Abstract
Introduction
People undergoing maintenance dialysis are at high risk for fractures, but less is known about fracture incidence and associated outcomes in earlier stages of chronic kidney disease (CKD).
Methods
We conducted an observational analysis from the Stockholm Creatinine Measurement project, a Swedish health care utilization cohort during 2006–11. We identified all adults with confirmed CKD Stages 3–5 and no documented history of fractures and extracted information on comorbid history, ongoing medication, cardiovascular events and death. We studied incidence rates of fractures (overall and by location), with the estimated glomerular filtration rate (eGFR) as time-dependent exposure. We then studied hazard ratios [HRs and 95% confidence intervals (CIs)] for the events of death and major adverse cardiac events (MACE) using Cox regression with fracture as time-varying exposure.
Results
We identified 68 764 individuals with confirmed CKD (mean age 79 years, 56% women). During a median follow-up of 2.7 years, 9219 fractures occurred, of which 3105 were hip fractures. A more severe CKD stage was associated with a higher risk of fractures, particularly hip fractures: compared with CKD Stage 3a, the adjusted HR was 1.10 (95% CI 1.02–1.19), 1.32 (1.17–1.49) and 2.47 (1.94–3.15) for CKD Stage 3b, 4 and 5, respectively. Spline curves suggested a linear association with fracture risk with an eGFR <30 mL/min/1.73 m2. Compared with non-fracture periods, incident fracture was associated with a 4-fold increased mortality within 90 days [HR 4.21 (95% CI 3.95–4.49)]. The risk remained elevated beyond 90 days [HR 1.47 (95% CI 1.40–1.54)] and was stronger after hip fractures. Post-fracture MACE risk was also highest in the first 90 days [HR 4.02 (95% CI 3.73–4.33)], particularly after hip fractures, and persisted beyond 90 days [HR 1.20 (95% CI 1.10–1.30)].
Conclusion
Our findings highlight the commonness of fractures and the increased risk for subsequent adverse outcomes in CKD patients. These results may inform clinical decisions regarding post-fracture clinical surveillance and fracture prevention strategies.
Keywords: chronic kidney disease, cardiovascular events, epidemiology, fractures, mineral bone disorder
INTRODUCTION
Mineral and bone disorders (MBDs) are inherent in advanced chronic kidney disease (CKD), attributed to phosphate retention, disturbed calcium balance, vitamin D deficiency and secondary hyperparathyroidism [1]. These processes lead to derangements in bone turnover, mineralization and growth, resulting in reduced bone density and quality [2, 3], as well as cardiac hypertrophy, vascular calcification and arterial stiffness [4], particularly in end-stage kidney disease (ESKD) patients [5, 6]. Numerous studies have described an excess fracture risk in patients undergoing dialysis [7–14]. There are studies showing an excess fracture risk also in patients with CKD not on dialysis [8, 15–20], but evidence is mixed [17, 21, 22].
In ESKD, fractures have been associated with increased mortality [23, 24]. Health sequelae of fractures in earlier stages of CKD have been less studied. Previous studies have focused mostly on in-hospital and short-term mortality [8, 20, 25–28], leaving long-term mortality largely unexplored in CKD. Another plausible consequence of fractures is ischaemic cardiovascular disease (CVD), as recently suggested by general population studies [29–32]. We are not aware of studies evaluating the association between incident fractures and subsequent cardiovascular events in CKD patients, but we find this hypothesis conceivable given the features of CKD-MBD.
We aimed to comprehensibly characterize the incidence of fractures (overall and by location) in patients with manifest CKD Stages 3–5 not on dialysis in our region. We also explored sequalae of fractures, focusing on major adverse cardiovascular events (MACE) and death.
MATERIALS AND METHODS
Data source
The study population consisted of individuals drawn from the Stockholm Creatinine Measurement (SCREAM) project, a health care utilization cohort from the region of Stockholm, Sweden described in detail elsewhere [33]. In brief, SCREAM is a repository of all laboratory tests from any resident of Stockholm who had plasma or serum creatinine measured at least once during the years 2006–11. These lab tests were linked, via each citizen’s unique personal identification number, to regional and national administrative databases with complete information on demographics, health care use, dispensed drugs, validated renal outcomes, diagnoses and vital status, with no loss to follow-up. The Regional Ethical Review Board in Stockholm approved the study; informed patient consent was not deemed necessary since all data were de-identified at the government’s offices.
Participant selection and study design
For this observational study we included all adult (>18 years old) residents with confirmed CKD Stages 3–5, in accordance with the Kidney Disease: Improving Global Outcomes (KDIGO) criteria [34]. CKD was defined as having at least two consecutive outpatient creatinine measurements (>3 months and <2 years apart) indicating an estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2. The second measurement fitting this criterion was considered the index date. We then excluded participants with a history of fracture within 5 years before cohort entry and patients undergoing renal replacement therapy (RRT; as ascertained through linkage with the nationwide Swedish Renal Registry). A flow chart of the patient selection is depicted in Supplementary data, Figure S1.
This report contains two complementary study designs. First, we analysed incidence and fracture risk associated with eGFR. To minimize misclassification bias, eGFR was considered a time-dependent exposure using all subsequent eGFR determinations performed in outpatient health care. The outcome was the first encountered fracture. Second, we studied outcomes associated with incident fractures, with fractures considered a time-dependent exposure.
Study covariates
Study covariates included age, sex, laboratory values, comorbidities and concomitant medications. Comorbid conditions included hypertension, diabetes mellitus, ischaemic heart disease, congestive heart failure, peripheral vascular disease, cerebrovascular disease, cancer within 5 years prior to index, osteoporosis, arthritis, hypoparathyroidism, hyperparathyroidism, undernutrition, dementia, systemic inflammatory disease, chronic infections and psychoactive substance abuse, assessed using the International Classification of Diseases, Tenth Revision (ICD-10). Diagnostic codes are available since the implementation of ICD-10 in Sweden in 1997. Medications included renin–angiotensin–aldosterone system (RAAS) inhibitors, β-blockers, loop diuretics, statins, non-steroidal anti-inflammatory drugs (NSAIDs), vitamin D preparations, thiazides, opioids, anti-epileptic drugs, anti-depressants, corticosteroids, oestrogen supplementation, bisphosphonates and calcium salts. Information on drug dispensations was obtained from the Dispensed Drug Registry, a nationwide register with complete information on all prescribed drugs dispensed at Swedish pharmacies [35]. Drugs were assumed to be concomitant if there was a pharmacy dispensation at the time of or within the previous 5 months from index date or after 30 days. Covariate definitions are further detailed in Supplementary data, Tables S1 and S2.
Laboratory values considered were measurements of plasma creatinine ordered in outpatient care, performed with the enzymatic or corrected Jaffe method (alkaline picrate reaction), both methods being traceable to isotope dilution mass spectroscopy standards. Creatinine values <25 and >1500 μmol/L were considered implausible and were discarded. Plasma creatinine was used to estimate GFR with the Chronic Kidney Disease Epidemiology Collaboration equation [36]. The severity of CKD was categorized as eGFR 45–59 mL/min/1.73 m2 (CKD Stage 3a), 30–44 mL/min/1.73 m2 (CKD Stage 3b), 15–29 mL/min/1.73 m2 (CKD Stage 4) and <15 mL/min/1.73 m2 (CKD Stage 5 not on renal replacement therapy; RRT).
Study outcomes and statistical analyses
In the first part, we assessed fracture risk across CKD Stages 3–5. We followed patients until the occurrence of the first fracture, ascertained through relevant ICD-10 codes (S12–S92 and M80, excluding fractures of the face and skull). We stratified by fracture location (hip and non-hip). To reduce the risk of CKD misclassification bias, eGFR was considered a time-dependent exposure and covariates were updated at each creatinine sampling. Patients were followed until death, emigration, start of RRT or the end of follow-up (31 December 2011). Restricted cubic spline models were used to better assess the multivariable adjusted association between eGFR (as a continuous variable) and the incidence of fractures.
In the second part we considered health events associated with incident fractures, using Cox regression with fractures as the time-dependent exposure. Thus a patient suffering a fracture during follow-up contributed with time to the fracture-free group before the event and thereafter to the fracture exposed group. Covariates were time updated at the time of incident fracture. We estimated multivariable-adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) in fracture-exposed versus fracture-free periods for the events of death and MACE. Study outcomes were ascertained via linkage with the National Population Registry, with virtually no loss to follow-up for deaths or hospitalization diagnoses. MACE was defined as the composite of non-fatal ischaemic heart disease (I21, I22, I23, or I252), heart failure (I099, I110, I130, I132, I255, I420, I425-429, I43, I50), ischaemic stroke (H34.1, G45, G46, I60, I61, I63, I64) or death due to CVD (G45-46, H34.1, I00–I99). In the case of consecutive fractures, only the first one was considered for the time-updated exposure and the second was treated as a censoring event. To distinguish short-term versus long-term risk, we performed time-varying Cox regression analysis, splitting the follow-up of the exposed period in two intervals: <90 days and ≥90 days from incident fracture.
To evaluate the presence of residual confounding we included diverticulitis (K57) as a negative control outcome. Stratified analyses were performed to test the consistency of our results by age (<60 and ≥60 years), sex, presence/absence of diabetes mellitus or arthritis and eGFR strata (<30 or ≥30 mL/min/1.73 m2).
Categorical data were expressed using frequency (%) and continuous data were expressed as median with interquartile range (IQR). Statistical analyses were performed using R (R Foundation for Statistical Computing, Vienna, Austria; www.r-project.org). A two-sided P-value <0.05 was considered statistically significant.
RESULTS
Baseline characteristics
A total of 68 764 individuals with confirmed CKD Stages 3–5 met the inclusion criteria (Supplementary data, Figure S1). The median follow-up time was 2.7 years (IQR 1.3–4.1) and the median number of creatinine measurements per patient was 4 (IQR 2–9).
The general characteristics of study participants at cohort entry are described in Table 1. Women constituted 56% of participants, with a median age of 79 years (IQR 71–85) and median eGFR of 51 (IQR 43–56) mL/min/1.73 m2. Hypertension was the most common comorbidity, followed by congestive heart failure, cancer, diabetes mellitus and ischaemic heart disease. Consequently, RAAS inhibitors, β-blockers, loop diuretics and statins were the most frequently used medications. Bisphosphonate use was low and decreased with more severe CKD, while the use of vitamin D analogues increased. Generally age and common comorbidities increased with more severe CKD. Patient characteristics at the time of incident fracture are described in Supplementary data, Table S3. Briefly, patients who developed fractures seemed to be older, more often women, had more comorbidities and were more often using loop diuretics, opioids and calcium supplements.
Table 1.
Baseline characteristics, overall and by CKD strata
| Characteristics | Overall | CKD 3a | CKD 3b | CKD 4 | CKD 5 |
|---|---|---|---|---|---|
| Number of individuals | 68 764 | 48 145 | 15 700 | 4213 | 706 |
| Age (years), median (IQR) | 79 (71, 85) | 78 (71, 84) | 82 (75, 87) | 82 (73, 87) | 75 (63, 84) |
| Women, n (%) | 38 519 (56) | 26 930 (56) | 9048 (58) | 2232 (53) | 309 (44) |
| eGFR (mL/min/1.73 m2), median (IQR) | 51 (42–56) | 54 (50–57) | 39 (35–42) | 25 (21–27) | 11 (9–13) |
| Comorbidities, n (%) | |||||
| Hypertension | 43 819 (64) | 30 201 (63) | 10 260 (65) | 2853 (68) | 505 (72) |
| Diabetes mellitus | 15 137 (22) | 10 059 (21) | 3741 (24) | 1108 (26) | 229 (32) |
| Ischaemic heart disease | 11 845 (17) | 7467 (16) | 3210 (20) | 1029 (24) | 139 (20) |
| Congestive heart failure | 17 334 (25) | 10 168 (21) | 5227 (33) | 1725 (41) | 214 (30) |
| Peripheral vascular disease | 6213 (9) | 3865 (8) | 1702 (11) | 565 (13) | 81 (12) |
| Cerebrovascular disease | 11 989 (17) | 7766 (16) | 3190 (20) | 908 (22) | 125 (18) |
| Cancer | 15 219 (22) | 10 710 (22) | 3435 (22) | 923 (22) | 151 (21) |
| Osteoporosis | 3751 (5) | 2608 (5) | 851 (5) | 240 (6) | 52 (7) |
| Arthritis | 2234 (3) | 1662 (4) | 438 (3) | 111 (3) | 23 (3) |
| Hypoparathyroidism | 181 (0.3) | 100 (0.2) | 56 (0.4) | 16 (0.4) | 9 (1.3) |
| Hyperparathyroidism | 660 (1) | 401 (1) | 181 (1) | 68 (2) | 10 (1) |
| Undernutrition | 222 (0.3) | 143 (0.3) | 57 (0.4) | 20 (0.5) | 2 (0.3) |
| Dementia | 3472 (5) | 2240 (5) | 936 (6) | 256 (6) | 40 (6) |
| Systemic inflammatory disease | 4056 (6) | 2782 (6) | 947 (6) | 284 (7) | 43 (6) |
| Chronic infection | 727 (1) | 501 (1) | 153 (1) | 53 (1) | 20 (3) |
| Psychoactive substance abuse | 2316 (3) | 1641 (3) | 484 (3) | 161 (4) | 30 (4) |
| Ongoing medication, n (%) | |||||
| RAAS inhibitors | 37 626 (55) | 25 240 (52) | 9297 (59) | 2642 (63) | 447 (63) |
| β-blockers | 34 414 (50) | 23 214 (48) | 8436 (54) | 2351 (56) | 413 (59) |
| Loop diuretics | 23 726 (35) | 13 243 (28) | 7283 (46) | 2698 (64) | 502 (71) |
| Statins | 22 990 (33) | 16 528 (34) | 4926 (31) | 1315 (31) | 221 (31) |
| NSAIDs | 11 602 (17) | 8451 (18) | 2528 (16) | 574 (14) | 49 (7) |
| Vitamin D preparations | 1279 (2) | 246 (0.5) | 249 (2) | 449 (11) | 335 (48) |
| Thiazides | 5543 (8) | 3975 (8) | 1302 (8) | 249 (6) | 17 (2) |
| Opioids | 11 789 (17) | 7872 (16) | 2934 (18) | 832 (20) | 151 (21) |
| Anti-epileptic drugs | 2194 (3) | 1510 (3) | 511 (3) | 154 (4) | 19 (3) |
| Antidepressants | 9433 (14) | 6461 (13) | 2298 (15) | 589 (14) | 85 (12) |
| Corticosteroids | 7439 (11) | 5281 (11) | 1610 (10) | 485 (12) | 63 (9) |
| Oestrogen supplementation | 5781 (8) | 4447 (9) | 1075 (7) | 237 (6) | 22 (3) |
| Bisphosphonates | 3415 (5) | 2510 (5) | 741 (5) | 148 (4) | 16 (2) |
| Calcium salts | 8994 (13) | 6089 (13) | 1977 (13) | 650 (15) | 278 (39) |
Incidence of fractures and fracture types in CKD
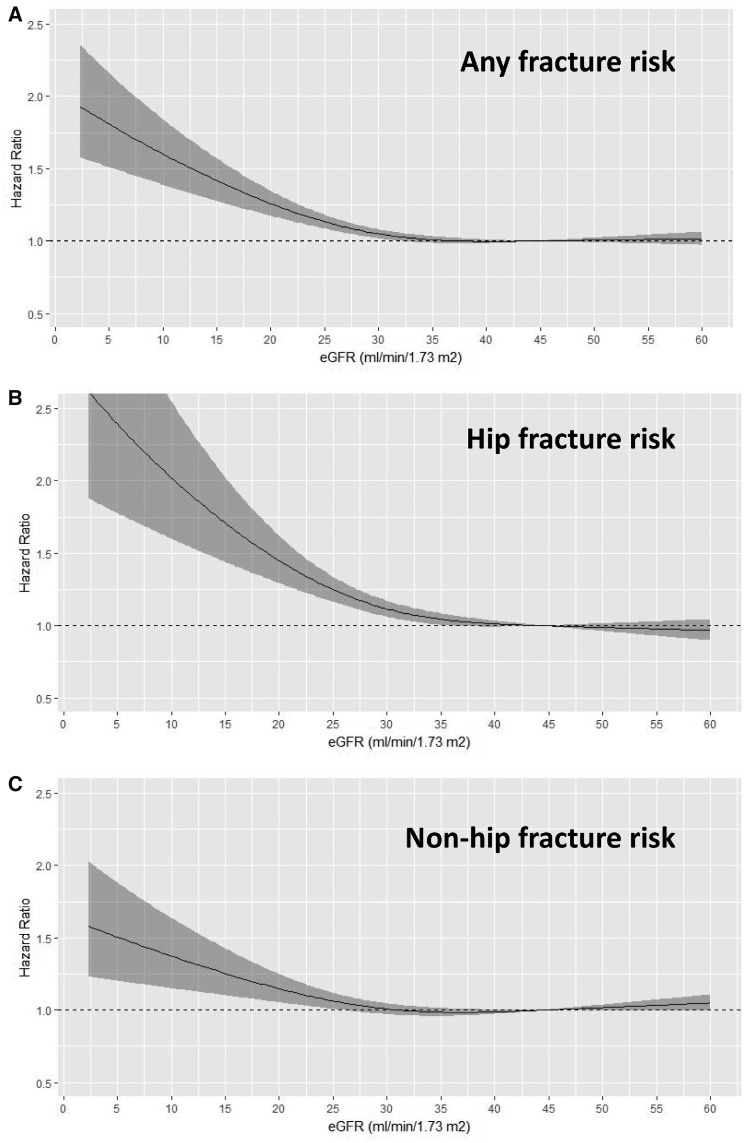
A total of 9219 first fractures were registered, giving an overall incidence rate (IR) of 49/1000 person-years (PY). Of those, 3105 (34%, 17/1000 PY) were fractures of the hip and the remaining 6114 (66%, 33/1000 PY) were grouped as ‘non-hip fractures’. Table 2 shows that the crude IR for all fractures and fracture subtypes increased in more severe CKD. Table 2 also describes HRs and 95% CIs for the risk of fracture associated with CKD stage. Compared with patients with CKD Stage 3a, the multivariable-adjusted risk of (any) fracture was significantly higher in patients with CKD Stage 4 [HR 1.20 (95% CI 1.11–1.29)] and CKD Stage 5 [HR 1.81 (95% CI 1.57–2.09)]. This increased risk was attributed to both hip and non-hip fractures, but the magnitude was greater for hip fractures [HR 1.10 (95% CI 1.02–1.19), 1.32 (1.17–1.49) and 2.47 (1.94–3.15) for CKD Stage 3b, 4 and 5, respectively]. Adjusted cubic splines (Figure 1) depict the multivariable-adjusted association between eGFR (as a continuous variable) and the risk of fractures, illustrating that the risk increases linearly when eGFR is ≤30 mL/min/1.73 m2. The full Cox models are described in Supplementary data, Table S4. Generally, older patients, women and the presence of comorbidities such as dementia, CVD, chronic infection or cancer history were associated with a higher risk of fractures.
Table 2.
IRs and HRs for fractures in relation to CKD severity stages
| CKD stage | No. of fractures | IR per 1000 PY (95% CI) | Adjusteda HR (95% CI) | Adjustedb HR (95% CI) | Adjustedc HR (95% CI) |
|---|---|---|---|---|---|
| Any fracture (n = 9219), IR 49 (95% CI 48.4–50.5) per 1000 PY | |||||
| CKD 3a | 5446 | 45.4 (44.2–46.7) | 1 | 1 | 1 |
| CKD 3b | 2693 | 54.4 (52.3–56.4) | 1.20 (1.14–1.25) | 1.04 (0.99–1.09) | 1.01 (0.96–1.06) |
| CKD 4 | 888 | 64.3 (60.2–68.6) | 1.41 (1.31–1.52) | 1.27 (1.18–1.37) | 1.20 (1.11–1.29) |
| CKD 5 | 192 | 59.2 (51.4–67.9) | 1.29 (1.12–1.50) | 1.89 (1.64–2.18) | 1.81 (1.57–2.09) |
| Hip fractures (n = 3105), IR 17 (95% CI 16.1–17.2) per 1000 PY | |||||
| CKD 3a | 1671 | 13.9 (13.3–14.6) | 1 | 1 | 1 |
| CKD 3b | 1017 | 20.5 (19.3–21.8) | 1.47 (1.36–1.60) | 1.15 (1.06–1.24) | 1.10 (1.02–1.19) |
| CKD 4 | 345 | 25.0 (22.5–27.7) | 1.79 (1.59–2.02) | 1.41 (1.25–1.59) | 1.32 (1.17–1.49) |
| CKD 5 | 72 | 22.2 (17.6–27.6) | 1.59 (1.26–2.02) | 2.59 (2.04–3.29) | 2.47 (1.94–3.15) |
| Other fractures (n = 6114), IR 33 (95% CI 32.0–33.6) per 1000 PY | |||||
| CKD 3a | 3775 | 31.5 (30.5–32.5) | 1 | 1 | 1 |
| CKD 3b | 1676 | 33.8 (32.3–35.5) | 1.07 (1.01–1.14) | 0.99 (0.93–1.05) | 0.96 (0.90–1.02) |
| CKD 4 | 543 | 39.3 (36.1–42.7) | 1.24 (1.13–1.36) | 1.18 (1.08–1.29) | 1.12 (1.02–1.23) |
| CKD 5 | 120 | 37.0 (31.0–43.9) | 1.16 (0.97–1.39) | 1.56 (1.30–1.87) | 1.50 (1.25–1.80) |
Adjusted for age and sex.
Adjusted for Model 1 plus comorbidities: hypertension, diabetes mellitus, ischaemic heart disease, congestive heart failure, peripheral vascular disease, cerebrovascular disease, cancer, osteoporosis, arthritis, hypoparathyroidism, hyperparathyroidism, undernutrition, dementia, systemic inflammatory disease, chronic infection and psychoactive substance abuse.
Adjusted for Model 2 plus ongoing medication: RAAS inhibitors, β-blockers, loop diuretics, statins, NSAIDs, vitamin D, thiazides, opioids, anti-epileptic drugs, antidepressants, corticosteroids, oestrogen supplementation, bisphosphonates and calcium supplements.
FIGURE 1.
Restricted cubic splines showing multivariable-adjusted HRs (95% CI) for any (A) fracture, (B) hip fracture and (C) non-hip fractures across eGFRs (continuous). Covariate adjustments as in Table 2, Model 3.
Subgroup analyses are detailed in Supplementary data, Table S5. Across CKD stages, the risk for hip fractures was marginally higher for men than for women (P for interaction = 0.049). No other interactions were observed.
Post-fracture outcomes
There were 18 993 deaths and 20 004 MACE registered during follow-up. Compared with fracture-free periods, incident fractures were associated with an increased risk of death in the short-term [<90 days, adjusted HR 4.21 (95% CI 3.95–4.49)] and after ≥90 days of follow-up [HR 1.47 (95% CI 1.4–1.54); Table 3]. Short- and long-term mortality was increased after both hip and non-hip fractures, but the risk was greater after a hip fracture.
Table 3.
Incident fractures and risk of subsequent adverse events before and after 90 days
| Event | Number of events | Non-fracture periods |
<90 days after fracture |
≥90 days after fracture |
||||
|---|---|---|---|---|---|---|---|---|
| Event rate (per 1000 PY) | Event rate (per 1000 PY) | Crude HR (95% CI) | Adjusted HR (95% CI) | Event rate (per 1000 PY) | Crude HR (95% CI) | Adjusted HR (95% CI) | ||
| Incident fracture (any) | ||||||||
| Death | 18 993 | 84 | 553 | 6.6 (6.2–7.0) | 4.2 (4.0–4.5) | 176 | 2.1 (2.0–2.2) | 1.5 (1.4–1.5) |
| MACE | 20 004 | 109 | 544 | 5.2 (4.9–5.6) | 4.0 (3.7–4.3) | 138 | 1.5 (1.4–1.6) | 1.2 (1.1–1.3) |
| Incident hip fracture | ||||||||
| Death | 17 150 | 84 | 961 | 11.5 (10.6–12.5) | 5.9 (5.4–6.4) | 248 | 3.0 (2.8–3.2) | 1.7 (1.5–1.8) |
| MACE | 18 703 | 109 | 991 | 9.6 (8.7–10.6) | 6.3 (5.7–7.0) | 163 | 1.8 (1.6–1.9) | 1.2 (1.1–1.3) |
| Incident non-hip fracture | ||||||||
| Death | 17 504 | 84 | 364 | 4.3 (4.0–4.8) | 3.09 (2.8–3.4) | 148 | 1.8 (1.7–1.9) | 1.4 (1.3–1.4) |
| MACE | 19 143 | 109 | 360 | 3.5 (3.1–3.8) | 2.9 (2.6–3.2) | 129 | 1.4 (1.3–1.5) | 1.2 (1.1–1.3) |
| Negative control outcome | ||||||||
| Diverticulitis | 2931 | 15 | 20 | 1.4 (1.0–1.9) | 1.3 (0.9–1.8) | 13 | 1.0 (0.8–1.2) | 0.9 (0.8–1.1) |
Adjusted for age, sex, eGFR, hypertension, diabetes mellitus, ischaemic heart disease, congestive heart failure, peripheral vascular disease, cerebrovascular disease, cancer, osteoporosis, arthritis, hypoparathyroidism, hyperparathyroidism, undernutrition, dementia, systemic inflammatory disease, chronic infection and psychoactive substance abuse.
Compared with fracture-free periods, the short-term risk of MACE was significantly elevated after fracture occurrence [4.02 (95% CI 3.73–4.33)], particularly after a hip fracture [6.3 (95% CI 5.66–7)]. This risk persisted after 90 days. In contrast, no association was observed between fractures and our negative control outcome.
Subgroup analyses are detailed in Supplementary data, Tables S6 and S7. The association between hip fracture and mortality was stronger for men than women (P interaction = 0.01), for patients with eGFR >30 mL/min/1.73 m2 versus <30 mL/min/1.73 m2 (P interaction = 0.004), and for non-diabetics versus diabetics (P for interaction = 0.003). The association between incident fractures and MACE was also stronger for non-diabetics than for diabetics (P for interaction <0.001).
DISCUSSION
In this large, region-representative sample of patients with CKD Stages 3–5, we observed a gradual increase in (hip) fracture risk with lower eGFR, particularly with eGFR <30 mL/min/1.73 m2. Incident fractures were associated with untoward sequelae such as increased risk of MACE and death, with risks being highest in the short term, but also extending beyond 90 days.
Our results are in agreement with some prior studies suggesting an excess risk of any fracture [15] and hip fracture [8, 18–21, 37] with lower eGFR. However, previous evidence may have had some limitations, such as the use of single eGFR measurements to define CKD, the inclusion of women only [17, 18], reliance on ICD diagnoses of CKD to identify cases [8], self-reports of comorbidities and medications or the inability to adjust for these important confounders [15, 16, 18, 19, 21, 25, 26]. Some studies excluded pathological fractures from their analysis [8, 25] and others adjusted for bone mineral density [17], which may lie within the causal pathway. This may have altogether underestimated the true fracture burden. Elliot et al. [38] found no relationship between eGFR and fracture incidence in a large Canadian health care–based study, possibly explained by a less rigid CKD definition (single GFR estimation) and by the use of eGFR >90 mL/min/1.73 m2 as a reference. The inaccuracies of creatinine-based eGFR, particularly among the elderly affected by comorbidities, frailty and muscle debilitating conditions, may have obscured the association if these individuals with inaccurately high eGFR are considered referent. This study and ours coincide, however, in the observation of an increased fracture incidence when eGFR is <30 mL/min/1.73 m2, which is the range where CKD-MBD alterations become clinically manifest [11].
A novelty is our analysis of health events following incident fractures. Using fractures as time-dependent exposure allowed us to dissect short- and long-term risks, considering the background event risk in non-exposed periods. We confirm previous studies showing an increased short-term death risk in patients with fractures [8, 25–28] and add novel evidence of a long-term risk. This association was somehow expected for hip fractures [39, 40], but was not reported before for ‘minor fractures’.
Interestingly, in our interaction analysis, we observed a stronger association between CKD and fractures in men than women, which agrees with previous findings of a stronger risk of vertebral fractures in men undergoing haemodialysis [41]. We also observe a stronger association between incident hip fractures and mortality in men. We are not aware of studies of CKD patients reporting such sex differences, which needs to be confirmed in further investigations. Because previous reports have focused on women, or on patients with a history of fractures, men may have been underrepresented or died before they could be included in previously investigated cohorts. Nonetheless, such findings are also consistent with recent epidemiological data from the UK general population demonstrating greater post-fracture mortality in men [42]. Our multivariable analyses (Supplementary data, Table S4) also illustrate the multiple conditions associated with fracture risk in this multimorbid and complex population, including diabetes, cardiovascular comorbidity or, as evidenced by earlier reports [43], the presence of dementia.
The observed association between incident fractures and MACE in CKD is novel in nephrology but is in line with recent evidence in the general population indicating a bidirectional relationship between CVD and poor bone health [29–31, 44, 45]. A recent meta-analysis found consistency across published studies with regard to CVD risk in patients with low (versus high) bone mineral density [pooled HR 1.33 (95% CI 1.27–1.38)] or a history of fractures [HR 1.25 (95% CI 1.05–1.48)] [45]. The underlying mechanisms remain inadequately elucidated, but this association is consistent with the observation in haemodialysis populations that the prevalence of vertebral fractures assessed by quantitative morphometry is strongly associated with vascular calcifications [41]. Osteoporosis and CVD share risk factors, such as a lack of physical activity, smoking, low-grade inflammation, alcohol use, glucocorticoid use and diabetes [45, 46]. In renal insufficiency, CKD-MBD leads, on the one hand, to arterial stiffness, vascular calcification and cardiac hypertrophy and, on the other hand, to heightened bone turnover, loss of bone quality and susceptibility to fragility fractures [1, 2, 4, 47, 48]. Therefore the observed associations may solely reflect the presence of such common risk factors. The increased cardiovascular risk could also relate to complications of the fractures per se, such as immobility, surgical procedures, nosocomial infections and a more liberal use of NSAIDs.
Compared with previous reports, our analysis offers some methodological strengths, starting with complete regional coverage by a single health care provider and universal health care access. The identification of CKD patients with two consecutive eGFR measurements and the use of eGFR as a time-dependent exposure reduces misclassification bias. We did not exclude pathological fractures, since CKD-induced fractures are, by nature, pathological, and we evaluated all incident fractures except for those of the skull and face (viewing them as more associated with trauma than to underlying bone disease), thereby providing a more comprehensive characterization of the burden of fractures in CKD. Our study also has limitations, including our inability to infer causality and the lack of information on important confounders such as albuminuria, body mass index, smoking or alcohol use and the lack of biomarkers such as alkaline phosphatase, 25-hydroxy vitamin D or parathyroid hormone (PTH). Nonetheless, our negative control outcome analysis suggests a potentially low risk of residual confounding.
Even if our observations mainly reflected underlying morbidity, they highlight the commonness of fractures and the susceptibility for adverse outcomes in CKD patients and thereby emphasize the need for fracture prevention and post-fracture clinical surveillance. Implementation of the fracture risk assessment tool has recently been shown to predict major osteoporotic fractures and hip fractures in CKD patients not on dialysis with equal accuracy irrespective of kidney function [49, 50]. The 2017 updated KDIGO guidelines recommend bone density assessment for all CKD patients [11, 51]. KDIGO also recommends the use of bisphosphonates for osteoporosis patients with CKD G3a-G3b who have normal PTH and a high risk of fractures [11, 51]. We note a small proportion of patients with CKD Stage 3 in our study using bisphosphonates, possibly suggesting underuse. Conversely, we also note a small proportion of patients with CKD Stages 4–5 using bisphosphonates, which may indicate off-label use. Other pharmacological treatments, such as osteoblast-stimulating agents, are also often discouraged in advanced CKD [52]. Of late, denosumab, a biologically active osteoclast inhibitor, has appeared as a new prevention tool for selected CKD patients [11]. Fall preventive efforts seem called for, particularly among the elderly, as well as counselling on physical activity to increase muscle strength, improve bone resistance and ameliorate cardiovascular fitness. Efforts towards phosphate, calcium and PTH control, together with supplementation of vitamin D, surely play a role, but we are not aware of strong evidence that this is the case.
In summary, the risk of fractures in non-dialysis-dependent CKD patients increased gradually with lower kidney function. The occurrence of fractures was associated with untoward, sometimes fatal sequalae, in the form of MACE and death, both in the immediate 90-day period and thereafter. Although our observations need to be confirmed in independent cohorts, we find it reasonable to propose that CKD patients suffering fractures should be closely monitored for signs and symptoms of cardiovascular complications and be (re-)evaluated for CVD prevention.
SUPPLEMENTARY DATA
Supplementary data are available at ndt online.
Supplementary Material
ACKNOWLEDGEMENTS
The authors acknowledge support from the Stockholm County Council and the Martin Rind and Westman Foundations. Baxter Novum is the result of a grant from Baxter Healthcare to the Karolinska Institutet.
CONFLICT OF INTEREST STATEMENT
B.L. is employed by Baxter Novum.
REFERENCES
- 1. Llach F. Secondary hyperparathyroidism in renal failure: the trade-off hypothesis revisited. Am J Kidney Dis 1995; 25: 663–679 [DOI] [PubMed] [Google Scholar]
- 2. Moe S, Drueke T, Cunningham J et al. Definition, evaluation, and classification of renal osteodystrophy: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 2006; 69: 1945–1953 [DOI] [PubMed] [Google Scholar]
- 3. Moe SM, Nickolas TL. Fractures in patients with CKD: time for action. Clin J Am Soc Nephrol 2016; 11: 1929–1931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hruska KA, Sugatani T, Agapova O et al. The chronic kidney disease – mineral bone disorder (CKD-MBD): advances in pathophysiology. Bone 2017; 100: 80–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Burton JO, Goldsmith DJ, Ruddock N et al. Renal association commentary on the KDIGO (2017) clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of CKD-MBD. BMC Nephrol 2018; 19: 240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aleksova J, Ng KW, Jung C et al. Bone health in chronic kidney disease–mineral and bone disorder a clinical case seminar and update. Intern Med J 2018; 48: 1435. [DOI] [PubMed] [Google Scholar]
- 7. Miller PD. Bone disease in CKD: a focus on osteoporosis diagnosis and management. Am J Kidney Dis 2014; 64: 290–304 [DOI] [PubMed] [Google Scholar]
- 8. Kim SM, Long J, Montez-Rath M et al. Hip fracture in patients with non-dialysis-requiring chronic kidney disease. J Bone Miner Res 2016; 31: 1803–1809 [DOI] [PubMed] [Google Scholar]
- 9. Pimentel A, Urena-Torres P, Zillikens MC et al. Fractures in patients with CKD—diagnosis, treatment, and prevention: a review by members of the European Calcified Tissue Society and the European Renal Association of Nephrology Dialysis and Transplantation. Kidney Int 2017; 92: 1343–1355 [DOI] [PubMed] [Google Scholar]
- 10. Ball AM, Gillen DL, Sherrard D et al. Risk of hip fracture among dialysis and renal transplant recipients. JAMA 2002; 288: 3014–3018 [DOI] [PubMed] [Google Scholar]
- 11. Damasiewicz MJ, Nickolas TL. Rethinking bone disease in kidney disease. JBMR Plus 2018; 2: 309–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Alem AM, Sherrard DJ, Gillen DL et al. Increased risk of hip fracture among patients with end-stage renal disease. Kidney Int 2000; 58: 396–399 [DOI] [PubMed] [Google Scholar]
- 13. Jadoul M, Albert JM, Akiba T et al. Incidence and risk factors for hip or other bone fractures among hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study. Kidney Int 2006; 70: 1358–1366 [DOI] [PubMed] [Google Scholar]
- 14. Maravic M, Ostertag A, Torres PU et al. Incidence and risk factors for hip fractures in dialysis patients. Osteoporos Int 2014; 25: 159–165 [DOI] [PubMed] [Google Scholar]
- 15. Naylor KL, McArthur E, Leslie WD et al. The three-year incidence of fracture in chronic kidney disease. Kidney Int 2014; 86: 810–818 [DOI] [PubMed] [Google Scholar]
- 16. Chen H, Lips P, Vervloet MG et al. Association of renal function with bone mineral density and fracture risk in the Longitudinal Aging Study Amsterdam. Osteoporos Int 2018; 29: 2129–2138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ensrud KE, Lui LY, Taylor BC et al. Renal function and risk of hip and vertebral fractures in older women. Arch Intern Med 2007; 167: 133–139 [DOI] [PubMed] [Google Scholar]
- 18. LaCroix AZ, Lee JS, Wu L et al. Cystatin-C, renal function, and incidence of hip fracture in postmenopausal women. J Am Geriatr Soc 2008; 56: 1434–1441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fried LF, Biggs ML, Shlipak MG et al. Association of kidney function with incident hip fracture in older adults. J Am Soc Nephrol 2007; 18: 282–286 [DOI] [PubMed] [Google Scholar]
- 20. Robertson L, Black C, Fluck N et al. Hip fracture incidence and mortality in chronic kidney disease: the GLOMMS-II record linkage cohort study. BMJ Open 2018; 8: e020312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nickolas TL, McMahon DJ, Shane E. Relationship between moderate to severe kidney disease and hip fracture in the United States. J Am Soc Nephrol 2006; 17: 3223–3232 [DOI] [PubMed] [Google Scholar]
- 22. Elliott MJ, James MT, Quinn RR et al. Estimated GFR and fracture risk: a population-based study. Clin J Am Soc Nephrol 2013; 8: 1367–1376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mittalhenkle A, Gillen DL, Stehman-Breen CO. Increased risk of mortality associated with hip fracture in the dialysis population. Am J Kidney Dis 2004; 44: 672–679 [PubMed] [Google Scholar]
- 24. Beaubrun AC, Kilpatrick RD, Freburger JK et al. Temporal trends in fracture rates and postdischarge outcomes among hemodialysis patients. J Am Soc Nephrol 2013; 24: 1461–1469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pajulammi HM, Luukkaala TH, Pihlajamaki HK et al. Decreased glomerular filtration rate estimated by 2009 CKD-EPI equation predicts mortality in older hip fracture population. Injury 2016; 47: 1536–1542 [DOI] [PubMed] [Google Scholar]
- 26. Singh Mangat K, Mehra A, Yunas I et al. Is estimated peri-operative glomerular filtration rate associated with post-operative mortality in fractured neck of femur patients? Injury 2008; 39: 1141–1146 [DOI] [PubMed] [Google Scholar]
- 27. Khan SK, Rushton SP, Courtney M et al. Elderly men with renal dysfunction are most at risk for poor outcome after neck of femur fractures. Age Ageing 2013; 42: 76–81 [DOI] [PubMed] [Google Scholar]
- 28. Frisch NB, Wessell N, Jildeh TR et al. Early-stage chronic kidney disease and hip fracture mortality. J Surg Orthop Adv 2018; 27: 226–230 [PubMed] [Google Scholar]
- 29. Paccou J, D’Angelo S, Rhodes A et al. Prior fragility fracture and risk of incident ischaemic cardiovascular events: results from UK Biobank. Osteoporos Int 2018; 29: 1321–1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tanko LB, Christiansen C, Cox DA et al. Relationship between osteoporosis and cardiovascular disease in postmenopausal women. J Bone Miner Res 2005; 20: 1912–1920 [DOI] [PubMed] [Google Scholar]
- 31. Chiang CH, Liu CJ, Chen PJ et al. Hip fracture and risk of acute myocardial infarction: a nationwide study. J Bone Miner Res 2013; 28: 404–411 [DOI] [PubMed] [Google Scholar]
- 32. Kang JH, Chung SD, Xirasagar S et al. Increased risk of stroke in the year after a hip fracture: a population-based follow-up study. Stroke 2011; 42: 336–341 [DOI] [PubMed] [Google Scholar]
- 33. Runesson B, Gasparini A, Qureshi AR et al. The Stockholm CREAtinine Measurements (SCREAM) project: protocol overview and regional representativeness. Clin Kidney J 2016; 9: 119–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chapter 1: Definition and classification of CKD. Kidney Int Suppl (2011) 2013; 3: 19–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wettermark B, Hammar N, Fored CM et al. The new Swedish Prescribed Drug Register—opportunities for pharmacoepidemiological research and experience from the first six months. Pharmacoepidem Drug Safe 2007; 16: 726–735 [DOI] [PubMed] [Google Scholar]
- 36. Levey AS, Stevens LA, Schmid CH et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Perez-Saez MJ, Prieto-Alhambra D, Barrios C et al. Increased hip fracture and mortality in chronic kidney disease individuals: the importance of competing risks. Bone 2015; 73: 154–159 [DOI] [PubMed] [Google Scholar]
- 38. Elliott MJ, James MT, Quinn RR et al. Estimated GFR and fracture risk: a population-based study. Clin J Am Soc Nephrol 2013; 8: 1367–1376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Katsoulis M, Benetou V, Karapetyan T et al. Excess mortality after hip fracture in elderly persons from Europe and the USA: the CHANCES project. J Intern Med 2017; 281: 300–310 [DOI] [PubMed] [Google Scholar]
- 40. Farahmand BY, Michaelsson K, Ahlbom A et al. Survival after hip fracture. Osteoporos Int 2005; 16: 1583–1590 [DOI] [PubMed] [Google Scholar]
- 41. Fusaro M, Tripepi G, Noale M et al. High prevalence of vertebral fractures assessed by quantitative morphometry in hemodialysis patients, strongly associated with vascular calcifications. Calcif Tissue Int 2013; 93: 39–47 [DOI] [PubMed] [Google Scholar]
- 42. Klop C, van Staa TP, Cooper C et al. The epidemiology of mortality after fracture in England: variation by age, sex, time, geographic location, and ethnicity. Osteoporos Int 2017; 28: 161–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Maravic M, Ostertag A, Urena P et al. Dementia is a major risk factor for hip fractures in patients with chronic kidney disease. Osteoporos Int 2016; 27: 1665–1669 [DOI] [PubMed] [Google Scholar]
- 44. Vassalle C, Mazzone A. Bone loss and vascular calcification: a bi-directional interplay? Vascul Pharmacol 2016; 86: 77–86 [DOI] [PubMed] [Google Scholar]
- 45. Veronese N, Stubbs B, Crepaldi G et al. Relationship between low bone mineral density and fractures with incident cardiovascular disease: a systematic review and meta-analysis. J Bone Miner Res 2017; 32: 1126–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lello S, Capozzi A, Scambia G. Osteoporosis and cardiovascular disease: an update. Gynecol Endocrinol 2015; 31: 590–594 [DOI] [PubMed] [Google Scholar]
- 47. Dhayat NA, Ackermann D, Pruijm M et al. Fibroblast growth factor 23 and markers of mineral metabolism in individuals with preserved renal function. Kidney Int 2016; 90: 648–657 [DOI] [PubMed] [Google Scholar]
- 48. Mathew S, Tustison KS, Sugatani T et al. The mechanism of phosphorus as a cardiovascular risk factor in CKD. J Am Soc Nephrol 2008; 19: 1092–1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Whitlock RH, Leslie WD, Shaw J et al. The Fracture Risk Assessment Tool (FRAX®) predicts fracture risk in patients with chronic kidney disease. Kidney Int 2019; 95: 447–454 [DOI] [PubMed] [Google Scholar]
- 50. Naylor KL, Garg AX, Zou G et al. Comparison of fracture risk prediction among individuals with reduced and normal kidney function. Clin J Am Soc Nephrol 2015; 10: 646–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ketteler M, Block GA, Evenepoel P et al. Executive summary of the 2017 KDIGO chronic kidney disease–mineral and bone disorder (CKD-MBD) guideline update: what’s changed and why it matters. Kidney Int 2017; 92: 26–36 [DOI] [PubMed] [Google Scholar]
- 52. Black DM, Rosen CJ, Clinical P. Postmenopausal osteoporosis. N Engl J Med 2016; 374: 254–262 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.