Abstract
Background
Fatigue prevalence and severity have been assessed in a variety of studies, yet, not in a standardized way, and predominantly in breast cancer patients. Systematic, comparative investigations across a broad range of cancer entities are lacking.
Methods
The FiX study systematically enrolled 2244 cancer patients across 15 entities approximately 2 years after diagnosis. Fatigue was assessed with the multidimensional EORTC QLQ‐FA12 questionnaire. Physical, emotional, cognitive, and total fatigue were compared across entities and with normative values of the general population. Differences in patients' characteristics and cancer therapy between entities were taken into account using analyses of covariance models.
Results
Across all entities, mean physical fatigue levels were significantly higher than age‐ and sex‐matched means of the general population for all cancer entities (all Bonferroni‐Holm adjusted P < .01). For most entities also emotional and cognitive fatigue levels were significantly higher than normative values. Age‐ and sex‐standardized physical fatigue prevalence ranged from 31.8% among prostate to 51.7% among liver cancer patients. Differences between entities could not be fully explained by sex, age, BMI, or cancer therapy. Adjusted for these factors, mean physical fatigue was higher for stomach (P = .0004), lung (P = .034), kidney (P = .0011), pancreas (P = .081), and endometrium (P = .022) compared to breast cancer patients. Adjusted means of emotional fatigue were also lowest in breast cancer patients and significantly higher in stomach (P = .0047), bladder (P = .0036), and rectal (P = .0020) cancer patients.
Conclusions
Physical, emotional, and cognitive fatigue is prevalent in all 15 investigated cancer entities even 2 years after diagnosis. Fatigue in breast cancer patients, the so‐far most studied group, is in the lowest range among all entities, suggesting that the extent of fatigue is still insufficiently determined. Entity‐specific problems might need to be considered in the treatment of fatigue.
Keywords: breast cancer, cancer survivorship, cancer‐related fatigue, gastrointestinal cancer, prevalence, quality of life
The FiX study compared fatigue in 2244 cancer patients across 15 tumor entities. Fatigue was prevalent even 2 years after diagnosis across all entities, but with different manifestation. We identified entity‐specific issues to be considered in the treatment of fatigue.

1. INTRODUCTION
Cancer‐related fatigue is a frequent and burdensome symptom that has been observed across different cancer entities and therapies. 1 Fatigue can manifest in various dimensions such as physical, emotional and cognitive exhaustion. It varies in intensity as well as in the temporal course. 2 Reported prevalence of fatigue during cancer treatment ranged from 25% to 99%, and in one quarter to one third of cancer survivors fatigue persisted for up to 10 years after end of therapy. 1 , 3 So‐far, breast cancer was the most frequent entity included in studies on fatigue, 4 , 5 , 6 but there is ample evidence that other patient groups may also be in need for fatigue management. To better determine those groups, comparable information on fatigue prevalence and severity are needed. Many studies have presented fatigue prevalence within their study populations. However, comparability of these data is hindered by several aspects: (1) Heterogeneity of assessment: Clear objective measures for fatigue do not exist. Instead, a variety of questionnaires have been typically used to assess fatigue. (2) Cut‐off points: Fatigue is not a dichotomous “yes/no‐variable” but exerts its intensity on a continuous scale. Thus, fatigue prevalence depends on the respective selected cut‐off. (3) Differences in study populations: Reporting of fatigue depends on the time point of assessment in relation to cancer therapy, the type of treatment, and individual patient characteristics such as age, sex, physical, or psychological condition.
To the best of our knowledge there is only one publication that reported uniformly determined fatigue prevalence for more than four cancer entities. 7 This study enrolled 1494 patients between 2002 and 2004, from only one hospital in Germany. Other studies exist that compared only three or four cancer types. 8 , 9 , 10
Therefore, our FiX study aimed to systematically assess and compare prevalence of fatigue across the 15 most frequent cancer entities. Hereby, different time points and dimensions of fatigue were considered. Moreover fatigue scores were compared to normative values of the general German population.
2. METHODS
2.1. Study population
The FiX study (Fatigue in Germany ‐ Examination of prevalence, severity, and state of screening and treatment) recruited patients between March 2018 and May 2019 via the Epidemiological Cancer Registry of Baden‐Württemberg, Germany. Patients aged 18+ years were eligible if diagnosed with a primary tumor of following entities: stomach (C16, D00.2), colon (C18, D01.0), rectum (C19‐20/D01.1‐1.2), liver (C22/D01.5), pancreas (C25/D01.7), lung (C33‐34/D02.1‐2.2), malignant melanoma (C43/D03), breast (C50/D05), endometrium (C54.1/D07.0), ovaries/cervix (C56/C53/D06), prostate (C61), kidney (C64), bladder (C67/D09.0), non‐Hodgkin lymphoma (C82‐88), leukemia (C91‐C95). To assess fatigue in the longer run, patients were recruited about 2 years after diagnosis. A population‐based sample of the cancer registry stratified by entity was drawn in two batches. To reach proximally balanced numbers across entities, sampling of the second batch was based on the return from the first batch. As in the first batch many liver, lung, and pancreas cancer patients had died (death notices to the cancer registry were still pending at time of sampling), these entities were excluded from second sampling to reduce the emotional burden for the relatives caused by receiving letters for the deceased family member. Following data protection laws, the cancer registry sent letters to the sampled patients, asking for permission to transfer contact data to the study center. Of 11113 sampled patients, 1277 could not be reached (eg invalid/unknown address) and in 1415 cases it turned out that the patient had already died (Figure 1). Thus, 8421 patients may have been reached by the cancer registry, however, from 2976 of these patients no feedback was received whereas 2694 actively refused the transfer of contact data to the study center. Of the remaining patients, 2508 gave informed consent to participate (29.8% of the potentially reached patients). We excluded 248 patients from the prevalence analysis, due to primary cancers in multiple entities. Further 16 patients did not complete the fatigue questionnaires, leaving 2244 patients included for the final analyses.
Figure 1.
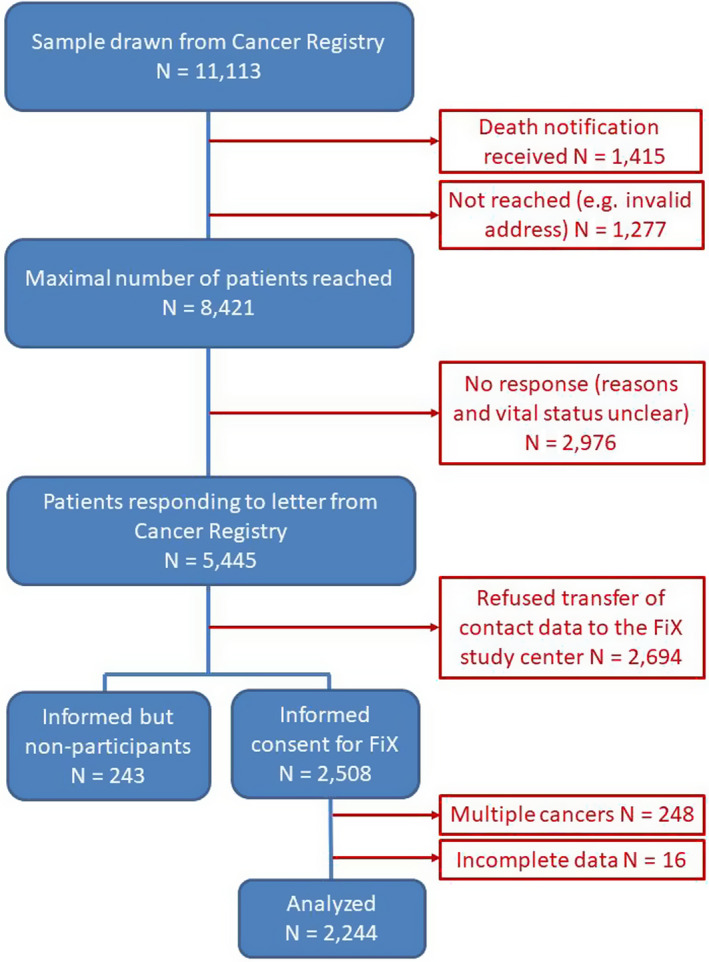
CONSORT diagram
2.2. Data collection
Fatigue was self‐reported by the patients using the EORTC QLQ‐FA12, a 12‐item, multidimensional questionnaire developed for cancer patients. 11 It covers the physical, emotional, and cognitive dimensions of fatigue and total fatigue, has good test‐retest reliability and internal consistency and been proved to identify clinically significant changes in fatigue in the course of cancer treatment. 11 , 12 Body‐mass index (BMI) was calculated from self‐reported weight and height. Cancer therapy was self‐reported and supplemented by cancer registry data. Age, sex, and cancer entity was derived from registry data. Normative values of the EORTC QLQ‐FA12 are available from 2411 individuals, representatively selected from the German general population, stratified by sex and age. 13
2.3. Statistical methods
Physical, emotional, cognitive, and total fatigue scores were derived from the EORTC QLQ‐FA12 and transformed to the range 0‐100 according to the EORTC scoring manual. Higher numbers indicate higher degrees of fatigue. Fatigue scores were compared between entities with three approaches. First, raw physical, emotional, cognitive, and total fatigue scores were presented in Box‐Whisker‐plots and compared been entities using Kruskal‐Wallis tests. Additionally, the raw fatigue scores were compared with the age‐ and sex‐matched mean scores of the general German population 13 using paired t tests. We adjusted for multiple testing across all entities using the Bonferroni‐Holm method. Secondly, age‐ and sex‐standardized prevalences were calculated. The cut‐off for abnormal high EORTC QLQ‐FA12 scores was defined by the age‐ and sex‐specific 75th percentile from the general population 13 according to Singer et al 7 Hence, we chose it to enable comparisons of our data with those previous findings. Thirdly, fatigue differences between entities adjusted for age (linear and quadratic term), sex (male, female), BMI (<18.5, 18.5‐<25, 25‐<30, 30‐<35, ≥35), surgery, chemo‐, radio‐, targeted, and endocrine therapy (never, within the last 4 weeks, more than 4 weeks ago) were explored using analyses of covariance (ANCOVA). Adjusted means enable comparison of fatigue across cancer entities irrespective of different patients' characteristics. The ANCOVA was generally explorative in nature, however, we in addition considered ANCOVA results using adjustment for multiple testing according to Dunnett‐Hsu. Since the number of missing values is below 4%, ANCOVA models were based on complete cases without missing imputations. All tests were two‐sided using 5% significance level, and SAS version 9.4.
3. RESULTS
The mean age of the study population was 65.5 years, gender was evenly distributed, and the mean BMI of 26.9 is similar to the mean BMI of the general population, and gender was evenly distributed (Table 1). Although we had aimed for about N = 200 patients per entity, the final numbers differed due to response rate and rate of the already deceased. Breast cancer was the most frequent entity (N = 230, response rate 40%, deceased 4%), whereas only 125 stomach cancer patients could be included (response rate 25%, deceased 30%).
Table 1.
Characteristics of study population (n = 2244)
| Characteristics | |
| Age at enrolment, mean (SD) | 65.6 (11.9) |
| <40 years | 51 (2.3%) |
| 40‐<50 years | 154 (6.9%) |
| 50‐<60 years | 519 (23.1%) |
| 60‐<70 years | 662 (29.5%) |
| 70‐<80 years | 620 (27.6%) |
| ≥80 years | 238 (10.6%) |
| Sex | |
| Male | 1131 (50.4%) |
| Female | 1105 (49.2%) |
| Missing | 8 (0.4%) |
| BMI (kg/m2), mean (SD) | 26.9 (5.5) |
| <18.5 | 38 (1.7%) |
| 18.5‐25 | 871 (38.8%) |
| 25‐30 | 798 (35.6%) |
| 30‐35 | 337 (15.0%) |
| ≥35 | 148 (6.6%) |
| Missing | 52 (2.3%) |
| Years since diagnosis, mean (SD) | 1.8 (0.4) |
| >1‐1.5 years | 458 (20.4%) |
| >1.5‐2 years | 1027 (45.8%) |
| >2‐2.5 years | 656 (29.2%) |
| >2.5‐3 years | 81 (3.6%) |
| >3‐3.5 years | 9 (0.4%) |
| >3.5‐5 years | 13 (0.6%) |
| Entity of cancer disease | |
| Breast | 230 (10.2%) |
| Prostate | 220 (9.8%) |
| Kidney | 206 (9.2%) |
| Non‐Hodgkin lymphoma | 204 (9.1%) |
| Rectum | 191 (8.5%) |
| Colon | 185 (8.2%) |
| Endometrium | 174 (7.8%) |
| Malignant melanoma | 166 (7.4%) |
| Leukemia | 158 (7.0%) |
| Ovaries/Cervix | 147 (6.6%) |
| Bladder | 139 (6.2%) |
| Stomach | 125 (5.6%) |
| Lung | 37 (1.6%) |
| Pancreas | 33 (1.5%) |
| Liver | 29 (1.3%) |
| Cancer treatment | |
| Chemotherapy | |
| Never | 1312 (58.5%) |
| In the past | 798 (35.6%) |
| Recent/current | 115 (5.1%) |
| Missing | 19 (0.8%) |
| Radiotherapy | |
| Never | 1620 (72.2%) |
| In the past | 587 (26.2%) |
| Recent/current | 22 (1.0%) |
| Missing | 15 (0.7%) |
| Targeted therapy | |
| Never | 1810 (80.7%) |
| In the past | 304 (13.5%) |
| Recent/current | 113 (5.0%) |
| Missing | 17 (0.8%) |
| Endocrine therapy | |
| Never | 1871 (83.4%) |
| In the past | 216 (9.6%) |
| Recent/current | 140 (6.2%) |
| Missing | 17 (0.8%) |
| Surgery | |
| Never | 379 (16.9%) |
| In the past | 1796 (80.0%) |
| Recent/current | 36 (1.6%) |
| Missing | 33 (1.5%) |
Figure 2 presents the distributions of the raw fatigue scores across the different entities. Physical (P = .0001), emotional (P = .0059), cognitive (P = .036), and total (P = .0002) fatigue scores differed significantly between entities. The median (Q1, Q3) physical fatigue ranged from 26.7 (13.3, 53.3) in prostate cancer patients to 46.7 (26.7, 66.7) and 46.7 (26.7, 73.3) in stomach and pancreas cancer patients, respectively. Emotional and total fatigue levels were also lowest among prostate cancer patients. Mean physical fatigue levels were significantly higher than age‐ and sex‐matched means of the general population for all cancer entities (paired t test, all Bonferroni‐Holm adjusted P < .01). Mean emotional fatigue levels were significantly higher for all entities except liver cancer (paired t test, Bonferroni‐Holm adjusted P = .16), and mean cognitive fatigue for all entities except pancreas (Bonferroni‐Holm adjusted P = .059), leukemia (P = .059), liver (P = .18), lung (P = .18), and malignant melanoma (P = .18). The cognitive fatigue score reported in the general population is predominantly zero, indicating no cognitive exhaustion (80% of male and 78% of female of age 60+). Cognitive fatigue was also rated as zero for over 50% of cancer patients. Since the cognitive fatigue score (based on only 2 items) is not very distinctive, no further analyses are presented for the cognitive fatigue dimension. The distributions of physical, emotional as well as total fatigue are presented for sex and age subgroups in Tables [Link], [Link], [Link]. The mean fatigue values and differences from age‐ and sex‐matched normative values are presented by entity in Table S4.
Figure 2.
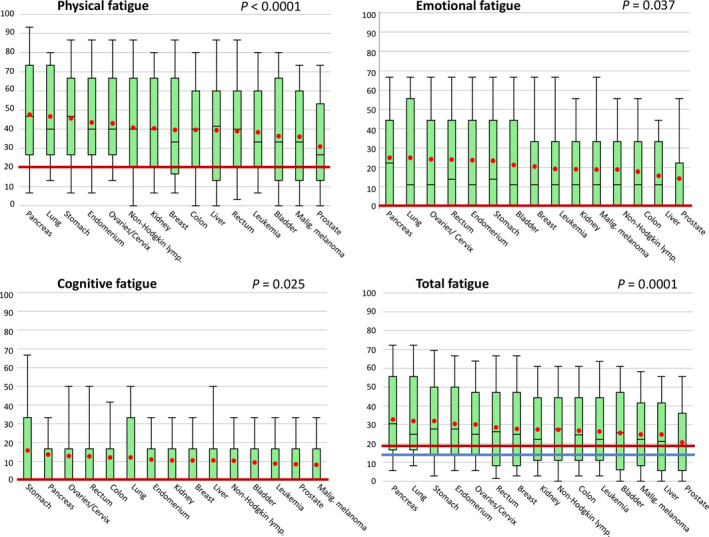
Box‐Whisker plots of raw physical, emotional, cognitive, and total fatigue scores. Boxes represents 25th to 75th percentiles with middle line in box at median, Whisker ends at 10th and 90th percentiles; red dots represent mean values. The median normative fatigue values of the German population of age 60+ years are presented by a blue line for males, and red line for females or both sexes, if median identical
Age‐ and sex‐standardized fatigue prevalence (Table 2) differs across tumor entities (physical fatigue P Chi = 0.092, emotional P Chi = 0.0038, total fatigue P Chi = 0.089). For physical fatigue, prevalence ranged from 31.8% (95% CI: 25.6%‐38.0%) among prostate cancer patients to 51.7% (33.5%‐69.9%) among liver cancer patients.
Table 2.
Age‐ and sex‐standardized prevalence a of different fatigue dimensions by tumor entity
| Entity b | N | Prevalence (%) with 95% confidence interval | ||
|---|---|---|---|---|
| Physical fatigue | Emotional fatigue | Total fatigue | ||
| Liver | 29 | 51.7 (33.5, 69.9) | 34.5 (17.2, 51.8) | 48.3 (30.1, 66.5) |
| Stomach | 121 | 51.6 (42.8, 60.5) | 45.5 (36.6, 54.3) | 50.0 (41.1, 58.9) |
| Lung | 35 | 51.4 (34.9, 68.0) | 40.0 (23.8, 56.2) | 45.7 (29.2, 62.2) |
| Pancreas | 33 | 48.5 (31.4, 65.5) | 48.5 (31.4, 65.5) | 54.5 (37.6, 71.5) |
| Kidney | 205 | 45.4 (38.6, 52.2) | 38.0 (31.4, 44.7) | 42.9 (36.2, 49.7) |
| Non‐Hodgkin lymph. | 203 | 45.3 (38.5, 52.2) | 41.9 (35.1, 48.7) | 45.3 (38.5, 52.2) |
| Rectum | 188 | 45.3 (38.2, 52.3) | 45.3 (38.2, 52.3) | 46.8 (39.7, 53.9) |
| Colon | 182 | 44.0 (36.7, 51.2) | 34.1 (27.2, 41.0) | 45.1 (37.8, 52.3) |
| Ovaries/Cervix | 145 | 42.8 (34.7, 50.8) | 35.2 (27.4, 42.9) | 44.8 (36.7, 52.9) |
| Malignant melanoma | 162 | 42.0 (34.4, 49.6) | 33.3 (26.1, 40.6) | 40.7 (33.2, 48.3) |
| Endometrium | 172 | 41.9 (34.5, 49.2) | 36.3 (29.1, 43.5) | 47.1 (39.6, 54.6) |
| Leukemia | 157 | 39.9 (32.2, 47.5) | 36.7 (29.2, 44.2) | 44.9 (37.2, 52.7) |
| Breast | 228 | 39.9 (33.6, 46.3) | 31.0 (25.0, 37.0) | 41.0 (34.7, 47.4) |
| Bladder | 138 | 38.4 (30.3, 46.5) | 49.3 (40.9, 57.6) | 44.9 (36.6, 53.2) |
| Prostate | 217 | 31.8 (25.6, 38.0) | 29.7 (23.6, 35.7) | 31.1 (24.9, 37.2) |
Patients with scores above the age‐ and sex‐specific 75% percentile of the general German population are considered fatigued.
Sorted by prevalence of physical fatigue.
Multivariate, adjusted ANCOVA analyses are summarized in Table 3. Breast cancer patients showed the lowest adjusted mean physical fatigue values, and compared to them the levels in stomach (P = .0004), lung (P = .034), kidney (P = .0011), pancreas (P = .081), and endometrium (P = .022) were markedly higher. When adjusting for multiple testing, the differences between stomach and kidney cancer compared to breast cancer still remained statistically significant. Adjusted means of emotional as well as total fatigue were also lowest for breast cancer and highest for stomach cancer. Bladder and rectum cancer patients reported significantly higher emotional fatigue, and renal cancer patients significantly higher total fatigue than breast cancer patients.
Table 3.
Physical, emotional, and total fatigue adjusted by age, sex, BMI and cancer treatment
| Entity a | Physical fatigue | Emotional fatigue | Total fatigue | |||
|---|---|---|---|---|---|---|
| Adjusted means (95% CI) | P (difference to breast cancer) | Adjusted means (95% CI) | P (difference to breast cancer) | Adjusted means (95% CI) | P (difference to breast cancer) | |
| Stomach | 69.4 (61.6, 77.2) | 0.0004 * | 42.4 (35.0, 49.9) | 0.0047 * | 50.0 (43.5, 56.4) | 0.0013 * |
| Lung | 66.9 (55.8, 78.0) | 0.034 | 39.5 (28.9, 50.1) | 0.13 | 46.9 (37.7, 56.0) | 0.10 |
| Kidney | 66.6 (59.4, 73.8) | 0.0011 * | 40.7 (33.9, 47.6) | 0.0069 | 47.8 (41.9, 53.7) | 0.0038 * |
| Pancreas | 65.0 (53.5, 76.5) | 0.081 | 37.1 (26.1, 48.1) | 0.31 | 45.9 (36.4, 55.4) | 0.16 |
| Endometrium | 62.7 (55.3, 70.1) | 0.022 | 37.7 (30.6, 44.8) | 0.056 | 44.7 (38.6, 50.8) | 0.052 |
| Liver | 61.9 (50.4, 73.4) | 0.23 | 33.3 (22.3, 44.3) | 0.72 | 41.8 (32.3, 51.3) | 0.58 |
| Leukemia | 61.7 (53.9, 69.4) | 0.10 | 39.1 (31.7, 46.6) | 0.056 | 44.5 (38.1, 50.9) | 0.13 |
| Ovaries/Cervix | 61.5 (53.8, 69.1) | 0.079 | 38.2 (30.9, 45.5) | 0.063 | 43.5 (37.2, 49.8) | 0.17 |
| Colon | 61.5 (54.2, 68.8) | 0.07 | 36.6 (29.6, 43.6) | 0.14 | 43.9 (37.8, 49.9) | 0.12 |
| Bladder | 60.8 (53.2, 68.4) | 0.12 | 42.4 (35.1, 49.6) | 0.0036 * | 44.8 (38.5, 51.1) | 0.082 |
| Rectum | 60.7 (53.6, 67.7) | 0.097 | 41.9 (35.1, 48.6) | 0.0020 * | 45.4 (39.5, 51.2) | 0.034 |
| Malignant melanoma | 60.4 (53.1, 67.8) | 0.12 | 38.4 (31.3, 45.4) | 0.046 | 43.1 (37.0, 49.2) | 0.19 |
| Non‐Hodgkin lymphoma | 59.2 (52.5, 66.0) | 0.22 | 35.5 (29.1, 41.9) | 0.23 | 41.8 (36.2, 47.3) | 0.38 |
| Prostate | 55.8 (48.9, 62.8) | 0.76 | 36.2 (29.6, 42.8) | 0.14 | 40.6 (34.9, 46.3) | 0.59 |
| Breast | 54.8 (47.8, 61.7) | Ref. | 31.3 (24.7, 37.9) | Ref. | 39.1 (33.4, 44.8) | Ref. |
Significant differences to breast cancer (P < .05) are marked as bold values.
Abbreviations: BMI, Body mass index; CI, Confidence interval.
Ordered by adjusted means of physical fatigue.
Significant (P < .05) after Dunnett‐Hsu adjustment for multiple testing.
4. DISCUSSION
The Fix study systematically assessed and compared fatigue prevalence and severity for 15 cancer entities among 2,244 cancer patients, differentiating by dimension of fatigue. For all entities, physical fatigue scores in cancer patients 2 years after diagnosis were significantly higher than in the general German population, and for the vast majority of entities significant differences were also seen for emotional and cognitive fatigue. Yet, our results indicated clear differences between entities. Physical fatigue prevalence ranged from 31.8% among prostate to 51.7% among liver cancer patients. Differences between entities were not fully explained by sex, age, BMI, or type and timing of cancer therapy, as after adjusting for those factors fatigue still differed significantly among tumor entities, with highest adjusted mean levels of physical, emotional, and total fatigue in stomach and lowest levels in breast cancer patients.
The considerable observed physical fatigue prevalence of 40% among breast cancer patients about 2 years after diagnosis indicates that an effective fatigue management and treatment is not yet established. The prevalence is in similar magnitude as published from another study with comparable prevalence calculation (36%). 7 Compared to breast cancer, fatigue values were higher in most other investigated entities. Thus, improvements in fatigue management might be needed even more urgently for patients with other types of cancer. Given the fact that most research on cancer‐related fatigue so far has been conducted with breast cancer patients, the scope of the problem and potential therapies likely have not yet been fully explored. Likewise, randomized controlled trials considering the so‐far promising treatment approaches for fatigue, that is, physical exercise, yoga or other mind‐body exercise, and psychosocial interventions such as cognitive behavioral therapy or mindfulness‐based stress reduction, have predominantly included breast cancer patients. 14 , 15 , 16 , 17 , 18 , 19 Overall, there is convincing evidence that physical activity and exercise is beneficial for breast cancer patients in the adjuvant setting. Survivors of (non‐metastasized) breast cancer often have less functional restrictions than patients with other tumor entities, are known to frequently use rehabilitation and continue to engage in physical activities. Thus, future research should focus on patients with cancers of other entities where evidence on fatigue therapies is weak.
One reason that adjusted physical fatigue levels were significantly higher among patients with stomach, lung, pancreas, and kidney cancer compared to breast cancer might be the specific course of disease: Since we collected data concerning fatigue approximately 2 years after diagnosis, for these cancers with poorer prognosis it is conceivable that patients are more likely to be in worse condition due to disease progression and might have already received several lines of therapy until this time point. This in return has been shown to be associated with increased fatigue levels. 20 However, also entity specific factors may need to be taken into consideration, as discussed in the following.
Stomach cancer patients showed high physical and emotional fatigue about 2 years after diagnosis. A study investigating 374 disease‐free stomach cancer patients also found a high fatigue prevalence of 51.3% (determined as global BFI score of ≥4). 21 Stomach cancer patient may suffer from postgastrectomy syndrome, which can result in malnutrition, loss of skeletal muscle mass, and anemia, and thus contribute to physical fatigue. Similar problems can also arise after pancreatic cancer, and likewise might contribute to the reported high physical fatigue levels.
Renal and lung cancer was associated with high physical fatigue, too. This is possibly caused by tyrosine kinase inhibitors (TKIs) used for therapy of renal cell carcinoma and non‐small cell lung cancer as fatigue has been shown to be a major side effect of several TKIs. 22 , 23 , 24
Furthermore, after adjusting for the different treatment modalities and patient characteristics, physical fatigue levels were significantly higher among endometrial compared to breast cancer patients. Although the difference failed statistical significance after adjusting for multiple testing (P = .17), it might be worth considering that fatigue in endometrial cancer has been found to be associated with menopausal symptoms, which are a common consequence of the surgical procedures for this type of cancer that result in estrogen deficiency. 25 Thus, hormonal pathways may be more relevant in fatigue etiology with this type of cancer than in many other cancer types.
Surprisingly, bladder cancer patients had the highest prevalence of emotional fatigue (49%) although prevalence for physical fatigue (38%) was among the lowest of all considered entities. The adjusted mean emotional fatigue was significantly higher than among breast cancer patients. A recent review found that bladder cancer often suffer from depression and anxiety, 26 possibly because social participation and emotional well‐being are impacted by incontinence, frequent and painful urination, embarrassment, and fear of catheter insertion or removal. 27 Similar problems might contribute to the high adjusted mean emotional fatigue among rectal cancer patients who often suffer from stool or urinary incontinence and partly may need a stoma, which can impact mental health. 28 , 29
Overall, all potential causes or contributing factors for fatigue may need more attention regarding the management of fatigue. Up‐to‐date, recommendations usually do not differentiate by entity or individual patients' and treatment characteristics. Likewise, intervention studies typically follow a one‐fits‐all approach. However, fatigue management and treatment may need to be more individualized, for example, taking entity‐specific problems, cancer therapy, nutritional status, physical condition, and psycho‐social factors into account. Additionally, our results showing that fatigue is prevalent even 2 years after diagnosis across all investigated entities underlines the need to integrate the recommendations for a systematic fatigue management into long‐term aftercare.
Limitations and strengths of our study need to be considered. We cannot exclude a selection bias due to the limited response rate, which could result in (1) underestimation, as patients with fatigue might have been too exhausted to participate, as well as in (2) overestimation as patients without fatigue might not have been interested to participate, because they were not affected by this problem. However, low response was also caused by the formal two‐step procedure required by legislation for data protection issues. The first step, that is, asking by postal mail for patient's consent to transfer his or her contact data to the study center, was a major hurdle: 31% of contacted patients did not agree. Furthermore, from 36% of contacted patients—especially with cancer entities that tend to have worse prognosis—the cancer registry received no response at all. It can be speculated that one major reason for nonresponse may have been poor health status or being already in a palliative situation. However, as advanced tumor stage, the presence of metastases, and a poorer performance status have been shown previously to be associated with higher fatigue levels, 30 our study finding of differential fatigue across cancer entities, with low fatigue among breast cancer and highest fatigue among stomach cancer, might be rather conservative than biased into a false direction. This was supported by sensitivity analyses estimating the “true” fatigue prevalence under different scenarios, for example, assuming a high fatigue prevalence of 60% among non‐participants. Overall, although bias due to low response rate cannot be excluded, we believe that the study data still yields reliable and valuable results. Yet, for lung, pancreas, and liver cancer the results should be interpreted with caution due to low sample size. Furthermore, we had only limited data on metastases. Thus, we could not stratify fatigue prevalence by disease stage. Moreover we got feedback from several contacted persons or their relatives that participation was declined due to poor physical or mental health, for example, dementia. Patients with insufficient German language skills also could not participate. Cognitive fatigue might be underestimated, since patients with severe cognitive fatigue may have been less willing or able to participate. Moreover to limit the length of the survey, we did not collect information on other potential confounders such as physical activity or social support. Strengths of the study include the systematic and comparable assessment of fatigue across a variety of common cancer entities (albeit with limited sample size for some entities), the systematic, representative sampling via a population‐based statewide cancer registry, the relatively high sample size, and the consideration of different fatigue dimensions.
5. CONCLUSIONS
Our study among cancer patients showed that physical, emotional, and cognitive fatigue is prevalent in all 15 investigated entities even approximately 2 years after diagnosis. Yet, there are differences between entities, which are not solely attributable to differences in sex, age, BMI, and cancer therapies. For most cancer types, fatigue levels were above those of breast cancer patients—the latter being the group investigated most in respect to fatigue so far. Thus, the extent of this burdensome problem is probably still insufficiently determined and recognized.
CONFLICT OF INTEREST
None.
AUTHOR CONTRIBUTIONS
Martina Schmidt contributed to conceptualization, supervision, formal analysis, and writing—original draft. Silke Hermann contributed to resources and writing—review and editing. Volker Arndt contributed to resources and writing—review and editing. Karen Steindorf contributed to conceptualization and writing—review and editing.
COMPLIANCE WITH ETHICAL STANDARDS
This study was conducted in accordance with the ethical standards of the Helsinki Declaration. The study was approved by the Ethic Committee of the Medical Faculty of the University of Heidelberg. All patients have given written informed consent.
Supporting information
Table S1
Table S2
Table S3
Table S4
ACKNOWLEDGMENTS
The authors thank Sabine Holzmeier for data management and support of study conduct, and Susanne Bergbold from the Epidemiological Cancer Registry for supporting the recruitment and data extraction. Furthermore, the authors thank Dagmar Schuldt and Gudrun Wöhr from the Trust Center of the Cancer Registry of Baden‐Württemberg for requesting informed consent from the patient sample and providing us with contact information of those that agreed to this data transferal.
Schmidt ME, Hermann S, Arndt V, Steindorf K. Prevalence and severity of long‐term physical, emotional, and cognitive fatigue across 15 different cancer entities. Cancer Med. 2020;9:8053–8061. 10.1002/cam4.3413
Funding information
No external funding.
DATA AVAILABILITY STATEMENT
Data available on request due to privacy/ethical restrictions.
REFERENCES
- 1. Hofman M, Ryan JL, Figueroa‐Moseley CD, Jean‐Pierre P, Morrow GR. Cancer‐related fatigue: the scale of the problem. Oncologist. 2007;12(Suppl 1):4‐10. [DOI] [PubMed] [Google Scholar]
- 2. Schmidt ME, Chang‐Claude J, Vrieling A, Heinz J, Flesch‐Janys D, Steindorf K. Fatigue and quality of life in breast cancer survivors: temporal courses and long‐term pattern. J Cancer Surviv. 2012;6(1):11‐19. [DOI] [PubMed] [Google Scholar]
- 3. Bower JE. Cancer‐related fatigue–mechanisms, risk factors, and treatments. Nature Reviews Clinical Oncology. 2014;11(10):597‐609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mustian KM, Alfano CM, Heckler C, et al. Comparison of pharmaceutical, psychological, and exercise treatments for cancer‐related fatigue: a meta‐analysis. JAMA Oncology. 2017;3(7):961‐968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Network NCC . NCCN Clinical Practice Guidelines in Oncology: cancer‐related fatigue, version 2.2018. 2018.
- 6. Wu C, Zheng Y, Duan Y, et al. Nonpharmacological interventions for cancer‐related fatigue: a systematic review and Bayesian network meta‐analysis. Worldviews Evid Based Nurs. 2019;16(2):102‐110. [DOI] [PubMed] [Google Scholar]
- 7. Singer S, Kuhnt S, Zwerenz R, et al. Age‐ and sex‐standardised prevalence rates of fatigue in a large hospital‐based sample of cancer patients. Br J Cancer. 2011;105(3):445‐451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jones JM, Olson K, Catton P, et al. Cancer‐related fatigue and associated disability in post‐treatment cancer survivors. J Cancer Surviv. 2016;10(1):51‐61. [DOI] [PubMed] [Google Scholar]
- 9. Wang XS, Zhao F, Fisch MJ, et al. Prevalence and characteristics of moderate to severe fatigue: a multicenter study in cancer patients and survivors. Cancer. 2014;120(3):425‐432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tan SY, Turner J, Kerin‐Ayres K, et al. Health concerns of cancer survivors after primary anti‐cancer treatment. Support Care Cancer. 2019;27(10):3739‐3747. [DOI] [PubMed] [Google Scholar]
- 11. Weis J, Tomaszewski KA, Hammerlid E, et al. International psychometric validation of an EORTC quality of life module measuring cancer related fatigue (EORTC QLQ‐FA12). J Natl Cancer Inst. 2017;109(5):1‐8. 10.1093/jnci/djw273 [DOI] [PubMed] [Google Scholar]
- 12. Weis J, Wirtz MA, Tomaszewski KA, et al. Sensitivity to change of the EORTC quality of life module measuring cancer‐related fatigue (EORTC QlQ‐Fa12): results from the international psychometric validation. Psycho‐oncology. 2019;28(8):1753‐1761. [DOI] [PubMed] [Google Scholar]
- 13. Hinz A, Weis J, Brahler E, Mehnert A. Fatigue in the general population: German normative values of the EORTC QLQ‐FA12. Qual Life Res. 2018;27(10):2681‐2689. [DOI] [PubMed] [Google Scholar]
- 14. Furmaniak AC, Menig M, Markes MH. Exercise for women receiving adjuvant therapy for breast cancer. Cochrane Database Syst Rev. 2016;9:CD005001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cramer H, Lauche R, Klose P, Lange S, Langhorst J, Dobos GJ. Yoga for improving health‐related quality of life, mental health and cancer‐related symptoms in women diagnosed with breast cancer. Cochrane Database Syst Rev. 2017;1:CD010802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cobeanu O, David D. Alleviation of side effects and distress in breast cancer patients by cognitive‐behavioral interventions: a systematic review and meta‐analysis. J Clin Psychol Med Settings. 2018;25(4):335‐355. [DOI] [PubMed] [Google Scholar]
- 17. Schell LK, Monsef I, Wockel A, Skoetz N. Mindfulness‐based stress reduction for women diagnosed with breast cancer. Cochrane Database Syst Rev. 2019;3:CD011518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hilfiker R, Meichtry A, Eicher M, et al. Exercise and other non‐pharmaceutical interventions for cancer‐related fatigue in patients during or after cancer treatment: a systematic review incorporating an indirect‐comparisons meta‐analysis. Br J Sports Med. 2018;52(10):651‐658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Campbell KL, Winters‐stone KM, Wiskemann J, et al. Exercise guidelines for cancer survivors: consensus statement from international multidisciplinary roundtable. Med Sci Sports Exerc. 2019;51(11):2375‐2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zabernigg A, Giesinger JM, Pall G, et al. Quality of life across chemotherapy lines in patients with cancers of the pancreas and biliary tract. BMC Cancer. 2012;12:390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hwang IC, Yun YH, Kim Y‐W, et al. Factors related to clinically relevant fatigue in disease‐free stomach cancer survivors and expectation‐outcome consistency. Support Care Cancer. 2014;22(6):1453‐1460. [DOI] [PubMed] [Google Scholar]
- 22. Zhao F, Cella D, Manola J, DiPaola RS, Wagner LI, Haas NSB. Fatigue among patients with renal cell carcinoma receiving adjuvant sunitinib or sorafenib: patient‐reported outcomes of ECOG‐ACRIN E2805 trial. Support Care Cancer. 2018;26(6):1889‐1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ghatalia P, Je Y, Nguyen PL, Trinh QD, Choueiri TK, Sonpavde G. Fatigue with vascular endothelial growth factor receptor tyrosine kinase inhibitors and mammalian target of rapamycin inhibitors in patients with renal cell carcinoma (RCC) and other malignancies: a meta‐analysis of randomized clinical trials. Crit Rev Oncol Hematol. 2015;95(2):251‐263. [DOI] [PubMed] [Google Scholar]
- 24. Li J, Sun W. Fatigue with epidermal growth factor receptor tyrosine kinase inhibitors in cancer patients: a meta‐analysis of randomized controlled trials. J Chemother. 2018;30(6–8):323‐331. [DOI] [PubMed] [Google Scholar]
- 25. Li CC, Tsai YF, Chang TC, Chen L. Associations among menopausal symptoms, sleep and fatigue in Taiwanese women with endometrial cancer. Eur J Cancer Care (Engl). 2017;26(5):e12559. [DOI] [PubMed] [Google Scholar]
- 26. Pham H, Torres H, Sharma P. Mental health implications in bladder cancer patients: a review. Urol Oncol. 2019;37(2):97‐107. [DOI] [PubMed] [Google Scholar]
- 27. Van Hemelrijck M, Sparano F, Josephs D, Sprangers M, Cottone F, Efficace F. Patient‐reported outcomes in randomised clinical trials of bladder cancer: an updated systematic review. BMC Urol. 2019;19(1):86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nasvall P, Dahlstrand U, Lowenmark T, Rutegard J, Gunnarsson U, Strigard K. Quality of life in patients with a permanent stoma after rectal cancer surgery. Qual Life Res. 2017;26(1):55‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Downing A, Glaser AW, Finan PJ, et al. Functional outcomes and health‐related quality of life after curative treatment for rectal cancer: a population‐level study in England. Int J Radiat Oncol Biol Phys. 2019;103(5):1132‐1142. [DOI] [PubMed] [Google Scholar]
- 30. Hinz A, Weis J, Brahler E, Harter M, Geue K, Ernst J. Fatigue in cancer patients: comparison with the general population and prognostic factors. Support Care Cancer. 2020;28(9):4517‐4526. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Table S2
Table S3
Table S4
Data Availability Statement
Data available on request due to privacy/ethical restrictions.


