Abstract
Background
Symptomatic urinary tract infection (UTI) is the most common infectious complication in renal transplant recipients (RTRs). Fosfomycin (FOS) is an attractive alternative for prophylaxis because it does not interact with immunosuppressants; although 90% is excreted unchanged in the urine, it does not require adjustment for renal function for single dose prophylaxis.
Methods
RTRs were recruited into this randomized, double-blind, placebo-controlled trial. Participants were randomized (1:1) to receive one 4 g dose of FOS disodium intravenously 3 h (FOS group) or placebo (placebo group) before placement and removal of a urinary catheter and before removal of a double-J ureteral stent. All participants received prophylaxis with trimethoprim/sulfamethoxazole. The main outcome was a comparison of the mean number of symptomatic UTI and asymptomatic bacteriuria (AB) episodes per patient during a 7-week follow-up period. The study was registered at ClinicalTrials.gov, NTC03235947.
Results
Eighty-two participants were included (41 in the FOS group and 41 in placebo group). The mean number of AB or symptomatic UTI episodes per patient was lower in the FOS group [intention-to-treat (ITT) 0.29 versus 0.60, P = 0.04]. The incidence of symptomatic UTI was lower in the FOS group (ITT, 7.3% versus 36.6%, P = 0.001), and there was no difference in the incidence of AB between both groups. The incidence of adverse events was similar in both groups.
Conclusions
FOS addition is an effective and safe strategy to reduce the number of symptomatic UTIs during the first 7 weeks after renal transplant.
Keywords: fosfomycin, kidney transplantation, prophylaxis, urinary tract infection
INTRODUCTION
Symptomatic urinary tract infection (UTI) is the most common infectious complication in renal transplant recipients (RTRs) during the first year, with a prevalence between 7% and 80% [1, 2]. Asymptomatic bacteriuria (AB) and symptomatic UTI are associated with graft pyelonephritis, sepsis, acute rejection and long-term graft dysfunction [3–5]. Recently, recurrent symptomatic UTI episodes have been associated with worse patient and graft survival compared with the absence of recurrent infection [6]. New guidelines recommend prophylaxis with trimethoprim/sulfamethoxazole (TMP/SMX) with the aim of decreasing symptomatic UTI and bacteremia episodes in this population [7].
In our tertiary care center, the incidence of symptomatic UTIs in RTRs has remained between 30% and 36% during the first year after renal transplant (RT) [8]; this incidence is partially explained by a high rate (>80%) of resistance to TMP/SMX in clinical isolates of Escherichia coli recovered from urine. High rates of resistance to TMP/SMX in urine isolates from RTRs are a worldwide problem, since it has been reported in other centers [9, 10]. Therefore, we thought it necessary to implement new prophylaxis strategies for procedures associated with an increased risk of symptomatic UTI in the early post-RT period. Our group reported a median time of 7 days after RT to development of the first symptomatic UTI episode [11]. Although a causal relationship could not be defined, it is conceivable that infectious episodes were associated with manipulation and the presence of devices in the urinary tract [12].
Fosfomycin (FOS) is an antibiotic active against most enterobacteria isolated from the urine of RTRs, even multidrug-resistant (MDR) bacteria [10]. FOS has a unique mechanism of action, different from other antibiotics, and lacks a mechanism of cross-resistance. Moreover, FOS efficacy has been confirmed in several clinical trials as an effective prophylactic agent in urological procedures in the general population [13]. Thus, our aim was to evaluate the efficacy and safety of FOS in preventing episodes of symptomatic UTI and AB in RTRs compared with standard TMP/SMX prophylaxis during the first 7 weeks after RT.
MATERIALS AND METHODS
We conducted a randomized, double-blind, placebo-controlled clinical trial between September 2016 and November 2017 at a tertiary care center in Mexico City. We included RTRs (living or deceased donor) >18 years of age, excluding those with a known allergy to TMP/SMX or FOS. Once the study started, subjects who retained the urinary catheter (UC) and the double-J ureteral stent (DJS) >15 and 30 days after RT, respectively, were eliminated. The study was evaluated and approved by the local Ethics and Research Committees (Institutional Review Board ref. 1649). Informed consent was obtained from each subject under the Declaration of Helsinki, and the study was registered at ClinicalTrials.gov, NTC03235947.
Randomization and blinding
Participants were randomized to receive FOS (FOS group) or placebo (placebo group). An external group of collaborators oversaw participant randomization through an online software program assigning participants to each group at a 1:1 ratio and in sets of four, stratified by gender.
The institutional pharmacy confidentially secured the randomization sheet and the group to which each participant was assigned. Bags of 0.9% saline solution (100 mL) labeled with the number of each subject were used, and when the assignment corresponded to the experimental arm, a 4 g FOS disodium flask was diluted in the solution bag using a sterile technique. In the end, the appearance of the infusion bags of both groups was indistinguishable, and the bags were given to the nursing staff for administration. The medical, nursing and laboratory staff, along with the statistical analyst and patients, were blinded during the study.
Intervention and follow-up
Each FOS group member received one 4 g dose of FOS disodium intravenously (IV) 3 h before the following procedures: placement of the UC immediately before the RT surgery, removal of the UC and removal of the DJS. The placebo group received saline solution 0.9% IV with the same scheme.
All RTRs received cephalothin (1 g, IV single dose) for prophylaxis before RT surgery; the UC was placed using a sterile technique, and the patients received a DJS. The type of ureteral reimplantation (extra or intravesical) was decided by the surgical team.
All participants received TMP/SMX prophylaxis at 160/800 mg (PO) daily when the estimated glomerular filtration rate, calculated according to the formula proposed by the Chronic Kidney Disease (CKD) Epidemiology Collaboration [14], was ≥ 30 mL/min/1.73 m2. It was defined as the date of DJS removal since Day 14 and UC removal since Day 2.
RTRs with high immunological risk (presence of preformed donor-specific antibodies, deceased donor or retransplant) received induction with antithymocyte globulin (Thymoglobulin®, Sanofi-Genzyme, Lyon, France) at a dose of 4.5 mg/kg divided into three daily doses given on consecutive days. Low-risk RTRs received induction with 20 mg basiliximab (Simulect®, Novartis, Basel, Switzerland) on Days 0 and 4 after RT. Immunosuppression maintenance consisted of tacrolimus, mycophenolic acid and prednisone. All RTRs were assessed at Weeks 1, 4, 6 and 7 after RT through questioning, physical examination, evaluation of FOS- or TMP/SMX-related adverse events, a TMP/SMX tablet count to assess adherence, and midstream urine culture, in addition to an assessment of the immunosuppressive treatment for RT.
We obtained urine cultures in the following order: the first at 48 h after UC removal; the second before DJS removal, between Days 14 and 30; and the third, fourth and fifth on Days 28, 42 and 49 after RT, respectively. Additional urine cultures were performed for subjects with symptoms or signs of symptomatic UTI; in those taking antibiotics at the time of the visit, the urine culture was deferred until the end of antibiotic treatment. RTRs with AB or symptomatic UTI were treated according to antimicrobial susceptibility for 7 or 14 days, respectively. AB was treated following the recommendations of the latest clinical guidelines during the first 3 months post-RT [7, 15].
Clinical definitions
We defined significant bacteriuria in women as two consecutive urine cultures with the same clinical isolate and in men as a single urine culture with a count of ≥105 colony-forming units per milliliter (CFU/mL). AB was defined as the presence of significant bacteriuria without clinical manifestations. We considered symptomatic UTI any symptomatic episode (dysuria, frequency, bladder tenesmus, suprapubic pain and/or fever) plus a positive urine culture with ≥105 CFU/mL. Acute pyelonephritis was defined as fever, positive urine culture with ≥105 CFU/mL, with or without bacteremia, greater graft pain or urinary symptoms [16].
We considered bacteriological cure in the absence of the clinical isolate recovered from the urine culture prior to treatment. We defined MDR isolates as those resistant to at least one agent from three or more families of antibiotics [17].
RTRs requiring dialysis during the first week after RT were considered as cases with delayed graft function. We defined symptomatic UTI-related hospitalization as any episode of symptomatic UTI warranting hospital management or hospitalization for another reason along with simultaneous symptomatic UTI development.
Microbiological methods
The urine cultures were processed in the first hour after being obtained [11].
Outcomes
The primary outcome was a comparison of the mean number of symptomatic UTI or AB episodes per patient in both treatment groups during the first 7 weeks after RT. We chose the total number of episodes (new and recurrent) because recurrent episodes are associated with worse outcomes than single episodes [3, 6].
Secondary outcomes were the time to the first symptomatic UTI or AB episode; the incidence of symptomatic UTI, AB, pyelonephritis, bacteremia, symptomatic UTI hospitalization, Clostridioides difficile infection and MDR bacterial infection. Safety outcomes were adverse events related to volume overload, hematological and gastrointestinal events, incidence of acute rejection, graft loss and death.
Sample size
The mean number of episodes per patient in the first 7 weeks after RT was 0.83 episodes/patient during the year prior to the study. With the hypothesis of a reduction to 0.40 episodes per patient in the experimental arm, using the comparison of means formula with a power of 80% and a type I error of 0.05, we calculated 40 subjects per treatment arm; assuming a 2% loss to follow-up, we estimated a total sample size of 82 subjects. An intermediate analysis to evaluate safety and efficacy was scheduled once half of the estimated sample size of patients was enrolled.
Statistical analysis
All included and randomized RTRs were assessed via intention-to-treat (ITT) analysis, and the subjects who strictly and completely adhered to the intervention were analyzed per protocol (PP). The following descriptive statistics were used: relative frequencies and percentages for categorical variables, in addition to mean and SD or median and interquartile range (IQR); and minimum and maximum values for continuous variables according to their distribution. For comparison between groups, a Chi-squared test or Fisher’s exact test was used as appropriate. Continuous variables with normal and nonnormal distribution were analyzed with Student’s t-test and Mann–Whitney U test, respectively. Time-dependent analyses are presented by Kaplan–Meier curves, and the results were compared using the log-rank method. A two-tailed P < 0.05 was considered significant for all analyses. The STATA version 13 statistical package (College Station, TX, USA) was used.
RESULTS
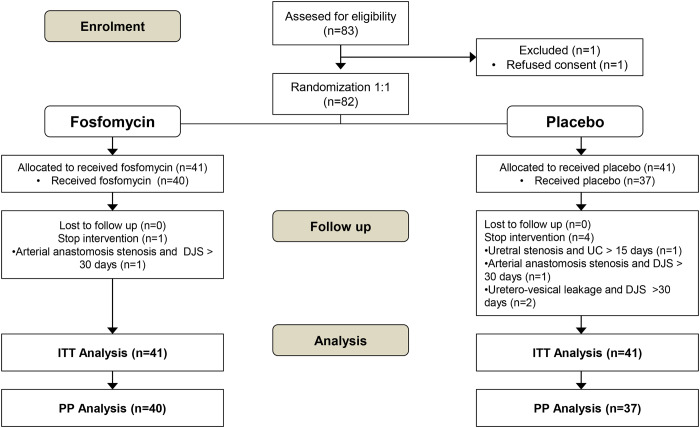
We invited 83 potentially eligible subjects to participate in the study, and only one declined. A total of 82 subjects were randomized in two groups: FOS group (n = 41) and placebo group (n = 41). Five subjects were eliminated after randomization; the reasons for elimination and patient flow are described in Figure 1. Therefore, for the PP analysis, 40 subjects were included in the FOS group and 37 subjects in the placebo group. There were no losses to follow-up.
FIGURE 1.
Patient flow diagram.
The mean age of the participants was 41 years (range: 19–75 years), and 63.4% were men. Overall, the median time for UC removal was 3 days (range 2–8 days), the initiation of TMP/SMX prophylaxis was 4 days (range 1–19 days) and the duration of DJS was 22 days (range 14–30 days); differences in these parameters were not observed between the two groups. Other baseline conditions of the population and comparisons between groups are shown in Table 1.
Table 1.
Baseline population characteristics at time of transplant (ITT population)
| Characteristic | Global (n = 82) | Fosfomycin (n = 41) | Placebo (n = 41) | P-value |
|---|---|---|---|---|
| Age, mean (SD), years | 41 ± 15 | 41.9 ± 16 | 40.2 ± 14 | 0.6 |
| Women, n (%) | 30 (36.6) | 14 (34) | 16 (39) | 0.64 |
| Cause of CKD, n (%) | ||||
| Unknown | 46 (56.1) | 20 (48.8) | 26 (63.4) | 0.3 |
| Diabetic nephropathy | 15 (18.3) | 9 (21.9) | 6 (14.6) | – |
| Lupus nephritis | 5 (6.1) | 4 (9.8) | 1 (2.4) | – |
| Polycystic kidney | 5 (6.1) | 4 (9.8) | 1 (2.4) | – |
| Glomerulonephritis | 4 (4.9) | 1 (2.4) | 3 (7.3) | – |
| Other | 7 (8.5) | 3 (7.3) | 4 (9.8) | – |
| Diabetes mellitus before RT, n (%) | 18 (21.9) | 10 (24.3) | 8 (19.5) | 0.59 |
| Previous renal transplantation, n (%) | 10 (12.2) | 2 (4.8) | 8 (19.5) | 0.088 |
| Months on dialysis, median (IQR) | 32 (18–48) | 24 (12–48) | 36 (24–72) | 0.069 |
| Donor age, mean (SD), years | 40.8 ± 14 | 43.1 ± 13.7 | 38.5 ± 14 | 0.13 |
| Deceased donor, n (%) | 44 (53.7) | 23 (56.1) | 21 (51.2) | 0.65 |
| Cold ischemia time, median (IQR), min | 750 (46–1200) | 840 (44–1378) | 720 (49–992) | 0.29 |
| Donor-specific antibodies before RT, n (%) | 27 (32.9) | 19 (46.3) | 8 (19.5) | 0.01 |
| Thymoglobulin induction, n (%) | 56 (68.3) | 31 (75.6) | 25 (60.9) | 0.15 |
| High risk for CMV, n (%) | 8 (9.8) | 4 (9.8) | 4 (9.8) | 1.0 |
| Days of hospital stay for RT surgery, median (min–max) | 7 (5–21) | 7 (5–21) | 7 (5–15) | 0.84 |
| Delayed graft function, n (%) | 7 (8.5) | 2 (8.5) | 5 (12.2) | 0.43 |
| Days of UC use, median (min–max) | 3 (2–8) | 3 (2–6) | 3 (2–8) | 0.53 |
| Days of prophylaxis initiation with TMP/SMX, median (min–max) | 4 (1–19) | 5 (1–17) | 4 (2–19) | 0.058 |
| Days of DJS use, median (min–max), days | 22 (14–30) | 22 (14–30) | 22 (15–29) | 0.894 |
CMV, cytomegalovirus; min, minimum; max, maximum.
Outcomes
Among the entire cohort, 28 (34%) RTRs presented AB or symptomatic UTI as the outcome, and among these 28 patients, 19 (68%) had a single infectious episode [8 (42%) in the FOS group and 11 (58%) in the placebo group] and 9 (32%) had recurrent events [2 (22%) in the FOS group and 7 (78%) in the placebo group]. We identified 37 infectious episodes (16 AB and 21 symptomatic UTI), corresponding to an incidence of 0.45 episodes/patient. The median time to the first episode of AB or UTI was 25.5 (4–45) days in the entire cohort, 25 (4–33) days in the FOS group and 25.5 (5–45) days in the placebo group. Some clinical and microbiological data of the episodes of symptomatic UTI occurred during the study period are described in more detail in Supplementary data, Tables S1 and S2.
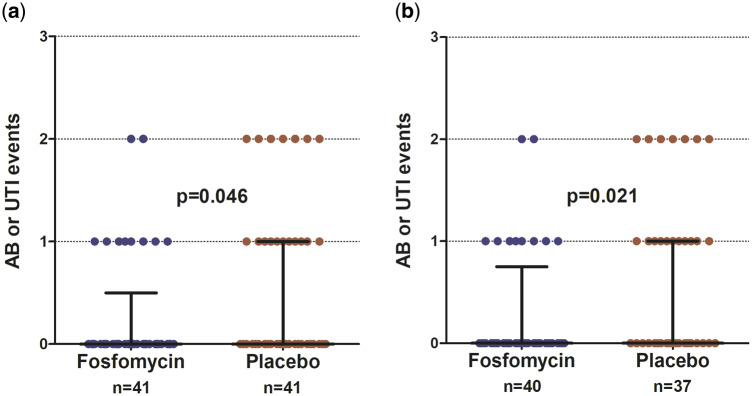
The mean number of AB or symptomatic UTI episodes per patient was significantly lower in the FOS group than in the placebo group (ITT, 0.29 episodes/patient versus 0.60 episodes/patient, P = 0.04; PP, 0.3 episodes/patient versus 0.67 episodes/patient, P = 0.02) (Figure 2).
FIGURE 2.
Number of episodes of AB or symptomatic UTI by intervention group, FOS versus placebo. (a) ITT analysis and (b) PP analysis.
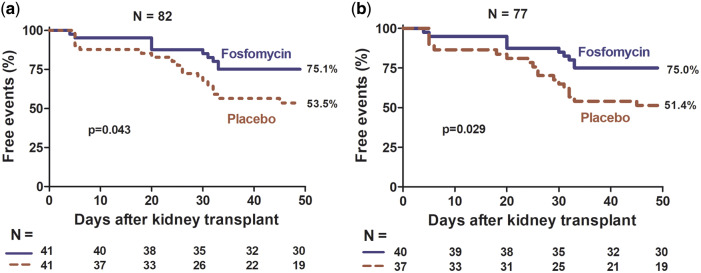
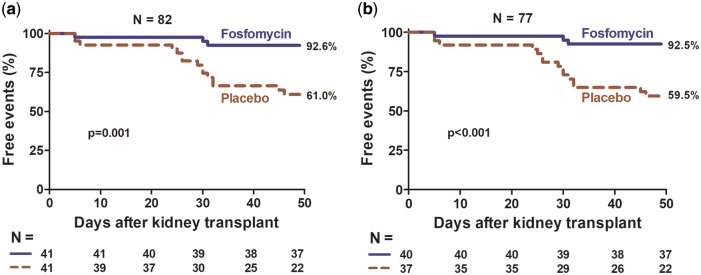
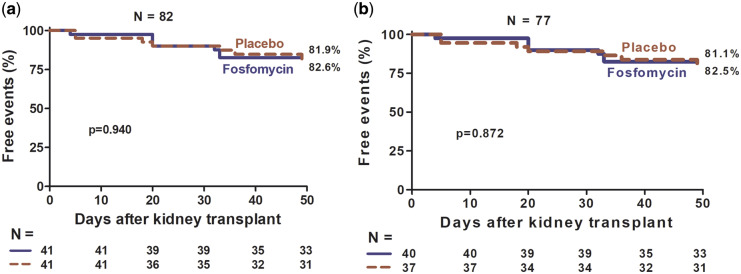
The FOS group had more event-free time (AB and symptomatic UTI) than the placebo group (ITT, P = 0.043; PP, P = 0.029, Figure 3), and this difference was at the expense of a longer symptomatic UTI-free time (ITT, P = 0.001; PP, P < 0.001, Figure 4); there was no difference in AB-free time (ITT, P = 0.940; PP, P = 0.872, Figure 5). The incidence of symptomatic UTI in the entire study population was 21.9% and was significantly lower in the FOS group (ITT, 7.3% versus 36.6%, P = 0.001; PP, 7.5% versus 40.5%, P = 0.001). The incidence of AB in the entire population was 17%, with no difference between the two groups (ITT, 17%, versus 17%, P = 1; PP, 17.5% versus 18.9%, P = 0.87).
FIGURE 3.
Percentage of participants free of AB or symptomatic UTI by intervention group, FOS versus placebo. (a) ITT analysis and (b) PP analysis.
FIGURE 4.
Percentage of participants free of symptomatic UTI by intervention group, FOS versus placebo. (a) ITT analysis and (b) PP analysis.
FIGURE 5.
Percentage of participants free of AB by intervention group, FOS versus placebo. (a) ITT analysis and (b) PP analysis.
The incidence of symptomatic UTI hospitalization in the entire cohort was 9.8%, with a lower incidence in the FOS group (ITT, 2.4% versus 17.1%, P = 0.057; PP, 2.5% versus 18.9%, P = 0.025).
There were seven cases of acute pyelonephritis (overall incidence of 8.5%), with a lower tendency in the FOS group (ITT, 2.4% versus 14.5%, P = 0.1; PP, 2.5% versus 16.2%, P = 0.051). For the other outcomes, see Table 2.
Table 2.
Primary and secondary outcomes
| Outcomes | ITT analysis |
PP analysis |
||||||
|---|---|---|---|---|---|---|---|---|
| Global (n = 82) | FOS (n = 41) | Placebo (n = 41) | P-value | Global (n = 77) | FOS (n = 40) | Placebo (n = 37) | P-value | |
| Number of AB and symptomatic UTI episodes (mean episodes/patient) | 37 (0.45) | 12 (0.29) | 25 (0.60) | 0.044 | 37 (0.48) | 12 (0.3) | 25 (0.67) | 0.02 |
| Incidence of symptomatic UTI, n (%) | 18 (21.9) | 3 (7.3) | 15 (36.6) | 0.001 | 18 (23.8) | 3 (7.5) | 15 (40.5) | 0.001 |
| Incidence of AB, n (%) | 14 (17) | 7 (17) | 7 (17) | 1 | 14 (18) | 7 (17.5) | 7 (18.9) | 0.87 |
| Incidence of pyelonephritis, n (%) | 7 (8.5) | 1 (2.4) | 6 (14.6) | 0.1 | 7 (9) | 1 (2.5) | 6 (16.2) | 0.051 |
| Incidence of bacteremia, n (%) | 2 (2.4) | – | 2 (4.9) | 0.49 | 2 (2.6) | – | 2 (5.4) | 0.22 |
| Incidence of symptomatic UTI hospitalization, n (%) | 8 (9.8) | 1 (2.4) | 7 (17.1) | 0.057 | 8 (10.3) | 1 (2.5) | 7 (18.9) | 0.025 |
| Incidence of infection with MDR bacteria, n (%) | 15 (18.3) | 6 (14.6) | 9 (21.9) | 0.36 | 15 (19.5) | 6 (15) | 9 (24.3) | 0.3 |
Adverse events
There were no cases of graft loss or death. Adherence to standard TMP/SMX prophylaxis was 98.7% in the FOS group and 96.6% in the placebo group (P = 0.22). There were eight cases of diarrhea, six in the FOS group and two in the placebo group (P = 0.20); two cases were caused by C. difficile (one in each group) and were resolved with oral vancomycin for 7 days. There were two episodes of volume overload; one subject in the FOS group had pulmonary edema and respiratory acidosis in the postoperative period, which were improved with noninvasive mechanical ventilation; one subject in the control group had pericardial effusion without hemodynamic compromise, which was improved after fluid restriction. The rest of the adverse events are described in Table 3.
Table 3.
Adverse events observed
| Adverse events | Fosfomycin (n = 41) | Placebo (n = 41) | P-value |
|---|---|---|---|
| Volume overload, n (%) | 1 (2.4) | 1 (2.4) | 1 |
| Clostridioides difficile infection, n (%) | 1 (2.4) | 1 (2.4) | 1 |
| Leukopenia, n (%) | 9 (21.9) | 6 (14.6) | 0.39 |
| Hyperkalemia, n (%) | 1 (2.4) | – | 1 |
| Hypertransaminasemia, n (%) | – | 1 (2.4) | 1 |
| Diarrhea, n (%) | 6 (14.6) | 2 (4.8) | 1 |
| Nausea, n (%) | 2 (4.8) | 2 (4.8) | 1 |
| Disruption of anastomosis of the urinary tract, n (%) | – | 2 (4.8) | 0.49 |
| Renal artery stenosis, n (%) | 1 (2.4) | 1 (2.4) | 1 |
| Infection of the surgical wound, n (%) | – | 1 (2.4) | 1 |
| Gastrointestinal bleeding, n (%) | 1 (2.4) | – | – |
Microbiological findings
The antimicrobial susceptibility of all isolates to FOS was 72% (FOS group 62% and placebo group 77%, P = 0.45), and susceptibility to TMP/SMX was 5% (FOS group 8% and placebo group 4%, P = 1), see Supplementary data.
We recovered 19 (48.7%) extended-spectrum β-lactamase (ESBL)-producing isolates in the entire cohort (6/13 in the FOS group and 13/26 in the placebo group, P = 0.82).
Ten of the 15 E. coli isolates (66.7%) were ESBL-producing isolates, which were distributed equally between the groups (2/3 in the FOS group and 8/12 in the placebo group, P = 1). All ESBL-producing and -nonproducing E. coli isolates were susceptible to FOS, and only one isolate from the placebo group was susceptible to TMP/SMX. Nine of the 15 Klebsiella spp. isolates were ESBL-producing isolates (60%), 4/5 in the FOS group and 5/10 in the placebo group (P = 0.26). Among these, three isolates from the FOS group and three from the placebo group were sensitive to FOS; no ESBL-producing Klebsiella spp. isolate was susceptible to TMP/SMX.
DISCUSSION
The results of this controlled clinical trial show that FOS administration before urinary tract procedures (transplant surgery, UC removal and DJS removal) in addition to standard TMP/SMX prophylaxis reduces the incidence of symptomatic UTI (P < 0.001), the incidence of symptomatic UTI hospitalization (P = 0.025) and the incidence of pyelonephritis (P = 0.051), although without statistical significance in the latter. In a previous study, we identified that the highest incidence of symptomatic UTI in RTRs occurred during the first week, and we observed a close association between symptomatic UTI occurrence and manipulation events and the presence of devices (UC and DJS) in the urinary tract [11]. Therefore, we conducted this new clinical trial, which showed that the standard prophylaxis recommended by international guidelines does not prevent symptomatic UTI after RT in individuals during the period of greatest risk [7]. Thus, as part of the management of these patients, we administered FOS before urinary tract manipulation events during the first 7 weeks after RT and obtained favorable results.
The tip of the UC is colonized even though it remains in place for only a short time, and its removal can be accompanied by the release of bacteria [18]. In the general population, it is estimated that the risk of symptomatic UTI increases from 3% to 7% per day of UC use; in RTRs, duration longer than 7 days is a risk factor [5, 19]. In a meta-analysis in the nontransplant population (n = 1 520), an absolute reduction of 5.8% in the number of symptomatic UTI episodes was observed in subjects receiving prophylaxis at the time of UC removal; in addition, the subgroup of individuals who underwent surgery received the greatest benefit [20].
Additionally, DJS has been associated with an increased risk of symptomatic UTI; in a recent clinical trial comparing early DJS removal without cystoscopy versus late removal with cystoscopy in RTRs (5 days versus 6 weeks after RT, respectively), the early removal group showed a lower incidence of UTI (7.6% versus 24.6%) in the PP analysis, although in the ITT analysis only a slight difference was observed [21], without significant statistical value.
Recently, Bonkat et al. [22] published a brief report about sonication of the removed DJS to detect early colonization possibly associated with symptomatic UTI; however, we did not sonicate DJS. In fact, as Bonkat et al. pointed out, the sonication procedure seemed to be a poor diagnostic tool since the microorganisms recovered were Gram-positives and only one of them would have a pathologic significance in that population (Enterococcus spp.). In addition, they delayed the removal of DJS (median time 6.3 weeks), which facilitates colonization.
There are no clinical trials assessing the use of antimicrobials before DJS removal in RTRs. A recent retrospective study reported that antimicrobial prophylaxis did not decrease the incidence of symptomatic UTI after DJS removal [23]. However, the guidelines of the American Society of Urology recommend antimicrobial prophylaxis before cystourethroscopy in subjects with risk factors such as urinary tract abnormalities or concomitant immunosuppressive therapy [24]. Therefore, we believe antimicrobial prophylaxis is necessary before DJS removal in RTRs when there is a history of recent urological surgery and patients under immunosuppressive therapy.
In RTRs, there is no consensus regarding the indication for perioperative prophylaxis; some reports suggest that ceftriaxone may be effective in reducing the number of UTI episodes [25]. However, this strategy is associated with the appearance of ESBL-producing bacteria and may not be effective in patients from institutions with high rates of resistance [25, 26].
A major concern regarding symptomatic UTI episodes in RTRs is a change in the susceptibility pattern of the causative organisms. In a report by Origüen et al. [10], FOS was the only antibiotic that maintained activity against most enterobacteria that cause symptomatic UTI, with susceptibility rates between 85% and 90% over 11 years. In our cohort, susceptibility to FOS was 72% and that to TMP/SMX was only 5%, supporting the success of this strategy.
Strikingly, the experimental strategy did not impact the incidence and the clinical significance of AB at the end of follow-up; it is possible that AB could correspond to an independent phenomenon that cannot be modified with antimicrobial prophylaxis. We included episodes of AB as part of the composite outcome because the latest guidelines of clinical practice of the American Society of Transplantation recommend treating such episodes during the first 8 weeks after RT [7]. However, our findings do not seem to support the idea that AB is associated with adverse long-term outcomes [7, 27].
The incidence of adverse events was similar in both groups, with an incidence of C. difficile infection of 2.4%, very similar to that in a recent report (5.7% in the treatment group versus 8.5% in the control group, P = 0.72) [15]. However, our study has insufficient power to conclude that FOS prophylaxis does not increase the risk of C. difficile infection.
Finally, one limitation of this study is the short follow-up time, which focused on monitoring infectious episodes related to urinary tract manipulation during the first 7 weeks after RT. However, we acknowledge that immunosuppression is powerful during the first 90 days after RT, and thus, there is an increased risk of infections. On the other hand, we cannot generalize our results to RT centers where DJS placement is not part of the routine, because in these patients a low incidence of symptomatic UTI might be expected due to less manipulation of the urinary tract. Another limitation might be the lack of availability of IV FOS in some countries. Our group preferred to use IV FOS presentation to ensure adherence and avoid risks associated with the oral route prior to anesthesia procedures (surgery and DJS removal).
In conclusion, adding IV FOS to the standard TMP/SMX prophylaxis strategy at the time of manipulation of the urinary tract is an effective and safe procedure to reduce the number of UTI episodes within the first 7 weeks after renal transplantation.
SUPPLEMENTARY DATA
Supplementary data are available at ndt online.
FUNDING
This study was conducted with the support of Laboratorios Senosiain S.A. de C.V., Mexico. J.S.-O. has received research grants from Senosiain, Pfizer, Merck Sharp & Dhome, Sanofi Pasteur, AstraZeneca and BioMérieux. L.E.M.-B has received personal fees from Sanofi Aventis, Boehringer Ingelheim, AstraZeneca, Lilly, Servier and Novartis, and a travel grant from Sanofi Aventis. F.T.R.-C. has received consulting and lecture fees from Janssen, Bayer and Ferring, and a travel grant from Ipsen. The results presented in this article have not been published previously in whole or part, except in abstract form.
AUTHORS’ CONTRIBUTIONS
The study design was performed by R.R.-C., L.E.M.-B., J.M.A.-G. and J.S.-O. Study conduct was done by R.R.-C., I.P.-A., R.C.-M., F.T.R.-C., E.C.-M., E.M.-F., C.K.-O and J.S.-O. Data collection was carried out by R.R.-C., I.P.-A., J.T.-M. and M.T.-M. Data interpretation was done by R.R.-C., I.P.-A., L.E.M.-B. and J.S.-O. J.S.-O was responsible for the integrity of the data analysis. Qualified academic investigators may request participant-level, de-identified clinical data and supporting documents pertaining to this study by request to the corresponding author.
CONFLICT OF INTEREST STATEMENT
None declared.
Supplementary Material
REFERENCES
- 1. Alangaden GJ, Thyagarajan R, Gruber SA et al. Infectious complications after kidney transplantation: current epidemiology and associated risk factors. Clin Transplant 2006; 20: 401–409 [DOI] [PubMed] [Google Scholar]
- 2. Hollyer I, Ison MG. The challenge of urinary tract infections in renal transplant recipients. Transpl Infect Dis 2018; 20: e12828. [DOI] [PubMed] [Google Scholar]
- 3. Fiorante S, Lopez-Medrano F, Lizasoain M et al. Systematic screening and treatment of asymptomatic bacteriuria in renal transplant recipients. Kidney Int 2010; 78: 774–781 [DOI] [PubMed] [Google Scholar]
- 4. Golebiewska JE, Debska-Slizien A, Rutkowski B. Treated asymptomatic bacteriuria during first year after renal transplantation. Transpl Infect Dis 2014; 16: 605–615 [DOI] [PubMed] [Google Scholar]
- 5. Lee JR, Bang H, Dadhania D et al. Independent risk factors for urinary tract infection and for subsequent bacteremia or acute cellular rejection: a single-center report of 1166 kidney allograft recipients. Transplantation 2013; 96: 732–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Britt NS, Hagopian JC, Brennan DC et al. Effects of recurrent urinary tract infections on graft and patient outcomes after kidney transplantation. Nephrol Dial Transplant 2017; 32: 1758–1766 [DOI] [PubMed] [Google Scholar]
- 7. Goldman JD, Julian K. Urinary tract infections in solid organ transplant recipients: guidelines from the American Society of Transplantation infectious diseases community of practice. Clin Transplant 2019; 33: e13507. [DOI] [PubMed] [Google Scholar]
- 8. Sorto R, Irizar SS, Delgadillo G et al. Risk factors for urinary tract infections during the first year after kidney transplantation. Transplant Proc 2010; 42: 280–281 [DOI] [PubMed] [Google Scholar]
- 9. Singh R, Bemelman FJ, Hodiamont CJ et al. The impact of trimethoprim-sulfamethoxazole as Pneumocystis jiroveci pneumonia prophylaxis on the occurrence of asymptomatic bacteriuria and urinary tract infections among renal allograft recipients: a retrospective before-after study. BMC Infect Dis 2016; 16: 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Origüen J, Fernandez-Ruiz M, Lopez-Medrano F et al. Progressive increase of resistance in Enterobacteriaceae urinary isolates from kidney transplant recipients over the past decade: narrowing of the therapeutic options. Transpl Infect Dis 2016; 18: 575–584 [DOI] [PubMed] [Google Scholar]
- 11. Arreola-Guerra JM, Rosado-Canto R, Alberu J et al. Fosfomycin trometamol in the prophylaxis of post-kidney transplant urinary tract infection: a controlled, randomized clinical trial. Transpl Infect Dis 2018; 20: e12980. [DOI] [PubMed] [Google Scholar]
- 12. Figueroa-Sánchez GE, Arreola-Guerra JM, Morales-Buenrostro LE. Time of presentation and antimicrobial resistance pattern of urinary tract infection in the early period after kidney transplantation. Rev Mex Trasplant 2016; 5: 20–26 [Google Scholar]
- 13. Wagenlehner FM, Thomas PM, Naber KG. Fosfomycin trometamol (3,000 mg) in perioperative antibiotic prophylaxis of healthcare-associated infections after endourological interventions: a narrative review. Urol Int 2014; 92: 125. [DOI] [PubMed] [Google Scholar]
- 14. Levey AS, Stevens LA, Schmid CH et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Origuen J, Lopez-Medrano F, Fernandez-Ruiz M et al. Should asymptomatic bacteriuria be systematically treated in kidney transplant recipients? Results from a randomized controlled trial. Am J Transplant 2016; 16: 2943–2953 [DOI] [PubMed] [Google Scholar]
- 16. Nicolle LE, Bradley S, Colgan R et al. Infectious diseases society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clin Infect Dis 2005; 40: 643–654 [DOI] [PubMed] [Google Scholar]
- 17. Magiorakos AP, Srinivasan A, Carey RB et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 2012; 18: 268–281 [DOI] [PubMed] [Google Scholar]
- 18. Wolters HH, Palmes D, Lordugin E et al. Antibiotic prophylaxis at urinary catheter removal prevents urinary tract infection after kidney transplantation. Transplant Proc 2014; 46: 3463–3465 [DOI] [PubMed] [Google Scholar]
- 19. Lo E, Nicolle L, Classen D et al. Strategies to prevent catheter-associated urinary tract infections in acute care hospitals. Infect Control Hosp Epidemiol 2008; 29: S41–S50 [DOI] [PubMed] [Google Scholar]
- 20. Marschall J, Carpenter CR, Fowler S et al. Antibiotic prophylaxis for urinary tract infections after removal of urinary catheter: meta-analysis. BMJ 2013; 346: f3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Patel P, Rebollo-Mesa I, Ryan E et al. Prophylactic ureteric stents in renal transplant recipients: a multicenter randomized controlled trial of early versus late removal. Am J Transplant 2017; 17: 2129–2138 [DOI] [PubMed] [Google Scholar]
- 22. Bonkat G, Rieken M, Siegel FP et al. Microbial ureteral stent colonization in renal transplant recipients: frequency and influence on the short-time functional outcome. Transpl Infect Dis 2012; 14: 57–63 [DOI] [PubMed] [Google Scholar]
- 23. Gregg JR, Kang CL, Talbot TR et al. Symptomatic urinary tract infections in renal transplant recipients after cystoscopy for ureteral stent removal. Urol Pract 2017; 4: 405–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wolf JS Jr, Bennett CJ, Dmochowski RR et al. Best practice policy statement on urologic surgery antimicrobial prophylaxis. J Urol 2008; 179: 1379–1390 [DOI] [PubMed] [Google Scholar]
- 25. Capocasale E, de Vecchi E, Mazzoni MP et al. Surgical site and early urinary tract infections in 1000 kidney transplants with antimicrobial perioperative prophylaxis. Transplant Proc 2014; 46: 3455–3458 [DOI] [PubMed] [Google Scholar]
- 26. Pouladfar G, Jafarpour Z, Hosseini SA et al. Antibiotic selective pressure and development of bacterial resistance detected in bacteriuria following kidney transplantation. Transplant Proc 2015; 47: 1131–1135 [DOI] [PubMed] [Google Scholar]
- 27. Coussement J, Scemla A, Abramowicz D et al. Antibiotics for asymptomatic bacteriuria in kidney transplant recipients. Cochrane Database Syst Rev 2018; 2: CD011357. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.