Abstract
Background
Limited data exist regarding the efficacy of piperacillin-tazobactam (TZP) for the management of nonbacteremic pyelonephritis caused by extended-spectrum β-lactamase (ESBL)-producing organisms.
Methods
We conducted a multicenter observational study comparing clinical outcomes of adults hospitalized with ESBL-producing pyelonephritis who were receiving TZP versus carbapenems, using an inverse probability of treatment weighted propensity score analysis. Patients were eligible for inclusion if all of the following criteria were met: (1) urine cultures growing Escherichia coli, Klebsiella pneumoniae, Klebsiella oxytoca, or Proteus mirabilis at ≥50 000 colony-forming units/mL; (2) identification of an ESBL gene; (3) pyuria (≥10 white blood cells per high powered field in the urine); and (4) dysuria and fever plus at least 1 of the following symptoms: emesis, rigors, hypotension, or flank pain.
Results
There were 186 patients included in the propensity score–weighted cohort; 45 (24%) received TZP and 141 (76%) received a carbapenem. Of these 186 patients, 27% were admitted to the intensive care unit, 48% were immunocompromised, and 45% had underlying urologic abnormalities. There were no differences between the 2 groups in the proportion of patients (20% vs 25%) with recurrent cystitis or pyelonephritis with the same ESBL-producing organism within 30 days (odds ratio, 0.75; 95% confidence interval, .31–1.81; P = .52). There were no differences in the resolution of clinical symptoms by Day 7 or in 30-day mortality. There was 1 (2%) patient in the TZP arm and 11 (8%) patients in the carbapenem arm who had incident carbapenem-resistant organisms isolated within 30 days (P = .09).
Conclusions
TZP may be a reasonable alternative to carbapenems for the management of ESBL-producing pyelonephritis and may mitigate the risk of emergence of carbapenem-resistant organisms, compared with carbapenem therapy.
Keywords: UTI, carbapenem, ESBL, urinary tract infection
In a propensity score–weighted multicenter cohort, we found no differences in the clinical outcomes of patients with extended-spectrum β-lactamase–producing pyelonephritis treated with piperacillin-tazobactam versus carbapenems. Patients treated with carbapenems trended towards more carbapenem-resistant organisms on subsequent clinical cultures.
Pyelonephritis is among the most common conditions leading to hospitalization in the United States, resulting in over 100 000 hospital admissions annually [1]. The proportion of urinary tract infections (UTIs) caused by extended-spectrum β-lactamase (ESBL)-producing bacteria has been steadily increasing, both in the United States and globally [2–5]. Although regional variation exists, between 5–20% and 3–35% of Escherichia coli and Klebsiella pneumoniae in the United States, respectively, are ESBL-producing [6, 7]. Few studies have estimated the prevalence of ESBL-production among Proteus mirabilis isolates in the United States, but available data suggest these numbers are in the range of 10–17% [8, 9].
The continued increase in ESBL-producing bacterial infections, in which the β-lactam ring of most noncarbapenem β-lactam agents (eg, penicillins, cephalosporins, and aztreonam) is inactivated in the setting of ESBL enzymes, has limited effective treatment options for these infections. Despite the disproportionate burden of ESBL-producing organisms causing UTIs compared to other sites of infection, the majority of large, adequately powered studies examining the optimal management of ESBL-producing infections have focused on bloodstream infections [10–14]. The MERINO study was a multinational, randomized, controlled trial of patients with ESBL-producing bacteremia [13]. It demonstrated that, when compared to meropenem, the administration of piperacillin-tazobactam (TZP) for ESBL-producing bloodstream infections increased 30-day mortality. Although this was a pivotal study that addressed important gaps in knowledge in the management of ESBL-producing bloodstream infections, it remains unclear whether patients with ESBL-producing pyelonephritis, in the absence of bacteremia, still require carbapenem therapy. The few studies comparing the role of TZP and carbapenems for ESBL-producing UTIs are limited by small numbers of patients receiving either agent [15–19], unclear distinctions between cystitis versus pyelonephritis [17–19], or no molecular confirmation of ESBL status [15, 16, 19, 20]. Our objective was to compare the clinical outcomes of hospitalized patients with molecularly confirmed ESBL-producing pyelonephritis without associated bacteremia who received TZP versus carbapenem therapy.
METHODS
Study Population
All hospitalized patients with a urine culture growing Escherichia coli, Klebsiella pneumoniae, Klebsiella oxytoca, or Proteus mirabilis at 3 hospitals within The Johns Hopkins Health System (The Johns Hopkins Hospital, Bayview Medical Center, and Howard County General Hospital) between January 2014 and December 2016 were considered for inclusion. Patients meeting all of the following criteria were categorized as having pyelonephritis: (1) urine cultures with at least 50 000 colony-forming units/mL with E. coli, K. pneumoniae, K. oxytoca, or P. mirabilis; (2) pyuria (greater than 10 white blood cells per high powered field in the urine); (3) identification of an ESBL gene in the uropathogen, as outlined below; (4) a temperature of at least 38.5°C and dysuria (except in cases of a neurogenic bladder for the later), plus at least 1 of the following symptoms: emesis, rigors, hypotension, or flank pain; and (5) receipt of either TZP or a carbapenem within 48 hours from the time the initial urine culture was obtained and for at least the subsequent 72 hours. Patients who did not remain on the study drug (ie, TZP or a carbapenem) for the duration of therapy were included only if they transitioned to ciprofloxacin, levofloxacin, or trimethoprim-sulfamethoxazole (TMP-SMX) and the isolate was susceptible to the selected agent. Patients transitioned to antibiotics to which the uropathogen was not susceptible, to antibiotics not expected to reach appropriate levels in the kidney parenchyma (eg, nitrofurantoin), to antibiotics the alternate arm (eg, TZP to ertapenem) after 72 hours, or patients who did not have a blood culture obtained within 48 hours of collection of their urine culture were excluded. Furthermore, patients with prostatitis, concomitant bacteremia, or renal abscesses were also excluded. Eligibility was determined based on independent adjudication by two physicians (S. L. S. and P. D. T.) through chart review.
Data Collection
Data on demographics, preexisting medical conditions, severity of illness, microbiological results, antibiotic treatments, and outcomes were collected by manual chart review for all patients. The primary exposure was receipt of TZP. The primary outcome was recurrent cystitis or pyelonephritis with the same ESBL-producing organism (based on the same genus and species and confirmatory ESBL testing) within 30 days. Secondary outcomes included the resolution of symptoms by Day 7, 30-day mortality, or identification of an incident carbapenem-resistant organism (a Gram-negative organism resistant to ertapenem, meropenem, or imipenem) in the 30 days following antibiotic initiation. The Epic Care Everywhere network, which includes inpatient and outpatient records from a large number of health care facilities in the United States, was reviewed for all patients to identify relevant pre- and postdischarge data. Similarly, the Chesapeake Regional Information System for Our Patients, which includes inpatient, outpatient, and emergency department information for patients in the state of Maryland and the District of Columbia, was also reviewed. If patients were discharged before Day 7 and no follow-up health care encounters documenting treatment failure were identified, the assumption was made that clinical resolution occurred. The study was approved by The Johns Hopkins University School of Medicine Institutional Review Board. A waiver of informed consent was granted.
Microbiological Testing
Bacterial identification and antimicrobial susceptibility testing results were identified using matrix-assisted laser-desorption ionization time-of-flight mass spectrometry (Bruker Daltonics Inc., Billerica, MA) and the BD Phoenix Automated System (BD Diagnostics, Sparks, MD). Urinary isolates growing E. coli, K. pneumoniae, K. oxytoca, and P. mirabilis with ceftriaxone minimum inhibitory concentrations (MICs) of >1 mcg/mL (but susceptible to ertapenem, meropenem, and imipenem) underwent evaluation with ESBL ETESTs (bioMérieux, Durham, NC). These results were reported to treating clinicians. Isolates with positive ESBL ETESTs were stored at −80°C in glycerol for future evaluation for the presence of ESBL genes. Specific ESBL genes identified were not reported to clinicians and were performed for research use only.
After isolates were subcultured twice from frozen stock to tryptic soy agar with 5% blood agar, genomic DNA was extracted using the DNeasy Blood & Tissue Kit (QIAGEN, Inc., Valencia, CA). Identification of β-lactamase–encoding genes was assessed utilizing a DNA microarray-based assay, Check-MDR CT101 (Check-Points, Wageningen, The Netherlands), according to the manufacturer’s protocol. The Check-Points assay detects a number of blaCTX-MESBL genes (blaCTX-M-1 group, blaCTX-M-2 group, blaCTX-M-8 & -25 group, and blaCTX-M-9 group), as well as single nucleotide polymorphisms that correspond to relevant amino acid changes, resulting in blaTEM ESBL genes (TEM E104K, TEM R164S, TEM R164H, and TEM G238S) and blaSHV ESBL genes (SHV G238S, SHV G238A, and SHV E240K).
Statistical Analysis
Baseline categorical data were compared using the Chi-square test and continuous data were compared using the Wilcoxon rank-sum test. Inverse probability of treatment weighting was performed to balance differences in baseline characteristics between the 2 groups. Using multivariable logistic regression, with the dependent variable being exposure to TZP, propensity scores were created for each patient. The covariates used to generate propensity scores included age, gender, chronic kidney disease, hemodialysis dependency, cirrhosis, diabetes, hypotension requiring vasopressors, active urologic abnormalities (neurogenic bladder, benign prostatic hypertrophy, nephrolithiasis, ureteral stent, urinary catheter, suprapubic catheter, nephrostomy tube, persistence of urinary hardware, including urinary catheters not removed during the treatment course, or urologic surgery), immunocompromised status (chemotherapy within the past 6 months, hematopoietic stem cell transplant within the past 12 months, solid organ transplant recipient, or immunosuppressive therapy within the previous 30 days), intensive care unit (ICU) status on Day 1 of pyelonephritis, and pathogens recovered from the urine.
Patients who received TZP were weighted by the inverse of the propensity score, and those receiving carbapenem antibiotics were weighted by the inverse of 1 minus the propensity score. A new pseudo-population was created, in which an individual who received the “unexpected” antibiotic (ie, a patient who received meropenem when the predicted probability—also known as the propensity score—was high that the patient would receive TZP) was given an increased weight. In comparison, an individual who received the “expected” treatment (ie, a patient who received TZP and who also had a high predicted probability of receiving TZP) was given a decreased weight, as this patient would already be adequately represented in that exposure group. Weights were stabilized to reduce the influence of extreme weights and visual inspection was performed to determine whether trimming was needed. The baseline characteristics were considered balanced if the standardized mean difference values were less than 10%. In the final analysis, odds ratios (ORs) and 95% confidence intervals (CIs) for the primary outcome (ie, recurrent cystitis or pyelonephritis with the same ESBL-producing organism within 30 days) were estimated using regression analysis, adjusting for variables with standardized mean differences greater than 10%. A 2-sided P value < .05 was considered statistically significant for all tests. The statistical analysis was completed using STATA version 13.0 (StataCorp, College Station, TX).
RESULTS
Description of Full Cohort
Overall, there were 188 unique hospitalized patients whose urine cultures grew an ESBL-producing Enterobacterales and who met eligibility criteria (Table 1). The TZP group consisted of 47 patients (25%) and the carbapenem group consisted of 141 patients (75%). The median age was 63 years (interquartile range [IQR], 49–74) and 127 patients (68%) were female. Almost half (47%) of the cohort had immunocompromising conditions and 88 (46%) patients had underlying urologic abnormalities. In total, 49 (26%) patients were admitted to the ICU for at least 24 hours for symptoms related to their UTI. The organisms identified included E. coli (56%), K. pneumoniae (30%), P. mirabilis (10%), and K. oxytoca (3%). We identified blaCTX-M-type genes in 87% of the ESBL uropathogens, whereas blaSHV-type and blaTEM-type ESBL genes were identified in 24% and 2%, respectively; some organisms contained more than 1 ESBL gene. The median MIC of TZP was 2 mcg/mL (IQRs, 2–8 and 2–16, respectively) for isolates in the TZP group as well as the carbapenem group.
Table 1.
Baseline Characteristics
| Full Cohort | Propensity Score–Weighted Cohorta | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Piperacillin-Tazobactam, n = 47; 25% | Carbapenem, n = 141; 75% | P Value | Standardized Mean Differences | Piperacillin-Tazobactam, n = 45; 24% | Carbapenem, n = 141; 76% | P Value | Standardized Mean Differences |
| Age, years, median (IQR) | 62 (48–73) | 63 (49–74) | .986 | 0.003 | 64 (50–72) | 63 (49–74) | .886 | 0.023 |
| Female gender (%) | 32 (68.1) | 95 (67.4) | .928 | −0.047 | 32 (71.1) | 96 (68.1) | .756 | −0.055 |
| Race/ethnicity | ||||||||
| Black | 15 (31.9) | 44 (31.2) | .928 | 0.030 | 16 (35.6) | 44 (31.2) | .643 | 0.084 |
| White | 24 (51.1) | 74 (52.5) | .866 | −0.049 | 22 (48.9) | 74 (52.5) | .705 | −0.068 |
| Asian | 5 (10.6) | 11 (7.8) | .546 | 0.105 | 4 (8.9) | 10 (7.1) | .602 | 0.079 |
| Latino | 2 (4.3) | 8 (5.7) | .707 | −0.060 | 2 (4.4) | 8 (5.7) | .715 | −0.063 |
| Other | 1 (2.1) | 4 (2.8) | .794 | −0.042 | 1 (2.2) | 4 (2.8) | .582 | −0.092 |
| Preexisting medical conditions | ||||||||
| Diabetes | 11 (23.4) | 41 (29.1) | .451 | −0.116 | 13 (28.9) | 39 (27.7) | .970 | 0.007 |
| Chronic kidney disease | 6 (12.8) | 21 (14.9) | .719 | −0.053 | 5 (11.1) | 20 (14.2) | .741 | −0.057 |
| Cirrhosis | 2 (4.3) | 10 (7.1) | .491 | −0.118 | 2 (4.4) | 9 (6.4) | .666 | −0.076 |
| Renal transplant | 4 (8.5) | 16 (11.3) | .585 | −0.088 | 5 (11.1) | 15 (10.6) | .960 | −0.009 |
| Solid organ transplant, other than renal transplant | 4 (8.5) | 18 (12.8) | .432 | −0.131 | 5 (11.1) | 16 (11.3) | .818 | −0.043 |
| Bone marrow transplant within past 12 months | 1 (2.1) | 4 (2.8) | .794 | −0.042 | 2 (4.4) | 4 (2.8) | .806 | 0.053 |
| Chemotherapy within past 6 months | 3 (6.4) | 10 (7.1) | .868 | −0.022 | 4 (8.9) | 10 (7.1) | .823 | 0.045 |
| Immunosuppressive therapy within the past 30 daysb | 5 (10.6) | 23 (16.3) | .344 | −0.158 | 7 (15.6) | 21 (14.9) | .997 | −0.001 |
| Urologic abnormalities | ||||||||
| Foley catheter, not removed | 5 (10.6) | 17 (12.1) | .793 | −0.037 | 5 (11.1) | 16 (11.3) | .939 | −0.014 |
| Intermittent catheterization | 8 (17.0) | 32 (22.7) | .410 | −0.132 | 8 (17.8) | 30 (21.3) | .756 | −0.058 |
| Nephrostomy tube or suprapubic catheter | 3 (6.4) | 2 (1.4) | .067 | 0.261 | 1 (2.2) | 4 (2.8) | .963 | 0.006 |
| Nephrolithiasis | 1 (2.1) | 8 (5.7) | .324 | −0.180 | 2 (4.4) | 7 (5.0) | .783 | −0.061 |
| Prior genitourinary surgery | 4 (8.5) | 6 (4.3) | .260 | 0.180 | 3 (6.7) | 8 (5.7) | .799 | 0.040 |
| Persistence of urinary hardware throughout antibiotic treatment | 5 (10.6) | 14 (9.9) | .889 | 0.031 | 4 (8.9) | 15 (10.6) | .854 | −0.034 |
| ICU admission for pyelonephritis | 11 (23.4) | 38 (27.0) | .684 | −0.069 | 13 (28.9) | 38 (27.0) | .825 | 0.042 |
| Vasopressors | 2 (4.3) | 8 (5.7) | .729 | −0.060 | 2 (4.4) | 7 (5.0) | .625 | −0.075 |
| Pathogen | ||||||||
| Escherichia coli | 29 (61.7) | 77 (54.6) | .396 | 0.126 | 25 (55.6) | 79 (56.0) | .970 | 0.007 |
| Klebsiella pneumoniae | 13 (27.7) | 44 (31.2) | .647 | −0.064 | 15 (33.3) | 43 (30.5) | .704 | 0.072 |
| Klebsiella oxytoca | 2 (4.3) | 4 (2.8) | .632 | 0.081 | 1 (2.2) | 4 (2.8) | .990 | 0.002 |
| Proteus mirabilis | 3 (6.4) | 16 (11.3) | .328 | −0.169 | 3 (6.7) | 14 (9.9) | .469 | −0.129 |
Data are for patients hospitalized with ESBL-producing Enterobacterales pyelonephritis without concomitant bacteremia who were treated with piperacillin-tazobactam versus carbapenem therapy. Data are shown as n (%), unless otherwise indicated.
Abbreviations: ESBL, extended-spectrum β-lactamase; ICU, intensive care unit; IQR, interquartile range.
aPropensity score–weighted numbers were rounded to whole numbers for ease of interpretation. All patients from the eligible population (ie, full cohort) were included in the propensity score–weighted cohort but the process of weighting led to a slightly decreased sample size in the propensity score–weighted cohort.
bOther than for the conditions described in the table.
Weighted Cohort
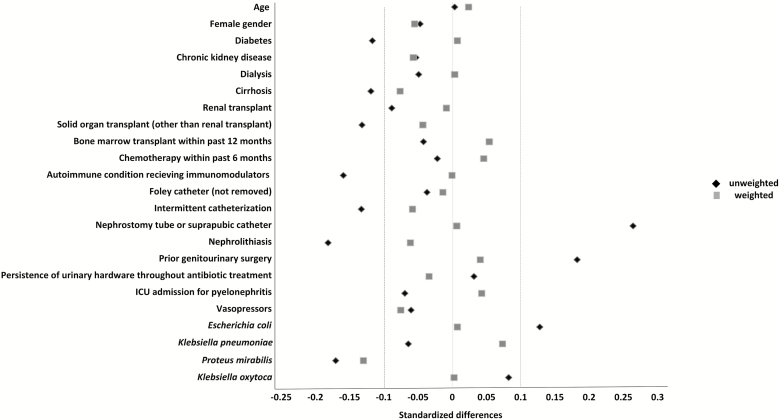
Because of differences in the distribution of baseline variables between the 2 treatment groups, propensity score generation and inverse probability of treatment weighting were pursued (Table 1). There were 186 patients included in the propensity score–weighted cohort; 45 (24%) received TZP and 141 (76%) received a carbapenem. After propensity score weighting, standardized mean differences were below 0.10 for most measured variables, except for the identification of Proteus mirabilis in urine, indicating similar distributions of variables in the TZP and carbapenem groups (Figure 1).
Figure 1.
Standardized mean differences comparing the full, unweighted cohort and the propensity score–weighted cohort after inverse probability of treatment weighting in a study of 188 adults with ESBL-producing Enterobacterales pyelonephritis. The dotted vertical lines represent standardized mean differences at −0.10 and 0.10, with the gray squares inside this range representing an adequate variable balance in the weighted cohort. Abbreviations: ESBL, extended-spectrum β-lactamase; ICU, intensive care unit.
Antibiotic Treatment Regimens
Table 2 describes antibiotic treatment regimens administered to the TZP and carbapenem groups in the weighted cohort. Common antibiotics administered on the day the urine culture was obtained are shown in Table 2. The carbapenem group consisted of 69 (49%) patients receiving ertapenem, 59 (42%) patients receiving meropenem, and 13 (9%) patients who were switched between the 2 carbapenems during their treatment course. There were 9 (20%) patients in the TZP group that transitioned to oral therapy (ie, a fluoroquinolone or TMP-SMX) after at least 72 hours of receiving TZP, whereas 11 (8%) patients in the carbapenem group transitioned to oral therapy after at least 72 hours of receiving a carbapenem antibiotic (P = .03). The median duration of TZP in the TZP group was 8 days (IQR, 7–10) and the median duration of a carbapenem in the carbapenem group was 8 days (IQR, 7–13; P = .08). The overall durations of antibiotic therapy for the TZP and carbapenem groups were 9 days (IQR, 7–12) and 10 days (IQR, 7–14), respectively (P = .07).
Table 2.
Antibiotic Treatment Regimens
| Piperacillin-Tazobactam, n = 45; 24% | Carbapenem, n = 141; 76% | P Value | |
|---|---|---|---|
| Antibiotic initiated within 24 hours of urine culture collection | |||
| Ceftriaxone | 2 (4.4) | 14 (9.9) | .34 |
| Cefepime | 0 | 11 (7.8) | .05 |
| Piperacillin-tazobactam | 43 (95.5) | 14 (9.9) | <.01 |
| Ertapenem | 0 | 51 (36.2) | <.01 |
| Meropenem | 0 | 47 (33.3) | <.01 |
| Fluoroquinolone | 0 | 4 (2.8) | <.01 |
| Oral step-down therapy | |||
| Trimethoprim-sulfamethoxazole | 3 (6.7) | 3 (2.1) | .19 |
| Ciprofloxacin | 6 (13.3) | 8 (5.7) | .12 |
| Duration of therapy | |||
| Total duration, median (IQR) | 9 (7–12) | 10 (7–14) | .07 |
| Duration of study drug, median (IQR) | 8 (7–10) | 8 (7–13) | .08 |
Data are for regimens administered to a cohort of patients hospitalized with ESBL-producing Enterobacterales pyelonephritis without concomitant bacteremia, after inverse probability of treatment weighting. Data are shown as n (%) unless otherwise indicated.
Abbreviations: ESBL, extended-spectrum β-lactamase; IQR, interquartile range.
The most commonly prescribed antibiotic dosages were as follows: TZP at either 3.375 grams (81%) or 4.5 grams (19%) intravenously (IV) every 6 hours; ertapenem at 1 gram IV every 24 hours; and meropenem at 1 gram IV every 8 hours, with adjustment based on renal function as needed. Of note, based on accumulating evidence that a higher dosage of TZP may be warranted for the effective treatment of ESBL-producing infections [21], in the fall of 2016, our local guidelines were revised to recommend TZP at 4.5 grams IV every 6 hours for the treatment of ESBL-producing UTIs in the absence of bacteremia [22].
Outcomes
In the weighed cohort, 44 patients (24%) developed recurrent cystitis or pyelonephritis with the same ESBL organism within 30 days; 9 patients (20%) were in the TZP group and 35 patients (25%) were in the carbapenem group (OR, 0.75; 95% CI, .31–1.81; P = .52). There were no differences in the secondary outcome of resolution of symptoms within 7 days of the first positive urine culture between the TZP and carbapenem groups (42 [93%] vs 125 [89%], respectively; OR, 1.79; 95% CI, .50–6.46; P = .37). There were 2 deaths in the TZP group (4%) and 10 deaths (7%) in the carbapenem group within 30 days, with no differences between the groups (OR, 0.38; 95% CI, .05–3.06; P = .36).
Finally, there was no significant difference in the proportion of patients with an incident carbapenem-resistant organism recovered within 30 days of antibiotic initiation between the 2 treatment groups (OR, 0.16; 95% CI, .02–1.29; P = .09). In the TZP treatment group, 1 patient (2%) had an incident carbapenem-resistant organism recovered, which was a carbapenem-resistant Pseudomonas aeruginosa. In the carbapenem treatment group, 11 patients (8%) had subsequent carbapenem-resistant organisms recovered, including 3 carbapenem-resistant E. coli, 4 carbapenem-resistant K. pneumoniae, 3 carbapenem-resistant P. aeruginosa, and 1 carbapenem-resistant Acinetobacter baumannii. Of the 11 patients who experienced infections with carbapenem-resistant organisms after receiving carbapenem therapy, 5 (45%) had received ertapenem and 6 (55%) had received meropenem.
DISCUSSION
Our findings suggest that in the absence of bacteremia, TZP therapy may result in similar clinical outcomes as carbapenem therapy for the treatment of hospitalized patients with pyelonephritis caused by ESBL-producing organisms. We also observed no difference in the resolution of symptoms within 7 days of treatment initiation or in 30-day all-cause mortality between the 2 treatment groups. Although not achieving statistical significance, 2% of patients receiving TZP, compared to 8% of patients receiving a carbapenem, had an incident carbapenem-resistant organism recovered from clinical cultures within 30 days of antibiotic initiation. This study highlights the potential utility of TZP as a carbapenem-sparing agent for the management of ESBL-producing pyelonephritis.
There have been limited investigations addressing the use of TZP for pyelonephritis caused by ESBL-producing bacteria. The international expert panel assembled to develop the 2010 Infectious Diseases Society of America Guidelines for the “Treatment of Acute Uncomplicated Cystitis and Pyelonephritis in Women” highlighted optimal treatment regimens for ESBL-producing uropathogens as an important gap in knowledge that needs to be further evaluated [23]. Similarly, when discussing the generalizability of their findings, the investigators of the landmark MERINO trial mention that “whether piperacillin-tazobactam remains effective for urinary infections caused by ESBL producers in patients without BSI [bloodstream infection] … remains uncertain” [13]. Previous studies that have attempted to compare the role of TZP and carbapenems for the treatment of pyelonephritis were plagued with small sample sizes [15–19], hazy distinctions between upper and lower urinary tract disease [17–19], or no molecular confirmation that the organism was indeed an ESBL producer [15, 16, 19, 20]. No studies addressing this question have been conducted in the United States.
Both fluoroquinolones and TMP-SMX have robust data supporting their efficacy for pyelonephritis, and provide convenient, oral treatment options for ESBL-producing organisms [19, 24–28]. Regrettably, the declining activity of these agents against Gram-negative organisms by several commonly identified chromosomal mutations (eg, parC and gyrA mutations) and acquired resistance genes (eg, dfr, sul, and qnr genes) restricts effective non–β-lactam options for pyelonephritis, often making hospitalization for patients with ESBL pyelonephritis with administration of β-lactam agents unavoidable [29].
The rising burden of carbapenem-resistant organisms is well recognized as a global crisis [30]. Several studies have identified preceding carbapenem use as a risk factor for the subsequent development of infections with carbapenem-resistant, Gram-negative organisms [31–33]. Although not statistically significant, our study also suggests a trend towards an increased risk of harboring carbapenem-resistant organisms with previous carbapenem exposure. This is particularly worrisome, as a number of studies have indicated that patients who previously received antibiotics to treat bacteria isolated from the urine—regardless of whether antibiotics were indicated—are at an increased risk of a subsequent UTI [34–37], with estimates of affected patients in the range of 24–44% in the subsequent 6–12 months after antibiotic use. Moreover, the subsequent UTI is more likely to be caused by a drug-resistant organism [38, 39]. As almost half of our cohort had underlying urologic abnormalities and are likely to have future UTIs, this finding underscores the importance of judicious antibiotic prescribing for the treatment of UTIs.
There are several limitations to consider when interpreting our findings. First, this was an observational study and, despite our attempts to account for the inherent selection bias this poses by analyzing data using inverse probability of treatment weighting with propensity scores, the potential for unmeasured confounding remains. Second, there may have been relevant data (eg, additional outpatient visits, subsequent drug-resistant urinary isolates, etc) that we did not capture. Although we used the Epic Care Everywhere network and the Chesapeake Regional Information System for Our Patients to increase the likelihood of obtaining postdischarge data, this does not resolve the possibility of residual missing data. However, there does not appear to be a reason to believe missing data was more likely in one treatment group compared to the other. Additionally, the misclassification of pyelonephritis as cystitis or vice versa may have occurred despite adjudication by two physicians, although we do not have reason to believe it occurred asymmetrically between the two treatment groups. Furthermore, we were unable to differentiate recurrent ESBL infections from persistent infections. Our modest sample size may have precluded the ability to identify poorer outcomes in patients receiving TZP. Although this possibility remains, none of the primary or secondary outcomes suggest a trend towards inferior outcomes with TZP compared to carbapenem therapy, in agreement with all published studies to date that have evaluated the role of TZP for ESBL-producing pyelonephritis. Finally, our study was limited to E. coli, Klebsiella spp., and P. mirabilis and to blaCTX-M-type, blaSHV-type, and blaTEM-type genes from 1 region. We did not test for the presence of blaOXA-1genes. It is unknown whether these findings can be extended to other members of the Enterobacterales family that are ESBL-producing or to regions where OXA-1 production may be more predominant.
In conclusion, our study suggests that TZP may be a suitable alternative to carbapenem therapy for the management of pyelonephritis by ESBL-producing organisms without bacteremia. Adequately powered and appropriately designed randomized, controlled trials are required to confirm these findings.
Notes
Financial support. This work was supported by the National Institutes of Health (grant number K23-AI127935 to P. D. T.).
Potential conflicts of interest. S. E. C. has received personal fees from Novartis, Theravance, and Basilea, outside of the submitted work. P. J. S. has received funds paid to her institution from Accelerate Diagnostics, BD Diagnostics, Affinity Biosensors, Opgen Inc., bioMerieux, Check Points Diagnostics BV, and Hardy Diagnostics; and has received personal fees from Accelerate Diagnostics, Roche Diagnostics, Opgen Inc, and Cosmos ID, outside the submitted work. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Ramakrishnan K, Scheid DC. Diagnosis and management of acute pyelonephritis in adults. Am Fam Physician 2005; 71:933–42. [PubMed] [Google Scholar]
- 2. Almomani BA, Hayajneh WA, Ayoub AM, Ababneh MA, Al Momani MA. Clinical patterns, epidemiology and risk factors of community-acquired urinary tract infection caused by extended-spectrum beta-lactamase producers: a prospective hospital case-control study. Infection 2018; 46:495–501. [DOI] [PubMed] [Google Scholar]
- 3. Meier S, Weber R, Zbinden R, Ruef C, Hasse B. Extended-spectrum β-lactamase-producing Gram-negative pathogens in community-acquired urinary tract infections: an increasing challenge for antimicrobial therapy. Infection 2011; 39:333–40. [DOI] [PubMed] [Google Scholar]
- 4. Pitout JD, Laupland KB. Extended-spectrum beta-lactamase-producing Enterobacteriaceae: an emerging public-health concern. Lancet Infect Dis 2008; 8:159–66. [DOI] [PubMed] [Google Scholar]
- 5. Thaden JT, Fowler VG, Sexton DJ, Anderson DJ. Increasing incidence of extended-spectrum β-lactamase-producing Escherichia coli in community hospitals throughout the southeastern United States. Infect Control Hosp Epidemiol 2016; 37:49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McDanel J, Schweizer M, Crabb V, et al. Incidence of extended-spectrum β-lactamase (ESBL)-producing Escherichia coli and Klebsiella infections in the United States: a systematic literature review. Infect Control Hosp Epidemiol 2017; 38:1209–15. [DOI] [PubMed] [Google Scholar]
- 7. Castanheira M, Farrell SE, Krause KM, Jones RN, Sader HS. Contemporary diversity of β-lactamases among Enterobacteriaceae in the nine US census regions and ceftazidime-avibactam activity tested against isolates producing the most prevalent β-lactamase groups. Antimicrob Agents Chemother 2014; 58:833–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Saurina G, Quale JM, Manikal VM, Oydna E, Landman D. Antimicrobial resistance in Enterobacteriaceae in Brooklyn, NY: epidemiology and relation to antibiotic usage patterns. J Antimicrob Chemother 2000; 45:895–8. [DOI] [PubMed] [Google Scholar]
- 9. Hoban DJ, Badal R, Bouchillon S, et al. In vitro susceptibility and distribution of beta-lactamases in Enterobacteriaceae causing intra-abdominal infections in North America 2010-2011. Diagn Microbiol Infect Dis 2014; 79:367–72. [DOI] [PubMed] [Google Scholar]
- 10. Palacios-Baena ZR, Gutierrez-Gutierrez B, Calbo E, et al. Empiric therapy with carbapenem-sparing regimens for bloodstream infections due to extended-spectrum beta-lactamase-producing Enterobacteriaceae: results from the increment cohort. Clin Infect Dis 2017; 65(10):1615–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gutiérrez-Gutiérrez B, Bonomo RA, Carmeli Y, et al. ; REIPI/ESGBIS/INCREMENT Group. Ertapenem for the treatment of bloodstream infections due to ESBL-producing Enterobacteriaceae: a multinational pre-registered cohort study. J Antimicrob Chemother 2016; 71:1672–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tamma PD, Han JH, Rock C, et al. ; Antibacterial Resistance Leadership Group Carbapenem therapy is associated with improved survival compared with piperacillin-tazobactam for patients with extended-spectrum β-lactamase bacteremia. Clin Infect Dis 2015; 60:1319–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Harris PNA, Tambyah PA, Lye DC, et al. ; MERINO Trial Investigators and the Australasian Society for Infectious Disease Clinical Research Network (ASID-CRN). Effect of piperacillin-tazobactam vs meropenem on 30-day mortality for patients with E coli or Klebsiella pneumoniae bloodstream infection and ceftriaxone resistance: a randomized clinical trial. JAMA 2018; 320:984–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gutiérrez-Gutiérrez B, Pérez-Galera S, Salamanca E, et al. A multinational, preregistered cohort study of β-lactam/β-lactamase inhibitor combinations for treatment of bloodstream infections due to extended-spectrum-β-lactamase-producing Enterobacteriaceae. Antimicrob Agents Chemother 2016; 60:4159–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. de La Blanchardiere A, Dargere S, Guerin F, et al. Non-carbapenem therapy of urinary tract infections caused by extended-spectrum beta-lactamase-producing Enterobacteriaceae. Med Mal Infect 2015; 45(5):169–72. [DOI] [PubMed] [Google Scholar]
- 16. Asakura T, Ikeda M, Nakamura A, Kodera S. Efficacy of empirical therapy with non-carbapenems for urinary tract infections with extended-spectrum beta-lactamase-producing Enterobacteriaceae. Int J Infect Dis 2014; 29:91–5. [DOI] [PubMed] [Google Scholar]
- 17. Seo YB, Lee J, Kim YK, et al. Randomized controlled trial of piperacillin-tazobactam, cefepime and ertapenem for the treatment of urinary tract infection caused by extended-spectrum beta-lactamase-producing Escherichia coli. BMC Infect Dis 2017; 17:404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gavin PJ, Suseno MT, Thomson RB Jr, et al. Clinical correlation of the CLSI susceptibility breakpoint for piperacillin-tazobactam against extended-spectrum-beta-lactamase-producing Escherichia coli and Klebsiella species. Antimicrob Agents Chemother 2006; 50:2244–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Park SH, Choi SM, Chang YK, et al. The efficacy of non-carbapenem antibiotics for the treatment of community-onset acute pyelonephritis due to extended-spectrum β-lactamase-producing Escherichia coli. J Antimicrob Chemother 2014; 69:2848–56. [DOI] [PubMed] [Google Scholar]
- 20. Yoon YK, Kim JH, Sohn JW, Yang KS, Kim MJ. Role of piperacillin/tazobactam as a carbapenem-sparing antibiotic for treatment of acute pyelonephritis due to extended-spectrum β-lactamase-producing Escherichia coli. Int J Antimicrob Agents 2017; 49:410–5. [DOI] [PubMed] [Google Scholar]
- 21. Tamma PD, Rodriguez-Bano J. The use of noncarbapenem β-lactams for the treatment of extended-spectrum β-lactamase infections. Clin Infect Dis 2017; 64:972–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cosgrove SE, Avdic E. JHH-BMC for antibiotic use app. Unbound Med 2019. [Google Scholar]
- 23. Gupta K, Hooton TM, Naber KG, et al. ; Infectious Diseases Society of America; European Society for Microbiology and Infectious Diseases International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European society for microbiology and infectious diseases. Clin Infect Dis 2011; 52:e103–20. [DOI] [PubMed] [Google Scholar]
- 24. Fox MT, Melia MT, Same RG, Conley AT, Tamma PD. A seven-day course of TMP-SMX may be as effective as a seven-day course of ciprofloxacin for the treatment of pyelonephritis. Am J Med 2017; 130:842–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Talan DA, Klimberg IW, Nicolle LE, Song J, Kowalsky SF, Church DA. Once daily, extended release ciprofloxacin for complicated urinary tract infections and acute uncomplicated pyelonephritis. J Urol 2004; 171:734–9. [DOI] [PubMed] [Google Scholar]
- 26. Talan DA, Stamm WE, Hooton TM, et al. Comparison of ciprofloxacin (7 days) and trimethoprim-sulfamethoxazole (14 days) for acute uncomplicated pyelonephritis pyelonephritis in women: a randomized trial. JAMA 2000; 283:1583–90. [DOI] [PubMed] [Google Scholar]
- 27. Peterson J, Kaul S, Khashab M, Fisher AC, Kahn JB. A double-blind, randomized comparison of levofloxacin 750 mg once-daily for five days with ciprofloxacin 400/500 mg twice-daily for 10 days for the treatment of complicated urinary tract infections and acute pyelonephritis. Urology 2008; 71:17–22. [DOI] [PubMed] [Google Scholar]
- 28. Cronberg S, Banke S, Bergman B, et al. Fewer bacterial relapses after oral treatment with norfloxacin than with ceftibuten in acute pyelonephritis initially treated with intravenous cefuroxime. Scand J Infect Dis 2001; 33:339–43. [DOI] [PubMed] [Google Scholar]
- 29. Karlowsky JA, Lagacé-Wiens PRS, Adam HJ, et al. In vitro susceptibility of urinary Escherichia coli isolates to first- and second-line empirically prescribed oral antimicrobials: CANWARD surveillance study results for Canadian outpatients, 2007-2016. Int J Antimicrob Agents 2019; 54:62–8. [DOI] [PubMed] [Google Scholar]
- 30. Guidelines for the prevention and control of carbapenem-resistant Enterobacteriaceae, Acinetobacter baumannii and Pseudomonas aeruginosa in health care facilities. Available at: apps.who.int/iris/bitstream/handle/10665/259462/9789241550178-eng.pdf;jsessionid=EABF4E588E4FBD2552E0D20407D8D04A?sequence=1. Accessed 8 October 2019. [PubMed]
- 31. Richter SE, Miller L, Needleman J, et al. Risk factors for development of carbapenem resistance among Gram-negative rods. Open Forum Infect Dis 2019; 6:ofz027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Muggeo A, Guillard T, Barbe C, et al. ; CARBAFREST Group. Factors associated with carriage of carbapenem-non-susceptible Enterobacteriaceae in North-Eastern France and outcomes of infected patients. J Antimicrob Chemother 2017; 72:1496–501. [DOI] [PubMed] [Google Scholar]
- 33. Chotiprasitsakul D, Srichatrapimuk S, Kirdlarp S, Pyden AD, Santanirand P. Epidemiology of carbapenem-resistant Enterobacteriaceae: a 5-year experience at a tertiary care hospital. Infect Drug Resist 2019; 12:461–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cai T, Mazzoli S, Mondaini N, et al. The role of asymptomatic bacteriuria in young women with recurrent urinary tract infections: to treat or not to treat? Clin Infect Dis 2012; 55:771–7. [DOI] [PubMed] [Google Scholar]
- 35. Ikäheimo R, Siitonen A, Heiskanen T, et al. Recurrence of urinary tract infection in a primary care setting: analysis of a 1-year follow-up of 179 women. Clin Infect Dis 1996; 22:91–9. [DOI] [PubMed] [Google Scholar]
- 36. Foxman B, Gillespie B, Koopman J, et al. Risk factors for second urinary tract infection among college women. Am J Epidemiol 2000; 151:1194–205. [DOI] [PubMed] [Google Scholar]
- 37. Foxman B. Urinary tract infection syndromes: occurrence, recurrence, bacteriology, risk factors, and disease burden. Infect Dis Clin North Am 2014; 28:1–13. [DOI] [PubMed] [Google Scholar]
- 38. Cai T, Nesi G, Mazzoli S, et al. Asymptomatic bacteriuria treatment is associated with a higher prevalence of antibiotic resistant strains in women with urinary tract infections. Clin Infect Dis 2015; 61:1655–61. [DOI] [PubMed] [Google Scholar]
- 39. Sundvall PD, Elm M, Gunnarsson R, et al. Antimicrobial resistance in urinary pathogens among Swedish nursing home residents remains low: a cross-sectional study comparing antimicrobial resistance from 2003 to 2012. BMC Geriatr 2014; 14:30. [DOI] [PMC free article] [PubMed] [Google Scholar]