishmania spp. infection, a few studies have been conducted by different laboratories. Despite these studies, whether NLRP3 plays a positive or a negative role during Leishmania spp. infection remains unresolved. Some studies suggest a positive role for NLRP3 inflammasome in controlling Leishmania spp. and resolving infection, while others suggest a negative role for NLRP3 inflammasome in promoting Leishmania spp. survival and pathogenic inflammation. In this section, we provide an in-depth review these studies and offer our perspective on some potential reasons for these discrepant results.
4.1 |. Review of studies demonstrating a protective role for the NLRP3 inflammasome during Leishmania spp. infection
Lima-Junior et al56 conducted one of the first studies demonstrating a protective role for the NLRP3 inflammasome during Leishmania spp. infection in mice. The authors primarily used new-world species of Leishmania spp. such as L braziliensis, L amazonensis, and L infantum chagasi to infect BMDM and mice of C57BL/6 strain. Primarily working with L amazonensis, the authors showed that LPS-primed BMDM infected with L amazonensis induce IL-1β production. Furthermore, L amazonensis-induced caspase-1 activation and IL-1β production is defective in BMDM deficient in NLRP3, ASC or caspase-1 suggesting activation of the NLRP3 inflammasome. Compared to WT BMDM, Nlrp3−/−, Asc−/−, or Casp1−/− BMDM do not show any defect in uptake of L amazonensis; however, Nlrp3−/−, Asc−/−, or Casp1−/− BMDM failed to restrict the growth of L amazonensis in vitro, as demonstrated by increased numbers of intracellular amastigotes 24, 48, and 72 hours postinfection. These results demonstrate a central role for the NLRP3 inflammasome in restricting L amazonensis growth in BMDM. L amazonensis-induced ear swelling and lesion size are significantly increased in Nlrp3−/−, Asc−/−, or Casp1−/− mice, when compared to C57BL/6 WT mice. Importantly, the increased ear swelling observed in vivo is due to increased parasite burden in the NLRP3 inflammasome-deficient mice. In agreement with the role of NLRP3 inflammasome in promoting IL-1β cytokine, ex vivo cultures of lymph node and spleen cells from L amazonensis-infected C57BL/6 mice produce significant IL-1β cytokines, which are abrogated in the lymph node and spleen cultures from Nlrp3−/−, Asc−/−, or Casp1−/− mice. Moreover, similar to inflammasome-deficient mice (ie, mice deficient in NLRP3, ASC, or caspase-1), Il1r−/− mice also show increased ear swelling, lesion size, and parasite burden following L amazonensis infection in vivo. Mechanistically, this study showed that L amazonensis-induced nitric oxide synthase (NOS) expression and nitric oxide (NO) in WT BMDM are significantly reduced in Casp1−/− BMDM, suggesting a role for the NLRP3 inflammasome in the induction of NO. Supporting a role for the inflammasome, IL-1β addition to WT BMDM during L amazonensis infection increased NO production in a dose-dependent manner. Nos2−/− mice, defective in NO production, failed to control L amazonensis growth in vitro and in vivo. Overall, this study proposes that during L amazonensis infection of C57BL/6 mice, NLRP3 inflammasome-dependent IL-1β production by macrophages drives IL-1β-dependent NO production to limit parasite growth and replication (Figure 3A).
FIGURE 3.
Leishmania spp.-induced activation of the NLRP3 inflammasome. A, Protective role of the NLRP3 inflammasome during Leishmania spp. infection. Ear infection of C57BL/6 or mice deficient in NLRP3 inflammasome components has demonstrated that macrophage sense Leishmania spp. and activate the NLRP3 inflammasome. Mechanistically, NLRP3 inflammasome-induced production of IL-1β by these macrophages is signaled in an autocrine manner via IL-1R to induce nitric oxide synthase (NOS) and nitric oxide (NO) production. NO is required to promote parasite clearance in macrophages in vitro and limit parasite growth and lesion development in vivo. B, Pathogenic role of the NLRP3 inflammasome during Leishmania spp. infection. Upper panel: L major infection of BALB/c footpads activates the NLRP3 inflammasome and subsequent production of IL-18. In the BALB/c model system, IL-18 cytokines produced by L major-infected macrophages promote a Th2-biased immune response that results in reduced IFN-γ and increased IL-4 production. Middle panel: L major Seidman (Sd) strain infection promotes non-healing cutaneous ear lesions in C57BL/6 mice. L major Sd infection activates NLRP3 inflammasome activation and production of IL-1β cytokine. IL-1β cytokines produced L major-infected macrophages recruit neutrophil to the site of the infection, which then promotes a Th2-biased immune response. Thus, both IL-1β and IL-18 produced by macrophages during L major infection promote a Th2-biased immune response that results in parasite growth and lesion development. Lower panel: Rag−/− mice reconstituted with CD8+ T cells (Rag−/− + CD8) or C57BL/6 mice co-infected with LCMV (lymphocytic choriomeningitis virus) provide a unique model system to study CD8+ T cell-induced immunopathology during Leishmania spp. infection. During Leishmania spp. infection of ears in these mice, CD8+ T cells promote NLRP3 inflammasome activation in myeloid cells. Activated myeloid cells produce IL-1β to promote immunopathology and development of lesions. Interestingly, in these model systems, IL-1β-mediated immunopathology and lesion development is not associated with parasite control
In concurrence with the protective role of inflammasome and IL-1β, Zamboni and colleagues have further reported a positive role of the NLRP3 inflammasome in providing resistance to Leishmania spp. infection. A recent study by de Carvalho et al88 showed that LRV is present within L guyanensis and activates TLR3 to dampen caspase-1 and IL-1β production, that is, LRV+ve L guyanensis induces significantly lower levels of caspase-1 activation and IL-1β production by BMDM in vitro. In vivo ear infection of C57BL/6 mice with LRV+ L guyanensis resulted in increased swelling and parasite burden when compared to LRV-ve L guyanensis; however, these differences between LRV+ve and LRV-ve L guyanensis are not observed during infection of Nlrp3−/− mice. These data indirectly suggest a protective role for NLRP3 and IL-1β during L guyanensis infection. In a follow-up study, Lima-Junior et al76 demonstrated that Dectin-1-deficient mice are also susceptible to L amazonensis infection in vitro and in vivo. Mechanistically, Dectin-1 sensing of L amazonensis activates SYK to promote ROS production, which then activated the NLRP3 inflammasome (Figure 2). Indeed, an independent study by Lefevre et al75 also showed that Dectin-1 is required for controlling L infantum-mediated visceral leishmaniasis in vitro and in vivo. They demonstrated Dectin-1/SYK-mediated ROS production in promoting inflammasome activation and IL-1β production, although the role of inflammasome and IL-1β were not evaluated in this study. These studies altogether suggest that Leishmania spp. sensing via Dectin-1/SYK pathway promotes ROS production to activate the NLRP3 inflammasome. In addition to the Dectin-1/SYK pathway, de Carvalho demonstrated that intracellular LPG from Leishmania spp. activate caspase-11 dependent non-canonical NLRP3 inflammasome (Figure 2).64 Compared to C57BL/6 WT mice, both Nlrp3−/− and Casp11−/− mice show significantly increased ear swelling, lesions, and parasite burden following L amazonensis infection in vivo. The protective role of caspase-11 is also evident in BMDM, as Casp11−/− BMDM failed to restrict L major, L braziliensis, and L amazonensis growth in vitro. Another study demonstrated a similar protective role for caspase-11 following footpad injection with L amazonensis, where Casp11−/− mice presented with significantly larger footpad lesions and higher parasite burden compared to WT mice.82
Gupta et al89 showed that L donovani hijacks negative regulatory proteins to limit NLRP3 inflammasome activation. Treatment of L donovani-infected BALB/c mice with the anti-leishmanial drug amphotericin B (AmpB) reduces parasitic burden in the liver and spleen, and importantly, this protection is dependent on IL-1β. Mechanistically, L donovani suppresses NLRP3 inflammasome activation and IL-1β production by employing two negative regulators, A20 and mitochondrial uncoupling protein 2 (UCP2). Treatment of L donovani-infected BALB/c mice with either A20 or UCP2 short hairpin (sh)RNA (to reduce their expression) ameliorate the visceral disease as demonstrated by reduced parasite burden in the liver and spleen. The L major- and L mexicana-associated metalloprotease GP63 have been shown to inhibit silica, asbestos or malarial hemozoin-induced NLRP3 inflammasome activation.90 Mechanistically, GP63 blocked ROS production and cleaved NLRP3 to inhibit activation of the NLRP3 inflammasome in this study. L infantum wash has been shown to inhibit nigericin- or amyloid-beta 4 (Aβ4)-induced NLRP3 inflammasome activation.91 Similarly, L amazonensis was shown to inhibit ATP-induced NLRP3 inflammasome activation in macrophages by modulating H3 histone activation marks to regulate both priming and activating steps.92 These data provide indirect evidence that NLRP3 inflammasome-dependent processes provide protection against Leishmania spp. infection and that Leishmania spp. have evolved mechanisms to inhibit activation of the NLRP3 inflammasome.
The overall findings on protective roles of the NLRP3 inflammasome during Leishmania spp. infection are presented in Figure 3A. Briefly, two upstream sensing mechanisms have been shown to activate the NLRP3 inflammasome: first, through the Dectin/SYK/ROS sensing pathway and, second, through the LPG-caspase-11 activation pathway. Mechanistically, Leishmania spp.-induced activation of the NLRP3 inflammasome provokes IL-1β production by macrophages, which signals in an autocrine manner via IL-1R to induce NO to limit intracellular parasite growth. Surprisingly, this is all that is known regarding mechanisms behind the protective role of the NLRP3 inflammasome during Leishmania spp. infection. It is well established that at chronic time points, Th1 responses that promote IFN-γ production are important in resolving the infection 93; however, how NLRP3 deficiency affects Th1 responses is not known.
4.2 |. Review of studies that demonstrate a pathogenic role for the NLRP3 inflammasome during Leishmania spp. infection
Using a few different model systems, three groups have shown a pathogenic role for the NLRP3 inflammasome during Leishmania spp. infection (Figure 3B).12,19,53 Gurung et al19 used a cutaneous model system with L major infection of WT and mutant mice on a BALB/c background, that is, WT BALB/c mice were used as controls, and NLRP3 inflammasome-deficient mice were backcrossed to BALB/c to generate NLRP3inflammasome-deficient mice on a BALB/c background. In vitro infection of LPS-primed BMDM from WT BALB/c mice with L major induce IL-1β and IL-18 production in a dose-dependent manner. L major-induced IL-1β and IL-18 production in BALB/c BMDM are driven by the NLRP3 inflammasome, since L major-infected Nlrp3−/−, Asc−/−, and Casp1−/− (also deficient in caspase-11) BMDM fail to produce IL-1β and IL-18. Similarly, the NLRP3 inflammasome-dependent caspase-1 activation, and IL-1β and IL-18 production were also observed in the L major-infected footpads in vivo. Interestingly, the clearance of intracellular L major by Asc−/− BMDM is comparable to WT BMDM. These results suggest that the NLRP3 inflammasome is required for production of IL-1β and IL-18 by L major-infected BMDM; however, clearance of the internalized L major does not require inflammasome activation. L major infection of the footpads of WT BALB/c mice provoked significant footpad swelling and L major burden, which is attenuated in the absence of NLRP3, ASC, or caspase-1. Analysis of the infected footpads from WT, Nlrp3−/−, Asc−/−, and Casp1−/− mice demonstrated that protection in the NLRP3 inflammasome-deficient footpads correlate with increased Th1 (IFN-γ) and reduced Th2 (IL-4, IL-5) cytokines. Furthermore, analysis of T cells from popliteal lymph nodes of L major-infected mice showed increased frequency of IFN-γ+ T cells in Nlrp3−/−, Asc−/−, and Casp1−/− mice, when compared to WT controls. In vitro anti-CD3 stimulation of T cells in the presence of recombinant IL-18, but not IL-1β, reduced IFN-γ and increased IL-4 production, implicating IL-18 in promoting susceptibility to L major infection. In agreement with in vitro data, IL-18BP treatment of WT BALB/c mice to neutralize IL-18 during L major infection in vivo rescued IL-4 levels detected in the infected footpads and ameliorated footpad swelling. In summary, this study shows that in BALB/c WT background, the NLRP3 inflammasome-induced IL-18 cytokine supports a Th2 response to promote susceptibility to L major infection.
Unlike BALB/c mice, C57BL/6 mice develop self-healing cutaneous lesions when infected with L major.94 Given that Lima-Junior et al56 did not observe any difference between C57BL/6 WT and Nlrp3-deficient mice on a C57BL/6 background following a self-healing L major infection, Charmoy et al12 compared immune responses of C57BL/6 WT mice following infection with a self-healing L major Friedlin (Fn) vs non-healing L major Seidman (Sd) infection. Infection of C57BL/6 WT with L major Sd strain induced chronic non-healing infection as observed in BALB/c WT mice following L major infection.12 The authors observed that L major Sd infection of C57BL/6 WT ears induce significantly more IL-1β when compared to L major Fn strain. Given the positive correlation of IL-1β production with chronic non-healing infection observed in C57BL/6 mice following L major Sd infection, Charmoy et al next investigated the role of the NLRP3 inflammasome during L major Sd infection. In vitro infection of LPS-primed C57BL/6 WT BMDM with L major Sd induce robust IL-1β production, which is abrogated in Nlrp3−/− or Casp1−/− BMDM. Furthermore, L major Sd infection of C57BL/6 WT mice ears provoked chronic non-resolving lesions, which were ameliorated in Nlrp3−/−, Asc−/−, Casp1−/−, and Il1b−/− and Il1r−/− mice. Resolution of L major Sd infection-induced pathologic lesions (determined based on ulcer and erosion of the ears, scale of 0–3) in the NLRP3 inflammasome-deficient mice correlated with reduced parasite burden. Mechanistically, this study demonstrated that the NLRP3 inflammasome-dependent IL-1β signaling accentuates neutrophil recruitment to the site of infection during L major Sd infection. CD4+ T cells from L major Sd-infected Nlrp3−/− and Il1r−/− mice produced significantly increased IFN-γ and reduced IL-4 when compared to C57BL/6 mice. Neutropenic Genista mice are resistant to L major Sd infection as demonstrated by their ability to resolve infection. This protection in Genista mice also correlated with increased IFN-γ and decreased IL-4 production by CD4+ T cells; however, the level of IL-1β in Genista mice was similar to C57BL/6 WT mice, which suggests that IL-1β is required for neutrophil recruitment. In addition to the role of NLRP3-driven IL-1β in recruiting neutrophils, Dey et al55 further show that the NLRP3 inflammasome within neutrophils promotes IL-1β to drive disease pathogenesis in a sandfly infection model, where C57BL/6 mice are naturally infected with L donovani. Thus, during non-healing L major infection in C57BL/6 mice, the NLRP3 inflammasome-dependent IL-1β recruits neutrophils, and excessive neutrophils at the site of infection promote a Th2-biased response to promote lesion development.
In addition to the above studies, Novais et al also demonstrated a pathogenic role for the NLRP3 inflammasome during L braziliensis and L major infection.53 These authors developed a unique mouse model system to specifically study the role of CD8+ T cells during L braziliensis infection.95 As demonstrated by Mombaerts and colleagues, Rag−/− mice (on a C57BL/6 background), deficient in mature T and B cells, do not develop any lesions when infected with L braziliensis96; however, Rag−/− mice reconstituted with CD8+ T cells (Rag−/− + CD8 mice) develop significant lesions, suggesting a pathogenic role for CD8+ T cells.53,95 The development of lesions in Rag−/− + CD8 mice positively correlate with an increase in mRNA and protein levels of IL-1β in the lesions. Flow cytometry analysis has demonstrated that most IL-1β producing cells in lesions are CD11b+, suggesting myeloid cells as the major source of IL-1β.12 Given that Rag−/− mice do not have T or B cells,96 Novais et al employed a complimentary approach that included co-infection of L major-infected C57BL/6 mice with Lymphocytic choriomeningitis virus (LCMV). Crosby et al showed that co-infection of C57BL/6 mice with L major and LCMV induces non-healing lesions that are provoked by pathogenic CD8+ T cells.97 The pathogenic lesions in L major + LCMV co-infected C57BL/6 mice are significantly blunted in Nlrp3−/− and Casp1−/−/11−/− mice.53 In support of a pathogenic role for the NLRP3 inflammasome, lesions observed in co-infected C57BL/6 mice are ameliorated by MCC950 or glyburide,53 both well-established inhibitors of the NLRP3 inflammasome.98 The pathologic lesions in L major+ LCMV co-infected C57BL/6 mice correlate with an increase in neutrophils, which are decreased in the MCC950 or glyburide-treated mice.53 Interestingly, Novais et al found that the parasite burden in the protected mice (mice with NLRP3 inflammasome inhibition—genetically or chemically) were similar to that in mice with severe lesions, suggesting that uncontrolled inflammation but not parasite burden contribute to lesion pathology.53 In agreement, Santos et al found that phagocytosis and clearance of L braziliensis by monocyte-derived macrophages from healthy individuals were not affected by addition (recombinant human IL-1β) or neutralization (anti-IL-1β antibody) of IL-1β.99 Altogether, these studies propose an entirely unique role for CD8+ T cells in driving inflammasome activation, which in turn provokes L major-induced pathology in mice. Mechanistically, Novais et al found that during L major infection, CD8+ T cells drive NLRP3 inflammasome activation in CD11b+ myeloid cells to produce IL-1β, which subsequently recruited neutrophils to promote immunopathology and lesion development.53 In agreement, Santos et al found that PBMC from L braziliensis-infected patients have a higher frequency of IL-1β expressing myeloid cells and produce higher levels of IL-1β when compared to PBMC from normal humans.99 Importantly, ex vivo IL-1β secretion by PBMC of L braziliensis-infected patients are significantly reduced when treated with NLRP3 inhibitor glyburide.53,100 In-depth flow cytometric analysis of PBMCs taken from L brazilliensis-infected patients showed that CD14+ CD16+ intermediate monocytes are the major producers of IL-1β.99 More recently, Amorim et al101 showed that lesions from patients with cutaneous leishmaniasis express significantly higher levels of IL-1β. Importantly, IL-1β levels directly correlate with success of the anti-leishmanial treatment with higher levels of IL-1β, demonstrating poor treatment prognosis.101 Taken together, these studies clearly suggest a pathogenic role for the NLRP3 inflammasome and IL-1β during L braziliensis infection.
The overall findings on the pathogenic role of the NLRP3 inflammasome during Leishmania spp. infection hint at three different mechanisms: (a) NLRP3 inflammasome-mediated IL-18 cytokine production promotes a Th2-biased response during L major infection of susceptible BALB/c mice, which results in parasite persistence and lesion pathology, (b) NLRP3 inflammasome activation and IL-1β production during L major infection of C57BL/6 mice promotes neutrophil infiltration, which supports a Th2-biased response to promote parasite persistence and cutaneous lesions, and (c) CD8+ T cell responses during L braziliensis or L major infection provokes NLRP3 inflammasome activation in myeloid cells to drive excessive IL-1β production and immunopathology.
5 |. PERSPECTIVES AND CONCLUSIONS
The current state of our understanding of whether NLRP3 inflammasome plays a protective or a pathogenic role during Leishmania spp. infection is far from clear. Historic evaluations of papers in the field of Leishmania spp. have demonstrated that the outcome of infection depends on several factors, including the genetic strain of the host, the genetic strain of the parasite, and the experimental model system.44,102 To add to this complexity, the microbial diversity of the gut microbiota within the mouse colony of different laboratories vary greatly and very well could have an impact on the outcomes of each study.103 While discussing the studies of the NLRP3 inflammasome and Leishmania spp. infection, we have highlighted the genetic strain of the mammalian host, genetic strain of the leishmania parasite, and the model system that was used so the readers can make an informed decision about the advantages and limitations of each systems.
To understand how the NLRP3 inflammasome may drive a protective vs a pathogenic response in a mammalian host, we have to think about the known molecular events downstream of NLRP3. As is well established, and briefly reviewed in Figure 1, activation of the NLRP3 inflammasome drives production of two major pro-inflammatory cytokines, IL-1β and IL-18, and induces pyroptotic cell death.37,38 However, several prior studies investigating the role of IL-1β and IL-18 have found mixed results for these cytokines, with some studies reporting a protective role and others a pathogenic role.102 Leishmania spp.-induced BMDM cell death determined by propidium iodide uptake or lactate dehydrogenase release was found to be dependent on caspase-11 and only partially dependent on NLRP3.64 Given that caspase-11 induces pyroptotic cell death,104 it is likely that Leishmania spp. induces pyroptotic cell death downstream of caspase-11 activation. Active caspase-11 and caspase-1 cleaves GSDMD to release N-terminal fragment of GSDMD, which then assembles pores in the cell membrane to promote pyroptotic cell death.105 It not yet known whether GSDMD is required during Leishmania spp. infection. Studies with WT and Gsdmd−/− mice will help elucidate the role of pyroptotic cell death during Leishmania spp. infection.
In reviewing all known articles that show a protective role for the NLRP3 inflammasome,56,64,76,82,88,89 we noted that these studies exclusively use C57BL/6 mice, and NLRP3 inflammasome-deficient mice backcrossed to C57BL/6 as mammalian hosts. Regarding Leishmania spp., these studies mostly use new-world Leishmania spp. that include L amazonensis, L braziliensis, and L infantum chagasi. Importantly, all studies demonstrating a protective role for the NLRP3 inflammasome and IL-1β were conducted by one group.56,64,76,88 An independent study also suggests a protective role for the NLRP3 inflammasome during L donovani infection of BALB/c mice; however, this study does not use NLRP3-inflammasome-deficient mice, and thus, the role for NLRP3 inflammasome was inferred based on the ability of L donovani to inhibit the activation of LPS+ nigericin-induced NLRP3 inflammasome.89 Mechanistically, IL-1β signaling in macrophages induces NO production to limit Leishmania spp. proliferation and cutaneous leishmaniasis.56 Thus, downstream of the NLRP3 inflammasome, only IL-1β has been implicated in providing protection and the roles for IL-18 or pyroptotic cell death remain unknown.
In contrast, several laboratories (mainly three groups12,19,53) have shown a pathogenic role for the NLRP3 inflammasome during Leishmania spp. infection. It is important to note that even though these studies agree on the overall pathogenic role of the NLRP3 inflammasome during Leishmania spp. infection, the proposed mechanisms vary greatly. In support of a pathogenic role for inflammasome, NLRP1 and AIM2 inflammasomes have also been implicated to promote lesion development during L braziliensis infection52,106; although the downstream mechanisms remain unexplored. Miltefosine, an FDA approved drug used for treating leishmaniasis,107 inhibits LPS + ATP-induced NLRP3 inflammasome activation and IL-1β release—suggesting NLRP3 targeting may provide specific drug alternatives to the currently available drugs.108 In preliminary experiments conducted in mice, inhibiting NLRP3 activation with MCC950 or glyburide protects mice from developing significant lesions during L braziliensis infection.53
For studies demonstrating a pathogenic role for NLRP3 inflammasome-dependent IL-1β during Leishmania spp. infection, two distinct mechanisms have been proposed for how IL-1β drives cutaneous pathology downstream of NLRP3 activation.12,53 These studies use mice on a C57BL/6 background as mammalian host, and L major or L braziliensis for infection. Charmoy et al12 propose that IL-1β supports L major growth and lesion development by recruiting neutrophils, which subsequently promote a Th2-biased response, whereas Novais et al suggest that CD8+ T cells drive myeloid cells to promote IL-1β-dependent inflammation and lesion development during L braziliensis infection.53 It is important to note that unlike Charmoy et al, Novais et al did not observe any difference in parasite burden, suggesting a direct role for IL-1β in promoting immunopathology. There are two main distinctions between the two studies that could explain the potential differences— (a) The model system: Charmoy et al directly infected WT or genetic KO mice whereas Novais et al use Rag−/− mice that received CD8+ T cells or C57BL/6 co-infected with LCMV, to specifically study the pathogenic role of CD8+ T cells and (b) The parasite: Charmoy et al used a non-healing L major Sd strain and Novais et al used L braziliensis or a self-healing L major. Regardless of the differences in mechanisms, these studies support a pathogenic role for NLRP3-induced IL-1β in leishmaniasis. In addition to IL-1β, NLRP3 inflammasome-induced IL-18 has also been demonstrated to promote Leishmania spp. pathology in susceptible BALB/c mice.19 Mechanistically, IL-18 produced by myeloid cells promotes a Th2-biased response to promote L major infection and lesion development in BALB/c mice. Whether NLRP3 inflammasome-dependent IL-18 also plays a role in other model systems has not been studied and needs further investigation.
In conclusion, both protective and pathogenic roles have been reported for NLRP3 inflammasome during Leishmania spp. infection. In this regard, NLRP3 inhibition has been proposed as a potential treatment option for leishmaniasis by both groups. This presents a hurdle, when thinking about translational potential of targeting NLRP3 in humans suffering from leishmaniasis. Thus, future studies that incorporate all of these experimental systems together to understand these discrepancies are of the utmost importance.
ACKNOWLEDGEMENTS
We thank Dr Kristina K. Greiner for help with scientific editing. Figures were generated using BioRender (www.biorender.com). VH was supported by NIGHMS R25GM116686 Grant awarded to Dr Donna Hammond. This work was supported by NIAID (R21AI148904-01) and The University of Iowa Startup funds to PG.
Funding information
National Institute of General Medical Sciences, Grant/Award Number: R25GM116686; University of Iowa; National Institute of Allergy and Infectious Diseases, Grant/Award Number: R21 AI148904 01
Footnotes
CONFLICT OF INTEREST
None.
REFERENCES
- 1.WHO. World Health Organization; Leishmaniasis in high-burden countries: an epidemiological update based on data reported in 2014. Weekly Epidemiol Record. 2016;91(22):287–296. [PubMed] [Google Scholar]
- 2.Alvar J, Velez ID, Bern C, et al. Leishmaniasis worldwide and global estimates of its incidence. PLoS One. 2012;7(5):e35671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akhoundi M, Kuhls K, Cannet A, et al. A historical overview of the classification, evolution, and dispersion of Leishmania parasites and sandflies. PLoS Negl Trop Dis. 2016;10(3):e0004349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maroli M, Khoury C. Prevention and control of leishmaniasis vectors: current approaches. Parassitologia. 2004;46(1–2):211–215. [PubMed] [Google Scholar]
- 5.Quinnell RJ, Courtenay O. Transmission, reservoir hosts and control of zoonotic visceral leishmaniasis. Parasitology. 2009;136(14):1915–1934. [DOI] [PubMed] [Google Scholar]
- 6.Chang KP, Dwyer DM. Multiplication of a human parasite (Leishmania donovani) in phagolysosomes of hamster macrophages in vitro. Science. 1976;193(4254):678–680. [DOI] [PubMed] [Google Scholar]
- 7.CDC. Center for Disease Control and Prevention; Parasites-Leishmaniasis Biology, 2019. [Google Scholar]
- 8.Sousa LM, Carneiro MB, Resende ME, et al. Neutrophils have a protective role during early stages of Leishmania amazonensis infection in BALB/c mice. Parasite Immunol. 2014;36(1):13–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen L, Zhang ZH, Watanabe T, et al. The involvement of neutrophils in the resistance to Leishmania major infection in susceptible but not in resistant mice. Parasitol Int. 2005;54(2):109–118. [DOI] [PubMed] [Google Scholar]
- 10.Lima GM, Vallochi AL, Silva UR, Bevilacqua EM, Kiffer MM, Abrahamsohn IA. The role of polymorphonuclear leukocytes in the resistance to cutaneous Leishmaniasis. Immunol Lett. 1998;64(2–3):145–151. [DOI] [PubMed] [Google Scholar]
- 11.Tacchini-Cottier F, Zweifel C, Belkaid Y, et al. An immunomodulatory function for neutrophils during the induction of a CD4+ Th2 response in BALB/c mice infected with Leishmania major. J Immunol. 2000;165(5):2628–2636. [DOI] [PubMed] [Google Scholar]
- 12.Charmoy M, Hurrell BP, Romano A, et al. The Nlrp3 inflammasome, IL-1beta, and neutrophil recruitment are required for susceptibility to a nonhealing strain of Leishmania major in C57BL/6 mice. Eur J Immunol. 2016;46(4):897–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ribeiro-Gomes FL, Otero AC, Gomes NA, et al. Macrophage interactions with neutrophils regulate Leishmania major infection. J Immunol. 2004;172(7):4454–4462. [DOI] [PubMed] [Google Scholar]
- 14.Heyde S, Philipsen L, Formaglio P, et al. CD11c-expressing Ly6C+CCR2+ monocytes constitute a reservoir for efficient Leishmania proliferation and cell-to-cell transmission. PLoS Pathog. 2018;14(10):e1007374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol. 2009;27:451–483. [DOI] [PubMed] [Google Scholar]
- 16.Tomiotto-Pellissier F, Bortoleti B, Assolini JP, et al. Macrophage polarization in Leishmaniasis: broadening horizons. Front Immunol. 2018;9:2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soong L, Henard CA, Melby PC. Immunopathogenesis of non-healing American cutaneous Leishmaniasis and progressive visceral Leishmaniasis. Semin Immunopathol. 2012;34(6):735–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DiPiro JT. Cytokine networks with infection: mycobacterial infections, leishmaniasis, human immunodeficiency virus infection, and sepsis. Pharmacotherapy. 1997;17(2):205–223. [PubMed] [Google Scholar]
- 19.Gurung P, Karki R, Vogel P, et al. An NLRP3 inflammasome-triggered Th2-biased adaptive immune response promotes leishmaniasis. J Clin Invest. 2015;125(3):1329–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tacchini-Cottier F, Weinkopff T, Launois P. Does T helper differentiation correlate with resistance or susceptibility to infection with L major? Some insights from the murine model. Front Immunol. 2012;3:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Turchetti AP, da Costa LF, Romao Ede L, Fujiwara RT, da Paixao TA, Santos RL. Transcription of innate immunity genes and cytokine secretion by canine macrophages resistant or susceptible to intracellular survival of Leishmania infantum. Vet Immunol Immunopathol. 2015;163(1–2):67–76. [DOI] [PubMed] [Google Scholar]
- 22.Place DE, Kanneganti TD. Recent advances in inflammasome biology. Curr Opin Immunol. 2018;50:32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen H, Jiang Z. The essential adaptors of innate immune signaling. Protein Cell. 2013;4(1):27–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mathur A, Hayward JA, Man SM. Molecular mechanisms of inflammasome signaling. J Leukoc Biol. 2018;103(2):233–257. [DOI] [PubMed] [Google Scholar]
- 25.Evavold CL, Kagan JC. How inflammasomes inform adaptive immunity. J Mol Biol. 2018;430(2):217–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Broz P, Dixit VM. Inflammasomes: mechanism of assembly, regulation and signalling. Nat Rev Immunol. 2016;16(7):407–420. [DOI] [PubMed] [Google Scholar]
- 27.Gross CJ, Mishra R, Schneider KS, et al. K(+) Efflux-independent NLRP3 inflammasome activation by small molecules targeting mitochondria. Immunity. 2016;45(4):761–773. [DOI] [PubMed] [Google Scholar]
- 28.Sanman LE, Qian Y, Eisele NA, et al. Disruption of glycolytic flux is a signal for inflammasome signaling and pyroptotic cell death. Elife. 2016;5:e13663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gurung P, Malireddi RK, Anand PK, et al. Toll or interleukin-1 receptor (TIR) domain-containing adaptor inducing interferon-beta (TRIF)-mediated caspase-11 protease production integrates Toll-like receptor 4 (TLR4) protein- and Nlrp3 inflammasome-mediated host defense against enteropathogens. J Biol Chem. 2012;287(41):34474–34483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kayagaki N, Warming S, Lamkanfi M, et al. Non-canonical inflammasome activation targets caspase-11. Nature. 2011;479(7371):117–121. [DOI] [PubMed] [Google Scholar]
- 31.Rathinam VA, Vanaja SK, Waggoner L, et al. TRIF licenses caspase-11-dependent NLRP3 inflammasome activation by gram-negative bacteria. Cell. 2012;150(3):606–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aachoui Y, Leaf IA, Hagar JA, et al. Caspase-11 protects against bacteria that escape the vacuole. Science. 2013;339(6122): 975–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hagar JA, Powell DA, Aachoui Y, Ernst RK, Miao EA. Cytoplasmic LPS activates caspase-11: implications in TLR4-independent endotoxic shock. Science. 2013;341(6151):1250–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kayagaki N, Wong MT, Stowe IB, et al. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science. 2013;341(6151):1246–1249. [DOI] [PubMed] [Google Scholar]
- 35.Shi J, Zhao Y, Wang Y, et al. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature. 2014;514(7521):187–192. [DOI] [PubMed] [Google Scholar]
- 36.Boucher D, Monteleone M, Coll RC, et al. Caspase-1 self-cleavage is an intrinsic mechanism to terminate inflammasome activity. J Exp Med. 2018;215(3):827–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kelley N, Jeltema D, Duan Y, He Y. The NLRP3 inflammasome: an overview of mechanisms of activation and regulation. Int J Mol Sci. 2019;20(13):3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Poudel B, Gurung P. An update on cell intrinsic negative regulators of the NLRP3 inflammasome. J Leukoc Biol. 2018;103(6):1165–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bauernfeind FG, Horvath G, Stutz A, et al. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol. 2009;183(2):787–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fernandes-Alnemri T, Kang S, Anderson C, Sagara J, Fitzgerald KA, Alnemri ES. Cutting edge: TLR signaling licenses IRAK1 for rapid activation of the NLRP3 inflammasome. J Immunol. 2013;191(8):3995–3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Juliana C, Fernandes-Alnemri T, Kang S, Farias A, Qin F, Alnemri ES. Non-transcriptional priming and deubiquitination regulate NLRP3 inflammasome activation. J Biol Chem. 2012;287(43):36617–36622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin KM, Hu W, Troutman TD, et al. IRAK-1 bypasses priming and directly links TLRs to rapid NLRP3 inflammasome activation. Proc Natl Acad Sci USA. 2014;111(2):775–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Py BF, Kim MS, Vakifahmetoglu-Norberg H, Yuan J. Deubiquitination of NLRP3 by BRCC3 critically regulates inflammasome activity. Mol Cell. 2013;49(2):331–338. [DOI] [PubMed] [Google Scholar]
- 44.Gurung P, Kanneganti TD. Innate immunity against Leishmania infections. Cell Microbiol. 2015;17(9):1286–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de Veer MJ, Curtis JM, Baldwin TM, et al. MyD88 is essential for clearance of Leishmania major: possible role for lipophosphoglycan and Toll-like receptor 2 signaling. Eur J Immunol. 2003;33(10):2822–2831. [DOI] [PubMed] [Google Scholar]
- 46.Flandin JF, Chano F, Descoteaux A. RNA interference reveals a role for TLR2 and TLR3 in the recognition of Leishmania donovani promastigotes by interferon-gamma-primed macrophages. Eur J Immunol. 2006;36(2):411–420. [DOI] [PubMed] [Google Scholar]
- 47.Becker I, Salaiza N, Aguirre M, et al. Leishmania lipophosphoglycan (LPG) activates NK cells through toll-like receptor-2. Mol Biochem Parasitol. 2003;130(2):65–74. [DOI] [PubMed] [Google Scholar]
- 48.Karmakar S, Bhaumik SK, Paul J, De T. TLR4 and NKT cell synergy in immunotherapy against visceral leishmaniasis. PLoS Pathog. 2012;8(4):e1002646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Paul J, Karmakar S, De T. TLR-mediated distinct IFN-gamma/IL-10 pattern induces protective immunity against murine visceral leishmaniasis. Eur J Immunol. 2012;42(8):2087–2099. [DOI] [PubMed] [Google Scholar]
- 50.Liese J, Schleicher U, Bogdan C. TLR9 signaling is essential for the innate NK cell response in murine cutaneous leishmaniasis. Eur J Immunol. 2007;37(12):3424–3434. [DOI] [PubMed] [Google Scholar]
- 51.Schleicher U, Liese J, Knippertz I, et al. NK cell activation in visceral leishmaniasis requires TLR9, myeloid DCs, and IL-12, but is independent of plasmacytoid DCs. J Exp Med. 2007;204(4):893–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gupta G, Santana AKM, Gomes CM, et al. Inflammasome gene expression is associated with immunopathology in human localized cutaneous leishmaniasis. Cell Immunol. 2019;341:103920. [DOI] [PubMed] [Google Scholar]
- 53.Novais FO, Carvalho AM, Clark ML, et al. CD8+ T cell cytotoxicity mediates pathology in the skin by inflammasome activation and IL-1beta production. PLoS Pathog. 2017;13(2):e1006196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ives A, Ronet C, Prevel F, et al. Leishmania RNA virus controls the severity of mucocutaneous leishmaniasis. Science. 2011;331(6018):775–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dey R, Joshi AB, Oliveira F, et al. Microbes egested during bites of infected sand flies augment severity of leishmaniasis via inflammasome-derived IL-1beta. Cell Host Microbe. 2018;23(1):134–143 e136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lima-Junior DS, Costa DL, Carregaro V, et al. Inflammasomederived IL-1beta production induces nitric oxide-mediated resistance to Leishmania. Nat Med. 2013;19(7):909–915. [DOI] [PubMed] [Google Scholar]
- 57.Yu M, Morita SY, Daniel SR, Chapital A, Waxman K, Severino R. Transcutaneous pressure of oxygen: a noninvasive and early detector of peripheral shock and outcome. Shock. 2006;26(5):450–456. [DOI] [PubMed] [Google Scholar]
- 58.Hemmi H, Takeuchi O, Kawai T, et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408(6813):740–745. [DOI] [PubMed] [Google Scholar]
- 59.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413(6857):732–738. [DOI] [PubMed] [Google Scholar]
- 60.Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303(5663):1529–1531. [DOI] [PubMed] [Google Scholar]
- 61.Gao D, Wu J, Wu YT, et al. Cyclic GMP-AMP synthase is an innate immune sensor of HIV and other retroviruses. Science. 2013;341(6148):903–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sun L, Wu J, Du F, Chen X, Chen ZJ. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science. 2013;339(6121):786–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wu J, Sun L, Chen X, et al. Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science. 2013;339(6121):826–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.de Carvalho RVH, Andrade WA, Lima-Junior DS, et al. Leishmania lipophosphoglycan triggers caspase-11 and the non-canonical activation of the NLRP3 inflammasome. Cell Rep. 2019;26(2):429–437 e425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.He Y, Zeng MY, Yang D, Motro B, Nunez G. NEK7 is an essential mediator of NLRP3 activation downstream of potassium efflux. Nature. 2016;530(7590):354–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schmid-Burgk JL, Chauhan D, Schmidt T, et al. A Genome-wide CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) screen identifies NEK7 as an essential component of NLRP3 inflammasome activation. J Biol Chem. 2016;291(1):103–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shi H, Wang Y, Li X, et al. NLRP3 activation and mitosis are mutually exclusive events coordinated by NEK7, a new inflammasome component. Nat Immunol. 2016;17(3):250–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Samir P, Kesavardhana S, Patmore DM, et al. DDX3X acts as a liveor-die checkpoint in stressed cells by regulating NLRP3 inflammasome. Nature. 2019;573(7775):590–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gross O, Poeck H, Bscheider M, et al. Syk kinase signalling couples to the Nlrp3 inflammasome for anti-fungal host defence. Nature. 2009;459(7245):433–436. [DOI] [PubMed] [Google Scholar]
- 70.Lupfer C, Thomas PG, Anand PK, et al. Receptor interacting protein kinase 2-mediated mitophagy regulates inflammasome activation during virus infection. Nat Immunol. 2013;14(5):480–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dostert C, Petrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320(5876):674–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cruz CM, Rinna A, Forman HJ, Ventura AL, Persechini PM, Ojcius DM. ATP activates a reactive oxygen species-dependent oxidative stress response and secretion of proinflammatory cytokines in macrophages. J Biol Chem. 2007;282(5):2871–2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Martinon F Signaling by ROS drives inflammasome activation. Eur J Immunol. 2010;40(3):616–619. [DOI] [PubMed] [Google Scholar]
- 74.Horta MF, Mendes BP, Roma EH, et al. Reactive oxygen species and nitric oxide in cutaneous leishmaniasis. J Parasitol Res. 2012;2012:203818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lefevre L, Lugo-Villarino G, Meunier E, et al. The C-type lectin receptors dectin-1, MR, and SIGNR3 contribute both positively and negatively to the macrophage response to Leishmania infantum. Immunity. 2013;38(5):1038–1049. [DOI] [PubMed] [Google Scholar]
- 76.Lima-Junior DS, Mineo TWP, Calich VLG, Zamboni DS. Dectin-1 activation during Leishmania amazonensis phagocytosis prompts Syk-dependent reactive oxygen species production to trigger inflammasome assembly and restriction of parasite replication. J Immunol. 2017;199(6):2055–2068. [DOI] [PubMed] [Google Scholar]
- 77.Underhill DM, Rossnagle E, Lowell CA, Simmons RM. Dectin-1 activates Syk tyrosine kinase in a dynamic subset of macrophages for reactive oxygen production. Blood. 2005;106(7):2543–2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kayagaki N, Stowe IB, Lee BL, et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature. 2015;526(7575):666–671. [DOI] [PubMed] [Google Scholar]
- 79.Ruhl S, Broz P. Caspase-11 activates a canonical NLRP3 inflammasome by promoting K(+) efflux. Eur J Immunol. 2015;45(10):2927–2936. [DOI] [PubMed] [Google Scholar]
- 80.de Assis RR, Ibraim IC, Nogueira PM, Soares RP, Turco SJ. Glycoconjugates in New World species of Leishmania: polymorphisms in lipophosphoglycan and glycoinositolphospholipids and interaction with hosts. Biochim Biophys Acta. 2012;1820(9):1354–1365. [DOI] [PubMed] [Google Scholar]
- 81.Lupfer CR, Anand PK, Liu Z, et al. Reactive oxygen species regulate caspase-11 expression and activation of the non-canonical NLRP3 inflammasome during enteric pathogen infection. PLoS Pathog. 2014;10(9):e1004410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chaves MM, Sinflorio DA, Thorstenberg ML, et al. Non-canonical NLRP3 inflammasome activation and IL-1beta signaling are necessary to L. amazonensis control mediated by P2X7 receptor and leukotriene B4. PLoS Pathog. 2019;15(6):e1007887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chaves MM, Marques-da-Silva C, Monteiro AP, Canetti C, Coutinho-Silva R. Leukotriene B4 modulates P2X7 receptor-mediated Leishmania amazonensis elimination in murine macrophages. J Immunol. 2014;192(10):4765–4773. [DOI] [PubMed] [Google Scholar]
- 84.Anand PK, Malireddi RK, Kanneganti TD. Role of the nlrp3 inflammasome in microbial infection. Front Microbiol. 2011;2:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Coll RC, Robertson AA, Chae JJ, et al. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat Med. 2015;21(3):248–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gordon R, Albornoz EA, Christie DC, et al. Inflammasome inhibition prevents alpha-synuclein pathology and dopaminergic neurodegeneration in mice. Sci Transl Med. 2018;10(465):eaah4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Perera AP, Fernando R, Shinde T, et al. MCC950, a specific small molecule inhibitor of NLRP3 inflammasome attenuates colonic inflammation in spontaneous colitis mice. Sci Rep. 2018;8(1):8618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.de Carvalho RVH, Lima-Junior DS, da Silva MVG, et al. Leishmania RNA virus exacerbates Leishmaniasis by subverting innate immunity via TLR3-mediated NLRP3 inflammasome inhibition. Nat Commun. 2019;10(1):5273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gupta AK, Ghosh K, Palit S, Barua J, Das PK, Ukil A Leishmania donovani inhibits inflammasome-dependent macrophage activation by exploiting the negative regulatory proteins A20 and UCP2. FASEB J. 2017;31(11):5087–5101. [DOI] [PubMed] [Google Scholar]
- 90.Shio MT, Christian JG, Jung JY, Chang KP, Olivier M. PKC/ROS-Mediated NLRP3 inflammasome activation is attenuated by leishmania zinc-metalloprotease during infection. PLoS Negl Trop Dis. 2015;9(6):e0003868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Saresella M, Basilico N, Marventano I, et al. Leishmania infantum infection reduces the amyloid beta42-stimulated NLRP3 inflammasome activation. Brain Behav Immun. 2020. 10.1016/j.bbi.2020.04.058. [DOI] [PubMed] [Google Scholar]
- 92.Lecoeur H, Prina E, Rosazza T, et al. Targeting macrophage histone H3 modification as a leishmania strategy to dampen the NF-kappaB/NLRP3-mediated inflammatory response. Cell Rep. 2020;30(6):1870–1882 e1874. [DOI] [PubMed] [Google Scholar]
- 93.Kaye P, Scott P. Leishmaniasis: complexity at the host-pathogen interface. Nat Rev Microbiol. 2011;9(8):604–615. [DOI] [PubMed] [Google Scholar]
- 94.Soares MB, David JR, Titus RG. An in vitro model for infection with Leishmania major that mimics the immune response in mice. Infect Immun. 1997;65(7):2837–2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Novais FO, Carvalho LP, Graff JW, et al. Cytotoxic T cells mediate pathology and metastasis in cutaneous leishmaniasis. PLoS Pathog. 2013;9(7):e1003504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S, Papaioannou VE. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992;68(5):869–877. [DOI] [PubMed] [Google Scholar]
- 97.Crosby EJ, Clark M, Novais FO, Wherry EJ, Scott P. Lymphocytic choriomeningitis virus expands a population of NKG2D+CD8+ T cells that exacerbates disease in mice coinfected with Leishmania major. J Immunol. 2015;195(7):3301–3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zahid A, Li B, Kombe AJK, Jin T, Tao J. Pharmacological inhibitors of the NLRP3 inflammasome. Front Immunol. 2019;10:2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Santos D, Campos TM, Saldanha M, et al. IL-1beta production by intermediate monocytes is associated with immunopathology in Cutaneous Leishmaniasis. J Invest Dermatol. 2018;138(5):1107–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Carvalho AM, Novais FO, Paixao CS, et al. Glyburide, a NLRP3 inhibitor, decreases inflammatory response and is a candidate to reduce pathology in Leishmania braziliensis infection. J Invest Dermatol. 2020;140(1):246–249 e242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Amorim CF, Novais FO, Nguyen BT, et al. Variable gene expression and parasite load predict treatment outcome in cutaneous leishmaniasis. Sci Transl Med. 2019;11(519):eaax4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gurung P, Kanneganti TD. Immune responses against protozoan parasites: a focus on the emerging role of Nod-like receptors. Cell Mol Life Sci. 2016;73(16):3035–3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Alegre ML. Mouse microbiomes: overlooked culprits of experimental variability. Genome Biol. 2019;20(1):108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mazgaeen L, Gurung P. Recent advances in lipopolysaccharide recognition systems. Int J Mol Sci. 2020;21(2):379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kesavardhana S, Malireddi RKS, Kanneganti TD. Caspases in cell death, inflammation, and gasdermin-induced pyroptosis. Annu Rev Immunol. 2020;38:567–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Moreira RB, Pirmez C, de Oliveira-Neto MP, et al. AIM2 inflammasome is associated with disease severity in tegumentary leishmaniasis caused by Leishmania (V.) braziliensis. Parasite Immunol. 2017;39(7):e12435. [DOI] [PubMed] [Google Scholar]
- 107.Sundar S, Rosenkaimer F, Makharia MK, et al. Trial of oral miltefosine for visceral leishmaniasis. Lancet. 1998;352(9143):1821–1823. [DOI] [PubMed] [Google Scholar]
- 108.Iacano AJ, Lewis H, Hazen JE, Andro H, Smith JD, Gulshan K. Miltefosine increases macrophage cholesterol release and inhibits NLRP3-inflammasome assembly and IL-1beta release. Sci Rep. 2019;9(1):11128. [DOI] [PMC free article] [PubMed] [Google Scholar]