Abstract
Conventionally fractionated chemoradiation (CRT) or chemotherapy (CHT) are considered as standard options in locally advanced pancreatic cancer (LAPC) while stereotactic body radiotherapy (SBRT) is an emerging treatment in this setting. The aim of this study was to compare two cohorts of LAPC patients treated with SBRT ± CHT vs CRT ± CHT in terms of local control (LC), distant metastases‐free survival (DMFS), progression‐free survival (PFS), overall survival (OS), and toxicity. Eighty patients were included. Patients in the two cohorts were matched according to: age ≤/>65 years, tumor diameter (two cut‐offs: </≥3.0 and </≥3.9 cm), clinical tumor stage and clinical nodal stage, neoadjuvant CHT, and adjuvant CHT. Median prescribed total dose was 30.0 Gy (range: 18.0‐37.5) and 54.0 Gy (18.0‐63.0) in SBRT and CRT cohorts, respectively. Toxicity was evaluated by CTCAE v4.0 scale. Survival curves were calculated by Kaplan‐Meier method. For hypothesis testing an equivalence and a non‐inferiority test was calculated. No statistically significant differences in terms of acute and late toxicity, DMFS, PFS, and OS were recorded among the two cohorts. Median, 1‐, and 2‐year LC was: 16.0 months, 53.1%, and 40.5% in the CRT cohort and 22.0 months, 80.4%, and 49.8% in the SBRT cohort, respectively (P: .017). A statistically non‐inferiority significance was recorded in terms of OS between CRT and SBRT (P = .031). Patients treated with SBRT showed higher LC rate and similar OS compared to CRT. Therefore, the design of confirmatory randomized studies comparing SBRT and CRT seems justified.
Keywords: conventionally fractionated radiotherapy, pancreatic cancer, radiation therapy, stereotactic body radiotherapy
An equivalence of stereotactic body radiotherapy (SBRT) and chemoradiation (CRT) has been only suggested in some retrospective studies in pancreatic cancer (PC). This is the first matched case‐control study comparing SBRT and CRT in PC considering several endpoints. An equivalence in terms of overall survival, disease free survival, and metastasis free survival among the two treatments and an advantage of SBRT in terms of local control were recorded. Therefore, SBRT is almost comparable to standard CRT.
1. INTRODUCTION
Pancreatic cancer (PC) is a dismal disease with 8% 5‐year overall survival (OS) rate. 1 It represents the fourth leading cause of mortality in the USA. Epidemiological studies predict that in 2030 PC will rise to second place in the same country. 2 Moreover, only 20% of highly selected patients have a potentially resectable disease whereas 30%‐40% of patients present at diagnosis with nonmetastatic unresectable locally advanced PC (LAPC) 3 .
Chemotherapy (CHT) and/or chemoradiation (CRT) are considered as treatment options for LAPC 4 despite conflicting results from the randomized trials that compared these two strategies. 5 , 6 , 7 Particularly, median OS of LAPC patients treated with CRT plus CHT ranges from 9 to 16 months in the randomized trials published since 2000. 5 , 6 , 8 , 9
Stereotactic body radiotherapy (SBRT) is an emerging radiotherapy technique, that was pioneered in the LAPC setting by the Stanford group since 2004. 10 The highly conformed dose distribution achievable with SBRT allows the delivery of high biologically effective doses (BED) with the potential to overcome the PC radio‐resistance and therefore improving local control (LC). 11 , 12 , 13 Moreover considering the short duration, SBRT favors the sequential combination with CHT. In fact, SBRT can be completed in a few days unlike standard CRT whose duration is generally between 4‐5 weeks. Based on these potential advantages, studies comparing SBRT and CRT seem to be justified. However, only few retrospective analyses are currently available. 14 , 15 , 16 , 17
Therefore, we performed a matched case‐control study comparing two cohorts of LAPC patients treated with SBRT ± CHT or CRT ± CHT in terms of LC, progression‐free survival (PFS), distant metastases‐free survival (DMFS), and OS. The aim of this report was to present the results of this analysis.
2. MATERIALS AND METHODS
2.1. Study design
This is a multicentric, retrospective, case‐control study. On behalf of the AIRO (Italian Association of Radiation Oncology and Clinical Oncology) Gastrointestinal Study Group, we collected clinical data on 419 patients from 15 Italian centers. In our database, LAPC patients could have been treated with every possible combination and schedules of CHT and radiotherapy delivered with any technique.
For the purpose of this analysis we selected all LAPC patients (56) treated with SBRT from six different Italian centers. We then matched these 56 SBRT patients with the ones treated with CRT (298) according to the following criteria: age ≤/>65 years, tumor diameter (</≥3 cm, and </≥3.9 cm), clinical tumor stage (cT), clinical nodal stage (cN), administration of neoadjuvant and adjuvant CHT. Matching was performed, blinded to patient outcome, in a 1:1 ratio and when multiple patients matched, one was selected at random. At the end of this selection we obtained two cohorts of 40 patients each, treated with SBRT or CRT, respectively.
2.2. Endpoints
The purpose of this analysis was to compare SBRT ± CHT and CRT ± CHT in LAPC patients in terms of different outcomes: LC, DMFS, PFS, and OS. Our aim was also to test the non‐inferiority of SBRT compared to CRT.
2.3. Eligibility
LAPC patients without metastatic disease and not previously treated with surgery due to PC or with abdominal radiotherapy were included in this study.
2.4. Treatment
Details about SBRT treatment were previously described. 18 CRT patients were planned and treated in supine position using a customized foam cradle. CT‐simulation was performed with intravenous and oral contrast. CRT was delivered using three‐dimensional conformal radiotherapy (70.0%), intensity modulated radiotherapy (IMRT) (20.0%), or volumetric modulated arc therapy (VMAT) (10.0%). The clinical target volume (CTV) was defined as the gross tumor volume plus a 1‐2 cm margin in the pancreatic parenchyma. Regional nodes were included in the CTV based on the tumor site. The planning target volume was defined as the CTV plus an anisotropic margin of 0.5‐1 cm radially and 1‐2 cm in cranial‐caudal direction in most patients. In 57% of patients, the planning target volume was defined using a 4D‐CT‐simulation. Dose specification and prescription were based on ICRU (International Commission on Radiation Units & Measurements) report 62 and 83 for three‐dimensional conformal radiotherapy and IMRT/VMAT, respectively. All patients were treated with conventionally fractionated radiotherapy (1.8‐2 Gy/fraction) plus concurrent CHT.
2.5. Follow‐up
The first follow‐up visit was carried out 3 weeks after the end of radiotherapy. Further evaluations were planned with 3 months intervals. Patients were monitored with standard blood tests, medical history, physical examination, and contrast enhanced CT scans of chest and abdomen.
2.6. Statistical analysis
Descriptive statistics included median and percentages for continuous and categorical variables, respectively. Categorical variables were compared using the Pearson's Chi‐square test. For hypothesis testing an equivalence and a non‐inferiority test was calculated. Survival curves were calculated using the Kaplan‐Meier method 19 and compared using the log‐rank test. 20 A multivariable Cox model 21 was built to test if some clinical and pathological factors could influence outcomes. All tests were two‐sided and a P < .05 was considered significant. All endpoints were calculated from the date of radiotherapy start. Statistical analysis was performed with IBM SPSS Version 22.0 (IBM Corp) and Statgraphics software systems (full system 5.25 version 4.0‐ Graphics system by Statistical Graphics Corporation Ed, United States, 1989). Toxicity was scored using the CTCAE v. 4.0 scale.
2.7. Ethical issues
All enrolled patients signed a written informed consent. The study (PAULA‐1: Pooled Analysis in Unresectable Locally Advanced pancreatic cancer) was approved by our institutional review board (201/2015/O/OssN).
3. RESULTS
The characteristics of patients and treatment in the two cohorts are shown in Table 1. Median follow‐up was 15 months (range: 3‐70). Median total dose, median dose per fraction, and median total BEDα/β10Gy, were 30.0 Gy (range: 18.0‐37.5), 6.0 Gy (range: 5.0‐10.0), and 48.0 Gy (range: 28.8‐65.6) in the SBRT cohort while the corresponding values were 50.4 Gy (range: 18.0‐63.0), 1.8 Gy (range: 1.8‐2.1), and 59.4 Gy (range: 21.2‐76.2) in the CRT cohort.
TABLE 1.
Comparison between the two cohorts of patients treated with chemoradiation and SBRT
| Variable | Value | CRT | SBRT | P |
|---|---|---|---|---|
| Age (y) | Median (range) | 67 (36‐89) | 67 (36‐83) | |
| ≤65 | 17 (42.5) | 17 (42.5) | .589 | |
| >65 | 23 (57.5) | 23 (57.5) | ||
| Gender | Male | 24 (60.0) | 27 (67.5) | .321 |
| Female | 16 (40.0) | 13(32.5) | ||
| ECOG PS | 0 | 22 (55.0) | 20 (50.0) | .493 |
| 1 | 16 (40.0) | 15 (37.5) | ||
| 2 | 2 (5.0) | 5 (12.5) | ||
| Tumor site | Head | 28 (70.0) | 24 (60.0) | .638 |
| Body | 10 (25.0) | 13 (32.5) | ||
| Tail | 2 (5.0) | 3 (7.5) | ||
| Tumor diameter (cm) | Median (range) | 4.0 (1.2‐8.7) | 4.0 (2.0‐7.0) | |
| <3.0 | 5 (12.5) | 5 (12.5) | .631 | |
| ≥3.0 and <3.9 | 18 (32.5) | 18 (32.5) | ||
| ≥3.9 | 22 (55.0) | 22 (55.0) | ||
| cT stage | 3 | 11 (27.5) | 11 (27.5) | .599 |
| 4 | 29 (72.5) | 29 (72.5) | ||
| cN stage | 0 | 22 (55.0) | 22 (55.0) | .589 |
| 1 | 18 (45.0) | 18 (45.0) | ||
| Biliary stent | No | 15 (37.5) | 19 (47.5) | .078 |
| Yes | 23 (57.5) | 13 (32.5) | ||
| Unknown | 2 (5.0) | 8 (20.0) | ||
| Neoadjuvant chemotherapy | No | 16 (40.0) | 16 (40.0) | .590 |
| Yes | 24 (60.0) | 24 (60.0) | ||
| Neoadjuvant chemotherapy regimen | Gemcitabine | 8 (33.3) | 3 (12.5) | .002* |
| Folfox | 1 (4.2) | 1 (4.2) | ||
| Folfirinox | 2 (8.3) | 6 (25.0) | ||
| Gemcitabine + Nab‐placlitaxel | 0 (0.0) | 9 (37.5) | ||
| Gemcitabine + Oxaliplatinum | 13 (54.2) | 5 (20.8) | ||
| Adjuvant chemotherapy | No | 31 (77.5) | 31 (77.5) | .605 |
| Yes | 9 (22.5) | 9 (22.5) | ||
| Adjuvant chemotherapy regimen | Gemcitabine | 7 (77.8) | 2 (22.2) | .073 |
| 5‐Fluorouracil | 0 (0.0) | 1 (11.1) | ||
| Folfirinox | 1 (11.1) | 4 (44.4) | ||
| Gemcitabine + Nab‐placlitaxel | 0 (0.0) | 2 (22.2) | ||
| Gemcitabine + Oxaliplatinum | 1 (11.1) | 0 (0.0) | ||
| Acute gastrointestinal toxicity | 0 | 24 (60.0) | 31 (77.5) | .175 |
| 1 | 12 (30.0) | 8 (20.0) | ||
| 2 | 4 (10.0) | 1 (2.5) | ||
| Late gastrointestinal toxicity | 0 | 35 (92.1) | 39 (97.5) | .244 |
| 1 | 1 (2.6) | 0 (0.0) | ||
| 2 | 2 (5.3) | 0 (0.0) | ||
| 3 | 0 (0.0) | 1 (2.5) |
Abbreviation: CHT, chemotherapy; CRT, chemoradiotherapy; ECOG PS, Eastern Cooperative Oncology Group performance status; SBRT, stereotactic body radiotherapy.
Significant P value.
The prescribed concurrent CHT regimens were gemcitabine‐ (80.0%) or capecitabine‐based (20.0%). In both cohorts, 60.0% and 22.5% patients underwent neoadjuvant and adjuvant CHT, respectively. Details on the CHT regimens used before and after radiotherapy in the two cohorts are shown in Table 1.
There were no statistically significant differences neither in terms of acute (P = .175) nor late gastrointestinal toxicity (P = .244) comparing LAPC patients treated with SBRT or CRT, respectively. Only one case (2.5%) of gastrointestinal bleeding was recorded 9 months after SBRT.
At univariate analysis, there were no differences between SBRT and CRT treatment in terms of OS (P = .470), PFS (P = .749) and DMFS (P = .610) (Table 2). Patients treated with SBRT had a statistically significant LC improvement (Figure 1) compared to those treated with CRT (median LC: 22 months vs 16 months, respectively; P = .017).
TABLE 2.
Characteristics and main findings of studies comparing SBRT +/− CHT vs CRT +/− CHT in locally advanced pancreatic cancer
| Author, year | Study design | Patients | No patients of the compared treatment | Main findings |
|---|---|---|---|---|
| Lin, 2015 14 | Retrospective | 41 | 20 SBRT +/− cCHT vs 21 IMRT +/− cCHT |
Median, 1‐y OS: 20.0 mo vs 13.0 mo, 80.0% vs 70.7% (P = .127) Median, 1‐y LC: 17.5 mo vs 10.0 mo, 70% vs 37.0% (P = .004) |
| Park, 2017 16 | Retrospective unmatched cohort | 270 | 44 SBRT +/− iCHT vs 226 IMRT +/− iCHT +/− cCHT |
1‐ and 2‐y OS: 56.2%, 25.7% vs 59.6%, 27.2% (P = .75) 1‐ and 2‐y LF: 34.4%, 48.7% vs 30.2%, 45.5% (P = .51) 1‐y DF: 61.7% vs 52.4% (P = .25) 1‐y DF + LF: 71.5% vs 63.5% (P = .18) G2‐G3 GI acute toxicity: 7% vs 24% (P = .008); 0% vs 2% (P = 1.00) Resection rate: 7% vs 17% (P = .11) |
| de Geus, 2017 15 | Registry study (NCDB) unmatched cohort | 14 331 | 5464 CHT vs 6418 CRT vs 322 SBRT + CHT vs 2127 IMRT + cCHT | Median OS: 9.9 mo vs 10.9 mo vs 13.9 mo vs 12.0 mo, (P < .001) |
| matched cohort a | 644 |
322 SBRT + CHT vs 322 CHT 322 SBRT + multiagent CHT vs 322 multiagent CHT 322 SBRT + CHT vs 322 CRT 322 SBRT + CHT vs 322 IMRT + cCHT |
Median OS: 13.9 mo vs 10.2 mo, (P < .001) Median OS: 14.8 mo vs 12.9 mo (P = .095) Median OS: 13.9 mo vs 11.6 mo, (P = .018) Median OS: 13.9 mo vs 12.2 mo, (P = .049) |
|
| Zhong, 2017 17 | Registry study (NCDB) unmatched cohort | 8450 | 631 SBRT vs 7819 CRT |
Resection rate: 10.8% vs 9.2% (P = .410) Negative resection margin: 92% vs 84% (P = .062) 2‐y OS: 20.3% vs 16.3% (P < .001) |
| matched cohort b | 988 | 494 SBRT vs 494 CRT |
Median OS: 13.9 mo vs 11.6 mo, (P < .001) 2‐y OS: 21.7% vs 16.5% (P = .001) |
|
| Chapman, 2018 22 | Retrospective unmatched cohort | 29 | 22 SBRT + iCHT vs 7 IMRT + iCHT |
Median PFS: 8.6 mo vs 12.5 mo (P = .349) Median OS: 19.7 mo vs 21.1 mo (P = .966) |
| Present study | Retrospective matched cohort c | 80 | 40 SBRT +/− CHT vs 40 CRT +/− CHT |
Median, 1‐ and 2‐y OS: 16.0 mo vs 21.0 mo, 79.8% vs 73.8%, 14.7% vs 40.1% (P = .470) Median, 1‐ and 2‐y LC: 22.0 mo vs 16.0 mo, 80.4% vs 53.1%, 49.8% vs 40.5% (P = .017) Median, 1‐y and DMFS: 16.0 mo vs 12.0 mo, 64.5% vs 49.3%, 20.3% vs 41.7% (P = .610) Median, 1‐ and 2‐y PFS: 14.0 mo vs 12.0 mo, 59.1% vs 49.2, 59.1% vs 32.4% (P = .749) GI acute toxicity: G1: 20.0% vs 30.0%; G2: 2.5% vs 10.0% (P = .175) GI late toxicity: G1: 0.0% vs 2.6%; G2: 0.0% vs 5.3%; G3 2.5% vs 0.0% (P = .244) |
Abbreviations: cCHT, concomitant chemotherapy; CHT, chemotherapy; CRT, chemoradiotherapy; DF, distant failure; DMFS, distant metastases‐free survival; G, grade; GI, gastrointestinal; iCHT, induction chemotherapy; IMRT, intensity‐modulated radiation therapy; LC, local control; LF, local failure; NCDB, National Cancer Database; OS, overall survival; PFS, progression‐free survival; SBRT, stereotactic body radiotherapy.
By: age, sex, race, comorbidity, insurance, type of treatment center, tumor location (head or body), clinical stage.
By: age, Charlson score, AJCC clinical T and N staging, median tumor size, CT use, year of diagnosis, receipt of surgery.
By: age, AJCC clinical T and N staging, tumor diameter, neoadjuvant CT use, adjuvant CT use.
FIGURE 1.
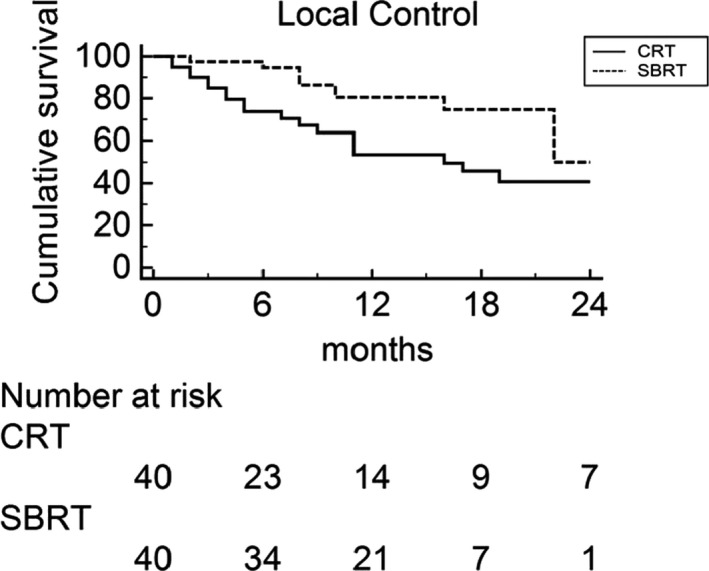
Local control of the two cohorts of patients treated with external beam chemoradiation (CRT) vs stereotactic body radiotherapy (SBRT)
Figures 2 and 3 represent the multivariate sub‐group analyses of the effects of patients' demographics, disease characteristics, and treatment details of both treatment impact on OS and LC. SBRT was associated with improved LC in the subsets of patients with tumor diameter ≤ 3.9 cm, tumor diameter ≥ 3.0 cm, cT4 and cN0 stage, while in no subset was there any advantage in terms of OS from the two therapeutic modalities.
FIGURE 2.
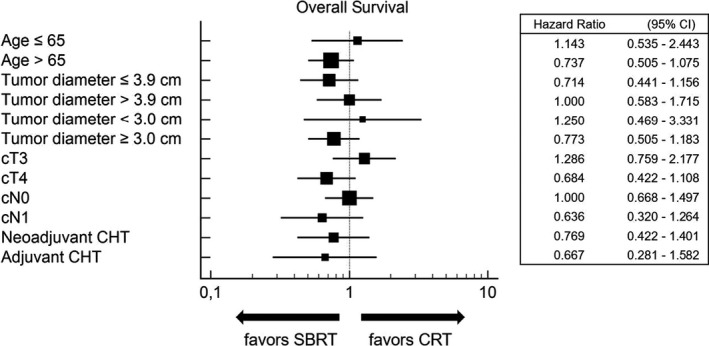
Multivariate subgroup analyses of the effects of patient characteristics on overall survival, comparing patients treated with external beam chemoradiation (CRT) vs stereotactic body radiotherapy (SBRT)
FIGURE 3.
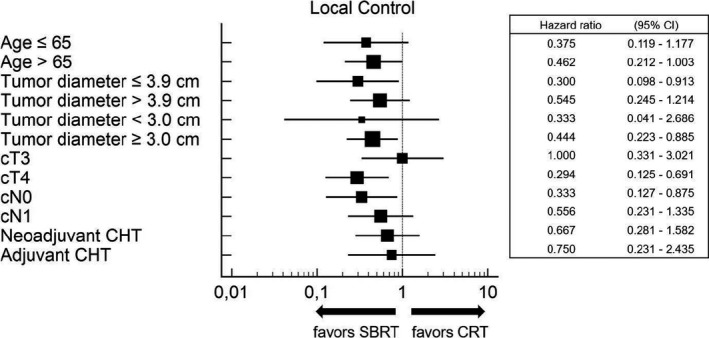
Multivariate subgroup analyses of the effects of patient characteristics on local control, comparing patients treated with external beam chemoradiation (CRT) vs stereotactic body radiotherapy (SBRT)
Finally, a statistically significant non‐inferiority in terms of OS was demonstrated between patients treated with SBRT and CRT (P = .031).
4. DISCUSSION
To the best of our knowledge, this is the first matched case‐control study in LAPC patients comparing conventionally fractionated CRT and SBRT in terms of different clinical outcomes. No differences in terms of OS, PFS, and DMFS were recorded while an improved LC in the SBRT cohort was registered.
Previously, some nonmatched studies 16 , 22 directly compared these two treatments reporting no significant differences in terms of outcomes. However, de Geus et al 15 and Zhong et al 17 compared matched cohorts treated with SBRT and CRT reporting improved median OS in the SBRT patients' group (Table 2). In fact, de Geus et al, 15 in a registry study from the National Cancer Data Base on LAPC, reported higher median OS after SBRT plus CHT compared to CHT alone (P < .001), to standard radiotherapy plus CHT (P = .018), and to IMRT plus CHT (P = .049). In another analysis also from the National Cancer Data Base, 17 a higher 2‐year OS rate was recorded in the SBRT ± CHT cohort, compared to conventionally fractionated radiotherapy ± CHT (P < .001) (Table 2). Similarly, in the meta‐analysis of Tchelebi et al 23 , including nine studies on SBRT and 11 studies on CRT in LAPC (1147 patients), an improved 2‐year OS in SBRT patients was reported (26.9% vs 13.7%, respectively; P = .004).
Unlike the studies mentioned above, 15 , 17 , 23 our study did not show significant differences between SBRT and CRT in terms of OS. This difference could be due to the relatively small sample size of our series and to the relatively low BEDα/β10Gy delivered in our SBRT cohort. In fact, median BEDα/β10Gy was significantly lower in the latter compared to the CRT cohort (48.0 Gy vs 59.4 Gy, respectively; P < .001). The significant correlation recently reported by our group among BEDα/β10Gy ≥ 48 Gy and improved OS in SBRT of LAPC 18 seems to confirm that the lack of improved OS in our SBRT cohort could depend on the relatively low BEDα/β10Gy.
As mentioned above, the most interesting result of our analysis is the higher LC rate in patients undergoing SBRT compared to CRT, despite the lower median BEDα/β10Gy in the SBRT cohort. This difference could be explained by the extremely shorter duration of SBRT compared to CRT which could prevent tumor repopulation during therapy. Similarly, in their retrospective unmatched study, Lin et al 14 reported significantly improved LC for LAPC patients treated with SBRT plus CHT compared to IMRT plus CHT. On the contrary, in their unmatched comparison, Park et al 16 did not observe significant differences in terms of LC between SBRT ± induction CHT and IMRT ± induction CHT. These conflicting results (Table 2) justify the design of randomized studies which may clarify this topic.
More generally, the results recorded in our two cohorts are similar to the ones reported in other studies on SBRT or CRT in LAPC. In fact, 1‐year LC was 80.4% in our SBRT cohort, which is consistent with the pooled 1‐year LC (72.3%) reported in the systematic review of Petrelli et al 24 on 1009 patients treated with SBRT in LAPC. Similarly, the median LC was 16 months in our CRT cohort, hence consistent with the median LC reported in the two arms of the SCALOP trial on conventionally fractionated CRT in LAPC (12.0 and 14.6 months). 9 Similar analogies can be observed in terms of OS. Our result in terms of median OS in the SBRT cohort (16 months) is similar to that of the two systematic reviews of Petrelli et al 24 (17 months) and Brunner et al 25 (11 months). Moreover our results in terms of median OS (21.0 months) in the CRT cohort were at least not inferior to those reported in the SCALOP 9 and LAP07 6 randomized trials (13.4‐15.2 months). Beyond the case‐control design of our analysis, the relative analogy between the results recorded by us with those reported in literature makes the findings of our comparison further reliable.
Our analysis showed no significant differences in terms of both acute and late toxicity between SBRT and CRT. This result contrasts with those reported in other studies. Indeed, Park et al 16 recorded significantly lower acute gastrointestinal toxicity grade ≥ 2 rates using SBRT compared to IMRT (P = .008). Moreover the metanalysis of Tchelebi et al 23 showed a significantly higher grade 3‐4 acute toxicity in patients treated with standard radiotherapy compared to SBRT, while no differences between the two treatments were recorded in terms of late toxicity. The lack of difference in terms of toxicity observed in our series may be due to several factors such as the small sample size and the retrospective study design. In fact, the latter could have led to an incomplete recording of adverse events. Moreover the impact of the small sample size on the failure to detect differences in toxicity seems confirmed by the enrolment of only 40 patients in a study reporting similar adverse event rates between SBRT and IMRT (Table 2). 14 Obviously, also this topic deserves further investigations.
As in any retrospective analysis our study has intrinsic limitations. Even if we used several matching criteria, the assignment to SBRT or CRT was not randomized. Therefore, we cannot rule out that our analysis is affected by bias. Particularly, although the percentage of patients undergoing neoadjuvant and adjuvant CHT was the same in the two cohorts, the used regimens were different among them. Furthermore, the relatively small sample size may have limited the possibility to detect significant differences, particularly in the subset analyses.
In conclusion, our comparison between SBRT and CRT suggests the equivalence in terms of most outcomes among the two techniques. Furthermore, for the first time using a case‐control methodology, an advantage of SBRT in terms of LC was recorded. This result, together with the logistical advantage of SBRT shorter duration, makes this technique an acceptable option in the treatment of LAPC in combination with CHT. Prospective trials are needed to better compare these two treatments. Moreover considering that in most cases LAPC treatment has a palliative purpose, these studies should include an accurate assessment of quality of life and symptoms control, especially in terms of pain relief. In fact, both conventional radiotherapy 26 and SBRT 27 are able to improve this symptom but direct comparisons of their relative effectiveness are lacking. Finally, considering that the only possibility of cure for patients with LAPC is to achieve a tumor downstaging to allow a radical surgical resection, the rate of resectability after SBRT and CRT should represent another relevant end point.
CONFLICT OF INTEREST
No actual or potential conflicts of interest do exist regarding this paper.
AUTHOR CONTRIBUTIONS
Conceptualization and design were performed by AGM, AA, GM, and MB. FB, AG, FD, SC, VS, MER, ID, SP, GCM, FC, MF, PB, LB, RMN, PG, NS, MDM, and RM contributed to data collection. Analysis and interpretation of data were performed by AA, AGM, MB, AG, FB, MDM, GM, RM, and FD. AA, AGM, MB, RM and AG contributed to manuscript writing, and NS, RM, FC, GCM, PG, PB, VS, LB, GM, and MDM contributed to the critical review of the manuscript. All Authors read and approved the final manuscript and gave consent to publication.
ACKNOWLEDGMENTS
The authors sincerely acknowledge AIRO (Italian Association of Radiation Oncology and Clinical Oncology) Group for Gastrointestinal Cancer for supporting the study.
Arcelli A, Buwenge M, Macchia G, et al; the AIRO (Italian Association of Radiation Oncology and Clinical Oncology) Gastrointestinal Study Group . Stereotactic body radiotherapy vs conventionally fractionated chemoradiation in locally advanced pancreatic cancer: A multicenter case-control study (PAULA-1). Cancer Med. 2020;9:7879–7887. 10.1002/cam4.3330
Renzo Mazzarotto and Alessio Giuseppe Morganti contributed equally.
Funding Information:
This research received no external funding.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics. CA Cancer J Clin. 2018;68:7‐30. [DOI] [PubMed] [Google Scholar]
- 2. Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913‐2921. [DOI] [PubMed] [Google Scholar]
- 3. Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605‐1617. [DOI] [PubMed] [Google Scholar]
- 4. NCCN clinical practice guidelines in oncology, pancreatic adenocarcinoma. Version 1.2020—published in November 26, 2019. https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf. Accessed May 2, 2020.
- 5. Chauffert B, Mornex F, Bonnetain F, et al. Phase III trial comparing intensive induction chemoradiotherapy (60 Gy, infusional 5‐FU and intermittent cisplatin) followed by maintenance gemcitabine with gemcitabine alone for locally advanced unresectable pancreatic cancer. Definitive results of the 2000–01 FFCD/SFRO study. Ann Oncol. 2008;19:1592‐1599. [DOI] [PubMed] [Google Scholar]
- 6. Hammel P, Huguet F, van Laethem JL, et al. Effect of chemoradiotherapy vs chemotherapy on survival in patients with locally advanced pancreatic cancer controlled after 4 months of gemcitabine with or without erlotinib: the LAP07 randomized clinical trial. JAMA. 2016;315:1844‐1853. [DOI] [PubMed] [Google Scholar]
- 7. Loehrer PJ Sr, Feng Y, Cardenes H, et al. Gemcitabine alone versus gemcitabine plus radiotherapy in patients with locally advanced pancreatic cancer: an Eastern Cooperative Oncology Group trial. J Clin Oncol. 2011;29:4105‐4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Louvet C, Labianca R, Hammel P, et al. Gemcitabine in combination with oxaliplatin compared with gemcitabine alone in locally advanced or metastatic pancreatic cancer: results of a GERCOR and GISCAD phase III trial. J Clin Oncol. 2005;23:3509‐3516. [DOI] [PubMed] [Google Scholar]
- 9. Mukherjee S, Hurt CN, Bridgewater J, et al. Gemcitabine‐based or capecitabine‐based chemoradiotherapy for locally advanced pancreatic cancer (SCALOP): a multicentre, randomised, phase 2 trial. Lancet Oncol. 2013;14:317‐326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Koong AC, Le QT, Ho A, et al. Phase I study of stereotactic radiosurgery in patients with locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2004;58:1017‐1021. [DOI] [PubMed] [Google Scholar]
- 11. Gurka MK, Collins SP, Slack R, et al. Stereotactic body radiation therapy with concurrent full‐dose gemcitabine for locally advanced pancreatic cancer: a pilot trial demonstrating safety. Radiat Oncol. 2013;8:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Herman JM, Chang DT, Goodman KA, et al. Phase 2 multiinstitutional trial evaluating gemcitabine and stereotactic body radiotherapy for patients with locally advanced unresectable pancreatic adenocarcinoma. Cancer. 2015;121:1128–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pollom EL, Alagappan M, von Eyben R, et al. Single‐ versus multifraction stereotactic body radiation therapy for pancreatic adenocarcinoma: outcomes and toxicity. Int J Radiat Oncol Biol Phys. 2014;90:918‐925. [DOI] [PubMed] [Google Scholar]
- 14. Lin JC, Jen YM, Li MH, Chao HL, Tsai JT. Comparing outcomes of stereotactic body radiotherapy with intensity‐modulated radiotherapy for patients with locally advanced unresectable pancreatic cancer. Eur J Gastroenterol Hepatol. 2015;27:259‐264. [DOI] [PubMed] [Google Scholar]
- 15. de Geus SWL, Eskander MF, Kasumova GG, Ng SC, Kent TS, Mancias JD, Callery MP, Mahadevan A, Tseng JF. Stereotactic body radiotherapy for unresected pancreatic cancer: a nationwide review. Cancer. 2017;123:4158‐4167. [DOI] [PubMed] [Google Scholar]
- 16. Park JJ, Hajj C, Reyngold M, et al. Stereotactic body radiation vs. intensity‐modulated radiation for unresectable pancreatic cancer. Acta Oncol. 2017;56:1746‐1753. [DOI] [PubMed] [Google Scholar]
- 17. Zhong J, Patel K, Switchenko J, et al. Outcomes for patients with locally advanced pancreatic adenocarcinoma treated with stereotactic body radiation therapy versus conventionally fractionated radiation. Cancer. 2017;123:3486‐3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Arcelli A, Guido A, Buwenge M, et al. Higher biologically effective dose predicts survival in SBRT of pancreatic cancer: a multicentric analysis (PAULA‐1). Anticancer Res. 2020;40:465‐472. [DOI] [PubMed] [Google Scholar]
- 19. Kaplan E, Meier P. Nonparametric estimation from incomplete observations. Am J Stat Assoc. 1958;53:457‐481. [Google Scholar]
- 20. Peto R, Peto J. Asymptotically efficient rank invariant procedures. J R Stat Soc. 1972;135:185‐207. [Google Scholar]
- 21. Cox DR. Regression models and life tables (with discussion). J R Stat Soc B. 1972;74:187‐220. [Google Scholar]
- 22. Chapman BC, Gleisner A, Rigg D, et al. Perioperative outcomes and survival following neoadjuvant stereotactic body radiation therapy (SBRT) versus intensity‐modulated radiation therapy (IMRT) in pancreatic adenocarcinoma. J Surg Oncol. 2018;117:1073‐1083. [DOI] [PubMed] [Google Scholar]
- 23. Tchelebi LT, Lehrer EJ, Trifiletti DM, Sharma NK, Gusani NJ, Crane CH, Zaorsky NG. Conventionally fractionated radiation therapy versus stereotactic body radiation therapy for locally advanced pancreatic cancer (CRiSP): an international systematic review and meta‐analysis. Cancer. 2020;126:2120–2131. [DOI] [PubMed] [Google Scholar]
- 24. Petrelli F, Comito T, Ghidini A, Torri V, Scorsetti M, Barni S. Stereotactic body radiation therapy for locally advanced pancreatic cancer: a systematic review and pooled analysis of 19 trials. Int J Radiat Oncol Biol Phys. 2017;97:313‐322. [DOI] [PubMed] [Google Scholar]
- 25. Brunner TB, Nestle U, Grosu AL, Partridge M. SBRT in pancreatic cancer: what is the therapeutic window? Radiother Oncol. 2015;114:109‐116. [DOI] [PubMed] [Google Scholar]
- 26. Morganti AG, Trodella L, Valentini V, et al. Pain relief with short‐term irradiation in locally advanced carcinoma of the pancreas. J Palliat Care. 2003;19:258‐262. [PubMed] [Google Scholar]
- 27. Buwenge M, Macchia G, Arcelli A, et al. Stereotactic radiotherapy of pancreatic cancer: a systematic review on pain relief. J Pain Res. 2018;11:2169‐2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


