Abstract
Objective
To compare the survival outcomes of local ablation (LA) and partial nephrectomy (PN) for T1N0M0 renal cell carcinoma (RCC).
Method
We identified 38,155 T1N0M0 RCC patients treated with PN or LA in 2004‐2016 from the retrospective Surveillance, Epidemiology, and End Results databases. Among them, there were 4656 LA and 33,499 PN. A Cox proportional hazards regression model, cause‐specific Cox regression and Fine and Gray sub‐distribution hazard ratio (sHR) with inverse probability of treatment weighting (IPTW) adjusting was utilized to compare the effects of LA vs PN on all‐, RCC‐, and non‐RCC–caused mortality.
Results
Within the IPTW analysis, patients who underwent PN experienced a better overall survival (OS) (HR, 1.56; 95% CI, 1.40‐1.74; P < .001) and cancer‐specific survival (CSS) (HR, 2.21; 95% CI, 1.62‐2.98; P < .001) than LA patients. In the subgroup of patients >85 years (HR, 1.14; 95% CI, 0.73‐1.79, P = .577), chromophobe RCC (HR, 1.68; 95% CI, 0.94‐3.00, P = .078), and tumor size <2 cm (HR, 1.21; 95% CI, 0.95‐1.53, P = .126), the OS showed no significant difference between LA and PN. No significant difference in CSS between LA and PN was observed in the subgroup of chromophobe RCC (HR, 0.34; 95% CI, 0.03‐3.97, P = .389), and tumor size <2 cm (HR, 1.83; 95% CI, 0.92‐3.64, P = .084). For patients >85 years (sHR, 0.89; 95% CI, 0.52‐1.27, P = .520) and tumor size <2 cm (sHR, 1.14; 95% CI, 0.94‐1.38, P = .200), the non‐RCC–specific mortality was not significantly different in PN and LA cohorts, however, for the chromophobe RCC, the LA showed a worse non‐RCC mortality than PN (HR, 1.72; 95% CI, 1.06‐2.79, P = .028).
Conclusion
PN showed a better prognosis than LA in T1N0M0 RCC treatment, but LA and PN showed a comparable OS in elderly patients (>85), small RCC (<2 cm) and chromophobe RCC.
Keywords: ablation, outcomes, partial nephrectomy, renal cell carcinoma, SEER, surgery
To compare the prognosis of local ablation (LA) and partial nephrectomy (PN) for T1N0M0 renal cell carcinoma (RCC) in the era of active surveillance and minimally invasive PN. Overall, PN showed a better OS and CSS than LA in T1N0M0 RCC treatment, but for elder age (>85 years), small tumors (<2 cm), and chromophobe RCC, both approaches showed no significant difference.

1. INTRODUCTION
The incidence rates of renal cell carcinoma (RCC) have been increasing in recent decades, especially with more detection of incidental small RCC due to ubiquitous abdominal imaging in current clinical practice. 1 In the past, radical nephrectomy (RN) by open or laparoscopic approach was the standard traditional treatment for RCC. With an increased understanding of the natural history and biology of RCC, guidelines have recently shifted toward the adoption of partial nephrectomy (PN) for the treatment of T1a and expanding selected T1b RCC when the technique is feasible 2 because PN can provide an equivalent oncological control or superior kidney functional outcome. 3 , 4 , 5 , 6 , 7 With the rapid development of laparoscopic and robot‐assisted surgical techniques, PN is mainly minimally invasive, which further promotes its application.
However, given the complications 8 , 9 induced by surgical intervention and potential overtreatment for localized small kidney masses, 10 more effort should be made to minimize the risk of intraoperative complications during PN, and alternative approaches are necessary for high‐risk patients. Local ablation (LA), mainly radiofrequency ablation and cryoablation, 2 has gradually gained acceptance as an option for localized small RCC. It tends to be used in patients who are elderly, have a severe cardiopulmonary disease, one kidney, hereditary RCC, or those who are poor candidates for PN or RN. 11 , 12 , 13 Although some studies with small sample sizes reported that LA was an effective and safe alternative treatment for T1a 14 , 15 and even suitable for T1b RCC, 16 , 17 there is a lack of sufficient evidence and guidelines to support the use of LA as a standard treatment, so treatment selection remains an empirical process.
The primary purpose of this study was to compare the survival prognoses following PN and LA for local T1N0M0 RCC. Given individual differences in demographical and clinical characteristics and the heterogeneity of biological characteristics among RCCs, we also conducted subgroup analyses and prognostic risk assessment for patients with local T1N0M0 RCC and compared the survival outcomes of LA and PN in different prognostic risk groups. The goal of this is to better screen people who are ideal candidates for LA with survival equivalent to or better than PN and to expand the indications of LA to benefit more people.
2. PATIENTS AND METHODS
2.1. Database and patient identification
In our retrospective study, all cohorts were obtained from the Surveillance, Epidemiology, and End Results (SEER) cancer database sponsored by the United States National Cancer Institute covering cancer patients’ demographical and clinical characteristics, cancer incidence, treatment, and survival outcomes from different cancer registries. The SEER 18 registries were used for patient selection, representing ~28% of the US population, and the patients' characteristics are comparable to the general population (https://seer.cancer.gov/). All case lists were identified and selected using SEER *Stat software (version 8.3.6). Since SEER data are anonymized, the need for institutional review board approval was waived.
Figure 1 presents a flowchart of data selection from the SEER database. All histologically confirmed T1N0M0 RCC (ICD‐O‐3 code C64.9) patients who underwent LA (RX Summ‐‐Surg Prim Site code in SEER database: 13, 15, and 23) or PN (RX Summ‐‐Surg Prim Site code in SEER database: 30) between 2004 and 2016 were included in the present study. Patients were excluded from the analysis for the following reasons: (a) age <18 years old; (b) no histological diagnosis or only by the autopsy/death certification; (c) tumor laterality was unknown or bilateral; (d) histology of tumor suspected origin from the renal pelvis, such as translational cell type; (e) lacking detailed information on tumor size, follow‐up, cause of death, or patients who underwent RN. For individual patient IDs with multiple records, the first was included. Derived American Joint Committee on Cancer 6th (2004‐2009), 7th (2010‐2015), and SEER Combined Stage (2016+) were used for RCC tumor node metastasis (TNM) staging classification in our study.
FIGURE 1.
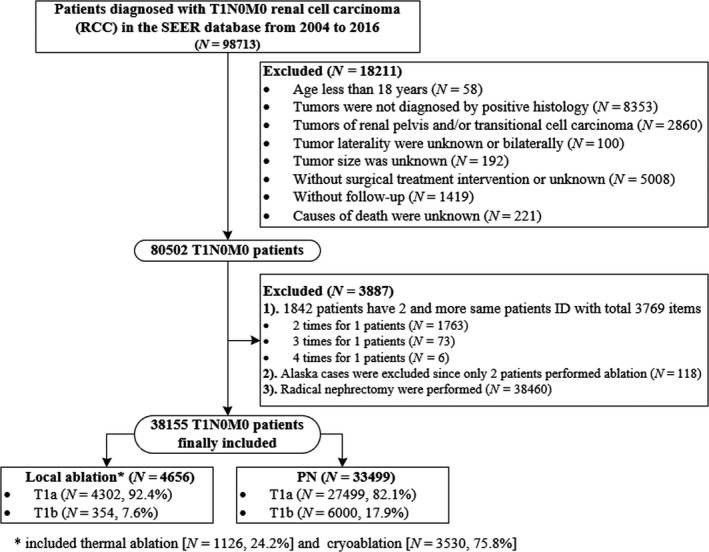
Flow chart for the data screening
2.2. Study covariables
We collected several demographical and clinical variables: the year of diagnosis, family income quartile, population, region, marital status, population density, age at diagnosis, sex, race/ethnicity (White, Black, and Others [American Indian/Alaska Native, Asian Native, and Asian/Pacific Islander]), and history of malignancy prior to primary RCC diagnosis. The tumor‐related characteristics included tumor size (cm) and histological cell type for RCC (clear cell, papillary, chromophobe, and other undefined cell types), tumor grade (well‐differentiated [grade 1], moderately differentiated [grade 2], poorly differentiated [grade 3], and undifferentiated [grade 4]), and tumor laterality.
2.3. Outcomes for analysis
The primary outcome of interest was overall survival (OS) and cancer‐specific survival (CSS, in the study, is RCC‐specific survival). The cause of death was determined following the cases list from the SEER database. Patients who died from non‐RCC causes were identified as competing for events for mortality by RCC. The survival interval was defined as the time from the date of RCC diagnosis to the date of death (events occurred) or last contact (censor).
2.4. Statistical analysis
Continuous variables and categorical variables are described as the mean (SD) and frequencies (%), respectively. The Student's t‐test or Wilcoxon rank‐sum test was used to compare continuous variables between groups, depending on whether the continuous variable data were normally distributed or not, respectively. The categorical variables were compared by the chi‐square test or Fisher's exact test. The reverse Kaplan‐Meier method was used to calculate the median follow‐up time.
In the nonrandomized studies, the effect of treatment on outcomes can be impacted by treatment‐selection bias in which the treated cohort systematically differs from the control cohort. To account for section bias and cofounding factors between the LA vs PN groups when comparing outcomes, weighted propensity score (PS) analysis was performed to balance differences in baseline demographical and clinical variables between patients who received LA and PN. First, a PS for each individual was calculated as the predicted probability of intervention with LA compared to PN from a multivariable logistic regression that included baseline confounding factors associated with survival outcomes. Then, we included all baseline characteristics for weighted PS analysis. The inverse probability of treatment weighting (IPTW) approach was used to generate the propensity model. 18 The weights are based on each individual's probability of receiving LA given the confounders, which is known as the PS, the weights are 1/PS for the LA participants and 1/(1 − PS) for the PN participants. The IPTW method is based on comparing the distribution of measured baseline covariates between treated and control groups in the sample weighted by the estimated inverse probability of treatment. 19 In brief, the IPTW method uses the principle of the standardization method to assign a corresponding weight to each research object through the PS value, so that the PS distributions are comparable between groups. This approach is a standardized method based on individuals that reduce the influence of confounding factors. Comparing the difference between the groups in the IPTW sample was made using standardized mean differences (SMDs), with a threshold <0.1 indicating a nonclinically meaningful difference.
The Kaplan‐Meier method using log‐rank statistics was used to compare OS and CSS between the LA and PN groups for the unweighted and IPTW populations. An IPTW Cox proportional hazard regression model and cause‐specific regression analysis were used to evaluate the risk factors of overall and cause‐specific mortality with and without adjusting confounders. Finally, we performed subgroup analyses for the impact of different treatments (PN vs LA) in each subgroup population for the overall and cancer‐specific mortality of patients with T1N0M0 RCC. In the subgroup analysis, we deleted incomplete data. Each subgroup was adjusted by IPTW to ensure that there was no statistical difference in basic data between the PN and LA groups. We also applied the sensitivity analysis for subgroup data without deleting the incomplete information.
All analyses were conducted using the R statistical package (v.3.6.3; R Foundation for Statistical Computing; https://www.r‐project.org). All P values are two sided, and P < .05 indicates statistical significance.
3. RESULTS
3.1. Patient baseline characteristics and treatments
Of the 98,713 T1N0M0 RCC patients in the databases between 2004 and 2016, 38,155 met the selection criteria; 4656 and 33,499 underwent LA and PN, respectively (Figure 1). The proportion of LA increased with the year of diagnosis, from 8.1% in 2004 to 14.9% in 2016 (Figure 2A). Table 1 lists patients’ demographical and clinicopathologic characteristics before and after propensity adjustment. Before IPTW adjustment, compared to the cohort of PN, patients who underwent LA were older (mean age 67.6 vs 58.8 years, P < .001), more 75 + years old (30.6% vs. 9.3%; P < .001), had a history of at least one prior cancer diagnosis (30.8% vs 18.6%; P < .001), were male (64.0% vs 61.9%; P < .006), had smaller tumor size (mean tumor size, 2.71 vs 2.95; P < .001), had tumor size >4 cm (7.6% vs 17.9%; P < .001), had less RCC with nuclear grade III+IV (5.8% vs 21.3%; P < .001), and more histology of RCC were other type/unknown (24.7% vs 16.6%; P < .001). Population density, adjusted median family incomes, and tumor laterality were not significantly different between groups (P = .390 for population density, P = .379 for adjusted family incomes, and P = .474 for laterality, respectively). After IPTW adjustment, there was no significant difference between groups, with SMDs < 10% for all covariables, indicating an excellent balance of baseline demographical and clinicopathologic characteristics between the PN and LA groups.
FIGURE 2.
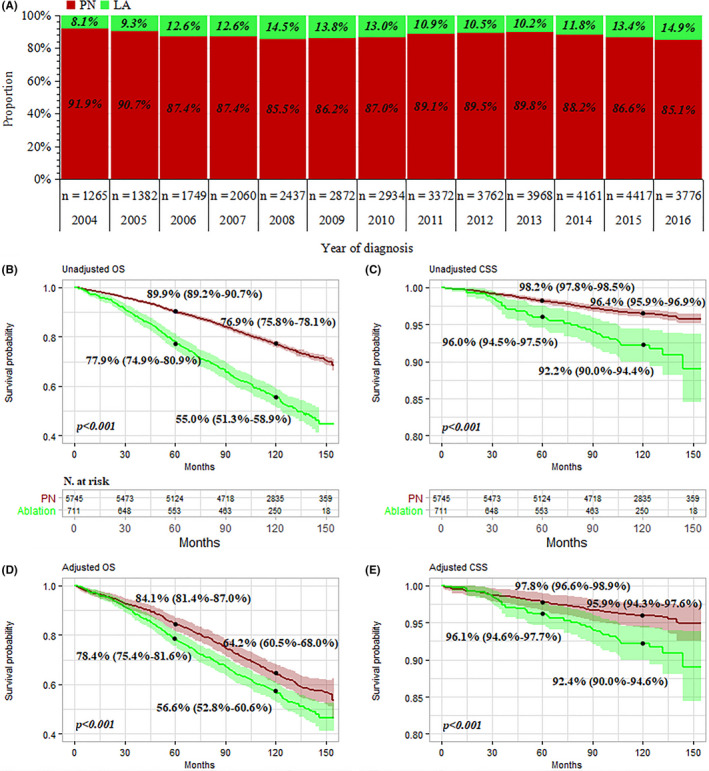
Surgery distribution, overall survival (OS) and cancer‐specific survival (CSS) of patients with T1N0M0 renal cell carcinoma underwent partial nephrectomy (PN) vs local ablation (LA). A, Proportion of surgery with the year at diagnosis; (B,C) unadjusted OS, and CSS, respectively; D,E, adjusted OS and CSS, respectively (renal cell carcinoma diagnoses between 2004 and 2007 with at least 10 y follow‐up were analyzed using the Kaplan‐Meier method)
TABLE 1.
Baseline characteristics of patients (n = 38 155) who received PN or LA
| Covariables | Overall (N = 38 155) | Unweighted population | Inverse probability of imputed treatment weighted population a | ||||||
|---|---|---|---|---|---|---|---|---|---|
| PN group (N = 33 499) | LA group b (N = 4656) | P‐value | SMD | PN group | LA group a | P‐value | SMD | ||
| Median follow‐up time, mo | 55 | 54 | 55 | — | — | 55 | 56 | — | — |
| Year at diagnosis | 0.004 | 0.053 | 0.373 | 0.025 | |||||
| 2004‐2007 | 6456 (16.9%) | 5745 (17.1%) | 711 (15.3%) | 16.3% | 15.5% | ||||
| 2008‐2012 | 15 377 (40.3%) | 13 487 (40.3%) | 1890 (40.6%) | 40.6% | 40.5% | ||||
| 2013‐2016 | 16 322 (42.8%) | 14 267 (42.6%) | 2055 (44.1%) | 43.0% | 44.0% | ||||
| Region | <0.001 | 0.235 | 0.994 | 0.005 | |||||
| East | 16 001 (41.9%) | 14 376 (42.9%) | 1625 (34.9%) | 36.0% | 35.9% | ||||
| Northern plains | 3874 (10.2%) | 3131 (9.3%) | 743 (16.0%) | 15.5% | 15.4% | ||||
| Pacific coast | 16 926 (44.4%) | 14 850 (44.3%) | 2076 (44.6%) | 44.0% | 44.2% | ||||
| Southwest | 1354 (3.5%) | 1142 (3.4%) | 212 (4.6%) | 4.4% | 4.5% | ||||
| Adjusted median family income | 0.379 | 0.014 | 0.636 | 0.009 | |||||
| $(~74 400] | 17 999 (47.2%) | 15 774 (47.1%) | 2225 (47.8%) | 51.4% | 51.8% | ||||
| $(74 400~) | 20 156 (52.8%) | 17 725 (52.9%) | 2431 (52.2%) | 48.6% | 48.2% | ||||
| Insurance | <0.001 | 0.146 | 0.750 | 0.019 | |||||
| Any medicaid | 3137 (8.2%) | 2678 (8.0%) | 459 (9.9%) | 9.6% | 9.8% | ||||
| Insured | 29 418 (77.1%) | 25 760 (76.9%) | 3658 (78.6%) | 78.1% | 78.3% | ||||
| Uninsured | 719 (1.9%) | 686 (2.0%) | 33 (0.7%) | 0.8% | 0.8% | ||||
| Unknown | 4881 (12.8%) | 4375 (13.1%) | 506 (10.9%) | 11.6% | 11.1% | ||||
| Population density | 0.390 | 0.014 | 13.9% | 13.8% | 0.860 | 0.003 | |||
| Counties | 32 857 (86.1%) | 28 828 (86.1%) | 4029 (86.5%) | ||||||
| Urban/rural | 5298 (13.9%) | 4671 (13.9%) | 627 (13.5%) | ||||||
| Prior cancer diagnosis | <0.001 | 0.296 | 0.882 | 0.009 | |||||
| No | 30 487 (79.9%) | 27 265 (81.4%) | 3222 (69.2%) | 70.9% | 71.3% | ||||
| 1 only | 6458 (16.9%) | 5323 (15.9%) | 1135 (24.4%) | 23.5% | 23.2% | ||||
| 2 or more | 1210 (3.2%) | 911 (2.7%) | 299 (6.4%) | 5.6% | 5.6% | ||||
| Marital status | <0.001 | 0.181 | 0.976 | 0.008 | |||||
| Married | 24 161 (63.3%) | 21 359 (63.8%) | 2802 (60.2%) | 60.9% | 60.9% | ||||
| Never married | 5719 (15.0%) | 5123 (15.3%) | 596 (12.8%) | 13.3% | 13.2% | ||||
| Separated/widowed/divorced | 6346 (16.6%) | 5291 (15.8%) | 1055 (22.7%) | 21.6% | 21.5% | ||||
| Unknown | 1929 (5.1%) | 1726 (5.2%) | 203 (4.4%) | 4.2% | 4.4% | ||||
| Age at diagnosis, y | <0.001 | 0.74 | 0.986 | <0.001 | |||||
| Mean (SD) | 59.8 (12.5) | 58.8 (12.3) | 67.6 (11.6) | 66.4 (10.9) | 66.4 (11.5) | ||||
| Median (IQR) | 61.0 (52.0‐69.0) | 60.0 (51.0‐68.0) | 69.0 (60.0‐76.0) | 67.0 (59.0‐75.0) | 67.0 (59.0‐75.0) | ||||
| Age at diagnosis, y | <0.001 | 0.704 | 0.984 | 0.007 | |||||
| ≦59 | 17 627 (46.2%) | 16 570 (49.5%) | 1057 (22.7%) | 25.2% | 25.4% | ||||
| 60‐74 | 15 986 (41.9%) | 13 811 (41.2%) | 2175 (46.7%) | 48.5% | 48.4% | ||||
| 75‐84 | 4101 (10.7%) | 2913 (8.7%) | 1188 (25.5%) | 22.9% | 22.7% | ||||
| 85+ | 441 (1.2%) | 205 (0.6%) | 236 (5.1%) | 3.1% | 3.1% | ||||
| Race | <0.001 | 0.087 | 0.998 | 0.0043 | |||||
| White | 31 242 (81.9%) | 27 372 (81.7%) | 3870 (83.1%) | 82.7% | 82.8% | ||||
| Black | 4350 (11.4%) | 3797 (11.3%) | 553 (11.9%) | 12.1% | 12.0% | ||||
| Other | 2214 (5.8%) | 2005 (6.0%) | 209 (4.5%) | 4.7% | 4.7% | ||||
| Unknown | 349 (0.9%) | 325 (1.0%) | 24 (0.5%) | 0.5% | 0.5% | ||||
| Sex | 0.006 | 0.044 | 0.949 | 0.001 | |||||
| Female | 14 431 (37.8%) | 12 756 (38.1%) | 1675 (36.0%) | 63.2% | 63.3% | ||||
| Male | 23 724 (62.2%) | 20 743 (61.9%) | 2981 (64.0%) | 36.8% | 36.7% | ||||
| Grade | <0.001 | 0.824 | 0.959 | 0.005 | |||||
| I + II | 26 568 (69.6%) | 23 859 (71.2%) | 2709 (58.2%) | 65.7% | 65.5% | ||||
| III + IV | 7419 (19.4%) | 7149 (21.3%) | 270 (5.8%) | 6.6% | 6.6% | ||||
| Unknown | 4168 (10.9%) | 2491 (7.4%) | 1677 (36.0%) | 27.7% | 27.9% | ||||
| Laterality | 0.474 | 0.011 | 51.3% | 51.2% | 0.909 | 0.002 | |||
| Left | 18 468 (48.4%) | 16 191 (48.3%) | 2277 (48.9%) | ||||||
| Right | 19 687 (51.6%) | 17 308 (51.7%) | 2379 (51.1%) | ||||||
| Histological type | <0.001 | 0.277 | 0.958 | 0.015 | |||||
| ccRCC | 22 441 (58.8%) | 19 936 (59.5%) | 2505 (53.8%) | 55.8% | 56.3% | ||||
| paRCC | 6520 (17.1%) | 5719 (17.1%) | 801 (17.2%) | 17.6% | 17.4% | ||||
| chRCC | 2473 (6.5%) | 2275 (6.8%) | 198 (4.3%) | 4.9% | 4.7% | ||||
| Other RCC | 1389 (3.6%) | 1277 (3.8%) | 112 (2.4%) | 2.7% | 2.6% | ||||
| Undefined | 5332 (14.0%) | 4292 (12.8%) | 1040 (22.3%) | 19.1% | 19.0% | ||||
| Tumor size, cm | <0.001 | 0.206 | 0.389 | 0.015 | |||||
| Mean (SD) | 2.92 (1.26) | 2.95 (1.29) | 2.71 (0.943) | 2.7 (1.10) | 2.7 (0.96) | ||||
| Median (IQR) | 2.70 (2.00‐3.60) | 2.70 (2.00‐3.70) | 2.60 (2.00‐3.20) | 2.5 (2.00‐3.30) | 2.6 (2.00‐3.25) | ||||
| Tumor size group, cm | <0.001 | 0.332 | 0.878 | 0.015 | |||||
| ≦2 | 11 067 (29.0%) | 9757 (29.1%) | 1310 (28.1%) | 29.3% | 28.9% | ||||
| 2‐3 | 12 829 (33.6%) | 10 902 (32.5%) | 1927 (41.4%) | 40.7% | 40.5% | ||||
| 3‐4 | 7905 (20.7%) | 6840 (20.4%) | 1065 (22.9%) | 21.9% | 22.4% | ||||
| 4‐7 | 6354 (16.7%) | 6000 (17.9%) | 354 (7.6%) | 8.1% | 8.3% | ||||
All the range of follow‐up time was 1‐155 mo. Wilcoxon rank‐sum test for the variables of age and tumor size; Chi‐square test for categorical variables.
Abbreviations: ccRCC, clear cell renal cell carcinoma; chRCC, chromophobe renal cell carcinoma; CI, confidence intervals; IQR, interquartile range; LA, Local ablation; paRCC, papillary renal cell carcinoma; PN, partial nephrectomy; SD, standardized difference; SMD, standardized mean difference.
Propensity scores were estimated in multivariable logistic regression models with all covariables, and inverse probability of imputed treatment weighted data was then created.
Including 1126 thermal ablation and 3530 cryoablation.
3.2. Follow‐up and survival outcomes
The median follow‐up was 54 months in the PN group vs 55 months in the LA group, the range was 1‐155 months. A total of 961 (20.6%) patients in the LA group and 3182 (9.4%) in the PN group died, and 155 (3.3%) in the LA group were RCC‐specific mortality compared with 474 (1.4%) in the PN group. To accurately estimate survival, RCC diagnoses between 2004 and 2007 with at least 10 years follow‐up were further analyzed using the Kaplan‐Meier method (Figure 2B‐E, the survival curve of 2004‐2016 was presented in the Figure S1). Before propensity adjustment, within the entire cohort, the median OS was significantly higher with PN compared with LA (NA vs 134 months; P < .001, Figure 2B). The OS rates at 5 and 10 years were 89.9% and 76.9% with PN and 77.9% and 55.0% with LA, respectively (Figure 2B). After IPTW adjustment, PN was still associated with improved OS. The OS rates at 5 and 10 years were 84.1% and 64.2% with PN vs 78.4% and 56.6% with LA, respectively (Figure 2D). The 5‐ and 10‐year CSS rates were >90.0% for both groups with and without IPTW adjustment, but the PN group fared better than the LA group (P < .001, Figure 2C,E).
Within different subgroups (before IPTW‐adjusted data presented in Table S1 and after IPTW‐adjusted data presented in Table S2). The 5‐ and 10‐year CSS rates were excellent with >80%, and most >90% for patients undergoing either PN or LA with or without IPTW adjustment. However, the OS was impacted by higher age, larger tumor size, and history of prior cancer, which were associated with 10‐year OS < 40%. For OS estimated by the Kaplan‐Meier method, all subgroups had significant differences in CSS for PN and LA (P < .001), except subgroups of patients with any Medicaid (P = .079), age >85 years (P = .420), and histology of chromophobe renal carcinoma (P = .240).
3.3. Treatment as a predictor for survival outcomes
Prognostic factors associated with OS and CSS in the overall cohort before IPTW adjustment are listed in Table S3. Compared with PN, LA was significantly associated with shorter OS and CSS (adjusted HR for OS, 1.66; 95% CI, 1.3‐1.81; P < .001; adjusted HR for CSS, 2.41; 95% CI, 1.95‐2.97; P < .001). After IPTW adjustment, LA had 1.56‐ and 2.21‐fold risk of all‐cause mortality and RCC‐caused mortality, respectively, in comparison with PN after adjusting all other variables (adjusted HR for all‐cause mortality, 1.56; 95% CI, 1.40‐1.74; P < .001; adjusted HR for CSS, 2.21; 95% CI, 1.63‐2.98; P < .001) (Table S4). Patients with lower family income, no insurance, a history of cancer, unmarried/separated/widowed/divorced, elder age, male gender, higher tumor grade, histology of papillary RCC, and increased tumor size had significantly increased risk of all‐cause mortality. However, insurance, marital status, and sex were not independent predictors for RCC‐caused mortality.
The effect of PN was consistent across subgroups before IPTW (Table S5). However, after IPTW adjustment, within subgroups of patients older than 85, histology of chromophobe RCC, and tumor size <2 cm, patients who underwent LA showed no significant difference for overall mortality compared with PN treatment, besides, there was no significant difference in CSS between LA and PN treatment in the subgroup of chromophobe RCC (HR adjusted, 0.34; 95% CI, 0.03‐3.97, P = .389), and tumor size <2 cm (HR adjusted, 1.83; 95% CI, 0.92‐3.64, P = .084) (Figure 3). Table 2 presents the results of non‐RCC–specific death for LA compared with PN in the subgroup of age, tumor size and histological types. For patients >85 years (sHR, 0.89; 95% CI, 0.52‐1.27, P = .520) and tumor size <2 cm (sHR, 1.14; 95% CI, 0.94‐1.38, P = .200), the non‐RCC–specific mortality was not significantly different between the PN and LA cohorts. And for the chromophobe RCC, the LA still showed a worse non‐RCC mortality risk than PN (HR adjusted, 1.72; 95% CI, 1.06‐2.79, P = .028). Similar results were obtained with the IPTW‐adjusted cause‐specific Cox regression model.
FIGURE 3.
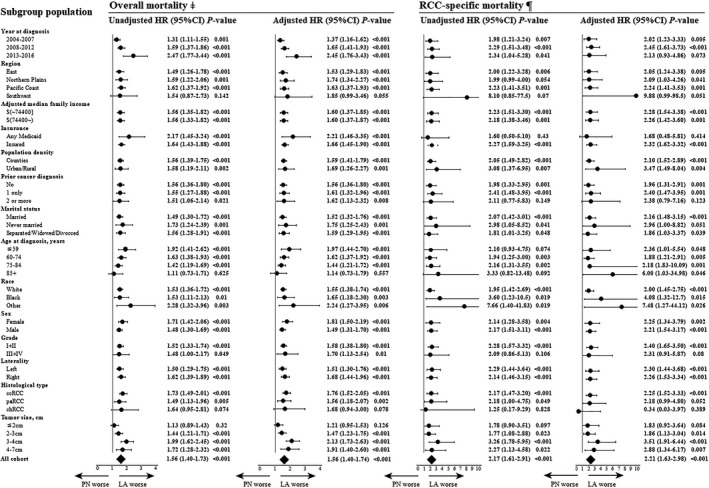
Subgroup analysis for the risk of overall and cancer‐specific mortality between different treatments of partial nephrectomy (PN) (as a Ref. [1]) vs local ablation (LA) in patients with T1N0M0 renal cell carcinoma (inverse probability of imputed treatment weighted population‐based on surgery for each subgroup). ccRCC, clear cell renal cell carcinoma; chRCC, chromophobe renal cell carcinoma; CI, confidence interval; HR, hazard ratio; paRCC, papillary renal cell carcinoma; sHR, sub‐distribution hazard ratio. ‡Univariable (unadjusted model) and multivariate (full covariables adjusted model) Cox regression analysis in each subgroup cohort. ¶Univariable (unadjusted model) and multivariate (full covariables adjusted model) cause‐specific Cox regression analysis in each subgroup cohort
TABLE 2.
Subgroup analysis for the impact of different treatments of partial nephrectomy (as a Ref. [1]) vs local ablation in subgroup population on non‐RCC–specific mortality (a competing death) of patients with T1N0M0 renal cell carcinoma
| Model 1 a | Model 2 b | |||
|---|---|---|---|---|
| Adjusted sHR (95% CI) | P‐value | Adjusted HR (95% CI) | P‐value | |
| All cohort | 1.51 (1.38‐1.65) | <0.001 | 1.56 (1.43‐1.71) | <0.001 |
| Age at diagnosis, y | ||||
| ≦59 | 2.14 (1.70‐2.68) | <0.001 | 1.92 (1.50‐2.46) | <0.001 |
| 60‐74 | 1.62 (1.42‐1.84) | <0.001 | 1.58 (1.37‐1.82) | <0.001 |
| 75‐84 | 1.37 (1.17‐1.59) | <0.001 | 1.36 (1.15‐1.60) | <0.001 |
| 85+ | 0.89 (0.62‐1.27) | 0.520 | 0.96 (0.64‐1.43) | 0.837 |
| Tumor size, cm | ||||
| ≦2 | 1.14 (0.94‐1.38) | 0.200 | 1.12 (0.91‐1.37) | 0.279 |
| 2‐3 | 1.44 (1.24‐1.67) | <0.001 | 1.41 (1.21‐1.64) | <0.001 |
| 3‐4 | 1.92 (1.61‐2.28) | <0.001 | 1.97 (1.64‐2.36) | <0.001 |
| 4‐7 | 1.82 (1.41‐2.33) | <0.001 | 1.73 (1.31‐2.28) | <0.001 |
| Histological type | ||||
| ccRCC | 1.72 (1.52‐1.94) | <0.001 | 1.66 (1.47‐1.89) | <0.001 |
| paRCC | 1.42 (1.15‐1.77) | 0.002 | 1.0.48 (1.16‐1.88) | 0.001 |
| chRCC | 1.72 (1.06‐2.79) | 0.028 | 1.77 (1.06‐2.97) | 0.030 |
Abbreviations: ccRCC, clear cell renal cell carcinoma; chRCC, chromophobe renal cell carcinoma; CI, confidence interval; HR, Hazard ratio; paRCC, papillary renal cell carcinoma; sHR, Sub‐distribution hazard ratio.
Results were computed by Fine and Grey regression using the data of crude unweighted population for each subgroup.
Results were computed by cause‐special Cox regression using the data of inverse probability of imputed treatment weighted population for each subgroup.
4. DISCUSSION
PN has become the most common surgery for localized RCC. 20 , 21 With advances in laparoscopic and robotic‐assisted technologies, most operations can be performed in a minimally invasive manner. Laparoscopic or robot‐assisted PN has been widely recognized and recommended by the surgical community. 22 Although laparoscopic or robot‐assisted PN offers a good trade‐off between minimally invasive and organ‐sparing procedures to achieve a win‐win effect, surgery still needs to be performed under general anesthesia. This may be associated with serious complications in patients with more comorbidities and poor physical tolerance. A prior study showed that about 5% of patients undergoing PN for a clinically localized renal tumor developed an intraoperative complication, 8 and higher American Anesthesiologists Score, complex RCC (eg, endophytic RCC), and the surgical technique were independent predictors of trifecta outcomes. 9 To reduce complications caused by PN and improve the quality of life for RCC patients, LA has gradually gained acceptance as an alternative to PN for patients with localized small RCC. 2 Our research validates the trend that the application of LA has increased over time, from 8.1% in 2004 to 14.9% in 2016. Previous studies compared the early or long‐term oncological outcomes, renal function, and complications between patients treated with LA and PN 12 , 14 , 16 , 17 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 ; some are summarized in Table 3. These studies suggested that when selecting patients, those treated with LA or PN showed comparable oncological outcomes and equivalent or better kidney function preservation.
TABLE 3.
Overview of current literature on local ablation and PN outcomes
| Study | Years of pooled pts. | Treatment | No. of pts | Mean/median age (y) | Size (cm) | Follow‐up (mo) | Conclusions |
|---|---|---|---|---|---|---|---|
| a , b Yu et al 24 | 2006‐2017 |
MWA LPN |
185 1770 |
63.2 50.9 |
2.3 2.3 |
40.6 |
1. No significant differences regarding oncologic outcomes or complications between percutaneous microwave ablation and laparoscopic PN for patients with cT1a renal cell carcinoma. 2. Percutaneous microwave ablation led to smaller renal function changes and reduced blood loss. |
| b Shapiro et al 25 | 2000‐2018 |
MWA PN RN |
40 74 211 |
69 58 59 |
4.4 4.7 5.0 |
34 35 49 |
No difference in 5‐y metastasis‐free survival or cancer‐specific survival was found among microwave ablation, partial nephrectomy, or radical nephrectomy. |
| a , b Rembeyo et al 26 | 2010‐2016 |
RPN CRA RFA |
36 55 11 |
60 72 84 |
4.5 4.6 4.2 |
23.7 19.9 51.3 |
1. No significant difference in renal preservation between the groups. 2. The adjusted hazard ratio for local recurrence‐free survival was significantly shorter for the cryoablation group. |
| b Zhou et al 27 | 2006‐2016 |
RFA CRA MWA |
244 26 27 |
73 68 69 |
2.4 2.4 2.2 |
24 | Radiofrequency ablation, cryoablation, and microwave ablation are equivalent at 2 y for the treatment of T1a renal cell carcinoma for the therapeutic outcome, renal function stability, and low adverse event rate. |
| a , b Pecoraro et al 29 | 2004‐2015 |
CRA PN |
242 5521 |
71 61 |
4.69 5.1 |
38 | A 2.5‐fold increase in cancer‐specific mortality when cryoablation was performed in patients with T1b renal cell carcinoma. |
| b Park et al 30 | 2005‐2014 |
RFA PN |
62 53 |
58 53 |
2.14 2.75 |
60 68 |
The groups did not differ in terms of eGFR change 1‐2 wk after surgery or at the last follow‐up or 5‐y survival rates. |
| a , b Kitley et al 31 | 1998‐2012 |
CRA PN |
6701 51 135 |
68 58 |
2.5 2.4 |
NA | Cryotherapy had lower overall survival than PN for tumors > 2 cm. |
| a , b Andrews et al 32 | 2000‐2011 |
CRA RFA PN |
187 180 1055 |
72 72 62 |
2.8 1.9 2.4 |
75.6 90 112.8 |
1. For cT1a patients, clinically relevant differences between PN and ablation are unlikely, and treatment choice should involve shared decision making. 2. For cT1b patients, death from renal cell carcinoma was more common with cryoablation, and large differences in this outcome cannot be ruled out. |
| c Alam et al 33 | 2009‐2018 |
PN RN AT AS |
231 41 27 339 |
61.3 69.3 71.8 70.6 |
2.4 3.1 2.1 1.8 |
36 |
1. PN and ablation are preferred over radical nephrectomy when intervention is indicated for small renal mass. 2. Active surveillance is a reasonable option for select patients, given comparable oncologic and mental health outcomes. |
| b Zhou et al 34 | 2006‐2016 |
RFA CRA MWA |
305 41 38 |
72 72 69 |
2.7 2.9 2.5 |
NA | CT‐guided percutaneous microwave ablation is comparable to radiofrequency ablation or cryoablation for the treatment of stage T1N0M0 renal cell carcinoma (concerning treatment response) and is associated with shorter treatment times and less sedation than radiofrequency ablation or cryoablation. |
| b Xing et al 35 | 2002‐2011 |
PN RN TA AS |
2820 4522 898 1978 |
71.9 73.9 75.9 NA |
NA | NA | For T1aN0M0 renal cell carcinoma, thermal ablation confers cancer‐specific and overall survival rates similar to those seen with surgical management, with significantly fewer adverse outcomes at 1 y after the procedure and similar rates of secondary cancer events compared with surgery. |
| b Talenfeld et al 12 | 2006‐2011 |
AT PN RN |
456 1748 2106 |
NA | NA |
44 51 55 |
1. Percutaneous ablation may result in oncologic outcomes similar to those of RN, but with less long‐term renal insufficiency and markedly fewer periprocedural complications. 2. Compared with partial nephrectomy, percutaneous ablation may be associated with slightly shorter disease‐specific survival but fewer periprocedural complications. |
| a , b Park et al 37 | 2008‐2016 |
RPN RFA |
63 63 |
NA | NA |
24.6 21 |
Partial nephrectomy provides a higher recurrence‐free survival rate than radiofrequency ablation. |
| a , b Caputo et al 38 | 1999‐2014 |
CRA PN |
31 161 |
68 61 |
4.3 5.0 |
30.1 13.0 |
Patients treated with cryoablation for cT1b renal tumors had a significantly higher rate of local cancer recurrence at 1 y compared to those treated with partial nephrectomy. |
| b Pantelidou et al 39 | 2005‐2013 |
RFA RPN |
63 63 |
61 54 |
2.11 2.88 |
47.5 18.5 |
Radiofrequency ablation demonstrated fewer perioperative complications and better preservation of renal function, whereas PN had an insignificantly lower local recurrence rate. |
| b Larcher et al 40 | 2000‐2009 |
AT PN |
561 2289 |
76 72 |
2.8 2.6 |
35 | Ablative therapies offer a short‐term protective effect from acute kidney injury. The short‐term rates of any dialysis treatment are similar after either ablative therapies or partial nephrectomy. At the long‐term assessment, renal function detriment rates are not different between ablative therapies and partial nephrectomy. |
| b Thompson et al 16 | 2000‐2011 |
PN RFA CRA |
1057 180 187 |
62 72 72 |
2.4 1.9 2.8 |
60 35 16.8 |
Recurrence‐free survival was similar for PN and percutaneous ablation patients. Metastasis‐free survival was superior for PN and cryoablation patients when compared with radiofrequency ablation for cT1a patients. Overall survival was superior after PN. |
| b Chang et al 17 | 2006‐2010 |
RFA PN |
27 29 |
64.0 56.9 |
4.7 5.2 |
65.9 70.2 |
Radiofrequency ablation is an effective treatment option that provides 5‐y overall survival, disease‐specific survival, and disease‐free survival rates comparable to that of PN, as well as better renal function preservation than PN for T1b renal cell carcinoma. |
| b Olweny et al 14 | 1998‐2005 |
RFA PN |
37 37 |
63.8 54.8 |
2.1 2.5 |
78 73.2 |
Radiofrequency ablation yields comparable long‐term oncologic outcomes to nephron‐sparing surgery. |
Abbreviations: AS, active surveillance; AT, ablative therapies; CRA, cryoablation; CT, computed tomography; eGFR, estimated glomerular filtration rate; MWA, microwave ablation, laparoscopic partial nephrectomy; PA, percutaneous ablation; PN, partial nephrectomy; RFA, radiofrequency ablation; RPN, robot‐assisted partial nephrectomy; TA, thermal ablation.
Propensity score matching study.
Retrospective study.
Prospective cohort study.
The primary aim of our study was to compare the prognoses of LA and PN after controlling for clinical baseline characteristics using IPTW adjustment. It was found that the patients undergoing PN still have a longer OS or CSS, but for patients >85 years, RCC <2 cm, and histology of chromophobe RCC, the LA cohort did not have significant differences for OS and CSS compared with the PN cohort. Patient selection is crucial for treatment decision making, and a prior study concluded that patients at high risk of complications (elder age, higher CCI, acute/chronic kidney injury, larger tumor size) may benefit the most from LA. 41 Also, both general and treatment‐specific complications can occur following LA, the increased risk of complications was attributed to patient‐related (increased age and higher CCI) and tumor‐related (increased size and next to renal sinus) factors, and included both urological and nonurological etiologies. 2 The incidence of complications was reported to be lower or comparable to that of PN 11 , 35 , 38 , 42 ; nevertheless, some studies have found no statistical differences between PN and LA surgical complications and postoperative kidney function changes. 13 , 38 Major complications of ablation occurred in 3.1%‐7.4% of cases, and overall complication rates were about 14%, but adverse effect reporting has not been standardized and is prone to bias in predominantly retrospective series. 2 Among all complications, bleeding is the most common, and cryoablation procedures showed a higher bleeding rate compared with radiofrequency ablation (4.9% vs 1.2%). 2
Older patients often have conditions affecting other organs and systems, especially cardiopulmonary diseases, which greatly increase the risk of noncancer death. For elderly patients with short life expectancy, considering that PN or RN surgery may be poorly tolerated and carry an increased risk of postoperative complications and competing mortalities, patients and physicians tend to choose LA. The current literature on the comparison of PN and LA surgical methods are usually concentrated on the elderly patient population with a median age of ~60 years. 11 , 16 , 35 We found that the prognosis of young patients receiving PN was better than that of LA patients. Besides, the patients included in previous studies were mainly elderly patients >65, with limited age stratification for older groups. We performed further stratification analysis on patients >60 and found that patients aged 60‐85 can still benefit more from PN than LA concerning OS and CSS, but there is no difference in OS for patients over 85. Age alone does not fully reflect the patient's physical health; other comorbidities and the CCI need to be considered. This is an important risk factor that affects the prognosis and was emphasized in prior studies. LA is recommended for RCC patients with CCI > 2. 41 , 42
Tumor size is an important sign of solid tumor TNM staging and determining surgery complexity. Our study found that only for RCC < 2 cm, there were no significant differences in OS and CSS between LA and PN surgery. LA is recommended by the American Urological Association 43 and the European Association of Urology 5 guidelines in patients with T1a RCC without lymph node invasion and distant metastasis. At present, there is still much controversy about LA usage for larger‐diameter tumors, such as T1bN0M0 patients undergoing LA. 16 , 17 , 29 , 38 In a high‐quality, comparative study concerning the efficacy of local cryoablation vs PN for T1b RCC treatment conducted by Caputo et al 38 renal cryoablation had a higher rate of local cancer recurrence; however, there was no significant difference in RCC‐specific mortality or overall mortality between the cryoablation and PN groups. One of the limitations of that study is the relatively small sample size, with a total of 31 patients undergoing LA, but at a single‐center, 31 complete T1b RCC patients undergoing LA surgery provide valuable data. Besides, they observed differences in the outcomes of interest between the two surgical methods, which met certain research objectives.
A recently published study by Pecoraro suggests that in T1b RCC, LA can increase the risk of RCC‐specific death of patients by about twofold compared to PN. 29 Studies have suggested that the application of new treatment techniques cannot take the "extreme" path, and indications should be determined using strong, evidence‐based medical data. In clinical practice, patients with T1bRCC should undergo PN as first‐line treatment whenever possible. PN operation for complex RCC can benefit from the advancement and application of robot technology. Many centers around the world can treat RCC with minimally invasive PN, which can obtain good results with regard to complications and patient quality of life. RCC size also affects the success rate of LA treatment. It has been reported that 26.5% of patients need to undergo two or more LA operations to achieve successful treatment, especially for large RCC; for tumors >4.5 cm, approximately 7.4% of patients undergo LA treatment. 44 Mauri et al 45 reported that among 149 patients treated with thermal ablations with a median follow‐up of 54 months, 18.1% received multiple successful ablations due to incomplete ablation, local tumor progression, distant tumor progression, or multiple tumor foci. Simultaneous use of ultrasound and computed tomography in ablation surgery can improve the ability to immediately detect RCC tissue that has not been sufficiently ablated, thereby guiding immediate secondary ablation. One of the disadvantages of image‐guided ablation is that incomplete ablation may occur, especially if the RCC is large or centrally located. 45 It should be noted that these results are mostly from large medical institutions. In these studies, patient enrollment, technical equipment, and surgeon experience may offer certain advantages, so it is necessary to interpret these conclusions carefully.
We found no statistical difference in oncologic control between the two surgical methods in the chromophobe RCC population. One group concluded that the prognosis of clear cell RCC after LA was worse than that of nonclear cell carcinoma, which is consistent with previous research. 46 In their study, 229 patients were included (181 clear cell RCC and 48 papillary RCC). After LA, the 5‐year disease‐free survival rates were 89.7% for clear cell RCC and 100% for papillary RCC, but there was no significant difference with respect to OS (88.4% vs 89.6%, P = .764). However, in LA group, the histology of clear cell RCC showed no significant difference in CSS and OS compared with nonclear cell RCC (papillary RCC vs clear cell RCC: HROS, 0.88, 95% CI 0.73‐1.07, HRCSS, 0.91, 95% CI 0.57‐1.47; chromophobe RCC vs clear cell RCC: HROS, 0.74, 95% CI 0.52‐1.06, HRCSS, 0.26, 95% CI 0.06‐1.07, data not shown). These results indicate that although the pathological type may not have a statistically significant effect on the prognosis of patients receiving LA, the prognosis of nonclear cell RCC is better, albeit not significantly.
A comparative study of laparoscopic LA and PN and a meta‐analysis provided fair evidence that oncologic outcomes are substantially worse for laparoscopic LA than for laparoscopic/robot‐assisted laparoscopic PN, but laparoscopic LA may be associated with improved perioperative outcomes. 23 However, with the widespread application of laparoscopic technology and increased surgeon experience, complications can be controlled and decreased, and some anatomically complex and large volume RCC can be treated with PN in a short time, and kidney functions damage can be minimalized through techniques such as blocking and nonblocking of renal artery branches. RCC enucleation can also reduce excessive normal healthy renal parenchyma loss. Because both laparoscopic LA and PN require general anesthesia, the benefits of LA in the era of mature laparoscopic technology still need further study. However, image‐guided percutaneous LA can indeed avoid the harm caused by general anesthesia. It is a good approach for some patients who are not suitable to undergo laparoscopic PN.
In the era of active surveillance and minimally invasive PN, evidence supporting the effectiveness and safety of ablation techniques continues to be refined. 35 , 42 , 45 There are still some difficulties in the promotion of LA; for example, ablation technology and equipment are not widely available in less developed areas. However, standard laparoscopic equipment and PN technology are increasingly common, and complications are decreased compared with the development period of the PN technique. Another issue is that an image follow‐up plan after ablation has not been established.
Our research also has certain limitations. The most important consideration is that our research data are retrospective, which has an inherent bias. However, to control the selection bias as much as possible and conduct case‐control studies, we used the PS weighting method to correct the baseline data in both groups. In our study, we could not adjust the CCI since the SEER database did not report it, but it is fundamental to correctly schedule the patients for a specific treatment; therefore, we used other competing mortality as a surrogate of CCI and to weight the competing risk analysis. We found that for subgroups of patients >85 years and tumor size <2 cm, non‐RCC–specific death was not significantly different, which suggested that the risks of competing events for the PN and LA cohorts were comparable in these two subgroups. However, we must interpret our results carefully, because the total cohort data suggested the LA group had a higher risk of non‐RCC–specific death compared with the PN group. This also indicated, to a certain extent, that our data still have potential biases between the two groups, even after IPTW adjustment of baseline characteristics. Since the records of surgical complications were incomplete, the article does not discuss the complications, but our main purpose was not to compare complications between methods. Rather, we focused on the comparison of OS and CSS. With the advancement of PN and LA technologies, the incidence of perioperative complications is now much lower, and the research pays more attention to long‐term patient prognosis, which is conducive to risk assessment and postoperative health consultation.
In conclusion, both PN and LA are valuable treatments for local small RCC treatment. Considering that the prognosis is generally worse following LA, we need to be cautious selecting patients to maximize the benefit from treatment. Our results indicate that age, tumor size, and histological type are important determinants for PN and LA surgical decision making.
CONFLICT OF INTEREST
The authors declare to have no competing interest.
AUTHORS CONTRIBUTIONS
Lei Shi and Zhixian Wang conceived the research and wrote the manuscript. Zhixian Wang registry the data. Lei Shi, Yan He, and Zhixian Wang analysis the data and prepare the figures and tables. All the authors were involved in approval of the final version.
Supporting information
Figure S1
Table S1‐S5
ACKNOWLEDGMENT
We thank SEER database supported by the Surveillance Research Program in the National Cancer Institute's Division of Cancer Control and Population Sciences.
Shi L, He Y, Liu C, Qian X, Wang Z. Local ablation vs partial nephrectomy in T1N0M0 renal cell carcinoma: An inverse probability of treatment weighting analysis. Cancer Med. 2020;9:7988–8003. 10.1002/cam4.3433
DATA AVAILABILITY STATEMENT
The raw data of this study are derived from the SEER database (https://seer.cancer.gov/), which is a publicly available database. All detailed data included in the study are available upon request by contact with the corresponding author (Zhixian Wang, SEER Username: 10062‐Nov2018).
REFERENCES
- 1. Znaor A, Lortet‐Tieulent J, Laversanne M, et al. International variations and trends in renal cell carcinoma incidence and mortality. Eur Urol. 2015;67:519. [DOI] [PubMed] [Google Scholar]
- 2. Ginzburg S, Tomaszewski JJ, Kutikov A. Focal ablation therapy for renal cancer in the era of active surveillance and minimally invasive partial nephrectomy. Nat Rev Urol. 2017;14:669. [DOI] [PubMed] [Google Scholar]
- 3. Capitanio U, Terrone C, Antonelli A, et al. Nephron‐sparing techniques independently decrease the risk of cardiovascular events relative to radical nephrectomy in patients with a T1a–T1b renal mass and normal preoperative renal function. Eur Urol. 2015;67:683. [DOI] [PubMed] [Google Scholar]
- 4. Kim SP, Campbell SC, Gill I, et al. Collaborative review of risk benefit trade‐offs between partial and radical nephrectomy in the management of anatomically complex renal masses. Eur Urol. 2017;72:64. [DOI] [PubMed] [Google Scholar]
- 5. Ljungberg B, Albiges L, Abu‐Ghanem Y, et al. European Association of Urology guidelines on renal cell carcinoma: the 2019 update. Eur Urol. 2019;75:799. [DOI] [PubMed] [Google Scholar]
- 6. Ljungberg B, Bensalah K, Canfield S, et al. EAU guidelines on renal cell carcinoma: 2014 update. Eur Urol. 2015;67:913. [DOI] [PubMed] [Google Scholar]
- 7. Mir MC, Derweesh I, Porpiglia F, et al. Partial nephrectomy versus radical nephrectomy for clinical T1b and T2 renal tumors: a systematic review and meta‐analysis of comparative studies. Eur Urol. 2017;71:606. [DOI] [PubMed] [Google Scholar]
- 8. Minervini A, Mari A, Borghesi M, et al. The occurrence of intraoperative complications during partial nephrectomy and their impact on postoperative outcome: results from the RECORd1 project. Minerva Urol Nefrol. 2019;71:47. [DOI] [PubMed] [Google Scholar]
- 9. Bianchi L, Schiavina R, Borghesi M, et al. Which patients with clinical localized renal mass would achieve the trifecta after partial nephrectomy? The impact of surgical technique. Minerva Urol Nefrol. 2020;72:339. [DOI] [PubMed] [Google Scholar]
- 10. McIntosh AG, Ristau BT, Ruth K, et al. Active surveillance for localized renal masses: tumor growth, delayed intervention rates, and >5‐yr clinical outcomes. Eur Urol. 2018;74:157. [DOI] [PubMed] [Google Scholar]
- 11. Bhindi B, Mason RJ, Haddad MM, et al. Outcomes after cryoablation versus partial nephrectomy for sporadic renal tumors in a solitary kidney: a propensity score analysis. Eur Urol. 2018;73:254. [DOI] [PubMed] [Google Scholar]
- 12. Talenfeld AD, Gennarelli RL, Elkin EB, et al. Percutaneous ablation versus partial and radical nephrectomy for T1a renal cancer: a population‐based analysis. Ann Intern Med. 2018;169:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mason RJ, Atwell TD, Lohse C, et al. Renal functional outcomes in patients undergoing percutaneous cryoablation or partial nephrectomy for a solitary renal mass. BJU Int. 2017;120:544. [DOI] [PubMed] [Google Scholar]
- 14. Olweny EO, Park SK, Tan YK, et al. Radiofrequency ablation versus partial nephrectomy in patients with solitary clinical T1a renal cell carcinoma: comparable oncologic outcomes at a minimum of 5 years of follow‐up. Eur Urol. 2012;61:1156. [DOI] [PubMed] [Google Scholar]
- 15. Ma Y, Bedir S, Cadeddu JA, et al. Long‐term outcomes in healthy adults after radiofrequency ablation of T1a renal tumours. BJU Int. 2014;113:51. [DOI] [PubMed] [Google Scholar]
- 16. Thompson RH, Atwell T, Schmit G, et al. Comparison of partial nephrectomy and percutaneous ablation for cT1 renal masses. Eur Urol. 2015;67:252. [DOI] [PubMed] [Google Scholar]
- 17. Chang X, Zhang F, Liu T, et al. Radio frequency ablation versus partial nephrectomy for clinical T1b renal cell carcinoma: long‐term clinical and oncologic outcomes. J Urol. 2015;193:430. [DOI] [PubMed] [Google Scholar]
- 18. Mansournia MA, Altman DG. Inverse probability weighting. BMJ. 2016;352:i189. [DOI] [PubMed] [Google Scholar]
- 19. Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34:3661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jabaji RB, Fischer H, Kern T, et al. Trend of surgical treatment of localized renal cell carcinoma. Perm J. 2019;23:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. White V, Marco DJT, Bolton D, et al. Trends in the surgical management of stage 1 renal cell carcinoma: findings from a population‐based study. BJU Int. 2017;120:6‐14. [DOI] [PubMed] [Google Scholar]
- 22. Han KS, Song GH, You D, et al. Comparison of hand‐assisted laparoscopic vs robot‐assisted laparoscopic vs open partial nephrectomy in patients with T1 renal masses. J Endourol. 2017;31:374. [DOI] [PubMed] [Google Scholar]
- 23. Klatte T, Shariat SF, Remzi M. Systematic review and meta‐analysis of perioperative and oncologic outcomes of laparoscopic cryoablation versus laparoscopic partial nephrectomy for the treatment of small renal tumors. J Urol. 2014;191:1209. [DOI] [PubMed] [Google Scholar]
- 24. Yu J, Zhang X, Liu H, et al. Percutaneous microwave ablation versus laparoscopic partial nephrectomy for cT1a renal cell carcinoma: a propensity‐matched cohort study of 1955 patients. Radiology. 2020;294:698. [DOI] [PubMed] [Google Scholar]
- 25. Shapiro DD, Wells SA, Best SL, et al. Comparing outcomes for patients with clinical T1b renal cell carcinoma treated with either percutaneous microwave ablation or surgery. Urology. 2020;135:88. [DOI] [PubMed] [Google Scholar]
- 26. Rembeyo G, Correas JM, Jantzen R, et al. Percutaneous ablation versus robotic partial nephrectomy in the treatment of cT1b renal tumors: oncologic and functional outcomes of a propensity score‐weighted analysis. Clin Genitourin Cancer. 2020;18:138. [DOI] [PubMed] [Google Scholar]
- 27. Zhou W, Herwald SE, McCarthy C, et al. Radiofrequency ablation, cryoablation, and microwave ablation for T1a renal cell carcinoma: a comparative evaluation of therapeutic and renal function outcomes. J Vasc Interv Radiol. 2019;30:1035. [DOI] [PubMed] [Google Scholar]
- 28. Wu J, Chang J, Bai HX, et al. A comparison of cryoablation with heat‐based thermal ablation for treatment of clinical T1a renal cell carcinoma: a national cancer database study. J Vasc Interv Radiol. 2019;30:1027. [DOI] [PubMed] [Google Scholar]
- 29. Pecoraro A, Palumbo C, Knipper S, et al. Cryoablation predisposes to higher cancer specific mortality relative to partial nephrectomy in patients with nonmetastatic pT1b kidney cancer. J Urol. 2019. 10.1097/JU.0000000000000460 [DOI] [PubMed] [Google Scholar]
- 30. Park JM, Yang SW, Shin JH, et al. Oncological and functional outcomes of laparoscopic radiofrequency ablation and partial nephrectomy for T1a renal masses: a retrospective single‐center 60 month follow‐up cohort study. Urol J. 2019;16:44. [DOI] [PubMed] [Google Scholar]
- 31. Kitley W, Sulek J, Sundaram C, et al. Treatment trends and long‐term survival associated with cryotherapy and partial nephrectomy for small renal masses in the national cancer database using propensity score matching. J Endourol. 2019;33:408. [DOI] [PubMed] [Google Scholar]
- 32. Andrews JR, Atwell T, Schmit G, et al. Oncologic outcomes following partial nephrectomy and percutaneous ablation for cT1 renal masses. Eur Urol. 2019;76:244. [DOI] [PubMed] [Google Scholar]
- 33. Alam R, Patel HD, Osumah T, et al. Comparative effectiveness of management options for patients with small renal masses: a prospective cohort study. BJU Int. 2019;123:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhou W, Arellano RS. Thermal ablation of T1c renal cell carcinoma: a comparative assessment of technical performance, procedural outcome, and safety of microwave ablation, radiofrequency ablation, and cryoablation. J Vasc Interv Radiol. 2018;29:943. [DOI] [PubMed] [Google Scholar]
- 35. Xing M, Kokabi N, Zhang D, et al. Comparative effectiveness of thermal ablation, surgical resection, and active surveillance for T1a renal cell carcinoma: a surveillance, epidemiology, and end results (SEER)‐medicare‐linked population study. Radiology. 2018;288:81. [DOI] [PubMed] [Google Scholar]
- 36. Uhlig J, Kokabi N, Xing M, et al. Ablation versus resection for stage 1A renal cell carcinoma: national variation in clinical management and selected outcomes. Radiology. 2018;288:889. [DOI] [PubMed] [Google Scholar]
- 37. Park BK, Gong IH, Kang MY, et al. RFA versus robotic partial nephrectomy for T1a renal cell carcinoma: a propensity score‐matched comparison of mid‐term outcome. Eur Radiol. 2018;28:2979. [DOI] [PubMed] [Google Scholar]
- 38. Caputo PA, Zargar H, Ramirez D, et al. Cryoablation versus partial nephrectomy for clinical T1b renal tumors: a matched group comparative analysis. Eur Urol. 2017;71:111. [DOI] [PubMed] [Google Scholar]
- 39. Pantelidou M, Challacombe B, McGrath A, et al. Percutaneous radiofrequency ablation versus robotic‐assisted partial nephrectomy for the treatment of small renal cell carcinoma. Cardiovasc Intervent Radiol. 2016;39:1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Larcher A, Meskawi M, Valdivieso R, et al. Comparison of renal function detriments after local tumor ablation or partial nephrectomy for renal cell carcinoma. World J Urol. 2016;34:383. [DOI] [PubMed] [Google Scholar]
- 41. Larcher A, Fossati N, Tian Z, et al. Prediction of complications following partial nephrectomy: implications for ablative techniques candidates. Eur Urol. 2016;69:676. [DOI] [PubMed] [Google Scholar]
- 42. Morkos J, Porosnicu Rodriguez KA, Zhou A, et al. percutaneous cryoablation for stage 1 renal cell carcinoma: outcomes from a 10‐year prospective study and comparison with matched cohorts from the National Cancer Database. Radiology. 2020. 10.1148/radiol.2020192325 [DOI] [PubMed] [Google Scholar]
- 43. Campbell S, Uzzo RG, Allaf ME, et al. Renal mass and localized renal cancer: AUA guideline. J Urol. 2017;198:520. [DOI] [PubMed] [Google Scholar]
- 44. Dvorak P, Hoffmann P, Brodak M, et al. Percutaneous radiofrequency and microwave ablation in the treatment of renal tumors—10 years of experience. Wideochir Inne Tech Maloinwazyjne. 2017;12:394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mauri G, Mistretta FA, Bonomo G, et al. Long‐term follow‐up outcomes after percutaneous US/CT‐guided radiofrequency ablation for cT1a‐b renal masses: experience from single high‐volume referral center. Cancers. 2020;12(5):1183 10.3390/cancers1205118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lay AH, Faddegon S, Olweny EO, et al. Oncologic efficacy of radio frequency ablation for small renal masses: clear cell vs papillary subtype. J Urol. 2015;194:653. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
Table S1‐S5
Data Availability Statement
The raw data of this study are derived from the SEER database (https://seer.cancer.gov/), which is a publicly available database. All detailed data included in the study are available upon request by contact with the corresponding author (Zhixian Wang, SEER Username: 10062‐Nov2018).


