Abstract
Among mouse mammary tumor models, syngeneic cell lines present an advantage for the study of immune response. However, few of these models are well characterized. The tumor line EO771 is derived from spontaneous breast cancer of C57BL/6 mice. These cells are widely used but are referenced under different names: EO771, EO 771, and E0771. The characteristics of the EO771 cells are well described but some data are contradictory. This cell line presents the great interest of developing an immunocompetent neoplastic model using an orthotopic implantation reflecting the mammary tumors encountered in breast cancer patients. This review presents the phenotype characteristics of EO771 and its sensitivity to nutrients and different therapies such as radiotherapy, chemotherapy, hormone therapy, and immunotherapy.
Keywords: antineoplastic agents, breast neoplasms, hormonal receptors, mice, murine cell line, inbred C57BL
The EO771 cell line presents a molecular pattern ERα‐, ERβ +, PR+ and ErbB2+ and could be considered as a luminal B subtype. The EO771 tumour‐bearing mouse model presents the great interest of developing an immunocompetent mammary neoplastic model using an orthotopic injection.
1. INTRODUCTION
Breast cancer is the most common cause of cancer deaths among women (522 000 deaths, WHO 2013) and the most frequently diagnosed woman cancer in the world. 1 Many studies are interested in breast cancer (348,598 results for “mammary cancer” and 405,294 results for “breast cancer” on pubmed, 7 April 2020). This cancer is subdivided into several types, which are well characterized taking into account the cell type of origin, its mutations and its gene expression profile. Before performing clinical trials, experimental models in vitro and in vivo must be used. Thus in preclinical approaches, it is important to choose the right experimental model to know what type of tumor is studied and what type of patient this model could match.
For in vivo experimental approaches, the use of murine models is often done. Among the mice models, the C57BL/6 strain seems to be the most used (about 300 000 publications are found during a pubmed search, 7 April 2020, depending on the way it is spelled) in front of the BALB/c strain (about 200 000 publications found on pubmed, 7 April 2020). The C57BL/6J mouse is the most widely used inbred strain and the first to have genome sequenced. 2 Although this strain is refractory to many tumors, it is a permissive background for maximal expression of most mutations. However, despite this massive use, few breast tumor lines result from C57BL/6 genetic background. Indeed, only 10 lines are derived from C57BL/6 mice (Table 1): 34T, 3 AT‐3, 4 EO771 5 and its derivative EO771.LMB, 6 M158, 7 MG1361, 8 MMT060562, 9 Py230, 10 Py8119, 10 WT145, 11 and WT276. 11 Of the 10 lines, only the EO771 line and its derivated line EO771.LMB come from spontaneous tumors, not induced by the addition of a transgene (as 34T, AT‐3, M158, MG1361, MMT060562, Py230, and Py8119) or a chemical agent DMBA (7,12‐dimethyl‐benzanthracene) (as WT145 and WT276).
TABLE 1.
C57BL/6 mammary cancer cell lines
| Mouse mammary cancer cell lines | Mouse strains | Tumor induction | References |
|---|---|---|---|
| EO771 | C57BL/6 | Spontaneous | Sugiura and Stock. 5 |
| EO771.LMB derived from EO771 | C57BL/6 | Spontaneous | Johnstone et al 6 |
| AT‐3 | C57BL/6 MMTV‐PyMT | Transgene addition | Stewart and Abrams. 4 |
| 34T | C57BL/6 × 129/SvJ | Transgene addition | Upadhyay et al 3 |
| M158 | CD‐1 x C57BL/6J MMTV‐c‐myc transgenic mice | Transgene addition | Stewart et al 7 |
| MG1361 | (C57BL/6xDBA)F1 x CD‐1 MMTV‐neu transgenic | Transgene addition | Sacco et al 8 |
| MMT060562 | (C57BL/6xDBA)F1 x CD‐1 MMTV‐neu transgenic | Transgene addition | Akatsu et al 9 |
| Py230 | C57BL/6 MMTV‐PyMT | Transgene addition | Gibby et al 10 |
| Py8119 | C57BL/6 MMTV‐PyMT | Transgene addition | Gibby et al 10 |
| WT145 | C57BL/6J | Chemical agent DMBA | Zinser et al 11 |
| WT276 | C57BL/6J | Chemical agent DMBA | Zinser et al 11 |
Breast tumour lines resulted from C57BL/6 genetic background. Among them, only the EO771 line and its derivated line EO771. LMB come from spontaneous tumours. The 34T, AT‐3, M158, MG1361, MMT060562, Py230, and Py8119 cell lines were obtained by addition of a transgene. WT145 and WT276 cell lines were obtained by exposure to the chemical agent DMBA (7,12‐dimethyl‐benzanthracene).
C57BL/6J is the parental substrain; “J” is the laboratory code for The Jackson Laboratory.
Therefore, this review is dedicated to the EO771 mammary cancer cell line. According to the spelling used to search for this lineage in pubmed, numbers of associated publications found are different. A total of 122 publications are identified following pubmed research on 7 April 2020 (Table 2). However, publications using these cells but not indicating the lineage in their title or abstract are not identified. 12 , 13 , 14 These cells have been used for many years and the publication of Sugiura and Stock 5 from 1952 is frequently presented as the original publication, but earlier articles using this line exist as Homburger's (1948). 15
TABLE 2.
Various spelling used to identify EO771 mammary cancer cell line in literatur
| Mouse mammary cancer cell lines | Number of Pubmed results |
|---|---|
| EO 771 | 25* |
| EO771 | 38 |
| E0771 | 79 |
| EO771.LMB | 1 |
According to the spelling used the numbers of associated publications found are different. A total of 122 publications are identified following pubmed research on 7 April 2020.
EO 771:25 results but 5 do not concern the tumor line. Research online 7 April 2020.
2. EO771 CELL PHENOTYPE
Despite their isolation in the late 1940s, characterization of hormonal receptors and classification of EO771 line remains controversial. Indeed, its classification diverges according to the authors, which is considered as triple negative in 10 publications 6 , 12 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 and as ERα+ in 19 publications. 13 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 Some authors prefer to mention the unclear status of this lineage. 42 , 43 Other publications do not report information on the expression of ERα. However, among the 30 publications considering EO771 cells as triple negative or ERα+, only 3 articles analyzed the expression of ERα. 6 , 40 , 41 Contreras‐Zárate et al have considered EO771 cells as triple negative and have showed that the proliferation of EO771 cells is independent of the estradiol presence. 23 But in the same time, they showed that estradiol drives the signaling for brain metastasis of EO771 cells. 23 Therefore, Hiraga et al have observed that the gene encoding ERα is transcribed in EO771 cells 41 and Gu et al have observed the protein expression of ERα by western blot. 40 However, they observed that this ERα expression is much weaker than that found in MCF‐7 cells (considered as ERα+). In addition, Johnstone et al have observed ERα by immunohistochemistry in EO771 cells but have considered these cells as ERα‐ because this receptor is only found in the cytoplasm but not in the nuclear compartment 6 which is the localization found in primary human breast cancers. Thus, based on these publications, the cells could be considered as ERα‐ because the expression of this receptor is very weak and not at the nuclear level.
Few publications have investigated the status of ERβ, the progesterone receptor and ErbB2 in EO771 cells. The immunohistochemical analysis performed by Johnstone et al on primary EO771 and EO771.LMB tumors did not detect the expression of ERβ. 6 Thus, Hiraga et al and Johnstone et al have not found gene transcription or protein expression of the progesterone receptor. 6 , 41 Similarly, the ErbB2 status for this line is poorly described and contradictory. Indeed, Johnstone et al have not found expression of ErbB2 by immunohistochemistry in the tumors formed after injection of EO771 cells 6 in vivo whereas Hiraga et al and Zou et al found respectively a transcription of ErbB2 and a protein expression highlighted by westernblot. 44
As for hormone receptors, the expression of claudin, a marker for the classification of triple negative cancers, is poorly determined. Some authors consider this lineage as claudin‐low without checking it. 31 On the contrary, Bousquenaud et al have found an expression of claudin 1, 7, and 10 in EO771 cells. 28 However, their expressions are greatly decreased when the mice are fed with a high‐fat diet (HFD), and are associated with a decrease in estrogen receptor and ErbB2 expression. This suggests that a hyperlipid diet would induce a triple negative phenotype for EO771 cells. 28
Finally, the expression of many other markers from EO771 cells has been characterized such as p53, Notch receptors and ligand, EGFR, PD1 (Programmed Cell Death 1), PDL1 (Programmed Cell Death Ligand 1), etc (Table 3, Figure 1).
Modified EO771 cell lines
TABLE 3.
Characterisation of hormonal receptors and protein patterns of EO771 line in literature
| Biomarker | Expressed | Low to moderate expression | Not expressed | References |
|---|---|---|---|---|
| Estrogen receptor alpha (ERα) | ER‐α | ER‐α |
Johnstone et al 6 |
|
| Estrogen receptor beta (ERβ) | ER‐β | Johnstone et al 6 | ||
| Progesterone receptor (PR) | PR | Johnstone et al 6 | ||
| Epidermal Growth Factor Receptor‐2 (ErbB2) | ErbB2 | ErbB2 |
Johnstone et al 6 |
|
| Claudin |
Claudin 1, 7, 10 decreased by HFD |
Bousquenaud et al 28 | ||
| Tumor suppressor | Mutant p53 | Johnstone et al 6 | ||
| Notch receptors |
Notch 2 Notch 3 Notch 4 |
Notch 1 but increased by leptin | Battle et al 24 | |
| Notch ligands |
Jagged 1 Delta‐like 4 |
Battle et al 24 | ||
| Matrix Metalloprotease | MMP4 | Ager et al 113 | ||
| MHC‐I molecules |
H‐2Kb H‐2Db |
Tu et al 46 | ||
| Ligands for NK activation |
Clr‐b RAE1 |
Tu et al 46 | ||
| Biomarker for breast cancer | Placental‐specific protein 1 (PLAC1) | Yuan et al 111 | ||
| Histamine receptors |
Receptor H1 Receptor H2 |
Vila‐Leahey et al 101 | ||
| BDNF/TrkB's oncogenic pathway | TrkB |
TrkA TrkC |
Contreras‐Zárate et al 23 | |
| Epidermal growth factor receptor | EGFR | EGFR |
Johnstone et al 6 Contreras‐Zárate et al 23 |
|
| Cytokeratin 5/6 | KRT5/6 | Johnstone et al 6 | ||
| Immunomodulator protein |
PD1 PDL1 |
Gray et al 20 |
List of publications with partial determination of proteins and hormonal receptors expression. Characterisation of phenotype and the expression of many other markers from EO771 cells were generally not done. Among the 30 publications considering EO771 cells, only 3 articles analysed the expression of ERα 6 , 40 , 41 . That’s why classification of EO771 line remains controversial.
FIGURE 1.
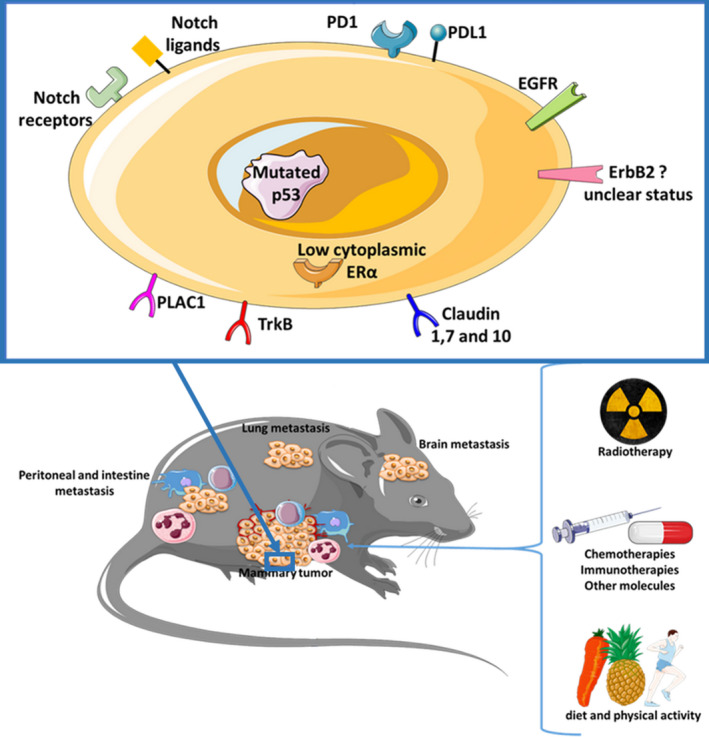
Characteristics of the EO771 cell line and tumor bearing in C57BL/6 mice. Numerous publications have made it possible to refine the phenotype of the EO771 line. This cell line could be considered as a luminal B type mammary cancer. Tumor‐bearing mice models are well‐known and sensitive to explore numerous therapeutic strategies. After injection of EO771 cells, the major metastatic dissemination sites are in the brain, the lung, the intestine, and the peritoneal cavity
The EO771 cell line can be genetically modified to follow their growth by fluorescence, for example by expression of green fluorescent protein (GFP), 13 , 14 , 23 , 33 , 45 or luciferase 13 as well as to modify the expression of genes by the CRISPR/Cas9 technology such as the EO771 line devoid of MHC I or MHC II or expressing the tumor antigen NY‐BR‐1. 46 , 47
3. EO771 MAMMARY TUMOR MOUSE MODELS
The C57BL/6J mouse is the most widely used inbred strain and the first to have genome sequenced. 2 Although this strain is refractory to many tumors, it is a permissive background for maximal expression of most mutations. It is also susceptible to diet‐induced obesity, type 2 diabetes, and atherosclerosis (https://www.jax.org/strain/000664). Due to the wide use of this mouse strain, many modified strains exist (transgenic, knock out (KO), knock down (KD), overexpression, etc) to evaluate tumor development under various conditions. Indeed, the advantage of having a tumor line from the most used mouse strain, C57BL/6, makes it possible to have a lot of genetically modified models of this strain. Thus, EO771 cell tumor growth can be evaluated i) in KO mice for E2 (estradiol), 23 apolipoprotein E and aromatase, 42 IFN‐γ receptor, 48 natural killer lytic‐associated molecule (NKLAM), 33 MMP13, 49 calcium‐independent phospholipase A2 β, 50 IGFBP‐3, 21 , 51 , 52 DUSP1, 53 GDF2, 54 BMP10, 54 and mKIAA1462 55 but also ii) in transgenic models such as mice overexpressing IL‐15, 56 obese, 13 , 35 NKCKD (exhibiting silenced Ly49 expression in NK cells), 46 GFP‐LC3 (LC3 linked to GFP expressing mice), 57 mice transgenically modified to express the HER2 proto‐oncogene (ERBB2) 58 or FAT‐1 (gene leading to the endogenous formation of ω3‐Polyunsaturated fatty acid from ω6‐Polyunsaturated fatty acid). 44 , 59
The use of EO771 cells in mouse models has the advantage of having tumor uptake close to 100%, in particular when an orthotopic mammary implantation is used. 33 This model is really close to human breast cancer (Figure 1). In reality, mammary cell line EO771 (5 × 10 5 cells) in suspension into Matrigel™ Matrix orthotopically transplanted into the fourth right mammary fat pad leads to large tumors within weeks. 38 In fact, implantation into the mammary gland provides a neoplastic model with a complete microenvironment promoting the intercellular dialogue. Syngeneic models have the advantage of being immunocompetent. Thus, communication between different cell types can be explored including the immune response. Therefore, injection of EO771 cells into C57BL/6 mice provides a model of mammary cancer in which the role of host and tumor immunity can be investigated.
In vivo proliferation and dissemination capacities of EO771 cells
The tumor proliferation of EO771 cells seems to be similar to other transplantable murine and human tumors, especially in relation to the duration of the S phase. 60 The size of the EO771 tumor is correlated with the cell density but also with the proportion of necrotic tissue. 60 However, the growth curve of EO771 tumors is independent of the number of cells injected but formulas have been proposed to estimate tumor growth. 61 The tumor growth can also be influenced by the characteristics of its host. Indeed, the tumor growth is linked to the weight of the mouse injected with EO771 cells with a higher initial growth rate followed by a faster growth deceleration phase in mice with higher body weight compared to mice with lower body weight. 62
In addition to their proliferation capacity, the ability of EO771 cells to disseminate has also been studied. In mice, a metastatic spread is found for this cell line, with characteristics similar to the human disease. 63 The sites of metastatic disseminations are multiple from EO771 tumors. Its preferential release seems to be the lungs 6 , 12 , 22 , 26 , 28 , 33 , 36 , 49 , 50 , 54 , 59 , 64 , 65 , 66 , 67 , 68 but other localizations such as the peritoneum, 26 , 36 , 64 , 68 bones, 6 , 41 brain, 23 diaphragm, 36 and intestines 36 are observed (Figure 1). In addition, EO771 cells express the CD73 antigen (ecto‐5'‐nucleotidase), associated with a pro‐metastatic phenotype in breast cancer. 63
In vivo cellular interactions in EO771 mammary tumor mouse model
Innate immune cells, such as macrophages, neutrophils, and dendritic cells, appear to be relatively uninvolved in the growth of the primary tumor but, on the other hand, appear to be important in the metastatic dissemination. Indeed, tumor‐associated macrophages and neutrophils and tumor‐infiltrating dendritic cells are less present in the metastatic microenvironment after splenectomy and this decrease was associated with a decrease in the number of mammary cancer lung metastases. 67
Similarly, the role of Natural Killer (NK) cells seems important in the control of mammary tumor development and metastatic dissemination. Indeed, the use of an anti‐NK1.1, causing NK cell depletion, leads to an increase in tumor growth compared to untreated mice. 46 Similarly, the KO of the NK lytic‐associated molecule (NKLAM), which plays an important role in the cytotoxic activity of NK, causes a very large increase of tumor cells in the blood and lungs of mice after orthotopic injection of EO771 cells, compared to wild‐type (WT) mice for NKLAM. 33 The effect of NK does not appear to be mediated by Toll‐like receptor 3 because its deficiency has a small impact on the tumor growth of EO771 cells. 69 Interestingly, a study showing that a prior immunization with the injection of another type of tumor cell (Colon adenocarcinoma line Colon38) resulted in an absence of EO771 tumor growth. This immunoprevention is mediated mainly by NK1.1 positive cells. 70
Finally, the evolution of T lymphocytes and their role during tumor development is also studied in the model of EO771 tumor‐bearing mice. Thus, an increase in the CD4 + and CD8 + populations is observed when the size of the tumor increases. However, the CD4/CD8 ratio differs during tumor development with a predominance of CD8 + T lymphocytes (cytotoxic lymphocytes) in the early stages whereas CD4 + lymphocytes become predominant in late stages. The accumulation of CD8 + T lymphocytes, which may involve IGFBP‐3 (Insulin‐like Growth Factor Binding Protein‐3), is an immune population with a strong antitumor role as shown by the decrease in tumor growth. 51 Experiments inducing a CD8 + T cell depletion exacerbates EO771 tumor growth, emphasizing the importance of CD8 + T cells in controlling tumor growth. 71 Within CD4 + cells, the proportions of population subtypes evolve during tumor development with, in the early stages, a predominant Th1 lymphocyte population, known to stimulate antitumor immune responses, and then evolve into subtypes of Treg, associated with a tolerogenic profile, and Th17 cells, in more advanced stages. 72
In addition to the applications already described, these cells have also been used with other cell types to study possible interactions. Thus, the interactions of EO771 cells with adipocytes or fibroblasts led to protumoral effects. An increase in EO771 cell proliferation is induced by secretions of senescent fibroblasts 73 or adipocytes. 74 Finally, this model has also been used to evaluate the effect of the tumor on memory loss, 75 muscle damage, 76 or tumor development when associated with a viral infection. 77
Impact of obesity and physical activity in EO771 tumor‐bearing mice
A sedentary lifestyle and obesity are studied because of their association with breast cancer 78 incidence and recurrence. On the contrary, physical activity is inversely associated with breast cancer risk. 78 To study these parameters, the use of the immunocompetent model is essential because obesity, inducing chronic low‐grade inflammation, and physical activity are able to modulate the immune system. Therefore, the use of a syngeneic model such as orthotopic injection of EO771 cells in C57BL/6 mice represents a relevant model of breast cancer to study the effect of overweight/obesity and physical activity.
The obesity of the C57BL/6 mice, induced either by taking a HFD 28 , 32 , 35 , 36 , 51 , 52 , 79 or by genetic alteration, 13 , 35 leads to a protumoral effect by increasing tumor growth, 13 , 28 , 32 , 52 promoting tumor angiogenesis, 28 , 32 having an immunomodulatory effect 28 as well as activating pro‐tumor pathways such as AKT/mTOR. 13
Nachat‐Kappes et al have used an environmental enrichment that leads to spontaneous physical activity in mice. 38 Environmental enrichment resulted in a decrease in COX‐2, which may suggest a decrease in inflammation, and a decrease in tumor volume and weight associated with a decrease in the proliferation index Ki67. 38 Physical exercise, whether spontaneous 25 , 38 or forced, 31 leads to a slowing down of tumor growth but also makes it possible to modulate angiogenesis 25 , 31 and to increase sensitivity to chemotherapy 25 compared with controlled mice.
Physical activity also induces changes in the cytokine environment, including adipokines, by increasing the plasma ratio of adiponectin/leptin levels. 38 This ratio of adipokines is decreased in a situation of obesity, which is a major risk of breast cancer. 78
Impact of phytonutrients in EO771 tumor‐bearing mice
Various phytonutrients from usual vegetables have been tested in mammary cancer models using EO771 cells. Bioactive compounds such as naringenin (citrus flavonoid), 80 [10] ‐Gingerol (a major phenolic constituent of ginger root), 16 secoisolariciresinol diglucoside (polyphenolic plant lignan), 17 meroxest (synthetic merosesquiterpenes), 29 , 30 EGCG (Epigallocatechin Gallate, a major green tea catechin) 81 and emodin (a Chinese herb‐derived compound) 34 , 65 induced antitumor effects against E0771 cells by several ways such as: 1) inducing cell death 16 , 80 ; 2) inhibiting the cell cycle 16 , 80 ; 3) modulating the immune system including macrophages 17 , 34 , 65 or leukocytes 29 , 34 ; 4) decreasing pro‐angiogenic markers such as expression of VEGF 17 , 29 , 81 ; 5) modulating signaling pathways such as NF‐κB, 17 , 81 IRF4, 34 STAT6, 34 and C/EBPβ. 34
4. SENSITIVITY TO DIFFERENT THERAPIES
The sensitivity of the EO771 line has been tested against many treatments such as radiotherapy, cytotoxic agents, antiangiogenics, hormone therapy, immunotherapy, gene therapy, and bioactive compounds.
Sensitivity to radiotherapy
Breast cancer treatment is multimodal and includes radiotherapy. 82 Therefore, EO771 cells are exposed to radiotherapy in animal models. 83 , 84 The EO771 tumor line seems resistant to irradiation by 30 Gy of Cobalt 60 because, despite a transient effect in tumor cell number, the day after irradiation, the effect is limited to a growth delay without tumor regression. 84
The radioactivity can also be used as a tracer in EO771 tumor‐bearing C57BL/6 mice, such as the use of 125I‐labeled 5‐iodo‐2'‐deoxyuridine, which allows tumor progression to be monitored. 85 , 86
Sensitivity to cytotoxic agents
4.1. Inhibitors of topoisomerase II and alkylating agents
Anthracyclines and topoisomerase II inhibitors, are among the conventional chemotherapies used in breast cancer, whether in the United States where AC regimen (A = doxorubicin, (anthracycline) and C = cyclophosphamide) is commonly used, or in Europe where FEC100 (combining F = 5‐fluorouracil, E = epirubicin (anthracycline) and C = cyclophosphamide) is frequently used. 87 Doxorubicin is tested on EO771 cells in vitro 73 and in vivo, in mouse models to test its antitumor efficacy and its toxicity. Studies on animal models have shown that this line is sensitive to doxorubicin and that the activity of the latter could be improved either by conjugating it to nanoparticles 27 , 88 or peptides 37 , 89 or by associating it with other molecules such as IL‐2, 64 TNF, 90 rapamycin, 57 or FTY720 (a sphingosine‐1‐phosphate receptor functional antagonist). 35 These models also tested the toxicity of doxorubicin in mouse mammary cancer models. This anthracyclin causes skeletal muscle dysfunction and increases mitochondrial H2O2 production inducing a decrease on the mitochondrial respiratory complex supported by complex I (pyruvate/glutamate/malate) and complex II (succinate) substrates. 76 This toxicity of doxorubicin, in particular at cardiac level, is not modified with the addition of IL‐2 64 but is decreased when doxorubicin is conjugated with nanoparticles 27 , 88 and peptides 64 , 89 or associated with rapamycin. 57
Cyclophosphamide, an alkylating agent, frequently used in breast cancer therapy as in the FEC100 and AC regimen protocols, leads to an initial significant cytotoxic activity but limited in time as the tumor growth restarts. 60 , 91
4.2. Other cytotoxic agents
In addition to these commonly used chemotherapies, other cytotoxic drugs can be used in the therapeutic arsenal of breast cancer. Thus, methotrexate, an antifolate, is tested in the mouse model of EO771 mammary cancer showing a sensitivity of this tumor to this drug. 92 This animal model is also tested for the development of a novel antifolate family as analogues of aminopterin 91 , 93 , 94 , 95 , 96 , 97 , 98 , 99 whose antitumor activity is superior to methotrexate in EO771 tumor‐bearing mice.
Antipyrimidic drugs such as cytosine arabinoside 100 and gemcitabine 101 are also active on EO771 tumors. The vinca alkaloids, such as vinblastine, navelbine, and vindesine induced increase in animal survival. 98 Similarly, paclitaxel, 102 cisplatin, 91 and melphalan 91 have antitumor activity against EO771 tumors. In contrast, 5‐fluorouracil appears to be of low activity on EO771 tumors. 91
Other compounds such as ranitidine, 45 , 101 , 103 a substituted 3‐(5‐imidazo[2,1‐b] thiazolylmethylene)‐2‐indolinones 104 or EB‐3D (a choline kinase 1 inhibitor) 105 have shown antitumor effects in EO771 mammary cancer models.
Sensitivity to antiangiogenic agents
Angiogenesis plays an important role in tumor progression and metastatic dissemination. 106 Thus, the development of antiangiogenic therapy has generated a great enthusiasm in recent years. The EO771 tumor‐bearing mouse model is used to evaluate the role of angiogenesis and to test molecules modulating angiogenesis, especially since this cell line expresses vascular endothelial growth factor (VEGF) receptors 1 and 2. 40 Tumor development seems to be dependent on angiogenesis. A decrease in neovascularization is found in a mouse model using mKIAA1462‐/‐ mice resulting in a loss of “junctional protein associated with coronary artery disease,” and is associated with a decrease in tumor volume compared with the control group of EO771 tumor‐bearing mice. 55 Antiangiogenic agents targeting VEGF or its receptors have been tested in this model. The SU11248 39 is a selective protein kinase inhibitor inducing inhibition of, among others, VEGFR types 1‐3 found in human breast cancer. SU11248, 39 but also pyrrolidine dithiocarbamate, 40 in EO771 tumor model modulated neoangiogenesis by decreasing intratumoral microvessel density. This effect is associated with a decrease in tumor weight compared to the control group. 39 , 40 The effect of pyrrolidine dithiocarbamate passed in particular through the inhibition of autocrine and paracrine VEGF effects by decreasing its expression and reducing NFkB activation. 40 This molecule pyrrolidine also inhibited the growth and migration of EO771 cells and had a synergistic effect with the VEGF receptor inhibitor SU5416. 40 Lu et al have showed that EO771 tumor growth is increased by stimulating VEGF‐dependent angiogenesis but that is inhibited by the use of SU5416. 107 The antioxidant N‐acetylcysteine has also been studied in models using EO771 cells. However, despite the fact that N‐acetylcysteine prevented Hif‐1α stabilization under hypoxia in vitro, it did not reduce in vivo the tumor growth or the survival of EO771 tumor‐bearing mice but rather, increased the metastatic burden. 108 Finally, vascular endothelial protein tyrosine phosphatase has also been investigated. Inhibition of the latter resulted in a delay in tumor growth during tumor establishment, but had no impact once the tumor is well established. 68
Sensitivity to hormone therapy
Since the status of EO771 cells in hormone receptor expression remains controversial, few studies have evaluated the impact of hormone therapy in this model. Johnstone et al have observed a reduction in the growth of EO771 tumors during treatment with tamoxifen, 6 which may therefore suggest an expression of estrogen receptors in these cells.
Sensitivity to immunotherapy
As previously mentioned, the EO771 tumor‐bearing mouse model is immunocompetent and of interest for evaluating the efficacy of immunotherapies such as the use of cytokines or immune checkpoint inhibitors. Indeed, it represents a model of choice because the EO771 cells express immunomodulatory molecules as PD1 and PDL1 in the basal state. 20 Moreover, in the presence of IFNγ, the expression of PD1 and PDL1 is increased in EO771 cells 20 and the overexpression of mucin 1 in these tumor cells resulted in an increase in PDL1 expression. 19
Therefore, therapies such as immune checkpoint inhibitors have been tested in this model. The use of anticytotoxic T lymphocyte‐associated protein 4 (CTLA4) or antiprogrammed cell death 1 (PD1) therapy allowed an increase in the number of circulating CD8 + T cells and IFN‐γ leading to T lymphocyte‐mediated antitumor response. 48 However, anti‐PD1 alone leads to partial antitumor activity in the model of EO771 tumor‐bearing mice. 22 Thus, it seems interesting to associate them with other treatments, such as surgery, chemotherapy, or even other immunotherapies. Liu et al have showed that neoadjuvant immunotherapy combining anti‐PD1 and anti‐CD137 enhanced therapeutic efficacy compared to surgery alone or surgery followed by this immunotherapy. 12 This increase in therapeutic efficacy is associated with an increase in CD8 + lymphocytes in the blood, spleen, liver, and lungs. 12 However, this neoadjuvant immunotherapy must be performed shortly before surgery because, after 10 days, the benefits of this treatment are lost. 18 Anti‐PD1 has also been associated with a Tyro3, Axl, and Mertk inhibitor (BMS‐777607), which are tyrosine kinase receptors having immunomodulatory properties. Their association allowed to significantly reduced tumor growth and incidence of lung metastasis, associated with an increase in proinflammatory cytokines and an infiltration of antitumor effector T cells. 22 The use of phosphatidylserine‐targeting antibodies decreases tumor growth and increases the antitumor efficacy of anti‐PD1 in a syngeneic model using EO771 cells by promoting tumor infiltration of T cells and increasing the production of proinflammatory cytokines. 20
Proinflammatory cytokines, such as interleukin (IL) 2 and tumor necrosis factor (TNF), have also been investigated in models using EO771. Treatment with IL‐2 resulted in prolonged survival of EO771 tumor‐bearing mice 109 , 110 and improved the efficacy of doxorubicin chemotherapy. 64 This effect required the action of lymphocytes, especially CD8 +. 64 , 109 , 110 Similarly, the presence of TNF led to the stimulation of CD8 + cytotoxic T lymphocytes and NK cells, allowing the complete remission of EO771 tumor‐bearing mice, when this cytokine was associated with doxorubicin chemotherapy. 90
Other immunomodulatory agents acting on other immune cells have also been studied in the mouse model of EO771 mammary cancer. For example, the use of a Cxcr2 antagonist decreased tumor growth by acting on different immune populations. In fact, it led in the tumor to a decrease in immunosuppressive cells such as myeloid‐derived suppressor cells (MDSC) and regulatory T cells (Treg) and, on the contrary, an increase in antitumor cells such as cytotoxic lymphocytes, NK cells, dendritic cells, and macrophages. 111 These latter have an ambivalent role on tumorigenesis depending of their polarization. Indeed, M1 macrophages have tumoricidal activity, while M2 macrophages exhibit low amounts of antigen presentation and suppress antitumor immunity. 112 Treatment with an anti‐MMP14 inhibitory antibody (DX‐2400) increased the tumor‐associated macrophage number and polarized them toward an antitumoral M1 phenotype. 113
Sensitivity to gene therapy
Gene therapy has also been tested in the EO771 tumor‐bearing mouse model. A reduction in tumor growth of the primary tumor but also at the metastatic level is observed when using adenovirus inducing the expression “brain‐derived neurotrophic factor” 36 or MBP‐1 66 (Myelin Basic Protein 1).
5. CONCLUSION
Therefore, the characteristics of the EO771 cells are well described but some data are contradictory. Thus, their molecular classification remains controversial at the present time between the luminal and triple negative subtype, close to the luminal B phenotype. However, the luminal A phenotype could be excluded considering the articles showing them as ERα‐. More accurate phenotyping of this cell line is needed as well as for other murine cell lines since very few of them are clearly defined in terms of expression of estrogen, progesterone, and ErbB2 receptors. The use of this cell line has been carried out in 2D, 3D and in vivo models. 3D cell culture techniques are now a way of using cancer cell lines. Even if there is currently only one publication on this subject, 107 the use of this line in 3D models is possible. The establishment of 3D models with EO771 cells will provide valuable data for understanding breast cancer.
The EO771 cell line presents the great interest of developing an immunocompetent neoplastic model using an orthotopic injection reflecting the mammary tumors encountered in breast cancer patients. This EO771 tumor‐bearing mouse model has been well characterized considering its sensitivity to various antineoplastic treatments and even other therapic approaches including nutritional interventions and physical activity (Figure 1).
Despite some uncertainties, all these data lead us to consider the EO771 cell line as a very relevant candidate for providing an experimental mammary tumor model very close to human breast cancer.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
AUTHOR CONTRIBUTIONS
Augustin Le Naour is the major contributor in writing the manuscript and analyzing the literature. Adrien Rossary and Marie‐Paule Vasson participated in the scientific discussions for the manuscript writing and during the revision of the manuscript. All the authors approved the final version of the manuscript.
ACKNOWLEDGMENTS
Not applicable.
Le Naour A, Rossary A, Vasson M-P. EO771, is it a well-characterized cell line for mouse mammary cancer model? Limit and uncertainty. Cancer Med. 2020;9:8074–8085. 10.1002/cam4.3295
Funding information
This work was supported by funding from the Institut National du Cancer (INCA: MammAdipo project; PLBIO 13‐106) and the Comité de l’Allier de la Ligue Contre le Cancer.
DATA AVAILABILITY STATEMENT
Not applicable.
REFERENCES
- 1. Winters S, Martin C, Murphy D, Shokar NK. Breast cancer epidemiology, prevention, and screening. Prog Mol Biol Transl Sci. 2017;151:1‐32. [DOI] [PubMed] [Google Scholar]
- 2. Mouse Genome Sequencing Consortium . Initial sequencing and comparative analysis of the mouse genome. Nature. 2002;420(6915):520‐562. [DOI] [PubMed] [Google Scholar]
- 3. Upadhyay G, Yin Y, Yuan H, Li X, Derynck R, Glazer RI. Stem cell antigen‐1 enhances tumorigenicity by disruption of growth differentiation factor‐10 (GDF10)‐dependent TGF‐ signaling. Proc Natl Acad Sci. 2011;108(19):7820‐7825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stewart TJ, Abrams SI. Altered immune function during long‐term host‐tumor interactions can be modulated to retard autochthonous neoplastic growth. J Immunol. 2007;179(5):2851‐2859. [DOI] [PubMed] [Google Scholar]
- 5. Sugiura K, Stock CC. Studies in a tumor spectrum. I. Comparison of the action of methylbis (2‐chloroethyl)amine and 3‐bis(2‐chloroethyl)aminomethyl‐4‐methoxymethyl ‐5‐hydroxy‐6‐methylpyridine on the growth of a variety of mouse and rat tumors. Cancer. 1952;5(2):382‐402. [DOI] [PubMed] [Google Scholar]
- 6. Johnstone CN, Smith YE, Cao Y, et al. Functional and molecular characterisation of EO771.LMB tumours, a new C57BL/6‐mouse‐derived model of spontaneously metastatic mammary cancer. Dis Model Mech. 2015;8(3):237‐251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stewart TA, Pattengale PK, Leder P. Spontaneous mammary adenocarcinomas in transgenic mice that carry and express MTV/myc fusion genes. Cell. 1984;38(3):627‐637. [DOI] [PubMed] [Google Scholar]
- 8. Sacco MG, Gribaldo L, Barbieri O, et al. Establishment and characterization of a new mammary adenocarcinoma cell line derived from MMTV neu transgenic mice. Breast Cancer Res Treat. 1998;47(2):171‐180. [DOI] [PubMed] [Google Scholar]
- 9. Akatsu T, Ono K, Katayama Y, et al. The mouse mammary tumor cell line, MMT060562, produces prostaglandin E2 and leukemia inhibitory factor and supports osteoclast formation in vitro via a stromal cell‐dependent pathway. J Bone Miner Res. 1998;13(3):400‐408. [DOI] [PubMed] [Google Scholar]
- 10. Gibby K, You W‐K, Kadoya K, et al. Early vascular deficits are correlated with delayed mammary tumorigenesis in the MMTV‐PyMT transgenic mouse following genetic ablation of the NG2 proteoglycan. Breast Cancer Res BCR. 2012;14(2):R67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zinser GM, McEleney K, Welsh J. Characterization of mammary tumor cell lines from wild type and vitamin D3 receptor knockout mice. Mol Cell Endocrinol. 2003;200(1–2):67‐80. [DOI] [PubMed] [Google Scholar]
- 12. Liu J, Blake SJ, Yong MCR, et al. Improved efficacy of neoadjuvant compared to adjuvant immunotherapy to eradicate metastatic disease. Cancer Discov. 2016;6(12):1382‐1399. [DOI] [PubMed] [Google Scholar]
- 13. Fuentes‐Mattei E, Velazquez‐Torres G, Phan L, et al. Effects of obesity on transcriptomic changes and cancer hallmarks in estrogen receptor‐positive breast cancer. J Natl Cancer Inst. 2014;106(7). 10.1093/jnci/dju158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Goetz J, Minguet S, Navarro‐Lérida I, et al. Biomechanical remodeling of the microenvironment by stromal caveolin‐1 favors tumor invasion and metastasis. Cell. 2011;146(1):148‐163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Homburger F. Studies on hypoproteinemia: III. Lymphoid hyperplasia and redistribution of nitrogen caused in mice by transplanted tumors (sarcoma 180 and breast adenocarcinoma EO 771). Science. 1948;107(2790):648‐649. [DOI] [PubMed] [Google Scholar]
- 16. Bernard MM, McConnery JR, Hoskin DW. [10]‐Gingerol, a major phenolic constituent of ginger root, induces cell cycle arrest and apoptosis in triple‐negative breast cancer cells. Exp Mol Pathol. 2017;102(2):370‐376. [DOI] [PubMed] [Google Scholar]
- 17. Bowers LW, Lineberger CG, Ford NA, et al. The flaxseed lignan secoisolariciresinol diglucoside decreases local inflammation, suppresses NFκB signaling, and inhibits mammary tumor growth. Breast Cancer Res Treat. 2019;173(3):545‐557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu J, O’Donnell JS, Yan J, et al. Timing of neoadjuvant immunotherapy in relation to surgery is crucial for outcome. OncoImmunology. 2019;8(5):e1581530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Maeda T, Hiraki M, Jin C, et al. MUC1‐C induces PD‐L1 and immune evasion in triple‐negative breast cancer. Cancer Res. 2018;78(1):205‐215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gray MJ, Gong J, Hatch MMS, et al. Phosphatidylserine‐targeting antibodies augment the anti‐tumorigenic activity of anti‐PD‐1 therapy by enhancing immune activation and downregulating pro‐oncogenic factors induced by T‐cell checkpoint inhibition in murine triple‐negative breast cancers. Breast Cancer Res. 2016;18(1):50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Scully T, Scott CD, Firth SM, Pintar JE, Twigg SM, Baxter RC. Contrasting effects of IGF binding protein‐3 expression in mammary tumor cells and the tumor microenvironment. Exp Cell Res. 2019;374(1):38‐45. [DOI] [PubMed] [Google Scholar]
- 22. Kasikara C, Davra V, Calianese D, et al. Pan‐TAM tyrosine kinase inhibitor BMS‐777607 enhances anti–PD‐1 mAb efficacy in a murine model of triple‐negative breast cancer. Cancer Res. 2019;79(10):2669‐2683. [DOI] [PubMed] [Google Scholar]
- 23. Contreras‐Zárate MJ, Day NL, Ormond DR, et al. Estradiol induces BDNF/TrkB signaling in triple‐negative breast cancer to promote brain metastases. Oncogene. 2019;38(24):4685‐4699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Battle M, Gillespie C, Quarshie A, et al. Obesity induced a leptin‐Notch signaling axis in breast cancer: obesity induced a leptin‐Notch signaling axis in BC. Int J Cancer. 2014;134(7):1605‐1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Betof AS, Lascola CD, Weitzel D, et al. Modulation of Murine Breast Tumor Vascularity, Hypoxia, and Chemotherapeutic Response by Exercise. JNCI. 2015;107(5): 10.1093/jnci/djv040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ewens A, Mihich E, Ehrke MJ. Distant metastasis from subcutaneously grown E0771 medullary breast adenocarcinoma. Anticancer Res. 2005;25(6B):3905‐3915. [PubMed] [Google Scholar]
- 27. Prados J, Cabeza L, Ortiz R, et al. Enhanced antitumor activity of doxorubicin in breast cancer through the use of poly(butylcyanoacrylate) nanoparticles. Int J Nanomed. 2015;1291 10.2147/IJN.S74378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bousquenaud M, Fico F, Solinas G, Rüegg C, Santamaria‐Martínez A. Obesity promotes the expansion of metastasis‐initiating cells in breast cancer. Breast Cancer Res. 2018;20(1):104 10.1186/s13058-018-1029-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Carrasco E, Garrido JM, Álvarez PJ, et al. Meroxest improves the prognosis of immunocompetent C57BL/6 mice with allografts of E0771 mouse breast tumor cells. Arch Med Sci. 2016;5:919‐927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Carrasco E, Álvarez PJ, Melguizo C, et al. Novel merosesquiterpene exerts a potent antitumor activity against breast cancer cells in vitro and in vivo. Eur J Med Chem. 2014;79:1‐12. [DOI] [PubMed] [Google Scholar]
- 31. Glass OK, Bowie M, Fuller J, et al. Differential response to exercise in claudin‐low breast cancer. Oncotarget. 2017;8(60):100989‐101004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gu J‐W, Young E, Patterson SG, et al. Postmenopausal obesity promotes tumor angiogenesis and breast cancer progression in mice. Cancer Biol Ther. 2011;11(10):910‐917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hoover RG, Gullickson G, Kornbluth J. Natural killer lytic‐associated molecule plays a role in controlling tumor dissemination and metastasis. Front Immunol. 2012;3 10.3389/fimmu.2012.00393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Iwanowycz S, Wang J, Hodge J, Wang Y, Yu F, Fan D. Emodin inhibits breast cancer growth by blocking the tumor‐promoting feedforward loop between cancer cells and macrophages. Mol Cancer Ther. 2016;15(8):1931‐1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Katsuta E, Yan LI, Nagahashi M, et al. Doxorubicin effect is enhanced by sphingosine‐1‐phosphate signaling antagonist in breast cancer. J Surg Res. 2017;219:202‐213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Liu X, McMurphy T, Xiao R, Slater A, Huang W, Cao L. Hypothalamic gene transfer of BDNF inhibits breast cancer progression and metastasis in middle age obese mice. Mol Ther. 2014;22(7):1275‐1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Moktan S, Perkins E, Kratz F, Raucher D. Thermal targeting of an acid‐sensitive doxorubicin conjugate of elastin‐like polypeptide enhances the therapeutic efficacy compared with the parent compound in vivo. Mol Cancer Ther. 2012;11(7):1547‐1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nachat‐Kappes R, Pinel A, Combe K, et al. Effects of enriched environment on COX‐2, leptin and eicosanoids in a mouse model of breast cancer. Coleman WB, ed. PLoS One. 2012;7(12):e51525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Young E, Miele L, Tucker KB, Huang M, Wells J, Gu J‐W. SU11248, A selective tyrosine kinases inhibitor suppresses breast tumor angiogenesis and growth via targeting both tumor vasculature and breast cancer cells. Cancer Biol Ther. 2010;10(7):703‐711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gu J‐W, Young E, Busby B, Covington J, Johnson JW. Oral administration of Pyrrolidine Dithiocarbamate (PDTC) inhibits VEGF expression, tumor angiogenesis, and growth of breast cancer in female mice. Cancer Biol Ther. 2009;8(6):514‐521. [DOI] [PubMed] [Google Scholar]
- 41. Hiraga T, Ninomiya T. Establishment and characterization of a C57BL/6 mouse model of bone metastasis of breast cancer. J Bone Miner Metab. 2019;37(2):235‐242. [DOI] [PubMed] [Google Scholar]
- 42. Buss LA, Mandani A, Phillips E, Scott NJA, Currie MJ, Dachs GU. Characterisation of a mouse model of breast cancer with metabolic syndrome. Vivo. 2018;32(5):1071‐1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Buss LA, Dachs GU. Voluntary exercise slows breast tumor establishment and reduces tumor hypoxia in ApoE −/− mice. J Appl Physiol. 2018;124(4):938‐949. [DOI] [PubMed] [Google Scholar]
- 44. Zou Z, Bellenger S, Massey KA, et al. Inhibition of the HER2 pathway by n‐3 polyunsaturated fatty acids prevents breast cancer in fat‐1 transgenic mice. J Lipid Res. 2013;54(12):3453‐3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rogers D, Vila‐Leahey A, Pessôa AC, Oldford S, Marignani PA, Marshall JS. Ranitidine inhibition of breast tumor growth is b cell dependent and associated with an enhanced antitumor antibody response. Front Immunol. 2018;9:1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tu MM, Rahim MMA, Sayed C, Mahmoud AB, Makrigiannis AP. Immunosurveillance and immunoediting of breast cancer via class I MHC receptors. Cancer Immunol Res. 2017;5(11):1016‐1028. [DOI] [PubMed] [Google Scholar]
- 47. Das K, Eisel D, Lenkl C, et al. Generation of murine tumor cell lines deficient in MHC molecule surface expression using the CRISPR/Cas9 system. Fujii H, ed. PLoS One. 2017;12(3):e0174077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zheng X, Fang Z, Liu X, et al. Increased vessel perfusion predicts the efficacy of immune checkpoint blockade. J Clin Invest. 2018;128(5):2104‐2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Perry SW, Schueckler JM, Burke K, Arcuri GL, Brown EB. Stromal matrix metalloprotease‐13 knockout alters Collagen I structure at the tumor‐host interface and increases lung metastasis of C57BL/6 syngeneic E0771 mammary tumor cells. BMC Cancer. 2013;13(1):411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. McHowat J, Gullickson G, Hoover RG, Sharma J, Turk J, Kornbluth J. Platelet‐activating factor and metastasis: calcium‐independent phospholipase A 2 β deficiency protects against breast cancer metastasis to the lung. Am J Physiol‐Cell Physiol. 2011;300(4):C825‐C832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Scully T, Scott CD, Firth SM, et al. Enhancement of mammary tumour growth by IGFBP‐3 involves impaired T cell accumulation. Endocr Relat Cancer. 2018;25(2):111‐122. [DOI] [PubMed] [Google Scholar]
- 52. Scully T, Firth SM, Scott CD, et al. Insulin‐like growth factor binding protein‐3 links obesity and breast cancer progression. Oncotarget. 2016;7(34):55491‐55505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhang X, Hyer JM, Yu H, D’Silva NJ, Kirkwood KL. DUSP1 phosphatase regulates the proinflammatory milieu in head and neck squamous cell carcinoma. Cancer Res. 2014;74(24):7191‐7197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ouarné M, Bouvard C, Boneva G, et al. BMP9, but not BMP10, acts as a quiescence factor on tumor growth, vessel normalization and metastasis in a mouse model of breast cancer. J Exp Clin Cancer Res. 2018;37(1):209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hara T, Monguchi T, Iwamoto N, et al. Targeted disruption of JCAD (Junctional Protein Associated With Coronary Artery Disease)/KIAA1462, a coronary artery disease‐associated gene product, inhibits angiogenic processes in vitro and in vivo. Arterioscler Thromb Vasc Biol. 2017;37(9):1667‐1673. [DOI] [PubMed] [Google Scholar]
- 56. Bohlen J, McLaughlin SL, Hazard‐Jenkins H, et al. Dysregulation of metabolic‐associated pathways in muscle of breast cancer patients: preclinical evaluation of interleukin‐15 targeting fatigue: skeletal muscle transcriptome of breast cancer patients. J Cachexia Sarcopenia Muscle. 2018;9(4):701‐714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sishi BJN, Loos B, van Rooyen J, Engelbrecht A‐M. Autophagy upregulation promotes survival and attenuates doxorubicin‐induced cardiotoxicity. Biochem Pharmacol. 2013;85(1):124‐134. [DOI] [PubMed] [Google Scholar]
- 58. Wang SH, Lu L, Fan Y, et al. Characterization of a novel transgenic mouse tumor model for targeting HER2+ cancer stem cells. Int J Biol Sci. 2014;10(1):25‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yun E‐J, Song K‐S, Shin S, et al. Docosahexaenoic acid suppresses breast cancer cell metastasis by targeting matrix‐metalloproteinases. Oncotarget. 2016;7(31):49961‐49971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Maurer‐Schultze B, Bassukas ID, Loer E. Growth and proliferation of a transplantable mouse tumor and of human tumors growing in nude mice. Acta Histochem Suppl. 1990;39:81‐91. [PubMed] [Google Scholar]
- 61. Bassukas ID, Maurer‐Schultze B. The recursion formula of the Gompertz function: a simple method for the estimation and comparison of tumor growth curves. Growth Dev Aging GDA. 1988;52(3):113‐122. [PubMed] [Google Scholar]
- 62. Bassukas ID, Maurer‐Schultze B. Relationship between preimplantation host weight and growth of the mouse adenocarcinoma EO 771. Vivo Athens Greece. 1992;6(1):93‐96. [PubMed] [Google Scholar]
- 63. Stagg J, Divisekera U, McLaughlin N, et al. Anti‐CD73 antibody therapy inhibits breast tumor growth and metastasis. Proc Natl Acad Sci. 2010;107(4):1547‐1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ewens A, Luo L, Berleth E, et al. Doxorubicin plus Interleukin‐2 chemoimmunotherapy against breast cancer in mice. Cancer Res. 2006;66(10):5419‐5426. [DOI] [PubMed] [Google Scholar]
- 65. Jia X, Yu F, Wang J, et al. Emodin suppresses pulmonary metastasis of breast cancer accompanied with decreased macrophage recruitment and M2 polarization in the lungs. Breast Cancer Res Treat. 2014;148(2):291‐302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kanda T, Raychoudhuri A, Steele R, Sagartz JE, West C, Ray RB. MBP‐1 inhibits breast cancer growth and metastasis in immunocompetent mice. Cancer Res. 2009;69(24):9354‐9359. [DOI] [PubMed] [Google Scholar]
- 67. Stöth M, Freire Valls A, Chen M, et al. Splenectomy reduces lung metastases and tumoral and metastatic niche inflammation. Int J Cancer. 2019;145(9):2509‐2520. [DOI] [PubMed] [Google Scholar]
- 68. Goel S, Gupta N, Walcott BP, et al. Effects of vascular‐endothelial protein tyrosine phosphatase inhibition on breast cancer vasculature and metastatic progression. JNCI. 2013;105(16):1188‐1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Guillerey C, Chow MT, Miles K, et al. Toll‐like receptor 3 regulates NK cell responses to cytokines and controls experimental metastasis. OncoImmunology. 2015;4(9):e1027468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Sedlacek AL, Gerber SA, Randall TD, van Rooijen N, Frelinger JG, Lord EM. Generation of a dual‐functioning antitumor immune response in the peritoneal cavity. Am J Pathol. 2013;183(4):1318‐1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Karkeni E, Morin SO, Bou Tayeh B, et al. Vitamin D controls tumor growth and CD8+ T cell infiltration in breast cancer. Front Immunol. 2019;10:1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Huang YI, Ma C, Zhang Q, et al. CD4+ and CD8+ T cells have opposing roles in breast cancer progression and outcome. Oncotarget. 2015;6(19):17462‐17478. 10.18632/oncotarget.3958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Fourie C, Davis T, Kriel J, Engelbrecht A‐M. The paracrine effects of fibroblasts on doxorubicin‐treated breast cancer cells. Exp Cell Res. 2019;381(2):280‐287. [DOI] [PubMed] [Google Scholar]
- 74. Xiong Y, Russell DL, McDonald LT, Cowart LA, LaRue AC. Hematopoietic stem cell‐derived adipocytes promote tumor growth and cancer cell migration. Int J Cancer Res Mol Mech. 2017;3(1). 10.16966/2381-3318.130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Walker AK, Chang A, Ziegler AI, Dhillon HM, Vardy JL, Sloan EK. Low dose aspirin blocks breast cancer‐induced cognitive impairment in mice. Samant R, ed. PLOS ONE. 2018;13(12). 10.1371/journal.pone.0208593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Gilliam LAA, Lark DS, Reese LR, et al. Targeted overexpression of mitochondrial catalase protects against cancer chemotherapy‐induced skeletal muscle dysfunction. Am J Physiol‐Endocrinol Metab. 2016;311(2):E293‐E301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Yang Z, Tang X, Meng G, et al. Latent cytomegalovirus infection in female mice increases breast cancer metastasis. Cancers. 2019;11(4):447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Kerr J, Anderson C, Lippman SM. Physical activity, sedentary behaviour, diet, and cancer: an update and emerging new evidence. Lancet Oncol. 2017;18(8):e457‐e471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Margolis M, Perez O, Martinez M, et al. Phospholipid makeup of the breast adipose tissue is impacted by obesity and mammary cancer in the mouse: results of a pilot study. Biochimie. 2015;108:133‐139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Ke J‐Y, Banh T, Hsiao Y‐H, et al. Citrus flavonoid naringenin reduces mammary tumor cell viability, adipose mass, and adipose inflammation in obese ovariectomized mice. Mol Nutr Food Res. 2017;61(9). 10.1002/mnfr.201600934 [DOI] [PubMed] [Google Scholar]
- 81. Gu J‐W, Makey KL, Tucker KB, et al. EGCG, a major green tea catechin suppresses breast tumor angiogenesis and growth via inhibiting the activation of HIF‐1α and NFκB, and VEGF expression. Vasc Cell. 2013;5(1):9 10.1186/2045-824X-5-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Krug D, Baumann R, Budach W, et al. Current controversies in radiotherapy for breast cancer. Radiat Oncol Lond Engl. 2017;12(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Bornschein R, Porschen R, Porschen W, Mühlensiepen H, Feinendegen LE. Cell loss from viable and necrotic tumor regions after local gamma irradiation measured by 125I‐UdR. Strahlenther Onkol Organ Dtsch Rontgengesellschaft Al. 1987;163(2):114‐122. [PubMed] [Google Scholar]
- 84. Walter J, Maurer‐Schultze B. Regrowth, tumor cell proliferation and morphological alterations of the adenocarcinoma EO 771 following a single dose of 30 Gy 60Co gamma‐rays. Strahlenther Onkol Organ Dtsch Rontgengesellschaft Al. 1987;163(10):687‐694. [PubMed] [Google Scholar]
- 85. Porschen R, Porschen W, Mühlensiepen H, Feinendegen LE. Evaluation of radio‐ and chemotoxic effects of 125I‐UdR on tumour growth and host survival. Radiat Environ Biophys. 1985;24(3):219‐226. [DOI] [PubMed] [Google Scholar]
- 86. Porschen R, Porschen W, Mühlensiepen H, Feinendegen LE. Cell loss from viable and necrotic tumour regions measured by 125I‐UdR. Cell Tissue Kinet. 1983;16(6):549‐556. [PubMed] [Google Scholar]
- 87. Hassan . Chemotherapy for breast cancer (Review). Oncol Rep. 2010;24(5). 10.3892/or_00000963 [DOI] [PubMed] [Google Scholar]
- 88. Cabeza L, Ortiz R, Prados J, et al. Improved antitumor activity and reduced toxicity of doxorubicin encapsulated in poly(ε‐caprolactone) nanoparticles in lung and breast cancer treatment: an in vitro and in vivo study. Eur J Pharm Sci. 2017;102:24‐34. [DOI] [PubMed] [Google Scholar]
- 89. Walker L, Perkins E, Kratz F, Raucher D. Cell penetrating peptides fused to a thermally targeted biopolymer drug carrier improve the delivery and antitumor efficacy of an acid‐sensitive doxorubicin derivative. Int J Pharm. 2012;436(1–2):825‐832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Mihich E, Ehrke MJ. Anticancer drugs plus cytokines: immunodulation based therapies of mouse tumors. Int J Immunopharmacol. 2000;22(12):1077‐1081. [DOI] [PubMed] [Google Scholar]
- 91. Schmid FA, Sirotnak FM, Otter GM, DeGraw JI. Combination chemotherapy with a new folate analog: activity of 10‐ethyl‐10‐deaza‐aminopterin compared to methotrexate with 5‐fluorouracil and alkylating agents against advanced metastatic disease in murine tumor models. Cancer Treat Rep. 1987;71(7–8):727‐732. [PubMed] [Google Scholar]
- 92. Zhao SC, Banerjee D, Mineishi S, Bertino JR. Post‐transplant methotrexate administration leads to improved curability of mice bearing a mammary tumor transplanted with marrow transduced with a mutant human dihydrofolate reductase cDNA. Hum Gene Ther. 1997;8(8):903‐909. [DOI] [PubMed] [Google Scholar]
- 93. Piper JR, Malik ND, Rhee MS, Galivan J, Sirotnak FM. Synthesis and antifolate evaluation of the 10‐propargyl derivatives of 5‐deazafolic acid, 5‐deazaaminopterin, and 5‐methyl‐5‐deazaaminopterin. J Med Chem. 1992;35(2):332‐337. [DOI] [PubMed] [Google Scholar]
- 94. Piper JR, Johnson CA, Maddry JA, et al. Studies on analogues of classical antifolates bearing the naphthoyl group in place of benzoyl in the side chain. J Med Chem. 1993;36(26):4161‐4171. [DOI] [PubMed] [Google Scholar]
- 95. Piper JR, Ramamurthy B, Johnson CA, Otter GM, Sirotnak FM. Analogues of 10‐deazaaminopterin and 5‐alkyl‐5,10‐dideazaaminopterin with the 4‐substituted 1‐naphthoyl group in the place of 4‐substituted benzoyl. J Med Chem. 1996;39(2):614‐618. [DOI] [PubMed] [Google Scholar]
- 96. Sirotnak FM, DeGraw JI, Schmid FA, Goutas LJ, Moccio DM. New folate analogs of the 10‐deaza‐aminopterin series. Further evidence for markedly increased antitumor efficacy compared with methotrexate in ascitic and solid murine tumor models. Cancer Chemother Pharmacol. 1984;12(1):26‐30. [PubMed] [Google Scholar]
- 97. Sirotnak FM, Schmid FA, Samuels LL, DeGraw JI. 10‐Ethyl‐10‐deaza‐aminopterin: structural design and biochemical, pharmacologic, and antitumor properties. NCI Monogr Publ Natl Cancer Inst. 1987;5:127‐131. [PubMed] [Google Scholar]
- 98. Otter GM, Sirotnak FM. Effective combination therapy of metastatic murine solid tumors with edatrexate and the vinca alkaloids, vinblastine, navelbine and vindesine. Cancer Chemother Pharmacol. 1994;33(4):286‐290. [DOI] [PubMed] [Google Scholar]
- 99. Sirotnak FM, Otter GM, Schmid FA. Markedly improved efficacy of edatrexate compared to methotrexate in a high‐dose regimen with leucovorin rescue against metastatic murine solid tumors. Cancer Res. 1993;53(3):587‐591. [PubMed] [Google Scholar]
- 100. Kreis W, Hession C, Soricelli A, Scully K. Combinations of tetrahydrouridine and cytosine arabinoside in mouse tumors. Cancer Treat Rep. 1977;61(7):1355‐1364. [PubMed] [Google Scholar]
- 101. Vila‐Leahey A, Oldford SA, Marignani PA, Wang J, Haidl ID, Marshall JS. Ranitidine modifies myeloid cell populations and inhibits breast tumor development and spread in mice. OncoImmunology. 2016;5(7):e1151591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Bourgeois‐Daigneault M‐C, St‐Germain LE, Roy DG, et al. Combination of Paclitaxel and MG1 oncolytic virus as a successful strategy for breast cancer treatment. Breast Cancer Res. 2016;18(1):83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Vila‐Leahey A, Rogers D, Marshall JS. The impact of ranitidine on monocyte responses in the context of solid tumors. Oncotarget. 2016;7(10):10891‐10904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Morigi R, Locatelli A, Leoni A, et al. Synthesis, in vitro and in vivo biological evaluation of substituted 3‐(5‐imidazo[2,1‐b]thiazolylmethylene)‐2‐indolinones as new potent anticancer agents. Eur J Med Chem. 2019;166:514‐530. [DOI] [PubMed] [Google Scholar]
- 105. Mariotto E, Viola G, Ronca R, et al. Choline Kinase Alpha inhibition by EB‐3D triggers cellular senescence, reduces tumor growth and metastatic dissemination in breast cancer. Cancers. 2018;10(10):391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86(3):353‐364. [DOI] [PubMed] [Google Scholar]
- 107. Lu Y, Ni F, Xu M, et al. Alcohol promotes mammary tumor growth through activation of VEGF‐dependent tumor angiogenesis. Oncol Lett. 2014;8(2):673‐678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Sceneay J, Liu MCP, Chen A, Wong CSF, Bowtell DDL, Möller A. The antioxidant N‐acetylcysteine prevents HIF‐1 stabilization under hypoxia in vitro but does not affect tumorigenesis in multiple breast cancer models in vivo. PLoS One. 2013;8(6):e66388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. de Zoeten E, Carr‐Brendel V, Markovic D, Taylor‐Papadimitriou J. Cohen EP. Treatment of breast cancer with fibroblasts transfected with DNA from breast cancer cells. J Immunol Baltim Md. 1999;162(11):6934‐6941. [PubMed] [Google Scholar]
- 110. Deshmukh P, Glick RP, Lichtor T, Moser R, Cohen EP. Immunogene therapy with interleukin‐2‐secreting fibroblasts for intracerebrally metastasizing breast cancer in mice. J Neurosurg. 2001;94(2):287‐292. [DOI] [PubMed] [Google Scholar]
- 111. Yuan H, Wang X, Shi C, et al. Plac1 is a key regulator of the inflammatory response and immune tolerance in mammary tumorigenesis. Sci Rep. 2018;8(1):5717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25(12):677‐686. [DOI] [PubMed] [Google Scholar]
- 113. Ager EI, Kozin SV, Kirkpatrick ND, et al. Blockade of MMP14 activity in murine breast carcinomas: implications for macrophages, vessels, and radiotherapy. J Natl Cancer Inst. 2015;107(4): 10.1093/jnci/djv017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


