Abstract
Objective
The purpose of this umbrella review was to assess the associations between sarcopenia and adverse health‐related outcomes.
Design
An umbrella review of meta‐analyses of observational studies.
Setting and Participants
Patients with sarcopenia and controls without sarcopenia were included.
Measures
The PubMed, Web of Science and Embase were searched for relevant systematic review and meta‐analysis. AMSTAR and GRADE system were used for methodological quality and evidence quality assessments, respectively.
Results
Totally 54 outcomes extracted from 30 meta‐analyses were analyzed. Twenty out of 21 prognostic outcomes indicated that sarcopenia was significantly associated with poorer prognosis of gastric cancer, hepatocellular cancer, urothelial cancer, head and neck cancer, hematological malignancy, pancreatic cancer, breast cancer, colorectal cancer, lung cancer, esophageal cancer, and ovarian cancer. Besides, 10 out of 16 postoperative outcomes suggested that sarcopenia significantly increased the risk of multiple postoperative complications and prolonged the length of hospitalization of patients with digestive cancer. In age‐related outcomes, sarcopenia significantly increased the risk of dysphagia, cognitive impairment, fractures, falls, hospitalization, and all‐cause mortality of elderly populations. Moreover, sarcopenia was also associated with higher level of albuminuria, risk of depression, and several metabolic diseases.
Conclusions and Implications
Sarcopenia significantly affected a wide range of adverse health‐related outcomes, particularly in patients of tumor and elderly populations. Because evidences of most outcomes were rated as “low” and “very low,” more prospective cohort studies are required in the future.
Keywords: AMSTAR, GRADE, health‐related outcomes, sarcopenia, umbrella review
Sarcopenia significantly affected a wide range of adverse health‐related outcomes, particularly in patients of tumor and elderly populations. Besides, associations between sarcopenia and risk of metabolic diseases, depression and albuminuria were also noticeable.

1. INTRODUCTION
Sarcopenia was first described as an age‐related decline in lean body mass in the 1980s. 1 With sarcopenia research continuing for more than 30 years, recently the European Working Group on Sarcopenia in Older People (EWGSOP) revised the definition of sarcopenia as a progressive and generalized skeletal muscle disorder that is characterized by low muscle strength, low muscle quantity or quality, and low physical performance. 2 Sarcopenia is a common disease worldwide, which is mainly associated with aging and older people, and it is also secondary to a systemic disease such as malignancy. It was suggested that the prevalence of sarcopenia was 10% in general elderly population worldwide. 3 For specific populations, the prevalence of sarcopenia was 14.7% in hospitalized older patients, 41% to 59% in older nursing home residents, 12.9% to 40.4% in community living older adults, and 38.6% in cancer patients. 4 , 5 , 6 , 7
Sarcopenia is such a highly prevalent disease that might promote several adverse health‐related outcomes. Previous studies suggested that cancer patients with pre‐therapeutic sarcopenia had higher risk of postoperative complications, chemotherapy‐induced toxicity, and poorer survival than those without sarcopenia, 6 and elderly people with sarcopenia were associated with functional decline, higher rate of hospitalizations, falls, and fractures. 8 A few meta‐analyses have investigated the associations between sarcopenia and various health‐related outcomes, in which some results were inconsistent. For example, a meta‐analysis of seven studies 9 suggested that sarcopenia was not associated with higher risk of major postoperative complications in patients of liver cancer, while another meta‐analysis of 28 studies 10 indicated that sarcopenia significantly increased the risk of major postoperative complications in patients with gastrointestinal (GI) cancer. Recently, we also noticed an umbrella review that investigated the associations between sarcopenia and health‐related outcomes in older people. 11 However, this umbrella review contained only six meta‐analyses with 14 outcomes, and current meta‐analyses about sarcopenia and prognostic outcomes of tumor, metabolic outcomes, and risk of depression were not included.
To better understand this issue, we systematically searched all the relevant meta‐analyses and provided an overview about the associations between sarcopenia and adverse health‐related outcomes in this study, and unified evidence assessments were also performed for all the outcomes reported currently.
2. METHODS
2.1. Literature search and eligibility criteria
For reviewing the existing meta‐analyses about sarcopenia and health‐related outcomes, we conducted this umbrella review according to the standardized procedures described previously. 12 , 13 The PubMed, Web of Science, and Embase were searched from the inception of the databases to April 2020. The following terms were used for search: (sarcopenia* OR sarcopenic* OR muscle*) AND (systematic review* OR meta‐analysis*), and detailed search strategies were shown in the Figure S1. Besides, we also reviewed the references of related studies for identifying potential meta‐analyses that were possibly missed in the initial search. Two authors reviewed the identified studies independently, and the inclusion criteria were: (a) published meta‐analysis or systematic review and meta‐analysis in English language, (b) investigating the associations between sarcopenia and health‐related outcomes, and (c) the summary effect size with 95% confidence intervals (CI) were reported. Systematic reviews without meta‐analysis and animal studies were excluded. All differences were discussed and resolved by consensus.
2.2. Data extraction
The data in each meta‐analysis were extracted by two authors independently. Briefly, the data we extracted were as follows: health‐related outcomes, the first author, year of publication, population characteristics, follow‐up, assessment of skeletal muscle, the number of studies and participants, metric of effect size, effects model of meta‐analysis, effect size with 95% CI, value of I 2, and publication bias. When a meta‐analysis contained multiple outcomes, each outcome would be extracted separately. Besides, if multiple meta‐analyses investigated a same outcome, usually we chose the newest meta‐analysis with the largest number of studies.
2.3. Methodological quality and evidence quality assessment
AMSTAR and the GRADE system were used for assessing the methodological quality of meta‐analysis and evidence quality of health‐related outcomes, respectively. AMSTAR was a measurement tool consisting of 11 items that has been shown to have good agreement, reliability, construct validity, and feasibility for methodological quality assessment, 14 , 15 and the GRADE system was an approach that offers a transparent and structured process for developing and presenting the summaries of evidence. 16 In AMSTAR, the methodological quality was usually categorized as high (8‐11 items achieved), moderate (4‐7 items achieved), and low (0‐3 items achieved). 17 In GRADE system, according to the assessment of risk of bias, inconsistence, indirectness, imprecision, and publication bias, the evidence quality was divided into four categories (high, moderate, low, and very low). 18
2.4. Data analysis
Instead of searching the primary studies in meta‐analysis and reanalyzing the summary estimates with 95% CI, we just extracted the existing effect size and 95% CI for each health‐related outcome. 12 When both random effects model and fixed effects model were performed for a same outcome, we primarily chose the one with random effects model as the final outcome. The value of I 2 and P value of Egger's or Begg's test in related meta‐analysis were extracted as the measures of heterogeneity and publication bias, respectively. If these data were lacked in meta‐analysis, we would calculate the I2 statistic to assess heterogeneity when detailed original data were available, and we also performed the Egger's test for assessing the publication bias when the health‐related outcome contained at least 10 studies. 19 , 20 A value of I2 > 50% was regarded as significant heterogeneity, and P value of <.1 for Egger's test indicated statistically significant publication bias. If P value of Egger's test <0.1, it could be an evidence of small‐study effects (whether smaller studies tend to give substantially larger estimates of effect size compared with larger studies) when the effect size of the largest study was more conservative than the summary effect size of the random effects meta‐analysis. 21
3. RESULTS
3.1. Search results and study characteristics
We identified 3442 articles from PubMed, 10 480 articles from the Web of Science, and 3372 articles from Embase by the initial search. Additionally, nine articles were identified by reviewing the references of the related studies. Flowchart of the selection process was showed in Figure S2. Totally 54 studies met the inclusion criteria and were included for further assessment (references of the 54 studies were showed in supplementary material). Because there were several meta‐analyses investigating the same health‐related outcomes, we compared these meta‐analyses according to their publication year and number of included studies. Then, we chose the newest meta‐analysis with the largest number of studies. Finally, 54 health‐related outcomes extracted from 30 meta‐analyses 10 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 were reported in this umbrella review. These 54 outcomes were mainly about prognostic outcomes of tumor, postoperative outcomes, age‐related outcomes, metabolic outcomes, and other outcomes. Among the 54 outcomes, median number of included studies was 6 (range 2‐28), and the median number of participants was 1851 (range 485‐23 061) (Table 1).
TABLE 1.
Associations between sarcopenia and adverse health‐related outcomes
| Outcome | Author; year | Follow‐up | Assessment of skeletal muscle | No. of studies; participants | Metric of MA | Effects model | Effect size | 95% CI | I 2 % | Publication bias | Small‐study effects | Quality of evidence |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prognostic outcomes of tumor | ||||||||||||
| OS (head and neck cancer) | Wong, A., et al, 2020 | Range from 11 to 68 mo | CT | 10; 2181 | HR | REM | 1.98 | 1.64‐2.39 | 0.00 | Yes | Yes | Low |
| All‐cause mortality (breast cancer) | Zhang, X. M., et al, 2020 | Range from 1.9 to 12 y | CT and DXA | 6; 5497 | HR | REM | 1.71 | 1.25‐2.33 | 59.10 | None | None | Low |
| Non‐relapse mortality (hematological malignancy) | Jia, S., et al, 2020 | NR | CT | 3; 1123 | OR | REM | 1.97 | 1.45‐2.68 | 0.00 | NR | NR | Very low |
| OS (GI cancer) | Su, H., et al, 2019 | NR | CT | 20; 6232 | HR | REM | 1.60 | 1.37‐1.87 | 59.50 | None | None | Low |
| DFS (GI cancer) | Su, H., et al, 2019 | NR | CT | 11; 4640 | HR | FEM | 1.46 | 1.30‐1.65 | 0.00 | None | None | Moderate |
| OS (pancreatic cancer) | Bundred, J., et al, 2019 | NR | CT and BIA | 8; NR | OR | REM | 1.95 | 1.35‐2.81 | 92.00 | None | None | Very low |
| OS (gastric cancer) | Kamarajah, S. K., et al, 2019 | NR | CT | 9; 4236 | HR | FEM | 2.12 | 1.89‐2.38 | 37.00 | None | None | Moderate |
| RFS (gastric cancer) | Kamarajah, S. K., et al, 2019 | NR | CT | 3; 1851 | HR | FEM | 2.12 | 1.82‐2.47 | 40.00 | None | None | Low |
| CSS (gastric cancer) | Kamarajah, S. K., et al, 2019 | NR | CT | 3; 1741 | HR | FEM | 2.00 | 1.54‐2.59 | 0.00 | None | None | Low |
| OS (esophageal cancer) | Deng, H. Y., et al, 2019 | Range from 20 to 39.3 mo | CT | 11; 1520 | HR | FEM | 1.58 | 1.35‐1.85 | 23.50 | None | None | Moderate |
| DFS (esophageal cancer) | Deng, H. Y., et al, 2019 | Range from 20 to 39.4 mo | CT | 4; 561 | HR | FEM | 1.46 | 1.12‐1.90 | 0.00 | NR | NR | Very low |
| OS (urothelial cancer) | Hu, X., et al, 2019 | Range from 6 to 227 mo | CT | 11; 1816 | HR | REM | 1.87 | 1.43‐2.45 | 54.30 | None | None | Low |
| CSS (urothelial cancer) | Hu, X., et al, 2019 | Range from 6 to 227 mo | CT | 10; 1513 | HR | REM | 1.98 | 1.43‐2.75 | 39.40 | None | None | Moderate |
| OS (lung cancer) | Deng, H. Y., et al, 2019 | Range from 0 to 145 mo | CT | 6; 1213 | RR | REM | 1.63 | 1.13‐2.33 | 73.10 | None | None | Low |
| DFS (lung cancer) | Deng, H. Y., et al, 2019 | Range from 0 to 146 mo | CT | 3; 577 | RR | REM | 1.14 | 0.59‐2.17 | 72.10 | NR | NR | Very low |
| OS (ovarian cancer) | Ubachs, J., et al, 2019 | NR | CT | 6; 1198 | HR | FEM | 1.11 | 1.03‐1.20 | 38.00 | NR | NR | Low |
| OS (colorectal cancer) | Sun, G., et al, 2018 | NR | CT | 6; 4279 | HR | REM | 1.63 | 1.24‐2.14 | 48.40 | None | None | Moderate |
| DFS (colorectal cancer) | Sun, G., et al, 2018 | NR | CT | 5; 1809 | HR | REM | 1.70 | 1.24‐2.32 | 31.20 | NR | NR | Low |
| CSS (colorectal cancer) | Sun, G., et al, 2018 | NR | CT | 3; 2792 | HR | REM | 1.62 | 1.16‐2.27 | 17.60 | NR | NR | Very low |
| All‐cause mortality (hepatocellular cancer) | Chang, K. V., et al, 2018 | NR | CT | 11; 2794 | HR | REM | 2.04 | 1.75‐2.38 | <0.001 | None | None | Moderate |
| Recurrence (hepatocellular cancer) | Chang, K. V., et al, 2018 | NR | CT | 6; 862 | HR | REM | 1.85 | 1.45‐2.38 | <0.001 | None | None | Low |
| Postoperative outcomes | ||||||||||||
| Postoperative pulmonary complications (esophageal cancer) | Wang, P. Y., et al, 2020 | NR | CT and BIA | 13; 2267 | OR | REM | 2.14 | 1.50‐3.04 | 46.40 | None | None | Moderate |
| Anastomotic leakage (esophageal cancer) | Wang, P. Y., et al, 2020 | NR | CT and BIA | 12; 2163 | OR | FEM | 1.29 | 0.99‐1.67 | 7.90 | None | None | Moderate |
| Overall postoperative complications (esophageal cancer) | Wang, P. Y., et al, 2020 | NR | CT | 11; 1972 | OR | REM | 1.42 | 1.08‐1.88 | 41.60 | None | None | Moderate |
| Rate of readmission (digestive cancer) | Hua, H., et al, 2019 | NR | CT and BIA | 5; 919 | RR | FEM | 2.53 | 1.66‐3.85 | 0.00 | NR | NR | Very low |
| Length of hospitalization (digestive cancer) | Hua, H., et al, 2019 | NR | CT and BIA | 9; 2174 | RR | REM | 4.61 | 1.84‐7.39 | 65.00 | NR | NR | Very low |
| Major complications (GI cancer) | Simonsen, C., et al, 2018 | NR | CT | 28; 6883 | RR | REM | 1.40 | 1.20‐1.64 | 52.00 | Yes | None | Very low |
| Major complications (patients of GI cancer with ERAS care) | Simonsen, C., et al, 2018 | NR | CT | 4; 703 | RR | REM | 1.29 | 0.91‐1.83 | 12.00 | NR | NR | Very low |
| Major complications (patients of GI cancer without ERAS care) | Simonsen, C., et al, 2018 | NR | CT | 24; 6180 | RR | REM | 1.44 | 1.21‐1.71 | 56.00 | Yes | None | Very low |
| Total complications (GI cancer) | Simonsen, C., et al, 2018 | NR | CT | 12; 3051 | RR | REM | 1.35 | 1.12‐1.61 | 60.00 | None | None | Low |
| Postoperative pneumonia (gastric cancer) | Yang, Z., et al, 2018 | NR | CT | 6; 1563 | OR | FEM | 6.24 | 3.38‐11.51 | 0.00 | NR | NR | Low |
| Postoperative ileus (gastric cancer) | Yang, Z., et al, 2018 | NR | CT | 5; 1464 | OR | FEM | 5.83 | 2.59‐13.08 | 21.00 | NR | NR | Low |
| Postoperative intra‐abdominal infection (gastric cancer) | Yang, Z., et al, 2018 | NR | CT | 7; 1720 | OR | FEM | 1.15 | 0.64‐2.05 | 0.00 | NR | NR | Low |
| Postoperative anastomotic leakage (gastric cancer) | Yang, Z., et al, 2018 | NR | CT | 7; 1720 | OR | FEM | 1.16 | 0.58‐2.33 | 0.00 | NR | NR | Low |
| Postoperative delayed gastric emptying (gastric cancer) | Yang, Z., et al, 2018 | NR | CT | 4; 994 | OR | FEM | 1.22 | 0.45‐3.26 | 44.00 | NR | NR | Very low |
| Postoperative infection (colorectal cancer) | Sun, G., et al, 2018 | NA | CT | 5; 1179 | OR | REM | 2.21 | 1.50‐3.25 | 0.00 | NR | NR | Low |
| Postoperative anastomotic leakage (colorectal cancer) | Sun, G., et al, 2018 | NA | CT | 6; 2106 | OR | REM | 0.73 | 0.51‐1.05 | 0.00 | NR | NR | Low |
| Age‐related outcomes | ||||||||||||
| Rate of hospitalization (people over 65 y old) | Zhao, Y., et al, 2019 | Range from 0.5 to 7 y | BIA and DXA | 8; 8174 | RR | REM | 1.40 | 1.04‐1.89 | 67.40 | NR | NR | Very low |
| Rate of readmission (hospitalized people over 65 y old) | Zhao, Y., et al, 2019 | Range from 0.5 to 3 y | BIA and DXA | 4; 1302 | RR | REM | 1.75 | 1.01‐3.03 | 76.00 | NR | NR | Very low |
| Length of hospitalization (community living people over 65 y old) | Zhao, Y., et al, 2019 | Range from 3 to 7 y | BIA and DXA | 4; 6276 | OR | REM | 1.21 | 0.90‐1.63 | 75.40 | NR | NR | Very low |
| Risk of falls (community living people over 65 y old) | Yeung, S. S. Y., et al, 2019 | NR | BIA and DXA | 16; 23 061 | OR | REM | 1.75 | 1.55‐1.97 | 7.00 | None | None | Moderate |
| Risk of falls (people over 60 y old in nursing home) | Zhang, X., et al, 2019 | NR | BIA and DXA | 3; 996 | OR | FEM | 1.12 | 0.84‐1.51 | 16.90 | NR | NR | Very low |
| Risk of fractures (people over 65 y old) | Yeung, S. S. Y., et al, 2019 | NR | BIA and DXA | 12; 18 944 | OR | REM | 1.84 | 1.30‐2.62 | 91.00 | None | None | Low |
| All‐cause mortality (elderly people in nursing home) | Zhang, X., et al, 2018 | Range from 6 to 24 mo | BIA | 6; 1494 | HR | FEM | 1.86 | 1.42‐2.45 | 0.00 | None | None | Moderate |
| All‐cause mortality (community living people over 65 y old) | Liu, P., et al, 2017 | Range from 3 to 14.4 mo | BIA and DXA | 6; 7367 | HR | REM | 1.60 | 1.24‐2.06 | 27.80 | None | None | Moderate |
| Risk of cognitive impairment (community living people over 60 y old) | Cabett Cipolli, G., et al, 2019 | NR | NR | 6; 7045 | OR | REM | 2.50 | 1.26‐4.92 | 84.00 | NR | NR | Very low |
| Risk of dysphagia (people over 60 y old) | Zhao, W. T., et al, 2018 | NR | CT and BIA | 5; 913 | OR | FEM | 6.17 | 3.81‐10.00 | 15.97 | NR | NR | Very low |
| Metabolic outcomes | ||||||||||||
| Hepatic encephalopathy (patients with liver cirrhosis) | Chang, K. V., et al, 2019 | NR | CT | 6; 1795 | OR | REM | 2.74 | 1.87‐4.01 | 54.97 | Yes | Yes | Very low |
| Metabolic syndrome (middle‐aged and older nonobese adults) | Zhang, H., et al, 2018 | NR | DXA | 13; 4427 | OR | REM | 2.01 | 1.63‐2.47 | 79.20 | None | None | Low |
| Steatohepatitis (patients with nonalcoholic fatty liver disease) | Yu, R., et al, 2018 | NR | BIA and DXA | 2; 534 | OR | FEM | 2.35 | 1.45‐3.81 | 0.00 | NR | NR | Very low |
| Risk of nonalcoholic fatty liver disease | Pan, X., et al, 2018 | NR | BIA and DXA | 7; 18 654 | OR | REM | 1.29 | 1.12‐1.49 | 61.00 | None | None | Low |
| Mortality of liver cirrhosis (patients with liver cirrhosis) | Kim, G., et al, 2017 | NR | CT | 4; 485 | OR | REM | 3.23 | 2.08‐5.01 | 32.00 | None | None | Low |
| Other outcomes | ||||||||||||
| Albuminuria (patients with diabetes) | Ida, S., et al, 2019 | NR | DXA | 5; 1958 | OR | REM | 2.11 | 1.55‐2.88 | 45.00 | NR | NR | Low |
| Risk of depression | Chang, K. V., et al, 2017 | NR | BIA and DXA | 10; 23 051 | OR | REM | 1.64 | 1.25‐2.16 | 64.38 | Yes | Yes | Very low |
Abbreviations: BIA, bioimpedance analysis; CI, confidence intervals; CSS, cancer‐specific survival; CT, computed tomography; DFS, disease‐free survival; DXA, dual x‐ray absorptiometry; FEM, fixed effects model; GI cancer, gastrointestinal cancer; HR, hazard ratios; MA, meta‐analysis; NR, not reported; OR, odds ratios; OS, overall survival; REM, random effects model; RFS, recurrence‐free survival; RR, relative risk.
3.2. Prognostic outcomes of tumor
There were totally 21 prognostic outcomes of over 12 kinds of tumors reported in this umbrella review 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 36 (Table 1). Associations between sarcopenia and overall survival (OS) were investigated in head and neck cancer, GI cancer, pancreatic cancer, gastric cancer, esophageal cancer, urothelial cancer, lung cancer, ovary cancer, and colorectal cancer, and sarcopenia was significantly associated with poorer OS of all these tumors. Besides, compared to those without sarcopenia, breast cancer and hepatocellular cancer patients with sarcopenia had 71% and 104% increased all‐cause mortality, respectively, and sarcopenia also increased the risk of recurrence of hepatocellular cancer (HR, 1.85; 95% CI 1.45‐2.38). Prognostic outcomes of disease‐free survival (DFS) were reported in four kinds of tumors, in which sarcopenia significantly decreased the DFS of GI cancer, esophageal cancer, and colorectal cancer, while no significant association was showed in lung cancer. Cancer‐specific survival (CSS) of gastric cancer, urothelial cancer, and colorectal cancer and recurrence‐free survival (RFS) of gastric cancer all had significantly inverse correlations with sarcopenia. For hematological malignancy, sarcopenia leaded to a 97% increment of non‐relapse mortality (OR, 1.97; 95% CI 1.45‐2.68).
In summary, among the 21 prognostic outcomes of tumor, 20 (95%) outcomes had significant associations with sarcopenia. According to the effect size, prognosis of gastric cancer was most affected by sarcopenia (Figure 1).
FIGURE 1.
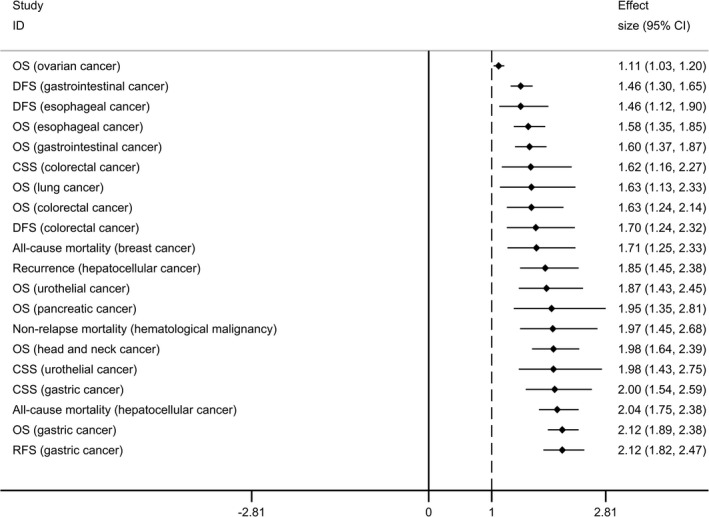
Forest plot of prognostic outcomes of tumor having significant associations with sarcopenia
3.3. Postoperative outcomes
Totally 16 postoperative outcomes of tumors were reported. 10 , 25 , 33 , 34 , 35 For esophageal cancer, patients with sarcopenia had significantly higher risk of overall postoperative complications and pulmonary complications, while no association was found with anastomotic leakage. In patients of digestive cancer, sarcopenia significantly increased the rate of readmission (RR, 2.53; 95% CI 1.66‐3.85) and prolonged the length of hospitalization (RR, 4.61; 95% CI 1.84‐7.39). Both major postoperative complications and total postoperative complications were increased by 40% and 35% in patients of GI cancer with sarcopenia, respectively. Moreover, subgroup analysis found that in patients of GI cancer with Enhanced Recovery after Surgery (ERAS) care, sarcopenia had no associations with the major complications (RR, 1.29; 95% CI 0.91‐1.83), whereas sarcopenia was also associated with increased major complications in those without ERAS care (RR, 1.44; 95% CI 1.21‐1.71). Additionally, sarcopenia was associated with increased postoperative pneumonia and ileus in patients of gastric cancer and increased postoperative infection in patients of colorectal cancer, respectively. However, no significant associations were showed between sarcopenia and postoperative intra‐abdominal infection, anastomotic leakage, and delayed gastric emptying in gastric cancer, and sarcopenia neither had association with postoperative anastomotic leakage in colorectal cancer.
In summary, 10 out of 16 postoperative outcomes (63%) had significant associations with sarcopenia. According to the effect size, total complications and major complications of GI cancer were comparatively less affected by sarcopenia, while the postoperative pneumonia and ileus of gastric cancer were most affected by sarcopenia (Figure 2).
FIGURE 2.
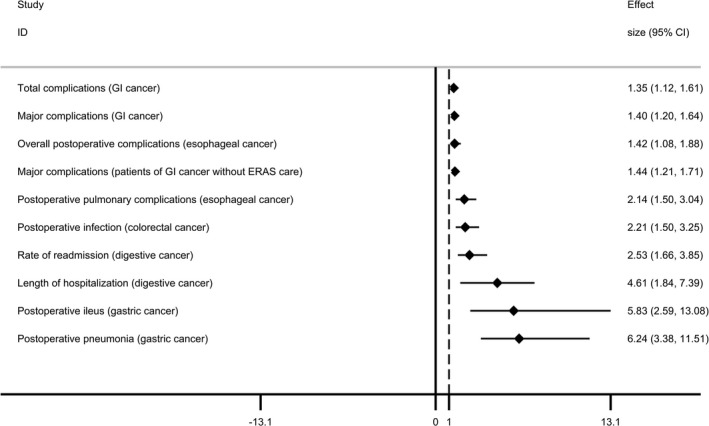
Forest plot of postoperative outcomes having significant associations with sarcopenia
3.4. Age‐related outcomes
There were totally 10 age‐related outcomes. 37 , 38 , 39 , 40 , 41 , 42 , 43 In people over 65 years old, sarcopenia leaded to increased rate of hospitalization (RR 1.40, 95% CI 1.04‐1.89) and risk of fractures (OR 1.84, 95% CI 1.30‐2.62). Moreover, hospitalized people over 65 years old with sarcopenia had higher rate of readmission (RR 1.75, 95% CI 1.01‐3.03). In community living people over 65 years old, sarcopenia was associated with higher risk of falls and all‐cause mortality, while no association was showed with length of hospitalization. In people over 60 years old and community living people over 60 years old, those with sarcopenia had significantly higher risk of dysphagia and cognitive impairment, respectively. In nursing home, elderly people with sarcopenia had significantly higher all‐cause mortality, while there was no association between sarcopenia and risk of falls.
In summary, eight out of 10 age‐related outcomes (80%) had significant associations with sarcopenia. Compared with people over 65 years old with sarcopenia in community, elderly people with sarcopenia in nursing home had higher all‐cause mortality. Moreover, the risk of dysphagia in people over 60 years old was most affected by sarcopenia (Figure 3).
FIGURE 3.
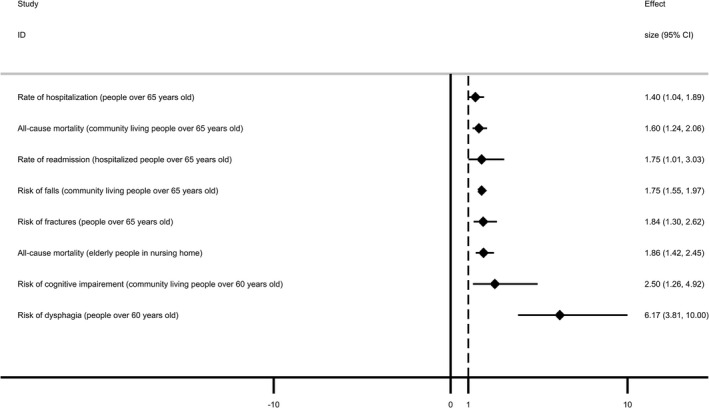
Forest plot of age‐related outcomes having significant associations with sarcopenia
3.5. Metabolic outcomes
Five meta‐analyses included in this study reported five metabolic outcomes. 44 , 45 , 46 , 47 , 48 In middle‐aged and older nonobese adults, sarcopenia significantly increased the risk of metabolic syndrome (OR 2.01, 95% CI 1.63‐2.47). Besides, people with sarcopenia had a 29% increased risk of nonalcoholic fatty liver disease, and in patients with nonalcoholic fatty liver disease, sarcopenia was associated with higher risk of steatohepatitis (OR 2.35, 95% CI 1.45‐3.81). Sarcopenia also leaded to increased risk of hepatic encephalopathy and mortality in patients with liver cirrhosis. In summary, all the five metabolic outcomes had significant associations with sarcopenia, in which the mortality in patients with liver cirrhosis was most affected by sarcopenia (Figure 4).
FIGURE 4.
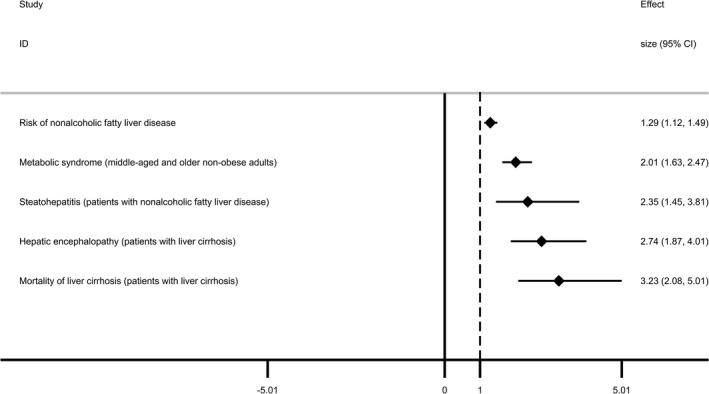
Forest plot of metabolic outcomes having significant associations with sarcopenia
3.6. Other outcomes
There were two single outcomes. 49 , 50 One reported that sarcopenia had positive correlation with albuminuria in patients with diabetes (OR 2.11, 95% CI 1.55‐2.88), and the other one showed that people with sarcopenia had higher risk of depression (OR 1.64, 95% CI 1.25‐2.16).
3.7. AMSTAR assessment and GRADE classification
The methodological quality of included meta‐analyses was assessed by AMSTAR which contained 11 items for scoring. Among the 30 included meta‐analyses, the median AMSTAR score was 8 (range 6‐11). Twenty‐two meta‐analyses (73%) had high methodological quality, and eight meta‐analyses (27%) had moderate methodological quality (Table S1).
Evidence quality assessment of the 54 health‐related outcomes was based on the GRADE system. Twelve outcomes (22%) were rated as “moderate,” 22 outcomes (41%) were rated as “low,” and 20 outcomes (37%) were rated as “very low.” Because all meta‐analyses in this umbrella review contained only observational studies, the risk of bias could be serious, and there was no outcome meeting a high quality of evidence. Moreover, high heterogeneity, small number of included studies or participants and significant publication bias also decreased the evidence quality of outcomes in this umbrella review. Detailed evidence quality assessments of the 54 outcomes were showed in Table S2.
4. DISCUSSION
In this umbrella review, we analyzed 30 current meta‐analyses and developed an overview of the associations between sarcopenia and 54 adverse health‐related outcomes. Particularly, the associations between sarcopenia and prognosis of tumor accounted for the largest percentage (39%) of the 54 outcomes. Although the evidences of majority prognostic outcomes were rated as “low” and “very low,” 95% of them had significant associations with sarcopenia, which indicating that sarcopenia was associated with poorer prognosis of diverse tumors. In postoperative outcomes, the tumors were mainly located at digestive tract, and sarcopenia was significantly associated with increased major postoperative complications, total postoperative complications, and several specific postoperative complications. Besides, about one thirds of specific postoperative outcomes were not associated with sarcopenia. Interestingly, we noticed that in patients of GI cancer with ERAS care, sarcopenia had no associations with the major postoperative complications. However, in patients of GI cancer without ERAS care, sarcopenia significantly increased the major postoperative complications. Although evidences of these two outcomes were rated as “very low,” we supposed that ERAS care might be helpful to improving the sarcopenia‐related postoperative complications, which needs more studies to verify in the future. Associations between sarcopenia and age‐related outcomes were also noticeable. Sarcopenia significantly affected a wide range of adverse outcomes such as all‐cause mortality, risk of falls, cognitive impairment, and dysphagia in different elderly populations, which seriously impaired the quality of life of the elderly. Moreover, sarcopenia was associated with several metabolic diseases and other outcomes including albuminuria and risk of depression in diverse populations, indicating that sarcopenia was a systematic medical condition and affected the human body more than the skeletal muscles themselves.
Sarcopenia was characterized by low muscle strength plus low muscle mass, so it might increase risk of falls and fractures in elderly people. Besides, decline of muscle function could affect the swallowing and breath and thereby increased the risk of dysphagia and postoperative pneumonia. Sarcopenia in cancer patients was commonly accompanied with malnutrition and disabled immune function, and it was also associated with higher chemotherapy toxicity and less efficacy of immunotherapy, 51 , 52 therefore, leading to higher postoperative complications and worse survival. In elderly people, some studies found that sarcopenia were closely associated with several comorbidities such as peptic ulcer disease, chronic obstructive pulmonary disease, osteoporosis, Parkinson's disease, and diabetes mellitus, 53 , 54 , 55 , 56 , 57 which may explain why sarcopenia was associated with a wide range of age‐related outcomes such as higher all‐cause mortality, risk of hospitalization, readmission, and cognitive impairment. Skeletal muscle is an important organ for insulin‐mediated glucose uptake. Loss of skeletal muscle mass could lead to metabolism changes including decrease of insulin sensitivity, upregulation of gluconeogenesis, enhanced lipolysis, and generation of free fatty acids. Then, liver may take up the elevated fatty liver acids and excess glucose, which increased the risk of metabolic diseases. 58 , 59 , 60
Current preventions and treatments for sarcopenia mainly included nutrition support and physical exercise. For healthy older populations, studies found that fish oil‐derived omega‐3 PUFA intake, high protein intake, resistance exercise training, and vitamin D3 supplements can be helpful for improving muscle mass and functions as well as preventing sarcopenia. 61 , 62 , 63 , 64 , 65 Nitrate‐rich diets and oral nutritional support combined with exercise were also associated with better muscle functions. 66 , 67 Moreover, beta‐Hydroxy‐beta‐methylbutyrate supplements, high‐intensity resistance training, and dairy protein intake could be useful therapies for improving sarcopenia, and fat and fish dietary pattern might be associated with lower risk of sarcopenia in patients with GI cancer. 68 , 69 , 70 , 71 Although drug therapies such as testosterone, myostatin antibodies, and activin receptor antibodies might have potential effects on sarcopenia treatment, 72 and recently a randomized controlled study reported that treatment with bimagrumab over 16 weeks increased muscle mass and strength in older adults with sarcopenia. 73 Evidences of drug therapy for sarcopenia were still limited, and more studies about this issue are required.
There were several strengths in our study. We developed an overview of associations between sarcopenia and adverse health‐related outcomes in different populations. Totally we analyzed 30 meta‐analyses and reported 54 outcomes. The methodological quality of included studies and evidence quality of reported outcomes were assessed by unified method, and we found that sarcopenia significantly affected a wide range of adverse health‐related outcomes. There were also some limitations in this study. Meta‐analyses in this umbrella review contained only observational studies, which could decrease the quality of evidence. Besides, the methods for assessing the skeletal muscle were inconsistent, and CT, BIA, and DXA were applied in different meta‐analyses, which might increase the risk of bias.
5. CONCLUSIONS AND IMPLICATIONS
In conclusion, sarcopenia significantly affected a wide range of adverse health‐related outcomes, particularly in patients of tumor and elderly populations. Besides, associations between sarcopenia and risk of metabolic diseases, depression and albuminuria were also noticeable. Considering that evidences of most outcomes were rated as “low” and “very low,” more prospective cohort studies are required in the future.
CONFLICT OF INTEREST
There was no conflict of interest.
AUTHOR CONTRIBUTIONS
LX, RZ, and QYW contributed equally in this study. LX, RZ, QYW, YZ, YW, YPC, and XDS contributed to the data collection and analysis. LX, RZ, QYW, and YTW wrote the manuscript under the guidance of XTW. All the authors have read manuscript, and XTW approved the final manuscript.
ETHICAL APPROVAL
This is an umbrella review of meta‐analysis, and ethical approval is not applicable.
Supporting information
Supplementary Material
Xia L, Zhao R, Wan Q, et al. Sarcopenia and adverse health‐related outcomes: An umbrella review of meta‐analyses of observational studies. Cancer Med. 2020;9:7964–7978. 10.1002/cam4.3428
Funding information
This work was supported by Sichuan Province Science and Technology Support Project (2018SZ0189).
DATA AVAILABILITY STATEMENT
All data generated or analyzed during this study are included in this published article.
REFERENCES
- 1. Cruz‐Jentoft AJ, Sayer AA. Sarcopenia. Lancet. 2019;393(10191):2636‐2646. [DOI] [PubMed] [Google Scholar]
- 2. Cruz‐Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(1):16‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shafiee G, Keshtkar A, Soltani A, Ahadi Z, Larijani B, Heshmat R. Prevalence of sarcopenia in the world: a systematic review and meta‐analysis of general population studies. J Diabetes Metab Disord. 2017;16:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mayhew AJ, Amog K, Phillips S, et al. The prevalence of sarcopenia in community‐dwelling older adults, an exploration of differences between studies and within definitions: a systematic review and meta‐analyses. Age Ageing. 2019;48(1):48‐56. [DOI] [PubMed] [Google Scholar]
- 5. Martone AM, Bianchi L, Abete P, et al. The incidence of sarcopenia among hospitalized older patients: results from the Glisten study. J Cachexia Sarcopeni. 2017;8(6):907‐914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pamoukdjian F, Bouillet T, Levy V, Soussan M, Zelek L, Paillaud E. Prevalence and predictive value of pre‐therapeutic sarcopenia in cancer patients: a systematic review. Clin Nutr. 2018;37(4):1101‐1113. [DOI] [PubMed] [Google Scholar]
- 7. Shen YJ, Chen J, Chen XY, Hou LS, Lin XF, Yang M. Prevalence and associated factors of sarcopenia in nursing home residents: a systematic review and meta‐analysis. J Am Med Directors Ass. 2019;20(1):5‐13. [DOI] [PubMed] [Google Scholar]
- 8. Beaudart C, Zaaria M, Pasleau F, Reginster JY, Bruyere O. Health outcomes of sarcopenia: a systematic review and meta‐analysis. PLoS One. 2017;12(1):e0169548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang G, Meng S, Li R, Ye J, Zhao L. Clinical significance of sarcopenia in the treatment of patients with primary hepatic malignancies, a systematic review and meta‐analysis. Oncotarget. 2017;8(60):102474‐102485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Simonsen C, de Heer P, Bjerre ED, et al. Sarcopenia and postoperative complication risk in gastrointestinal surgical oncology: a meta‐analysis. Ann Surg. 2018;268(1):58‐69. [DOI] [PubMed] [Google Scholar]
- 11. Veronese N, Demurtas J, Soysal P, et al. Sarcopenia and health‐related outcomes: an umbrella review of observational studies. Eur Geriatr Med. 2019;10(6):853‐862. [DOI] [PubMed] [Google Scholar]
- 12. Aromataris E, Fernandez R, Godfrey CM, Holly C, Khalil H, Tungpunkom P. Summarizing systematic reviews: methodological development, conduct and reporting of an umbrella review approach. Int J Evid Based Healthc. 2015;13(3):132‐140. [DOI] [PubMed] [Google Scholar]
- 13. Ioannidis JP. Integration of evidence from multiple meta‐analyses: a primer on umbrella reviews, treatment networks and multiple treatments meta‐analyses. CMAJ. 2009;181(8):488‐493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shea BJ, Hamel C, Wells GA, et al. AMSTAR is a reliable and valid measurement tool to assess the methodological quality of systematic reviews. J Clin Epidemiol. 2009;62(10):1013‐1020. [DOI] [PubMed] [Google Scholar]
- 15. Shea BJ, Grimshaw JM, Wells GA, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol. 2007;7:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction‐GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64(4):383‐394. [DOI] [PubMed] [Google Scholar]
- 17. Veronese N, Demurtas J, Celotto S, et al. Is chocolate consumption associated with health outcomes? An umbrella review of systematic reviews and meta‐analyses. Clin Nutr. 2018. [DOI] [PubMed] [Google Scholar]
- 18. Solmi M, Köhler CA, Stubbs B, et al. Environmental risk factors and nonpharmacological and nonsurgical interventions for obesity: an umbrella review of meta‐analyses of cohort studies and randomized controlled trials. Eur J Clin Invest. 2018;48(12):e12982. [DOI] [PubMed] [Google Scholar]
- 19. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ. 2003;327(7414):557‐560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629‐634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Belbasis L, Bellou V, Evangelou E, Ioannidis JP, Tzoulaki I. Environmental risk factors and multiple sclerosis: an umbrella review of systematic reviews and meta‐analyses. Lancet Neurol. 2015;14(3):263‐273. [DOI] [PubMed] [Google Scholar]
- 22. Zhang XM, Dou QL, Zeng Y, Yang Y, Cheng ASK, Zhang WW. Sarcopenia as a predictor of mortality in women with breast cancer: a meta‐analysis and systematic review. BMC Cancer. 2020;20(1):172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wong A, Zhu D, Kraus D, Tham T. Radiologically defined sarcopenia affects survival in head and neck cancer: a meta‐analysis. Laryngoscope. 2020. [DOI] [PubMed] [Google Scholar]
- 24. Ubachs J, Ziemons J, Minis‐Rutten IJG, et al. Sarcopenia and ovarian cancer survival: a systematic review and meta‐analysis. J Cachexia Sarcopenia Muscle. 2019;10(6):1165‐1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sun G, Li Y, Peng Y, et al. Can sarcopenia be a predictor of prognosis for patients with non‐metastatic colorectal cancer? A systematic review and meta‐analysis. Int J Colorectal Dis. 2018;33(10):1419‐1427. [DOI] [PubMed] [Google Scholar]
- 26. Su H, Ruan J, Chen T, Lin E, Shi L. CT‐assessed sarcopenia is a predictive factor for both long‐term and short‐term outcomes in gastrointestinal oncology patients: a systematic review and meta‐analysis. Cancer Imaging. 2019;19(1):82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kamarajah SK, Bundred J, Tan BHL. Body composition assessment and sarcopenia in patients with gastric cancer: a systematic review and meta‐analysis. Gastric Cancer. 2019;22(1):10‐22. [DOI] [PubMed] [Google Scholar]
- 28. Jia S, Qiao R, Xiao Y, et al. Prognostic value of sarcopenia in survivors of hematological malignances undergoing a hematopoietic stem cell transplantation: a systematic review and meta‐analysis. Support Care Cancer. 2020;28(8):3533‐3542. [DOI] [PubMed] [Google Scholar]
- 29. Hu X, Dou WC, Shao YX, et al. The prognostic value of sarcopenia in patients with surgically treated urothelial carcinoma: a systematic review and meta‐analysis. Eur J Surg Oncol. 2019;45(5):747‐754. [DOI] [PubMed] [Google Scholar]
- 30. Deng HY, Zha P, Peng L, Hou L, Huang KL, Li XY. Preoperative sarcopenia is a predictor of poor prognosis of esophageal cancer after esophagectomy: a comprehensive systematic review and meta‐analysis. Dis Esophagus. 2019;32(3). [DOI] [PubMed] [Google Scholar]
- 31. Deng HY, Hou L, Zha P, Huang KL, Peng L. Sarcopenia is an independent unfavorable prognostic factor of non‐small cell lung cancer after surgical resection: a comprehensive systematic review and meta‐analysis. Eur J Surg Oncol. 2019;45(5):728‐735. [DOI] [PubMed] [Google Scholar]
- 32. Chang KV, Chen JD, Wu WT, Huang KC, Hsu CT, Han DS. Association between loss of skeletal muscle mass and mortality and tumor recurrence in hepatocellular carcinoma: a systematic review and meta‐analysis. Liver Cancer. 2018;7(1):90‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yang Z, Zhou X, Ma B, Xing Y, Jiang X, Wang Z. Predictive value of preoperative sarcopenia in patients with gastric cancer: a meta‐analysis and systematic review. J Gastrointest Surg. 2018;22(11):1890‐1902. [DOI] [PubMed] [Google Scholar]
- 34. Wang PY, Xu LD, Chen XK, et al. Sarcopenia and short‐term outcomes after esophagectomy: a meta‐analysis. Ann Surg Oncol. 2020;27(8):3041‐3051. [DOI] [PubMed] [Google Scholar]
- 35. Hua H, Xu X, Tang Y, Ren Z, Xu Q, Chen L. Effect of sarcopenia on clinical outcomes following digestive carcinoma surgery: a meta‐analysis. Support Care Cancer. 2019;27(7):2385‐2394. [DOI] [PubMed] [Google Scholar]
- 36. Bundred J, Kamarajah SK, Roberts KJ. Body composition assessment and sarcopenia in patients with pancreatic cancer: a systematic review and meta‐analysis. HPB (Oxford). 2019;21(12):1603‐1612. [DOI] [PubMed] [Google Scholar]
- 37. Zhao Y, Zhang Y, Hao Q, Ge M, Dong B. Sarcopenia and hospital‐related outcomes in the old people: a systematic review and meta‐analysis. Aging Clin Exp Res. 2019;31(1):5‐15. [DOI] [PubMed] [Google Scholar]
- 38. Zhao WT, Yang M, Wu HM, Yang L, Zhang XM, Huang Y. Systematic review and meta‐analysis of the association between sarcopenia and dysphagia. J Nutr Health Aging. 2018;22(8):1003‐1009. [DOI] [PubMed] [Google Scholar]
- 39. Zhang X, Wang C, Dou Q, Zhang W, Yang Y, Xie X. Sarcopenia as a predictor of all‐cause mortality among older nursing home residents: a systematic review and meta‐analysis. BMJ Open. 2018;8(11):e021252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhang X, Huang P, Dou Q, et al. Falls among older adults with sarcopenia dwelling in nursing home or community: a meta‐analysis. Clin Nutr. 2019. [DOI] [PubMed] [Google Scholar]
- 41. Yeung SSY, Reijnierse EM, Pham VK, et al. Sarcopenia and its association with falls and fractures in older adults: a systematic review and meta‐analysis. J Cachexia Sarcopenia Muscle. 2019;10(3):485‐500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Liu P, Hao Q, Hai S, Wang H, Cao L, Dong B. Sarcopenia as a predictor of all‐cause mortality among community‐dwelling older people: a systematic review and meta‐analysis. Maturitas. 2017;103:16‐22. [DOI] [PubMed] [Google Scholar]
- 43. Cabett Cipolli G, Sanches Yassuda M, Aprahamian I. Sarcopenia is associated with cognitive impairment in older adults: a systematic review and meta‐analysis. J Nutr Health Aging. 2019;23(6):525‐531. [DOI] [PubMed] [Google Scholar]
- 44. Zhang H, Lin S, Gao T, et al. Association between sarcopenia and metabolic syndrome in middle‐aged and older non‐obese adults: a systematic review and meta‐analysis. Nutrients. 2018;10(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yu R, Shi Q, Liu L, Chen L. Relationship of sarcopenia with steatohepatitis and advanced liver fibrosis in non‐alcoholic fatty liver disease: a meta‐analysis. BMC Gastroenterol. 2018;18(1):51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pan X, Han Y, Zou T, et al. Sarcopenia contributes to the progression of nonalcoholic fatty liver disease‐ related fibrosis: a meta‐analysis. Dig Dis. 2018;36(6):427‐436. [DOI] [PubMed] [Google Scholar]
- 47. Kim G, Kang SH, Kim MY, Baik SK. Prognostic value of sarcopenia in patients with liver cirrhosis: a systematic review and meta‐analysis. PLoS One. 2017;12(10):e0186990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chang KV, Chen JD, Wu WT, Huang KC, Lin HY, Han DS. Is sarcopenia associated with hepatic encephalopathy in liver cirrhosis? A systematic review and meta‐analysis. J Formos Med Assoc. 2019;118(4):833‐842. [DOI] [PubMed] [Google Scholar]
- 49. Ida S, Kaneko R, Imataka K, Murata K. Association between sarcopenia and renal function in patients with diabetes: a systematic review and meta‐analysis. J Diabetes Res. 2019;2019:1365189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chang KV, Hsu TH, Wu WT, Huang KC, Han DS. Is sarcopenia associated with depression? A systematic review and meta‐analysis of observational studies. Age Ageing. 2017;46(5):738‐746. [DOI] [PubMed] [Google Scholar]
- 51. Prado CMM, Baracos VE, McCargar LJ, et al. Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clin Cancer Res. 2009;15(8):2920‐2926. [DOI] [PubMed] [Google Scholar]
- 52. Nishioka N, Uchino J, Hirai S, et al. Association of sarcopenia with and efficacy of Anti‐PD‐1/PD‐L1 therapy in non‐small‐cell lung cancer. J Clin Med. 2019;8(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Souza ABF, Nascimento DAC, Rodrigues IJM, et al. Association between sarcopenia and diabetes in community dwelling elderly in the Amazon region ‐ Viver Mais Project. Arch Gerontol Geriat. 2019;83:121‐125. [DOI] [PubMed] [Google Scholar]
- 54. Peball M, Mahlknecht P, Werkmann M, et al. Prevalence and associated factors of sarcopenia and frailty in Parkinson's disease: a cross‐sectional study. Gerontology. 2019;65(3):216‐228. [DOI] [PubMed] [Google Scholar]
- 55. Hayashi M, Abe K, Fujita M, Okai K, Takahashi A, Ohira H. Association between sarcopenia and osteoporosis in chronic liver disease. Hepatol Res. 2018;48(11):893‐904. [DOI] [PubMed] [Google Scholar]
- 56. de Blasio F, Di Gregorio A, de Blasio F, Bianco A, Bellofiore B, Scalfi L. Malnutrition and sarcopenia assessment in patients with chronic obstructive pulmonary disease according to international diagnostic criteria, and evaluation of raw BIA variables. Resp Med. 2018;134:1‐5. [DOI] [PubMed] [Google Scholar]
- 57. Choi YI, Park DK, Chung JW, Kim JH. Sarcopenia is independently associated with an increased risk of peptic ulcer disease. Gastroenterology. 2019;156(6):S751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Skov‐Jensen C, Skovbro M, Flint A, Helge JW, Dela F. Contraction‐mediated glucose uptake is increased in men with impaired glucose tolerance. Appl Physiol Nutr Me. 2007;32(1):115‐124. [DOI] [PubMed] [Google Scholar]
- 59. Lee SW, Youm Y, Lee WJ, et al. Appendicular skeletal muscle mass and insulin resistance in an elderly Korean population: the Korean social life, health and aging project‐health examination cohort. Diabetes Metab J. 2015;39(1):37‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Polyzos SA, Kountouras J, Zavos C. Nonalcoholic fatty liver disease: the pathogenetic roles of insulin resistance and adipocytokines. Curr Mol Med. 2009;9(3):299‐314. [DOI] [PubMed] [Google Scholar]
- 61. Ten Haaf DSM, Eijsvogels TMH, Bongers C, et al. Protein supplementation improves lean body mass in physically active older adults: a randomized placebo‐controlled trial. J Cachexia Sarcopenia Muscle. 2019;10(2):298‐310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Smith GI, Julliand S, Reeds DN, Sinacore DR, Klein S, Mittendorfer B. Fish oil‐derived n‐3 PUFA therapy increases muscle mass and function in healthy older adults. Am J Clin Nutr. 2015;102(1):115‐122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Liao CD, Tsauo JY, Wu YT, et al. Effects of protein supplementation combined with resistance exercise on body composition and physical function in older adults: a systematic review and meta‐analysis. Am J Clin Nutr. 2017;106(4):1078‐1091. [DOI] [PubMed] [Google Scholar]
- 64. Coelho‐Junior HJ, Milano‐Teixeira L, Rodrigues B, Bacurau R, Marzetti E, Uchida M. Relative protein intake and physical function in older adults: a systematic review and meta‐analysis of observational studies. Nutrients. 2018;10(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Antoniak AE, Greig CA. The effect of combined resistance exercise training and vitamin D3 supplementation on musculoskeletal health and function in older adults: a systematic review and meta‐analysis. BMJ Open. 2017;7(7):e014619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Wright J, Baldwin C. Oral nutritional support with or without exercise in the management of malnutrition in nutritionally vulnerable older people: A systematic review and meta‐analysis. Clin Nutr. 2018;37(6):1879‐1891. [DOI] [PubMed] [Google Scholar]
- 67. Sim M, Lewis JR, Blekkenhorst LC, et al. Higher dietary nitrate intake is associated with better muscle function in older women. J Cachexia Sarcopenia Muscle. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Velho S, Moço S, Ferreira A, et al. Dietary patterns and their relationships to sarcopenia in Portuguese patients with gastrointestinal cancer: an exploratory study. Nutrition. 2019;63–64:193‐199. [DOI] [PubMed] [Google Scholar]
- 69. Hanach NI, McCullough F, Avery A. The impact of dairy protein intake on muscle mass, muscle strength, and physical performance in middle‐aged to older adults with or without existing sarcopenia: a systematic review and meta‐analysis. Adv Nutr. 2019;10(1):59‐69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Beckwee D, Delaere A, Aelbrecht S, et al. Exercise interventions for the prevention and treatment of sarcopenia. a systematic umbrella review. J Nutr Health Aging. 2019;23(6):494‐502. [DOI] [PubMed] [Google Scholar]
- 71. Bear DE, Langan A, Dimidi E, et al. beta‐Hydroxy‐beta‐methylbutyrate and its impact on skeletal muscle mass and physical function in clinical practice: a systematic review and meta‐analysis. Am J Clin Nutr. 2019;109(4):1119‐1132. [DOI] [PubMed] [Google Scholar]
- 72. Morley JE. Treatment of sarcopenia: the road to the future. J Cachexia Sarcopeni. 2018;9(7):1196‐1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Rooks D, Praestgaard J, Hariry S, et al. Treatment of sarcopenia with bimagrumab: results from a Phase II, randomized, controlled. Proof‐of‐concept study. J Am Geriatr Soc. 2017;65(9):1988‐1995. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
All data generated or analyzed during this study are included in this published article.


