Editor—Several recommendations have been written for the management of acute respiratory distress syndrome (ARDS) over the last decade. The latest ones issued from a French group1 suggest individualising positive end-expiratory pressure (PEEP) settings, reserving higher PEEP for patients in whom oxygenation improves without deterioration of respiratory system compliance (CRS) or haemodynamic status. In contrast, coronavirus 2019 (COVID-19) guidelines2 suggest using higher PEEP (>10 cm H2O), although the risk of haemodynamic deterioration is not mentioned. However, it has been suggested that a significant proportion of intubated COVID-19 patients respond poorly to recruitment,3 and some experts have pointed out the risks of haemodynamic deterioration and congestion from the beginning of the pandemic in these patients.4 COVID-19 ARDS can be associated with an increase in intrapulmonary shunt fraction (Qs/Qt) related to abnormal pulmonary vasodilation and increased perfusion in some lung areas5 that might be related to endothelial and vascular dysfunction.6 Thus, although arterial oxygenation may improve through alveolar recruitment, Qs/Qt reduction as a result of the decrease in cardiac output caused by higher PEEP7 may be another mechanism to consider. Some studies have suggested that haemodynamic deterioration may occur when higher PEEP is used in COVID-19 ARDS,8 , 9 but the consequences on cardiac output and thus oxygen delivery (DaO2) have not been evaluated.
This single-centre retrospective study aimed to evaluate the haemodynamic impact of PEEP increase in COVID-19 ARDS and its consequences on DaO2. The study was conducted in a 36-bed ICU (Hôpital Lariboisière, Paris, France). Medical records of patients admitted between March 14, 2020 and April 22, 2020 were reviewed. Inclusion criteria were: ICU admission for respiratory failure, diagnosis of ARDS according to the Berlin criteria, laboratory confirmed SARS-CoV-2 infection, and at least one PEEP trial with cardiac output monitoring. The primary endpoints were the consequences of increasing PEEP on oxygenation, haemodynamics and oxygen delivery, and respiratory mechanics. The secondary endpoint was the association between higher PEEP (>10 cm H2O) and improvement of oxygenation and oxygen delivery. This study was approved by the Institutional Review Board (IRB-00006477) of HUPNVS, Paris 7 University.
Patients were managed according to our previously described local protocol10 based on the latest guidelines.1 The PEEP trial protocol is described in the Supplementary data. The effect of PEEP level on the dependent variables was tested in linear mixed-models with PEEP as a fixed effect and PEEP trial nested by patient as random effects to deal with the fact that a patient may have had multiple PEEP trials, and that a PEEP trial includes more than one PEEP level. The proportions of patients who benefited from higher PEEP (>10 cm H2O) were compared with a χ2 test. Statistical analyses were performed using R statistical software version 3.6.1 (R Core Team, 2019, R Foundation for Statistical Computing, Vienna, Austria, https://www.R-project.org). A p-value <0.05 was considered significant.
Of 89 patients admitted to our ICU during the study period with a diagnosis of COVID-19 ARDS, 30 patients met inclusion criteria and were analysed (age 61 [54–59] yr, sex ratio M/F 2.3, BMI 28 [26–31]kg m−2). The median number of PEEP trials performed per patient was two (one to five). The median time from ICU admission was 6 (3–9) days.
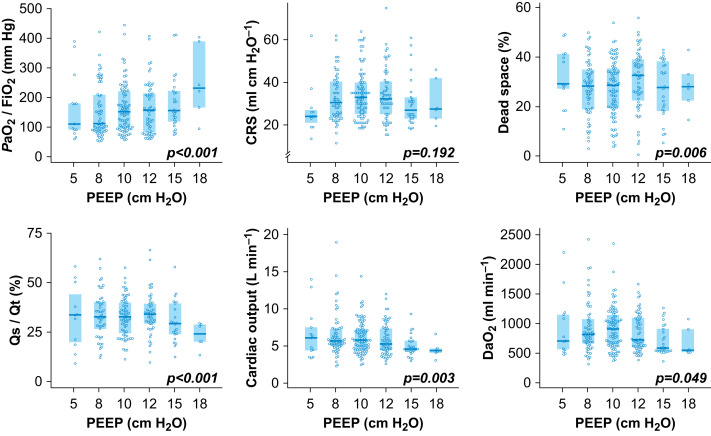
The effects of increasing PEEP levels on respiratory mechanics, oxygenation, haemodynamic status, and oxygen delivery are presented in Figure 1 . Increased PEEP was associated with an increase in Pao 2/FiO2 (P<0.001) and a decrease in Qs/Qt (P<0.001), without changes in CRS (P=0.192) but with decreases in cardiac output (P=0.003) and DaO2 (P=0.049). For each cm H2O of PEEP increase, Pao 2/FiO2 increased by 10 mm Hg (95% confidence interval [CI] 8–13) and Qs/Qt decreased by −1% (95% CI −1 to −2), but cardiac output and DaO2 decreased by −92 ml min−1 (95% CI −152 to −33) and −8.5 ml min−1 (95% CI −17.1 to 0.1), respectively (results for all variables in Supplementary Table S2). In our cohort, 43% of PEEP trials were associated with a positive response to higher PEEP (10 cm H2O) for Pao 2/FiO2. For DaO2, the positive response to higher PEEP decreased to 27% (P=0.031).
Fig 1.
Effect of PEEP level on oxygenation, respiratory system compliance, dead space, intrapulmonary shunt, cardiac output, and oxygen delivery. CRS, respiratory system compliance; DaO2, arterial oxygen delivery; Pao2/FiO2, arterial oxygen partial pressure (Pao2) to fraction of inspired oxygen (FiO2) ratio with FiO2=1; Qs/Qt, intrapulmonary shunt. The values are presented in Supplementary Table S1.
We showed that higher PEEP was associated with an increase in Pao 2/FiO2 ratio without improvement in oxygen delivery because of a decrease in cardiac output. When haemodynamic status and oxygen delivery were considered, most patients did not benefit from higher PEEP (>10 cm H2O). Interestingly, higher PEEP had a poor impact on CRS, and therefore was unlikely to have prevented ventilation-induced lung injury.
Higher Pao 2/FiO2 ratio associated with higher PEEP does not only rely on alveolar recruitment and improvement of lung mechanics. The correction of the ventilation-perfusion mismatch, which may result partly from reduced Qs/Qt associated with reduced cardiac output, may contribute to the improvement of arterial oxygenation.7 This observation is in line with a report of high interindividual variability of potential for lung recruitment,3 and ventilation–perfusion mismatch likely related to blood flow redistribution rather than non-ventilated units.9 The relative contribution of increased pleural pressure and increased transpulmonary pressure with PEEP increase, resulting from decreased right ventricle preload and increased right ventricular afterload (eventually leading to the decrease in cardiac output), were not specifically evaluated in this study. Their co-existence has been suggested by others as well.8
Even though this is a small study with potential selection bias, our data suggest that interpretation of results of PEEP titration in COVID-19 ARDS should not rely only on Pao 2/FiO2. CRS and cardiac output should be considered simultaneously to identify the patient-centred effect of PEEP level on alveolar recruitment and haemodynamic effect. When haemodynamic effect is preeminent, the apparent increase in Pao 2/FiO2 may not be associated with more oxygen delivery to the patient.
In COVID-19 ARDS, higher PEEP may lead to a decrease in cardiac output without increases in DaO2, despite an increase in Pao 2/FiO2. Higher PEEP could be unbeneficial to a significant proportion of patients. These results require a cautious and multimodal approach including cardiac output monitoring when using higher PEEP.
Authors' contributions
Designed the study: RBa, VB, RBo, BGC
Participated in the collection of data: RBa, VB, RBo, BGC, MC, ALG, AH
Performed the statistics: RBa, VB, RBo, BGC
Participated in interpretation of the data: RBa, VB, RBo, BGC, EG, AM
Participated in drafting of the manuscript: RBa, VB, RBo, BGC, MC, ALG, AH, EG, AM
Read and approved the final manuscript: all authors
Declarations of interest
RBa reports non-financial support from Vygon and from Getinge France. EG received lecture fees from Edwards Lifescience and research grants from Philips and Radiometer. AM received speaker's honoraria from Novartis, Orion, and Servier and fees as a member of the advisory board, steering committee, or both from Adrenomed, Sanofi, Roche, Abbott, and 4TEEN4. BGC received fees as a member of an advisory board from Roche Diagnostics. The other authors declare that they have no conflicts of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bja.2020.10.026.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Papazian L., Aubron C., Brochard L. Formal guidelines: management of acute respiratory distress syndrome. Ann Intensive Care. 2019;9:69. doi: 10.1186/s13613-019-0540-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alhazzani W., Møller M.H., Arabi Y.M. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19) Intensive Care Med. 2020;46:854–887. doi: 10.1007/s00134-020-06022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pan C., Chen L., Lu C. Lung recruitability in COVID-19-associated acute respiratory distress syndrome: a single-center observational study. Am J Respir Crit Care Med. 2020;201:1294–1297. doi: 10.1164/rccm.202003-0527LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gattinoni L., Coppola S., Cressoni M., Busana M., Rossi S., Chiumello D. COVID-19 does not lead to a ‘typical’ acute respiratory distress syndrome. Am J Respir Crit Care Med. 2020;201:1299–1300. doi: 10.1164/rccm.202003-0817LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lang M., Som A., Mendoza D.P. Hypoxaemia related to COVID-19: vascular and perfusion abnormalities on dual-energy CT. The Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30367-4. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Varga Z., Flammer A.J., Steiger P. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suter P.M., Fairley B., Isenberg M.D. Optimum end-expiratory airway pressure in patients with acute pulmonary failure. N Engl J Med. 1975;292:284–289. doi: 10.1056/NEJM197502062920604. [DOI] [PubMed] [Google Scholar]
- 8.Tsolaki V., Zakynthinos G.E., Makris D. The ARDSnet protocol may be detrimental in COVID-19. Crit Care. 2020;24:351. doi: 10.1186/s13054-020-03081-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mauri T., Spinelli E., Scotti E. Potential for lung recruitment and ventilation-perfusion mismatch in patients with the acute respiratory distress syndrome from coronavirus disease 2019. Crit Care Med. 2020;48:1129–1134. doi: 10.1097/CCM.0000000000004386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barthélémy R., Blot P.-L., Tiepolo A. Efficacy of almitrine in the treatment of hypoxemia in Sars-Cov-2 acute respiratory distress syndrome. Chest. 2020;158:2003–2006. doi: 10.1016/j.chest.2020.05.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.