Abstract
The European Commission asked EFSA to update its previous Opinion on nickel in food and drinking water, taking into account new occurrence data, the updated benchmark dose (BMD) Guidance and newly available scientific information. More than 47,000 analytical results on the occurrence of nickel were used for calculating chronic and acute dietary exposure. An increased incidence of post‐implantation loss in rats was identified as the critical effect for the risk characterisation of chronic oral exposure and a BMDL 10 of 1.3 mg Ni/kg body weight (bw) per day was selected as the reference point for the establishment of a tolerable daily intake (TDI) of 13 μg/kg bw. Eczematous flare‐up reactions in the skin elicited in nickel‐sensitised humans, a condition known as systemic contact dermatitis, was identified as the critical effect for the risk characterisation of acute oral exposure. A BMDL could not be derived, and therefore, the lowest‐observed‐adverse‐effect‐level of 4.3 μg Ni/kg bw was selected as the reference point. The margin of exposure (MOE) approach was applied and an MOE of 30 or higher was considered as being indicative of a low health concern. The mean lower bound (LB)/upper bound (UB) chronic dietary exposure was below or at the level of the TDI. The 95th percentile LB/UB chronic dietary exposure was below the TDI in adolescents and in all adult age groups, but generally exceeded the TDI in toddlers and in other children, as well as in infants in some surveys. This may raise a health concern in these young age groups. The MOE values for the mean UB acute dietary exposure and for the 95th percentile UB raises a health concern for nickel‐sensitised individuals. The MOE values for an acute scenario regarding consumption of a glass of water on an empty stomach do not raise a health concern.
Keywords: Nickel, tolerable daily intake (TDI), margin of exposure (MOE), food, dietary exposure, sensitisation, toxicity
Summary
The European Commission asked the European Food Safety Authority (EFSA) to update the previous EFSA Scientific Opinion on the risks to public health related to the presence of nickel in food and drinking water (EFSA CONTAM Panel, 2015), taking into account the new occurrence data, the updated benchmark dose (BMD) Guidance and any newly available scientific information. The CONTAM Panel developed the draft scientific Opinion which underwent a public consultation from 4 June until 15 July 2020. The comments received and how they were taken into account when finalising the scientific Opinion were published in an EFSA Technical Report (EFSA, 2020).
Nickel is a widespread component of Earth's crust and is ubiquitous in the biosphere. Its presence in food and drinking water can arise from both natural and anthropogenic sources. Nickel occurs in different oxidation states. In food and drinking water, nickel generally occurs in the divalent form, which is the most stable oxidation state.
Nickel is usually measured in food as total nickel and there are only few studies of nickel speciation in food. It is generally assumed that nickel occurs in food in the form of complex bound organic nickel, which has different physico‐chemical and possibly also different biological properties than inorganic nickel.
Hazard identification and characterisation
Nickel absorption from the gastrointestinal tract is dependent on the chemical form and thus, the solubility of the nickel compound. Absorption may be decreased by binding or chelating substances, competitive inhibitors or redox reagents. On the other hand, absorption is often enhanced by substances that increase pH, solubility or oxidation, or by chelating agents that are actively absorbed.
In humans, the bioavailability of nickel following ingestion also depends on the solubility of the administered nickel compound, the dosing vehicle and the fasting state of the subject. A low absorption (0.7–2.5%) was reported when nickel was ingested in the presence of food or under a non‐fasted state, whereas a higher absorption (25–27%) was reported when nickel was ingested via drinking water in the absence of food, or under a fasted state. The number of individuals examined in the relevant human studies was low. There was also a considerable inter‐individual variability in these studies. Thus, a precise estimate of the oral bioavailability of nickel in humans under different conditions cannot be established for the acute risk characterisation.
A study in rats showed an absorption of around 10% when soluble nickel compounds were administered in a 5% starch saline solution as a vehicle. Such a condition is considered as being representative for dietary exposure via food and beverages for the chronic risk characterisation.
After absorption, nickel is widely distributed in the organism. Nickel was shown to cross the placenta in mice. Nickel can also be transported across the blood–brain barrier. Absorbed nickel is excreted mainly via the urine. During lactation, nickel can also be excreted in the breast milk. An elimination half‐life of 28 ± 9 h was estimated in human volunteers.
The divalent metal transporter 1 (DMT1) mediates the transport of nickel and other divalent metal ions such as iron from the lumen of the intestine into the enterocyte and also mediates apical uptake of divalent cations in the kidney. DMT1 is known to be involved in the transport of divalent iron into the cytosol of endosomal cells prior to transport across the blood–brain barrier by ferroportin. Since nickel is also a substrate for DMT1, this transporter is likely to also be involved in nickel uptake into the brain.
The major effects observed in the short‐term repeated dose toxicity studies in rodents and dogs following oral administration were decreased body weight and effects in the liver and kidney (changes in organ weights and histopathological changes). Effects on bone and on gut microbiota have also been reported in a few recent studies.
A few studies indicate that nickel can disturb neurobehavioural functions in mice and rats as indicated by impaired spatial memory performance and effects on locomotor activity. Neurodegeneration in adult rats has also been reported.
In mice, different reproductive effects such as decreased male sex organ weights and histopathological changes in these organs, disturbed spermatogenesis, decreased sperm motility and sperm damage have been reported after oral exposure to soluble nickel compounds. The reproductive effects were responsible for a decreased fertility in mice. A recent short‐term toxicity study (28 days) with limited reporting suggested that nickel may also cause testicular degeneration in rats. Mice appear to be more sensitive than rats regarding reproductive effects.
There is consistent evidence of developmental toxicity in rats in the form of increased pup mortality (stillbirth or post implantation loss/perinatal lethality) and decreased pup weight after oral exposure to soluble nickel compounds. Developmental toxicity was also observed in mice (decreased fetal weight, malformations) but at higher doses than for rats suggesting that rats appear to be more sensitive than mice regarding developmental toxicity. Based on the available data, the CONTAM Panel considers that the increased incidence of post‐implantation loss in rats is the critical effect for the risk characterisation of chronic oral exposure to nickel. This is in agreement with the previous Opinion.
Nickel compounds are inactive in almost all bacterial mutagenicity tests and are weakly mutagenic in cultured mammalian cells. Nickel ions may be co‐mutagenic, which is likely due to interference with DNA repair processes. Nickel compounds can induce sister chromatid exchanges, chromosomal aberrations and micronuclei at high (mM), cytotoxic levels in different mammalian cell systems; these effects are likely due to aneugenic as well as clastogenic actions. Nickel compounds have been shown to induce DNA single‐strand breaks (SSBs), DNA–protein cross‐links and oxidative DNA damage in mammalian test systems in vitro. Induction of chromosomal aberrations and micronuclei in rodents treated with different nickel compounds is not consistent across studies and both positive and negative results have been reported after oral administration, and intraperitoneal or subcutaneous injection. Nickel compounds give rise to both DNA SSBs and DNA–protein cross‐links in vivo after oral administration or subcutaneous injection.
No tumours have been observed in the carcinogenicity studies in experimental animals after oral administration of soluble nickel compounds.
Nickel has different types of effects on the immune system. It is a sensitiser; hence exposure may lead to adverse hypersensitivity reactions. Oral exposure studies to investigate sensitisation to nickel by the oral route are scant. Oral exposure to nickel is not known to cause sensitisation, but nickel may elicit eczematous flare‐up reactions in the skin of nickel‐sensitised individuals that suffer from a condition known as systemic contact dermatitis (SCD). The CONTAM Panel concludes that SCD elicited by oral intake of nickel in humans already sensitive to nickel is the critical effect for the risk assessment of acute effects of nickel. However, there are uncertainties associated with information regarding adverse reactions in humans after ingestion of nickel. The evaluation is based on 3 individual studies, all with a limited number of nickel‐sensitised individuals. The degree of sensitivity of these individuals is not known. The outcomes of these studies were expressed in different ways, i.e. as flare‐up reactions of already eczematous skin lesions, or as flare‐up reactions in addition to new skin reactions, which makes comparison of these studies difficult. Individuals were fasted before oral exposure to nickel and subsequent monitoring of the effects, which may not represent all types of nickel intake. Nevertheless, the CONTAM Panel considers, in agreement with the previous Opinion, that SCD is the critical effect for the risk characterisation of acute oral exposure to nickel.
In the previous Opinion, the CONTAM Panel concluded that the data from the available epidemiological studies do not support an association between oral exposure to nickel and reproductive and developmental effects in humans. From the small number of studies published since the previous opinion, a few suggest that there may be an association between nickel exposure and adverse reproductive and developmental outcomes.
No studies on neurotoxicity in humans were identified in the previous Opinion. In the few studies published since then, no clear signs of neurotoxicity were reported.
No data linking cancer in humans with oral exposure to nickel are available.
It is evident that oxidative stress and an elevation of reactive oxygen species (ROS) are involved in the toxicity of nickel. A contribution of oxidative stress is evident in relation to reproductive toxicity, genotoxicity, immunotoxicity and neurotoxicity. It has also been postulated that nickel might exert some of its effects via perturbation of iron homeostasis since divalent nickel competes with the transport of divalent iron into cells via DMT1 and possibly could also compete with iron sites on enzymes like the prolyl hydroxylases that modify hypoxia inducible factor‐1α (HIF‐1α).
Nickel has been demonstrated to disturb regulation of mammalian reproductive function at several levels. Mice appear more sensitive than rats and this was associated with a higher level of oxidative stress in mouse testes compared to testes of rats. A part of this higher sensitivity of mice appears to be due to the formation of a complex between nickel and protamine 2 in sperm chromatin, which further elevates ROS production. Oxidative stress and nickel complexation with protamine 2 may both contribute to infertility. Rats have very low levels of protamine 2 in contrast to mice and humans, which have much higher levels of this protein. The fact that protamine 2 is expressed in humans might suggest that the mouse is a better model than the rat in predicting the ability of nickel to induce human male infertility. However, the relative level of the antioxidant status of human testes will be an important determinant of susceptibility based on the role of ROS.
The genotoxicity of nickel is likely due to indirect effects including inhibition of DNA repair and ROS production. In addition, chromatin changes may occur following dysregulation of signalling pathways and alteration of the epigenetic landscape.
The ability of nickel to bind to proteins is responsible for the induction of specific immune responses, leading to allergic reactions. These may be evident in the skin but can also occur elsewhere in the body. Nickel has also a non‐specific activity on the immune system, such as the induction of inflammatory reactions through toll like receptors and nucleic factor kappa B signalling pathways that may be involved in the adverse reactions, including the allergic reactions. Even though predominant reactions to nickel occur after skin exposure, oral exposure to nickel may potentially induce these effects as well, and especially may elicit flare‐up reactions in already sensitised individuals suffering from systemic contact dermatitis. In addition, nickel may also interfere with immunity through causing apoptosis of monocytes as observed in vitro, and thus may have an impact on host resistance.
Nickel causes deficits in neurobehavioural performance in rodents and neuronal cell toxicity in vivo and in vitro. These effects are associated with oxidative stress and disturbance of mitochondrial aerobic metabolism evidently involving HIF‐1α.
Nickel is classified as a human carcinogen via inhalation. No data linking cancer in humans with oral exposure to nickel are available. No tumours have been observed in the carcinogenicity studies in experimental animals after oral administration of soluble nickel compounds. Therefore, the CONTAM Panel considers it unlikely that dietary exposure to nickel results in cancer in humans.
For chronic oral exposure to nickel, the critical effect is the increased incidence of post‐implantation loss in rats observed in the one‐ and two‐generation studies. The CONTAM Panel noted that other toxic effects, including neurotoxic effects reported in the experimental animal studies were observed at higher dose levels than those resulting in developmental toxicity, i.e. post‐implantation loss. From the BMD analysis, the BMDL10 of 1.3 mg Ni/kg body weight (bw) per day was selected as the reference point for the establishment of the tolerable daily intake (TDI). A TDI of 13 μg/kg bw was established by applying the default uncertainty factor of 100 to account for intra‐ and interspecies differences.
For acute oral exposure to nickel, the critical effect is eczematous flare‐up reactions in the skin (SCD) elicited in nickel‐sensitised humans. The dose–response modelling showed that a BMDL could not be derived from the available data by applying the current BMD guidance. Therefore, the reference point was based on the no‐observed‐adverse‐effect‐level (NOAEL)/lowest‐observed‐adverse‐effect‐level (LOAEL) approach. In the absence of a NOAEL, a LOAEL of 4.3 μg Ni/kg bw was identified. In accordance with the previous Opinion, the data were considered insufficient to derive an acute reference dose (ARfD) and an margin of exposure (MOE) approach was applied for the acute risk assessment. The CONTAM Panel considered that an MOE of 30 or higher would indicate a low health concern.
Occurrence/exposure for the EU population
More than 47,000 analytical results on the occurrence of nickel in food and drinking water were used for the chronic and acute dietary exposure assessment. The highest mean nickel concentrations were measured for the food category ‘Legumes, nuts and oilseeds’ and for the food category ‘Products for special nutritional use’.
The mean lower bound (LB)/upper bound (UB) chronic dietary exposure to nickel across the different dietary surveys and age classes ranged from 1.57/1.89 μg/kg bw per day in elderly to 12.5/14.6 μg/kg bw per day in toddlers. The 95th percentile LB/UB chronic dietary exposure to nickel ranged from 3.35/3.93 μg/kg bw per day in very elderly to 28.1/29.9 μg/kg bw per day in infants. The food category, ‘grains and grain‐based products’ was the most important contributor to the mean LB chronic dietary exposure to nickel in all age classes.
The mean UB acute exposure ranged from 1.89 μg/kg bw per day in the elderly to 14.6 μg/kg bw per day in toddlers. The 95th percentile UB acute exposure ranged from 5.35 μg/kg bw per day in the elderly to 40.8 μg/kg bw per day in toddlers. The most relevant food categories for the 95th percentile UB acute dietary exposure varied between age classes and surveys. Beans, coffee, ready‐to‐eat soups, chocolate and breakfast cereals were the most relevant food categories in most of the surveys.
The acute dietary exposure to nickel from consumption of a small bottle of water (500 mL) containing a high concentration of nickel was estimated to be 0.04 μg/kg bw from tap water and 0.08 μg/kg from bottled water.
Risk characterisation
The mean LB and UB chronic dietary exposure was below the TDI and thus, does not indicate a concern. However, for one survey in toddlers, the mean chronic dietary exposure was at the level of the TDI (LB/UB: 12.5/14.6 μg/kg bw per day) and this may indicate a health concern.
The 95th percentile LB chronic dietary exposure exceeded the TDI in toddlers in 10 out of 14 dietary surveys and in other children in 11 out of 19 dietary surveys. Also in infants, an exceedance of the TDI was observed in some surveys. The 95th percentile LB chronic dietary exposure was below the TDI in adolescents and in all adult age groups. Thus, the 95th percentile chronic dietary exposure to nickel may raise a health concern for infants, toddlers and other children.
The CONTAM Panel noted that the risk characterisation for chronic dietary exposure is conservative and thus will overestimate the risk, as the critical effect for the TDI, post‐implantation loss, is not a relevant effect for young age groups. The TDI is also protective for effects that might occur in these age groups as no effects of relevance for young age groups have been reported at the reference point identified for the derivation of the TDI.
Comparison of the estimated mean UB acute dietary exposure with the acute reference point of 4.3 μg Ni/kg bw resulted in MOE values ranging from 0.3 to 2.3, across dietary surveys and age classes. The MOE values when using the 95th percentile UB acute dietary exposure ranged from 0.1 to 0.8 across dietary surveys and age classes. Thus, these MOE values raise a health concern for nickel‐sensitised individuals.
For the scenario regarding consumption of a small bottle of drinking water, the MOE values of 120 and 55 for tap water and bottled water, respectively do not raise a health concern.
Uncertainty analysis
The CONTAM Panel concluded that the uncertainties in the risk assessment of acute exposure to nickel in food and drinking water are larger than for the chronic exposure. The CONTAM Panel considered that the use of fasting condition in the pivotal study is a major source of uncertainty and therefore the assessment is more likely to overestimate than to underestimate the risk.
Recommendations
In order to improve the risk assessment and reduce the uncertainties, the CONTAM Panel recommends the generation of more information on oral bioavailability of nickel in humans under different dosing regimens (i.e. vehicle, fasting/non‐fasting condition). In addition, it is recommended to perform new studies with larger numbers of nickel‐sensitised individuals and different dosing regimens and dose levels included to allow a better characterisation of the dose–response and facilitate a BMD approach. Such studies would form the basis for a more precise risk assessment of skin and systemic reactions to nickel exposure via food and drinking water in nickel‐sensitised individuals. Information on the potential presence of nickel nanoparticles in food and drinking water is also needed.
1. Introduction
1.1. Background and terms of reference as provided by the requestor
Background
On 22 January 2015, EFSA's Scientific Panel on Contaminants in the Food Chain (CONTAM) adopted a Scientific Opinion on the risks to public health related to the presence of nickel in food and drinking water, in which it established a tolerable daily intake (TDI) of 2.8 μg/kg Ni/kg body weight (bw) per day and concluded that on the basis of the available occurrence data the current chronic dietary exposure raises health concerns for all age groups and that the acute exposure is of concern for nickel‐sensitised individuals. The CONTAM Panel noted the need for mechanistic studies to assess the human relevance of the effects on reproduction and development that had been observed in experimental animals and for additional studies on human absorption of nickel from food; for example, in combination with duplicate diet studies.
In its Opinion, EFSA considered occurrence data on nickel in food and drinking water, which were collected in 15 different European countries. However, as 80% of the total collected data were collected in just one Member State, a geographically more widespread data set would be needed to verify the occurrence of nickel in food throughout the EU. Furthermore, for certain food groups, considered as main contributors to dietary exposure in the EFSA Scientific Opinion, only limited occurrence data were available. In order to discuss possible future risk management measures, a better view of the nickel content in food commodities belonging to these food groups was needed. Therefore, by means of Recommendation (EU) 2016/11111, Member States were asked to collect additional occurrence data for several foodstuffs in 2016, 2017 and 2018.
On 17 November 2016, EFSA adopted its updated guidance on the use of the benchmark dose (BMD) approach in risk assessment, which might impact on the previously established TDI for nickel.
It is therefore appropriate to request EFSA to update the EFSA Scientific Opinion on the risks to public health related to the presence of nickel in food and drinking water, taking into account the new occurrence data, the updated BMD Guidance and any newly available scientific information.
Terms of reference
In accordance with Art 29 (1) of Regulation (EC) No 178/20022, the European Commission asks the European Food Safety Authority for an updated Scientific Opinion on the risks to public health related to the presence of nickel in food and drinking water, taking into account the new occurrence data, the updated BMD Guidance and any newly available scientific information.
1.2. Interpretation of the terms of reference
The CONTAM Panel concluded that this Opinion should comprise:
an evaluation of the toxicity of nickel for humans, considering all relevant toxicological endpoints;
an estimation of the dietary exposure of the EU population to nickel from food and drinking water, including the consumption patterns of specific groups of the population; and
an assessment of the human health risks to the EU population, including specific (vulnerable) groups of the population, as a consequence of the estimated dietary exposure.
In the context of human exposure to nickel via the diet and drinking water, water‐soluble nickel compounds are the most relevant. This Scientific Opinion is therefore confined to water‐soluble nickel compounds (i.e. nickel (II), nickel chloride, nickel sulfate, nickel dinitrate and nickel acetate). Non‐ or low‐soluble nickel compounds such as nickel sulfide, nickel oxide and nickel carbonate are not considered in the current assessment.
Nickel can also be present in the environment as nickel nanoparticles. In the absence of evidence that nickel nanoparticles occur in food and/or drinking water, studies on the toxicity of nickel nanoparticles were not considered in the present assessment.
As outlined in the terms of reference, the current risk assessment is an update of the previous Opinion, published in 2015. The literature search for the latter was conducted in 2013. Therefore, papers published since 2013 were taken into account for the current risk assessment when not yet included in the previous Opinion.
1.3. Supporting information for the assessment
This section is an adapted and amended version of the corresponding sections in the previous Opinion on nickel in food and drinking water (EFSA CONTAM Panel, 2015).
1.3.1. Chemistry
The chemistry of nickel (CAS registry No. 7440‐02‐0) and nickel compounds is described in many general scientific references (e.g. IARC, 1990, 2012; Health Canada, 1994; ATSDR, 2005, EU RAR 2008; Nielsen and Larsen, 2013). Only the main relevant information is presented here.
Nickel is a silver‐white metal with typical metallic properties and has an atomic number of 28 and atomic weight of 58.71. It has five naturally occurring stable isotopes, with mass numbers 58 (68.07%), 60 (26.23%), 61 (1.14%), 62 (3.63%) and 64 (0.93%). Although it has oxidation states of –1, 0, +1, +2, +3 and +4, the most common valence state in the environment is the divalent oxidation state (Ni2+ or Ni (II)). In the absence of strong complexing agents, nickel (II) occurs mostly as the green hexaquonickel ion [Ni(H2O)6]2+ in natural waters at pH 5–9. Simple inorganic complexes (salts) with common ligands, such as HCO3 −, Cl−, OH−, NH3, SO4 2−, are formed to a minor degree in this pH range. The most water‐soluble nickel salts are nickel chloride hexahydrate (NiCl2(H2O)6; 2,500 g/L), nickel dinitrate hexahydrate (Ni(NO3)2(H2O)6; 2,400 g/L), nickel sulfate hexahydrate (NiSO4(H2O)6; 660 g/L), nickel sulfate heptahydrate (NiSO4(H2O)7;760 g/L) and nickel acetate (Ni(CH3CO2)2(H2O)4; 170 g/L). Less‐soluble nickel compounds include nickel hydroxide (Ni(OH)2; 0.13 g/L) and nickel carbonate (NiCO3; 0.09 g/L). Nickel sulfides and oxides are practically insoluble in water.
Since nickel is usually measured in food as total nickel, limited information is available on the content or dietary intake of different chemical species of nickel in food. It is generally assumed that it occurs in the form of complex bound organic nickel, which has different physico‐chemical and possibly also different biological properties than inorganic nickel (EU RAR, 2008). However, there are only a few studies of nickel speciation in food. The majority of studies in this field were made of nickel fractionation in different samples of tea, soybean flour and human milk (Schaumlöffel, 2005; Scancar et al., 2013). In tea, nickel is present as nickel (II) or as complexes with large organic molecules (4–6 kDa) or flavonoid components or mainly associated with quinic acid (Scancar et al., 2013). In soybean flour, 66% of the total nickel was extractable and was present mainly as complexes of 2–3 kDa size. In human milk, nickel was found to be associated with high molecular mass biomolecules, probably caseins, lactotransferrin, serum albumin or immunoglobulins. Recently, nickel (II), nickel gluconate and nickel citrate complexes were found in cocoa infusions (Peeters et al., 2017). Nickel citrate and nickel malate complexes account for 99% of the nickel present in pea root nodule cytoplasm fraction (Cacho et al., 2010).
1.3.2. Environmental fate and sources of food and drinking water contamination
The CONTAM Panel extensively reviewed the environmental fate and sources of food and drinking water contamination in 2015. The conclusions from this review are repeated below. Further details are available in EFSA CONTAM Panel (2015).
‘Nickel occurs in environmental compartments and in the biosphere with highly variable levels, normally as nickel (II) compounds or complexes. The metal presence is determined by natural as well as anthropogenic factors, the latter generically identifiable with industrial and technological sources. A wide variability characterizes ambient nickel concentrations reflecting the influence of nickel emissions from different types of sources.
In air, nickel occurs mostly as fine respirable particles that are removed by wet and dry deposition. Anthropogenic sources of air‐borne nickel account for more than 80% of the atmospheric nickel burden; the remainder to 100% is accounted for by natural sources. In non‐industrialized areas, background nickel concentrations are generally around or below 3 ng/m3 (yearly averages), although higher levels have also been observed; in urban and industrialized areas nickel concentrations in air can be considerably higher (up to tens or hundreds of ng/m3). In rainwater, nickel concentrations are on average measured in the range < 1 μg/L, although greater levels have been detected depending on location.
Surface runoff, deposition from air, and release of municipal and industrial waste waters are sources of nickel in surface waters. Under anaerobic conditions, typical of deep waters, nickel can be segregated from the environment as insoluble sulfide. Although in surface waters total nickel may be present at levels greater than a few μg/L, in general the element is detected at average concentrations in the order of 3 μg/L or lower, rivers being more contaminated than lakes and sea water’. Fish and seafood are consequently another source of nickel in the diet. ‘Total nickel concentrations in ground water and water from drinking water sources/supplies may range from less than 1 μg/L up to few tens of μg/L, although cases of a high nickel occurrence (up to hundreds of μg/L) have also been reported.
Nickel is released to soils from smelting and refining operations, disposal of sewage sludge, or use of sludge as a fertilizer; secondary anthropogenic sources include emissions from motor vehicles and electric power utilities. Weathering and erosion of geological materials are natural sources of nickel to soils. Typical average background concentrations of nickel in topsoil are in the order of few tens of mg/kg (namely, < 50 mg/kg): these values are consistent with nickel levels that on a local basis can be even remarkably higher, and with concentration ranges of two or three orders of magnitude. Reflecting the extent of anthropogenic impact, nickel concentrations are on average higher in agricultural soils while reaching the highest values in soils proximal to industrial activities’.
Uptake of nickel by plants results in another source of nickel in the diet. For example, root vegetables like carrots, potatoes and onions accumulate nickel when grown in contaminated soil or irrigated with contaminated water (Stasinos et al., 2014). The same has been observed in plants grown in paddy fields (Rahman et al., 2018).
‘Sediments are an important sink for nickel in water. In general, nickel concentrations detected in such matrix show similarities with those detected in topsoil: in particular, nickel content in sediments is expected to be high near sources of nickel emissions.
Migration from food contact material could represent an additional source for the presence of nickel in food and drinking water. The CONTAM Panel concluded that the extent of nickel migration into food and drinking water due to the use of good quality stainless steel cookware, tableware, and in general food contact materials has likely little or no relevance compared to the dietary exposure determined by the intrinsic presence of nickel in diet constituents. However, leaching of nickel into food may not be negligible for food contact materials made of poor quality stainless steel, or of other metal alloys containing nickel’.
1.3.3. Analytical methods
Flame or graphite furnace with atomic absorption spectrometry (F‐ or GF‐AAS), and, increasingly, inductively coupled plasma‐optical/atomic emission spectrometry (ICP‐OES/ICP‐AES) or inductively coupled plasma‐mass spectrometry (ICP‐MS) are the most common analytical techniques suitable for the determination of total nickel in foods and drinking water. The limits of detection (LODs) in water samples range from 0.05 to 1.0 μg/L depending on the analytical techniques used. In foods, there is a wide variation of LODs ranging from 2 to 290 μg/kg and from 0.006 to 117 μg/L, depending on the detection techniques used and the type of food (EFSA CONTAM Panel, 2015).
Four European standardised methods for the determination of total nickel in water are available by F‐ or GF‐AAS or ICP‐(OES or MS) techniques with LODs ranging from < 0.1 to 1 μg/L (ISO 8288:1986; EN ISO 17294‐2:2016; EN ISO 15586:2004; EN ISO 11885:2009). Only one standardised method is available for food, namely for animal and vegetable fats and oils by GF‐AAS and no LOD or limit of quantification (LOQ) is reported (ISO 8294:1999).
Sample preparation for the analysis of total nickel should be performed in accordance with Standard EN 13804:2013, ‘Foodstuffs — Determination of elements and their chemical species — General considerations and specific requirements’. Further details are provided in EFSA CONTAM Panel (2015).
To achieve analytical quality assurance, several standards, certified reference materials and regular proficiency testing schemes3 are available for total nickel in food and water.
1.3.4. Previous assessments
In 2015, The CONTAM Panel prepared a Scientific Opinion on the risks to public health related to the presence of nickel in food and drinking water. For the assessment of chronic effects of nickel, developmental toxicity in experimental animals was considered as the critical effect. A TDI of 2.8 μg Ni/kg bw per day was derived from a BMD lower confidence limit for an extra risk of 10% (BMDL10) of 0.28 mg/kg bw for post‐implantation loss per litter in rats based on the data from a dose‐range‐finding reproductive toxicity study (SLI, 2000a) and a 2‐generation reproductive toxicity study (SLI, 2000b). The default uncertainty factor of 100 was applied to establish the TDI. The dietary exposure to nickel raised concern when considering the mean and 95th percentile chronic exposure levels for all age classes. As the critical effect for the assessment of acute effects of nickel, the Panel selected the systemic contact dermatitis (SCD) elicited in nickel‐sensitised humans after oral exposure to nickel. BMD analyses were performed on data from three studies on human volunteers (Gawkrodger et al., 1986; Hindsén et al., 2001; Jensen et al., 2003). The lowest BMDL10 of 0.08 mg Ni per person, corresponding to 1.1 μg Ni/kg bw, calculated from the data by Jensen et al. (2003), was selected as a reference point for SCD elicited in Ni‐sensitive humans after acute oral exposure to nickel. The CONTAM Panel applied a margin of exposure (MOE) approach and considered an MOE of 10 to be indicative of a low health concern. The acute dietary exposure to nickel raised concern that nickel‐sensitised individuals may develop eczematous flare‐up skin reactions. The CONTAM Panel noted ‘the need for mechanistic studies to assess the human relevance of the effects on reproduction and development observed in experimental animals and for additional studies on human absorption of nickel from food, for example in combination with duplicate diet studies’ (EFSA CONTAM Panel, 2015).
Epidemiological studies have provided evidence for lung cancer related to specific nickel compounds or classes of compounds in humans exposed by inhalation: water‐soluble nickel compounds (e.g. nickel chloride, nickel sulfate), insoluble nickel compounds (e.g. nickel oxides and nickel sulfides) (IARC, 1990, 2012). Nickel and nickel compounds have been classified by the IARC as carcinogenic to humans (Group 1) causing cancers of the lung, nasal cavity and paranasal sinuses after inhalation.
Upon request from the Danish Environmental Protection Agency, Nielsen and Larsen (2013) evaluated the health hazards from exposure to nickel, inorganic and soluble salts to propose a health‐based quality criterion for nickel in drinking water. The assessment was finalised in 2010 and published in 2013. The assessment was based on the EU Risk Assessment Reports. A no‐observed‐adverse‐effect‐level (NOAEL) of 1.1 mg Ni/kg bw per day was identified for developmental toxicity in the two‐generation study (SLI, 2000b) with nickel sulfate. A TDI of 5.5 μg Ni/kg bw per day was calculated based on this NOAEL by applying an uncertainty factor of 200 to account for inter‐ and intraspecies variations (10 × 10) and a factor of two in order to consider the severity of effects (peri‐ and postnatal increased mortality) at only twice the dose level of the NOAEL value. A health‐based quality criterion in drinking water for repeated exposure to soluble inorganic nickel salts of 17 μg Ni/L was then calculated. A health‐based quality criterion in drinking water for acute exposure of 37 μg Ni/L was calculated based on a lowest‐observed‐adverse‐effect‐level (LOAEL) of 12 μg/kg bw for oral challenge of nickel‐sensitised individuals to nickel in drinking water on an empty stomach (Nielsen et al., 1999) and assuming an ingestion of 2.3 L of drinking water per day (90th percentile for adults, body weight: 70 kg). An uncertainty factor of 10 was applied because a LOAEL instead of a NOAEL was used and because the LOAEL would probably have been lower if the nickel status of the patients was not lowered by giving them a nickel‐poor diet during the last 2 days before the provocation test.
In the most recent version of the WHO Guidelines for Drinking‐water quality (WHO, 2017) the guideline value for nickel is 70 μg/L. The guideline value is based on a TDI of 12 μg/kg bw, derived from a LOAEL established after oral provocation of fasted patients with an empty stomach in the study by Nielsen et al. (1999). It is noted that the principal reference is WHO (2005) reporting the 2004 assessment, i.e. there has been no new WHO evaluation since the EFSA CONTAM Opinion from 2015.
1.3.5. Legislation
Currently, there are no maximum levels in the EU legislation for nickel as a contaminant in foodstuffs. The Framework Regulation EC 1935/20044 lays down general requirements for materials and articles intended to come in contact with food and Regulation EC 2023/20065 describes good manufacturing practices for these materials and articles. Commission Regulation (EU) No 10/20116 includes a specific migration limit for nickel of 0.02 mg/kg food or food simulant from plastic materials and articles. In addition, the Council of Europe published in 2013 a practical guide on metals and alloys used in food contact materials and articles, which set out a specific release limit (SRL) for nickel of 0.14 mg/kg food (EDQM, 2013).
EU Council Directive 98/83/EC7 on the quality of water intended for human consumption sets a parametric value for nickel at 20 μg/L (Annex I, Part B ‘Chemical parameters’); at the same time, it also indicates the minimum performance characteristics to be warranted by the method used for the analysis (Annex III). Within the Directive's scope, water intended for human consumption refers to:
all water either in its original state or after treatment, intended for drinking, cooking, food preparation or other domestic purposes, regardless of its origin and whether it is supplied from a distribution network, from a tanker, or in bottles or containers;
all water used in any food‐production undertaking for the manufacture, processing, preservation or marketing of products or substances intended for human consumption unless the competent national authorities are satisfied that the quality of the water cannot affect the wholesomeness of the foodstuff in its finished form.
The maximum limit for nickel in natural mineral water is regulated in the EU by Commission Directive 2003/40/EC8. In this Directive, nickel is listed in Annex I among the constituents naturally present in natural mineral water, with a maximum limit of 20 μg/L. As above, the Directive also indicates the performance characteristics to be warranted by the method used for the analysis (Annex II).
According to Annex VI of Regulation (EC) No 1272/20089 (Classification, Labelling and Packaging Regulation), nickel sulfate and nickel dinitrate are classified:
Carc. 1A H350i (May cause cancer by inhalation)
Muta. 2 H341 (Suspected of causing genetic effects)
Rep. 1B H360D (May damage the unborn child)
STOT RE 1 H372 (Causes damage to organs)
Acute Tox. 4 H302 (Harmful if swallowed)
Acute Tox. 4 H332 (Harmful if inhaled)
Skin Irrit. 2 H315 (Causes skin irritation)
Skin Sens. 1 H317 (May cause an allergic skin reaction)
Resp. Sens. 1 H334 (May cause allergy or asthma symptoms or breathing difficulties if inhaled)
Aquatic Acute. 1 H400 (Very toxic to aquatic life)
Aquatic Chronic 1 H410 (Very toxic to aquatic life with long lasting effects).
In addition, nickel dinitrate is also classified as Eye Dam. 1 H318 (causes serious eye damage). For nickel chloride there is no harmonised classification in the EU.
2. Data and methodologies
2.1. Supporting information for the assessment
The CONTAM Panel used its previous risk assessment on nickel in food and drinking water issued in 2015 as a starting point for drafting the supporting information. The data were summarised in a narrative way based on expert knowledge/judgement and updated when new information became available as identified in reviews and relevant scientific evaluations by national or international bodies. A search for previous assessments was carried out on the websites of the relevant organisations. In addition, three specific literature searches were conducted to identify scientific literature on previously reported occurrence and exposure data, on the occurrence of nickel nanoparticles in food and drinking water and on the migration of nickel from food contact materials into food. The literature search was performed in October and November 2019. Web of Science10 and PubMed11 were identified as databases appropriate for retrieving literature for the present evaluation. An overview of the search terms is given in Appendix A, Section A.1. The references obtained from the literature search were imported and saved using a software package (EndNote12) and screened based on title and abstract. The draft scientific Opinion underwent a public consultation from 4 June until 15 July 2020. The comments received and how they were taken into account when finalising the scientific Opinion were published in an EFSA Technical Report (EFSA, 2020).
2.2. Hazard identification and characterisation
The CONTAM Panel applied the general principles of the hazard identification and characterisation for chemicals in food as described by WHO/IPCS (2009) as well as the different EFSA guidance documents relevant to this step of the risk assessment (Appendix A, Section A.4).
2.2.1. Collection and selection of evidence
A comprehensive search for literature was conducted for peer‐reviewed original research pertaining to adverse health effects in experimental animals and humans following oral exposure. The search strategy was designed to identify scientific literature dealing with toxicokinetics, toxicity and mode of action. This Scientific Opinion is an update of the previous Scientific Opinion on nickel in food and drinking water published in 2015 and for which the literature search was conducted in 2013 (Casalegno et al., 2015). Therefore, the literature search for the current Opinion was restricted to papers published since 1 January 2013. It was decided not to restrict the literature search to publications in English.
The literature search was performed in June 2019. Web of Science,10 PubMed11 and SciFinder were identified as databases appropriate for retrieving literature for the present evaluation. An overview of the search terms is given in Appendix A, Section A.2. The references obtained from the literature search were imported and saved using a software package (EndNote12). The references obtained were screened based on title and abstract using Distiller SR to identify the relevant literature, and the exclusion criteria are shown in Appendix A, Section A.3.
Additionally, relevant scientific evaluations by national or international bodies and reviews were considered for the current risk assessment.
2.2.2. Appraisal of evidence
The information retrieved was screened and evaluated by relevant domain experts from the CONTAM working group on nickel in food and used for the present assessment. Limitations in the information used are documented in this Scientific Opinion.
The selection of the scientific papers for inclusion or exclusion was based on consideration of the extent to which the study was relevant to the assessment or on general study quality considerations (e.g. sufficient details on the methodology, performance and outcome of the study, on dosing, substance studied and route of administration and on statistical description of the results), irrespective of the results.
2.3. Occurrence data submitted to EFSA
2.3.1. Data collection and validation
Following a mandate from the European Commission to EFSA, a call for annual collection of chemical contaminant occurrence data in food and drinking water, including nickel, was issued in December 2010.13 European national authorities and similar bodies, research institutions, academia, food business operators and other stakeholders were invited to submit analytical data on nickel in food and drinking water. The data for the present assessment were provided by organisations from 26 European countries. In addition, for some samples, the EU was indicated as place of sampling without specification of the country while for other samples no information on sampling place was provided. All analytical results were reported as nickel without providing information on specific chemical forms.
The data submission to EFSA followed the requirements of the EFSA Guidance on Standard Sample Description for Food and Feed (EFSA, 2010a); occurrence data were managed following the EFSA standard operational procedures (SOPs) on ‘Data collection and validation’ and on ‘Data analysis of food consumption and occurrence data’.
Data on nickel in food and drinking water submitted to EFSA by the beginning of January 2020 were considered for the present assessment. Data received after that date were not included.
2.3.2. Data analysis
Following EFSA's SOP on ‘Data analysis of food consumption and occurrence data’ to guarantee an appropriate quality of the data used in the exposure assessment, the initial data set was carefully evaluated by applying several data cleaning and validation steps. Special attention was paid to identification of duplicates and to accuracy of different parameters such as ‘Sampling country’, ‘Sampling year’, ‘Sampling strategy’, ‘Analytical methods’, ‘Result express’, ‘Reporting unit’, ‘LOD/LOQ’, and the codification of analytical results under FoodEx classification (EFSA, 2011a). The outcome of the data analysis is presented in Section 3.2.1 and Annex C, Table C.1.
The left‐censored data (LCD) (results below LOD or below LOQ) were treated by the substitution method as recommended in the ‘Principles and methods for the risk assessment of chemicals in food’ (WHO/IPCS, 2009). The same method is indicated in the EFSA scientific report ‘Management of left‐censored data in dietary exposure assessment of chemical substances’ (EFSA, 2010b) as an option in the treatment of LCD. The guidance suggests that the lower bound (LB) and upper bound (UB) approach should be used for chemicals likely to be present in the food (e.g. naturally occurring contaminants, nutrients and mycotoxins). The LB is obtained by assigning a value of zero (minimum possible value) to all samples reported as lower than the LOD (< LOD) or LOQ (< LOQ). The UB is obtained by assigning the numerical value of the LOD to values reported as < LOD and the LOQ to values reported as < LOQ (maximum possible value), depending on whether the LOD or LOQ is reported by the laboratory.
2.4. Food consumption data
EFSA Comprehensive European Food Consumption Database (hereinafter referred to as the Comprehensive Database) provides a compilation of existing national information on food consumption at the individual level. It was first built in 2010 (EFSA, 2011b; Huybrechts et al., 2011; Merten et al., 2011). Details on how the Comprehensive Database is used have been published in an EFSA Guidance (EFSA, 2011b). The latest version of the Comprehensive Database updated in 2020 contains results from a total of 69 different dietary surveys carried out in 25 different Member States covering 134,929 individuals.
Within the dietary studies, subjects are classified in different age classes as follows:
Infants: < 12 months old
Toddlers: ≥ 12 months to < 36 months old
Other children: ≥ 36 months to < 10 years old
Adolescents: ≥ 10 years to < 18 years old
Adults: ≥ 18 years to < 65 years old
Elderly: ≥ 65 years to < 75 years old
Very elderly: ≥ 75 years old
Seven surveys provide information on specific population groups: ‘Pregnant women’ (≥ 15 years to ≤ 48 years old) and ‘Lactating women’ (≥ 18 years to ≤ 45 years old).
Overall, the food consumption data gathered by EFSA in the Comprehensive Database are the most complete and detailed data currently available in the EU. Consumption data were collected using single or repeated 24‐ or 48‐h dietary recalls or dietary records covering from three to seven days per subject. Owing to the differences in the methods used for data collection, direct country‐to‐country comparisons can be misleading.
Detailed information on the different dietary surveys used in the present evaluation is shown in Annex B Table B.1, including the number of subjects and days available for each age class.
2.5. Food classification
Consumption data were classified according to the FoodEx classification system (EFSA, 2011a). FoodEx is a food classification system developed by EFSA in 2009 with the objective of simplifying the linkage between occurrence and food consumption data when assessing the exposure to hazardous substances. The system consists of a large number of individual food items aggregated into food groups and broader food categories in a hierarchical parent–child relationship. It contains 20 main food categories (first level), which are further divided into subgroups having 140 items at the second level, 1,261 items at the third level and reaching about 1,800 endpoints (food names or generic food names) at the fourth level.
2.6. Exposure assessment
The CONTAM Panel estimated chronic and acute dietary exposure to nickel. In Annex B Table B.1, the number of available days for each age class used in the acute exposure assessment is described beside the number of subjects available for the chronic exposure assessment.
Some of the occurrence data were obtained for food products containing seaweed (e.g. pasta, biscuits, soups). Since no consumption data for such specific products are available, these data could not be used for the overall chronic and acute exposure to nickel. However, the exposure from pasta containing seaweed was covered in a separate acute exposure scenario (see below).
For calculating chronic dietary exposure to nickel, dietary surveys with only 1 day per subject were not considered as they are not adequate to assess repeated exposure (EFSA, 2011a). Similarly, subjects who participated in the dietary studies for only 1 day when the protocol prescribed more reporting days per individual, were also excluded for the chronic exposure assessment. When, for one particular country and age class, two different dietary surveys were available, only the most recent one was used.
Thus, for the chronic exposure assessment, food consumption data were used from 44 different and most recent dietary surveys carried out in 23 different European countries present in the latest version of the Comprehensive Database (Annex B, Table B.1).
For calculating chronic dietary exposure to nickel, food consumption and body weight data at the individual level were accessed in the Comprehensive Database. Occurrence data and consumption data were linked at the relevant FoodEx level. In addition, the different food commodities were grouped within each food category to better explain their contribution to the total dietary exposure to nickel. The food categories represented by either very low number of samples (< 6 samples) or for which all data were below the LOD or LOQ were considered not suitable and were not used for the exposure calculation.
The mean and the high (95th percentile) chronic dietary exposures were calculated by combining nickel mean occurrence values for food samples collected in different countries (pooled European occurrence data) with the average daily consumption for each food at an individual level in each dietary survey and age class. Consequently, individual average exposures per day and body weight were obtained for all individuals. On the basis of distributions of individual exposures, the mean and 95th percentile exposure were calculated per survey and per age class. Dietary exposure was calculated using overall European LB and UB mean occurrence of nickel.
Before linking the consumption data to the corresponding occurrence data, the following adjustments to the occurrence and consumption data were made to reduce uncertainty and reach more accurate exposure estimates:
Occurrence and consumption events for solid forms of certain foods (tea leaves, cocoa powder, cocoa powder preparations, cocoa beans, coffee powder, coffee beans, coffee imitates powder, concentrated/dehydrated/powdered fruit juices, dried milk and dehydrated soups) were adjusted by an appropriate dilution factor and these consumption events were reclassified to the liquid forms as this is considered more appropriate for the current assessment (EFSA, 2018b).
Occurrence data and consumption events for solid forms of infant formulas and follow‐on formulas were adjusted by a dilution factor of eight and reclassified to the liquid forms (as ready for feeding) as this is considered more appropriate for the current assessment.
The nickel contamination in water and milk used for the dilution was not taken into account since it was considered unlikely that the water and milk would always contain nickel. This could lead to an underestimation of the exposure.
Consumption events for cereal‐based food for infants and young children were adjusted by a factor of 0.25 (when reconstituted with water) or 0.15 (when reconstituted with milk) when the eating occasions were reported as consumed (liquid) since the occurrence data mainly referred to the analysis of the food as purchased. This correction was based on the information given by the data provider as to whether the product is reconstituted with milk or water (EFSA, 2018b).
Acute dietary exposure to nickel was estimated using a probabilistic approach based on the method of random sampling with replacement of occurrence data. The random sampling captures the variability in occurrence values. The consumption events were not randomly sampled because the CONTAM Panel considered it would not have had a major impact on the results since all food categories mostly contributing to the mean acute dietary exposure to nickel are regularly and widely consumed foods. In addition, it would have considerably increased the complexity and the computation time. A total of the 48 most recent dietary surveys carried out in 25 different European countries were used (Annex B, Table B.1). Acute exposure was assessed for each reporting day by multiplying the total consumption amount for each food category by one UB occurrence level randomly drawn among the individual results available for that food category. Respective intakes of the foods consumed that day were then summed and finally divided by the individual's body weight. To model the uncertainty, the process was iterated 1,000 times for each reporting day. The overall mean values of the 1,000 means and of the 1,000 P95 daily acute UB exposures per survey and per age class were then calculated. The 95% confidence interval (CI) defined as the interval between the 2.5th and 97.5th percentiles obtained from the 1,000 iterations was determined to indicate the uncertainty around the mean value.
In addition, the CONTAM Panel considered that it is of interest to also estimate an acute exposure from specific foods or occurring within particular circumstances. Therefore, three additional specific acute exposure scenarios were developed and calculated as follows:
Acute exposure from seaweed. The exposure was assessed on a per day basis by multiplying the mean and the highest reliable percentile consumption amount of each age class and survey by the 95th percentile occurrence level (6,269 μg/kg) of seaweed. It was noted that the 95th percentile UB and LB occurrence levels were equal. Due to the lack of consumption data, the exposure could be estimated only for a limited number of surveys.
Acute exposure from pasta containing seaweed. In the absence of consumption data for such specific food products, an amount of regular pasta was assumed as a proxy also for the pasta containing seaweed. The exposure was calculated on a per day basis by multiplying the mean and 95th percentile consumption amount of each age class and survey by the 75th percentile (the highest reliable percentile) occurrence level (1,521 μg/kg) of pasta containing seaweed. It was noted that the 75th percentile UB and LB occurrence levels were equal.
Acute exposure from water, considering an adult subject drinking a glass of tap water or bottled water in the morning on empty stomach. The exposure was assessed by multiplying 500 mL of tap water or bottled water by the 95th percentile UB occurrence level of 5.0 or 11 μg/kg, respectively. A standard body weight of 70 kg for adults was considered.
All analyses were run using the SAS Statistical Software (SAS enterprise guide 9.4).
2.7. Risk characterisation
The general principles of the risk characterisation for chemicals in food as described by WHO/IPCS (2009) were applied as well as the different EFSA guidance documents relevant to this step of the risk assessment (Appendix A, Section A.4).
3. Assessment
3.1. Hazard identification and characterisation
3.1.1. Toxicokinetics
3.1.1.1. Absorption, Distribution, Metabolism, and Excretion
According to the data presented in the previous Opinion (EFSA CONTAM Panel, 2015), the bioavailability of nickel following ingestion depends on the solubility of the administered nickel compound, the dosing vehicle and the fasting state of the subject. Solomons et al. (1982) reported that when nickel was given in drinking water to fasted individuals nickel plasma levels increased significantly compared to non‐exposed fasted individuals. The absorption of nickel when administered in meals was considerably lower, with plasma levels not being statistically significantly different from those in non‐exposed fasted individuals. When nickel was given via a soft drink to fasted subjects, the absorption was similar to that observed with drinking water, whereas a lower increase in plasma levels was observed following administration in whole milk, coffee, tea or orange juice. For healthy human volunteers, Sunderman et al. (1989) reported a mean absorption of 27 ± 17% of the administered nickel dose when administered in drinking water after a 12 h fasting period, versus a mean absorption of 0.7 ± 0.4% when administered in food; the absorption was estimated based on excretion of nickel in the urine. Nielsen et al. (1999) reported that the cumulative median amount of nickel excreted in urine within three days after dosing was 2.26% (1.03–4.71%) when nickel was ingested together with food or mixed into food. Increasing amounts of nickel were excreted in the urine as the interval between intake of water and meal increased, with a cumulative median amount of 25.8% (25.00 ± 11.02) excreted in urine when food was served 4 h prior to ingestion of nickel‐containing drinking water. Patriarca et al. (1997) reported, based on faecal excretion measurements, that 9–40% of nickel ingested in drinking water was absorbed in four fasted human volunteers. In laboratory animals, nickel was rapidly but poorly absorbed following ingestion, as suggested by the low urinary excretion observed in various studies (EFSA CONTAM Panel, 2015). A study in rats showed an absorption of around 10% when nickel sulfate or nickel chloride was administered in a 5% starch saline solution as vehicle (EFSA CONTAM Panel, 2015). After absorption, nickel is widely distributed in the organism of both animals and humans. Animal studies showed that nickel can be found in the peripheral nerve tissues and in the brain (EFSA CONTAM Panel, 2015). In studies with mice, nickel was shown to cross the placenta resulting in increased levels of nickel in the fetuses. There are some indications that the absorbed nickel can bind to serum proteins, in particular to albumin. Absorbed nickel is excreted mainly via the urine and to a lower extent in breast milk. An estimated elimination half‐life of 28 ± 9 h was calculated in human volunteers (EFSA CONTAM Panel, 2015).
Since the previous Opinion, Toman et al. (2014) investigated the distribution of nickel in selected organs of male Wistar rats after oral administration of nickel chloride hexahydrate in drinking water at a concentration of 100 mg/L for 90 days. This corresponds to a dose of nickel chloride hexahydrate of 9 mg/kg bw per day applying the default factor of 0.09 for a subchronic study in rats (EFSA Scientific Committee, 2012a), equivalent to 2 mg Ni/kg bw per day. An untreated group served as the control. The concentration of nickel was statistically significantly lower in the muscle of the treated group (0.20 ± 0.12 mg/kg) compared with the control group (1.18 ± 0.79 mg/kg). No significant differences were observed for the liver, kidney and testis. These results indicate that nickel does not accumulate in tissues following repeated oral ingestion.
The divalent metal transporter 1 (DMT1; encoded by the SLC11a2 gene which is polymorphic (Kayaalti et al., 2015)) mediates the transport of nickel and other divalent metal ions such as iron from the lumen of the intestine into the enterocyte and export of iron from endocytic vesicles. DMT1 also mediates apical uptake of divalent cations in the kidney, and has been shown to be involved in recovery of iron from recycling endosomes during transferrin receptor‐associated cellular uptake in various cell types (Mackenzie and Garrick, 2005). There is evidence for accumulation of nickel in the brain (see Section 3.1.2.7 Neurotoxicity). DMT1 is known to be involved in the transport of divalent iron into the cytosol of endosomal cells prior to transport across the blood–brain barrier by ferroportin (Skjørringe et al., 2015). Since nickel is also a substrate for DMT1, this transporter is likely to also be involved in nickel uptake into the brain.
3.1.1.2. Kinetic modelling
A toxicokinetic model developed for oral exposure to nickel by Sunderman et al. (1989) was described in the previous Opinion (EFSA CONTAM Panel, 2015). The model was based on two studies in eight human volunteers, in which levels of nickel in serum and faecal excretion were determined on the 2 days before and 4 days after administration of nickel sulfate at dose levels of 12, 18 or 50 μg Ni/kg bw in water or in food to the same subjects. The only estimated kinetic parameter that appeared significantly different between exposure in water and food was the fraction of the dose that was absorbed. The model was shown to adequately predict serum nickel levels.
Since the previous Opinion, one study of relevance for this mandate has been published (Dede et al., 2018). The aim of this study was to use physiologically based pharmacokinetic (PBPK) models to determine the optimal time for collecting biological samples in a longitudinal study to evaluate whether participants who consumed different foods had been exposed to arsenic, cadmium, chromium, nickel or lead. Only information relevant to nickel is presented here. The model was based on the parameters from experiment 2 in relation to the PBPK model developed by Sunderman et al. (1989) in which nickel levels were determined in serum, urine and faeces from eight human subjects who had been given an oral dose of nickel (as nickel sulfate) in food. As Sunderman et al. (1989) did not determine the rate of transfer from tissues to serum in experiment 2, Dede et al. (2018) used the nickel transfer from tissues to serum from experiment 1 in which nickel was administered in water. The unabsorbed fraction of nickel was accounted for by adding a faeces compartment to the model. The predictive performance of the modified model was tested by using data from two previous studies, i.e. Sunderman et al. (1989) and Nielsen et al. (1999). The predicted urinary excretion of nickel was shown to match closely with data from Sunderman et al. (1989). The mass fraction of the nickel dose absorbed from the gut was predicted to be 0.7 ± 0.4% by Sunderman et al. (1989) when nickel was ingested via food. However, a higher nickel absorption from food of 2.95 ± 1.32% was reported by Nielsen et al. (1999). In the Dede et al. (2018) model, the most sensitive parameters were related to oral absorption of nickel. The model also showed that the urinary elimination rate of nickel was an additional sensitive parameter.
3.1.1.3. Summary
The bioavailability of ingested nickel ranged from about 1% to about 30% in human volunteers when evaluated based on analyses of nickel in plasma or urine. A low absorption (0.7–2.5%) was observed when nickel was ingested in the presence of food or under non‐fasted state, whereas a higher absorption (25–27%) was observed when nickel was ingested via drinking water in the absence of food, or under a fasted state. The CONTAM Panel noted the low number of individuals examined in the three relevant human studies, as well as a considerable inter‐individual variability in the measured parameters precluding a precise estimate of the oral bioavailability of nickel. A study in rats showed an absorption of around 10% when nickel sulfate or nickel chloride was administered in a 5% starch saline solution as vehicle. After absorption, nickel is widely distributed in the organism. In a study with mice, nickel was shown to cross the placenta. There are also indications of transport across the blood–brain barrier. Absorbed nickel is excreted mainly via the urine and to a lower extent in breast milk. An estimated elimination half‐life of 28 ± 9 h was calculated in human volunteers. A recent PBPK model based on parameters from a previously published model showed that the most sensitive parameters were related to oral absorption of nickel. The model also showed that the urinary elimination rate of nickel was an additional sensitive parameter.
3.1.2. Toxicity in experimental animals
3.1.2.1. Acute toxicity (single exposure)
According to the data presented in the previous Opinion (EFSA CONTAM Panel, 2015), water‐soluble nickel compounds have shown moderate to high acute toxicity with LD50 values ranging from 39 to 190 mg Ni/kg bw for nickel sulfate, 43–130 mg Ni/kg bw for nickel chloride, > 404 mg Ni/kg bw for nickel nitrate and 116–325 mg Ni/kg bw for nickel acetate.
Since the previous Opinion, no acute toxicity studies of relevance for this mandate have been identified.
3.1.2.2. Short‐term toxicity (5–90 days)
According to the data presented in the previous Opinion (EFSA CONTAM Panel, 2015), the major effects observed in the short‐term repeated‐dose toxicity studies following oral administration were decreased body weight, changes in organ weight (liver and kidneys), and histopathological changes in the liver and the kidney.
Since the previous Opinion, nine short‐term toxicity studies of relevance for this mandate have been published (details are reported in Appendix B.1). The reporting of several studies does not allow the CONTAM Panel to evaluate the results and these studies are only reported in Appendix B.1.
In a study aiming to analyse the biochemical parameters of blood plasma, male Wistar rats were administered nickel chloride hexahydrate in the drinking water at concentrations of 0 or 100 mg/L (corresponding to 2 mg Ni/kg bw per day based on the default factor of 0.09 for a subchronic study in rats set by the EFSA Scientific Committee (2012a)) daily for 90 days (Toman et al., 2013). Potassium, calcium, magnesium, total proteins, cholesterol, bilirubin and glutamate dehydrogenase concentrations were significantly decreased when compared with the control values, and glucose and alkaline phosphatase concentrations were significantly increased.
In a study on the effects on bone composition, adult male mice were administered nickel sulfate or nickel nitrate by oral gavage daily for 40 days (0, 5.0, 15 or 40 or 5.0, 20 or 40 mg/kg bw per day, respectively) (Gathwan and Al‐Karkhi, 2015). Assuming that the doses are expressed as nickel salt, the corresponding doses of nickel are 1.9, 5.7 and 15.2 mg Ni/kg bw per day for nickel sulfate and 1.6, 6.4 and 12.8 mg Ni/kg bw per day for nickel nitrate. The control group was on a normal diet and water. The intake of feed and water was lower in treated mice as compared to the control group and, according to the authors, the decrease was dose dependent (no data presented in the article). The femur bone weight was significantly decreased in the mid‐ and high‐dose groups. Histopathologically, necrosis to layers of decalcified bone, i.e. periosteum, matrix and endosteum was observed with both nickel salts. The bone‐forming cells, lamellae and Haversian canals were also affected. The cortical width of bone section decreased dose dependently with both nickel salts. Such changes were also observed in samples of powdered dried bone with scanning electron microscopy (SEM). According to the authors, the effects of nickel sulfate were more severe than those of nickel nitrate. The CONTAM Panel noted that the doses causing effects, expressed as nickel, were higher for nickel sulfate than for nickel nitrate, which could explain the differences in toxicity reported by the authors.
In a more recent publication by the same group (Gathwan and Albir, 2019), effects on bone composition were also examined in adult male mice. The CONTAM Panel was not able to evaluate the results of this study based on the two‐page article without details.
The gut microbiota are critical for healthy functioning of the gut. In humans and animals, changes in the gut microbial population are associated with multiple health problems. In humans, this includes obesity and inflammatory bowel disease. The CONTAM Panel identified two studies investigating the effect of nickel on gut microbiota.
In a study aiming to gain a more comprehensive understanding of the effects of metal exposure on the gut microbiota, Richardson et al. (2018) exposed rats to nickel chloride. Sprague–Dawley rats were administered nickel chloride by oral gavage at doses of 0, 177, 232, or 300 mg/kg bw per day (corresponding to 0, 80, 105 or 136 mg Ni/kg bw per day) daily for five consecutive days. 16S ribosomal RNA (rRNA) gene sequencing was used to track changes in the gut microbiota composition. Significant dose‐dependent changes were observed in response to nickel. Bacteria with higher numbers of iron‐importing gene orthologs were overrepresented after exposure to nickel.
In a study examining the effect of oral nickel exposure on intestinal microflora, female mice were administered water containing 400 μM nickel sulfate hexahydrate for 21 days (Zhou et al., 2019). Based on the default factor of 0.18 for a subacute study in mice (EFSA Scientific Committee, 2012a) and the molecular weight of 262.85 g/mol for nickel sulfate hexahydrate, the corresponding dose is 4 mg Ni/kg bw per day. The control group received pure water. There was no significant difference in body weight between the treated group and the control group. The nickel concentration in the kidney of treated mice was significantly higher compared to the controls. Regarding the influence on gut microbiota, the authors concluded that orally administered nickel could change the intestinal flora in mice and thus could alter the interaction between the host and the intestinal flora.
In summary, the short‐term toxicity studies published since the previous Opinion have reported similar effects as the studies reported in the previous Opinion. Furthermore, effects on bone and on gut microbiota were reported.
3.1.2.3. Genotoxicity
In 2015, the CONTAM Panel concluded that ‘soluble nickel compounds are not mutagenic in bacterial cells and, in general, weakly mutagenic in mammalian cells in vitro. Chromosomal effects due to both aneugenic and clastogenic activity of soluble nickel compounds have been observed in mammalian cells in vitro. The evidence for in vivo induction of chromosomal alterations is inconsistent. There is evidence for the induction of DNA damage by soluble nickel compounds both in vitro and in vivo’. It was also shown that soluble nickel compounds can induce morphological transformation of mammalian cells in vitro.
Since the previous Opinion (EFSA CONTAM Panel, 2015), 12 new studies have been identified and they are summarised in Tables 1 and 2. The papers by Terpilowska and Siwicki (2018) and Czarnek et al. (2019) are not included in Table 1 due to the limited reporting and the unreliable results for the controls. The in vivo study by Mitkovska et al. (2017) is not included in Table 2 due to the lack of the identification of the compound tested and the absence of a validation of the methodology.
Table 1.
In vitro new genotoxicity studies on nickel
| Endpoint | Experimental test system | Test substance | Exposure conditions | Result | Comments | Reference |
|---|---|---|---|---|---|---|
| SSBs (Comet assay) | Primary normal human dermal fibroblasts | NiCl2 (purity: 99.99%) Negative and positive controls: substance not specified | 5,000, 10,000, 25,000 and 50,000 μM 2 h exposure | Increased SSBs only at 50,000 μM (tail moment) Positive | According to protocol by Singh et al. (1988) | Belliardo et al. (2018) |
| SSBs (Comet assay) | Human B lymphoblastoid cell line HMy2.CIR | NiCl2 (purity: not specified) Solvent: not specified Negative control: solvent | 0, 80, 160, 320 and 640 μM 24 or 48 h exposure | Increased SSBs only at 640 μM at 24 h and 48 h (% DNA in the tail) Positive 640 μM: increased ROS levels at 48 h but not at 24 h 160, 320 and 640 μM: increased MDA levels at 24 h and 48 h | According to protocol by Singh et al. (1988) 640 μM: modest inhibition of viability at 24 h 160, 320, 640 μM: inhibition of viability at 48 h | Lou et al. (2013) |
| DSBs (γ‐H2AX by western analysis) | Human Hep G2 (hepatoblastoma) and LS‐174T (colorectal adenocarcinoma) cells | NiCl2 (purity > 95%) Solvent: water Negative control: solvent Positive control: 1 μM benzo[a]pyrene | 100, 250, 500, 750 and 1,000 μM 24‐h exposure | Negative | Dose‐dependent decrease in cell viability (up to 50%) | Kopp et al. (2018) |
| Micronuclei, NPB, and NBUD (cytokinesis‐block micronucleus cytome test) | Immortalised human bronchial epithelial cell line (BEAS‐2B) | Water‐soluble nickel (II) chloride (NiCl2·6H2O) Negative control: untreated cells Positive control: mitomycin C | 1, 5 and 10 μg/mL Exposure: 48 h | The frequency of micronuclei in binucleated cells was significantly higher than for control cells for the two highest concentrations tested NiCl2 increased NPB and NBUD frequencies Positive | NiCl2 showed a significant cytostatic effect and also reduced the mitotic index | Di Bucchianico et al. (2018) |
| Chromosomal aberrations | Immortalised human bronchial epithelial cell line (BEAS‐2B) | Water‐soluble nickel (II) chloride (NiCl2·6H2O) Negative control: untreated cells Positive control: mitomycin C | 1, 5 and 10 μg/mL Exposure: 48 h | NiCl2 significantly increased the rate of chromatid‐type aberrations and induced both inter‐ and intra‐arm exchanges It also induced chromosome‐type aberrations, mainly the formation of dicentric chromosomes as well as endo‐reduplications. Various degrees of aneuploidy such as trisomy, and to a lesser extent monosomy, particularly involving chromosomes 1, 3, 14, 20 and 21 The mitotic index slightly decreased following NiCl2 exposures Positive | Di Bucchianico et al. (2018) | |
| SSBs, (Comet assay) | Immortalised human bronchial epithelial cell line (BEAS‐2B) | Water‐soluble nickel (II) chloride (NiCl2·6H2O) Negative control: untreated cells Positive control: H2O2 | 1, 5 and 10 μg/mL Exposure: 48 h | Modest increases of SSBs compared to control without clear dose response Increased ROS level NiCl2 caused a statistically significant increase in intracellular Ca2+ Positive | Di Bucchianico et al. (2018) | |
| DSB (Neutral Comet assay) | A549 cells: human lung carcinoma BEAS‐2B cells: non‐tumorigenic cells, immortalised cell line derived from normal human bronchial epithelium | Water‐soluble nickel (II) chloride (NiCl2) Negative control: water | A549 cells: 0, 100, 250 and 500 μM BEAS‐2B cells: 0, 100 and 250 μM Exposure : 45 h +/– irradiation (5 Gy IR) Harvesting: 24 h post irradiation | 0 μM NiCl2: no increase in DSB in irradiated cells (repair completed at 24 h) > 100 μM: concentration‐dependent increase in DSB persisting 24 h post‐irradiation in irradiated cells At 250 μM (BEAS‐2B) and 500 μM (A549): small increases in the median comet tail moment were observed in non‐irradiated cells Positive Nickel inhibits repair of IR‐induced DSB in tumorigenic and non‐tumorigenic lung cells | Scanlon et al. (2017) |
DSBs: double‐strand breaks; IR: irradiation; MDA: malondialdehyde; NiCl2: nickel chloride; ROS: reactive oxygen species; SSBs: single‐strand breaks; NPB: nucleoplasmic bridges (a biomarker of DNA misrepair and/or telomere end‐fusions); NBUD: nuclear buds (a biomarker of elimination of amplified DNA and/or DNA repair complexes).
Table 2.
In vivo new genotoxicity studies on nickel
| Endpoint and experimental system | Test substance | Exposure conditions | Result | Comments | Reference |
|---|---|---|---|---|---|
| Chromosomal aberrations Male mice bone marrow N = 5/group | NiCl2 Vehicle: not specified Positive control: endoxan Negative control: vehicle | Single i.p. treatment at 0, 2.62, 5.25, 10.5 and 21.0 mg/kg bw Harvesting: 24 h Repeated i.p. treatment at 2.62, 5.25 and 10.5 mg/kg bw per day for 1, 2 or 3 weeks | Single treatment: dose‐dependent increase in % abnormal metaphases (fragment/breaks, deletions, translocations, endomitosis) from 5.25 mg/kg bw onwards Positive Repeated treatment: 1 week: increased % of abnormal metaphases at the two highest doses second and third weeks: increased abnormal metaphases at all doses Positive | Cumulative effect of repeated dosing of NiCl2 | Fahmy et al. (2014) |
| Chromosomal aberrations Male mice spermatocytes N = 5/group | NiCl2 Vehicle: not specified Positive control: endoxan Negative control: vehicle | Single i.p. treatment at 0, 2.62, 5.25, 10.5 and 21.0 mg/kg bw Harvesting: 24 h Repeated i.p. treatment at 2.62, 5.25 and 10.5 mg/kg bw per day for 1, 2 or 3 weeks | Single treatment: dose‐dependent increase in % abnormal metaphases (separation of X‐Y and autosomal univalent, fragment/breaks) from 5.25 mg/kg bw Positive Repeated treatment: Dose‐ and time‐dependent increase in % abnormal metaphases at all doses Positive | The authors report a significant dose‐dependent increase in the % of sperm abnormalities (heads and tails) | Fahmy et al. (2014) |
| Chromosomal aberrations (structural and numerical) adult male Swiss albino mice Bone marrow N = 12/group | NiCl2 Vehicle: saline Positive control: not included Negative control: vehicle | 2.3, 4.7 and 7.0 mg/kg bw (s.c. injection) 24‐h exposure 800 cells scored | Dose‐related increase in % aberrant cells (without gaps) (significant only at the highest dose) (induction of gaps, breaks, fragments and exchanges) Increase incidence of aneuploidy in all groups. Ratio hypoploidy (38/39 chromosomes)/hyperploidy (41/42 chromosomes): between 2.1 and 3:1 Increased incidence of polyploidy (3 N) Positive | Clastogenic and aneugenic effects were associated with oxidative stress (increased lipid peroxidation and NO, decreased GSH levels) and cytotoxicity (Lactate dehydrogenase) | El‐Habit and Abdel (2014) |
| Micronuclei Adult male Swiss albino mice Bone marrow N = 12/group | NiCl2 Vehicle: saline Positive control: not included Negative control: vehicle | 2.3, 4.7 and 7.0 μmol/kg bw (s.c. injection) Harvesting; 24 h | Significant increase in MNPCE (4–9‐folds) at all doses Dose‐related decrease in PCE/NCE Positive | 500 PCE scored | El‐Habit and Abdel (2014) |
| SSBs (comet assay) Adult male Swiss albino mice Bone marrow N = 12/group | NiCl2 Vehicle: saline Positive control: not included Negative control: vehicle | 2.3, 4.7 and 7.0 μmol/kg bw (s.c. injection) | Dose‐dependent increase in SSBs at all doses Positive | El‐Habit and Abdel (2014) |
NiCl2: nickel chloride; i.p.: intraperitoneal; N: number of animals; PCE: polychromatic erythrocytes; NCE: normochromatic erythrocytes; MNPCE: micronucleated polychromatic erythrocytes; s.c.: subcutaneous; SSBs: single‐strand breaks; GSH: glutathione.
In vitro
In the previous EFSA Opinion, it was shown that nickel compounds are inactive in almost all bacterial mutagenicity tests and are weakly mutagenic in cultured mammalian cells. Several reports indicate that nickel ions may be co‐mutagenic (e.g. with alkylating agents or ultraviolet light). This is likely due to interference with DNA repair processes. It was demonstrated that nickel can alter gene expression by enhanced DNA methylation and compaction. It is important to note that most of the evidence of nickel mutagenesis in mammalian cells was obtained using transgenic cell lines (e.g. locus gpt in G12 and G10 cell lines, lac I in an embryonic fibroblast cell line) (Kargacin et al., 1993; Klein et al., 1994; Mayer et al., 1998; Kasprzak et al., 2003).
It was also shown that water‐soluble and poorly water‐soluble nickel compounds induce sister chromatid exchanges, chromosomal aberrations and micronuclei at high (mM), cytotoxic levels in different mammalian cell systems. These effects are likely due to aneugenic as well as clastogenic actions. It was reported that the chromosomal aberrations induced by nickel occurred predominantly in heterochromatic regions of the chromosomes. Water‐soluble as well as water insoluble nickel compounds have been shown to induce DNA single‐strand breaks (SSBs), DNA–protein cross‐links and oxidative DNA damage in mammalian test systems.
The genotoxicity data published since the previous EFSA assessment (see Table 1) confirm that soluble nickel compounds induce DNA damage in vitro as visualised in alkaline comet assays indicating the formation of SSBs (Lou et al., 2013; Belliardo et al., 2018). In a recent study, it was shown that nickel chloride induces micronuclei, chromosomal aberrations and SSBs in immortalised human bronchial epithelial cells (Di Bucchianico et al., 2018). However, no increase in double‐strand breaks (DSBs) as measured by a γ‐H2AX assay was observed in the human hepatoblastoma Hep G2 and colorectal adenocarcinoma LS‐174T cell lines (Kopp et al., 2018). Finally, Scanlon et al. (2017) investigated the biological consequences of the inhibition by nickel of homology‐dependent DSBs repair (HDR). By reducing this repair pathway, low doses of nickel increased ionising radiation‐induced DSBs (as measured by a neutral comet assay) in immortalised bronchial cells or in lung carcinoma cells. At high doses of nickel,, small increases of DSBs were also observed in non‐irradiated cells indicating a defective repair of spontaneous DSBs.
In vivo
As reported previously, in vivo mutation studies with nickel compounds were mostly conducted in Drosophila melanogaster and showed weakly positive effects. The mutagenic effects of nickel sulfide were tested in vivo in LacZ transgenic CD2F1 mice and in lacI transgenic F34 rats. In nasal mucosa and lung tissue, no increase in mutation frequencies was observed compared with negative controls (Mayer et al., 1998).
The induction of chromosomal aberrations and micronuclei in rodents treated with different nickel compounds is not consistent across studies. As reported previously, following oral, intraperitoneal (i.p.) or subcutaneous (s.c.) administration, both positive (Sobti and Gill, 1989; El‐Habit and Abdel, 2014) and negative (Deknudt and Leonard, 1982; Oller and Erexson, 2007) results were obtained. In the more recent publications (see Table 2), increased chromosomal aberrations and micronuclei were observed in mouse bone marrow after i.p. or s.c. exposure to nickel chloride, and chromosomal aberrations were also observed in spermatocytes after i.p. exposure.
Both soluble and insoluble nickel compounds give rise to both DNA breaks and DNA–protein cross‐links in vivo after oral exposure or s.c. injection (Saplakoglu et al., 1997; Kawanishi et al., 2002; Kasprzak et al., 2003; Danadevi et al., 2004). The formation of SSBs was confirmed in a new alkaline comet assay in mice after s.c. injection (Table 2).
As reported in the previous opinion, DNA damage and chromosomal alterations have been analysed in cells from nickel‐exposed workers (e.g. from an electrolytic nickel refinery or welders) with inconsistent findings since both positive (Werfel et al., 1998; Danadevi et al., 2004) and negative studies (Kiilunen et al., 1997) were reported. A positive association between nickel levels and the level of oxidative DNA lesions (fpg‐sensitive sites) was observed in an urban population in Germany (Merzenich et al., 2001). Increases in micronuclei have also been reported in the oral epithelial cells of children exposed to nickel via metal crowns (Morán‐Martínez et al., 2013).
The CONTAM Panel noted that several of the new studies had limitations in their design and/or reporting as indicated in the Tables.
In summary, new data confirm that soluble nickel compounds induce structural and numerical chromosomal aberrations and SSBs in vitro and in vivo. Based on the available data, the genotoxicity of nickel is likely due to indirect effects including inhibition of DNA repair and reactive oxygen species (ROS) production (see Section 3.1.4.1).
3.1.2.4. Long‐term toxicity (including carcinogenicity)
No tumours have been observed in the carcinogenicity studies in experimental animals after oral administration of soluble nickel compound (EFSA CONTAM Panel, 2015).
Since the previous Opinion, no long‐term toxicity studies or carcinogenicity studies of relevance for this mandate have been identified.
3.1.2.5. Reproductive and developmental toxicity
In the previous Opinion (EFSA CONTAM Panel, 2015), several findings were reported. In rats, oral administration of nickel compounds does not induce alterations in reproductive tissues and no adverse effects on fertility or reproductive performances were reported (Ambrose et al., 1976; RTI, 1988a; Smith et al., 1993; Obone et al., 1999; SLI 2000a,b). However, in mice, effects on male sex organ weights, histopathological changes in these organs, disturbed spermatogenesis, decreased sperm motility and sperm damage have been reported in studies after oral exposure to nickel compounds (Pandey et al., 1999; Pandey and Srivastava, 2000) at doses of ≥ 2.2 mg Ni/kg bw per day. These effects were responsible for a decrease in fertility. Limitations in these studies preclude their use for the establishment of a reference point.
There is consistent evidence of increased pup mortality (stillbirth or post‐implantation loss/perinatal lethality) after exposure of rats to nickel chloride or sulfate in several reproductive toxicity studies at doses ≥ 1.3 mg/kg bw per day (range‐finding reproductive toxicity study (SLI 2000a), two‐generation reproductive toxicity study (RTI 1988a,b, SLI 2000b); Smith et al., 1993). For developmental toxicity, nickel crosses the placental barrier, directly affecting the developing embryo or fetus. Decreases in fetal weight (at doses ≥ 92 mg Ni/kg bw per day in mice exposed from gestation day (GD) 6–13, Saini et al., 2013) or pup weight (at doses of 6.8 mg/kg bw per day in rats exposed during one generation, Smith et al., 1993) were observed at higher doses. In mice exposed to nickel chloride, malformations, reduced ossification and increased incidence of skeletal anomalies were observed at doses ≥ 92 mg Ni/kg bw per day in the presence of maternal toxicity. Microphthalmia was observed at 46 mg Ni/kg bw per day in the absence of maternal toxicity (Saini et al., 2013). Nickel is a developmental toxicant inducing fetotoxicity, embryotoxicity and teratogenicity.
In 2015, the CONTAM Panel concluded that the most suitable and reliable dose–response information for developmental and reproductive effects are those reported in the studies by SLI (2000a,b). The most relevant information is copied below and further details are provided in the previous Opinion (EFSA CONTAM Panel, 2015).
In a one‐generation dose‐range‐finding study, significant increases in post‐implantation loss14 were observed in the offspring of Sprague–Dawley rats administered ≥ 6.6 mg Ni/kg bw per day as nickel sulfate hexahydrate via gavage for 14 days prior to mating, during mating, and gestation (SLI, 2000a). The number of dead pups at delivery was significantly increased in all exposure groups except the 11 mg Ni/kg bw per day group, and at 17 mg Ni/kg bw per day, a decreased mean litter size was observed. No effect on the growth of surviving F1 pups during lactation and no effect on the survival or growth of F1 pups from postnatal day (PND) 22 for several weeks following weaning was observed (see Table 3). In 2015, the CONTAM Panel identified a NOAEL for parental toxicity of 17 mg Ni/kg bw per day (the highest dose tested) and a LOAEL of 2.2 mg Ni/kg bw per day for offspring toxicity, based on the number of dead pups at PND 0.
Table 3.
One‐generation dose range‐finding study in rats (SLI, 2000a)
| Dose (mg Ni/kg bw per day) | 0a | 2.2 | 4.4 | 6.6 | 11 | 17 |
|---|---|---|---|---|---|---|
| Mean post‐implantation loss | 0.4 | 2.6 | 1.5 | 2.3* | 2.7** | 4.8** |
| Number of litters with post‐implantation loss | 2/8 | 5/8 | 6/8 | 6/7 | 7/7 | 8/8 |
| Number of litters with at least three post‐implantations losses | 0/8 | 1/8 | 1/8 | 2/7 | 3/7 | 7/8 |
| Number of dead/live pups, at delivery | 1/128 | 12/100** | 10/106** | 10/92** | 4/89 | 23/80** |
bw: body weight; Ni: nickel.
Historical control: mean: 1.5 (0.88–2.31).
p < 0.05.
p < 0.01.
In addition, no clinical signs of toxicity or macroscopic changes in the examined organs and tissues were observed among the offspring surviving the peri‐natal period in this study (SLI, 2000a). The highest dose in this study of 17 mg Ni/kg bw per day can therefore be considered as a NOAEL for surviving pups.
In a two‐generation reproduction toxicity study, nickel sulfate hexahydrate was administered by gavage to Sprague–Dawley rats at levels of 0, 0.2, 0.6, 1.1 and 2.2 mg Ni/kg bw per day (SLI, 2000b). According to the authors, no effect on F1 or F2 pup viability and growth was observed in the offspring of rats administered up to the highest dose tested, 2.2 mg Ni/kg bw per day. The authors reported therefore a NOAEL for developmental toxicity of 2.2 mg/kg bw per day. The mean post‐implantation loss14 among the F1 offspring was higher at 2.2 mg Ni/kg bw per day. However, the difference was not statistically significant (Table 4). In the F2 offspring, the mean post‐implantation loss was similar to that in the F2 control group. Historical control group mean values for F0 post‐implantation loss from eight studies ranged from 0.88 to 2.3 per litter. The value of 2.1 per litter for the group exposed to 2.2 mg Ni/kg bw per day is within this range. There was no statistically significant effect on post‐implantation/perinatal lethality in the F2 offspring.
Table 4.
Two‐generation study in rats (SLI, 2000b)
| Dose (mg Ni/kg bw per day) | 0a | 0.2 | 0.6 | 1.1 | 2.2 |
|---|---|---|---|---|---|
| F0/F1 generation | |||||
| Mean post‐implantation loss | 0.9 | 1.5 | 1.2 | 1.3 | 2.1 |
| Number of litters with post‐implantation loss (%) | 13/25 (52) | 18/26 (69) | 15/25 (60) | 19/26 (73) | 19/28 (68) |
| Number of litters with at least three post‐implantation losses (%)b | 3/25 (12) | 3/26 (12) | 5/25 (20) | 5/26 (19) | 9/28 (32) |
| F1/F2 generation | |||||
| Mean post‐implantation loss | 0.9 | 1.9 | 1.3 | 1.3 | 1.2 |
| Number of litters with post‐implantation loss (%) | 13/24 (54) | 18/26 (69) | 16/25 (64) | 18/23 (78) | 14/24 (58) |
| Number of litters with at least three post‐implantation losses (%)b | 0/24 (0) | 4/26 (15) | 3/25 (12) | 3/23 (13) | 4/24 (17) |
bw: body weight; Ni: nickel.
Historical control: mean: 1.5 (0.88–2.31).
The cut‐off of three post‐implantation losses was based on the maximum value in the historical controls of 2.31.
In addition, no effect on F1 or F2 pup viability and growth was observed in the offspring of rats administered up to the highest dose tested, 2.2 mg Ni/kg bw per day (EFSA CONTAM Panel, 2015).No treatment‐related clinical signs of toxicity or histopathological changes in the examined organs and tissues were observed among the offspring surviving the peri‐natal period in this study performed according to OECD TG 41615 (SLI, 2000b). The highest dose in this study of 2.2 mg Ni/kg bw per day can therefore be considered as a NOAEL for surviving pups.
In 2015, the CONTAM Panel decided to apply a BMD approach to derive a reference point on the dose–response curve.
Since the previous Opinion, three studies of relevance for this mandate have been identified.
Reproductive toxicity
Adult Wistar rats were administered nickel chloride by gavage daily for 28 days at 0, 5.25, 10.5 and 21 mg/kg bw (assuming that the doses are expressed as nickel chloride, the corresponding doses of nickel are 1.0, 4.8 and 9.5 mg/kg bw per day) (Lambade et al., 2015; see also Appendix B.1). According to the authors, testes of mid‐ and high‐dosed rats showed severe testicular degeneration, which was described by the authors as ‘emptying of the seminiferous tubules’. Most of the seminiferous tubules contained necrotic cell debris and proteinaceous material besides degenerative changes in spermatogonia cells. The CONTAM Panel noted that except for a figure of a slide, the histopathological changes in the testes are only descriptive and no information on incidence and severity in the various groups is presented.
Developmental toxicity
Effects of nickel on the postnatal development of Swiss albino mice exposed during the three gestation periods were examined (Saini et al., 2014a). Nickel chloride hexahydrate was administered to pregnant females by gavage (46, 92, or 185 mg Ni/kg bw) during GD 0–5 (pre‐implantation period), GD 6–13 (organogenetic period) or GD 14–18 days (fetal period). The pregnant females were allowed to reach term, deliver normally, and raise their pups. The litter size and sex ratio of neonates were recorded. The offspring were examined for morphological anomalies, also the eye opening, pinna detachment, hair appearance, vaginal opening and testes descent. The weights of the offspring were recorded weekly up to the age of 6 weeks to determine their growth. A significant decrease in litter size was observed after exposure to 185 mg Ni/kg bw during all three gestation periods, as well as after exposure to 92 mg/kg bw during the pre‐implantation period in comparison with the control group. Mortality was observed at the highest dose administered during all three gestation periods, and also at the mid‐dose administered during the organogenetic and fetal periods. The mortality was higher in the fetal period as compared to the organogenetic period. Administration during the organogenetic period caused morphological anomalies in eye, limb, and tail at the highest dose and in eye at the mid‐dose. The gestation index16 was decreased following administration during the pre‐implantation period, but to the same extent at all three doses. The live birth index was decreased following administration of the highest dose during the pre‐implantation and organogenetic periods. The viability and weaning indices were decreased following administration of the highest dose during all three gestation periods, and of the mid‐dose during the organogenetic and fetal periods. A significant decrease in offspring body weight was observed at all doses administered during the organogenetic period, at the two highest doses during the fetal period and at the highest dose during the pre‐implantation period. The CONTAM Panel concluded that nickel affects both pre‐ and postnatal development in a dose‐dependent manner after pre‐natal exposure.
The effects of nickel on developmental parameters in Swiss albino mice during the pre‐implantation period were investigated (Saini et al., 2014b). Nickel chloride hexahydrate was administered to pregnant females by gavage (46, 92, or 185 mg Ni/kg bw) during GD 0–5. Dams were sacrificed by cervical dislocation on GD 18 and the uteri were examined. A significant decrease in maternal and fetal body weight was found for the mid‐ and highest dose groups. A dose‐dependent significant reduction in the number of implant sites and live fetuses per dam, increase in the number of resorptions, post‐implantation deaths and decrease in placental weight was reported. For the lowest dose, the skeleton of the fetuses exhibited reduced ossification of nasal, parietal, intraparietal (5.8%), metatarsals, and phalanges (11.7%) while some revealed absence of ossification. The degree of malformation was more pronounced at the highest dose (185 mg Ni/kg bw) with increased reduction of skull ossification (22.7%), reduced number of ribs (4.5%), sternebrae (13.6%), caudal vertebrae (4.5%), and absence/reduced ossification of forepaws and hind paws (27.2%).
Summary
Effects on male sex organ weights, histopathological changes in these organs, disturbed spermatogenesis, decreased sperm motility and sperm damage have been reported previously in studies in mice after oral exposure to nickel compounds. These effects were responsible for a decrease in fertility. A recent short‐term toxicity study (28 days) with limited reporting suggested that nickel also may cause damage to the testes (testicular degeneration) of rats.
There is consistent evidence in previous studies of developmental toxicity in rats in form of increased pup mortality (stillbirth or post‐implantation loss/perinatal lethality) and decreased pup weight after oral exposure to nickel compounds. Developmental toxicity was also observed in previous studies in mice (decreased fetal weight, malformations) but at higher doses than for rats. Two recent studies confirmed that nickel caused developmental toxicity in mice when administered during different gestational periods at doses higher than those resulting in developmental toxicity in rats.
3.1.2.6. Immunotoxicity
The Panel did not identify animal studies that would be suitable for risk assessment of allergic reactions after oral exposure to nickel. Animal studies that were identified were focused on mechanisms of immunotoxicity caused by nickel and are described in Section 3.1.4.4.
3.1.2.7. Neurotoxicity
Since the previous Opinion, three neurotoxicity studies of relevance for this mandate have been published.
Neurobehavioural changes induced by nickel were investigated in inbred male Kunming mice (6 or 8 animals per group, 25 ± 3 g, age not mentioned) administered nickel chloride hexahydrate orally by gavage in sterile water at doses of 0, 5 and 50 mg Ni/kg bw (He et al., 2013a). Spatial memory performance was evaluated by using the Morris water maze (escape latency) and locomotor activity was evaluated by the open field test (total distance travelled) at 1, 3, 12, 24 and 48 h after dosing. Nickel content was measured in the brain (left hemisphere) and the cerebral cortex (dissected from the right hemisphere). Spatial memory performance was affected in the high‐dose group as the escape latencies were statistically significantly increased at 1, 3 and 12 h, but were similar to the levels in the control group at 24 and 48 h. The escape latencies were slightly increased in the low‐dose group at 3 h (not statistically significant). The locomotor activity was also affected as the total distance travelled was lower in the high‐dose group at all time points, but only statistically significant at 1, 3 and 12 h. The total distance travelled was also lower in the low‐dose group (not statistically significant). The nickel content in the brain and the cerebral cortex of high‐dose animals was significantly increased at 3 and 24 h after dosing and was more than 10 times higher than the levels observed in the control group after 3 h. After 24 h, almost two‐thirds of the nickel was eliminated. The nickel content in the brain and cerebral cortex of low‐dose animals was slightly higher than in the control group at both time points (not statistically significant).
Nickel‐induced neurodegeneration was investigated in adult male Wistar rats (180 ± 20 g, number of animals per group and age not mentioned). They were administered nickel chloride hexahydrate by gavage in saline at doses of 0, 10 or 20 mg NiCl2/kg bw (corresponding to 0, 4.5 or 9.1 mg Ni/kg bw) for 4 weeks (Ijomone et al., 2018a). Ultrastructural changes were observed in neurons of the hippocampus, striatum and cortex. Mitochondria structural integrity in the neurons was also affected. In the hippocampus, changes at the high dose were graded as severe in nuclei, cell membrane, mitochondria and Golgi apparatus and as moderate in endoplasmic reticulum; no changes were observed at the low dose. In the striatum, changes in nuclei were graded as moderate at both dose levels and in mitochondria as severe at the high dose; no changes were observed in other structures. In the cortex, changes in nuclei were graded as mild at the high dose and in mitochondria as mild at both dose levels; no changes were observed in other structures.
The effect of administration (GD 1 until weaning) of 0.2% nickel sulfate to Wistar rats (10 males and 20 females/group) via drinking water on their neurobehavioural functions during gestation and lactation was investigated (Kahloula et al., 2014). The concentration in drinking water corresponds to a dose of 91 mg Ni/kg bw per day using a default factor of 0.12 for a subacute study in rats (EFSA Scientific Committee, 2012a) and the molecular weight of nickel sulfate. Impaired spatial learning performance was observed in the Morris water maze test. Locomotor hyperactivity was also observed in the open field test. An increase in the immobility time in the forced swimming test was observed, which suggests a depressive behaviour. These effects reflect an alteration in the neurodevelopmental process.
In summary, the studies indicate that nickel can disturb the neurobehavioural functions in rats and mice as indicated by impaired spatial memory performance and effects on locomotor activity. Neurodegeneration was also observed in adult rats.
3.1.3. Observations in humans
3.1.3.1. Human biomonitoring
In 2015, the CONTAM Panel concluded the following regarding biomonitoring:
‘In subjects exposed to the same species of Ni from the same absorption route, serum Ni (S‐Ni) and especially U‐Ni are useful biomarkers of exposure and can be used for bio‐monitoring purposes, as occurs in the case of occupational setting. However, too many variables give rise to individual concentrations in biological media, which makes translation into exposure data impossible. Such variables include the bio‐accessibility and bioavailability of ingested Ni, the route of entry and clearance (from the airways, the GI tract, and the skin). Once absorbed, Ni excretion rate (kinetics) depends on protein binding and renal function, which can modify both serum and urinary concentration in subjects with similar exposure. Finally, the sampling time selected to obtain blood or urinary spot samples is another variable crucial for data interpretation. As a result, it is not possible to back‐calculate the contribution of intake from food or drinking water to the concentration of Ni in accessible biological media’.
Deposition in hair has been reported after absorption of nickel (WHO, 2000). The CONTAM Panel noted that some studies have been published on nickel in hair or nails as a biomarker of exposure. Therefore, the Panel assessed the suitability of these matrices to be used as biomarkers.
Based on a review of scientific literature, ATSDR (2005) reported that determination of nickel in the urine, faeces, serum, hair and nasal mucosa has been used to demonstrate human exposure to nickel compounds. Alimonti and Mattei (2008) concluded that nickel measurements in the urine, serum or hair may serve as indices of exposure. Based on an extensive review of biological monitoring data, Sunderman (1993) concluded that serum and urine nickel levels were the most useful biomarkers of nickel exposure, especially if the route, sources, and duration of exposure are known, if the chemical identities and physical‐chemical properties of the nickel compounds are known, and if physiological information (e.g. renal function) of the exposed population is known.
In order to evaluate hair nickel concentration as a biomarker of exposure, an epidemiological cross‐sectional study was conducted in a Greek population that was exposed to nickel via food consumption. The study provides evidence of the suitability of hair analysis in assessing environmental exposure to nickel. The authors concluded that hair nickel content is a valuable and relatively inexpensive tool for biomonitoring and to identify people at risk for certain biochemical alterations (Sazakli and Leotsinidis, 2017).
In a study by Peters et al. (1991), concentrations of nickel in fingernails were suggested as a measure of occupational exposure to nickel. Nickel concentrations in fingernails from moderately and heavily exposed workers were significantly higher than in non‐exposed subjects (mean ± standard deviation (SD) and median ± standard error: 29.2 ± 55.7 μg/g and 13.8 ± 5.58 μg/g in moderately exposed (n = 83); 123 ± 289 μg/g and 29.9 ± 18 μg/g in heavily exposed (n = 51); vs 1.19 ± 1.61 μg/g and 0.488 ± 0.13 μg/g in non‐exposed (n = 95)). However, no correlation between the nickel concentration in fingernails and the duration of exposure was demonstrated.
In a more recent study in workers, concentrations of metals, including nickel, in toenails were suggested as a biomarker of occupational welding fume exposure (Grashow et al., 2014). The geometric mean nickel concentration in toenails was 2.53 ± 3.50 (mean ± SD) μg/g and the median was 2.19 μg/g (n = 48). The association between nickel concentrations in toenails and weld hours at 7–9 months prior to clipping of the analysed toenails approached significance (p = 0.06). A non‐exposed group was not included in this study.
In a study of Arab‐American residents living in a highly industrialised area (Detroit, Michigan), profiles of trace elements, including nickel, in toenails were evaluated as a tool in biomonitoring exposure history (Slotnick et al., 2005). The concentration of nickel toenails was 37 ± 109 μg/g (mean ± SD; n = 263). A non‐exposed group was not included in this study, but average nickel levels of 2.34 μg/g (n = 48), 1.19 μg/g (n = 95) and 2.70 μg/g (n = 34) for non‐occupationally exposed populations were cited in the paper.
In a more recent study of American residents, biological exposure was evaluated by concentrations of nickel in toenails from adults living in Appalachian Kentucky and compared with those of adults living in Jefferson, a non‐Appalachian, urban county (Johnson et al., 2011). The concentration of nickel in toenails tended to be higher among the Appalachian subjects (median: 0.28 μg/g in Appalachian Kentucky residents (n = 88) vs 0.18 μg/g in Jefferson residents (n = 151)).
Based on these four studies, the CONTAM Panel considers that nickel in toenails is not a suitable biomarker for evaluation of nickel exposure in humans. This is mainly due to the high variability in nickel nail concentrations among individuals as shown by the high standard deviations.
To summarise, serum and urine nickel levels are the most useful biomarkers of nickel exposure but some studies also demonstrated that hair could be used as a non‐invasive biomarkers of nickel exposure. However, the Panel considers that currently nickel in fingernails and toenails has not been sufficiently validated to serve as a biomarker of nickel exposure.
Levels of biomarkers of exposure in the European population
The text below describes studies that reported levels of nickel in human samples from the European population, without aiming to be complete. No recent studies reporting nickel concentrations in human milk samples from the European population were identified.
Pérez et al. (2018) studied the relationship between levels of 20 elements, including nickel, in children's urine and their diet. Subjects (6–11 years old) were from two cities in Spain (Alzira; n = 62 and Valencia; n = 58). No strong correlation was identified for nickel. The geometric means (95% CI) for the two cities were 3.9 (3.4–4.48) and 4.7 (4.03–5.48) μg/g creatinine, respectively. Roca et al. (2016) also measured nickel concentrations in urine samples from 120 children of the same age and from the same cities. The geometric mean (95% CI) was 4.3 (3.5–5.03) μg/g creatinine.
Protano et al. (2016) analysed the concentration of elements in urine from children from central Italy. The median (interquartile range (IQR)) nickel concentration was 6.71 (4.78–8.17) μg/L for children not exposed to environmental tobacco smoke and 6.81 (5.39–9.05) μg/L for exposed children.
Adults (n = 1,177) from industrially contaminated areas north of Rome were recruited in 1996 for a human biomonitoring study. The average nickel concentration in urine (corrected for creatinine) was 0.81 μg/L (95% CI: 0.77–0.85 μg/L) (Ancona et al., 2016).
In central Italy, 24‐h urine samples were collected from adults (18–60 years old) living in three areas: an area close to a recycling plant (n = 153), an urban area (n = 95) and an area in the countryside (n = 55). The median nickel levels (range) were 6.87 (0.78–22.27), 7.05 (1.05–42.68) and 4.96 μg/24 h (1.18–17.05), respectively (Chellini et al., 2017).
Non‐fasting spot urine samples from 460 males and 541 females (18–80 years old; living in Belgium) were analysed for the presence of 26 elements. The median nickel concentration was 1.79 μg/g creatinine (P2.5–P97.5: < LOD 4.88) (Hoet et al., 2013).
A total of 2,000 adults (982 men and 1,018 women) living in northern France participated in a biomonitoring study. Blood (n = 1,992) and urine (n = 1,910) samples were analysed for the presence of nickel and other metals and metalloids. In blood, nickel was detected in 99.95% of the samples and the geometric mean was 1.31 μg/L (95% CI: 1.28–1.34). In urine, nickel was detected in 98.38% of the samples and the geometric mean was 2.00 μg/L (95% CI: 1.93–2.08) (Nisse et al., 2017).
In Kosovo, the serum nickel concentration was determined in males between 31 and 64 years of age working in a thermal power plant (n = 70) and 27 male control subjects (30–65 years of age). The concentrations were 2.76 ± 0.4 and 2.18 ± 0.2 μg/L, respectively (Zeneli et al., 2015).
Sureda et al. (2017) selected the 25 most inactive and 25 most active individuals for each sex from a group of 280 older adults in Spain (55–80 years old) and analysed trace elements in toenail samples. No significant difference in nickel concentration was observed between active and inactive adults. The median nickel concentration in toenails was 989 μg/kg (IQR: 568–2,033 μg/kg) in men and 887 μg/kg (IQR: 360–1,660 μg/kg) in women.
3.1.3.2. Carcinogenicity
Based on i) the lack of epidemiological data suggesting that nickel compounds cause cancer after oral administration, ii) the lack of tumours in the carcinogenicity studies in experimental animals after oral administration of soluble nickel compounds and iii) the mode of action, the CONTAM Panel considered it unlikely that dietary exposure to nickel results in cancer in humans (EFSA CONTAM Panel, 2015).
No new data linking cancer in humans with oral exposure to nickel have been identified since the previous Opinion.
3.1.3.3. Reproductive and developmental toxicity
In its previous Scientific Opinion (EFSA CONTAM Panel, 2015), the CONTAM Panel concluded that the data from the available epidemiological studies did not support an association between oral exposure to nickel and reproductive and developmental effects in humans. Since that 2015 Opinion, a number of new studies have been published.
Reproductive toxicity
Zheng et al. (2014) investigated the status of heavy metals and trace elements in a Chinese population by collecting umbilical cord blood. No difference with statistical significance in the median nickel concentration was observed between the adverse pregnancy outcome group (e.g. fetal distress, premature births (infants born < 37 completed weeks of gestation) and macrosomia (birth weight ≥ 4,000 g)) and the reference group. The nickel concentrations (mean ± SD) were 46.32 ± 69.75 μg/L for the control group (n = 68) and 38.82 ± 92.36 μg/L for adverse cases (n = 58).
Serum concentrations of 11 trace elements in patients with polycystic ovary syndrome (PCOS) were investigated. A total of 369 women (including 96 patients with PCOS) were studied. Serum nickel levels were significantly higher in patients with PCOS compared with the control group. According to the authors, the results suggested that nickel, copper and zinc may play a role in the pathogenesis of PCOS related to reproductive hormone levels (Zheng et al., 2015).
Another study investigated prenatal exposure to nickel as a risk factor for pre‐term delivery (< 37 weeks) (Chen et al., 2018). Pregnant women (n = 7,291) were recruited in the longitudinal Healthy Baby Cohort in Wuhan, China. The mean age of the recruited pregnant women was 28.5 ± 3.7 years and 96.4% had detectable urinary concentrations of nickel. The mean urinary nickel concentration was 11.23 μg/g creatinine (median 5.05 μg/g creatinine; IQR of 2.65‐9.51 μg/g creatinine). Statistically significant higher urinary nickel concentrations were found for the pre‐term delivery mothers (median 7.12 μg/g creatinine; IQR 3.53–13.06 μg/g creatinine; n = 293) compared to the full‐term mothers (4.98 μg/g creatinine; IQR 2.63–9.31 μg/g creatinine; n = 6,998). A statistically significant decrease in gestational age was observed as maternal urinary nickel levels increased. Using adjusted models, each doubling of the nickel concentration was associated with an increase in the adjusted odds ratios (aORs) for pre‐term delivery with 16%. Similar results were obtained for both spontaneous and iatrogenic pre‐term delivery. The authors concluded that higher maternal urinary nickel concentrations are associated with an increased risk of pre‐term delivery.
The study of Bian et al. (2019) investigated the relationship between seminal quality and ion levels in seminal plasma. A total of 205 semen samples were collected from the Yangtze River Delta Region in eastern China and the samples were divided into two groups: normal sperm motility group (total motility > 40% (55.6 ± 8.67), n = 103) and abnormal sperm motility group (total motility < 40% (24.2 ± 9.51), n = 102) according to the WHO 2010 standard (WHO, 2010). The low sperm motility group showed distinctively reduced nickel concentration (5.69 ± 1.93 μg/L) in seminal plasma compared with the normal sperm motility group (10.22 ± 3.83 μg/L). According to these findings, the authors suggested that nickel increases sperm motility. This observation is in contradiction with the outcome of the study by Zafar et al. (2015) (see below).
In the same study, the effects of nickel on sperm total motility and progressive motility were studied in abnormal semen samples (total motility < 40%). The cells were incubated in vitro with nickel sulfate (control – 0; or 0.5, 1, 4, or 10 μM nickel sulfate; corresponding to 29, 59, 235 and 587 μg Ni/L) for 0.5–2 h (Bian et al., 2019). Both total sperm motility and progressive motility increased significantly in low activity samples treated with 0.5 or 1 μM nickel sulfate incubated for 0.5 or 1 h. On the other hand, a high nickel concentration (4 and 10 μM) dramatically decreased total sperm motility and progressive motility. According to the authors, the data suggest that a low nickel concentration increases total sperm motility and progressive motility in vitro. Another study provides data on the concentration of nickel in human seminal plasma (75 seminal plasma samples, categorised into three groups – normozoospermia, oligozoospermia and azoospermia; n = 25/group) in a Pakistani population (Zafar et al., 2015). The nickel concentrations in the seminal plasma (mean ± SD: 3.07 ± 1.63 (median: 2.57); 1.92 ± 0.77 (2.09); 10.49 ± 10.94 (6.94) μg/kg; respectively) were negatively correlated with sperm concentration and motility. The authors state that nickel concentration showed a significant difference in all three groups, indicating a key role in male infertility.
The relationship between human semen quality and the concentration of trace elements, including nickel was examined (Ali et al., 2017). The detection frequencies in normal (64 samples) and in abnormal (30 samples) semen specimens were similar. The concentration of nickel in seminal plasma and in the sperm DNA was slightly higher in abnormal semen (5.28 ± 2.4 μg/L and 0.34 ± 0.2 ng/μg) compared to normal semen (1.9 ± 6.8 μg/L and 0.04 ± 0.1 ng/μg). The CONTAM Panel noted that detection of nickel in sperm DNA is not necessarily an indication of an interaction with the DNA.
Halder et al. (2014) studied four cases of dark‐coloured semen with non‐obstructive azoospermia and without genital tract bleeding or spinal cord injury, which is rarely observed. Normal volume, pH, leucocyte count and azoospermia or oligoazoospermia count was observed in the semen samples. The samples did not contain red blood cells and haem pigment, but increased levels of lead, manganese and nickel were observed in serum samples of the cases. The concentrations of nickel in the serum of the four cases were 10.6, 14.2, 27.8 and 32.2 μg/kg, and the mean ± SD for the control group (n = 15) was 2.7 ± 2.3 μg/kg. The authors suggest that dark‐coloured semen can also be linked with metals.
A study focused on the associations between urinary metal concentrations and circulating testosterone in Chinese men (n = 118). After adjustment for age, body mass index (BMI), alcohol use, smoking status and income, men in the third quartile of nickel concentration had a significant decrease of 83.79 ng/dL in testosterone in serum compared with those in the first quartile, but there was a lack of dose–response trends (Zeng et al., 2013).
Developmental toxicity
A study evaluated the concentrations of selected essential and toxic elements in amniotic fluid and their relation to maternal and fetal parameters (Suliburska et al., 2016). The study was carried out in 39 pregnant women, aged 34.6 ± 4.7 years, between weeks 16 and 26 of gestation. Subjects in this study were divided into two groups according to age: those under 35 years old (n = 17) and those 35 years or older (n = 22). It was found that the concentration of nickel was markedly higher in the amniotic fluid of older women (median 3.98 vs 2.32 μg/L). Significant positive correlations between diastolic blood pressure and the level of nickel were observed. The authors also reported that high blood pressure in mothers correlated with higher concentrations of nickel in amniotic fluid.
Sun et al. (2018) described the possible link between prenatal nickel exposure and pre‐term low birth weight (PLBW). Nickel was analysed in urine samples from 408 pregnant women (102 PLBW; 306 controls) in China. A significantly higher median urine concentration was observed for PLBW cases (4.34 μg/g creatinine) compared to the controls (2.80 μg/g creatinine). Conditional logistic regression showed a significant association between higher maternal urinary Ni levels and risk of PLBW. For the highest tertile of the nickel urinary concentration, the aOR was 2.80 (95% CI: 1.44–5.44). The observed association was more apparent among female than male infants.
To explore the association of nickel exposure and occurrence of congenital heart defects (CHD), a case–control study with 490 controls and 399 cases was conducted in China (Zhang et al., 2019). The cases included septal defects, conotruncal defects, right and left ventricular outflow tract obstruction, anomalous pulmonary venous return and other heart defects. The concentrations of nickel in the hair of pregnant woman and fetal placental tissue were measured. Logistic regression analysis was used to explore the relationship between nickel exposure and risk of CHD in the offspring. In the CHD group, the median concentration of nickel in maternal hair was 0.629 (5th–95th percentile: 0.276–2.250; arithmetic mean = 0.857) and 0.178 ng/mg (0.012–0.851 ng/mg; arithmetic mean = 0.308) in fetal placental tissue. In the control group, the median concentration of nickel in maternal hair was 0.443 (0.182–1.710; arithmetic mean = 0.648) and 0.148 ng/mg (0.008–0.954; arithmetic mean = 0.242) in fetal placental tissue. The overall risk of CHD increased with nickel hair concentrations (aOR: 1.326; 95% CI: 1.003–1.757; p < 0.001). For fetal placental tissue, no significant trend was found (aOR: 2.204; 95% CI: 0.783–6.206), except when focusing on the group of other heart defects (aOR: 11.280; 95% CI: 1.621–78.512; p < 0.01).
A study investigated the associations between concentrations of As, Cd, Pb and Ni in umbilical cord tissues and risk of orofacial clefts (OFCs), and the interactions between each pair of metals on OFC risk in a case–control study (Ni et al., 2018). Concentrations above the median of all subjects was associated with an elevated OFC risk of 6.79‐fold for nickel. The median level of nickel in OFC cases (38.92 ng/g) was significantly higher than in controls (21.22 ng/g) and nickel concentration in the subtypes of OFC cases (cleft lip with cleft palate (CLP) or cleft lip only (CLO)) was significantly higher than those in the controls (p < 0.001). The authors also detected a significant association between the concentration of nickel and the risks of CLP and CLO. Finally, they concluded that in utero exposure to nickel may increase the risks for total OFCs, CLP, and CLO.
Summary
From the small number of studies published since the previous opinion, a few suggest that there may be an association between nickel exposure and adverse reproductive and developmental outcomes. One study reported an association between nickel and an increased risk of pre‐term delivery. Another study indicated that nickel concentrations in the seminal plasma were negatively correlated with sperm concentration and motility. One developmental toxicity study suggested that occurrence of CHD may be associated with nickel exposure. Another study reported that an increased risk of OFCs may be related to in utero exposure to nickel.
3.1.3.4. Immunotoxicity including sensitisation
As stated in the EFSA Opinion (EFSA CONTAM Panel, 2015), nickel has different types of effects on the immune system. It is a sensitiser, hence exposure may lead to adverse hypersensitivity reactions. Nickel allergic contact dermatitis has a prevalence of around 15% in the EU, Asia and the USA.
As indicated earlier (EFSA CONTAM Panel, 2015), oral exposure studies to investigate sensitisation to nickel by the oral route, or studies in which sensitised animals are orally exposed are scant. Animal studies have reported that oral exposure may lead to the induction of oral tolerance towards nickel. Also in humans, experimental studies have shown that repeated oral exposure to nickel may prevent or diminish sensitisation. On the other hand, the EFSA Opinion of 2015 also reviewed information that indicated that consumption of a nickel‐rich diet may elicit eczematous flare‐up reactions in the skin of sensitive individuals, a phenomenon called SCD or haematogenous contact eczema. The CONTAM Panel concluded that SCD elicited in nickel‐sensitised humans after oral exposure to nickel was the critical effect suitable for the assessment of acute effects of nickel.
In a study by Nielsen et al. (1990), nickel‐sensitised individuals and matched non‐sensitive controls (both groups having vesicular hand eczema of the pompholyx type) were exposed through drinking water to 12 μg Ni/kg bw. Nine of 20 nickel‐allergic eczema patients experienced aggravation of hand eczema after oral nickel administration, and three also developed a maculopapular exanthema, while no exacerbation was seen in the control group. A LOAEL of 12 μg/kg bw was identified after provocation. The guideline value for nickel in drinking water established by WHO (2005) is based on this study.
In 2015, the CONTAM Panel identified the data from Jensen et al. (2003), who investigated 60 fasted volunteers (40 nickel‐sensitised and 20 non‐sensitised), with incidences of clinically cutaneous reactions including flare‐up reactions of 1/10, 4/10, 4/10 and 7/10 at the oral doses 0, 0.3, 1, and 4 mg Ni per person, respectively, as the most sensitive ones. Nickel was given as nickel sulfate hexadydrate in a lactose capsule. At that time, the Panel derived a BMDL10 of 0.08 mg Ni per person, corresponding to 1.1 μg Ni/kg bw, as a reference point for SCD elicited in Ni‐sensitive humans after acute oral exposure to nickel (EFSA CONTAM Panel, 2015). The CONTAM Panel noted that this value of 1.1 μg Ni/kg bw was in the same range as the lower confidence bounds of the effective dose in 10% of the population (ED10) calculated in the meta‐analysis by Jensen et al. (2006).
Another study on which EFSA has performed a BMD analysis was published by Gawkrodger et al. (1986). These authors investigated 26 persons (24 females and two males; aged 19–67 years) in a double‐blind cross‐over study. The subjects were positive in patch testing to nickel, after oral uptake of 0.4, 2.5 or and 5.6 mg Ni per person (in the form of nickel sulfate heptahydrate in lactose in capsules). Worsening of previous clinical skin sites (flare‐ups) and new skin lesions was recorded. Reactions were mostly seen at the highest dose tested and the incidences were 5/10, 5/10, 6/6, respectively. Based on this study, the CONTAM Panel (2015) calculated a BMDL10 value of 0.18 mg Ni per person, corresponding to 2.6 μg Ni/kg bw. In this study, subjects were challenged with a placebo either the week before or the week after treatment with nickel. Reactions after placebo treatment were also seen (10/26). A possible explanation for some of these reactions may be the relative short period of the placebo treatments after the initial nickel treatment, and that these reactions may have been caused by the earlier nickel treatment which was not considered by the authors.
Hindsén et al. (2001) challenged 30 females (21–44 years old, 12 with atopy and pompholyx and 18 without atopy and hand eczema) after a night's fasting, to capsules containing 4.48 mg or 13.44 mg nickel sulfate hexahydrate in lactulose; corresponding to a dose of 1 or 3 mg Ni per person. In contrast to the studies by Jensen et al. (2003) and Gawkrodger et al. (1986), Hindsén et al. only recorded flare‐up reactions, i.e. the worsening of already eczematous lesions. The incidence was 0/10, 2/10, 9/9 for the dose groups 0, 1 and 3 mg Ni per person, respectively. The CONTAM Panel (2015) calculated, based on this study, a BMDL10 of 0.11 mg per person, corresponding to 1.6 μg Ni/kg bw.
In 2015, the CONTAM Panel noted that ‘Whereas contact allergy is the most frequent clinical pattern in nickel‐sensitized individuals, and resistance to infections may be influenced, many other clinical elements may demonstrate that the systemic absorption of nickel, e.g. by the oral route, is able to elicit gastrointestinal (e.g. abdominal pain, diarrhoea and/or constipation, nausea and/or vomiting), atypical systemic manifestations (e.g. headache, chronic fatigue) and chronic dermatological symptoms (e.g. urticaria‐angioedema), that are called systemic nickel allergy syndrome (SNAS)’. The EFSA 2015 Opinion concluded that the SNAS relationship with oral nickel exposure has not been firmly confirmed.
In addition to sensitisation and eliciting specific allergic reactions, the EFSA Opinion had also reviewed other, non‐specific effects of nickel on the immune system. Even if immunomodulatory effects of nickel have been noted, i.e. both a stimulation of antibody responses to antigens other than nickel, that may potentially enhance allergic responses, as well as depressed antibody responses, that may lead to suppressed resistance. Indeed, in humans, individuals suffering from nickel allergy show a higher incidence of Herpes labialis, genital candidiasis, and upper respiratory tract infections, which is supported by evidence showing reduced resistance to allogeneic tumour cells in rats (EFSA CONTAM Panel, 2015).
The CONTAM Panel considered, however, that allergenicity of nickel is more pronounced than its immunomodulatory influence.
Since the 2015 EFSA Opinion, a number of new studies have been published.
Ahlstrom et al. (2017) reviewed 46 studies on nickel allergy (10 in the general population and 36 in dermatitis patients), and concluded that since the implementation of Directive 94/27/EC17, the so‐called Nickel Directive to diminish exposure through the skin in order to minimise the prevalence of nickel allergy, the number of people suffering from nickel allergy has been reduced, but the prevalence remains high. A prevalence of nickel allergy was noted in 11.4% of the general population, with a prevalence of up to 20% in female dermatitis patients. Generally, a higher prevalence was noted in southern European countries than in the north. In obese patients the prevalence seems to be considerably higher (Lusi et al., 2015; Watanabe et al., 2018). In a study performed by Akan et al. (2015) of 134 children with atopic dermatitis (n = 45), 33.8% were positive to nickel skin patch testing.
In addition to skin flare‐up reactions after skin exposure to nickel in nickel‐sensitised individuals, as described in the EFSA Opinion in 2015 (EFSA CONTAM Panel, 2015), other skin symptoms may depend on nickel sensitisation. Cifci (2019) reported an association between nickel sensitivity and rosacea. Nickel sensitivity may be one of the underlying pathologies or a triggering factor of the rosacea.
Skin reactions do occur in sensitive individuals after consuming nickel‐containing foods such as pasta and cereals, as illustrated in a case study reported by Peredelskaya (2018). The patient was allergic to nickel and reacted severely to intravenous injection procedures, i.e. most likely to the nickel present in the injection needle, and these reactions were intensified by ingestion of nickel‐containing foods.
In an epidemiological study among Asian individuals, nickel contact allergy was found to be associated with occupational exposure to the metal, as well as with seafood and canned food consumption (Boonchai et al., 2014).
Büyükoztürk et al. (2015) studied patients with positive patch testing to nickel, by skin‐prick testing and measurement of interleukin (IL)‐10, IL‐4, IL‐5 and interferon (IFN)‐γ in supernatants of peripheral blood mononuclear cells stimulated by nickel during proliferation. Some patients were described as having reactions after placing dental devices in their mouth, others experienced symptoms after consuming foods with high levels of nickel, such as whole wheat, rye, cocoa, tea and green salads. The study suggests the presence of Type I hypersensitivity in addition to Type IV hypersensitivity. Lymphocyte proliferation, IL‐4 and IL‐10 were significantly elevated in patients having urticarial, angioedema and respiratory symptoms compared to patients who had only oral symptoms or systemic dermatitis. The results indicate that oral exposure to nickel triggers systemic symptoms in previously sensitive patients.
Ricciardi et al. (2014) performed an epidemiological study of the prevalence of SNAS in Italy. The authors report that nickel patch‐test‐positive patients showed flare‐up reactions after oral exposure to nickel. The authors state that foods particularly rich in nickel, such as peanuts, beans, lentils, peas, soybeans, oats, cocoa, chocolate, nuts, whole wheat, pears and mushrooms can trigger symptoms of SNAS, including flare‐up reactions and systemic dermatitis.
In addition to skin flare‐up reactions, exposure of mucosal surfaces to nickel is often the trigger of irritable bowel syndrome‐like gastrointestinal disorders: its ingestion may cause allergic contact mucositis, identifiable by means of oral mucosa patch testing (Borghini et al., 2016). All 22 nickel‐sensitised patients studied, challenged with a 5 mm paper disc saturated with a 5% solution of nickel sulfate in vaseline (0.4 mg nickel sulfate/8 mg vaseline corresponding to 0.15 mg nickel/8 mg vaseline) on the lower lip mucosa presented oral mucosa hyperaemia and/or oedema. Eight of the same 22 patients presented a local delayed vesicular reaction, unlike the remaining 14 patients. None of the 12 patients belonging to the control group showed any alteration.
In addition to specific reactions to nickel, exposure to nickel may also lead to non‐specific reactions of the immune system. For instance, Andrioli et al. (2015) suggested that individuals suffering from the SNAS as an immune‐mediated disease had an increased risk for thyroid autoimmunity. Yuk et al. (2015) reported that nickel allergy may be a risk factor for endometriosis.
Aslan et al. (2017) showed that the majority of foods that increase gastroesophageal reflux symptoms contain nickel. The purpose of this study was to evaluate the relationship between nickel sensitivity and gastroesophageal reflux disease. Forty‐eight subjects suffering from gastroesophageal reflux disease were also nickel‐sensitised, in contrast to 22 of the control group. Braga et al. (2013) showed that individuals on a diet with less than 50 μg nickel had significant improvement of their SNAS‐associated symptoms. Similar findings were made by Borghini et al. (2018) in nickel‐sensitised females suffering from endometriosis.
Overall, the studies published since the 2015 EFSA Opinion confirm the risk of flare‐up reactions after ingestion of nickel. In addition to flare‐up reactions in the skin, immune‐mediated systemic conditions may also be associated with oral exposure to nickel.
3.1.3.5. Neurotoxicity
In 2015, the CONTAM Panel did not identify studies on neurotoxicity in humans. Since then, three studies have been identified.
A study designed to investigate whether age‐related cognitive deficit is associated with oxidative damage, especially with inhibition of the enzyme δ‐aminolevulinate dehydratase (ALA‐D) activity and whether some metals, including nickel, influence the enzyme activity and cognitive performance (Baierle et al., 2014). Fifty elderly individuals (≥ 60 years old) and 20 young individuals (25–35 years old) from Porto Alegre, Brazil were examined. The study included three steps: 1) a questionnaire, 2) collection of blood samples, and 3) cognitive tests. The elderly group generally had a lower performance in the cognitive tests than the young group, as well as a lower activity of ALA‐D. No significant difference was observed in the serum level of nickel between the two age groups and the nickel level was within the reference interval. There was no relation between nickel and ALA‐D activity, but there was a negative association with ALA‐D reactivation.
Another study evaluated levels of some metals including nickel in biological samples along with measurement of cognitive ability and biomarkers of oxidative stress (ALA‐D and malondialdehyde (MDA)) in children (do Nascimento et al., 2014). Twenty children (9 girls and 11 boys, aged 8–14 years) from a rural area in southern Brazil and 20 children (10 girls and 10 boys, aged 8–14 years) from an urban zone in the same region were included. The nickel blood level in rural children was 4–5 times higher than that recommended by WHO, whereas the nickel hair level was very close to the reference level. The rural children had generally a relatively low performance of cognitive ability. The plasma MDA levels were statistically significantly higher in rural children than the levels in urban children. There was no significant difference in the blood ALA‐D activity between the two groups whereas the ALA‐D reactivation percentage was significantly higher in rural children compared to urban children. The CONTAM Panel noted that an association between nickel and cognitive decline, as well as with biomarkers of oxidative stress, cannot be evaluated from this study because of the co‐exposure to other neurotoxic metals such as lead and aluminium.
In a study that investigated the effects of low levels of some metals including nickel on three neurobehavioural domains (sustained attention, short‐term memory, and manual motor speed), 606 adolescents (13.6–17 years) were examined (Kicinski et al., 2015). The mean concentration of nickel in urine (n = 533) was approximately twice the reference value (0.88 μg/g creatinine) and the 95th percentile was approximately eight times higher than the reference value. There was no significant association between nickel in urine and the neurobehavioural parameters.
In summary, one study reported a negative association with ALA‐D reactivation and nickel exposure. Another study showed no significant association between nickel in urine and the neurobehavioural parameters.
3.1.3.6. Other
The role of metals in the development of different diseases has been the subject of several studies. These studies typically focus on several metals and trace elements, but the text below reports only the results on nickel.
No association was observed between nickel concentration in blood, plasma and urine and the height of children (Klatka et al., 2015). This association was also investigated in a case–control study with Georgian children, and no significant association between short stature and nickel in hair was observed (Tabatadze et al., 2015). The nickel concentration was measured in blood, plasma and urine from obese and non‐obese children and adolescents (aged 6–17 years); no correlation was found between the BMI and the concentration of nickel (Blażewicz et al., 2013).
Significantly lower nickel concentrations have been observed in the blood of rheumatoid arthritis patients than in controls (Irfan et al., 2017), and significantly higher nickel concentrations in the hair, blood and urine of hypertensive patients compared to controls (Afridi et al., 2014). Plasma levels of nickel were significantly higher in patients with Parkinson's disease (n = 225) than in healthy controls (n = 125) (Verma et al., 2016). Lower serum concentrations of nickel have been determined in patients with alcoholic liver cirrhosis (n = 62) compared to healthy individuals (n = 18) (Prystupa et al., 2016). The mean serum nickel concentration was 1.9 μg/L in the control group and the mean concentrations in patients with increasing severity of liver disease were 0.7, 0.5 and 0.3 μg/L. A significant difference was reported between the control and the dose groups with the highest severity. Another study reported a significantly higher mean nickel concentration in serum for Serbian hypothyroid patients (n = 23) compared to healthy volunteers (n = 70) (Stojsavljević et al., 2018). The mean concentrations (range) were 3.4 (0.1–21.16 μg/L) and 2.19 (1.01–7.78 μg/L), respectively. López‐Jornet et al. (2014) measured nickel in the saliva of patients with burning mouth syndrome (n = 28) and controls (n = 30) but no significant difference was observed (0.052 ± 0.071 mg/kg (mean ± SD) vs 0.009 ± 0.03 mg/kg).
In a cross‐sectional prospective study, a positive association has been reported for urinary nickel with the prevalence of Type 2 diabetes (Liu et al., 2015), as well as a positive association with albuminuria and β2‐microglobulinuria, indicators of glomerular or tubular kidney damage, in Chinese adults (Liu et al., 2016). In a cross‐sectional prospective study with Greek adults, using nickel in hair as a biomarker, men with a higher concentration of nickel in hair (upper quartile of the distribution) have a higher risk of abnormally high cholesterol, low‐density lipoproteins, albumin and calcium. In women, a higher concentration of nickel in hair is associated with abnormal glucose, triglycerides and low abnormal sodium (Sazakli and Leotsinidis, 2017).
No significant association has been found between high nickel blood levels and the risk of nasosinusal polyposis in a case–control study of Tunisian patients (Khlifi et al., 2015) and between serum nickel concentrations and brain damage markers and serum hormones in a case–control study with male Russian patients suffering from acute ischaemic stroke (Skalny et al., 2017). In Chinese pregnant women, a significant association was observed for nickel in blood with a decrease in free thyroxine using single‐metal models. However, in the multiple‐metals models, the trend was no longer significant (Guo et al., 2018).
There is a growing body of evidence that metals and trace elements, including nickel, have a role in the development of various diseases in humans. However, this evidence is sparse and the studies had methodological limitations, and therefore these studies cannot be used in risk assessment of nickel.
3.1.4. Mode of action
A recurring theme in the toxicity of nickel is the evidence for a role of oxidative stress and elevation of ROS. A contribution of oxidative stress is evident in relation to reproductive toxicity, genotoxicity, immunotoxicity and neurotoxicity (see below). Further evidence of oxidative stress comes from a study by Deng et al. (2016) who investigated the pulmonary toxicity induced by dietary exposure to nickel chloride in broiler chickens. Chickens were fed diets containing nickel chloride hexahydrate for 42 days. Dose‐ and time‐dependent lesions in the lung (swollen and exfoliated epithelial cells, thickened alveolar walls, infiltration of inflammatory cells and congestion) was associated with the generation of nitric oxide free radicals, oxidative damage to DNA (increased 8‐hydroxy deoxyguanosine) and lipid peroxidation (increased MDA in the lung). Nickel decreased the messenger RNA (mRNA) levels and activities of antioxidant enzymes in the lung. Glutathione (GSH) content in the lung decreased in the treated groups whereas oxidised glutathione content increased. Although not directly relevant to ingestion of nickel via food, further evidence of oxidative stress associated with an apoptotic mechanism of cell death comes from studies in nasal epithelia. Nickel acetate caused apoptosis in nasal epithelial RPMI‐2650 cells in association with increased caspase‐3/7 activity, increased annexin V binding, p53 and increased Bax/Bcl‐2 protein ratio. There was also a concentration‐dependent increase in ROS and mitochondrial depolarisation which was inhibited by the antioxidant N‐acetylcysteine (NAC) (Lee et al., 2016).
It has also been postulated that nickel might exert some of its effects via perturbation of iron homeostasis since divalent nickel competes with the transport of divalent iron into cells via DMT1 (see Section 3.1.1 on Toxicokinetics) and possibly could compete with iron sites on enzymes like the prolyl hydroxylases that modify hypoxia‐inducible factor‐1α (HIF‐1α) (Davidson et al., 2005). Nickel decreased the binding of the Von Hippel–Lindau protein to HIF‐1α, indicative of a decrease in prolyl hydroxylase activity. In addition, there was a concentration‐dependent inhibition of intestinal divalent iron absorption by divalent nickel, measured using gut sacs from freshwater rainbow trout (Oncorhynchus mykiss) in vitro. The relatively high sensitivity of the mucosal epithelium of the intestine to inhibition relative to the mucus or blood compartment, suggested to the authors that the interactions were likely to occur via DMT1 (Kwong and Niyogi, 2009).
3.1.4.1. Genotoxicity
The genotoxicity of nickel is likely due to indirect effects including inhibition of DNA repair and ROS production. In addition, chromatin changes may occur following dysregulation of signalling pathways and alteration of the epigenetic landscape.
In agreement with the conclusion drawn in 2015, the CONTAM Panel considers it unlikely that dietary exposure to nickel is carcinogenic to humans. Most of the new publications concern the mechanism of genotoxicity which may contribute to nickel‐induced carcinogenesis by inhalation.
Inhibition of DNA repair
The treatment of cells with soluble nickel (II) increases the DNA damage and mutagenicity of several agents evidently via inhibition of DNA repair (nucleotide excision repair, base excision repair and O6‐ methylguanine‐DNA methyltransferase).
Cells with reduced repair of DNA DSBs exhibit higher levels of baseline genomic instability and sensitivity to DNA damaging agents. Scanlon et al. (2017) have shown that nickel‐induced downregulation of HDR is consistent with the time course of nickel‐induced genetic lesions and the co‐carcinogenic effect of nickel with DNA damaging agents. They suggest that nickel can impact cellular DNA repair on multiple levels, ranging from direct enzyme inhibition to modulation of DNA repair factor expression. Nickel exposure (250 or 500 μM for 48 h) in human tumorigenic (lung carcinoma A549, cervical carcinoma HeLa, and breast carcinoma MCF7 cells) and non‐tumorigenic (BEAS‐2B cells, immortalised cell line derived from normal human bronchial epithelium) lung cells leads to transcriptional downregulation of the HDR proteins BRCA1, RAD51 and FANCD2 and the mismatch repair (MMR) protein MLH1, without downregulation of the non‐homologous end joining (NHEJ) factors. Treatment with nickel chloride (> 100 μM) in BEAS‐2B and A549 cells led to a concentration‐dependent increase in DNA DSBs persisting 24 h post‐irradiation in irradiated cells. At 250 μM and 500 μM nickel chloride in BEAS‐2B and A549 cells, respectively, small increases in the median comet tail moment were observed in non‐irradiated cells indicating that, at high concentrations, nickel‐induced repression of HDR may limit repair of spontaneous DNA DSBs within cells. They also showed that nickel represses MLH1 promoter activity; however, upon longer treatment, enhanced MLH1 promoter silencing did not persist. Their findings support a model in which nickel inhibits high‐fidelity DNA repair pathways through acute hypoxia‐mimetic transcriptional pathways, potentially contributing to nickel‐induced carcinogenesis by inhalation. The authors noted that the gene expression and functional changes in DNA repair in nickel‐treated cells have similarities to those induced by hypoxic stress. Hypoxia represses the high‐fidelity HDR and MMR pathways through multiple transcriptional and epigenetic mechanisms but not the error‐prone NHEJ pathway.
Oxidative stress
Treatment with soluble and insoluble Ni causes increases in ROS in many cell types and in animal models. ROS induction seems to be responsible for increased DNA SSBs, DNA–protein cross‐links and sister chromatid exchanges (EFSA CONTAM Panel, 2015). Since then few new publications have been identified.
Terpilowska and Siwicki (2019) demonstrate a concentration‐related increase in the intracellular ROS level and the concentration of MDA (a marker of lipid peroxidation) in both BALB/3T3 and HepG2 cells after exposure to 100–1400 μM nickel chloride hexahydrate. Superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GSH‐Px) activities significantly decreased after treatment, dependent upon concentration.
A correlation between the results of a comet assay and the results of oxidative stress assays was described by Lou et al. (2013). They showed that when human B lymphoblastoid cells were exposed for 24 h or 48 h to 640 μM nickel chloride the percentage of DNA in the tail of the comets was significantly higher for the exposed groups compared to the control group. After 48 h exposure, ROS levels in cells exposed to 640 μM nickel chloride were also significantly enhanced as compared to the controls. Furthermore, MDA levels in cells exposed to 160 to 640 μM nickel chloride for 24 or 48 h increased as compared with the controls (see Section 3.1.2.3 Genotoxicity).
The induction of ROS after treatment with nickel chloride may be responsible for inconsistent outcomes of genotoxicity tests in different systems (see Section 3.1.2.3 Genotoxicity). The susceptibility of test systems to genotoxicity caused by ROS is highly dependent upon the antioxidant capacity of the cells. Some systems are more prone to ROS, which may lead to positive genotoxicity results. However, some systems have relatively high antioxidant capacity that can scavenge excessively produced ROS, and therefore attenuate the damaging effects of ROS (Stannard et al., 2017).
Summary
The new studies confirm the previous conclusion (EFSA CONTAM Panel, 2015) that the genotoxicity of nickel is likely due to indirect effects including inhibition of DNA repair and ROS production.
3.1.4.2. Epigenetic effects
Chromatin
Nickel is able to silence genes near heterochromatin regions by initiating chromatin condensation (Sun et al., 2013).
Abnormal chromosomal forms were found in mammalian cells after treatment of cells with heavy metals including nickel. Polarisation and premature local heterochromatisation are the most characteristic signs of chromatin toxicity of nickel (II). It was shown that structural aberrations take place in chromatin organisation upon treatment with nickel. These changes may generate characteristic geometric distortions in the intermediates of chromatin condensation. Alterations in chromatin structures can lead to apoptosis. Injury‐specific chromatin changes that manifest at low concentrations suggest that pre‐apoptotic events are useful indicators of genotoxicity (Banfalvi, 2014).
Studies have shown that some metals are capable of binding to the chromatin and proteins and thereby inducing chromosomal aberrations, DNA‐protein cross‐links and DNA SSBs (Chen et al., 2017). In particular, this review highlights that nickel has the ability to induce cell transformation and epigenetic changes. However, the Panel noted that these effects are only relevant in the context of carcinogenesis of nickel by inhalation.
DNA methylation, histone acetylation and microRNA regulation
Studies performed in order to understand the mechanism of nickel carcinogenesis by inhalation point toward epigenetic alterations. Epigenetic alterations such as DNA methylation, histone modifications, and small non‐coding RNA are critical factors in inducing changes in the chromatin structure (Chen et al., 2017).
The epigenetic effects of nickel involve DNA hypermethylation and histone hypoacetylation resulting in the activation or silencing of certain genes, especially those involved in cellular response to hypoxia (Salnikow et al., 2000; Davidson et al., 2003, 2006; Kasprzak et al., 2003; Costa et al., 2005; Chen et al., 2010, 2017).
Water‐soluble and water‐insoluble nickel compounds are able to cause gene silencing. In the previous EFSA Opinion (EFSA CONTAM Panel, 2015), several experiments were described showing that nickel compounds influence DNA methylation, induce modification of histones and that microRNAs may play a role in nickel‐induced cell transformation and carcinogenesis. New data have become available since the previous EFSA Opinion, showing the key implication of epigenetic mechanisms in nickel carcinogenicity by inhalation (Ji et al., 2013; Zhang et al., 2013; Brocato and Costa, 2015).
Lee et al. (1995) have demonstrated that DNA methylation induced by nickel was found to inactivate the expression of a stably integrated reporter gene, gpt, near the telomeres of Chinese hamster cells.
Lee et al. (1998) have shown that nickel directly inhibits cytosine 5‐methyltransferase activity. This effect was only transient. Ni‐induced an initial hypomethylation of DNA but global DNA methylation was subsequently increased above basal levels and before any rebound of methyltransferase activity. The hypermethylation may be targeted towards tumour suppressor genes and/or senescence as part of its carcinogenesis mechanism by inhalation. Hypermethylation induced by nickel has been observed in vitro and in vivo (Govindarajan et al., 2002; Zhang et al., 2011; Sun et al., 2013). It has also been demonstrated that nickel selectively targets the inactive heterochromatin regions such as the long arm of chromosome X in CHO cells instead of targeting active euchromatic regions. It was also reported that nickel inhibits dioxygenases which result in an increase in DNA methylation marks (Brocato and Costa, 2015).
Nickel can also trigger silencing through histone modifications. It has been shown that in vitro and in vivo exposure to nickel reduce global histone acetylation levels (Broday et al., 2000; Golebiowski and Kasprzak, 2005; Ke et al., 2006). The reduction in histone acetylation may be due to inhibition of histone acetyltransferase (HAT) or through ROS generation (Broday et al., 2000; Koyama et al., 2000; Zoroddu et al., 2000; Bal and Kasprzak, 2002; Kang et al., 2003). It was also demonstrated that nickel is capable of inducing alpha‐helical conformation of the histone H4 tail rendering the transfer of an acetyl group by acetyltransferase inactive (Zoroddu et al., 2000).
Histone H3 lysine 9 dimethylation (H3K9me2) is involved in differentiation and maintaining cell identity and is associated with gene silencing. It is organised into large repressive domains that exist in proximity to active genes, which indicates the importance of maintenance of proper domain structure. Jose et al. (2014) treated immortalised non‐cancerous human bronchial epithelial BEAS‐2B cells with 500 μM nickel chloride for 72 h. They showed that nickel disrupted H3K9me2 domains, resulting in the spreading of H3K9me2 into active regions, which is associated with gene silencing. They demonstrate that nickel exposure can inhibit CTCF (insulator protein CCCTC‐binding factor) binding at the weak binding sites. It was suggested that inhibition of CTCF at the H3K9me2 domain boundaries is a potential reason for H3K9me2 domain disruption and downregulation of gene expression.
MicroRNA profiles significantly differ between tumour and normal tissues (Calin et al., 2002; Croce, 2009; Iorio and Croce, 2012). Chen et al. (2017) reported that miR‐21 expression levels dose dependently increased in nickel‐induced human lung cancers. Clinically, patients with high nickel exposure and high miR‐21 expression have a lower rate of survival. These data indicate that microRNA may play important roles in nickel‐induced lung cancer following inhalation.
In the context of cancer, these epigenetic changes would only be relevant to the inhalation route. Other potential consequences of epigenetic changes due to nickel exposure are currently unknown.
3.1.4.3. Reproductive and developmental toxicity
Nickel exposure dose dependently disturbs the regular ovarian cycle, inhibits ovulation, decreases the implantation frequency in early embryogenesis, increases the frequency of early and late resorptions and the frequency of stillborn and abnormal fetuses (EFSA CONTAM Panel, 2015).
Nickel also dose dependently degenerates testicular structures, reduces sperm motility and count, and increases the occurrence of abnormal spermatozoa in mice.
Reproductive toxicity
A study was designed to investigate the effect of nickel administration on the histology of the testes, sperm parameters, and the expression of CatSper 1 and CatSper 2 genes in adult male mice. CatSper 1 and CatSper 2 are proteins involved in the formation of calcium ion channels, essential for the correct functioning of sperm cells. The exposed group was injected i.p. with 5 mg/kg bw per day of nickel chloride (corresponding to 2.3 mg/kg bw per day Ni) for 2 weeks. Nickel caused a reduction in sperm parameters as well as a decrease in the thickness of the germinal epithelium. Histological examination of the testes showed congestion of blood vessels, disintegration of germ cells from their basement membrane, and distorted intratubular architecture. In addition, there were variable degrees of Leydig cell hyperplasia, maturation arrest in some tubules, and scattered apoptotic cells. The most common types of morphological abnormalities were sperm head deformity (49.75%). The expression of CatSper 2 in the exposed group was significantly lower compared to the control group, while no significant change was observed for CatSper 1 (Mohammadi et al., 2018).
Some studies in rats (Ambrose et al., 1976; Obone et al., 1999; and American Biogenics Corporation, 1988; see EFSA CONTAM Panel, 2015 for a summary of these studies) showed no alterations in reproductive tissues and no adverse effects on fertility or reproductive performances after oral administration of nickel compounds. However, in mice, decreased male sex organ weights, histopathological changes in these organs, disturbed spermatogenesis, decreased sperm motility and sperm damage have been reported in studies after oral exposure to nickel compounds. These effects were responsible for a decrease in fertility.
Taking into account the importance of free radical generation in the genotoxic effect of nickel (II) and knowing that GSH contributes to the reduction of damage to DNA, the primary aim of the study of Murawska‐Ciałowicz et al. (2012) was to study whether male infertility caused by nickel (II) may be a result of oxidative stress involving protamine 2 in sperm chromatin. Corzett et al. (2002) had previously observed the expression of protamine 1 in rat sperm with only very little protamine 2 (2‐5% of total protamine in Norwegian rats). This contrasted with different species of mice in which protamine 2 expression was 67–72% of total protamines in sperm. Thus Murawska‐Ciałowicz et al. (2012) hypothesised that rats are less sensitive to nickel (II)‐induced infertility due to a relative lack of protamine 2. The experiment was performed on male rats of the Buffalo strain (n = 10 in the control and n = 15 in the exposed group) and male mice of the Balb/c strain (n = 10 in the control and n = 15 in the exposed group). The exposed groups received one i.p. injection with nickel chloride at a dose of 5 mg Ni/kg bw. The concentration of lipid peroxidation markers (measurement of MDA + 4‐hydroxynonenal) in testicular homogenates of control mice are almost twice as high as the concentration measured in control rats. After exposure to nickel, there was a significant increase (over twofold) in lipid peroxidation in testicular homogenates of mice. In the group of rats exposed to nickel, concentration of peroxidation markers in testicular homogenates did not change. GSH concentration in testicular homogenates of exposed rats was not significantly affected, whereas in treated mice GSH concentration was significantly lowered (by 20%). This suggests that mice are more sensitive to the activity of nickel ions than rats. On the basis of these results, it appears that nickel at this dose can initiate oxidative stress in the testes of mice but not of rats. As a consequence of a reduced concentration of GSH, the antioxidant defence of the testes is reduced. The consequent elevation of ROS in the testes may contribute to infertility. At least part of the reason for a higher production of ROS in mouse testis compared to rat testis appears to be related to the much higher concentration of protamine 2 in mouse testes. The rat expresses only 2‐5% of the level of protamine 2 compared to the mouse (Bunick et al., 1990; Belokopytova et al., 1993). Thus, some of the ROS production appears to be a result of the formation of a complex between nickel and the N terminus of protamine 2 in species that express this protein. This mechanism of ROS production has been studied (Bal et al., 1997a,b) in relation to the forms of protamine 2 which are expressed in humans (Ammer et al., 1986; McKay et al., 1986). A synthesised peptide having the N‐terminal sequence of the human sperm protamine 2, binds nickel strongly and this leads to ROS production that damages DNA evidenced by the formation of 8‐oxo deoxyguanosine in vitro. In addition to the complex resulting in an elevation of ROS, it is well established that alteration of the level of the ratio of protamine 1 and protamine 2 (normally expressed at a similar level; Carrell et al., 2007) has been associated with human male infertility (Balhorn et al., 1988; Belokopytova et al., 1993; Oliva, 2006).
Zou et al. (2017) studied the role of nickel‐induced ROS generation in relation to apoptosis mediated by mitochondria and endoplasmic reticulum stress (ERS) pathways in rat Leydig cells. Leydig cells were seeded for 12 h with 0, 250, 500 and 1,000 μM nickel sulfate or were incubated for 0, 6, 12 and 24 h with 500 μM nickel sulfate. Cells were also incubated with 2 ROS scavengers, NAC (5 mM) or 2,2,6,6‐tetramethyl‐1‐piperidinyloxy (TEMPO; 1 mM), for 1 h before treatment with 500 μM nickel sulfate. Using the MTT assay, the authors showed a significantly decreased number of viable cells in a dose‐dependent manner. After the treatment for 6, 12 and 24 h, the viability of the Leydig cells decreased significantly in all groups. The viability of the cells remained no more than 50% after treatment with 500 and 1,000 μM nickel sulfate. The authors found twofold increases of dichlorofluorescein fluorescence intensity of Leydig cells compared with controls (12 h, 500 μM nickel sulfate). ROS generation was significantly alleviated by NAC and TEMPO indicating that ROS is involved in nickel‐induced cytotoxicity in rat Leydig cells. The percentage of early apoptotic Leydig cells was significantly increased after 12 h of treatment. Nickel also upregulated the mRNA expression of Bak,18 cytochrome c and caspase 9, indicative of an apoptotic mechanism, but these changes were reversed by both NAC and TEMPO (p < 0.05). The relative protein expression levels of GRP78, GADD153 and caspase 12 were upregulated significantly after nickel treatment for 12 h. The authors concluded that ROS‐dependent mitochondria and ERS‐mediated apoptotic signal pathways are involved in nickel‐induced apoptosis in rat Leydig cells.
To determine the concentration‐ and time‐effects of nickel on testosterone production and mitogen‐activated protein kinase (MAPK) phosphorylation, Leydig cells were treated with nickel sulfate (0, 250, 500 and 1,000 μM) for 0, 6, 12 and 24 h, respectively (Han et al., 2018). To clarify the roles of ROS in testosterone synthesis, cells were incubated with 5 mM NAC or 1 mM TEMPO for 1 h before treatment with the highest nickel concentration (1,000 μM nickel sulfate) for 24 h. To understand the roles of MAPKs in testosterone synthesis, cells were pre‐incubated with or without inhibitors of extracellular signal‐regulated kinase 1/2 (ERK1/2), p38 and c‐JUN NH2‐terminal protein kinase (JNK) for 0.5 h and then treated with 1,000 μM nickel sulfate for 24 h. The authors reported dose‐dependent decreases in testosterone levels in culture media (1,000 μM nickel sulfate; p < 0.05) and significant downregulation of mRNA and protein expression levels of steroidogenic acute regulatory protein (StAR), cytochrome P450 11A1 (CYP11A1), 3β‐hydroxysteroid dehydrogenase (3β‐HSD), CYP17A1 and 17β‐HSD. The reduction of testosterone concentrations and downregulated expression of testosterone synthetase were both reversed by NAC and by TEMPO (p < 0.05), indicating that ROS were involved in nickel‐induced reduction of testosterone synthesis in rat Leydig cells. The phosphorylation of MEK1/2 in Leydig cells significantly increased at 250, 500, 1,000 μM nickel sulfate and the phosphorylation of ERK1/2, MKK3, p38, MKK7 and JNK significantly increased at 500 and 1,000 μM nickel sulfate. According to these findings, the authors used only the highest concentration (1,000 μM) for further experiments. The results suggest that, at least, ERK1/2 and p38 MAPK signal pathways mediate nickel‐induced decrease in testosterone production by downregulating the expression of testosterone synthetase. The authors also conclude that nickel‐induced ROS generation and the activation of ERK1/2 and p38 MAPK pathways contributed to the downregulated mRNA and protein levels of StAR, CYP11A1, 3β‐HSD, CYP17A1 and 17β‐HSD, which ultimately reduced the testosterone content in rat Leydig cells (Han et al., 2018).
Developmental toxicity
The effect of nickel on embryo development was investigated in the mouse in vitro. Zygotes were treated with 50 μM or 100 μM of nickel chloride until the blastocyst stage. Nickel at 100 μM completely eliminated hatching (statistically significant) and the rate of hatching at 50 μM of nickel exposure was reduced (although not statistically significant) compared to the controls. The expression of pluripotent genes (Nanog, Oct4, Sox2 and Klf4) in blastocysts exposed to nickel were downregulated in a dose‐dependent manner compared to the controls. The authors concluded that nickel disrupted blastocyst hatching in a dose‐dependent manner (Wang et al., 2018; abstract only).
Summary
There is evidence for an effect of nickel on sperm quality, testicular histology and male fertility in mice. Testicular degeneration has also been reported in rats in a study with limited reporting. Mice appear to be more sensitive than rats. The mode of action appears to involve ROS, at least in part mediated by nickel complexation with protamine 2 which is expressed in sperm chromatin. Humans express both protamine 2 and protamine 1 at appreciable levels in sperm (43% protamine 2) and therefore may be more similar in this respect to the mouse (67–72% protamine 2 in various mouse species) than to the rat which expresses very little protamine 2 (2–5% in Norwegian rats). Thus, on the basis of protamine 2 expression levels, it appears that the susceptibility of humans to infertility might be more similar to that of the mouse rather than to that of the rat, although the relative level of antioxidant defence in sperm will undoubtedly also be a major determinant of susceptibility. There is also a possible interference with calcium ion channels. In vitro, Leydig cell toxicity and reduced testosterone production is related to increased ROS and there is evidence of altered ERK1/2 and p38 MAPK signalling which appears to inhibit testosterone synthetase.
3.1.4.4. Immunotoxic activity of nickel
The EFSA 2015 Opinion concluded that the combination of nickel with circulating or tissue protein gives rise to new antigens and acts as a contact allergen and causes sensitisation expressed either as Type I or Type IV hypersensitivity. Such Ni‐binding proteins may include immunomodulatory and nickel T cell activating human serum albumin (HSA‐Ni) or cytoskeletal proteins and stress proteins (HSP‐70, BiP, HSC‐70, HSP‐54 or TCP1/CCT), in human immune cells or in keratinocytes from human skin (Thierse et al., 2004; Heiss et al., 2005; Koppes et al., 2017). Moreover, a dominant binding of non‐classical hapten nickel to CDR3 histidine was shown by Thierse et al. (2005). These hypersensitivity reactions are mediated by IgE antibodies or by allergen‐specific T lymphocytes, respectively, which are associated with a wide range of cutaneous eruptions following dermal or systemic exposure. Alternatively, binding to the major histocompatibility complex (MHC) and/or to MHC‐bound peptides and T cell receptors leading to the activation of nickel‐specific T cells may result in sensitisation. Kuroishi et al. (2017) described chemokine ligand 4 (CXCL4) as a novel nickel‐binding protein. This is involved in hypersensitivity to nickel as well as in the adjuvant effect that nickel has been shown to exert. A recent study from Aparicio‐Soto et al. (2020) demonstrates the dominance of a specific T cell receptor alpha (TRAV9‐2) in Ni‐specific T cell activation in allergic and non‐allergic individuals, thus indicating immunologically a privileged recognition of nickel by the human immune system, thereby possibly co‐explaining high numbers of nickel‐reactive individuals, whether developing clinical symptoms or not.
New literature published since the EFSA Opinion in 2015 revealed more information on the mechanisms of nickel‐induced immunotoxicity. Such mechanisms may pertain to allergic responses that involve inflammatory processes but may also relate to immunodysregulation and immunosuppression.
Dyring‐Andersen et al. (2013) showed that CD4(+) T cells producing IL‐17, IL‐22 and IFN‐γ are important effector cells in the eczematous reactions of nickel‐induced allergic contact dermatitis in humans. Bechara et al. (2017, 2018) showed that toll‐like receptor 4 (TLR4), p38 MAPK and nuclear factor kappa B (NF‐κB) were involved in IL‐23 production induced by nickel. Jak‐signal transducer and activator of transcription seems to maintain the IL‐23/IL‐12p70 balance by limiting IL‐23 production and promoting Th1 polarisation. These results indicate that nickel‐induced Th17 cell development is dependent on the production of IL‐23 by human monocyte‐derived dendritic cells via toll‐like receptor 4 (TLR4), p38 MAPK, NF‐κB and Jak‐signal transducer and activator of transcription pathways. Similar observations on TLR4 and NF‐κB involvement were made by Zoroddu et al. (2014), Lin et al. (2016), Oblak et al. (2015) and Peana et al. (2017). Almogren et al. (2013) observed a predominance of a Th1 phenotype, based on cytokine expression including IL‐4 and IL‐10, of lymphocytes collected from patients suffering from nickel allergy, in line with commonly accepted mechanisms of contact sensitivity. Also another inflammatory mediator were shown to be induced by nickel: nickel chloride induced the expression of cyclooxygenase‐2 (COX‐2) mRNA in primary fibroblasts, neutrophils, RAW 264 cells, and THP‐1 cells, indicating that nickel ions can induce COX‐2 expression in various types of cells (Sato et al., 2016).
Oral exposure to nickel as a determining factor for flare‐ups in sensitive individuals has been reported (see Section 3.1.3). In humans, as in animal studies, repeated oral exposure to nickel may prevent or diminish skin sensitisation. As everybody is exposed to nickel, this may also occur in nickel‐sensitised individuals. For instance, Ricciardi et al. (2013) evaluated the efficacy of exposure to increasing doses of nickel in women suffering from SNAS. The study indicates the efficacy of the desensitisation treatment after a period of being on a low‐nickel diet, and confirms that oral exposure may induce oral tolerance, as previously reported (EFSA CONTAM Panel, 2015). Reduced IL‐10 levels were noted in the desensitised individuals, indicating a role for IL‐10 in the regulation of nickel‐specific responses after oral uptake of nickel in nickel‐sensitised individuals. Di Gioacchino et al. (2014) also studied the efficacy of oral desensitisation to nickel, and found that after desensitisation treatment, higher oral doses were required to induce flare‐up reactions. Gingival fibroblasts may be involved in the induction of oral tolerance through nickel‐induced alteration in NF‐κB and HIF‐1α regulation (Gölz et al., 2016). Increased IL1‐β, chemokine ligand 20 and vascular endothelial growth factor protein levels, as well as decreased IL‐10 levels, which predispose an individual to an inflammatory reaction in the skin due to nickel, seem to be inhibited in gingival/oral tissue. Not everybody will be sensitised to nickel, and of those sensitised not everybody will show flare‐up reactions to oral ingestion. Whereas processes that regulate the function of the immune system, and that suppress inappropriate strong responses to allergens (in the alimentary tract leading to oral tolerance), will be operational in all individuals, apparently this is not sufficient for everybody, as flare‐up reactions after oral ingestion in sensitised individuals do occur.
Monocytes are precursors of macrophages as well as dendritic cells, and are capable of antigen presentation. They can activate nickel‐specific T cells. Volke et al. (2013) studied lipopolysaccharide (LPS)‐stimulated and non‐stimulated RAW 264.7 macrophage cell lines incubated with nickel sulfate for 24 h. Nickel sulfate increased LPS‐induced nitrite production as well as the formation of l‐citrulline from l‐arginine. Correspondingly, the expression of the inducible nitric oxide synthase gene and protein was also remarkably enhanced. In contrast, nickel had an inhibitory effect on l‐arginine transport. These data indicate that nickel interferes with macrophages’ l‐arginine/NOS system on multiple levels.
Asakawa et al. (2015) also studied the effects of nickel (II) on the LPS‐induced production of cytokines in murine macrophage cell line RAW264, as well as in the air pouch‐type inflammation model in BALB/c mice. Nickel (II) inhibited LPS‐induced production of IL‐6, but not that of tumour necrosis factor‐α (TNF‐α) both in vivo and in vitro. In another study by the same group (Asakawa et al., 2018), it was shown that nickel ions bind to the heat‐shock protein 90‐β and enhance HIF‐1α‐mediated IL‐8 expression.
Freitas et al. (2013) demonstrated that that nickel nitrate kills neutrophils in vitro by an apoptotic mechanism most likely involving ROS production and increases in nicotinamide adenine dinucleotide phosphate oxidase. This toxicity may be important in relation to the immune system or in terms of bacterial defence if the toxic concentrations could be achieved in vivo.
Kim et al. (2013) performed a genome‐wide study on susceptibility loci for allergic nickel dermatitis. NTN4 (encoding for and extracellular matrix molecule) and PELI1 (involved in TLR/IL‐1R signalling) seem to be involved in nickel sensitisation. The claudin‐1 gene seems also to be involved (Ross‐Hansen et al., 2013). This implies that such genes may also be involved in the effects of oral exposure of sensitive individuals. Studies with macrophages performed by Ferko and Catelas (2018) have shown that nickel ions can activate the NLRP3 inflammasome, via oxidative stress and NF‐κB signalling. This is in line with studies by Li et al. (2013) and Li and Zhong (2014), indicating that nickel (II) activates the NLRP3‐ASC‐caspase‐1 immune signalling pathway in antigen‐presenting cells, leading to release of the pro‐inflammatory cytokine IL‐1β.
The inflammatory action of nickel was also shown in vitro, using neutrophils harvested from canine peripheral blood. Extracellular traps were formed, i.e. networks mainly consisting of DNA and decorated with neutrophil elastase and myeloperoxidase (MPO) (Wei et al., 2018).
Jakob et al. (2017), using a proteomic approach, identified in human monocytes harvested from peripheral blood and exposed in vitro to nickel (II), changes of protein expression linked to cell death, metal ion binding and cytoskeleton remodelling, indicative of apoptosis. Caspase‐3 and ‐7 independent cell death of monocytes was observed at concentrations of 250 μM and higher. Lower or same concentrations may result in activation of nickel‐specific T cells (Thierse et al., 2004; Aparicio‐Soto et al., 2020). This is in line with earlier studies performed in broilers. Tang et al. (2015) noted that nickel chloride in excess of 300 mg/kg inhibited thymocyte growth by arresting cell cycle in broilers, increasing apoptosis percentage, altering apoptotic protein mRNA expression levels, and downregulating cytokine expression levels. Apoptosis was also found in splenocytes from broilers (Huang et al., 2013). Dietary nickel chloride in excess of 300 mg/kg caused apoptosis, altered Bax, Bcl‐2 and caspase‐3 mRNA expression levels and induced oxidative stress in the spleen which are associated with apoptosis. Li et al. (2014) observed significant decreases in several haematological parameters (total erythrocyte counts, haemoglobin content and packed cell volume and osmotic fragility) in broilers. Also, the immune adherence function of erythrocytes, measured as the percentage of E‐C3bRR, was decreased.
Wu et al. (2014) noted suppressed mRNA expression of toll‐like receptors TLR2‐2 and TLR4 in the intestinal and cecal mucosa in broilers. The same group (Huang et al., 2014b) found decreased expression of IL4 and IL7 mRNA in the spleen of the broilers, as well as decreased levels of secretory immunoglobulin A, IgA, IgG and IgM in the small intestinal and cecal tonsil and in serum. The results were expanded by Huang et al. (2014a) to mRNA levels of IL‐2, IL‐6, IL‐10, IL‐12, TNFα and IFNγ. Also, IgG, IgA and IgM content was found to be reduced in the bursa of Fabricius by oral nickel exposure. The authors also observed a loss of lymphocyte cellularity by histopathology. Concurrent with these findings, SOD, GSH‐Px and the ability to inhibit hydroxyl radical and glutathione content were significantly decreased. These results extended earlier findings by Wu et al. (2013b), showing that the serum IL‐2, IL‐4, IL‐6, IL‐10, IFN‐γ and TNF‐α content was reduced. Collectively, these data indicate that the innate and acquired immune system of broilers is affected by exposure to nickel. Findings were further confirmed and extended by Yin et al. (2016a), who observed in broilers decreased cellularity lymphoid follicles with thinner cortices and wider medullae. Concurrently, the activities of SOD, CAT, GSH‐Px and the ability to inhibit hydroxyl radical and GSH contents were decreased in the bursa of Fabricius, while MDA content was increased in the nickel chloride‐treated groups. In accordance with earlier studies, serum IgG, IgM and bursa IgG and IgM contents were lower in the nickel chloride‐treated groups compared to the controls. This was further supported by the same group, which showed that dietary nickel suppressed the development of the bursa of Fabricius, characterised by the relative weight of this organ, decreased lymphocyte density, increased G0/G1 phase (a prolonged non‐dividing state), reduced S phase (DNA replication) and proliferating index, and increased percentages of apoptotic cells (Yin et al., 2016b). Also mRNA expression levels of Bax, cytochrome c, apoptotic peptidase activating factor 1, caspase‐3, caspase‐6, caspase‐7 and caspase‐9 were increased whereas the Bcl‐2 mRNA expression levels was decreased. This suppression of bursal development, and in particular, the reduction of the B lymphocyte population and B lymphocyte activity, led to impairment of humoral immunity in the broiler chicken. The above‐mentioned results show that in broilers, dietary nickel can cause histopathological lesions via oxidative damage, which finally impairs the function of the bursa of Fabricius and reduces the IgG and IgM content of the serum and the bursa of Fabricius.
Guo et al. (2015) showed that nickel chloride altered inflammatory mediators, and functional damage in the broiler's kidney, using biochemical methods, immunohistochemistry and reverse transcription quantitative polymerase chain reaction. Dietary nickel chloride at doses higher than 300 mg/kg resulted in renal inflammatory responses, expressed as increased mRNA expression levels of the pro‐inflammatory mediators including TNF‐α, COX‐2, IL‐1β, IL‐6, IL‐8 and IL‐18 through activation of NF‐κB, in addition to decreased mRNA expression levels of the anti‐inflammatory mediators including IL‐2, IL‐4 and IL‐13. These data suggest that activation of the NF‐κB pathway and reduction of anti‐inflammatory mediator expression are the main mechanisms of nickel chloride‐caused renal inflammatory responses after nickel chloride treatment. Several key inflammatory markers have been consistently associated with both obesity and risk of adverse outcomes in obesity‐associated diseases, suggesting that a persistent, low‐grade, inflammatory response is a potentially modifiable risk factor. The higher prevalence of nickel allergy in obese individuals (Lusi et al., 2015) was associated with a worse metabolic profile, while reducing the oral intake of nickel led to a considerable reduction in the BMI (Lusi et al., 2015; Watanabe et al., 2018). Chana et al. (2018) confirmed that nickel (II) amplifies LPS‐induced secretion of several pro‐inflammatory cytokines from monocytes. It is known that hyperglycaemic conditions also affect monocytic function. The study showed that that nickel (II) decreased mitochondrial activity in monocytic‐cells and macrophages under normal conditions, but hyperglycaemic conditions diminished the toxicity seen with nickel (II) exposure.
Collectively, these studies show that the ability of nickel to bind to proteins is responsible for the induction of specific immune responses, leading to allergic reactions. These may be evident in the skin but can also occur elsewhere in the body. Nickel also has a non‐specific activity on the immune system, such as the induction of inflammatory reactions through toll‐like receptors and NF‐κB signalling pathways, which may be involved in the adverse reactions, including the allergic reactions. On the other hand, these mechanisms may also lead to dysregulation of the immune system, for instance through apoptosis, resulting in reduced production of immunoglobulins, that may have an impact on host resistance. Even though predominant reactions to nickel occur after skin exposure, oral exposure to nickel may potentially induce these effects as well, and especially cause flare‐up reactions in already sensitised individuals.
3.1.4.5. Neurotoxicity
He et al. (2013a) reported that nickel exposure caused deficits in neurobehavioural performance in male mice administered nickel chloride hexahydrate orally by gavage and that nickel was deposited in the brain including the cerebral cortex (see Section 3.1.2.7 Neurotoxicity). They also examined nickel‐induced aerobic metabolic disturbances in the cerebral cortex. Oxygen consumption and adenosine triphosphate (ATP) concentration were significantly decreased in the high‐dose group at 3 h after dosing and lactate concentrations and the ratio of the reduced and oxidised form of nicotinamide adenine dinucleotide were significantly increased. These alterations returned to control levels at 24 h after dosing. In the high‐dose group, oxidative stress was evident from the elevation of MDA concentrations and reduced activity of SOD. Oxidative stress was also induced in the low‐dose group, but only the SOD activity was significantly decreased. The activity of two iron‐sulfur cluster‐dependent metabolic enzymes (ISCs), aconitase and complex I that are known to control aerobic metabolism was also measured. The aconitase activity was significantly decreased in both dose groups at 3 h after dosing while the activity of complex I was only significantly decreased in the high‐dose group. The activity of both enzymes was similar in all groups at 24 h. The expression of ISC assembly scaffold protein was significantly suppressed in the high‐dose groups at 3 h, but not at 24 h. According to the authors, these data suggest that aerobic metabolic disturbances may participate in the reported nickel‐induced neurobehavioural effects and that the inhibition of ISC‐containing metabolic enzymes may result in the disturbance of aerobic metabolism.
Ijomone et al. (2018b) reported that nickel compromised neurobehavioural performance (cognitive and motor behaviour), affected neuronal morphology in the brain and significantly decreased the percentage of intact neurons in both hippocampus and striatum in adult male Wistar rats administered nickel chloride hexahydrate via i.p. injections in normal saline for 21 days at doses of 0, 5, 10, 50 mg NiCl2/kg bw (corresponding to 0, 2.3, 4.5 and 22.6 mg Ni/kg bw). The activities of SOD, catalase, glutathione S‐transferase and GSH‐Px were significantly decreased at all dose levels. Furthermore, the levels of glutathione were significantly decreased and the levels of MPO, lipid peroxidation and nitric oxide were significantly increased at all dose levels. These data suggest that the compromised neurobehavioural performance and brain histomorphology is associated with an increase in oxidative stress. Ijomone et al. (2018a) reported ultrastructural changes in neurons of the hippocampus, striatum and cortex in adult male Wistar rats administered nickel chloride hexahydrate orally by gavage in saline at doses of 0, 10, 20 mg NiCl2/kg bw (corresponding to 0, 4.5 and 9.1 mg Ni/kg bw) for 4 weeks (see Section 3.1.2.7 Neurotoxicity). Caspase‐3 was markedly increased in CA3 and DG of the hippocampus and in the striatum in the high‐dose group. Alpha‐synuclein was also significantly increased in the cortex in the high‐dose group; no effect was noted in the hippocampus and striatum. According to the authors, these data implicate mitochondria in an apoptotic mechanism of nickel‐induced neurodegeneration.
Rats were treated with nickel chloride by daily i.p. injection (0.25–1 mg/kg bw). Neurobehavioural tests after 8 weeks showed increased anxiety‐like behaviour and depression‐like symptoms compared with controls. Spatial learning and memory were impaired in males at the top dose only. There were associated changes in enzymes involved in antioxidant response and evidence of oxidative stress, which the authors consider may be the causative mechanism (Lamtai et al., 2018). Further evidence of oxidative stress is evident from a report that nickel causes elevation of metallothionein and oxidative stress in mouse brain (Sadauskiene et al., 2013). However, this is reported only as an abstract and the dose levels are not given.
Previous studies demonstrated that nickel can cause a disruption of mitochondrial energy supply mediated by activation of HIF‐1α and which could potentially contribute to neurobehavioural changes in mice (He et al., 2013b). In neuro‐2a cells, nickel chloride caused a concentration‐dependent increased expression of the microRNA miR‐210 (which is known to be regulated by HIF‐1α) and a subsequent reduction of the protein that facilitates the assembly of the iron–sulfur cluster required for mitochondrial function. This further supports the role of HIF‐1α in nickel toxicity (He et al., 2014).
Several studies have focused on the effect of nickel in neuronal cell lines. Primary cultures of cortical neurons were exposed to 0.5, 1.0 and 2.0 mM nickel chloride. Cytotoxicity was indicated by the release of lactate dehydrogenase. Nickel reduced ATP production, disrupted mitochondrial membrane potential and reduced mitochondrial DNA content. These effects were associated with an increase in ROS production, decreased SOD activity and decreased concentration of GSH and were inhibited by pre‐treatment of cells with taurine which has antioxidant capacity. The findings support a role of oxidative stress in neuronal cell toxicity induced by nickel (Xu et al., 2015). A differentiating neuronal cell line (NT2) was treated with nickel (10 μM) which increased the expression of HIF‐1α and specific markers of neuronal differentiation in the absence of cytotoxicity. After 4 weeks of treatment, the expression of tyrosine hydroxylase as a marker of dopaminergic neurons was reduced, suggesting a potential to affect neurological development (Ceci et al., 2015).
Further evidence for the potential for nickel to cause neurotoxicity comes from the observation in cardiac neurons that 50 μM nickel (II) inhibited neuronal excitability mediated by pituitary adenylate cyclase polypeptide (Tompkins et al., 2015). In addition, nickel can interfere with calcium‐induced dimerisation of N‐cadherin through competition with the binding of calcium (Dukes et al., 2019). This shows the potential for an interference with adherens junctions including neurological synapses but the effects were seen at 1 mM and it is not clear whether the potency is high enough to achieve the same effects as the concentrations of nickel achieved in vivo.
In a study by Baierle et al. (2014) associations were sought between ALA‐D activity and various metals, including nickel, in human volunteers (see Section 3.1.3.5 for further details). Nickel had no effect on ALA‐D activity, but nickel inhibited the reductive reactivation of oxidised ALA‐D. This suggests a potential to inhibit the maintenance of reduced thiol groups in ALA‐D and is in accordance with nickel‐induced oxidative stress.
In summary, nickel causes deficits in neurobehavioural performance in rodents and neuronal cell toxicity in vivo and in vitro. These effects are associated with oxidative stress and disturbance of mitochondrial aerobic metabolism evidently involving HIF‐1α.
3.1.4.6. Other
The cytotoxic effects of nickel ions on osteocytes were investigated in vitro (Kanaji et al., 2014). Osteocytes from a murine long bone‐derived osteocytic cell line (MLO‐Y4) were treated with nickel chloride solutions at concentrations of 0, 0.05, 0.10 and 0.5 mM for 24 and 48 h. A significant cytotoxic effect was observed at 0.10 and 0.50 mM after 48 h of treatment. Significant higher levels of necrosis and apoptosis were observed at 0.50 mM after 24 h of treatment.
The effects of dietary nickel (diet supplemented with 300, 600 and 900 mg/kg of nickel chloride for 42 days) on the development of the small intestine in broilers were investigated. Doses ≥ 300 mg/kg bw reduce the intestinal villus height, crypt depth and villus/crypt ratio, as well as the number of small intestinal goblet cells. Decreases of the insulin‐like growth factor‐1 and the epidermal growth factor content were also observed. This indicates that the normal development and function of the small intestine was impaired in broilers (Wu et al., 2013a).
3.1.5. Considerations of critical effects and dose–response analysis
3.1.5.1. Considerations of critical effects
Chronic effects
In the previous Opinion (EFSA CONTAM Panel, 2015), the CONTAM Panel identified reproductive and developmental toxicity as the critical effect for the risk characterisation of chronic oral exposure to nickel. Different reproductive effects were reported in mice such as decreased male sex organ weight and histopathological changes, disturbed spermatogenesis, decreased sperm motility and sperm damage. These effects were responsible for a decrease in fertility in mice. Developmental toxicity included increased pup mortality (stillbirth or post‐implantation loss/perinatal lethality) and decreased pup weight in rats. Developmental toxicity was also observed in previous studies in mice (decreased fetal weight, malformations) but at higher doses than for rats. The effects were reported in a number of studies of varying quality. The most reliable dose–response information for reproductive and developmental effects was identified in a one‐generation dose‐range‐finding study performed with nickel sulfate hexahydrate in rats (SLI, 2000a) and in the subsequent main two‐generation study (SLI, 2000b), see Section 3.1.2.5. The CONTAM Panel identified the incidence of litters with post‐implantation loss per treatment group as the relevant and sensitive endpoint for the dose–response assessment. A recent short‐term toxicity study (28 days) with limited reporting suggested that nickel also may cause damage to the testes (testicular degeneration) of rats. Two recent studies confirmed that nickel caused developmental toxicity in mice when administered during different gestational periods at doses higher than those resulting in developmental toxicity in rats. For testicular effects, mice appear to be more sensitive than rats whereas for developmental toxicity rats appear to be more sensitive than mice. Human studies published since the previous Opinion suggest an association between nickel exposure and adverse reproductive and developmental outcomes.
The short‐term toxicity studies in experimental animals published since the previous Opinion have reported similar effects to those previously reported. Furthermore, effects on bone and on gut microbiota were reported. Recent studies in experimental animals have indicated that nickel can disturb the neurobehavioural functions in rats and mice and cause neurodegeneration in adult rats whereas no clear signs of neurotoxicity have been reported in the few human studies. None of these studies are adequate for the derivation of a reference point for the risk characterisation of chronic oral exposure to nickel.
A recurring theme in the toxicity of nickel is the evidence for a role of oxidative stress and ROS formation. A contribution of oxidative stress is evident in relation to reproductive toxicity, genotoxicity, immunotoxicity and neurotoxicity. Studies investigating the mode(s) of action underlying the adverse reproductive and developmental effects published since the previous Opinion support a contribution of oxidative stress and possible interference with calcium ion channels. In vitro, Leydig cell toxicity and reduced testosterone production is also related to increased ROS and there is evidence of altered ERK1/2 and p38 MAPK signalling which appears to inhibit testosterone synthetase.
Based on the available data, the CONTAM Panel still considered that the increased incidence of post‐implantation loss in rats is the critical effect for the risk characterisation of chronic oral exposure to nickel. The Panel concluded that the one‐ and two‐generation studies by SLI (2000a,b) are still the most suitable and reliable studies for dose–response modelling.
Acute effects
Exposure through the skin or by inhalation may lead to nickel sensitisation. Whereas oral exposure to nickel is not known to sensitise, oral absorption of nickel may elicit eczematous flare‐up reactions in the skin (SCD) in nickel‐sensitised individuals.
The CONTAM Panel affirms that SCD elicited in previously nickel‐sensitised individuals either via the skin or the respiratory tract after oral exposure to nickel is the critical effect suitable for the risk characterisation of acute oral exposure to nickel. In the current assessment, no new studies were identified as suitable for dose–response analysis and the CONTAM Panel used the same three studies as in 2015 (Gawkrodger et al., 1986; Hindsén et al., 2001; Jensen et al., 2003).
3.1.5.2. Dose–response analysis (including BMD modelling)
The BMD analysis performed followed the updated guidance of the Scientific Committee on BMD modelling (EFSA Scientific Committee, 2017). The detailed description of the BMD analysis performed by the Panel can be found in Appendix C and Annex A. Appendix C shows the detailed BMD analysis from which the reference point was selected, and all other BMD analyses are shown in Annex A. The BMD analyses were performed using the EFSA web tool, which is based on the R‐package PROAST 67.0.
Chronic effects
As described in Section 3.1.5.1, the CONTAM Panel considered the incidence of post‐implantation loss19 in rats as the critical effect following oral exposure to nickel. The CONTAM Panel concluded that the studies by SLI (2000a,b) are still the most suitable and reliable studies for dose–response modelling. These studies are particularly suitable for dose–response modelling as nine dose levels (0, 0.2, 0.6, 1.1, 2.2, 4.4, 6.6, 11 and 17 mg Ni/kg bw per day) were tested (see Tables 3 and 4). The data set from these studies consists of three subsets: the one‐generation dose‐range‐finding study (DRF), the F0/F1 generation of the two‐generation study (2GEN F0F1) and the F1/F2 generation of the two‐generation study (2GEN F1F2). In 2015, the CONTAM Panel used the incidence of litters with post‐implantation loss per treatment group and the incidence of litters with three or more post‐implantation losses per treatment group for BMD analysis. The individual data were not used to derive a reference point since the analysis of these nested dichotomous data‐response data using the software available at that time did not comply with the established goodness‐of‐fit criterion EFSA was using in 2015 in accordance with the previous BMD Guidance (EFSA, 2009). Considering the update of the BMD guidance (EFSA Scientific Committee, 2017) and the available software, the CONTAM Panel decided to use the individual data of post‐implantation loss per litter for the current assessment.
BMD analysis of the 2GEN F1F2 data showed that none of the models were accepted, indicating that there is no observable trend (see Annex A.1). The BMD analysis was therefore limited to the data from the DRF and the 2GEN F0F1.
For quantal data, the default benchmark response (BMR), as recommended by EFSA's guidance, is an extra risk of 10% compared with the background risk. The CONTAM Panel noted that the US EPA (2012) indicates that most reproductive and developmental studies with nested study designs support a BMR of 5%. Applying a BMR of 5%, using model averaging and using the study as covariate, the resulting BMDL05 values for post‐implantation loss were 0.06 and 0.12 mg Ni/kg bw per day for the DRF and the 2GEN F0F1 studies, respectively (see Table 5 and Annex A.2). Large BMDL05–BMDU05 CIs (0.06–5.17 and 0.12–4.18 mg Ni/kg bw per day, respectively) were observed. Therefore, the CONTAM Panel decided to apply the default BMR of 10% (see Appendix C.1). Applying a BMR of 10%, using model averaging and using the study as covariate, the resulting BMDL10 values for post‐implantation loss were 1.40 and 1.34 mg Ni/kg bw per day for the DRF and the 2GEN F0F1 studies, respectively (see Table 5). From this analysis, the CONTAM Panel selected the BMDL10 of 1.3 mg Ni/kg bw per day for the increase in post‐implantation loss in rats as a reference point for chronic effects caused by nickel.
Table 5.
Summary of the BMDL and BMDU values (mg Ni/kg bw per day) for post‐implantation loss calculated for the combined analysis of the dose‐range‐finding study (DRF) and the F0/F1 generation of the two‐generation study (2GEN F0F1), using model averaging and study as covariate
| Study | BMDL05 | BMDU05 | BMDL10 | BMDU10 |
|---|---|---|---|---|
| DRF | 0.06 | 5.17 | 1.40 | 10.7 |
| 2GEN F0F1 | 0.12 | 4.18 | 1.34 | 9.8 |
BMDL: benchmark dose lower confidence limit; BMDU: benchmark dose upper confidence limit.
Acute effects
The CONTAM Panel confirms its previous conclusion to use SCD elicited in nickel‐sensitised humans after oral exposure as the critical effect for acute oral exposure to nickel. In 2015, the CONTAM Panel identified three studies, namely Gawkrodger et al. (1986), Hindsén et al. (2001) and Jensen et al. (2003), as being suitable for the dose–response analysis.
In the current assessment, no other studies were identified as suitable for the dose–response analysis and the CONTAM Panel used the same three studies. It was noted that the study by Gawkrodger et al. (1986) had limitations, i.e. a high incidence of unexplained placebo reactions (See section 3.1.3.4) and that the study by Hindsén et al. (2001) includes one control group and only two exposed groups. As both experimental designs have important limitations for BMD analyses, the CONTAM Panel decided to use the study by Jensen et al. (2003), consisting of one control group and three exposed groups (see Table 6), for BMD analysis. The CONTAM Panel selected the default BMR of an extra risk of 10% compared with the background risk for quantal data. Using model averaging, the resulting BMDL10–BMDU10 interval for the incidence of clinically cutaneous reactions was 2.66 × 10−5–1.63 mg Ni/person (Annex A.3). The CONTAM Panel noted the large BMDL10–BMDU10 interval and that very low BMDL10 values (< 0.00001 mg Ni/person; see Annex A.3.5 Table A.4) were estimated for four models (i.e. the log‐logistic, the log‐probit, the Weibull and the gamma model). These models were restricted in the previous Opinion (EFSA CONTAM Panel, 2015). The new BMD Guidance (EFSA Scientific Committee, 2017) does not recommend constraining the steepness/shape parameter in the models. Therefore, if the shape of the dose–response curve is not sufficiently constrained by the data itself in the region of the BMR (due to the low number of dose groups, and/or the dose spacing, and/or limited sample size) a large BMD confidence interval can result as a consequence.
Table 6.
Incidence of cutaneous reactions to nickel following oral exposure in nickel‐sensitised persons as reported by Gawkrodger et al. (1986) and Jensen et al. (2003)
| Dose (mg Ni/person) | N | N with clinically cutaneous reactionsa | N with flare‐up of previous sites of dermatitis | Reference |
|---|---|---|---|---|
| 0 | 10 | 1 | 1 | Jensen et al. (2003) |
| 0.3 | 10 | 4 | 4 | Jensen et al. (2003) |
| 1 | 10 | 4 | 4 | Jensen et al. (2003) |
| 4 | 10 | 7 | 6 | Jensen et al. (2003) |
| 0.4 | 10 | 5 | n.r. | Gawkrodger et al. (1986) |
| 2.5 | 10 | 5 | n.r. | Gawkrodger et al. (1986) |
| 5.6 | 6 | 6 | n.r. | Gawkrodger et al. (1986) |
N: number of nickel‐sensitised persons; n.r.: not reported.
Flare‐up reactions and widespread clinical reactions, including any large or small clinical eruption on previously unaffected skin.
Based on this analysis, the CONTAM Panel concluded that it is not appropriate to perform the BMD analysis on the study by Jensen et al. (2003) alone and considered the possibility of combining data sets from the different studies. It was noted that both Hindsén et al. (2001) and Jensen et al. (2003) reported the incidence of flare‐up reactions only. However, BMD analysis of the incidence of flare‐up reactions reported by Jensen et al. (2003) showed that none of the models were accepted, indicating that there is no observable trend (see Annex A.4). Therefore, the CONTAM Panel did not further use the incidence of flare‐up reactions only in the assessment.
Both Gawkrodger et al. (1986) and Jensen et al. (2003) reported the incidence of flare‐up reactions together with the development of new physical signs (Table 6) and the populations studied by both research groups are comparable (based on comparison of age, sex, type of exposure and region where the study was conducted). Therefore, the CONTAM Panel decided to combine both data sets in one BMD analysis (not using the study as a covariate). Using model averaging, the resulting BMDL10–BMDU10 interval for the incidence of clinically cutaneous reactions was 0.0124–2.43 mg Ni/person (Annex A.5). The CONTAM Panel noted the large BMDL–BMDU interval and that BMDL10 of 0.0124 mg Ni/person is outside the dose range. The large uncertainty in the BMD can be related to the small group size even though several dose groups were used in this case. For these reasons, the Panel decided to identify the reference point based on the NOAEL/LOAEL approach instead of BMD modelling. From the study by Jensen et al. (2003), a LOAEL of 0.3 mg Ni/person, the lowest dose tested, was identified. This LOAEL corresponds to 4.3 μg Ni/kg bw, assuming a body weight of 70 kg.
3.1.6. Derivation of an HBGV/margin of exposure approach
Chronic effects
Previously, the CONTAM Panel established a TDI and the more recently available data do not provide a basis for changing this approach. Taking into account the revised BMD Guidance, the CONTAM Panel selected the BMDL10 of 1.3 mg Ni/kg bw per day for the increase in post‐implantation loss in rats as a reference point for chronic effects caused by nickel. Based on this BMDL10 value, the CONTAM Panel established a TDI of 13 μg/kg bw for nickel using the default uncertainty factor of 100 to account for intra‐ and interspecies differences. To apply the default factor of 100 in the derivation of the TDI is considered to be conservative. Nickel (II) is not biotransformed and therefore applying the default factors for interspecies and inter‐individual variability in toxicokinetics (4 and 3.16) is conservative as metabolic difference is the major contributor to the variability in toxicokinetics (ECHA, 2012). The available human data do not give a clear indication that nickel is a developmental toxicant in humans and therefore, applying the default factors for interspecies and inter‐individual variability in toxicodynamics (2.5 and 3.16) is conservative. Considering the conservatism in the derivation of the TDI the CONTAM Panel concluded that an additional uncertainty factor for severity of effects, due to the use of a BMR of 10%, is not needed.
Acute effects
Eczematous flare‐up reactions in the skin (SCD) following nickel exposure via food and drinking water have been reported to occur in nickel‐sensitised individuals (see Section 3.1.3.4). The CONTAM Panel decided to characterise the hazard for the acute effects based on the LOAEL of 4.3 μg Ni/kg bw as the reference point for acute oral exposure to nickel.
In 2015, the CONTAM Panel stated ‘It is generally accepted amongst scientists in the field of immunotoxicology and sensitization that contact sensitization as well as elicitation of responses in sensitized individuals follow dose response relationships and have a threshold (Friedmann, 2007; Kimber and Basketter, 2008). This is also true for hypersensitivity to nickel (Ross‐Hansen et al., 2014). For nickel ingested via the oral route, this implies that access of nickel molecules to the skin may lead to hypersensitivity reactions in the skin in a dose‐dependent fashion. On the other hand, thresholds have not been formally established for sensitization to most contact allergens, and information on thresholds of allergic reactions in sensitized individuals is even sparser’. Therefore, the CONTAM Panel decided at that time not to establish an acute reference dose (ARfD), but to apply an MOE approach to the risk characterisation of this critical acute effect.
No new information was identified since the previous opinion that supports a deviation from this approach and the Panel confirmed the previous conclusion to apply an MOE approach.
The Panel considered that an MOE of 30 or higher would indicate a low health concern. This MOE of 30 takes into account:
the extrapolation from a LOAEL to a NOAEL (a factor of 3 as recommended in the REACH Guidance Document for the majority of cases (ECHA, 2012); no specific factor recommended by EFSA);
high incidence of positive reactions at the LOAEL (40%);
only a limited number of individuals were included in the pivotal study;
that there is uncertainty regarding the threshold and that it can be expected that the threshold is low;
the effects of nickel exposure in nickel‐sensitised individuals have an impact on the quality of life, although not life‐threatening (overall factor of 10 covering points 2–5).
3.2. Occurrence data
3.2.1. Occurrence data on food submitted to EFSA
An initial number of 86,668 analytical results on nickel were available in the EFSA database. All analytical data were reported as ‘Total nickel’ without providing information on specific chemical forms. Considering only data on food and drinking water, approximately 52% of the samples were reported as food and 48% as drinking water samples.
The occurrence data for nickel on 43,915 food samples, 39,381 drinking water samples and 3,372 non‐food samples (i.e. feed, food contact materials etc.) is available on the EFSA Knowledge Junction community on Zenodo.20
Data were collected in 26 European countries. The analytical results were obtained between the years 2000 and 2019. However, in order to reflect current contamination levels, only the most recent data were used in the present exposure assessment (from 2009 onwards).
The occurrence data were carefully evaluated, and a list of validation steps was applied before being used to estimate dietary exposure (see Annex C, Table C.1 for further details). The final data set comprised 48,007 analytical results (63% for food and 37% for drinking water).
The presence of relatively high LODs/LOQs may have a significant influence on the UB scenario. Therefore, an evaluation of the reported LOQs was performed in order to reduce the impact of high LOQs reported, but without compromising the number of analytical results (EFSA, 2018a). Based on Council Directive 98/83/EC and Commission Directive 2003/40/EC, an LOD of 2 μg/L for the analysis of water samples was considered in this assessment. Applying a factor of three to calculate the LOQ, a value of 6 μg/L was used as an LOQ cut‐off for samples of water. For the other food categories, special attention was paid to those shown to be the most important contributors to the dietary exposure to nickel in previous assessments (EFSA CONTAM Panel, 2015) and for which the difference between the LB and UB was higher than 10%. For this purpose, different FoodEx level 2 food categories belonging to ‘Grains and grain‐based products’, ‘Vegetables and vegetable products’, ‘Milk and dairy products’ and ‘Non‐alcoholic beverages’ were identified. To identify the most appropriate LOQ cut‐off values, the distributions of quantified values (values above the LOQ) as well as the reported LOQs were evaluated. The 75th or 90th percentile of the LOQs derived from the quantified values was selected as a cut‐off value and subsequently applied to the LOQs reported (EFSA, 2018a). The outcome of this evaluation is reported in Annex C, Table C.2.
Approximately 78% of the data were obtained for samples collected within the official monitoring programmes, while the remaining samples came from private programmes conducted by industry and other programmes (e.g. diet studies, surveillance and national surveys).
Regarding the sampling method, a small number of the analytical results (< 1%) were obtained from pooled samples, meaning that the result represented an average of a number of samples taken in equal parts from different consignments/batches and pooled together before analysis. Since the level of aggregation for pooled samples matched the level of classification of the individual samples (only similar food matrices were pooled together), results from pooled samples were retained for further evaluation. To ensure a proportionate representation of the individual samples and thus an accurate use of occurrence data in assessing the dietary exposure, the mean concentrations per food category were calculated by weighting the reported analytical results for the number of samples pooled.
For analysis of total nickel, the sample is digested and consequently a recovery rate of about 100% is expected. Recovery rates were reported for 2% of the data and approximately 2% of the analytical results (n = 1,048) submitted to EFSA were corrected for recovery.
Based on the data cleaning (see Annex C, Table C.1) 38,661 nickel analytical results were excluded. The analytical results included in the final data set (n = 48,007) and considered for the dietary exposure to nickel were collected in 24 different European countries, most of them in Germany (66% of analytical results), while other countries contributed far less data (Figure 1). Approximately 1% of the data was sampled in the EU without specification of the country. For a few data (n = 51; 0.1%) no information on sampling place was available; however, it was indicated that these products may be available on the EU market. It should be noted that the origin of the samples was not always the European country reporting the data, i.e. the data set also contained samples originating from North and South America, Africa, Asia and Australia. The number of samples per year is presented in Figure 2.
Figure 1.
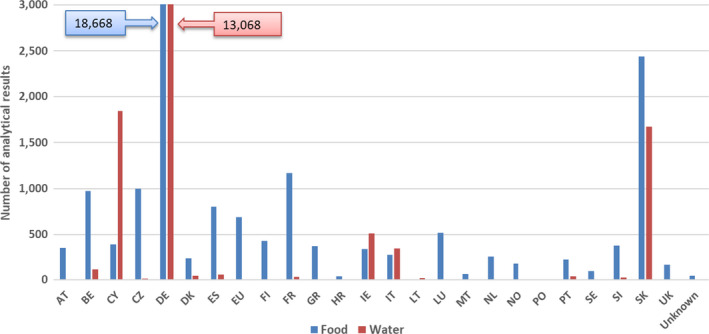
- AT: Austria; BE: Belgium; CY: Cyprus; CZ: Czechia; DE: Germany; DK: Denmark; ES: Spain; EU: European Union; FI: Finland; FR: France; GR: Greece; HR: Croatia; IE: Ireland; IT: Italy; LT: Lithuania; LU: Luxembourg; MT: Malta; NL: Netherlands; NO: Norway; PO: Poland; PT: Portugal; SE: Sweden; SI: Slovenia; SK: Slovakia; UK: United Kingdom.
Figure 2.
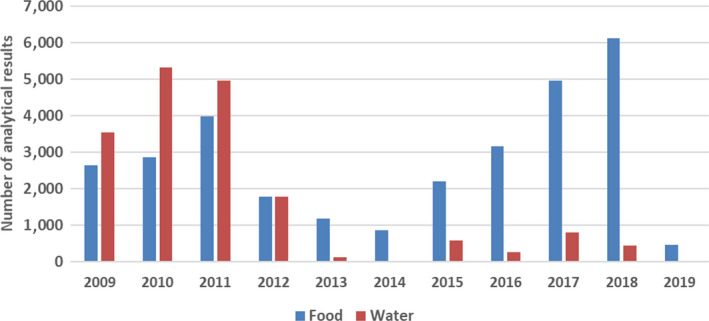
Distribution of analytical results of nickel in food and drinking water over the sampling years (after excluding non‐qualifying data)
Table 7 (see Section 3.2.1.2) shows the number of analytical results and the percentage of LCD per food category at FoodEx Level 1. The most frequently analysed food categories were ‘Drinking water’, ‘Vegetables and vegetable products’ and ‘Grains and grain‐based products’. A substantial amount of data was also available for many other food categories while others, e.g. ‘Non‐alcoholic beverages’, ‘Eggs and egg products’ were much less represented. The proportion of LCD ranged from 4% observed for the food category ‘Legumes, nuts and oilseeds’ to 85% observed for ‘Animal and vegetable fats and oils’ and the overall percentage of LCD was 53%.
Table 7.
Summary of the nickel occurrence data by food category (μg/kg)
| Food category, FoodEx Level 1 | N | %LCD | Mean | Mediana | P95 | |||
|---|---|---|---|---|---|---|---|---|
| LB | UB | LB | UB | LB | UB | |||
| Grains and grain‐based products | 5,221 | 23 | 311 | 331 | 160 | 160 | 1,250 | 1,250 |
| Vegetables and vegetable products | 6,476 | 25 | 731 | 741 | 50 | 54 | 5,100 | 5,100 |
| Starchy roots and tubers | 887 | 16 | 100 | 106 | 42 | 46 | 500 | 500 |
| Legumes, nuts and oilseeds | 2,368 | 4 | 2,236 | 2,250 | 1,342 | 1,342 | 7,490 | 7,490 |
| Fruit and fruit products | 2,130 | 34 | 81 | 107 | 29 | 50 | 274 | 440 |
| Meat and meat products | 2,322 | 70 | 105 | 144 | 0 | 50 | 202 | 500 |
| Fish and other seafood | 1,655 | 51 | 128 | 160 | 0 | 50 | 420 | 500 |
| Milk and dairy products | 1,067 | 55 | 82 | 100 | 0 | 25 | 500 | 515 |
| Eggs and egg products | 153 | 61 | 19 | 28 | 0 | 10 | 70 | 70 |
| Sugar and confectionary | 772 | 38 | 1,392 | 1,462 | 305 | 503 | 5,330 | 5,330 |
| Animal and vegetable fats and oils | 1,343 | 85 | 100 | 213 | 0 | 60 | 180 | 1,000 |
| Fruit and vegetable juices | 1,246 | 46 | 25 | 52 | 11 | 24 | 78 | 110 |
| Non‐alcoholic beverages | 88 | 30 | 49 | 58 | 12 | 20 | 180 | 180 |
| Alcoholic beverages | 1,512 | 68 | 12 | 40 | 0 | 20 | 36 | 100 |
| Drinking water | 17,831 | 81 | 2 | 3 | 0 | 1 | 7 | 7 |
| Herbs, spices and condiments | 982 | 20 | 1,176 | 1,201 | 361 | 460 | 4,640 | 4,640 |
| Food for infants and small children | 995 | 37 | 127 | 193 | 40 | 78 | 630 | 740 |
| Products for special nutritional use | 690 | 29 | 1,637 | 1,748 | 443 | 500 | 6,500 | 6,720 |
| Composite food | 160 | 19 | 117 | 141 | 60 | 64 | 340 | 500 |
| Snacks, desserts, and other foods | 109 | 48 | 133 | 168 | 40 | 100 | 630 | 630 |
| Total | 48,007 | 53 | ||||||
N: number of analytical results; % LCD: proportion of left‐censored data; P95: 95th percentile; LB: lower bound; UB: upper bound.
Due to the high proportion of left‐censored data (> 50%), the distribution of the LB concentrations is right‐skewed. Therefore, the LB median result is zero.
3.2.1.1. Analytical methods
Information on the analytical methods used to analyse nickel was provided for 43% of the data included in the final data set. Most of the analytical results (32%) were obtained using ICP‐based analytical methods with different detection techniques: ICP‐MS (low or high resolution MS) and ICP‐OES/ICP‐AES. Approximately 10% of data were measured by AAS, either reported without information or with information on the atomising unit, namely GF‐AAS. Electrochemical and spectroscopic methods were reported for less than 1%. For the remaining samples, no information on the analytical method was reported.
The distribution of the LOQs across the FoodEx Level 1 food categories is summarised in Annex C, Table C.3. A variety of median LOQs was noted across the food categories with the lowest median LOQ of 1.0 μg/kg observed for ‘Drinking water’ and the highest median LOQ of 150 μg/kg observed for ‘Products for special nutritional use’.
3.2.1.2. Occurrence data considered for dietary exposure assessment
An overview of the number of data points, the proportion of LCD as a percentage, the mean, median and 95th percentile (P95) concentration values of the FoodEx Level 1 food categories is presented in Table 7. More details on statistical description and according to lower FoodEx levels are reported in Annex C, Tables C.4–C.6.
The occurrence data on nickel covered 20 FoodEx Level 1 food categories. The highest nickel mean concentrations were measured for the category ‘Legumes, nuts and oilseeds’, in particular for soya beans, soya bean flour, chestnuts and cashew nuts, and for the food category ‘Products for special nutritional use’, in particular for plant extract formula and mineral supplements. High mean nickel concentration levels were also measured for food products belonging to the food categories ‘Sugar and confectionary’ (mainly driven by chocolate (cocoa) products), ‘Herbs, spices and condiments’ (mainly driven by different spices) and ‘Vegetables and vegetable products’ (mainly driven by cocoa beans/cocoa products, tea leaves and seaweed), while for other food categories the mean nickel levels were much lower.
It was noted that the mean LB nickel levels in drinking water are twice as high as those reported in the previous assessment (EFSA CONTAM Panel, 2015). This difference is associated with the selection of the LOQ cut‐off; for the current assessment the LOQ cut‐off of 6 μg/kg was selected (for more detail see Section 3.2.1) while the LOQ cut‐off of 4 μg/kg was selected for the previous assessment. Consequently, samples analysed using a method with an LOQ between 4 and 6 μg/kg were included in the current data set. It was noted that some of these samples, which were excluded in the previous assessment due to LOQs above 4 μg/kg, had a high nickel concentration measured. This explains the mean LB nickel levels in drinking water being twice as high as those reported in the previous assessment.
3.2.2. Previously reported occurrence data in the open literature
The CONTAM Panel reviewed previously reported occurrence data on nickel in food and drinking water in 2015 and concluded that in general, foods contained less than 500 μg Ni/kg. Foods with the highest mean concentrations of nickel were wild mushrooms, cocoa and cocoa products, beans, seeds, nuts and grains. In breast milk and waters, nickel concentrations are generally below 10 μg/L (EFSA CONTAM Panel, 2015).
For its dietary exposure assessment, the CONTAM Panel in 2015 used a data set of 18,885 food samples and 25,700 drinking water samples. The samples were collected between 2003 and 2012 in 15 different European countries, with almost 80% of the total collected in one Member State. In accordance with the scientific literature, high mean levels of nickel were reported for ‘Legumes, nuts and oilseeds’ (~ 2,000 μg/kg), certain types of chocolate (cocoa) products (3,800 μg/kg), and ‘Cocoa beans and cocoa products’ (9,500 μg/kg) (EFSA CONTAM Panel, 2015).
Babaahmadifooladi et al. (2020b) reviewed the scientific literature for data on the occurrence of nickel, including the EFSA Opinion from 2015 and concluded that the foods with high nickel content are mostly of plant‐based origin, e.g. legumes, soya‐based products and nuts, compared with foods of animal origin such as meat, fish, and honey, which have lower nickel concentrations.
In addition to this recent review, the CONTAM Panel noted that some new studies regarding the occurrence of nickel in food in Europe have become available. Examples of such studies are summarised below. When the CONTAM Panel was aware of duplication between data submitted to EFSA and data reported in the scientific literature, the data were only included in the data submitted to EFSA. However, no systematic check was done for possible duplication and this might have resulted in a partial overlap between Sections 3.2.1 and 3.2.2.
Different food samples (n = 291) were analysed in the French total diet study (TDS) on infants and toddlers to collect occurrence data on metals and metalloids (Chekri et al., 2019). Nickel was quantified in 37% of the samples. In infant foods, the highest mean nickel concentrations were observed in meat/fish‐based ready‐to‐eat meals (75.7 μg/kg), vegetable‐based ready‐to‐eat meals (71.5 μg/kg), soups/purees (57.7 μg/kg) and fruit purees (54.7 μg/kg). In common foods, the highest mean concentrations were found in sweet and savoury biscuits and bars (n = 1; 527 μg/kg), dairy‐based desserts (388 μg/kg), croissant‐like pastries (173 μg/kg) and hot beverages (n = 1; 96.2 μg/kg). These high mean concentrations were mainly due to the contribution of samples containing chocolate.
In a 2014, TDS carried out in the UK, the highest concentration of nickel was detected in nuts (2,140 μg/kg) and poultry (290 μg/kg). In the previous TDS, nuts also had the highest concentration with a level of 3,020 μg/kg (FERA, 2015).
Rubio et al. (2018) analysed 31 samples of Blue Jack mackerel muscle obtained from markets in the Canary Islands. Nickel was quantified in all samples, with a mean concentration of 110 μg/kg wet weight (ww) and ranging from 30 to 350 μg/kg. Nickel was also analysed in seafood samples collected from five marine ecosystems in Europe (Norway, Spain, Portugal, Italy and the Netherlands). It was only detected in mussels collected in the Po Delta with a mean concentration of 7,100 μg/kg dry weight (dw) (Maulvault et al., 2015). Fish samples from nine different species collected from local fish markets at the Romanian Black Sea coast were analysed for their metal content. Nickel was detected in the muscle of all fish species with the highest average concentration in Mediterranean horse mackerel (330 μg/kg ww) and European pilchard (300 μg/kg ww) (Plavan et al., 2017). A total of 50 mussel and 40 clam samples were collected at the Milan fish market. Nickel was detected in all mussel samples (mean concentration: 960 μg/kg) and in 39 clam samples (mean concentration of the positive samples: 1,230 μg/kg) (Chiesa et al., 2018). In another Italian study, fish and shellfish samples (n = 30/species) of the Gulf of Catania were analysed and mean concentrations ranged between 42 and 196 μg/kg ww (Copat et al., 2018). In Poland, three farmed fish species were analysed for their nickel content as fresh fish (n = 18) and after processing (smoking or marinating; n = 15). Nickel was in all the samples below the LOD (0.0105 μg/L) (Cieslik et al., 2018).
Nickel was analysed in samples of 11 different vegetables grown in Serbia. The highest average nickel concentrations were reported for spinach (2,200 μg/kg), broccoli (1,700 μg/kg) and tomatoes (1,500 μg/kg) (Pajević et al., 2018). Samples of 12 vegetables were collected in La Rochelle, France, and analysed for their nickel concentration. Nickel was detected in all samples and the concentration ranged from 200 to 1,050 μg/kg (Cherfi et al., 2016).
Beer samples (n = 148) taken at the Belgian market were analysed for their nickel content. The results were submitted to EFSA and are therefore not further discussed in this section. However, the authors performed further analyses which are reported below. No correlation was identified between the nickel content and the indicated alcohol percentage, nor between the nickel concentration and the type of brewing process (top‐fermented beers of high alcohol percentage, pilsner beers and sour beers). Further analysis of top‐fermented beers showed that the yeast fraction contained a higher amount of nickel than the supernatants which could be due to a bioaccumulation of nickel in the yeast cells (Babaahmadifooladi et al., 2020a).
Overall the results reported in the scientific literature are in line with the concentrations reported to EFSA.
Release of nickel from food contact materials
Nickel may be released from food contact materials, including packaging material, cooking utensils and storage containers, which may result in additional exposure.
Nickel can be released from coffee machines, generally at low concentration, however concentrations above the SRL (up to 780 μg/kg) have been reported after decalcification in 2 out of 8 machines (Müller et al., 2015). The authors pointed to the importance of sufficient rinsing after decalcification. Only low releases (maximum: 4.9 μg/L) were detected in the same study when water was boiled in electric kettles (n = 11).
Storage for 72 h of lemon juice in new and used cast iron containers resulted in nickel concentrations up to 28 and 514 μg/L, respectively. When storing water in the containers, the concentrations did not exceed the LOD (0.3 μg/L). (Khaniki et al., 2016). The CONTAM Panel noted the limited reporting regarding the used containers as well as the limited applicability of the procedures for actual use.
Nickel migration has been reported in canned vegetables (beans, chickpeas and okra). The nickel concentration was stable during the first half of the shelf‐life but significant small concentration increases were observed during the second half. For example, in one brand of fava beans, the concentration after 493 days of storage at 22°C was 500 μg/kg for a concentration of 410 μg/kg after 165 days. In one brand of chickpeas, the concentration after 493 days of storage was 1,110 μg/kg for a concentration of 770 μg/kg after 165 days (Noureddine El Moussawi et al., 2019). In another study, a large number of foods packed in different materials were sampled and analysed for their nickel content; no effect of the packaging material was found (Babaahmadifooladi et al., 2020a).
Nickel migration was also studied in tea brewed in traditional metallic and stainless steel teapots (19 samples of teapots; three items per sample; old and new samples). The nickel concentration in the tea ranged from 41 to 209 μg/L. However, in one sample, the concentration was 856 μg/L. In comparison, when using a pyrex glass recipient, the mean nickel concentration was 32 μg/L. Nickel was also measured in tea samples from Moroccan families (n = 14) and oriental tearooms (n = 11) in Brussels. The nickel concentration ranged from 14 to 187 μg/L and from 46 to 152 μg/L, respectively (Petit et al., 2013).
Guarneri et al. (2016) assessed the release of nickel from 18/1021 stainless steel pots during common cooking conditions. Tomato sauce and lemon marmalade were cooked for 1 h in used pots from three different stainless steel brands. The pots had been used in a domestic setting for 10–12 years. Cooking tomato sauce for 1 h resulted in releases of 66, 144 and 98 μg/L and the maximum concentration found after 1 h of cooking (basal level in the food and the release from the pots) was 148 μg/L. Cooking lemon marmalade for 1 h resulted in releases of 38, 77 and 34 μg/L and the maximum concentration found after 1 h of cooking (basal level in the food and the release from the pots) was 80 μg/L.
These results are in contradiction with the results from Flint and Packirisamy (1995, 1997) who only observed an increase in the nickel concentration for the first and/or second cooking operation when using new stainless steel pots for cooking acidic foods. The authors concluded that the contribution of stainless steel cooking utensils to nickel in the diet is negligible.
Szynal et al. (2016) reported the migration results from ceramic (n = 172) and glass (n = 52) tableware using food simulants. The frequency of positive results and the amount of Ni that leaches into the food simulants is low (i.e. one sample in which nickel was quantified (LOQ = 20 μg/L) at a concentration of 40 μg/L).
In addition to the information identified in the scientific literature, EFSA received results of migration tests using food simulants, from the Belgian and Swiss competent authorities. Belgium provided results from 198 analyses of materials in alloys and aluminium carried out in 2018 and 165 in 2019. Per sample, three items were analysed and one analysis corresponds to one sample. In both years, three analyses (corresponding to one sample) were non‐conform (i.e. concentration > SRL of 140 μg/kg). The detected concentration was for both samples 1,000 μg/kg (FASFC, 2019). Switzerland provided results on 100 samples (3 items/sample) of cooking utensils in metals and alloys and exceedance of the SRL was observed in 19 samples (concentration range: 170‐4,570 μg/kg) (FSVO, 2019).
In general, concentrations of nickel following migration are in the same order of magnitude as concentrations reported to occur in food (see Section 3.2.1.2). Differences are observed between studies, which may reflect a difference in quality of food contact materials. The CONTAM Panel considered the available database too limited to draw up a scenario on dietary exposure to nickel resulting from food contact material.
3.3. Dietary exposure assessment for humans
3.3.1. Current dietary exposure assessment
3.3.1.1. Current chronic dietary exposure assessment
The CONTAM Panel assessed the dietary exposure to nickel following the methodology described in Section 2.6.
A summary of the nickel occurrence data including the number of results, percentage of LCD and mean concentrations for the food categories at the FoodEx level as used for exposure assessment is presented in Annex C, Table C.7.
Overall, the CONTAM Panel noted that a high proportion of LCD was reported for some food categories. The exposure is likely to be underestimated with the LB approach and overestimated with the UB approach. This particularly applies to chronic dietary exposure estimates, whilst the acute dietary exposure estimates are overestimated as based on UB occurrence data only.
Mean and high chronic dietary exposure
Table 8 shows summary statistics for the assessment of chronic dietary exposure to nickel. Detailed mean and 95th percentile dietary exposure estimates calculated for each of the 44 dietary surveys are presented in Annex D, Table D.1.
Table 8.
Summary statistics for chronic dietary exposure to nickel (μg/kg bw per day) across European countries
| Age class | Minimum | Median | Maximum | |||
|---|---|---|---|---|---|---|
| LB | UB | LB | UB | LB | UB | |
| Mean dietary exposure in total population (μg/kg bw per day) | ||||||
| Infants | 3.05 | 4.25 | 4.40 | 6.14 | 8.31 | 9.71 |
| Toddlers | 6.23 | 7.77 | 8.53 | 10.1 | 12.5 | 14.6 |
| Other children | 4.69 | 5.42 | 7.05 | 8.16 | 8.97 | 10.1 |
| Adolescents | 2.40 | 2.80 | 3.58 | 4.27 | 5.56 | 6.44 |
| Adults | 1.83 | 2.20 | 2.90 | 3.41 | 3.65 | 4.19 |
| Elderly | 1.57 | 1.89 | 2.51 | 2.99 | 3.65 | 4.28 |
| Very elderly | 1.91 | 2.31 | 3.05 | 3.55 | 3.77 | 4.29 |
| 95th percentile dietary exposure in total population (μg/kg bw per day) | ||||||
| Infantsa | 6.19 | 7.91 | 9.81 | 12.8 | 28.1 | 29.9 |
| Toddlersa | 10.7 | 12.5 | 16.1 | 17.9 | 23.2 | 24.8 |
| Other children | 10.3 | 11.5 | 13.3 | 14.6 | 18.8 | 20.5 |
| Adolescentsa | 5.59 | 6.13 | 7.47 | 8.27 | 11.3 | 12.8 |
| Adults | 3.83 | 4.29 | 5.66 | 6.30 | 7.43 | 8.05 |
| Elderly | 3.55 | 4.12 | 4.98 | 5.56 | 6.83 | 7.69 |
| Very elderlya | 3.35 | 3.93 | 5.58 | 6.31 | 6.81 | 7.60 |
bw: body weight; LB: lower bound; UB: upper bound.
The 95th percentile estimates obtained on dietary surveys/age classes with fewer than 60 observations may not be statistically robust (EFSA, 2011b) and are therefore not included in this table.
The highest estimated chronic dietary exposure to nickel was in the young age groups. Concerning the mean dietary exposure, the highest estimated LB/UB exposure levels were in toddlers with a maximum exposure of 12.5/14.6 μg/kg bw per day. The highest 95th percentile LB/UB exposure was observed for infants with estimates of 28.1/29.9 μg/kg bw per day.
Dietary exposure in specific groups of the population, namely ‘Pregnant women’ and ‘Lactating women’, were within the range of exposure estimates for the adult population (see Annex D, Table D.1).
Contributions of different food groups to chronic dietary exposure
The contribution (%) of each food category to the total mean exposure of nickel was calculated for each age class and dietary survey. Estimations of exposure using the LB approach, which is considered to be less influenced by the value of the LOD/LOQ, were used to present the contribution of the different food categories. The contribution of individual food categories to the mean LB chronic dietary exposure to nickel varied between the dietary surveys. This is explained by the specific food consumption patterns in the individual European countries and even in different regions within a country.
The detailed contribution to the mean LB chronic dietary nickel exposure of the different food categories at FoodEx Level 1 and grouped by age class is shown in Annex D, Table D.2. The detailed contribution of the different food categories at the FoodEx Level as used for the exposure assessment and grouped by age class is shown in Annex D, Table D.3.
Overall, the food categories mainly contributing to the mean LB chronic dietary exposure to nickel across all age classes was ‘grains and grain‐based products’ with contributions reaching up to 49% in infants and toddlers. Bread and rolls had the highest contribution among the food subcategories belonging to ‘grains and grain‐based products’. The mean nickel concentration levels for ‘bread and rolls’ were not particularly high; therefore, it is likely that their high contribution is driven by high consumption rather than the presence of nickel. Also ‘fine bakery wares’ contributed considerably to the mean LB chronic dietary exposure to nickel, in particular in other children. The food category ‘non‐alcoholic beverages’ gave the second highest contribution except for young age groups, with a contribution reaching up to 47% in very elderly. For adult age groups, coffee beverages were the main contributor, and soft drinks and cocoa beverages for toddlers, other children and adolescents.
Several other food groups were also important contributors to the mean LB chronic dietary exposure to nickel. These included ‘Legumes, nuts and oilseeds’ contributing up to 36% in toddlers and within this food category in particular beans. The food category ‘Vegetables and vegetable products’ contributed up to 34% in infants, and among the sub‐categories pickled vegetables were an important contributor in particular for the adult age groups.
Among young age groups, the food category ‘Sugar and confectionary’ also made an important contribution, contributing up to 31% in adolescents. When analysing the subcategories, it was observed that this outcome was mostly driven by a contribution of chocolate (cocoa) products. In addition, for a few dietary surveys reporting high consumption of ready‐to‐eat soups, ‘Composite food’ was an important contributor to the mean LB chronic dietary exposure to nickel.
The contribution of ‘Drinking water’ was rather low (up to 3% in infants). When comparing the contribution from ‘bottled water’ and the other types of water (i.e. tap water, water ice, well water, drinking water unspecified), the contribution to the mean LB chronic dietary exposure to nickel from ‘bottled water’ was slightly higher.
The contribution of other food categories was minor. Despite relatively high nickel concentrations measured in ‘Herbs, spices and condiments’ and ‘Products for special nutritional use’, the exposure from these foods was small because of the low consumption recorded within the dietary surveys.
Exposure of infants through breastfeeding
In 2015, the CONTAM Panel estimated the exposure of breastfed infants from human milk (EFSA CONTAM Panel, 2015; see Section 3.3.2). No recent studies reporting nickel concentrations in human milk samples from the European population were identified and therefore, no new estimations were performed.
3.3.1.2. Current acute dietary exposure assessment
Mean and high acute dietary exposure
Table 9 summarises the range of mean and 95th percentile UB acute exposures to nickel across different age classes and dietary surveys. Detailed mean and 95th percentile UB acute exposure estimates for each dietary survey across age classes with their corresponding confidence intervals (2.5th and 97.5th percentiles) are described in Annex D, Table D.4.
Table 9.
Range of mean and 95th percentile acute dietary exposurea to nickel across European dietary surveys
| Age class | Number of dietary surveys | Range of mean acute exposure (μg/kg bw per day) | |
|---|---|---|---|
| Minimum | Maximum | ||
| Infants | 13 | 4.25 (4.06–4.45) | 9.17 (8.40–10.2) |
| Toddlers | 17 | 7.77 (7.51–8.06) | 14.6 (12.6–16.7) |
| Other children | 21 | 5.42 (5.17–5.67) | 10.5 (9.91–11.1) |
| Adolescents | 23 | 2.81 (2.61–3.03) | 7.08 (6.75–7.47) |
| Adults | 25 | 2.21 (2.03–2.48) | 4.69 (4.57–4.83) |
| Elderly | 22 | 1.89 (1.75–2.07) | 4.28 (4.07–4.52) |
| Very elderly | 17 | 2.31 (2.15–2.51) | 4.30 (4.12–4.48) |
| Range of 95th percentile acute exposure (μg/kg bw per day) | |||
| Minimum | Maximum | ||
| Infants | 13 | 12.0 (10.8–13.3) | 32.4 (28.0–38.0) |
| Toddlers | 17 | 18.1 (16.9–19.5) | 40.8 (30.6–54.1) |
| Other children | 21 | 15.1 (13.8–16.5) | 28.0 (25.8–30.4) |
| Adolescents | 23 | 8.26 (7.15–9.48) | 18.1 (15.5–20.9) |
| Adults | 25 | 6.04 (5.15–7.15) | 11.6 (10.9–12.3) |
| Elderly | 22 | 5.35 (4.54–6.40) | 11.8 (10.8–12.9) |
| Very elderly | 17 | 5.57 (4.95–6.30) | 12.0 (11.1–13.0) |
bw: body weight.
With their corresponding confidence intervals (2.5th and 97.5th percentiles).
Overall, the young age groups (infants, toddlers and other children) showed higher acute exposure to nickel than the other age classes. Mean acute exposure ranged from a minimum of 1.89 μg/kg bw per day estimated in the elderly up to a maximum of 14.6 μg/kg bw per day estimated in toddlers. The 95th percentile acute exposure ranged from a minimum of 5.35 μg/kg bw per day estimated in the elderly up to a maximum of 40.8 μg/kg bw per day estimated in toddlers.
Mean acute exposure estimates did not differ much from those calculated for the mean chronic exposure to nickel. This can be explained by the fact that nickel is present in many different foods which are regularly consumed.
Acute dietary exposure in the dietary surveys covering pregnant and lactating women were within the range of exposure estimates in the adult population (see Annex D, Table D.4).
Contributions of different food groups to acute dietary exposure
The food categories having the most important contribution to the acute dietary exposure to nickel were determined across age classes and dietary surveys based on the 95th percentile exposure levels estimated. The most relevant food categories varied considerably between the surveys and age classes, which is explained by the specific food consumption patterns in the individual European countries and age classes. For infants, the most relevant foods involved in the acute exposure to nickel were grain‐based products (in particular, breakfast cereals, oat milling products and cereal flakes), ready‐to‐eat meals and in some surveys also the infant formulae. For toddlers, the most relevant foods involved in the acute exposure to nickel were also very variable across the dietary surveys, including beans, ready‐to‐eat soups, chocolate, breakfast cereals and cereal flakes. More homogenous pattern was observed for other children and adolescents; for these age categories the most relevant food categories were beans and chocolate and in one survey also fruit and vegetable juices. Among adult population groups (adults, the elderly and very elderly), the most relevant foods involved in the acute exposure to nickel were beans, coffee, ready‐to‐eat soups, chocolate, breakfast cereals, and in one particular survey also pickled vegetables. The detailed 95th percentile acute exposure levels to nickel of the different food categories at the FoodEx level as used for the exposure assessment and grouped by age classes and dietary surveys are shown in Annex D, Table D.5.
In addition, the contributions (%) of food categories as used for the exposure assessment to the total mean acute exposure of nickel were calculated for each age class and dietary survey and the results are presented in Annex D, Table D.6. Overall, the main contributors were similar as those described for the chronic exposure.
3.3.1.3. Additional specific acute scenarios
Acute exposure from seaweed
The nickel concentrations measured in seaweed were particularly higher as compared to most other vegetable products (see Annex C, Tables C.5 and C.6). Such finding together with the increasing popularity of these specific food products in Europe may represent an important health issue. The exposure to nickel from seaweed may be of particular concern in case of large consumption within a very short period (one day). Therefore, the CONTAM Panel decided to evaluate the acute exposure to nickel from seaweed (for more detail see Section 2.6).
Given the limited number of consuming days available in the Comprehensive Database, the Panel focused only on the surveys where the seaweed consumption was recorded for at least 12 consuming days. The calculated high exposure levels were based on the 75th percentile identified as the highest reliable percentile. Finally, calculations were possible for four different dietary surveys in adults carried out in four European countries.
The 75th percentile estimates for acute dietary exposure to nickel from seaweed across dietary surveys ranged from 0.15 to 5.04 μg/kg bw per day. Detailed 75th percentile dietary exposure estimates calculated for each of the selected dietary surveys are presented in Annex D, Table D.7.
Given the limited consumption data availability the results are only indicative and do not allow to draw firm conclusions.
Acute exposure from pasta containing seaweed
In response to high concentration levels of nickel measured in seaweed and an increasing popularity of the specific types of pasta containing the seaweed in Europe, the CONTAM Panel considered it of interest to address the issue of nickel exposure from this food products (for more details, see Section 2.6).
The mean estimates for acute dietary exposure to nickel from pasta containing seaweed across dietary surveys and age classes ranged from 0.02 μg/kg bw per day observed in very elderly to 3.81 μg/kg bw per day observed in infants. The 95th percentile ranged from 0.20 μg/kg bw per day in the elderly to 10.4 μg/kg bw per day in toddlers. Detailed mean and 95th percentile dietary exposure estimates calculated for each of the selected dietary surveys are presented in Annex D, Table D.8.
Acute exposure from drinking water
Nickel bioavailability is higher under fasted condition and when nickel is ingested without food (see Section 3.1.1). Such conditions apply when drinking water on an empty stomach. Therefore, the CONTAM Panel estimated the dietary exposure to nickel from a small bottle of water (500 mL) containing a high concentration of nickel (for more detail see Section 2.6). Under this specific scenario, the acute exposure from tap water was 0.04 μg/kg bw and 0.08 μg/kg bw from bottled water. It was concluded that the exposure from drinking a small bottle of water is low.
3.3.2. Previously reported dietary exposure
In 2015, the CONTAM Panel reviewed previously reported dietary exposure assessments in European countries showing that mean adult exposure ranged from 90 to 361 μg/day. Assuming a body weight of 70 kg, this range corresponds to 1.3–5.2 μg/kg bw per day. The Panel also assessed chronic and acute dietary exposure using occurrence data submitted to EFSA. Mean chronic dietary exposure to nickel, across the different dietary surveys and age classes, ranged from 2.0 to 13.1 μg/kg bw per day (minimum LB–maximum UB). The 95th percentile dietary exposure ranged from 3.6 to 20.1 μg/kg bw per day (minimum LB–maximum UB). The highest chronic dietary exposure to nickel was observed for toddlers and other children. The main contributors were ‘Grain and grain‐based products’, ‘Non‐alcoholic beverages (except milk‐based beverages)’, ‘Sugar and confectionery’, ‘Legumes, nuts and oilseeds’, and ‘Vegetables and vegetable products (including fungi)’. ‘Milk and dairy products’ were also important contributors to the dietary exposure to nickel in the young population, in particular in toddlers. The contribution of ‘Drinking water’ was very small. Vegetarians seem to have slightly higher dietary exposure to nickel than the general population. However, it should be noted that the calculated exposures were based on very limited consumption data. Mean dietary acute exposure in the young populations (infants, toddlers, other children and adolescents) ranged from 3.4 (95% CI: 3.1–3.7) μg/kg bw in one survey for adolescents to 14.3 (95% CI: 13.2–15.5) μg/kg bw in one survey for toddlers. The 95th percentile ranged from 8.6 (95% CI 8.0–9.1) μg/kg bw in one survey for adolescents to 35.0 (95% CI 26.8–47.2) μg/kg bw in one survey for toddlers. In the adult populations, mean dietary acute exposure ranged from 2.5 (95% CI 2.2–2.9) μg/kg bw in one survey for elderly to 4.9 (95% CI: 4.6–5.5) μg/kg bw in one survey for adults. The 95th percentile ranged from 5.5 (95% CI: 5.1–6.0) μg/kg bw in one survey for elderly to 11.8 (95% CI: 10.6–13.8) μg/kg bw in one survey for adults. Possible exposure due to leaching of nickel into food from food contact material was not included in this dietary exposure assessment (EFSA CONTAM Panel, 2015).
The CONTAM Panel also estimated the exposure of infants via human milk (EFSA CONTAM Panel, 2015). An average daily milk consumption of 800 mL and a high consumption of 1,200 mL was used for an infant of three months (6.1 kg bw) that is exclusively breast‐fed. Considering the highest reported average concentration of nickel in human milk (43.9 μg/L), the mean dietary exposure was estimated to be 5.8 μg/kg bw per day. For breastfed infants with high milk consumption, the exposure was estimated to be 8.6 μg/kg bw per day. The CONTAM Panel noted that lower or similar exposure to nickel is expected in breastfed infants as compared to non‐breastfeeding infants.
Babaahmadifooladi et al. (2020b) reviewed the scientific literature for data on the dietary exposure to nickel. However, no recent studies conducted in Europe were included and so this review is not further discussed in this Opinion. The CONTAM Panel noted that some new studies regarding the dietary exposure of nickel from food in Europe have become available. Examples of such studies are summarised below.
A TDS was conducted in France between 2010 and 2016 to assess the dietary exposure of infants and toddlers (Sirot et al., 2018). The mean LB exposure of children under 3 years of age was between 0.4 and 2.7 μg/kg bw per day and the P90 LB between 1.3 and 4.6 μg/kg bw per day. Chocolate‐based products contributed 30–60% to the dietary exposure to nickel for 13–36 month‐old children (Sirot et al., 2018).
In the 2014 TDS carried out in the UK, a wide variety of foods was included to assess the dietary exposure to metals and other elements, including nickel. Children (1.5–3 years of age) had the highest mean and P97.5; being 4.4–5.2 μg/kg bw per day and 7.1–8.1 μg/kg bw per day, respectively. The food group with the highest contribution to the total mean exposure was the ‘Miscellaneous cereals’ group (FERA, 2015).
The Committee on Toxicology (COT) estimated dietary exposure of infants and children aged 1–5 years (COT, 2018). Chronic nickel exposure from exclusive breastfeeding of 0‐ to 6‐month‐old infants ranged from 0.01 to 6.4 μg/kg bw per day for average consumers (800 mL/day) and from 0.02 to 9.6 μg/kg bw per day for high consumers (1,200 mL/day). Mean estimated chronic exposure of children from infant formula, commercial infant foods and other foods was 1.3–5.6 μg/kg bw per day and the P97.5 was 2.8–8.7 μg/kg bw per day. The COT also estimated acute exposure from breast milk in 4–12‐month‐old infants up to 8.5 μg/kg bw per day. Overall, acute exposures of up to 12 μg/kg bw per day were calculated for children up to 5 years of age.
Dietary exposure of the Italian population to nickel has been assessed in the TDS carried out by the Istituto Superiore di Sanità in 2012–2014. The mean dietary exposure ranged from 1.5 to 4.6 μg/kg bw per day across age classes. The 95th dietary exposure ranged from 2.5 to 9.6 μg/kg bw per day. Both mean and 95th percentile dietary exposure was the highest for children and infants (Cubadda et al., 2020). The main contributors to the dietary exposure were cereals and cereal products (27%), sweet products (16%), vegetables (11%), potatoes (8%), fruit (7%) and pulses (6%) (Cubadda, 2020 personal communication).
The Finnish Food Authority assessed the dietary heavy metal and aluminium exposure of Finnish adults. The mean exposure to nickel was 2.53 and 2.16 μg/kg bw per day for people aged 25–64 years and 65–74 years, respectively. The main contributors to the dietary exposure of people aged 25–64 years were ‘cereals and cereal products’, ‘legumes, nuts and seeds’ and ‘sugar and sweets’. In the older age group (65–74 years), the main contributors were ‘cereals and cereal products’, ‘legumes, nuts and seeds’ and ‘fruit and berries’ (Suomi et al., 2020).
The dietary exposure of Polish students (n = 850) to nickel was assessed in 2006–2010 using 24‐h dietary recall and diet duplicates. In female students, the exposure was between 101 and 152 μg/day and in male students between 139 and 204 μg/day (Marzec et al., 2014). These exposures correspond to 1.68–2.42 μg/kg bw per day for females and 1.99–2.60 μg/kg bw per day for males (Koch, 2019 personal communication).
Koch et al. (2016) assessed the dietary exposure of 583 healthy adults aged 19–30 years living in the eastern part of Poland using 24‐h dietary recall and a market basket method. The study was performed in 2011–2013. The mean dietary exposure to nickel of females was 384 μg/day and for males 455 μg/day. The corresponding exposures on a body weight basis were 6.18–6.82 μg/kg bw per day (women; range) and 6.30–8.09 μg/kg bw per day (men; range) (Koch, 2020 personal communication).
In Poland, Bartos et al. (2014) observed a mean nickel intake of 227 μg/day in a group of students aged 20–25 years. The same study conducted among senior researchers aged 40–50 years from the same university indicated a mean nickel intake of 161 μg/day.
In addition, several scientific papers reporting dietary exposure from one or a few food groups in Europe were identified. However, these were not included in the Opinion.
3.3.3. Non‐dietary sources of exposure
For both smokers and non‐smokers not‐occupationally exposed to nickel, exposure by inhalation may be expected in general to represent a negligible or minor addition to the daily exposure via the diet (EFSA CONTAM Panel, 2015).
The COT estimated exposure to nickel from air of infants and young children and calculated an exposure ranging from 0.00014 to 0.042 μg/kg bw per day. In addition, ingestion of dust and soil may add to the oral exposure to nickel. The COT estimated for infants and young children a possible nickel exposure from dust to be between 0.18 and 0.55 μg/kg bw per day. Ingestion of soil may result in an exposure of these age groups ranging from 0.071 to 0.2μg/kg bw per day (COT, 2018).
Additional non‐dietary exposure may result from the use of nickel in the production of many varieties of iron–nickel alloys, in countless industrial and consumer products, in electroplating, in pigments and colours for ceramics and glassware, in marine anti‐fouling agents, and in alloys with aluminium, cobalt, chromium, copper, gold, lead, silver and titanium (EFSA CONTAM Panel, 2015). Nickel may be present in white gold and in inexpensive alloys used for fashion or jewellery, including piercings. A nickel flash may also be used in the silver or gold plating process of such jewellery (Bocca et al., 2007). The presence of nickel in these types of products may result in dermal exposure and consequently sensitisation.
3.4. Risk characterisation
3.4.1. Chronic effects
The mean and 95th percentile chronic dietary exposures to nickel (see Table 8) were compared with the TDI of 13 μg/kg bw.
Mean chronic dietary exposure was the highest for the young age groups and particularly for toddlers. The mean LB chronic dietary exposure for toddlers ranged from 6.23 to 12.5 μg/kg bw and the mean UB from 7.77 to 14.6 μg/kg bw per day, across dietary surveys. For one survey in toddlers, the mean chronic dietary exposure was at the level of the TDI (LB–UB: 12.5–14.6 μg/kg bw per day) and this may indicate a concern. However, the Panel noted that this particular survey included only 36 subjects (see Annex D, Table D.1) For all other age classes, the mean LB and UB chronic dietary exposure was below the TDI and does not indicate a concern.
The 95th percentile chronic dietary exposure was also the highest for the young age groups and particularly for toddlers. The 95th percentile LB chronic dietary exposure for toddlers ranged from 10.7 to 23.2 μg/kg bw and the 95th percentile UB from 12.5 to 24.8 μg/kg bw per day, across dietary surveys. The 95th percentile LB chronic dietary exposure exceeded the TDI in 10 out of 14 dietary surveys in toddlers and in 11 out of 19 dietary surveys in other children. Also in infants, an exceedance of the TDI was observed in some surveys. For adults, the 95th percentile LB chronic dietary exposure for toddlers ranged from 3.83 to 7.43 μg/kg bw per day and the 95th percentile UB from 4.29 to 8.05 μg/kg bw per day. In the adolescents and all adult age groups, the 95th percentile chronic dietary exposure was below the TDI. The 95th percentile chronic dietary exposure exceeds the TDI in several dietary surveys in the young age groups (infants, toddlers and other children). In general, the difference between LB and UB estimates is rather small and the exceedance of the TDI is not due to a high proportion of LCD and high LOQs. The CONTAM Panel concluded that the 95th percentile chronic dietary exposure to nickel may raise a health concern for the young age groups. The CONTAM Panel noted that this risk characterisation is conservative and thus will overestimate the risk, as the critical effect for the TDI, post‐implantation loss, is not a relevant effect for young age groups. The TDI is also protective for effects that might occur in these age groups as no effects of relevance for young age groups have been reported at the reference point identified for the derivation of the TDI.
3.4.2. Acute effect
The CONTAM Panel selected a LOAEL of 4.3 μg Ni/kg bw as the reference point for the acute oral exposure to nickel and decided to apply an MOE approach. The Panel considered that an MOE of 30 or higher would be indicative of a low health concern.
Comparison of the mean UB acute dietary exposure to nickel reported above (Table 9) to the LOAEL of 4.3 μg Ni/kg bw, results in MOE values that range from 0.3 to 2.3 across dietary surveys and age classes (Table 10). For the young age groups (i.e. infants, toddlers and other children) all calculated MOEs are equal to or below 1. The MOEs values when using the 95th percentile UB acute dietary exposure range from 0.1 to 0.8 across dietary surveys and age classes.
Table 10.
Margins of exposure based on acute dietary exposure across dietary surveys and age classes for SCD elicitation in nickel‐sensitised individuals
| Age class | MOE calculated from mean acute dietary exposure | MOE calculated from P95 acute dietary exposure | ||
|---|---|---|---|---|
| Minimuma | Maximumb | Minimuma | Maximumb | |
| Infants | 1.0 | 0.5 | 0.4 | 0.1 |
| Toddlers | 0.6 | 0.3 | 0.2 | 0.1 |
| Other children | 0.8 | 0.4 | 0.3 | 0.2 |
| Adolescents | 1.5 | 0.6 | 0.5 | 0.2 |
| Adults | 1.9 | 0.9 | 0.7 | 0.4 |
| Elderly | 2.3 | 1.0 | 0.8 | 0.4 |
| Very elderly | 1.9 | 1.0 | 0.8 | 0.4 |
MOE: margin of exposure; P95: 95th percentile.
MOE calculated based on minimum dietary exposure across dietary surveys.
MOE calculated based on maximum dietary exposure across dietary surveys.
The CONTAM Panel concluded that the calculated MOEs raise a health concern for nickel‐sensitised individuals.
The CONTAM Panel elaborated also a few scenarios of acute exposure, each representing a specific situation of dietary exposure to nickel, including a specific scenario on drinking water consumption (see Section 3.3.1.3).
Seaweed contains relatively high nickel concentrations (see Section 3.2.1.2 and Annex C, Table C.5 and C.6). The UB 75th percentile estimates for acute dietary exposure to nickel from seaweed ranged from 0.15 to 5.04 μg/kg bw per day across dietary surveys. The corresponding MOE range is 0.9–29.
Seaweed is also used for the production of seaweed products like seaweed pasta. Therefore, a scenario was elaborated to estimate the nickel exposure from this product. The mean UB estimates for acute dietary exposure to nickel from pasta containing seaweed across dietary surveys and age classes ranged from 0.02 to 3.81 μg/kg bw per day. The 95th percentile UB acute dietary exposure estimates across dietary surveys and age classes ranged from 0.20 to 10.4 μg/kg bw per day. The MOE values calculated from the mean UB acute dietary exposure for this scenario were in the range 1.1–215 and in the range 0.4–21.5 for the 95th percentile UB acute dietary exposure.
These scenarios indicate that high consumption of seaweed and seaweed pasta by nickel‐sensitised individuals would raise a health concern. However, the CONTAM Panel noted that these scenarios were elaborated with limited data and the results are therefore only indicative and do not allow to draw any firm conclusions.
Bioavailability of nickel under fasted conditions is higher compared to the ingestion with food. Therefore, a scenario was elaborated to estimate the dietary exposure when drinking a small bottle of water (500 mL) containing a high concentration of nickel (see Section 2.6) under fasted conditions. The acute exposure from tap water was 0.04 μg/kg bw and 0.08 μg/kg bw from bottled water. The corresponding MOE values were 120 and 55, respectively. These MOE values do not raise a health concern.
3.5. Uncertainty analysis
The evaluation of the inherent uncertainties in the assessment of exposure to nickel in food and drinking water has been performed following the guidance of the Opinion of the Scientific Committee related to uncertainties in dietary exposure assessment (EFSA, 2007). In addition, the report ‘Uncertainty and Data Quality in Exposure Assessment’ has been considered (WHO/IPCS, 2008). According to the guidance provided in the EFSA Opinion (2007) the following sources of uncertainty have been considered: assessment objectives, exposure scenario, exposure model, and model input (parameters).
3.5.1. Assessment objectives
The objectives of the assessment were clearly specified in the terms of reference.
3.5.2. Exposure scenario/exposure model
The exposure assessment was based on nickel occurrence data collected in numerous European countries; however, most of them (66%) were collected in only one country, while other countries contributed far less data. There is uncertainty around possible regional differences in nickel contamination and the data set is likely not fully representative of the EU market.
When considered appropriate, occurrence data and consumption events for solid forms of certain foods (e.g. coffee beans, infant formulas, etc.; for more detail see Section 2.6) were adjusted by an appropriate dilution factor. Assumptions applied for this conversion may, however, not be accurate and representative for all possible commercial products. This may lead to an overestimation or underestimation of exposure. For the adjusted data, it was not considered appropriate to assume that the water or milk used for dilution would always contain nickel. However, this could result in an underestimation of exposure.
Exposure from nickel released from food contact materials (including packaging material, cooking utensils and storage containers) was not considered due to lack of solid scientific information. This could result in an underestimation of exposure.
A high proportion of LCD was reported for some food categories. However, these food categories were not the main contributors to the dietary exposure and consequently the difference between LB and UB estimates is rather small. The use of the LB in this Opinion tends to underestimate, while the UB tends to overestimate the dietary exposure. This uncertainty particularly applies to chronic dietary exposure estimates. The acute dietary exposure estimates are based on UB occurrence data only and tend to be an overestimation. Given, the small difference between the LB and UB nickel concentrations for major contributors to the acute dietary exposure, this overestimation is considered to be low. The limited number of available analytical results for some food categories adds uncertainty to the representativeness of the mean concentration values used to estimate the exposure.
The results of the additional acute exposure scenarios for seaweed and seaweed pasta (see Section 3.3.1.3) should be considered as indicative due to the limited occurrence and consumption data.
Uncertainties and limitations related to the use of the EFSA Comprehensive Food Consumption Database have already been described by EFSA (2011b) and are not further detailed in this Opinion.
3.5.3. Model input (parameters)
Four European standardised methods for the determination of total nickel in water are available and only one standardised method for food, namely for animal and vegetable fats and oils. Several standards, certified reference materials and regular proficiency testing schemes are available for total nickel in food and water. The analytical results used for the exposure assessment were generated by different laboratories using different analytical methods with varying LODs and LOQs. These limitations may have added to the overall uncertainty of the analytical results.
3.5.4. Other uncertainties
Nickel is usually measured in food as total nickel and there are only few studies of nickel speciation in food. It is generally assumed that nickel occurs in food in the form of complex bound organic nickel. Nickel can also be present in the environment as nickel nanoparticles and possibly also in food; however no information is available. Complex bound organic nickel and nickel nanoparticles have different physico‐chemical and possibly different biological properties than inorganic nickel.
Nickel absorption from the gastrointestinal tract is dependent on the chemical form and thus, the solubility of the nickel compound. Absorption may be suppressed by binding or chelating substances, competitive inhibitors, or redox reagents. On the other hand, absorption is often enhanced by substances that increase pH, solubility, or oxidation, or by chelating agents that are actively absorbed. Limited data are available on oral bioavailability for humans and even more for experimental animals. The available human data indicate a lower oral bioavailability when nickel is administered in the presence of food (0.7–2.5%) compared with administration via drinking water in the absence of food, or in a fasted state (25–27%). However, the three relevant human studies only included a low number of individuals and furthermore, a considerable inter‐individual variability in the measured parameters was noted in these studies. Therefore, there is a high uncertainty regarding oral bioavailability of nickel from food and beverages including drinking water in humans. The pivotal study used for the acute hazard characterisation was conducted in fasted individuals. Consequently, the reference point used for the acute risk characterisation is representing a fasted condition while most dietary exposure results from food intake. This results in a high uncertainty in the acute risk assessment. Comparison of reported bioavailabilities under different conditions indicates that the acute risk might be overestimated.
A study in rats showed an absorption of around 10% when nickel sulfate or nickel chloride was administered in a 5% starch saline solution as vehicle. The pivotal study used for the chronic hazard characterisation is a gavage study in rats, which had free access to feed during the treatment period. Although such a condition is considered more representative for dietary exposure via food and beverages, comparison of reported bioavailabilities for humans and rats results in some uncertainty in the chronic risk assessment.
Regarding effects on male infertility, data indicate that rats are less sensitive than mice. Male infertility caused by exposure to nickel appears to be a result of oxidative stress, in part mediated by nickel complexation with protamine 2 in sperm chromatin which elevates ROS production. As well as oxidative stress, the modification of protamine 2 per se in sperm may also contribute to infertility. The fact that protamine 2 (and the ratio of protamine 2 to protamine 1) in human and mouse sperm is much higher compared to that of the rat might implicate the mouse to represent a better model than the rat in predicting the ability of nickel to induce human male infertility. However, the relative level of the antioxidant status of human testes will be an important determinant of susceptibility based on the role of ROS.
A few studies indicate that nickel can disturb the neurobehavioural functions in mice and rats. However, the CONTAM Panel noted that the dose levels resulting in neurotoxic effects in the experimental animal studies were higher than those resulting in developmental toxicity, i.e. the critical effect for the derivation of the reference point applied for the establishment of the TDI.
There are uncertainties associated with the information about adverse reactions in humans after ingestion of nickel. The outcome is based on three individual studies, all with a limited number of nickel‐sensitised individuals. The degree of sensitivity of these individuals is not known. The outcomes of these studies were expressed in different ways, i.e. as flare‐up reactions of already eczematous skin lesions, or as flare‐up reactions in addition to new skin reactions, which makes comparison of these studies difficult. Individuals were fasted before exposure to nickel and subsequent monitoring of the effects, which may not represent all types of nickel intake. The dose responses of these three studies could not be analysed using the BMD approach, and a LOAEL was used instead, giving rise to additional uncertainty. Finally, the generalised effects covered by the term SNAS have not been included in the risk assessment, as the symptoms are currently too undefined and no dose–response assessment is available. Whereas the pattern of nickel exposure may be different from drinking water after fasting, effects may be overestimated, not including SNAS may lead to an underestimation of the effects.
Regarding the mode of action, it is evident that oxidative stress and an elevation of ROS is involved in the range of toxicities of nickel observed. There is, however, an uncertainty regarding the level of oxidative stress required for adversity, which is dependent on the antioxidant status of the target cells. There is also uncertainty regarding the potential role of altered calcium ion channels and mitochondrial disturbance and associated apoptosis of Leydig cells contributing to male reproductive toxicity. These effects may be secondary to oxidative stress. The relative roles of a direct immune response vs an inflammatory response in immunotoxicity and specifically in allergenicity is unclear.
3.5.5. Summary of uncertainties
In Table 11, a summary of the uncertainty evaluation is presented, highlighting the main sources of uncertainty and indicating an estimate of whether the respective source of uncertainty might have led to an over‐ or underestimation of the exposure or the resulting risk.
Table 11.
Summary of qualitative evaluation of the impact of uncertainties on the risk assessment of nickel in food and drinking water
| Sources of uncertainty | Directiona |
|---|---|
| Extrapolation of the occurrence data to the whole of Europe | +/– |
| An additional nickel occurrence from water/milk used for dilution of solid foods not considered | – |
| An additional exposure from nickel released from food contact materials not considered | – |
| Limited number of subjects and lack of information on degree of sensitisation in the pivotal study for the acute risk assessment | +/– |
| Use of fasting condition in the pivotal study for the acute risk assessment | + |
| Not including systemic nickel allergy syndrome in the risk assessment | – |
| Uncertainty in the reference point for acute effects: use of LOAEL that results in a high incidence (40%) of skin reactions | – |
+ = uncertainty with potential to cause overestimation of exposure/risk; – = uncertainty with potential to cause underestimation of exposure/risk.
The CONTAM Panel concluded that the uncertainties in the risk assessment of acute exposure to nickel in food and drinking water are larger than for the chronic exposure. The CONTAM Panel considered that the use of fasting condition in the pivotal study is a major source of uncertainty and therefore the assessment is more likely to overestimate than to underestimate the risk.
4. Conclusions
Hazard identification and characterisation
Toxicokinetics
Limited data are available on bioavailability in humans and experimental animals.
In humans, the bioavailability of nickel following ingestion depends on the solubility of the administered nickel compound, the dosing vehicle and the fasting state of the subject. A low absorption (0.7–2.5%) was reported when nickel was ingested in the presence of food or under a non‐fasted state, whereas a higher absorption (25–27%) was reported when nickel was ingested via drinking water in the absence of food, or under a fasted state.
A study in rats showed an absorption of around 10% when soluble nickel compounds were administered in a 5% starch saline solution as vehicle.
After absorption, nickel is widely distributed in the organism.
In a study with mice, nickel was shown to cross the placenta.
There are indications of transport across the blood–brain barrier.
Absorbed nickel is excreted mainly via the urine
Nickel can be excreted in breast milk during lactation.
An elimination half‐life of 28 ± 9 h was estimated in human volunteers.
Toxicity in experimental animals
Water‐soluble nickel compounds are of moderate to high acute toxicity with LD50‐values ranging from 39 to > 404 mg Ni/kg bw.
The major effects observed in the short‐term repeated dose toxicity studies in rodents and dogs following oral administration were decreased body weight, changes in organ weights (liver and kidneys), and histopathological changes in the liver and the kidney. Effects on bone and on gut microbiota have also been reported.
Recent studies have indicated that nickel can disturb the neurobehavioural functions in rats and mice and cause neurodegeneration in adult rats.
In mice, different reproductive effects (decreased male sex organ weights and histopathological changes, disturbed spermatogenesis, decreased sperm motility and sperm damage) have been reported to be responsible for a decrease in fertility in mice. A recent short‐term toxicity study suggested that nickel may also cause testicular degeneration in rats. Mice appear to be more sensitive than rats regarding reproductive effects.
In rats, developmental toxicity included increased pup mortality (stillbirth or post‐implantation loss/perinatal lethality) and decreased pup weight. Developmental toxicity was also observed in mice (decreased fetal weight, malformations), but at higher doses than for rats suggesting that rats appear to be more sensitive than mice regarding developmental toxicity.
Soluble nickel compounds induce structural and numerical chromosomal aberrations and DNA SSBs in vitro and in vivo. The genotoxicity of nickel is likely due to indirect effects including inhibition of DNA repair and ROS production.
No tumours have been observed in the carcinogenicity studies in experimental animals after oral administration of soluble nickel compounds.
Observations in humans
Oral exposure to nickel is not known to sensitise, but nickel may elicit eczematous flare‐up reactions in the skin (SCD) in nickel‐sensitised individuals following oral ingestion.
From the small number of studies published since the previous opinion, a few suggest that there may be an association between nickel exposure and adverse reproductive and developmental outcomes.
No clear signs of neurotoxicity have been reported in the few available studies.
No data linking cancer in humans with oral exposure to nickel are available.
Mode of action
A recurring theme in the toxicity of nickel is the evidence for a role of oxidative stress and ROS formation. A contribution of oxidative stress is evident in relation to reproductive toxicity, genotoxicity, immunotoxicity and neurotoxicity.
The genotoxicity of nickel is likely due to indirect effects including inhibition of DNA repair and ROS production.
Hypoxia‐mimicking effects, dysregulation of cell signalling pathways and alterations of the epigenetic mechanisms have been observed. In the context of cancer, these epigenetic changes would only be relevant to the inhalation route. Other potential consequences of epigenetic changes due to nickel exposure are currently unknown.
HBGV/Margin of Exposure approach
Nickel is classified as a human carcinogen via inhalation. No data linking cancer in humans with oral exposure to nickel are available. No tumours have been observed in the carcinogenicity studies in experimental animals after oral administration of soluble nickel compounds. Therefore, the CONTAM Panel considers it unlikely that dietary exposure to nickel results in cancer in humans. For the chronic risk assessment, the critical effect is the increased incidence of post‐implantation loss in rats observed in the one‐ and two‐generation studies by SLI (2000a,b). From the dose–response modelling, the BMDL10 of 1.3 mg Ni/kg bw per day was selected as a reference point for the establishment of the TDI. A TDI of 13 μg/kg bw was established by applying the default uncertainty factor of 100 to account for intra‐ and interspecies differences.
For the acute risk assessment, the critical effect is eczematous flare‐up reactions in the skin (SCD) elicited in nickel‐sensitised humans after oral exposure. The dose–response modelling showed that a BMDL could not be derived from the available data. Therefore, the reference point was based on the NOAEL/LOAEL approach. In the absence of a NOAEL, a LOAEL of 4.3 μg Ni/kg bw was identified. The data were considered insufficient to derive an ARfD and an MOE approach was applied. The CONTAM Panel considered that an MOE of 30 or higher would indicate a low health concern.
Occurrence/exposure for the EU population
The highest mean nickel concentrations were measured for the food category ‘Legumes, nuts and oilseeds’, in particular for soya beans, soya beans flour, chestnuts and cashew nuts and for the food category ‘Products for special nutritional use’, in particular for plant extract formula and mineral supplements.
The mean LB/UB chronic dietary exposure to nickel across the different dietary surveys and age classes ranged from 1.57/1.89 μg/kg bw per day in elderly to 12.5/14.6 μg/kg bw per day in toddlers. The 95th percentile LB/UB chronic dietary exposure to nickel across the different dietary surveys and age classes ranged from 3.35/3.93 μg/kg bw per day in very elderly to 28.1/29.9 μg/kg bw per day in infants.
Overall, ‘grains and grain‐based products’ was the most important contributor to the mean LB chronic dietary exposure to nickel in all age classes. The subcategories driving the contribution of this food category were ‘bread and rolls’ and ‘fine bakery wares’.
Mean UB acute exposure ranged from 1.89 μg/kg bw per day estimated in the elderly to 14.6 μg/kg bw per day estimated in toddlers. The 95th percentile UB acute exposure ranged from 5.35 μg/kg bw per day estimated in the elderly to 40.8 μg/kg bw per day estimated in toddlers.
The most relevant food categories for the 95th percentile UB acute dietary exposure to nickel varied between age classes and surveys. Beans, coffee, ready‐to‐eat soups, chocolate and breakfast cereals were the most relevant food categories in most of the surveys.
The acute dietary exposure to nickel from a small bottle of water (500 mL) containing a high concentration of nickel was 0.04 μg/kg bw from tap water and 0.08 μg/kg from bottled water.
Risk characterisation
Except for one survey, the mean LB and UB chronic dietary exposure was below the TDI and does not indicate a concern. For one survey in toddlers, the mean chronic dietary exposure was at the level of the TDI (LB/UB: 12.5/14.6 μg/kg bw per day) and this may indicate a health concern.
The 95th percentile LB chronic dietary exposure exceeded the TDI in 10 out of 14 dietary surveys in toddlers and in 11 out of 19 dietary surveys in other children. Also in infants, an exceedance of the TDI was observed in some surveys. In the adolescents and all adult age groups, the 95th percentile LB chronic dietary exposure was below the TDI. The 95th percentile chronic dietary exposure to nickel may raise a health concern for infants, toddlers and other children.
The CONTAM Panel noted that the risk characterisation for chronic dietary exposure is conservative and thus will overestimate the risk, as the critical effect for the TDI, post‐implantation loss, is not a relevant effect for young age groups. The TDI is also protective for effects that might occur in these age groups as no effects of relevance for young age groups have been reported at the reference point identified for the derivation of the TDI.
Comparison of the estimated mean acute UB exposure levels with the acute reference point of 4.3 μg Ni/kg bw resulted in MOE values ranging from 0.3 to 2.3, across dietary surveys and age classes. The MOEs values when using the 95th percentile UB acute dietary exposure range from 0.1 to 0.8 across dietary surveys and age classes. These MOE values raise a health concern for nickel‐sensitised individuals.
For the scenario regarding the consumption of a small bottle of drinking water, the MOE values of 120 and 55 for tap water and bottled water, respectively do not raise a health concern.
5. Recommendations
More information on oral bioavailability of nickel in humans under different dosing regimens (i.e. vehicle, fasting/non‐fasting condition) is needed in order to reduce the uncertainties in the acute and chronic risk assessments.
It is recommended to perform new studies with larger numbers of nickel‐sensitised individuals and different dosing regimens and dose levels included to allow a better characterisation of the dose–response and facilitate a BMD approach. Such studies would form the basis for a more precise risk assessment of skin and systemic reactions to nickel exposure via food and drinking water in nickel‐sensitised individuals.
Information on the potential presence of nickel nanoparticles in food and drinking water is needed.
6. Documentation as provided to EFSA
SLI (Springborn Laboratories), 2000a. A one‐generation reproduction range‐finding study in rats with nickel sulfate hexahydrate. Spencerville, OH: Springborn Laboratories, Inc. SLI Study No. 3472.3.
SLI (Springborn Laboratories), 2000b. An oral (gavage) two‐generation reproduction toxicity study in Sprague–Dawley rats with nickel sulfate hexahydrate. Final Report. Volume 1 of 3. Spencerville, OH: Springborn Laboratories, Inc. SLI Study No. 3472.4.
RTI (Research Triangle Institute), 1988a. Two‐generation reproduction and fertility study of nickel chloride administered to CD rats in the drinking water: Fertility and reproductive performance of the P generation. Final study report (II of III). Research Triangle Park, NC: Office of Solid Waste Management, US Environmental Protection Agency.
RTI (Research Triangle Institute), 1988b. Two‐generation reproduction and fertility study of nickel chloride administered to CD rats in the drinking water: Fertility and reproductive performance of the F1 generation. Final study report (III of III). Research Triangle Park, NC: Office of Solid Waste Management, US Environmental Protection Agency.
FSVO (Federal Food Safety and Veterinary Office), 2019. Data on migration of nickel from food contact material; Monitoring 2019. Federal Department of Home Affairs FDHA Switzerland.
FASFC (Federal Agency for the Safety of the Food Chain), 2019. Data on migration of nickel from food contact material; Monitoring 2018 and 2019. Federal Agency for the Safety of the Food Chain Belgium.
Abbreviations
- 2GEN F0F1
F0/F1 generation of the two‐generation study
- 2GEN F1F2
F1/F2 generation of the two‐generation study
- 2GEN F1F2
F1/F2 generation of the two‐generation study
- 3β‐HSD
3β‐hydroxysteroid dehydrogenase
- AIC
Akaike information criterion
- ALA‐D
δ‐aminolevulinate dehydratase
- ALP
alkaline phosphatase
- aOR
adjusted odds ratio
- ARfD
acute reference dose
- ATP
adenosine triphosphate
- BMD
benchmark dose
- BMDL
benchmark dose lower confidence limit
- BMDU
benchmark dose upper confidence limit
- BMI
body mass index
- BMR
benchmark response
- bw
body weight
- CAS
Chemical Abstracts Service
- CAT
catalase
- CHD
congenital heart defects
- CI
confidence interval
- CLO
cleft lip only
- CLP
cleft lip with cleft palate
- CONTAM
Panel on Contaminants in the Food Chain
- COT
Committee on Toxicology
- COX‐2
cyclooxygenase‐2
- CXCL4
chemokine ligand 4
- CYP
cytochrome P450
- DMT1
divalent metal transporter 1
- DRF
dose‐range‐finding study
- DSBs
double‐strand breaks
- ED
effective dose
- ERK
extracellular signal‐regulated kinase
- ERS
endoplasmic reticulum stress
- F‐AAS
flame atomic absorption spectrometry
- GD
gestation day
- GF‐AAS
graphite furnace atomic absorption spectrometry
- GI
gastrointestinal
- GSH
glutathione
- GSH‐Px
glutathione peroxidase
- GST
glutathione S‐transferase
- HDR
homology‐dependent double‐strand break repair
- HIF‐1α
hypoxia‐inducible factor 1‐alpha
- i.p.
intraperitoneal
- IARC
International Agency for Research on Cancer
- ICP‐AES
inductively coupled plasma‐atomic emission spectrometry
- ICP‐MS
inductively coupled plasma‐mass spectrometry
- ICP‐OES
inductively coupled plasma‐optical emission spectrometry
- IFN
interferon
- IgA
immunoglobulin A
- IL
interleukin
- IQR
interquartile range
- ISC
iron‐sulfur cluster‐dependent metabolic enzyme
- JNK
c‐JUN NH2‐terminal protein kinase
- LB
lower bound
- LCD
left‐censored data
- LD50
lethal dose killing 50% of the animals
- LOAEL
lowest‐observed‐adverse‐effect‐level
- LOD
limit of detection
- LOQ
limit of quantification
- LPS
lipopolysaccharide
- MAPK
mitogen‐activated protein kinase
- MDA
malondialdehyde
- MHC
major histocompatibility complex
- MNPCE
micronucleated polychromatic erythrocytes
- MOE
margin of exposure
- MPO
myeloperoxidase
- mRNA
messenger RNA
- MS
mass spectrometry
- NAC
N‐acetylcysteine
- NBUD
nuclear buds
- NCE
normochromatic erythrocytes
- NF‐κB
nuclear factor kappa B
- NiCl2
nickel chloride
- NOAEL
no‐observed‐adverse‐effect‐level
- OFCs
orofacial clefts
- OR
odds ratio
- PBPK
physiologically based pharmacokinetic
- PCE
polychromatic erythrocytes
- PCOS
polycystic ovary syndrome
- PLBW
pre‐term low birth weight
- PND
postnatal day
- ROS
reactive oxygen species
- rRNA
ribosomal RNA
- s.c.
subcutaneous
- SCD
systemic contact dermatitis
- SD
standard deviation
- SEM
scanning electron microscopy
- SNAS
systemic nickel allergy syndrome
- SOD
superoxide dismutase
- SOP
standard operational procedure
- SRL
specific release limit
- SSB
single‐strand break
- StAR
steroidogenic acute regulatory protein
- TDI
tolerable daily intake
- TDS
total diet study
- TEMPO
2,2,6,6‐tetramethyl‐1‐piperidinyloxy
- TLR4
toll‐like receptor 4
- TNF
tumour necrosis factor
- UB
upper bound
- WHO
World Health Organization
- ww
wet weight
Appendix A – Identification and selection of evidence relevant for the risk assessment of nickel in food and drinking water
A.1 Literature search for supporting information for the assessment
A. Web of Science and PubMed
| Toxicokinetics | |
|---|---|
| Search terms | TOPIC (TITLE/ABSTRACT in PubMed): nickel* OR Ni AND TOPIC (All fields in PubMed): occurrence or exposure AND TOPIC (All fields in PubMed): food or drinking water or diet* Timespan = Last 5 years |
| Occurrence of nickel nanoparticles | |
| Search terms | TOPIC (TITLE/ABSTRACT in PubMed): nickel nanoparticle AND TOPIC (ALL FIELDS in PubMed): food or drinking water Timespan = All years |
| Migration of nickel from food contact material | |
| Search terms | TOPIC (TITLE/ABSTRACT in PubMed): food AND TOPIC (ALL FIELDS in PubMed): nickel AND TOPIC (ALL FIELDS in PubMed): migration or release Timespan = All years |
After removal of all duplicates, 1,165 papers were screened for relevance based on title and abstract.
A.2 Literature search for hazard identification and characterisation
A. Web of Science and PubMed
Limited to between 01/01/2013 and 25/06/2019
| Toxicokinetics | |
| Search terms | TOPIC (TITLE/ABSTRACT in PubMed): nickel* OR Ni AND TOPIC (All fields in PubMed): (absor* OR tissue* OR metaboli* OR excret* OR kinetic* OR toxicokinetic* OR pharmacokinetic* OR degrad* OR biotrans* OR eliminat* OR PBPK OR PBTK OR PBK) AND TOPIC (All fields in PubMed): (rat OR rats OR mouse OR mice OR rabbit* OR guinea OR hamster* OR primate* OR monkey* OR pig* OR minipig* OR dog* OR cat OR cats OR mink*) |
| Toxicity in experimental animals | |
| Search terms | TOPIC (TITLE/ABSTRACT in PubMed): nickel* OR Ni AND TOPIC (ALL FIELDS in PubMed): (acute OR chronic OR tox* OR cancer* OR carcino* OR tumor* OR tumour* OR organ* OR immun* OR neuro* OR developmental OR teratogen* OR repro* OR liver OR hepato* OR kidney* OR brain* OR lung OR lungs OR heart* OR thyroid* OR spermat* OR testes OR ovar* OR uterus) |
| AND TOPIC (ALL FIELDS in PubMed): (rat OR rats OR mouse OR mice OR rabbit* OR guinea OR hamster* OR primate* OR monkey* OR pig* OR minipig* OR dog* OR cat OR cats OR mink*) | |
| In vitro and in vivo genotoxicity and mode of action | |
| Search terms | TOPIC (TITLE/ABSTRACT in PubMed): nickel* OR Ni AND TOPIC (ALL FIELDS in PubMed): (“in vitro” OR “mode of action” OR endocrin* OR estrogen* OR oestrogen* OR androgen* OR “mechanism of action” OR apoptosis OR “oxidative stress” OR cytotox* OR genotox* OR mutagen* OR clastogen* OR aneugen* OR chromosom* OR chromatid) |
| Human observations (epi or biomo) | |
| Search terms | TOPIC (TITLE/ABSTRACT in WoS): nickel* OR Ni AND TOPIC (ALL FIELDS in PubMed): oral AND TOPIC (ALL FIELDS in PubMed): (epidemio* OR intervention OR exposure* OR “case study*” OR “case control*” OR “case report*” OR poison* OR cohort* OR cross‐sectional OR occupational OR “adverse effect*” OR “occupational case*” OR “biological marker” OR human health OR meta‐analys*) |
B. Sci Finder
| Nickel | |
|---|---|
| Search terms | SEARCH BY CAS NUMBER: 7440‐02‐0 REFINED FOR: adverse effect YEARS: 2013–2019 |
| Nickel sulfate | |
| Search terms | SEARCH BY CAS NUMBER: 7786‐81‐4/10101‐97‐0/10101‐98‐1 REFINED FOR: adverse effect YEARS: 2013–2019 |
| Nickel chloride | |
| Search terms | SEARCH BY CAS NUMBER: 7718‐54‐9 REFINED FOR: adverse effect YEARS: 2013 to 2019 |
After removal of all duplicates, 6,469 papers were screened for relevance based on title and abstract.
A.3 Exclusion criteria for the screening of titles and abstracts of papers related to the hazard identification and characterisation
The titles and abstracts of the references retrieved from the literature search were screened to identify the relevant papers for the sections on hazard identification and characterisation. Papers on the following subjects were excluded:
Papers not related to hazard identification and characterisation.
Papers reporting on environmental or occupational exposures in a human population, which did not involve oral routes.
Studies in experimental animals using routes of exposure other than oral.
Studies in which experimental animals are exposed to mixtures that include other substances in addition to nickel.
Studies designed to evaluate substances or extracts for medical treatment.
Nickel nanoparticles.
Exposure due to dental treatment.
Nickel as a treatment with the exception of papers on desensitisation.
A.4 EFSA guidance documents applied in the present assessment
Guidance of the Scientific Committee on a request from EFSA related to uncertainties in Dietary Exposure Assessment (EFSA, 2007);
Guidance of the Scientific Committee on transparency in the scientific aspects of risk assessments carried out by EFSA. Part 2: General principles (EFSA Scientific Committee, 2009);
Standard sample description for food and feed (EFSA, 2010a);
Management of left‐censored data in dietary exposure assessment of chemical substances (EFSA, 2010b);
Guidance of EFSA on the use of the EFSA Comprehensive European Food Consumption Database in exposure assessment (EFSA, 2011b);
Scientific opinion on genotoxicity testing strategies applicable to food and feed safety assessment (EFSA Scientific Committee, 2011);
Guidance on selected default values to be used by the EFSA Scientific Committee, Scientific Panels and Units in the absence of actual measured data (EFSA Scientific Committee, 2012a);
Scientific Opinion on Risk Assessment terminology (EFSA Scientific Committee, 2012b);
Update: use of the benchmark dose approach in risk assessment (EFSA Scientific Committee, 2017).
Appendix B – Hazard identification and characterisation
B.1 Short‐term toxicity
The following studies were reported in a limited way, which did not allow the CONTAM Panel to evaluate the results. For transparency, these studies are reported below.
Adult Wistar rats were administered nickel chloride by gavage daily for 28 days at 0, 5.25, 10.5 or 21 mg/kg bw (assuming that the doses are expressed as nickel chloride, the corresponding doses of nickel are 1.0, 4.8 and 9.5 mg/kg bw per day) (Lambade et al., 2015). The control group was administered deionised water. The average body weight was significantly decreased in both the mid‐ and high‐dose groups. Increased mean relative liver weights were observed in high‐dose males, decreased mean relative liver weights were observed in high‐dose females, mean relative kidney weights were decreased in both high‐dose males and females, mean relative lung weight was increased in high‐dose females and mean relative testis weight was decreased in all treated male groups. Histopathological changes were reported in all treated rats in a dose‐related manner. The changes comprised varying degrees of degenerative and vascular changes in various visceral organs. A higher severity and distribution was reported in mid‐ and high‐dose rats compared with low‐dose rats and the controls. According to the article, average weekly body weights were presented in Table 1 and relative organ weights of liver, lung, kidney and testis were presented in Table 2; however, the CONTAM Panel noted that no tables are included in the paper. The Panel also noted that except for a few figures of slides, the histopathological changes are only descriptive and no information on incidences and severity in the various groups is presented. Based on the poor reporting of results, the Panel considers that the reliability of this study is low.
In a study designed to analyse the biochemical parameters of blood plasma, male Wistar rats (10 per group) were administered nickel chloride hexahydrate in the drinking water at concentrations of 0 or 100 mg/L (corresponding to 2 mg Ni/kg bw per day based on the default factor of 0.09 for a subchronic study in rats (EFSA Scientific Committee, 2012a)) daily for 90 days (Toman et al., 2013). Animals were sacrificed and blood samples were collected. The parameters of mineral profile (calcium, phosphorus, magnesium, sodium, potassium and chlorides) and other parameters of energy, nitrogen and enzymatic profile (glucose, cholesterol, total proteins, triglycerides, urea, bilirubin, aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase (ALP), and glutamate dehydrogenase (GLDH)) were measured. Potassium, calcium and magnesium concentrations were significantly decreased when compared with the control values. Analysis of nitrogen and the energy profile showed a significant increase in concentrations of glucose and a decrease in total proteins, cholesterol, and bilirubin. There were changes in enzymatic activity in ALP and GLDH. The results showed, according to the authors, that nickel may have negative effects on the metabolism due to the disruption of certain metabolic processes.
Adult male mice (five per group, weight 30–35 g, age not mentioned) were administered nickel sulfate orally by gavage daily for 21 days at 0, 6.3, 25.8 or 45.1 mg/kg bw (assuming that the doses are expressed as nickel sulfate, the doses expressed as nickel are 0, 2.4, 9.8, 17.1 mg Ni/kg bw per day) (Gathwan, 2015a). The control group was on normal diet and water. After sacrifice, the liver was weighed and prepared for histopathological examination by light microscopy and SEM. The intake of feed and water was lower in treated mice than in the control group and according to the authors, the decrease was dose dependent (no data were presented in the article). The relative liver weight was significantly decreased in the mid‐ and high‐dose groups. The histopathological changes in the low‐dose group were described as ‘a few spaces were observed, the sinuses were broadened, the number of binucleated cells were increased, and the nuclear chromatin had a darker colour.’ In the mid‐ and high‐dose groups the above‐mentioned effects were, according to the authors, more prominent and furthermore, the intercellular membranes were lost, the number of Kupffer cells was increased, and vacuolisation in the hepatic cells increased. The SEM revealed that the microvilli of hepatic cells were damaged and the sinusoids had fewer Kupffer cells; the changes were dose dependent. The CONTAM Panel was not able to evaluate the results of this study due to the limited reporting.
Male rabbits (Oryctolagus cuniculus) (five per group, weight 1.5–1.8 kg, age not mentioned) were administered nickel chloride (concentration: 0, 250 or 500 mg/kg) orally by gavage for 90 days (Nadjiba et al., 2018). After sacrifice, the liver was weighed and stored for determination of liver proteins and oxidative stress parameters, i.e. GSH, GSH‐Px activity, CAT activity, MDA and glutathione S‐transferase (GST) activity were determined. The level of liver proteins was increased. Hepatic GSH and the activities of GST, GSH‐Px and CAT were decreased. The CONTAM Panel was not able to evaluate the results of this study based on the two‐page article without details.
In a study on histopathological changes in the kidney, adult male mice (five per group, weight 28–32 g, age not mentioned) were administered nickel chloride orally by gavage daily for 21 days at 0, 6 or 15 mg/kg bw (assuming that the doses are expressed as nickel chloride, the doses expressed as nickel are 0, 2.7 and 6.8 mg Ni/kg bw per day) (Gathwan, 2015b). The control group was on normal diet and water. The kidney was cut into two pieces and underwent histopathological examination with light microscopy. The intake of feed and water was lower in treated mice than in the control group and according to the authors, the decrease was dose dependent (no data were presented in the article). The histopathological changes at 6 mg/kg bw were described as spaces between the tubules and decreased density of Bowman's capsules and tubules when compared with controls. At 15 mg/kg bw the histopathological changes were described as increased spaces between the tubules, some glomeruli were damaged and the outer wall of the Bowman's capsule was also damaged. Furthermore, the lumen of some of proximal convoluted tubules was blocked and the boundaries of cells disappeared. The cells of some of the distal convoluted tubules also showed necrosis. The authors concluded that high doses caused severe nephrotoxicity as the histoarchitecture of glomeruli and proximal convoluted tubules showed necrosis. The CONTAM Panel was not able to evaluate the results of this study based on the one‐page article without details.
In a study on effects on bone composition, adult male mice (five per group, weight 32–35 g, age not mentioned) were administered different doses of two nickel compounds orally by gavage daily for 40 days (Gathwan and Al‐Karkhi, 2015). Nickel sulfate was administered at doses of 0, 5.0, 15 or 40 mg/kg bw per day and nickel nitrate was administered at doses of 5.0, 20 or 40 mg/kg bw per day (assuming that the doses are expressed as the nickel salt, the corresponding doses of nickel are 1.9, 5.7 and 15.2 mg Ni/kg bw per day for the groups exposed to nickel sulfate and 1.6, 6.4 and 12.8 mg Ni/kg bw per day for the groups exposed to nickel nitrate). The control group was on normal diet and water. After sacrifice of the animals, the femur bone was weighed and then decalcified in ethylenediaminetetraacetic acid for seven days and softness of the bones was tested by light microscopy and SEM. The intake of feed and water was lower in treated mice than in the control group and according to the authors, the decrease was dose dependent (no data were presented in the article). The femur bone weight was significantly decreased in the mid‐ and high‐dose groups. Histopathologically, necrosis to layers of decalcified bone, i.e. periosteum, matrix and endosteum was observed with both nickel salts. The bone‐forming cells, lamellae and Haversian canals were also affected. The cortical width of bone section decreased dose dependently with both nickel salts. Such changes were also observed on samples of powdered dried bone with SEM. According to the authors, the effects of nickel sulfate were more severe than those of nickel nitrate. The CONTAM Panel noted that the doses causing effects, expressed as nickel, were higher for nickel sulfate than for nickel nitrate, which could reflect the differences in toxicity reported by the authors.
In a study on the effects on bone composition, adult male mice (seven per group, weight 30–38 g, age not mentioned) were administered nickel sulfate orally (not further specified) daily for 21 days at 0, 5.1, 11.7 or 24.2 mg/kg bw (assuming that the doses are expressed as nickel sulfate, the doses expressed as nickel are 1.9, 4.4, 9.2 mg Ni/kg bw per day) (Gathwan and Albir, 2019). The control group was untreated. There was a significant decrease in both wet and dry weight of the femur bone in the mid‐ and high‐dose groups. The percentage change in both dry weight and wet weight were increased dose dependently. The CONTAM Panel was not able to evaluate the results of this study based on the two‐page article without details.
In a study designed to gain a more comprehensive understanding of the effects of metal exposure on the gut microbiota, Richardson et al. (2018) exposed rats to nickel chloride. Sprague–Dawley rats (five per group) were administered nickel chloride by oral gavage (5 mL/kg bw) at doses of 0, 177, 232, or 300 mg/kg bw per day (corresponding to 0, 80, 105 or 136 mg Ni/kg bw per day) daily for five consecutive days. Fresh faecal samples were collected prior to the initial dosing and 24 h after the final dosing. 16S ribosomal RNA (rRNA) gene sequencing was used to track changes in the gut microbiota composition. Significant dose‐dependent changes were observed in response to nickel. Bacteria with higher numbers of iron‐importing gene orthologs were overly represented after exposure to nickel.
In a study examining the effect of oral nickel exposure on intestinal microflora, female mice (10 per group, 7–8 weeks old, 25–30 g) were administered water containing 400 μM nickel sulfate hexahydrate for 21 days (Zhou et al., 2019). Based on the default factor of 0.18 for a subacute study in mice (EFSA Scientific Committee, 2012a) and the molecular weight of 262.85 g/mol for nickel sulfate hexahydrate, the corresponding dose is 4 mg Ni/kg bw per day. The control group received pure water. There was no significant difference in body weight between the treated group and the control group. The nickel concentration in the kidney of treated mice was significantly higher than in that of the controls. Regarding the influence on gut microbiota, there was a significantly higher relative abundance of Bacteroides and Intestinimonas, and a significantly lower relative abundance of Lachnospiraceae_NK4A136_group and Lachnospiraceae_UCG‐001_group in the treated group compared with the control group. Furthermore, the treated group had a significantly lower ratio of Firmicutes/Bacteroides. These results indicate, according to the authors, that orally administered nickel could change the intestinal flora in mice and thus could alter the interaction between the host and the intestinal flora.
Appendix C – Benchmark dose analysis
C.1 Post‐implantation loss DRF and 2GEN F0F1 studies; BMR 10%
C.1.1 Data description
The incidence of post‐implantation loss as reported for the DRF study (SLI, 2000a) and the F0/F1 generation in the 2‐generation study (SLI, 2000b) was used and the individual data are included in Section C.1.6 of this Appendix. The incidence of post‐implantation loss was calculated as follows: implantation scar count minus the number of live pups at delivery. The study was used as a covariate and the litter effect was taken into account.
C.1.2 Selection of the benchmark response
A default benchmark response (BMR) of 10% (extra risk) and a 90% confidence interval around the BMD were selected as recommended by EFSA Scientific Committee (2017).
C.1.3 Software used
Results are obtained using the EFSA web tool for BMD analysis, which uses the R‐package PROAST, version 67.0, for the underlying calculations.
C.1.4 Specification of deviations from default assumptions
General assumptions
No deviation from the recommended defaults (e.g. gamma distributional assumption instead of log‐normal, heteroscedasticity instead of homoscedasticity) was made.
Dose–response models
No deviation from the recommended defaults. Default set of fitted models:
| Model | Number of parameters | Formula | |
|---|---|---|---|
| Null | 1 |
|
|
| Full | No. of groups |
|
|
| Logistic | 2 |
|
|
| Probit | 2 |
|
|
| Log‐logistic | 3 |
|
|
| Log‐probit | 3 |
|
|
| Weibull | 3 |
|
|
| Gamma | 3 |
|
|
| Two‐stage | 3 |
|
|
| Exp model 3 | 3 |
|
|
| Exp model 5 | 4 |
|
|
| Hill model 3 | 3 |
|
|
| Hill model 5 | 4 |
|
For the Exp and Hill family, we fit models with 3 and 4 parameters as listed in the table. The 3‐parameter model is selected if the difference in Akaike information criterion (AIC) is smaller than 5, otherwise the 4‐parameter model is selected.
As a covariate is included in the analysis, these models will also be fitted assuming that some of the parameters (background response parameter (a), potency parameter (BMD) and/or variance (var)) depend on the subgroup defined by the covariate. Therefore the number of parameters in each model might be larger than indicated in the table above.
Procedure for selection of the BMDL
There was no deviation from the procedure described in the flow chart to obtain the final BMD confidence interval.
Figure C.1.
Flow chart for selection of BMDL
C.1.5 Results
Table C.1.
Results for the incidence of post‐implantation loss in rats studied in the F1/F2 generation of the two‐generation study using a BMR of 10%
| Model | No. par | Loglik | AIC | Accepted | BMDL | BMDU | BMD | sens.subgr | conv |
|---|---|---|---|---|---|---|---|---|---|
| null | 3 | –830.99 | 1,667.98 | NA | NA | NA | NA | ||
| full | 12 | –816.37 | 1,656.74 | NA | NA | NA | NA | ||
| two.stage | 4 | –824.67 | 1,657.34 | No | NA | NA | 5.80 | – | Yes |
| log.logist | 4 | –823.33 | 1,654.66 | Yes | 1.16 | 7.99 | 3.03 | – | Yes |
| Weibull | 4 | –823.27 | 1,654.54 | Yes | 1.18 | 7.90 | 3.05 | – | Yes |
| log.prob | 4 | –823.55 | 1,655.10 | Yes | 1.09 | 8.32 | 2.91 | – | Yes |
| gamma | 4 | –823.21 | 1,654.42 | Yes | 1.19 | 7.84 | 3.07 | – | Yes |
| logistic‐b | 4 | –824.20 | 1,656.40 | No | NA | NA | 3.06 | 2GEN | Yes |
| LVM: Expon. m3‐ | 4 | –823.04 | 1,654.08 | Yes | 1.35 | 7.77 | 3.20 | 2GEN | Yes |
| LVM: Hill m3‐ | 4 | –823.09 | 1,654.18 | Yes | 1.28 | 7.79 | 3.16 | 2GEN | Yes |
AIC: Akaike information criterion; BMDL: benchmark dose lower confidence limit; BMDU: benchmark dose upper confidence limit; BMR: benchmark response.
Confidence intervals for the BMD are based on generated data sets.
Estimated model parameters
two.stage
estimate for alfa‐ : 1.002
estimate for a‐2GEN : 0.08402
estimate for a‐DRF : 5.796
estimate for BMD‐2GEN : 1e‐06
estimate for BMD‐DRF : 1.002
estimate for c : 0.08402
log.logist
estimate for alfa‐ : 1.024
estimate for a‐2GEN : 0.05898
estimate for a‐DRF : 3.027
estimate for BMD‐2GEN : 0.4934
estimate for BMD‐DRF : 1.024
estimate for c : 0.05898
Weibull
estimate for alfa‐ : 1.025
estimate for a‐2GEN : 0.05882
estimate for a‐DRF : 3.054
estimate for BMD‐2GEN : 0.4714
estimate for BMD‐DRF : 1.025
estimate for c : 0.05882
log.prob
estimate for alfa‐ : 1.02
estimate for a‐2GEN : 0.05902
estimate for a‐DRF : 2.905
estimate for BMD‐2GEN : 0.2404
estimate for BMD‐DRF : 1.02
estimate for c : 0.05902
gamma
estimate for alfa‐ : 1.026
estimate for a‐2GEN : 0.05855
estimate for a‐DRF : 3.07
estimate for BMD‐2GEN : 0.4481
estimate for BMD‐DRF : 1.026
estimate for cc : 0.05855
logistic
estimate for alfa‐ : 1.03
estimate for a‐2GEN : ‐2.42
estimate for a‐DRF : 3.058
estimate for BMD‐2GEN : 8.298
estimate for BMD‐DRF : 1.03
EXP
estimate for alfa‐ : 1.029
estimate for a‐ : 1.481
estimate for CED‐ : 3.199
estimate for d‐ : 0.3373
estimate for th‐1(fixed) : 0
estimate for sigma(fixed) : 0.25
HILL
estimate for alfa‐ : 1.028
estimate for a‐ : 1.48
estimate for CED‐ : 3.164
estimate for d‐ : 0.3596
estimate for th‐1(fixed) : 0
estimate for sigma(fixed) : 0.25
Weights for model averaging
| two.stage | log.logist | Weibull | log.prob | gamma | logistic | EXP | HILL |
|---|---|---|---|---|---|---|---|
| 0.04 | 0.14 | 0.15 | 0.11 | 0.15 | 0.06 | 0.18 | 0.17 |
Final BMD values
| Subgroup | BMDL | BMDU |
|---|---|---|
| 2GEN | 1.34 | 9.8 |
| DRF | 1.40 | 10.7 |
Confidence intervals for the BMD are based on 1,000 bootstrap data sets.
Visualisation
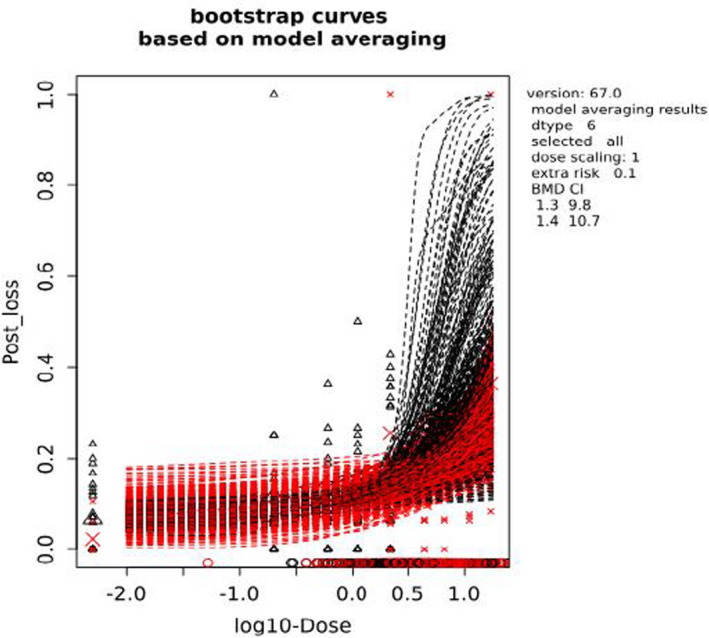
C.1.6 Data used for analysis
| Animal | Dose (mg Ni/kg bw per day) | Incidence of post‐implantation loss | Implantation scar count | Study |
|---|---|---|---|---|
| 48 | 0.0 | 0 | 6 | 2GEN |
| 49 | 0.0 | 1 | 17 | 2GEN |
| 50 | 0.0 | 0 | 14 | 2GEN |
| 51 | 0.0 | 2 | 15 | 2GEN |
| 52 | 0.0 | 2 | 17 | 2GEN |
| 53 | 0.0 | 0 | 13 | 2GEN |
| 54 | 0.0 | 2 | 14 | 2GEN |
| 55 | 0.0 | 1 | 14 | 2GEN |
| 56 | 0.0 | 3 | 16 | 2GEN |
| 57 | 0.0 | 0 | 16 | 2GEN |
| 58 | 0.0 | 0 | 5 | 2GEN |
| 59 | 0.0 | 0 | 14 | 2GEN |
| 60 | 0.0 | 0 | 13 | 2GEN |
| 61 | 0.0 | 1 | 14 | 2GEN |
| 62 | 0.0 | 0 | 16 | 2GEN |
| 63 | 0.0 | 3 | 13 | 2GEN |
| 64 | 0.0 | 0 | 14 | 2GEN |
| 65 | 0.0 | 0 | 14 | 2GEN |
| 66 | 0.0 | 1 | 6 | 2GEN |
| 67 | 0.0 | 1 | 14 | 2GEN |
| 68 | 0.0 | 0 | 17 | 2GEN |
| 69 | 0.0 | 1 | 14 | 2GEN |
| 70 | 0.0 | 3 | 15 | 2GEN |
| 71 | 0.0 | 2 | 16 | 2GEN |
| 72 | 0.0 | 0 | 12 | 2GEN |
| 73 | 0.2 | 2 | 12 | 2GEN |
| 74 | 0.2 | 2 | 13 | 2GEN |
| 75 | 0.2 | 0 | 8 | 2GEN |
| 76 | 0.2 | 1 | 13 | 2GEN |
| 77 | 0.2 | 0 | 13 | 2GEN |
| 78 | 0.2 | 0 | 13 | 2GEN |
| 79 | 0.2 | 1 | 15 | 2GEN |
| 80 | 0.2 | 11 | 11 | 2GEN |
| 81 | 0.2 | 4 | 16 | 2GEN |
| 82 | 0.2 | 4 | 16 | 2GEN |
| 83 | 0.2 | 1 | 16 | 2GEN |
| 84 | 0.2 | 1 | 15 | 2GEN |
| 85 | 0.2 | 2 | 17 | 2GEN |
| 86 | 0.2 | 1 | 13 | 2GEN |
| 87 | 0.2 | 0 | 13 | 2GEN |
| 88 | 0.2 | 1 | 16 | 2GEN |
| 89 | 0.2 | 0 | 14 | 2GEN |
| 90 | 0.2 | 0 | 14 | 2GEN |
| 91 | 0.2 | 1 | 15 | 2GEN |
| 92 | 0.2 | 2 | 16 | 2GEN |
| 93 | 0.2 | 1 | 14 | 2GEN |
| 94 | 0.2 | 1 | 18 | 2GEN |
| 95 | 0.2 | 0 | 16 | 2GEN |
| 96 | 0.2 | 0 | 16 | 2GEN |
| 97 | 0.2 | 2 | 18 | 2GEN |
| 98 | 0.2 | 1 | 18 | 2GEN |
| 99 | 0.6 | 2 | 16 | 2GEN |
| 100 | 0.6 | 0 | 14 | 2GEN |
| 101 | 0.6 | 0 | 5 | 2GEN |
| 102 | 0.6 | 1 | 15 | 2GEN |
| 103 | 0.6 | 1 | 12 | 2GEN |
| 104 | 0.6 | 4 | 11 | 2GEN |
| 105 | 0.6 | 3 | 16 | 2GEN |
| 106 | 0.6 | 3 | 15 | 2GEN |
| 107 | 0.6 | 0 | 15 | 2GEN |
| 108 | 0.6 | 1 | 14 | 2GEN |
| 109 | 0.6 | 1 | 17 | 2GEN |
| 110 | 0.6 | 4 | 15 | 2GEN |
| 111 | 0.6 | 0 | 14 | 2GEN |
| 112 | 0.6 | 4 | 17 | 2GEN |
| 113 | 0.6 | 1 | 15 | 2GEN |
| 114 | 0.6 | 1 | 12 | 2GEN |
| 115 | 0.6 | 0 | 7 | 2GEN |
| 116 | 0.6 | 1 | 12 | 2GEN |
| 117 | 0.6 | 1 | 15 | 2GEN |
| 118 | 0.6 | 1 | 14 | 2GEN |
| 119 | 0.6 | 0 | 16 | 2GEN |
| 120 | 0.6 | 0 | 15 | 2GEN |
| 121 | 0.6 | 0 | 16 | 2GEN |
| 122 | 0.6 | 0 | 13 | 2GEN |
| 123 | 0.6 | 0 | 12 | 2GEN |
| 124 | 1.1 | 3 | 14 | 2GEN |
| 125 | 1.1 | 1 | 13 | 2GEN |
| 126 | 1.1 | 1 | 15 | 2GEN |
| 127 | 1.1 | 1 | 11 | 2GEN |
| 128 | 1.1 | 3 | 6 | 2GEN |
| 129 | 1.1 | 2 | 14 | 2GEN |
| 130 | 1.1 | 1 | 16 | 2GEN |
| 131 | 1.1 | 2 | 15 | 2GEN |
| 132 | 1.1 | 1 | 13 | 2GEN |
| 133 | 1.1 | 1 | 14 | 2GEN |
| 134 | 1.1 | 1 | 15 | 2GEN |
| 135 | 1.1 | 0 | 11 | 2GEN |
| 136 | 1.1 | 0 | 13 | 2GEN |
| 137 | 1.1 | 1 | 16 | 2GEN |
| 138 | 1.1 | 0 | 14 | 2GEN |
| 139 | 1.1 | 4 | 15 | 2GEN |
| 140 | 1.1 | 1 | 15 | 2GEN |
| 141 | 1.1 | 3 | 12 | 2GEN |
| 142 | 1.1 | 0 | 14 | 2GEN |
| 143 | 1.1 | 0 | 14 | 2GEN |
| 144 | 1.1 | 1 | 13 | 2GEN |
| 145 | 1.1 | 0 | 14 | 2GEN |
| 146 | 1.1 | 0 | 16 | 2GEN |
| 147 | 1.1 | 2 | 14 | 2GEN |
| 148 | 1.1 | 3 | 13 | 2GEN |
| 149 | 1.1 | 1 | 17 | 2GEN |
| 150 | 2.2 | 1 | 16 | 2GEN |
| 151 | 2.2 | 1 | 12 | 2GEN |
| 152 | 2.2 | 5 | 14 | 2GEN |
| 153 | 2.2 | 1 | 6 | 2GEN |
| 154 | 2.2 | 0 | 15 | 2GEN |
| 155 | 2.2 | 0 | 10 | 2GEN |
| 156 | 2.2 | 2 | 15 | 2GEN |
| 157 | 2.2 | 4 | 12 | 2GEN |
| 158 | 2.2 | 2 | 14 | 2GEN |
| 159 | 2.2 | 3 | 16 | 2GEN |
| 160 | 2.2 | 0 | 5 | 2GEN |
| 161 | 2.2 | 0 | 13 | 2GEN |
| 162 | 2.2 | 6 | 14 | 2GEN |
| 163 | 2.2 | 0 | 14 | 2GEN |
| 164 | 2.2 | 1 | 15 | 2GEN |
| 165 | 2.2 | 0 | 16 | 2GEN |
| 166 | 2.2 | 0 | 14 | 2GEN |
| 167 | 2.2 | 5 | 14 | 2GEN |
| 168 | 2.2 | 0 | 16 | 2GEN |
| 169 | 2.2 | 6 | 19 | 2GEN |
| 170 | 2.2 | 1 | 16 | 2GEN |
| 171 | 2.2 | 6 | 16 | 2GEN |
| 172 | 2.2 | 1 | 16 | 2GEN |
| 173 | 2.2 | 5 | 16 | 2GEN |
| 174 | 2.2 | 0 | 4 | 2GEN |
| 175 | 2.2 | 6 | 15 | 2GEN |
| 176 | 2.2 | 1 | 13 | 2GEN |
| 177 | 2.2 | 2 | 13 | 2GEN |
| 1 | 0.0 | 0 | 17 | DRF |
| 2 | 0.0 | 1 | 17 | DRF |
| 3 | 0.0 | 0 | 16 | DRF |
| 4 | 0.0 | 0 | 13 | DRF |
| 5 | 0.0 | 0 | 17 | DRF |
| 6 | 0.0 | 2 | 19 | DRF |
| 7 | 0.0 | 0 | 16 | DRF |
| 8 | 0.0 | 0 | 16 | DRF |
| 9 | 2.2 | 0 | 6 | DRF |
| 10 | 2.2 | 1 | 17 | DRF |
| 11 | 2.2 | 0 | 18 | DRF |
| 12 | 2.2 | 16 | 16 | DRF |
| 13 | 2.2 | 0 | 14 | DRF |
| 14 | 2.2 | 2 | 17 | DRF |
| 15 | 2.2 | 1 | 15 | DRF |
| 16 | 2.2 | 1 | 18 | DRF |
| 17 | 4.4 | 1 | 15 | DRF |
| 18 | 4.4 | 1 | 16 | DRF |
| 19 | 4.4 | 1 | 16 | DRF |
| 20 | 4.4 | 0 | 13 | DRF |
| 21 | 4.4 | 5 | 17 | DRF |
| 22 | 4.4 | 0 | 16 | DRF |
| 23 | 4.4 | 2 | 9 | DRF |
| 24 | 4.4 | 2 | 16 | DRF |
| 25 | 6.6 | 5 | 17 | DRF |
| 26 | 6.6 | 0 | 0 | DRF |
| 27 | 6.6 | 2 | 14 | DRF |
| 28 | 6.6 | 2 | 18 | DRF |
| 29 | 6.6 | 1 | 16 | DRF |
| 30 | 6.6 | 5 | 15 | DRF |
| 31 | 6.6 | 0 | 13 | DRF |
| 32 | 6.6 | 1 | 15 | DRF |
| 33 | 11.0 | 6 | 15 | DRF |
| 34 | 11.0 | 1 | 13 | DRF |
| 35 | 11.0 | 2 | 18 | DRF |
| 36 | 11.0 | 4 | 16 | DRF |
| 37 | 11.0 | 1 | 14 | DRF |
| 38 | 11.0 | 4 | 16 | DRF |
| 39 | 11.0 | 1 | 16 | DRF |
| 40 | 17.0 | 6 | 15 | DRF |
| 41 | 17.0 | 3 | 17 | DRF |
| 42 | 17.0 | 6 | 15 | DRF |
| 43 | 17.0 | 3 | 17 | DRF |
| 44 | 17.0 | 5 | 17 | DRF |
| 45 | 17.0 | 1 | 12 | DRF |
| 46 | 17.0 | 6 | 17 | DRF |
| 47 | 17.0 | 8 | 8 | DRF |
Annex A – Benchmark dose analysis
1.
The Annex is provided as a separate pdf file containing the detailed results of the benchmark dose analyses from which no reference point was selected and is available on the EFSA Knowledge Junction community on Zenodo at: https://doi.org/10.5281/zenodo.4081872
Annex B – Dietary surveys per country and age group available in the EFSA Comprehensive Database, considered in the exposure assessment
1.
The Annex is provided as a separate Excel file containing the dietary surveys per country and age group and is available on the EFSA Knowledge Junction community on Zenodo at: https://doi.org/10.5281/zenodo.4081872
Annex C – Occurrence data on nickel in food and drinking water
1.
The Annex is provided as a separate Excel file containing summary statistics on occurrence data on nickel and is available on the EFSA Knowledge Junction community on Zenodo at: https://doi.org/10.5281/zenodo.4081872
Annex D – Chronic and acute dietary exposure to nickel and the contribution of different food groups to the dietary exposure
1.
The Annex is provided as a separate Excel file containing the chronic and acute dietary exposure to nickel per survey and the contribution of different food groups to the dietary exposure and is available on the EFSA Knowledge Junction community on Zenodo at: https://doi.org/10.5281/zenodo.4081872
Suggested citation: EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain) , Schrenk D, Bignami M, Bodin L, Chipman JK, del Mazo J, Grasl‐Kraupp B, Hogstrand C, Hoogenboom LR, Leblanc J‐C, Nebbia CS, Ntzani E, Petersen A, Sand S, Schwerdtle T, Vleminckx C, Wallace H, Guérin T, Massanyi P, Van Loveren H, Baert K, Gergelova P and Nielsen E, 2020. Scientific Opinion on the update of the risk assessment of nickel in food and drinking water. EFSA Journal 2020;18(11):6268, 101 pp. 10.2903/j.efsa.2020.6268
Requestor: European Commission
Question number: EFSA‐Q‐2019‐00214
Panel members: Margherita Bignami, Laurent Bodin, James Kevin Chipman, Jesús del Mazo, Bettina Grasl‐Kraupp, Christer Hogstrand, Laurentius (Ron) Hoogenboom, Jean‐Charles Leblanc, Carlo Stefano Nebbia, Elsa Nielsen, Evangelia Ntzani, Annette Petersen, Salomon Sand, Dieter Schrenk, Tanja Schwerdtle, Christiane Vleminckx and Heather Wallace
Acknowledgements: The Panel wishes to thank the following for the support provided to this scientific output: Elena Rovesti. The Panel wishes to acknowledge all European competent institutions, Member State bodies and other organisations that provided data for this scientific output.
Adopted: 24 September 2020
Notes
Commission Recommendation (EU) 2016/1111 of 6 July 2016 on the monitoring of nickel in food. C/2016/3858. OJ L 183, 8.7.2016, p. 70–71.
Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety. OJ L 31, 1.2.2002, p. 1–24.
Regulation (EC) No 1935/2004 of the European Parliament and of the Council of 27 October 2004 on materials and articles intended to come into contact with food and repealing Directives 80/590/EEC and 89/109/EEC. OJ L 338, 13.11.2004, p. 4–17.
Commission Regulation (EC) No 2023/2006 of 22 December 2006 on good manufacturing practice for materials and articles intended to come into contact with food (Text with EEA relevance). OJ L 384, 29.12.2006, p. 75–78.
Commission Regulation (EU) No 10/2011 of 14 January 2011 on plastic materials and articles intended to come into contact with food. OJ L 12, 15.1.2011, p. 1.
Council Directive 98/83/EC of 3 November 1998 on the quality of water intended for human consumption. OJ L 330, 5.12.1998, p. 1–28.
Commission Directive 2003/40/EC of 16 May 2003 establishing the list, concentration limits, and labelling requirements for the constituents of natural mineral waters and the conditions for using ozone‐enriched air for the treatment of natural mineral waters and spring waters. OJ L 126, 22.5.2003, p. 34–39.
Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on classification, labelling and packaging of substances and mixtures, amending and repealing Directives 67/548/EEC and 1999/45/EC, and amending Regulation (EC) No 1907/2006. OJ L 353, 31.12.2008, p. 1–1355.
Web of Science (WoS), formally ISI Web of Knowledge, Thomson Reuters. Available online: http://thomsonreuters.com/thomson-reuters-web-of-science/
PubMed, Entrez Global Query Cross‐Database Search System, National Center for Biotechnology Information (NCBI), National Library of Medicine (NLM), Department of the National Institutes of Health (NIH), United States Department of Health and Human Services. Available online: http://www.ncbi.nlm.nih.gov/pubmed/
EndNote X5, Thomson Reuters. Available at: http://endnote.com/
Calculated as implantation scar count minus the number of live pups at delivery.
The calculated indices were defined as follows: gestation index (%) = (number of females delivering live young/number of females with evidence of pregnancy) × 100; live birth index (%) = (number of live offspring/number of offspring delivered) × 100; viability index = (number of live offspring at PND 4, 7, 14/number of offspring delivered) × 100; weaning index (%) = (number of live offspring at day 21/number of live offspring born) × 100.
European Parliament and Council Directive 94/27/EC of 30 June 1994 amending for the 12th time Directive 76/769/EEC on the approximation of the laws, regulations and administrative provisions of the Member States relating to restrictions on the marketing and use of certain dangerous substances and preparations. OJ L 188, 22.7.1994, p. 1–2. This directive is no longer in force. The restriction is now incorporated into REACH (Annex XVII, Entry 27).
Members of the Bcl‐2 protein family are regulators in the mitochondrial pathway, and the multidomain group of pro‐apoptotic proteins in the Bcl‐2 family, including Bak, can result in the cytochrome c release from mitochondrial membrane.
The incidence of post‐implantation loss was calculated as follows: implantation scar count minus the number of live pups at delivery.
18/10 steel contains 18% chromium and 10% nickel.
References
- Afridi HI, Kazi TG, Talpur FN, Arain S, Arain SS, Kazi N and Panhwar AH, 2014. Distribution of arsenic, cadmium, lead, and nickel levels in biological samples of Pakistani hypertensive patients and control subjects. Clinical Laboratory, 60, 1309–1318. [DOI] [PubMed] [Google Scholar]
- Ahlstrom MG, Thyssen JP, Menne T and Johansen JD, 2017. Prevalence of nickel allergy in Europe following the EU Nickel Directive ‐ a review. Contact Dermatitis, 77, 193–200. [DOI] [PubMed] [Google Scholar]
- Akan A, Toyran M, Vezir E, Azkur D, Kaya A, Erkocoglu M, Civelek E, Dibek Misirlioglu E and Kocabas CN, 2015. The patterns and clinical relevance of contact allergen sensitization in a pediatric population with atopic dermatitis. Turkish Journal of Medical Sciences, 45, 1207–1213. 10.3906/sag-1309-62 [DOI] [PubMed] [Google Scholar]
- Ali S, Chaspoul F, Anderson L, Berge‐Lefranc D, Achard V, Perrin J, Gallice P and Guichaoua M, 2017. Mapping fifteen trace elements in human seminal plasma and sperm DNA. Biological Trace Element Research, 175, 244–253. 10.1007/s12011-016-0772-6 [DOI] [PubMed] [Google Scholar]
- Alimonti A and Mattei D, 2008. Biomarkers for human biomonitoring In: Conti ME. (ed.). Biological Monitoring: Theory & Applications. WIT Press, Southampton, Boston: pp. 163–211. [Google Scholar]
- Almogren A, Adam MH, Shakoor Z, Gadelrab MO and Musa HA, 2013. Th1 and Th2 cytokine profile of CD4 and CD8 positive peripheral blood lymphocytes in nickel contact dermatitis. Central European Journal of Immunology, 38, 100–106. 10.5114/ceji.2013.34365 [DOI] [Google Scholar]
- Ambrose AM, Larson PS, Borzelleca JF and Hennigar GR Jr, 1976. Long term toxicologic assessment of nickel in rats and dogs. Journal of Food Science and Technology, India, 13, 181–187. [Google Scholar]
- American Biogenics Corporation , 1988. Ninety day gavage study in albino rats using nickel. Draft Final Report submitted to Research Triangle Institute, Research Triangle Park, NC. [Google Scholar]
- Ammer H, Henschen A and Lee CH, 1986. Isolation and amino‐acid sequence analysis of human sperm protamines P1 and P2. Occurrence of two forms of protamine P2. Biol Chem Hoppe Seyler, 367, 515–522. 10.1515/bchm3.1986.367.1.515 [DOI] [PubMed] [Google Scholar]
- Ancona C, Bauleo L, Biscotti G, Bocca B, Caimi S, Cruciani F, Di Lorenzo S, Petrolati M, Pino A, Piras G, Pizzabiocca A, Rabbiosi S, Ruggieri F, Salatino C, Alimonti A and Forastiere F, 2016. A survey on lifestyle and level of biomarkers of environmental exposure in residents in Civitavecchia (Italy). Annali dell'Istituto Superiore di Sanità, 52, 488–494. 10.4415/ANN_16_04_05 [DOI] [PubMed] [Google Scholar]
- Andrioli M, Trimboli P, Maio D, Persani L and Minelli M, 2015. Systemic nickel allergic syndrome as an immune‐mediated disease with an increased risk for thyroid autoimmunity. Endocrine, 50, 807–810. 10.1007/s12020-015-0581-2 [DOI] [PubMed] [Google Scholar]
- Aparicio‐Soto M, Riedel F, Leddermann M, Bacher P, Scheffold A, Kuhl H, Timmermann B, Chudakov DM, Molin S, Worm M, Heine G, Thierse HJ, Luch A and Siewert K, 2020. TCRs with segment TRAV9‐2 or a CDR3 histidine are overrepresented among nickel‐specific CD4+ T cells. Allergy. 10.1111/all.14322 [DOI] [PubMed] [Google Scholar]
- Asakawa S, Kishimoto Y, Takano T, Okita K, Takakuwa S, Sato T, Hiratsuka M, Takeuchi O and Hirasawa N, 2015. Nickel ions selectively inhibit lipopolysaccharide‐induced interleukin‐6 production by decreasing its mRNA stability. PLoS ONE, 10, e0119428 10.1371/journal.pone.0119428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asakawa S, Onodera R, Kasai K, Kishimoto Y, Sato T, Segawa R, Mizuno N, Ogasawara K, Moriya T, Hiratsuka M and Hirasawa N, 2018. Nickel ions bind to HSP90 beta and enhance HIF‐1 alpha‐mediated IL‐8 expression. Toxicology, 395, 45–53. [DOI] [PubMed] [Google Scholar]
- Aslan N, Sezikli M and Erdal E, 2017. Nickel sensitivity in patients with gastroesophageal reflux disease. Cutaneous and Ocular Toxicology, 36, 347–350. [DOI] [PubMed] [Google Scholar]
- ATSDR (Agency for Toxic Substances and Disease Registry), 2005. Toxicological Profile for Nickel. Agency for Toxic Substances and Disease Registry, US Department of Health and Human Services. Atlanta, USA. Available online: http://www.atsdr.cdc.gov/toxprofiles/tp15.pdf
- Babaahmadifooladi M, Jacxsens L, De Meulenaer B and Du Laing G, 2020a. Nickel in foods sampled on the Belgian market: identification of potential contamination sources. Food Additives & Contaminants: Part A: Chemistry, Analysis, Control, Exposure & Risk Assessment, 37, 607–621. 10.1080/19440049.2020.1714751 [DOI] [PubMed] [Google Scholar]
- Babaahmadifooladi M, Jacxsens L, Van de Wiele T and Laing GD, 2020b. Gap analysis of nickel bioaccessibility and bioavailability in different food matrices and its impact on the nickel exposure assessment. Food Research International, 129, 108866 10.1016/j.foodres.2019.108866 [DOI] [PubMed] [Google Scholar]
- Baierle M, Charao MF, Goethel G, Barth A, Fracasso R, Bubols G, Sauer E, Campanharo SC, Rocha RC, Saint'Pierre TD, Bordignon S, Zibetti M, Trentini CM, Avila DS, Gioda A, Garcia SC, 2014. Are delta‐aminolevulinate dehydratase inhibition and metal concentrations additional factors for the age‐related cognitive decline? International Journal of Environmental Research and Public Health, 11, 10851–10867. 10.3390/ijerph111010851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bal W and Kasprzak KS, 2002. Induction of oxidative DNA damage by carcinogenic metals. Toxicology Letters, 127, 55–62. [DOI] [PubMed] [Google Scholar]
- Bal W, Jezowska‐Bojczuk M and Kasprzak KS, 1997a. Binding of nickel(II) and copper(II) to the N‐terminal sequence of human protamine HP2. Chemical Research in Toxicology, 10, 906–914. 10.1021/tx970028x [DOI] [PubMed] [Google Scholar]
- Bal W, Lukszo J and Kasprzak KS, 1997b. Mediation of oxidative DNA damage by nickel(II) and copper(II) complexes with the N‐terminal sequence of human protamine HP2. Chemical Research in Toxicology, 10, 915–921. 10.1021/tx970029p [DOI] [PubMed] [Google Scholar]
- Balhorn R, Reed S and Tanphaichitr N, 1988. Aberrant protamine 1/protamine 2 ratios in sperm of infertile human males. Experientia, 44, 52–55. 10.1007/bf01960243 [DOI] [PubMed] [Google Scholar]
- Banfalvi G, 2014. Apoptotic agents inducing genotoxicity‐specific chromatin changes. Apoptosis, 19, 1301–1316. 10.1007/s10495-014-1018-8 [DOI] [PubMed] [Google Scholar]
- Bartos A, Majak I and Leszczyńska J, 2014. Uptake and assimilability of nickel in the course of systemic allergy: implications for elimination diet. Food Research International, 55, 412–417. 10.1016/j.foodres.2013.11.044 [DOI] [Google Scholar]
- Bechara R, Antonios D, Azouri H and Pallardy M, 2017. Nickel sulfate promotes IL‐17A producing CD4D T cells by an IL‐23‐dependent mechanism regulated by TLR4 and Jak‐STAT pathways. Journal of Investigative Dermatology, 137, 2140–2148. [DOI] [PubMed] [Google Scholar]
- Bechara R, Nabhan M, Antonios D, Azouri H and Pallardy M, 2018. IL‐27 production and regulation in human dendritic cells treated with the chemical sensitizer NiSO4 . Chemical Research in Toxicology, 31, 1323–1331. [DOI] [PubMed] [Google Scholar]
- Belliardo C, Di Giorgio C, Chaspoul F, Gallice P and Berge‐Lefranc D, 2018. Direct DNA interaction and genotoxic impact of three metals: cadmium, nickel and aluminum. Journal of Chemical Thermodynamics, 125, 271–277. [Google Scholar]
- Belokopytova IA, Kostyleva EI, Tomilin AN and Vorob'ev VI, 1993. Human male infertility may be due to a decrease of the protamine P2 content in sperm chromatin. Molecular Reproduction and Development, 34, 53–57. 10.1002/mrd.1080340109 [DOI] [PubMed] [Google Scholar]
- Bian J, Shi X, Li Q, Zhao M, Wang L, Lee J, Tao M and Wu X, 2019. A novel functional role of nickel in sperm motility and eukaryotic cell growth. Journal of Trace Elements in Medicine and Biology, 54, 142–149. 10.1016/j.jtemb.2019.04.017 [DOI] [PubMed] [Google Scholar]
- Blażewicz A, Klatka M, Astel A, Partyka M and Kocjan R, 2013. Differences in trace metal concentrations (Co, Cu, Fe, Mn, Zn, Cd, and Ni) in whole blood, plasma, and urine of obese and nonobese children. Biological Trace Element Research, 155, 190–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocca B, Forte G, Senofonte O, Violante N, Paoletti L, De Berardis B, Petrucci F and Cristaudo A, 2007. A pilot study on the content and the release of Ni and other allergenic metals from cheap earrings available on the Italian market. The Science of the Total Environment, 388, 24–34. 10.1016/j.scitotenv.2007.07.027 [DOI] [PubMed] [Google Scholar]
- Boonchai W, Chaiwanon O and Kasemsarn P, 2014. Risk assessment for nickel contact allergy. Journal of Dermatology, 41, 1065–1068. 10.1111/1346-8138.12663 [DOI] [PubMed] [Google Scholar]
- Borghini R, Puzzono M, Rosato E, Di Tola M, Marino M, Greco F and Picarelli A, 2016. Nickel‐related intestinal mucositis in IBS‐like patients: laser doppler perfusion imaging and oral mucosa patch test in use. Biological Trace Element Research, 173, 55–61. [DOI] [PubMed] [Google Scholar]
- Borghini R, Simoncelli M, Marino M, Casale R, Porpora MG and Picarelli A, 2018. Relationship between nickel allergic contact mucositis and nickel‐rich diet in symptomatic women suffering from endometriosis. Gastroenterology, 154, S683. [Google Scholar]
- Braga M, Quecchia C, Perotta C, Timpini A, Maccarinelli K, Di Tommaso L and Di Gioacchino M, 2013. Systemic nickel allergy syndrome: nosologic framework and usefulness of diet regimen for diagnosis. International Journal of Immunopathology and Pharmacology, 26, 707–716. 10.1177/039463201302600314 [DOI] [PubMed] [Google Scholar]
- Brocato J and Costa M, 2015. 10th NTES conference: nickel and arsenic compounds alter the epigenome of peripheral blood mononuclear cells. Journal of Trace Elements in Medicine and Biology, 31, 209–213. 10.1016/j.jtemb.2014.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broday L, Peng W, Kuo MH, Salnikow K, Zoroddu M and Costa M, 2000. Nickel compounds are novel inhibitors of histone H4 acetylation. Cancer Research, 60, 238–241. [PubMed] [Google Scholar]
- Bunick D, Balhorn R, Stanker LH and Hecht NB, 1990. Expression of the rat protamine 2 gene is suppressed at the level of transcription and translation. Experimental Cell Research, 188, 147–152. 10.1016/0014-4827(90)90290-q [DOI] [PubMed] [Google Scholar]
- Büyükoztürk S, Gelincik A, Unal D, Demirturk M, Celik DD, Erden S, Colakoglu B and Erdem Kuruca S, 2015. Oral nickel exposure may induce type I hypersensitivity reaction in nickel‐sensitized subjects. International Immunopharmacology, 26, 92–96. 10.1016/j.intimp.2015.03.012 [DOI] [PubMed] [Google Scholar]
- Cacho C, Brito B, Palacios J, Perez‐Conde C and Camara C, 2010. Speciation of nickel by HPLC‐UV/MS in pea nodules. Talanta, 83, 78–83. 10.1016/j.talanta.2010.08.044 [DOI] [PubMed] [Google Scholar]
- Calin GA, Dumitru CD, Zhimizu M, Bichi R, Zupo S, Noch E, Aldler H, Rattan S, Keating M, Rai K, Rassenti L, Kipps T, Negrini M, Bullrich F and Croce CM, 2002. Frequent deletions and down‐regulation of micro‐RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proceedings of the National Academy of Sciences of the United States of America, 99, 15524–15529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrell DT, Emery BR and Hammoud S, 2007. Altered protamine expression and diminished spermatogenesis: what is the link? Hum Reprod Update, 13, 313–327. 10.1093/humupd/dml057 [DOI] [PubMed] [Google Scholar]
- Casalegno C, Schifanella O, Zennaro E, Marroncelli S and Briant R, 2015. Collate literature data on toxicity of Chromium (Cr) and Nickel (Ni) in experimental animals and humans. EFSA Supporting Publications 2015;EN‐478, 287 pp. 10.2903/sp.efsa.2015.EN-478 [DOI] [Google Scholar]
- Ceci C, Barbaccia ML and Pistritto G, 2015. A not cytotoxic nickel concentration alters the expression of neuronal differentiation markers in NT2 cells. NeuroToxicology, 47, 47–53. 10.1016/j.neuro.2015.01.001 [DOI] [PubMed] [Google Scholar]
- Chana M, Lewis JB, Davis R, Elam Y, Hobbs D, Lockwood PE, Wataha JC and Messer RL, 2018. Biological effects of Ni(II) on monocytes and macrophages in normal and hyperglycemic environments. Journal of Biomedical Material Research Part A, 106, 2433–2439. 10.1002/jbm.a.36437 [DOI] [PubMed] [Google Scholar]
- Chekri R, Le Calvez E, Zinck J, Leblanc J‐C, Sirot V, Hulin M, Noël L and Guérin T, 2019. Trace element contents in foods from the first French total diet study on infants and toddlers. Journal of Food Composition and Analysis, 78, 108–120. 10.1016/j.jfca.2019.02.002 [DOI] [Google Scholar]
- Chellini E, Maurello MT, Cortini B and Aprea C, 2017. Human bio‐monitoring study around a plant that recycles and refines precious metals in Central Italy. Science of the Total Environment, 584–585, 348–354. 10.1016/j.scitotenv.2016.12.178 [DOI] [PubMed] [Google Scholar]
- Chen H, Kluz T, Zhang R and Costa M, 2010. Hypoxia and nickel inhibit histone demethylase JMJD1A and repress Spry2 expression in human bronchial epithelial BEAS‐2B cells. Carcinogenesis, 31, 2136–2144. 10.1093/carcin/bgq197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen QY, Brocato J, Laulicht F and Costa M, 2017. Mechanisms of Nickel carcinogenesis In: Mudipalli A. and Zelikoff JT. (eds.). Essential and Non‐Essential Metals: Carcinogenesis, Prevention and Cancer Therapeutics. Springer International Publishing AG, Cham Switzerland: pp. 181–197. [Google Scholar]
- Chen X, Li Y, Zhang B, Zhou A, Zheng T, Huang Z, Pan X, Liu W, Liu H, Jiang Y, Sun X, Hu C, Xing Y, Xia W and Xu S, 2018. Maternal exposure to nickel in relation to preterm delivery. Chemosphere, 193, 1157–1163. [DOI] [PubMed] [Google Scholar]
- Cherfi A, Cherfi M, Maache‐Rezzoug Z and Rezzoug SA, 2016. Risk assessment of heavy metals via consumption of vegetables collected from different supermarkets in La Rochelle, France. Environmental Monitoring and Assessment, 188, 136 10.1007/s10661-016-5140-7 [DOI] [PubMed] [Google Scholar]
- Chiesa LM, Ceriani F, Caligara M, Di Candia D, Malandra R, Panseri S and Arioli F, 2018. Mussels and clams from the italian fish market. Is there a human exposition risk to metals and arsenic? Chemosphere, 194, 644–649. 10.1016/j.chemosphere.2017.12.041 Epub 2017 Dec 22. [DOI] [PubMed] [Google Scholar]
- Cieslik I, Migdal W, Topolska K, Gambus F, Szczurowska X and Cieslik E, 2018. Changes in the content of heavy metals (PB, CD, HG, AS, NI, CR) in freshwater fish after processing ‐ the consumer's exposure. Journal of Elementology, 23, 247–259. 10.5601/jelem.2017.22.2.1436 [DOI] [Google Scholar]
- Cifci N, 2019. Nickel sensitivity in rosacea patients: a prospective case control stud. Endocrine Metabolic & Immune Disorders ‐ Drug Targets, 19, 367–372. 10.2174/1871530319666190101120437 [DOI] [PubMed] [Google Scholar]
- Copat C, Grasso A, Fiore M, Cristaldi A, Zuccarello P, Signorelli SS, Conti GO and Ferrante M, 2018. Trace elements in seafood from the Mediterranean sea: an exposure risk assessment. Food and Chemical Toxicology, 115, 13–19. 10.1016/j.fct.2018.03.001 [DOI] [PubMed] [Google Scholar]
- Corzett M, Mazrimas J and Balhorn R, 2002. Protamine 1: protamine 2 stoichiometry in the sperm of eutherian mammals. Molecular Reproduction and Development, 61, 519–527. 10.1002/mrd.10105 [DOI] [PubMed] [Google Scholar]
- Costa M, Davidson TL, Chen H, Ke Q, Zhang P, Yan Y, Huang C and Kluz T, 2005. Nickel carcinogenesis: epigenetics and hypoxia signaling. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis, 592, 79–88. 10.1016/j.mrfmmm.2005.06.008 [DOI] [PubMed] [Google Scholar]
- COT (Committee on Toxicity), 2018. Statement on potential risks from nickel in the diet of infants aged 0 to 12 months and children aged 1 to 5 years. COT Statement 2018/02. 32 pp.
- Croce CM, 2009. Causes and consequences of microRNA dysregulation in cancer. Nature Review Genetics, 10, 704–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubadda F, 2020. R: Italian TDS_results on nickel. Message to Baert K., 8, May 2020. [Google Scholar]
- Cubadda F, Iacoponi F, Ferraris F, D'Amato M, Aureli F, Raggi A, Sette S, Turrini A and Mantovani A, 2020. Dietary exposure of the Italian population to nickel: the national Total Diet Study. Food and Chemical Toxicology, 146, December 2020, 111813. 10.1016/j.fct.2020.111813 [DOI] [PubMed] [Google Scholar]
- Czarnek K, Terpilowska S and Siwicki AK, 2019. Genotoxicity and mutagenicity of nickel(II) and iron(III) and interactions between these microelements. Trace Elements and Electrolytes, 36, 17–24. [Google Scholar]
- Danadevi K, Rozati R, Saleha Banu B and Grover P, 2004. In vivo genotoxic effect of nickel chloride in mice leukocytes using comet assay. Food and Chemical Toxicology, 42, 751–757. 10.1016/j.fct.2003.12.013 [DOI] [PubMed] [Google Scholar]
- Davidson T, Salnikow K and Costa M, 2003. Hypoxia inducible factor‐1 alpha‐independent suppression of aryl hydrocarbon receptor‐regulated genes by nickel. Molecular Pharmacology, 64, 1485–1493. 10.1124/mol.64.6.1485 [DOI] [PubMed] [Google Scholar]
- Davidson T, Chen H, Garrick MD, D'Angelo G and Costa M, 2005. Soluble nickel interferes with cellular iron homeostasis. Molecular and Cellular Biochemistry, 279, 157–162. 10.1007/s11010-005-8288-y [DOI] [PubMed] [Google Scholar]
- Davidson TL, Chen H, Di Toro DM, D'Angelo G and Costa M, 2006. Soluble nickel inhibits HIF‐prolyl‐hydroxylases creating persistent hypoxic signaling in A549 cells. Molecular Carcinogenesis, 45, 479–489. 10.1002/mc.20176 [DOI] [PubMed] [Google Scholar]
- Dede E, Tindall MJ, Cherrie JW, Hankin S and Collins C, 2018. Physiologically‐based pharmacokinetic and toxicokinetic models for estimating human exposure to five toxic elements through oral ingestion. Environmental Toxicology and Pharmacology, 57, 104–114. 10.1016/j.etap.2017.12.003 [DOI] [PubMed] [Google Scholar]
- Deknudt G and Leonard A, 1982. Mutagenicity tests with nickel salts in the male mouse. Toxicology, 25, 289–292. [DOI] [PubMed] [Google Scholar]
- Deng J, Guo H, Cui H, Fang J, Zuo Z, Deng J, Wang X and Zhao L, 2016. Oxidative stress and inflammatory responses involved in dietary nickel chloride (NiCl2)‐induced pulmonary toxicity in broiler chickens. Toxicology Research (Camb), 5, 1421–1433. 10.1039/c6tx00197a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Bucchianico S, Gliga AR, Akerlund E, Skoglund S, Wallinder IO, Fadeel B and Karlsson HL, 2018. Calcium‐dependent cyto‐ and genotoxicity of nickel metal and nickel oxide nanoparticles in human lung cells. Particle and Fibre Toxicology, 15, 32 10.1186/s12989-018-0268-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Gioacchino M, Ricciardi L, De Pita O, Minelli M, Patella V, Voltolini S, Di Rienzo V, Braga M, Ballone E, Mangifesta R and Schiavino D, 2014. Nickel oral hyposensitization in patients with systemic nickel allergy syndrome. Annals of Medicine, 46, 31–37. 10.3109/07853890.2013.861158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dukes MP, Rowe RK, Harvey T, Rangel W and Pedigo S, 2019. Nickel reduces calcium dependent dimerization in neural cadherin. Metallomics, 11, 475–482. 10.1039/c8mt00349a [DOI] [PubMed] [Google Scholar]
- Dyring‐Andersen B, Skov L, Lovendorf MB, Bzorek M, Sondergaard K, Lauritsen J‐PH, Dabelsteen S, Geisler C and Bonefeld CM, 2013. CD4(+) T cells producing interleukin (IL)‐17, IL‐22 and interferon‐gamma are major effector T cells in nickel allergy. Contact Dermatitis, 68, 339–347. [DOI] [PubMed] [Google Scholar]
- ECHA (European Chemicals Agency), 2012. Guidance on information requirements and chemical safety assessment. Chapter R.8: characterisation of dose [concentration]‐response for human health. 186 pp.
- EDQM (European Directorate for the Quality of Medicines & HealthCare), 2013. Metals and alloys used in food contact materials and articles. A practical guide for manufacturers and regulators. 218 pp.
- EFSA (European Food Safety Authority), 2007. Guidance of the Scientific Committee related to Uncertainties in Dietary Exposure Assessment. EFSA Journal 2007;5(1):438, 54 pp. 10.2903/j.efsa.2007.438 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2009. Guidance of the Scientific Committee on a request from EFSA on the use of the benchmark dose approach in risk assessment. EFSA Journal 2009;7(6):1150, 72 pp. 10.2903/j.efsa.2009.1150 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2010a. Standard sample description for food and feed. EFSA Journal 2010;8(1):1457, 54 pp. 10.2903/j.efsa.2010.1457 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2010b. Management of left‐censored data in dietary exposure assessment of chemical substances. EFSA Journal 2010;8(3):1557, 96 pp. 10.2903/j.efsa.2010.1557 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2011a. Evaluation of the FoodEx, the food classification system applied to the development of the EFSA Comprehensive European Food Consumption Database. EFSA Journal 2011;9(3):1970, 27 pp. 10.2903/j.efsa.2011.1970 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2011b. Use of the EFSA Comprehensive European Food Consumption Database in Exposure Assessment. EFSA Journal 2011;9(3):2097, 34 pp. 10.2903/j.efsa.2011.2097 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2020. Outcome of a public consultation on the draft update of the risk assessment of nickel in food and drinking water. EFSA Supporting Publications 2020;EN‐1940, 54 pp. 10.2903/sp.efsa.2020.EN-1940 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2015. Scientific Opinion on the risks to public health related to the presence of nickel in food and drinking water. EFSA Journal 2015;13(2):4002, 202 pp. 10.2903/j.efsa.2015.4002 [DOI] [Google Scholar]
- EFSA Scientific Committee, 2009. Guidance of the Scientific Committee on Transparency in the Scientific Aspects of Risk Assessments carried out by EFSA. Part 2: general Principles. EFSA Journal 2009;7(6):1051, 1051 pp. 10.2903/j.efsa.2009.1051 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2011. Scientific opinion on genotoxicity testing strategies applicable to food and feed safety assessment. EFSA Journal 2011;9(9):2379, 69 pp. 10.2903/j.efsa.2011.2379 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2012a. Guidance on selected default values to be used by the EFSA Scientific Committee, Scientific Panels and Units in the absence of actual measured data. EFSA Journal 2012;10(3):2579, 32 pp. 10.2903/j.efsa.2012.2579 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2012b. Scientific Opinion on Risk Assessment Terminology. EFSA Journal 2012;10(5):2664, 43 pp. 10.2903/j.efsa.2012.2664 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2017. Update: use of the benchmark dose approach in risk assessment. EFSA Journal 2017;15(1):4658, 41 pp. 10.2903/j.efsa.2017.4658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA , Arcella D and Gómez Ruiz J, 2018a. Technical report on use of cut‐off values on the limits of quantification reported in datasets used to estimate dietary exposure to chemical contaminants. EFSA supporting publication 2018;EN‐1452. 11 pp. 10.2903/sp.efsa.2018.en-1452. [DOI] [Google Scholar]
- EFSA , Arcella D, Ioannidou S and Sousa R, 2018b. Internal report on the harmonisation of dilution factors to be used in the assessment of dietary exposure. 10.5281/zenodo.1256085 [DOI]
- El‐Habit OH and Abdel Moneim AE, 2014. Testing the genotoxicity, cytotoxicity, and oxidative stress of cadmium and nickel and their additive effect in male mice. Biological Trace Element Research, 159, 364–372. 10.1007/s12011-014-0016-6 [DOI] [PubMed] [Google Scholar]
- EU RAR (European Union Risk Assessment Report), 2008. European Union Risk Assessment Report: Nickel and nickel compounds. Office for Official Publications of the European Communities, 1715 pp. Available online: http://echa.europa.eu/documents/10162/cefda8bc-2952-4c11-885f-342aacf769b3
- Fahmy MA, Hassan NHA, Melek FR, Hassan ZM and Al‐Ashaal HA, 2014. Studies on the genotoxic effect of nickel chloride in mice and the possible protective role of soybean seeds extracts. Global Journal of Pharmacology, 8, 625–634. [Google Scholar]
- FERA (The Food and Environment Research Agency), 2015. Total Diet Study of metals and other elements in food. 69 pp.
- Ferko MA and Catelas I, 2018. Effects of metal ions on caspase‐1 activation and interleukin‐1beta release in murine bone marrow‐derived macrophages. PLoS ONE, 13, e0199936 10.1371/journal.pone.0199936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint GN and Packirisamy S, 1995. Systemic nickel ‐ the contribution made by stainless‐steel cooking utensils. Contact Dermatitis, 32, 218–224. 10.1111/j.1600-0536.1995.tb00672.x [DOI] [PubMed] [Google Scholar]
- Flint GN and Packirisamy S, 1997. Purity of food cooked in stainless steel utensils. Food Additives and Contaminants, 14, 115–126. 10.1080/02652039709374506 [DOI] [PubMed] [Google Scholar]
- Freitas M, Barcellos‐de‐Souza P, Barja‐Fidalgo C and Fernandes E, 2013. Nickel induces apoptosis in human neutrophils. BioMetals, 26, 13–21. 10.1007/s10534-012-9590-2 [DOI] [PubMed] [Google Scholar]
- Friedmann PS, 2007. The relationships between exposure dose and response in induction and elicitation of contact hypersensitivity in humans. British Journal of Dermatology, 157, 1093–1102. 10.1111/j.1365-2133.2007.08162.x [DOI] [PubMed] [Google Scholar]
- Gathwan KH, 2015a. Comparative effect of orally fed nickel intoxicants on liver of male mice and observation under scanning electron microscope (SEM). World Journal of Pharmaceutical Research, 4, 179–185. [Google Scholar]
- Gathwan KH, 2015b. Histopathological on kidney of male mice orally intoxicated with nickel chloride. Elixir International Journal, 35400–35401. [Google Scholar]
- Gathwan KH and Albir AA, 2019. The effect of nickel sulphate on bone composition in mice. International Journal of Biosciences, 14, 183–185. [Google Scholar]
- Gathwan KH and Al‐Karkhi I, 2015. Histological and ultrastructural studies on bone of mice intoxicated with nickel salts. Elixir International Journal, 30799–30802. [Google Scholar]
- Gawkrodger DJ, Cook SW, Fell GS and Hunter JA, 1986. Nickel dermatitis: the reaction to oral nickel challenge. British Journal of Dermatology, 115, 33–38. [DOI] [PubMed] [Google Scholar]
- Golebiowski F and Kasprzak KS, 2005. Inhibition of core histones acetylation by carcinogenic nickel(II). Molecular and Cellular Biochemistry, 279, 133–139. [DOI] [PubMed] [Google Scholar]
- Gölz L, Buerfent BC, Hofmann A, Ruhl H, Fricker N, Stamminger W, Oldenburg J, Deschner J, Hoerauf A, Nothen MM, Schumacher J, Hubner MP and Jager A, 2016. Genome‐wide transcriptome induced by nickel in human monocytes. Acta Biomaterialia, 43, 369–382. 10.1016/j.actbio.2016.07.047 [DOI] [PubMed] [Google Scholar]
- Govindarajan B, Klafter R, Miller MS, Mansur C, Mizesko M, Bai XH, LaMontagne K and Arbiser JL, 2002. Reactive oxygen‐induced carcinogenesis causes hypermethylation of p16(Ink4a) and activation of MAP kinase. Molecular Medicine, 8, 1–8. [PMC free article] [PubMed] [Google Scholar]
- Grashow R, Zhang J, Fang SC, Weisskopf MG, Christiani DC and Cavallari JM, 2014. Toenail metal concentration as a biomarker of occupational welding fume exposure. Journal of Occupational and Environmental Hygiene, 11, 397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarneri F, Costa C, Cannavo SP, Catania S, Bua GD, Fenga C and Dugo G, 2016. Release of nickel and chromium in common foods during cooking in 18/10 (grade 316) stainless steel pots. Contact Dermatitis, 76, 40–48. 10.1111/cod.12692 [DOI] [PubMed] [Google Scholar]
- Guo H, Deng H, Cui H, Peng X, Fang J, Zuo Z, Deng J, Wang X, Wu B and Chen K, 2015. Nickel chloride (NiCl2)‐caused inflammatory responses via activation of NF‐kappaB pathway and reduction of anti‐inflammatory mediator expression in the kidney. Oncotarget, 6, 28607–28620. 10.18632/oncotarget.5759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, Lv N, Tang J, Zhang X, Peng L, Du X, Li S, Luo Q, Zhang D and Chen G, 2018. Associations of blood metal exposure with thyroid hormones in Chinese pregnant women: a cross‐sectional study. Environment International, 121, 1185–1192. 10.1016/j.envint.2018.10.038 [DOI] [PubMed] [Google Scholar]
- Halder A, Jain M, Chaudhary I, Kumar G, Das T and Gupta YK, 2014. Dark‐coloured semen in nonobstructive azoospermia: a report of four cases. Andrologia, 46, 316–321. 10.1111/and.12078 [DOI] [PubMed] [Google Scholar]
- Han A, Zou L, Gan X, Li Y, Liu F, Chang X, Zhang X, Tian M, Li S, Su L and Sun Y, 2018. ROS generation and MAPKs activation contribute to the Ni‐induced testosterone synthesis disturbance in rat Leydig cells. Toxicology Letters, 290, 36–45. 10.1016/j.toxlet.2018.03.016 [DOI] [PubMed] [Google Scholar]
- He M‐D, Xu S‐C, Zhang X, Wang Y, Xiong J‐C, Zhang X, Lu Y‐H, Zhang L, Yu Z‐P and Zhou Z, 2013a. Disturbance of aerobic metabolism accompanies neurobehavioral changes induced by nickel in mice. NeuroToxicology, 38, 9–16. [DOI] [PubMed] [Google Scholar]
- He M, Mao L, Yu Z and Zhou Z, 2013b. MiRNA‐210 modulates nickel‐induced hypoxic responses by repressing the iron‐sulfur cluster assembly proteins ISCU1/2. Toxicology Letters, 221, S154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He M, Lu Y, Xu S, Mao L, Zhang L, Duan W, Liu C, Pi H, Zhang Y, Zhong M, Yu Z and Zhou Z, 2014. MiRNA‐210 modulates a nickel‐induced cellular energy metabolism shift by repressing the iron‐sulfur cluster assembly proteins ISCU1/2 in Neuro‐2a cells. Cell Death and Disease, 5, e1090 10.1038/cddis.2014.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Health Canada , 1994. Nickel and Its Compounds. Priority Substances List Assessment Report. Canadian Environmental Protection Act. National Printers, Inc. Ottawa. Available online: http://www.hc-sc.gc.ca/ewh-semt/alt_formats/hecs-sesc/pdf/pubs/contaminants/psl1-lsp1/compounds_nickel_composes/nickel-eng.pdf
- Heiss K, Junkes C, Guerreiro N, Swamy M, Camacho‐Carvajal MM, Schamel WW, Haidl ID, Wild D, Weltzien HU and Thierse HJ, 2005. Subproteomic analysis of metal‐interacting proteins in human B cells. Proteomics, 5, 3614–3622. 10.1002/pmic.200401215 [DOI] [PubMed] [Google Scholar]
- Hindsén M, Bruze M and Christensen OB, 2001. Flare‐up reactions after oral challenge with nickel in relation to challenge dose and intensity and time of previous patch test reactions. Journal of the American Academy of Dermatology, 44, 616–623. 10.1067/mjd.2001.110873 [DOI] [PubMed] [Google Scholar]
- Hoet P, Jacquerye C, Deumer G, Lison D and Haufroid V, 2013. Reference values and upper reference limits for 26 trace elements in the urine of adults living in Belgium. Clinical Chemistry and Laboratory Medicine, 51, 839–849. 10.1515/cclm-2012-0688 [DOI] [PubMed] [Google Scholar]
- Huang J, Cui H, Peng X, Fang J, Zuo Z, Deng J and Wu B, 2013. The association between splenocyte apoptosis and alterations of Bax, Bcl‐2 and caspase‐3 mRNA expression, and oxidative stress induced by dietary nickel chloride in broilers. International Journal of Environmental Research and Public Health, 10, 7310–7326. 10.3390/ijerph10127310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Cui H, Peng X, Fang J, Zuo Z, Deng J, Wang X and Wu B, 2014a. Effect of dietary nickel chloride on splenic immune function in broilers. Biological Trace Element Research, 159, 183–191. [DOI] [PubMed] [Google Scholar]
- Huang J, Cui H, Peng X, Fang J, Zuo Z, Deng J, Wang X and Wu B, 2014b. Downregulation of TLR4 and 7 mRNA expression levels in broiler's spleen caused by diets supplemented with nickel chloride. Biological Trace Element Research, 158, 353–358. [DOI] [PubMed] [Google Scholar]
- Huybrechts I, Sioen I, Boon PE, Ruprich J, Lafay L, Turrini A, Amiano P, Hirvonen T, De Neve M, Arcella D, Moschandreas J, Westerlund A, Ribas‐Barba L, Hilbig A, Papoutsou S, Christensen T, Oltarzewski M, Virtanen S, Rehurkova I, Azpiri M, Sette S, Kersting M, Walkiewicz A, SerraMajem L, Volatier JL, Trolle E, Tornaritis M, Busk L, Kafatos A, Fabiansson S, De Henauw S and Van Klaveren J, 2011. Dietary exposure assessments for children in Europe (the EXPOCHI project): rationale, methods and design. Archives of Public Health, 69, 4 10.1186/0778-7367-1169-1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- IARC (International Agency for Research on Cancer), 1990. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Chromium, nickel, and welding. World Health Organization: Lyon; 257–445. Available online: http://monographs.iarc.fr/ENG/Monographs/vol49/index.php
- IARC (International Agency for Research on Cancer), 2012. Nickel and nickel compounds. IARC Monographs 100 C. World Health Organization: Lyon. Available online: http://monographs.iarc.fr/ENG/Monographs/vol100C/mono100C.pdf
- Ijomone OM, Olatunji SY, Owolabi JO, Naicker T and Aschner M, 2018a. Nickel‐induced neurodegeneration in the hippocampus, striatum and cortex; an ultrastructural insight, and the role of caspase‐3 and alpha‐synuclein. Journal of Trace Elements in Medicine and Biology, 50, 16–23. 10.1016/j.jtemb.2018.05.017 [DOI] [PubMed] [Google Scholar]
- Ijomone OM, Okori SO, Ijomone OK and Ebokaiwe AP, 2018b. Sub‐acute nickel exposure impairs behavior, alters neuronal microarchitecture, and induces oxidative stress in rats’ brain. Drug and Chemical Toxicology, 41, 377–384. 10.1080/01480545.2018.1437173 [DOI] [PubMed] [Google Scholar]
- Iorio MV and Croce CM, 2012. MicroRNA dysregulation in cancer: diagnostics, monitoring and therapeutics. A comprehensive review. EMBO Molecular Medicine, 4, 143–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irfan S, Rani A, Riaz N, Arshad M and Kashif Nawaz S, 2017. Comparative evaluation of heavy metals in patients with rheumatoid arthritis and healthy control in pakistani population. Iranian Journal of Public Health, 46, 626–633. [PMC free article] [PubMed] [Google Scholar]
- Jakob A, Mussotter F, Ohnesorge S, Dietz L, Pardo J, Haidl ID and Thierse HJ, 2017. Immunoproteomic identification and characterization of Ni(2+)‐regulated proteins implicates Ni(2+) in the induction of monocyte cell death. Cell Death and Disease, 8, e2684 10.1038/cddis.2017.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen CS, Menne T, Lisby S, Kristiansen J and Veien NK, 2003. Experimental systemic contact dermatitis from nickel: a dose‐response study. Contact Dermatitis, 49, 124–132. [DOI] [PubMed] [Google Scholar]
- Jensen CS, Menne T and Johansen JD, 2006. Systemic contact dermatitis after oral exposure to nickel: a review with a modified meta‐analysis. Contact Dermatitis, 54, 79–86. 10.1111/j.0105-1873.2006.00773.x [DOI] [PubMed] [Google Scholar]
- Ji W, Yang L, Yuan J, Yang L, Zhang M, Qi D, Duan X, Xuan A, Zhang W, Lu J, Zhuang Z and Zeng G, 2013. MicroRNA‐152 targets DNA methyltransferase 1 in NiS‐transformed cells via a feedback mechanism. Carcinogenesis, 34, 446–453. 10.1093/carcin/bgs343 [DOI] [PubMed] [Google Scholar]
- Johnson N, Shelton BJ, Hopenhayn C, Tucker TT, Unrine JM, Huang B, Christian W, Zhang Z, Shi X and Li L, 2011. Concentrations of arsenic, chromium, and nickel in toenail samples from Appalachian Kentucky residents. Journal of Environmental Pathology, Toxicology and Oncology, 30, 213–223. 10.1615/jenvironpatholtoxicoloncol.v30.i3.40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jose CC, Xu B, Jagannathan L, Trac C, Mallela RK, Hattori T, Lai D, Koide S, Schones DE and Cuddapah S, 2014. Epigenetic dysregulation by nickel through repressive chromatin domain disruption. Proceedings of the National Academy of Sciences of the USA, 111, 14631–14636. 10.1073/pnas.1406923111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahloula K, Adli DEH, Slimani M, Terras H and Achour S, 2014. Effect of nickel chronic exposure on the neurobehavioral functions in Wistar rats during the development period. Toxicologie Analytique et Clinique, 26, 186–192. [Google Scholar]
- Kanaji A, Orhue V, Caicedo MS, Virdi AS, Sumner DR, Hallab NJ, Yoshiaki T and Sena K, 2014. Cytotoxic effects of cobalt and nickel ions on osteocytes in vitro. Journal of Orthopaedic Surgery and Resarch, 9, 91 10.1186/s13018-014-0091-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J, Zhang Y, Chen J, Chen H, Lin J, Wang Q and Ou Y, 2003. Nickel‐induced histone hypoacetylation: the role of reactive oxygen species. Toxicological Sciences, 74, 279–286. [DOI] [PubMed] [Google Scholar]
- Kargacin B, Klein CB and Costa M, 1993. Mutagenic responses of nickel oxides and nickel sulfides in Chinese hamster V79 cell lines at the xanthine‐guanidine phosphoribosyl transferase locus. Mutation Research/Genetic Toxicology, 300, 63–72. 10.1016/0165-1218(93)90141-Y [DOI] [PubMed] [Google Scholar]
- Kasprzak KS, Sunderman FW Jr and Salnikow K, 2003. Nickel carcinogenesis. Mutation Research, 533, 67–97. [DOI] [PubMed] [Google Scholar]
- Kawanishi S, Oikawa S, Inoue S and Nishino K, 2002. Distinct mechanisms of oxidative DNA damage induced by carcinogenic nickel subsulfide and nickel oxides. Environmental Health Perspectives, 110(Suupl 5), 789–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayaalti Z, Akyuzlu DK and Soylemezoglu T, 2015. Evaluation of the effect of divalent metal transporter 1 gene polymorphism on blood iron, lead and cadmium levels. Environmental Research, 137, 8–13. 10.1016/j.envres.2014.11.008 [DOI] [PubMed] [Google Scholar]
- Ke Q, Davidson T, Chen H, Kluz T and Costa M, 2006. Alterations of histone modifications and transgene silencing by nickel chloride. Carcinogenesis, 27, 1481–1488. [DOI] [PubMed] [Google Scholar]
- Khaniki GJ, Khosravi R, Nazmara S and Foroushani AR, 2016. Migration of heavy metals into water and lemon juice stored in cast iron containers. Annals Food Science and Technology, 17, 394–403. [Google Scholar]
- Khlifi R, Olmedo P, Gil F, Hammami B and Hamza‐Chaffai A, 2015. Cadmium and nickel in blood of Tunisian population and risk of nasosinusal polyposis disease. Environmental Science and Pollution Resarch, 22, 3586–3593. [DOI] [PubMed] [Google Scholar]
- Kicinski M, Vrijens J, Vermier G, Hond ED, Schoeters G, Nelen V, Bruckers L, Sioen I, Baeyens W, Van Larebeke N, Viaene MK and Nawrot TS, 2015. Neurobehavioral function and low‐level metal exposure in adolescents. International Journal of Hygiene and Environmental Health, 218, 139–146. [DOI] [PubMed] [Google Scholar]
- Kiilunen M, Utela J, Rantanen T, Norppa H, Tossavainen A, Koponen M, Paakkulainen H and Aitio A, 1997. Exposure to soluble nickel in electrolytic nickel refining. Annals of Occupational Hygiene, 41, 167–188. [DOI] [PubMed] [Google Scholar]
- Kim DS, Kim DH, Lee H, Jee H, Lee Y, Chang M‐y, Kwak T‐j, Kim C‐H, Shin Y‐A, Lee J‐H, T‐j Yoon and Lee M‐G, 2013. A genome‐wide association study in Koreans identifies susceptibility loci for allergic nickel dermatitis. International Archives of Allergy and Immunology, 162, 86–88. 10.1159/000353235 [DOI] [PubMed] [Google Scholar]
- Kimber I and Basketter D, 2008. Thresholds, dose‐response relationships and dose metrics in allergic contact dermatitis. British Journal of Dermatology, 159, 1380–1381; author reply 1381–1382. 10.1111/j.1365-2133.2008.08868.x [DOI] [PubMed] [Google Scholar]
- Klatka M, Blazewicz A, Partyka M, Kollataj W, Zienkiewicz E and Kocjan R, 2015. Concentration of selected metals in whole blood, plasma, and urine in short stature and healthy children. Biological Trace Element Research, 166, 142–148. 10.1007/s12011-015-0262-2 [DOI] [PubMed] [Google Scholar]
- Klein CB, Kargacin B, Su L, Cosentino S, Snow ET and Costa M, 1994. Metal mutagenesis in transgenic Chinese hamster cell lines. Environmental Health Perspectives, 102(Suppl 3), 63–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch W, 2019. Dietary exposure to nickel. Message to Baert K. 5 December 2019.
- Koch W, 2020. RE: Dietary exposure to nickel. Message to Baert K. 17 January 2020.
- Koch W, Karim MR, Marzec Z, Miyataka H, Himeno S and Asakawa Y, 2016. Dietary intake of metals by the young adult population of Eastern Poland: results from a market basket study. Journal of Trace Elements in Medical Biology, 35, 36–42. 10.1016/j.jtemb.2016.01.007 [DOI] [PubMed] [Google Scholar]
- Kopp B, Zalko D and Audebert M, 2018. Genotoxicity of 11 heavy metals detected as food contaminants in two human cell lines. Environmental and Molecular Mutagenesis, 59, 202–210. 10.1002/em.22157 [DOI] [PubMed] [Google Scholar]
- Koppes SA, Engebretsen KA, Agner T, Angelova‐Fischer I, Berents T, Brandner J, Brans R, Clausen ML, Hummler E, Jakasa I, Jurakić‐Tončic R, John SM, Khnykin D, Molin S, Holm JO, Suomela S, Thierse HJ, Kezic S, Martin SF and Thyssen JP, 2017. Current knowledge on biomarkers for contact sensitization and allergic contact dermatitis. Contact Dermatitis, 77, 1–16. 10.1111/cod.12789 [DOI] [PubMed] [Google Scholar]
- Koyama Y, Adachi M, Sekiya M, Takekawa M and Imai K, 2000. Histone deacetylase inhibitors suppress IL‐2‐mediated gene expression prior to induction of apoptosis. Blood, 96, 1490–1495. [PubMed] [Google Scholar]
- Kuroishi T, Bando K, Tanaka Y, Shishido K, Kinbara M, Ogawa T, Muramoto K, Endo Y and Sugawara S, 2017. CXCL4 is a novel nickel‐binding protein and augments nickel allergy. Clinical and Experimental Allergy, 47, 1069–1078. 10.1111/cea.12926 [DOI] [PubMed] [Google Scholar]
- Kwong RW and Niyogi S, 2009. The interactions of iron with other divalent metals in the intestinal tract of a freshwater teleost, rainbow trout (Oncorhynchus mykiss). Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology, 150, 442–449. 10.1016/j.cbpc.2009.06.011 [DOI] [PubMed] [Google Scholar]
- Lambade PS, Joshi DV, Patel BJ, Patel JG, Raval SH and Patel JD, 2015. Clinicopathological studies of experimentally induced nickel chloride in Wistar rats (Rattus norvegicus). Indian Journal of Veterinary Pathology, 39, 41–45. [Google Scholar]
- Lamtai M, Chaibat J, Ouakki S, Zghari O, Mesfioui A, El Hessni A, Rifi E‐H, Marmouzi I, Essamri A and Ouichou A, 2018. Effect of chronic administration of nickel on affective and cognitive behavior in male and female rats: possible implication of oxidative stress pathway. brain sciences, 8 10.3390/brainsci8080141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YW, Klein CB, Kargacin B, Salnikow K, Kitahara J, Dowjat K, Zhitkovich A, Christie NT and Costa M, 1995. Carcinogenic nickel silences gene expression by chromatin condensation and DNA methylation: a new model for epigenetic carcinogens. Molecular and Cellular Biology, 15, 2547–2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YW, Broday L and Costa M, 1998. Effects of nickel on DNA methyltransferase activity and genomic DNA methylation levels. Mutation Research, 415, 213–218. 10.1016/s1383-5718(98)00078-3 [DOI] [PubMed] [Google Scholar]
- Lee Y‐J, Lim S‐S, Baek BJ, An J‐M, Nam H‐S, Woo K‐M, Cho M‐K, Kim S‐H and Lee S‐H, 2016. Nickel(II)‐induced nasal epithelial toxicity and oxidative mitochondrial damage. Environmental Toxicology and Pharmacology, 42, 76–84. 10.1016/j.etap.2016.01.005 [DOI] [PubMed] [Google Scholar]
- Li X and Zhong F, 2014. Nickel induces interleukin‐1beta secretion via the NLRP3‐ASC‐caspase‐1 pathway. Inflammation, 37, 457–466. 10.1007/s10753-013-9759-z [DOI] [PubMed] [Google Scholar]
- Li L, Drury JL, Zhang H, Sun J, DiJulio D, Chung WO and Wataha JC, 2013. Effect of Ni(II) on inflammatory gene expression in THP1 monocytic cells. Journal of Biomedical Materials Research Part B: Applied Biomaterials, 101A, 902–908. [DOI] [PubMed] [Google Scholar]
- Li J, Wu B, Cui H, Peng X, Fang J, Zuo Z, Deng J, Wang X, Tang K and Yin S, 2014. Effects of nickel chloride on the erythrocytes and erythrocyte immune adherence function in broilers. Biological Trace Element Research, 161, 173–179. [DOI] [PubMed] [Google Scholar]
- Lin C‐H, Chung C‐A, Wong J‐H, Chen B‐K, Chiu S‐J, Klahan S, Lee Y‐C and Chang W‐C, 2016. Involvement of L‐type Ca2+ channel and toll‐like receptor‐4 in nickel‐induced interleukin‐8 gene expression. Environmental Toxicology, 31, 5–12. [DOI] [PubMed] [Google Scholar]
- Liu G, Sun L, Pan A, Zhu M, Li Z, Wang Z, Liu X, Ye X, Li H, Zheng H, Ong CN, Yin H, Lin X and Chen Y, 2015. Nickel exposure is associated with the prevalence of type 2 diabetes in Chinese adults. International Journal of Epidemiology, 44, 240–248. [DOI] [PubMed] [Google Scholar]
- Liu G, Sun Q, Zhu M, Sun L, Wang Z, Li H, Li Z, Chen Y, Yin H and Lin X, 2016. Nickel exposure and prevalent albuminuria and beta 2‐microglobulinuria: evidence from a population‐based study. Journal of Epidemiology and Community Health, 70, 437–443. 10.1136/jech-2015-205994 [DOI] [PubMed] [Google Scholar]
- López‐Jornet P, Juan H and Alvaro PF, 2014. Mineral and trace element analysis of saliva from patients with BMS: a cross‐sectional prospective controlled clinical study. Journal of Oral Pathology and Medicine, 43, 111–116. 10.1111/jop.12105 [DOI] [PubMed] [Google Scholar]
- Lou J, Jin L, Wu N, Tan Y, Song Y, Gao M, Liu K, Zhang X and He J, 2013. DNA damage and oxidative stress in human B lymphoblastoid cells after combined exposure to hexavalent chromium and nickel compounds. Food and Chemical Toxicology, 55, 533–540. 10.1016/j.fct.2013.01.053 [DOI] [PubMed] [Google Scholar]
- Lusi EA, Di Ciommo VM, Patrissi T and Guarascio P, 2015. High prevalence of nickel allergy in an overweight female population: a pilot observational analysis. PLoS ONE, 10 10.1371/journal.pone.0123265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie B and Garrick MD, 2005. Iron Imports. II. Iron uptake at the apical membrane in the intestine. American Journal of Physiology‐Gastrointestinal and Liver Physiology, 289, G981–G986. 10.1152/ajpgi.00363.2005 [DOI] [PubMed] [Google Scholar]
- Marzec Z, Koch W, Marzec A and Zukiewicz‐Sobczak W, 2014. Dietary exposure to cadmium, lead and nickel among students from south‐east Poland. Annals of Agricultural and Environmental Medicine, 21, 825–828. 10.5604/12321966.1129941 [DOI] [PubMed] [Google Scholar]
- Maulvault AL, Anacleto P, Barbosa V, Sloth JJ, Rasmussen RR, Tediosi A, Fernandez‐Tejedor M, van den Heuvel FH, Kotterman M and Marques A, 2015. Toxic elements and speciation in seafood samples from different contaminated sites in Europe. Environmental Research, 143, 72–81. 10.1016/j.envres.2015.09.016 [DOI] [PubMed] [Google Scholar]
- Mayer C, Klein RG, Wesch H and Schmezer P, 1998. Nickel subsulfide is genotoxic in vitro but shows no mutagenic potential in respiratory tract tissues of BigBlue rats and Muta Mouse mice in vivo after inhalation. Mutation Research, 420, 85–98. [DOI] [PubMed] [Google Scholar]
- McKay DJ, Renaux BS and Dixon GH, 1986. Human sperm protamines. Amino‐acid sequences of two forms of protamine P2. European Journal of Biochemistry, 156, 5–8. 10.1111/j.1432-1033.1986.tb09540.x [DOI] [PubMed] [Google Scholar]
- Merten C, Ferrari P, Bakker M, Boss A, Hearty A, Leclercq C, Lindtner O, Tlustos C, Verger P, Volatier JL and Arcella D, 2011. Methodological characteristics of the national dietary surveys carried out in the European Union as included in the European Food Safety Authority (EFSA) Comprehensive European Food Consumption Database. Food additives & contaminants Part A, Chemistry, Analysis, Control, Exposure & Risk Assessment, 28, 975–995. 10.1080/19440049.2011.576440 [DOI] [PubMed] [Google Scholar]
- Merzenich H, Hartwig A, Ahrens W, Beyersmann D, Schlepegrell R, Scholze M, Timm J and Jöckel KH, 2001. Biomonitoring on carcinogenic metals and oxidative DNA damage in a cross‐sectional study. Cancer Epidemiology, Biomarkers and Prevention, 10, 515–522. [PubMed] [Google Scholar]
- Mitkovska VI, Dimitrov HA and Chassovnikarova TG, 2017. In vivo genotoxicity and cytotoxicity assessment of allowable concentrations of nickel and lead: comet assay and nuclear abnormalities in acridine orange stained erythrocytes of common. Carp (Cyprinus carpio L.). Acta Zoologica Bulgarica, 47–56. [Google Scholar]
- Mohammadi S, Gholamin M, Mansouri A, Mahmoodian RS, Babazadeh B, Kebriaei SM, Zibaei B, Roshanaei M, Daneshvar F, Khandehro M, Khodadadegan MA, Delshad A, Mohammadzadeh F, Mohammadi M, Sadeghi S, Shoeibi S, Boroumand‐Noughabi S, Ghayour‐Mobarhan M, Tavallaie S, Vafaei A and Ferns GAA, 2018. Effect of cadmium and nickel on expression of CatSper 1 and 2 genes in mice. Toxin Reviews, 37, 216–222. [Google Scholar]
- Morán‐Martínez J, Monreal‐de Luna KD, Betancourt‐Martínez ND, Carranza‐Rosales P, Contreras‐Martínez JG, López‐Meza MC and Rodríguez‐Villarreal O, 2013. Genotoxicity in oral epithelial cells in children caused by nickel in metal crowns. Genetics and Molecular Research, 12, 3178–3185. 10.4238/2013.August.29.1 [DOI] [PubMed] [Google Scholar]
- Müller FD, Hackethal C, Schmidt R, Kappenstein O, Pfaff K and Luch A, 2015. Metal release from coffee machines and electric kettles. Food Additives and Contaminants ‐ Part A Chemistry, Analysis, Control, Exposure and Risk Assessment, 32, 1959–1964. 10.1080/19440049.2015.1086929 [DOI] [PubMed] [Google Scholar]
- Murawska‐Ciałowicz E, Bal W, Januszewska L, Zawadzki M, Rychel J and Zuwala‐Jagiello J, 2012. Oxidative stress level in the testes of mice and rats during nickel intoxication. Scientific World Journal, 395741, 10.1100/2012/395741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadjiba T, Aya S and Rachid R, 2018. Evaluation of Hepatotoxicity: Nickel on an Indicator Model of Pollution (Oryctolagus cuniculus) In: Kallel A, Ksibi M, BenDhia H, Khelifi N. (eds.). Recent Advances in Environmental Science from the Euro‐Mediterranean and Surrounding Regions, Vols I and II. pp. 567–569. [Google Scholar]
- do Nascimento SN, Charao MF, Moro AM, Roehrs M, Paniz C, Baierle M, Brucker N, Gioda A, Barbosa Jr F, Bohrer D, Silva Ávila D and Garcia SC, 2014. Evaluation of toxic metals and essential elements in children with learning disabilities from a rural area of southern Brazil. International Journal of Environmental Research and Public Health, 11, 10806–10823. 10.3390/ijerph111010806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni W, Yang W, Yu J, Li Z, Jin L, Liu J, Zhang Y, Wang L and Ren A, 2018. Umbilical cord concentrations of selected heavy metals and risk for orofacial clefts. Environmental Science and Technology, 52, 10787–10795. [DOI] [PubMed] [Google Scholar]
- Nielsen E and Larsen P, 2013. Nickel, inorganic and soluble salts. Evaluation of health hazards and proposal of a health‐based quality criterion for drinking water. The Danish Environmental Protection Agency, Copenhagen, Denmark, 78 pp.
- Nielsen GD, Jepsen LV, Jorgensen PJ, Grandjean P and Brandrup F, 1990. Nickel‐sensitive patients with vesicular hand eczema: oral challenge with a diet naturally high in nickel. British Journal of Dermatology, 122, 299–308. [DOI] [PubMed] [Google Scholar]
- Nielsen GD, Soderberg U, Jorgensen PJ, Templeton DM, Rasmussen SN, Andersen KE and Grandjean P, 1999. Absorption and retention of nickel from drinking water in relation to food intake and nickel sensitivity. Toxicology and Applied Pharmacology, 154, 67–75. 10.1006/taap.1998.8577 [DOI] [PubMed] [Google Scholar]
- Nisse C, Tagne‐Fotso R, Howsam M, Richeval C, Labat L and Leroyer A, 2017. Blood and urinary levels of metals and metalloids in the general adult population of Northern France: The IMEPOGE study, 2008–2010. International Journal of Hygiene and Environmental Health, 220, 341–363. 10.1016/j.ijheh.2016.09.020 [DOI] [PubMed] [Google Scholar]
- Noureddine El Moussawi S, Ouaini R, Matta J, Chebib H, Cladiere M and Camel V, 2019. Simultaneous migration of bisphenol compounds and trace metals in canned vegetable food. Food Chemistry, 288, 228–238. 10.1016/j.foodchem.2019.02.116 [DOI] [PubMed] [Google Scholar]
- Oblak A, Pohar J and Jerala R, 2015. MD‐2 determinants of nickel and cobalt‐mediated activation of human TLR4. PLoS ONE. 10:e0120583/0120581‐e0120583/0120515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obone E, Chakrabarti SK, Bai CJ, Malick MA, Lamontagne L and Subramanian KS, 1999. Toxicity and bioaccumulation of nickel sulfate in Sprague‐Dawley rats following 13 weeks of subchronic exposure. Journal of Toxicology and Environmental Health‐Part A, 57, 379–401. 10.1080/009841099157593 [DOI] [PubMed] [Google Scholar]
- Oliva R, 2006. Protamines and male infertility. Human Reproduction Update, 12, 417–435. 10.1093/humupd/dml009 [DOI] [PubMed] [Google Scholar]
- Oller AR and Erexson G, 2007. Lack of micronuclei formation in bone marrow of rats after repeated oral exposure to nickel sulfate hexahydrate. Mutation Research, 626, 102–110. 10.1016/j.mrgentox.2006.09.001 [DOI] [PubMed] [Google Scholar]
- Pajević S, Arsenov D, Nikolic N, Borisev M, Orcic D, Zupunski M and Mimica‐Dukic N, 2018. Heavy metal accumulation in vegetable species and health risk assessment in Serbia. Environmental Monitoring and Assessment, 190, 459 10.1007/s10661-018-6743-y [DOI] [PubMed] [Google Scholar]
- Pandey R and Srivastava SP, 2000. Spermatotoxic effects of nickel in mice. Bulletin of Environmental Contamination and Toxicology, 64, 161–167. [DOI] [PubMed] [Google Scholar]
- Pandey R, Kumar R, Singh SP, Saxena DK and Srivastava SP, 1999. Male reproductive effect of nickel sulphate in mice. BioMetals, 12, 339–346. 10.1023/a:1009291816033 [DOI] [PubMed] [Google Scholar]
- Patriarca M, Lyon TDB and Fell GS, 1997. Nickel metabolism in humans investigated with an oral stable isotope. American Journal of Clinical Nutrition, 66, 616–621. [DOI] [PubMed] [Google Scholar]
- Peana M, Zdyb K, Medici S, Pelucelli A, Simula G, Gumienna‐Kontecka E and Zoroddu MA, 2017. Ni(II) interaction with a peptide model of the human TLR4 ectodomain. Journal of Trace Elements in Medicine and Biology, 44, 151–160. [DOI] [PubMed] [Google Scholar]
- Peeters K, Zuliani T, Zigon D, Milacic R and Scancar J, 2017. Nickel speciation in cocoa infusions using monolithic chromatography ‐ Post‐column ID‐ICP‐MS and Q‐TOF‐MS. Food Chemistry, 230, 327–335. 10.1016/j.foodchem.2017.03.050 [DOI] [PubMed] [Google Scholar]
- Peredelskaya M, 2018. Case of allergy to nickel against the background of its reception in food. Clinical and Translational Allergy, 8, 67. [Google Scholar]
- Pérez R, Domenech E, Conchado A, Sanchez A, Coscolla C and Yusa V, 2018. Influence of diet in urinary levels of metals in a biomonitoring study of a child population of the Valencian region (Spain). Science of the Total Environment, 618, 1647–1657. 10.1016/j.scitotenv.2017.10.011 [DOI] [PubMed] [Google Scholar]
- Peters K, Gammelgaard B and Menne T, 1991. Nickel concentrations in fingernails as a measure of occupational exposure to nickel. Contact Dermatitis, 25, 237–241. 10.1111/j.1600-0536.1991.tb01851.x [DOI] [PubMed] [Google Scholar]
- Petit D, El Houari W, Jacobs K, Baeyens W and Leermakers M, 2013. Trace element content in tea brewed in traditional metallic and stainless steel teapots. Environmental Monitoring and Assessment, 185, 8957–8966. 10.1007/s10661-013-3226-z [DOI] [PubMed] [Google Scholar]
- Plavan G, Jitar O, Teodosiu C, Nicoara M, Micu D and Strungaru SA, 2017. Toxic metals in tissues of fishes from the Black Sea and associated human health risk exposure. Environmental Science and Pollution Research International, 24, 7776–7787. 10.1007/s11356-017-8442-6 [DOI] [PubMed] [Google Scholar]
- Protano C, Astolfi ML, Canepari S and Vitali M, 2016. Urinary levels of trace elements among primary school‐aged children from Italy: the contribution of smoking habits of family members. Science of the Total Environment, 557–558, 378–385. 10.1016/j.scitotenv.2016.03.073 [DOI] [PubMed] [Google Scholar]
- Prystupa A, Blazewicz A, Kicinski P, Sak JJ, Niedzialek J and Zaluska W, 2016. Serum concentrations of selected heavy metals in patients with alcoholic liver cirrhosis from the lublin region in Eastern Poland. International Journal of Environmental Research and Public Health, 13 10.3390/ijerph13060582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman MS, Biswas PK, Al Hasan SM, Rahman MM, Lee SH, Kim KH, Rahman SM and Islam MR, 2018. The occurrences of heavy metals in farmland soils and their propagation into paddy plants. Environmental Monitoring and Assessment, 190, 201 10.1007/s10661-018-6577-7 [DOI] [PubMed] [Google Scholar]
- Ricciardi L, Carni A, Loschiavo G, Gangemi S, Tigano V, Arena E, Mannucci C and Calapai G, 2013. Systemic nickel allergy: oral desensitization and possible role of cytokines interleukins 2 and 10. International Journal of Immunopathology and Pharmacology, 26, 251–257. 10.1177/039463201302600127 [DOI] [PubMed] [Google Scholar]
- Ricciardi L, Arena A, Arena E, Zambito M, Ingrassia A, Valenti G, Loschiavo G, D'Angelo A and Saitta S, 2014. Systemic nickel allergy syndrome: epidemiological data from four Italian allergy units. International Journal of Immunopathology and Pharmacology, 27, 131–136. 10.1177/039463201402700118 [DOI] [PubMed] [Google Scholar]
- Richardson JB, Dancy BCR, Horton CL, Lee YS, Madejczyk MS, Xu ZZ, Ackermann G, Humphrey G, Palacios G, Knight R and Lewis JA, 2018. Exposure to toxic metals triggers unique responses from the rat gut microbiota. Scientific Reports, 8, 6578 10.1038/s41598-018-24931-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roca M, Sanchez A, Perez R, Pardo O and Yusa V, 2016. Biomonitoring of 20 elements in urine of children levels and predictors of exposure. Chemosphere, 144, 1698–1705. [DOI] [PubMed] [Google Scholar]
- Ross‐Hansen K, Linneberg A, Johansen JD, Hersoug LG, Brasch‐Andersen C, Menne T and Thyssen JP, 2013. The role of glutathione S‐transferase and claudin‐1 gene polymorphisms in contact sensitization: a cross‐sectional study. British Journal of Dermatology, 168, 762–770. 10.1111/bjd.12126 [DOI] [PubMed] [Google Scholar]
- Ross‐Hansen K, Johansen JD, Vølund A, Menné T and Thyssen JP, 2014. The nickel dose‐response relationship by filaggrin genotype (FLG). Contact Dermatitis, 71, 49–53. 10.1111/cod.12228 [DOI] [PubMed] [Google Scholar]
- RTI (Research Triangle Institute), 1988a. Two‐generation reproduction and fertility study of nickel chloride administered to CD rats in the drinking water: Fertility and reproductive performance of the P generation. Final study report (II of III). Research Triangle Park, NC: Office of Solid Waste Management, US Environmental Protection Agency. Unpublished report.
- RTI (Research Triangle Institute), 1988b. Two‐generation reproduction and fertility study of nickel chloride administered to CD rats in the drinking water: Fertility and reproductive performance of the F1 generation. Final study report (III of III). Research Triangle Park, NC: Office of Solid Waste Management, US Environmental Protection Agency.
- Rubio C, Acosta L, Luis‐Gonzalez G, Gonzalez‐Weller D, Revert C, Hardisson A and Gutierrez A, 2018. A limited survey of metal content in blue Jack Mackerel (Trachurus picturatus) obtained from markets in the Canary Islands. Journal of Food Protection, 81, 202–208. 10.4315/0362-028x.Jfp-17-181 [DOI] [PubMed] [Google Scholar]
- Sadauskiene I, Bernotiene R, Sulinskiene J, Ivanoviene L and Ivanov L, 2013. The content of metallothioneins and lipid peroxidation in mouse brain: effects of cadmium and nickel ions. FEBS Journal, 280, 248–249. [Google Scholar]
- Saini S, Nair N and Saini MR, 2013. Embryotoxic and teratogenic effects of nickel in Swiss albino mice during organogenetic period. Biomed Research International, 2013, 1–9. 10.1155/2013/701439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saini S, Nair N and Saini M, 2014a. Effects of gestational administration of nickel on postnatal development in Swiss albino mice. Human and Experimental Toxicology, 33, 1199–1208. 10.1177/0960327114527627 [DOI] [PubMed] [Google Scholar]
- Saini S, Nair N and Saini MR, 2014b. Prenatal exposure to nickel on pregnant Swiss albino mice and fetal development. Toxicological and Environmental Chemistry, 96, 650–659. [Google Scholar]
- Salnikow K, Blagosklonny MV, Ryan H, Johnson R and Costa M, 2000. Carcinogenic nickel induces genes involved with hypoxic stress. Cancer Research, 60, 38–41. [PubMed] [Google Scholar]
- Saplakoglu U, Iscan M and Iscan M, 1997. DNA single‐strand breakage in rat lung, liver and kidney after single and combined treatments of nickel and cadmium. Mutation Research, 394, 133–140. [PubMed] [Google Scholar]
- Sato T, Kishimoto Y, Asakawa S, Mizuno N, Hiratsuka M and Hirasawa N, 2016. Involvement of COX‐2 in nickel elution from a wire implanted subcutaneously in mice. Toxicology, 363–364, 37–45. 10.1016/j.tox.2016.07.013 [DOI] [PubMed] [Google Scholar]
- Sazakli E and Leotsinidis M, 2017. Hair biomonitoring and health status of a general population exposed to Nickel. Journal of Trace Elements in Medicine and Biology, 43, 161–168. 10.1016/j.jtemb.2017.02.001 [DOI] [PubMed] [Google Scholar]
- Scancar J, Zuliani T, Zigon D and Milacic R, 2013. Ni speciation in tea infusions by monolithic chromatography ‐ ICP‐MS and Q‐TOF‐MS. Analytical and Bioanalytical Chemistry, 405, 2041–2051. 10.1007/s00216-012-6611-5 [DOI] [PubMed] [Google Scholar]
- Scanlon SE, Scanlon CD, Hegan DC, Sulkowski PL and Glazer PM, 2017. Nickel induces transcriptional down‐regulation of DNA repair pathways in tumorigenic and non‐tumorigenic lung cells. Carcinogenesis, 38, 627–637. 10.1093/carcin/bgx038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaumlöffel D, 2005. Speciation of Nickel In: Cornelis R, Caruso J, Crews H. and Heumann K. (eds.). Handbook of Elemental Speciation II – Species in the Environment, Food, Medicine and Occupational Health. John Wiley & Sons, Chichester: pp. 310–326. [Google Scholar]
- Singh NP, McCoy MT, Tice RR and Schneider EL, 1988. A simple technique for quantitation of low levels of DNA damage in individual cells. Experimental Cell Research, 175, 184–191. 10.1016/0014-4827(88)90265-0 [DOI] [PubMed] [Google Scholar]
- Sirot V, Traore T, Guerin T, Noel L, Bachelot M, Cravedi JP, Mazur A, Glorennec P, Vasseur P, Jean J, Carne G, Gorecki S, Riviere G and Hulin M, 2018. French infant total diet study: exposure to selected trace elements and associated health risks. Food and Chemical Toxicology, 120, 625–633. 10.1016/j.fct.2018.07.062 [DOI] [PubMed] [Google Scholar]
- Skalny AV, Klimenko LL, Turna AA, Budanova MN, Baskakov IS, Savostina MS, Mazilina AN, Deyev AI, Skalnaya MG and Tinkov AA, 2017. Serum trace elements are interrelated with hormonal imbalance in men with acute ischemic stroke. Journal of Trace Elements in Medicine and Biology, 43, 142–147. 10.1016/j.jtemb.2016.12.018 [DOI] [PubMed] [Google Scholar]
- Skjørringe T, Burkhart A, Johnsen KB and Moos T, 2015. Divalent metal transporter 1 (DMT1) in the brain: implications for a role in iron transport at the blood‐brain barrier, and neuronal and glial pathology. Frontiers in Molecular Neuroscience, 8, 19 10.3389/fnmol.2015.00019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- SLI (Springborn Laboratories), 2000a. A one‐generation reproduction range‐finding study in rats with nickel sulfate hexahydrate. Spencerville, OH: Springborn Laboratories, Inc. SLI Study No. 3472.3. Unpublished report.
- SLI (Springborn Laboratories), 2000b. An oral (gavage) two‐generation reproduction toxicity study in Sprague–Dawley rats with nickel sulfate hexahydrate. Final Report. Volume 1 of 3. Spencerville, OH: Springborn Laboratories, Inc. SLI Study No. 3472.4. Unpublished report.
- Slotnick MJ, Nriagu JO, Johnson MM, Linder AM, Savoie KL, Jamil HJ and Hammad AS, 2005. Profiles of trace elements in toenails of Arab‐Americans in the Detroit area, Michigan. Biological Trace Element Research, 107, 113–126. 10.1385/bter:107:2:113 [DOI] [PubMed] [Google Scholar]
- Smith MK, George EL, Stober JA, Feng HA and Kimmel GL, 1993. Perinatal toxicity associated with nickel chloride exposure. Environmental Research, 61, 200–211. [DOI] [PubMed] [Google Scholar]
- Sobti RC and Gill RK, 1989. Incidence of micronuclei and abnormalities in the head of spermatozoa caused by the salts of a heavy metal, nickel. Cytologia, 54, 249–253. 10.1508/cytologia.54.249 [DOI] [Google Scholar]
- Solomons NW, Viteri F, Shuler TR and Nielsen FH, 1982. Bioavailability of nickel in man ‐ effects of foods and chemically‐defined dietary constituents on the absorption of inorganic nickel. Journal of Nutrition, 112, 39–50. [DOI] [PubMed] [Google Scholar]
- Stannard L, Doak SH, Doherty A and Jenkins GJ, 2017. Is nickel chloride really a non‐genotoxic carcinogen? Basic & Clinical Pharmacology & Toxicology, 121, 10–15. 10.1111/bcpt.12689 [DOI] [PubMed] [Google Scholar]
- Stasinos S, Nasopoulou C, Tsikrika C and Zabetakis I, 2014. The bioaccumulation and physiological effects of heavy metals in carrots, onions, and potatoes and dietary implications for Cr and Ni: a review. Journal of Food Science, 79, R765–R780. 10.1111/1750-3841.12433 [DOI] [PubMed] [Google Scholar]
- Stojsavljević A, Trifkovic J, Rasic‐Milutinovic Z, Jovanovic D, Bogdanovic G, Mutic J and Manojlovic D, 2018. Determination of toxic and essential trace elements in serum of healthy and hypothyroid respondents by ICP‐MS: a chemometric approach for discrimination of hypothyroidism. Journal of Trace Elements in Medicine and Biology, 48, 134–140. 10.1016/j.jtemb.2018.03.020 [DOI] [PubMed] [Google Scholar]
- Suliburska J, Kocylowski R, Komorowicz I, Grzesiak M, Bogdanski P and Baralkiewicz D, 2016. Concentrations of mineral in amniotic fluid and their relations to selected maternal and fetal parameters. Biological Trace Element Research, 172, 37–45. 10.1007/s12011-015-0557-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Shamy M and Costa M, 2013. Nickel and Epigenetic Gene Silencing. Genes, 4, 583–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Jiang Y, Xia W, Jin S, Liu W, Lin X, Liu H, Chen X, Peng Y, Li H, Lu B, Xu S, Li Y and Shen X, 2018. Association between prenatal nickel exposure and preterm low birth weight: possible effect of selenium. Environmental Science and Pollution Research, 25, 25888–25895. [DOI] [PubMed] [Google Scholar]
- Sunderman FW Jr, 1993. Biological monitoring of nickel in humans. Scandinavian Journal of Work, Environment and Health, 19(Suppl 1), 34–38. [PubMed] [Google Scholar]
- Sunderman FW, Hopfer SM, Sweeney KR, Marcus AH, Most BM and Creason J, 1989. Nickel absorption and kinetics in human volunteers. Proceedings of the Society for Experimental Biology and Medicine, 191, 5–11. [DOI] [PubMed] [Google Scholar]
- Suomi J, Valsta L, Suominen K and Tuominen P, 2020. Risk assessment on the dietary heavy metal exposure and aluminium exposure of Finnish adults. Finnish Food Authority Research Reports 1/2020, 78 pp. [Google Scholar]
- Sureda A, Bibiloni MDM, Julibert A, Aparicio‐Ugarriza R, Palacios‐Le Ble G, Pons A, Gonzalez‐Gross M and Tur JA, 2017. Trace element contents in toenails are related to regular physical activity in older adults. PLoS ONE, 12, e0185318 10.1371/journal.pone.0185318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szynal T, Rebeniak M and Mania M, 2016. Migration studies of nickel and chromium from ceramic and glass tableware into food simulants. Roczniki Państwowego Zakładu Higieny, 67, 247–252. [PubMed] [Google Scholar]
- Tabatadze T, Zhorzholiani L, Kherkheulidze M, Karseladze R and Ivanashvili T, 2015. Association between short stature and hair elements. Georgian Medical News, 25–30. [PubMed] [Google Scholar]
- Tang K, Guo H, Deng J, Cui H, Peng X, Fang J, Zuo Z, Wang X, Wu B, Li J and Yin S, 2015. Inhibitive effects of nickel chloride (NiCl2) on thymocytes. Biological Trace Element Research, 164, 242–252. [DOI] [PubMed] [Google Scholar]
- Terpilowska S and Siwicki AK, 2018. Interactions between chromium(III) and iron(III), molybdenum(III) or nickel(II): cytotoxicity, genotoxicity and mutagenicity studies. Chemosphere, 201, 780–789. 10.1016/j.chemosphere.2018.03.062 [DOI] [PubMed] [Google Scholar]
- Terpilowska S and Siwicki AK, 2019. Pro‐ and antioxidant activity of chromium(III), iron(III), molybdenum(III) or nickel(II) and their mixtures. Chemico‐Biological Interactions, 298, 43–51. 10.1016/j.cbi.2018.10.028 [DOI] [PubMed] [Google Scholar]
- Thierse HJ, Moulon C, Allespach Y, Zimmermann B, Doetze A, Kuppig S, Wild D, Herberg F and Weltzien HU, 2004. Metal‐protein complex‐mediated transport and delivery of Ni2+ to TCR/MHC contact sites in nickel‐specific human T cell activation. J Immunol., 172, 1926–1934. 10.4049/jimmunol.172.3.1926 [DOI] [PubMed] [Google Scholar]
- Thierse HJ, Gamerdinger K, Junkes C, Guerreiro N and Weltzien HU, 2005. T cell receptor (TCR) interaction with haptens: metal ions as non‐classical haptens. Toxicology, 209, 101–107. 10.1016/j.tox.2004.12.015 [DOI] [PubMed] [Google Scholar]
- Toman R, Lukac N, Massanyi P and Hajkova Z, 2013. Changes of blood parameters associated with nickel administration in rats. Animal Welfare, Ethology and Housing Systems, 9, 604–611. [Google Scholar]
- Toman R, Hluchy S, Hajkova Z and Bencsik I, 2014. Distribution of nickel in rat organs after an administration of nickel (II) chloride. Scientific Papers: Animal Science and Biotechnologies, 47, 352–356. [Google Scholar]
- Tompkins JD, Merriam LA, Girard BM, May V and Parsons RL, 2015. Nickel suppresses the PACAP‐induced increase in guinea pig cardiac neuron excitability. American Journal of Physiology – Cell Physiology, 308, C857–C866. 10.1152/ajpcell.00403.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- US EPA (United States Environmental Protection Agency), 2012. Benchmark Dose Technical Guidance. EPA/100/R‐12/001. Washington, DC, 99 pp. Available online: https://www.epa.gov/sites/production/files/2015-01/documents/benchmark_dose_guidance.pdf [Google Scholar]
- Verma AK, Keshari AK, Raj J, Kumari R, Kumar T, Sharma V, Singh TB, Srivastava S and Srivastava R, 2016. Prolidase‐associated trace elements (Mn, Zn Co, and Ni) in the patients with Parkinson's Disease. Biology Trace Element Research, 171, 48–53. 10.1007/s12011-015-0503-4 [DOI] [PubMed] [Google Scholar]
- Volke A, Runkorg K, Wegener G, Vasar E and Volke V, 2013. Dual effect of nickel on L‐arginine/nitric oxide system in RAW 264.7 macrophages. International Immunopharmacology, 15, 511–516. 10.1016/j.intimp.2013.01.019 [DOI] [PubMed] [Google Scholar]
- Wang F, Maxwell SM, Masbou AK, Bourroul FM and Keefe DL, 2018. Nickel exposure in vitro prevents hatching in mouse embryos by down‐regulating pluripotent genes and repressing trophectoderm cdx2 expression. Fertility and Sterility, 110, E172–E173. 10.1016/j.fertnstert.2018.07.512 [DOI] [Google Scholar]
- Watanabe M, Masieri S, Costantini D, Tozzi R, De Giorgi F, Gangitano E, Tuccinardi D, Poggiogalle E, Mariani S, Basciani S, Petrangeli E, Gnessi L and Lubrano C, 2018. Overweight and obese patients with nickel allergy have a worse metabolic profile compared to weight matched non‐allergic individuals. PLoS ONE, 13, e0202683 10.1371/journal.pone.0202683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Z, Zhang X, Wang Y, Fu Y and Yang Z, 2018. Nickel (II) nitrate hexahydrate triggered canine neutrophil extracellular traps release in vitro . Chemosphere, 208, 117–121. [DOI] [PubMed] [Google Scholar]
- Werfel U, Langen V, Eickhoff I, Schoonbrood J, Vahrenholz C, Brauksiepe A, Popp W and Norpoth K, 1998. Elevated DNA single‐strand breakage frequencies in lymphocytes of welders exposed to chromium and nickel. Carcinogenesis, 19, 413–418. [DOI] [PubMed] [Google Scholar]
- WHO (World Health Organization), 2000. WHO air quality guidelines for Europe, 2nd edition [CD ROM version]. WHO: Copenhagen. Available online: http://www.euro.who.int/en/health-topics/environment-and-health/air-quality/publications/pre2009/who-air-quality-guidelines-for-europe,-2nd-edition,-2000-cd-rom-version
- WHO (World Health Organization), 2005. Nickel in Drinking Water. WHO/SDE/WSH/05.08/55. Background document for development of WHO Guidelines for Drinking Water Quality. Available online: http://www.who.int/water_sanitation_health/gdwqrevision/nickel2005.pdf
- WHO (World Health Organization), 2010. WHO Laboratory Manual for the Examination and Processing of Human Semen, fifth edition. World Health Organization, 2010. 271 p. Available from: https://apps.who.int/iris/bitstream/handle/10665/44261/9789241547789_eng.pdf?sequence=1
- WHO (World Health Organization), 2017. Guidelines for drinking‐water quality, 4th edition, incorporating the 1st addendum. 631 pp. [PubMed] [Google Scholar]
- WHO/IPCS (World Health Organization/International Programme on Chemical Safety), 2008. Uncertainty and data quality in exposure assessment. Available online: http://www.who.int/iris/handle/10665/44017
- WHO/IPCS (World Health Organization/International Programme on Chemical Safety), 2009. Principles and Methods for the Risk Assessment of Chemicals in Food, International Programme on Chemical Safety, Environmental Health Criteria 240. Chapter 6: Dietary Exposure Assessment of Chemicals in Food. Available online: http://www.who.int/ipcs/food/principles/en/index1.html
- Wu B, Cui H, Peng X, Fang J, Zuo Z, Deng J and Huang J, 2013a. Dietary nickel chloride restrains the development of small intestine in broilers. Biological Trace Element Research, 155, 236–246. 10.1007/s12011-013-9792-7 [DOI] [PubMed] [Google Scholar]
- Wu B, Cui H, Peng X, Fang J, Zuo Z, Huang J, Luo Q, Deng Y, Wang H and Liu J, 2013b. Changes of the serum cytokine contents in broilers fed on diets supplemented with nickel chloride. Biological Trace Element Research, 151, 234–239. [DOI] [PubMed] [Google Scholar]
- Wu B, Cui H, Xi P, Fang J, Zuo Z, Deng J and Huang J, 2014. Analysis of the toll‐like receptor 2‐2 (TLR2‐2) and TLR4 mRNA expression in the intestinal mucosal immunity of broilers fed on diets supplemented with nickel chloride. International Journal of Environmental Research and Public Health, 11, 657 10.3390/ijerph110100657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S, He M, Zhong M, Li L, Lu Y, Zhang Y, Zhang L, Yu Z and Zhou Z, 2015. The neuroprotective effects of taurine against nickel by reducing oxidative stress and maintaining mitochondrial function in cortical neurons. Neuroscience Letters, 590, 52–57. [DOI] [PubMed] [Google Scholar]
- Yin S, Guo H, Cui H, Peng X, Fang J, Zuo Z, Deng J, Wang X, Tang K and Li J, 2016a. Nickel Chloride (NiCl2) induces histopathological lesions via oxidative damage in the Broiler's Bursa of Fabricius. Biological Trace Element Research, 171, 214–223. 10.1007/s12011-015-0528-8 [DOI] [PubMed] [Google Scholar]
- Yin S, Cui H, Peng X, Fang J, Zuo Z, Deng J, Wang X, Wu B and Guo H, 2016b. Toxic effect of NiCl2 on development of the bursa of Fabricius in broiler chickens. Oncotarget, 7, 125–139. 10.18632/oncotarget.6591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuk J‐S, Shin JS, Shin J‐Y, Oh E, Kim H and Park WI, 2015. Nickel allergy is a risk factor for endometriosis: an 11‐year population‐based nested case‐control study. PLoS ONE, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zafar A, Eqani SAMAS, Bostan N, Cincinelli A, Tahir F, Shah STA, Hussain A, Alamdar A, Huang Q, Peng S and Shen H, 2015. Toxic metals signature in the human seminal plasma of Pakistani population and their potential role in male infertility. Environmental Geochemistry and Health, 37, 515–527. [DOI] [PubMed] [Google Scholar]
- Zeneli L, Sekovanic A and Daci N, 2015. Chronic exposure to aluminum, nickel, thallium and uranium and their relationship with essential elements in human whole blood and blood serum. Journal of Environmental Science and Health Part A, Toxic/Hazardous Substances & Environmental Engineering, 50, 540–546. [DOI] [PubMed] [Google Scholar]
- Zeng Q, Zhou B, Feng W, Wang Y‐X, Liu A‐L, Yue J, Li Y‐F and Lu W‐Q, 2013. Associations of urinary metal concentrations and circulating testosterone in Chinese men. Reproductive Toxicology, 41, 109–114. [DOI] [PubMed] [Google Scholar]
- Zhang J, Zhang J, Li M, Wu Y, Fan Y, Zhou Y, Tan L, Shao Z and Shi H, 2011. Methylation of RAR‐beta 2, RASSF1A, and CDKN2A genes induced by nickel subsulfide and nickel‐carcinogenesis in rats. Biomedical and Environmental Sciences, 24, 163–171. 10.3967/0895-3988.2011.02.011 [DOI] [PubMed] [Google Scholar]
- Zhang J, Zhou Y, Wu YJ, Li MJ, Wang RJ, Huang SQ, Gao RR, Ma L, Shi HJ and Zhang J, 2013. Hyper‐methylated miR‐203 dysregulates ABL1 and contributes to the nickel‐induced tumorigenesis. Toxicology Letters, 223, 42–51. 10.1016/j.toxlet.2013.08.007 [DOI] [PubMed] [Google Scholar]
- Zhang N, Chen M, Li J, Deng Y, Li SL, Guo YX, Li N, Lin Y, Yu P, Liu Z and Zhu J, 2019. Metal nickel exposure increase the risk of congenital heart defects occurrence in offspring: a case‐control study in China. Medicine (Baltimore), 98, e15352 10.1097/md.0000000000015352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng G, Zhong H, Guo Z, Wu Z, Zhang H, Wang C, Zhou Y and Zuo Z, 2014. Levels of heavy metals and trace elements in umbilical cord blood and the risk of adverse pregnancy outcomes: a population‐based study. Biological Trace Element Research, 160, 437–444. 10.1007/s12011-014-0057-x [DOI] [PubMed] [Google Scholar]
- Zheng G, Wang L, Guo Z, Sun L, Wang L, Wang C, Zuo Z and Qiu H, 2015. Association of serum heavy metals and trace element concentrations with reproductive hormone levels and polycystic ovary syndrome in a chinese population. Biological Trace Element Research, 167, 1–10. 10.1007/s12011-015-0294-7 [DOI] [PubMed] [Google Scholar]
- Zhou X, Li J and Sun JL, 2019. Oral nickel changes of intestinal microflora in mice. Current Opinion in Microbiology, 76, 590–596. 10.1007/s00284-019-01664-1 [DOI] [PubMed] [Google Scholar]
- Zoroddu MA, Kowalik‐Jankowska T, Kozlowski HK, Molinari H, Salnikow K, Brody L and Costa M, 2000. Interaction of Ni (II) and Cu (II) with a metal binding sequence of histone H4: AKRHRK, a model of the H4 tail. Biochimica et Biophysica Acta, 1475, 163–168. [DOI] [PubMed] [Google Scholar]
- Zoroddu MA, Peana M, Medici S, Potocki S and Kozlowski H, 2014. Ni(II) binding to the 429‐460 peptide fragment from human Toll like receptor (hTLR4): a crucial role for nickel‐induced contact allergy? Dalton Transactions, 43, 2764–2771. 10.1039/C3DT52187G [DOI] [PubMed] [Google Scholar]
- Zou L, Su L, Sun Y, Han A, Chang X, Zhu A, Liu F, Li J and Sun Y, 2017. Nickel sulfate induced apoptosis via activating ROS‐dependent mitochondria and endoplasmic reticulum stress pathways in rat Leydig cells. Environmental Toxicology, 32, 1918–1926. 10.1002/tox.22414 [DOI] [PubMed] [Google Scholar]


