Abstract
Background
Campylobacter species are among the most common causes of enteric bacterial infections worldwide. Men who have sex with men (MSM) are at increased risk for sexually transmitted enteric infections, including globally distributed strains of multidrug-resistant Shigella species.
Methods
This was a retrospective study of MSM-associated Campylobacter in Seattle, Washington and Montréal, Québec with phenotypic antimicrobial resistance profiles and whole genome sequencing (WGS).
Results
We report the isolation of 2 clonal lineages of multidrug-resistant Campylobacter coli from MSM in Seattle and Montréal. WGS revealed nearly identical strains obtained from the 2 regions over a 4-year period. Comparison with the National Center for Biotechnology Information’s Pathogen Detection database revealed extensive Campylobacter species clusters carrying multiple drug resistance genes that segregated with these isolates. Examination of the genetic basis of antimicrobial resistance revealed multiple macrolide resistance determinants including a novel ribosomal RNA methyltransferase situated in a CRISPR (clustered regularly interspaced short palindromic repeats) array locus in a C. coli isolate.
Conclusions
As previously reported for Shigella, specific multidrug-resistant strains of Campylobacter are circulating by sexual transmission in MSM populations across diverse geographic locations, suggesting a need to incorporate sexual behavior in the investigation of clusters of foodborne pathogens revealed by WGS data.
Keywords: Campylobacter, MSM, antimicrobial resistance, Washington State, Québec
We show defined clonal lineages of likely sexually transmitted Campylobacter species associated with spread in men who have sex with men between Seattle and Montréal. We also report the first CRISPR-acquired antibiotic resistance gene in a clinical isolate.
Men who have sex with men (MSM) are at increased risk for sexually transmitted enteric infections including shigellosis, campylobacteriosis, giardiasis, and viral hepatitis [1]. Sexually transmitted enteric pathogens in MSM are becoming increasingly resistant to first-line antimicrobial agents. Global transmission of azithromycin-resistant Shigella flexneri serotype 3a associated with MSM has been shown across the United Kingdom, United States, Canada, and Australia, and possibly Latin America, during the past 20 years [2]. Outbreaks of Shigella sonnei resistant to ciprofloxacin and/or azithromycin associated with MSM have also been detected over the past decade in the United States, Canada, Taiwan, and Australia [3–6].
Campylobacter species (spp) are common causes of bacterial gastroenteritis worldwide that have been recognized as a cause of sexually transmitted infections in MSM since before the onset of the human immunodeficiency virus (HIV) epidemic [7–9]. Although gradually increasing antimicrobial resistance in clinical Campylobacter isolates has been noted [10], a specific association between multidrug-resistant Campylobacter and MSM has been reported only sporadically. Fluoroquinolone resistance was common among Campylobacter isolates from people living with HIV in France from 1989 to 1994, but macrolide resistance was infrequent [11]. During the past 2 decades, several outbreaks of multidrug-resistant Campylobacter have occurred in MSM in Québec, Canada. The first occurred in 1998–2001, involving 11 men with enterocolitis caused by erythromycin- and ciprofloxacin-resistant Campylobacter jejuni that was clonal by pulsed-field gel electrophoresis [12]. Two discrete clades of macrolide- and fluoroquinolone-resistant C. jejuni were noted to be persistent in the MSM community in Québec between 2003 and 2013 [13]. From 2010 to 2011, tetracycline- and ciprofloxacin-resistant Campylobacter coli pulsovar 1 was detected among 10 MSM in Montréal, while a C. coli pulsovar 15 outbreak involved 6 MSM in Montréal in 2015 [14, 15]. Most recently, a cluster of Campylobacter fetus infections occurred in Québec in 2014–2016, including several fluoroquinolone-resistant strains [16].
Over the past 3 years, we noted several multidrug-resistant Campylobacter isolates from patients in the Seattle, Washington area, some of whom were MSM. Here, we present the epidemiological correlates, antimicrobial resistance phenotypes, and genotypic characterization based on whole genome sequencing (WGS) of these isolates along with comparisons to 9 multidrug-resistant C. coli isolates obtained from MSM in Québec during 2015–2018.
METHODS
Isolate Description and Antimicrobial Susceptibility
This study was approved by the University of Washington Institutional Review Board and the Centre hospitalier de l’Université de Montréal Research Ethics Committee. For Washington State isolates, stools that were positive on the FilmArray Gastrointestinal Panel (BioFire Diagnostics, Salt Lake City, Utah) for Campylobacter spp were plated on Campylobacter CVA selective media and incubated for 3 days at 42°C in microaerophilic conditions. Following incubation, Campylobacter spp were identified by a matrix-assisted laser desorption/ionization–time of flight (MALDI-TOF) mass spectrometry system (Bruker Daltonics, Bremen, Germany).
All Campylobacter isolates were grown for 24 hours at 37°C in microaerophilic conditions before susceptibility testing. Campylobacter jejuni ATCC 33560 was used as a quality control. After 24 hours, several colonies were suspended in saline to a turbidity of 0.5 McFarland. Isolates were cultured on Mueller-Hinton agar with 5% sheep blood agar under microaerophilic conditions at 37°C for 24 hours. Etests (bioMérieux, Marcy-l’Étoile, France) containing ciprofloxacin, amoxicillin–clavulanic acid, erythromycin, fosfomycin, gentamicin, meropenem, tetracycline, rifampin, azithromycin, clindamycin, and chloramphenicol were used to determine minimum inhibitory concentrations (MICs). A portion of the antimicrobial susceptibility testing (AST) was performed retrospectively for nonclinical purposes. Dual macrolide- and fluoroquinolone-resistant isolates were chosen for WGS.
Campylobacter Sequencing and Analysis
DNA was extracted from Campylobacter pellets using the MoBio UltraPure kit. DNA was quantitated on a Qubit 3.0 fluorometer (Thermo Fisher) and 1 ng was used as input for tagmentation library preparation using two-fifths volumes of the Nextera XT kit protocol (Illumina). Libraries were amplified using dual-index primers with 17 cycles of polymerase chain reaction and purified using 0.7X Ampure beads. Libraries were quantitated using the Qubit 3.0 fluorometer and sequenced on a 2 × 300 bp run of the Illumina MiSeq. Sequencing reads and assembly contigs are available in the National Center for Biotechnology Information (NCBI) under BioProject PRJNA542889.
Additional Campylobacter isolates that fell into single-nucleotide polymorphism (SNP) clusters (based on single-linkage clusters with a maximum of 50 SNP differences) with the 18 sequenced isolates were identified using the NCBI Pathogen Detection browser (as of 15 June 2019) and included in downstream bioinformatic analyses. BioSample metadata were also downloaded for 8624 Campylobacter isolates in Pathogen Detection that were collected between June 2014 and January 2019 and derived from clinical material. Of these, 5039 isolates contained age ranges. Age ranges were set in 20-year bins and distribution between sequenced clinical Campylobacter isolates available in NCBI, and isolates clustering with our sequenced isolates were compared using Fisher exact test.
Paired-end sequencing reads were adapter- and quality-trimmed using trimmomatic (ILLUMINACLIP:NexteraPE-PE.fa:2:30:10 LEADING:3 TRAILING:3 SLIDINGWINDOW:4:30 MINLEN:20) and de novo assembled using SPAdes version 3.13 with default options followed by prokka version 1.13 annotation of contigs [17, 18]. Antibiotic resistance genes were identified using the NCBI AMRFinder [19]. Virulence genes were identified using VFanalyzer and a custom BLAST database constructed with corresponding genes from the C. jejuni NCTC11168 strain (NC_002163.1) as well as virB11 (NC_005012.1) and hcp (NZ_CP028373.1) [20]. The presence and absence of virulence genes are discussed in the Supplementary Materials. Multilocus sequence typing (MLST) was performed using PubMLST and Torsten Seeman’s MLST typing tool (https://github.com/tseemann/mlst) [21]. Initially unassigned sequence types and alleles were submitted to PubMLST to obtain definitions.
Core genome SNP phylogeny was performed as described previously and used to initially discover isolate clusters and confirm NCBI Pathogen Detection relationships [6, 22]. In brief, quality- and adapter-trimmed reads were aligned to a reference genome with BWA-MEM and SAMtools and filtered with VCFtools using the following parameters: minDP 10, minQ 200, and minGQ 10. The clinical C. jejuni NCTC11168 strain (NC_002163.1) was used as the reference genome for constructing the C. jejuni phylogeny [23]. The multidrug-resistant C. coli RM2228 strain (NZ_CP035927.1) was used as the reference genome for constructing C. coli phylogeny [24].
RESULTS
Bacterial Strains
Nine isolates of Campylobacter spp resistant to both fluoroquinolones and macrolides were isolated by the clinical microbiology laboratories at Harborview Medical Center (Seattle, Washington) or the University of Washington Medical Center between 2015 and 2018. Three were C. jejuni and 6 were C. coli, as identified by MALDI-TOF mass spectrometry. Review of the patient medical records associated with the isolates indicated the potential for sexual transmission among MSM for 5 of the 9 isolates, while 3 of the 4 non-MSM isolates were associated with recent travel to Asia (Table 1). Because of the well-documented outbreaks of multidrug-resistant Campylobacter spp in Québec [12, 15, 16], 9 multidrug-resistant C. coli isolates were also obtained from the Laboratoire de santé publique du Québec in Canada from the same time period. Seven of these 9 isolates from Canada were also associated with MSM. All isolates were then phenotypically confirmed, AST was performed as above, and WGS was performed to examine mechanisms of antimicrobial resistance, virulence factors, and epidemiological relatedness.
Table 1.
Epidemiological Information for the Isolates in This Study
| Location and Strain | Campylobacter Species | Date ofCollection | Age, y | Sex | MSM | HIV | Travel/Origin | Resistance |
|---|---|---|---|---|---|---|---|---|
| Québec | ||||||||
| 42478 | C. coli | Dec 2017 | 24 | M | Y | N | … | FQ, AZM, TET, GEN |
| 43371 | C. coli | Dec 2017 | 44 | M | Y | Y | … | FQ, AZM, TET, GEN |
| 48777 | C. coli | Jan 2018 | 40 | M | Y | Y | … | FQ, AZM, TET |
| 76331 | C. coli | May 2018 | 48 | M | Y | N | … | FQ, AZM, TET |
| 138449 | C. coli | Jan 2015 | 62 | M | Y | Y | … | FQ, AZM, TET |
| 143854 | C. coli | Sep 2015 | 29 | M | Y | N | … | FQ, AZM, TET |
| 143970 | C. coli | Sep 2015 | 59 | M | Y | Y | … | FQ, AZM, TET |
| 148558 | C. coli | Apr 2016 | 74 | M | N | NA | … | FQ, AZM, TET |
| 158403 | C. coli | Apr 2017 | 25 | M | NA | NA | … | FQ, AZM, TET |
| Washington State | ||||||||
| SP15-082 | C. coli | Jul 2015 | 68 | M | N | N | Malaysia | FQ, AZM, TET |
| SP16-070 | C. coli | Jun 2016 | 21 | M | N | N | Thailand | FQ, AZM, TET |
| SP17-196 | C. jejuni | Dec 2017 | 72 | M | N | N | Philippines | FQ, AZM, TET |
| HMC314 | C. jejuni | Jan 2018 | 55 | M | Y | N | … | FQ, AZM |
| SP18-054 | C. coli | Feb 2018 | 25 | M | Y | N | … | FQ, AZM, TET, GEN |
| SP18-090 | C. coli | Feb 2018 | 27 | M | Y | Y | … | FQ, AZM, TET, GEN |
| S871 | C. coli | Mar 2018 | 34 | M | Y | Y | … | FQ, AZM, TET |
| SP18-164 | C. jejuni | Jun 2018 | 22 | M | Y | N | … | FQ, AZM |
| SP18-232 | C. coli | Oct 2018 | 59 | F | N | N | … | FQ, AZM, TET |
| Pathogen Detection (NCBI) | ||||||||
| PNUSAC002907 | C. coli | Sep 2017 | 30-39 | … | … | … | Midwest US | … |
| PNUSAC000107 | C. coli | May 2015 | 20-29 | … | … | … | Midwest US | … |
| PNUSAC000108 | C. coli | Jul 2015 | 30-39 | … | … | … | Midwest US | … |
| PNUSAC000199 | C. coli | Nov 2015 | 50-59 | … | … | … | Midwest US | … |
| PNUSAC000219 | C. coli | Nov 2015 | 50-59 | … | … | … | Midwest US | … |
| PNUSAC006454 | C. coli | … | … | … | … | … | … | … |
| PNUSAC007077 | C. coli | … | … | … | … | … | … | … |
| PNUSAC008980 | C. coli | … | … | … | … | … | … | … |
| PNUSAC007971 | C. jejuni | … | … | … | … | … | … | … |
| NC05-27 | C. jejuni | 2005 | … | … | … | … | … | … |
| PNUSAC000631 | C. jejuni | Jun 2016 | 20-29 | … | … | … | Southwest US | … |
| PNUSAC001707 | C. jejuni | … | … | … | … | … | … | … |
| PNUSAC004578 | C. jejuni | May 2018 | 30-39 | … | … | … | Midwest US | … |
| PNUSAC005510 | C. jejuni | Jul 2018 | 20-29 | … | … | … | Midwest US | … |
| PNUSAC005955 | C. jejuni | Aug 2018 | 20-29 | … | … | … | Midwest US | … |
| PNUSAC006340 | C. jejuni | … | … | … | … | … | … | … |
| PNUSAC006599 | C. jejuni | Mar 2018 | 20-29 | … | … | … | Southeast US | … |
| PNUSAC006863 | C. jejuni | Jun 2018 | 30-39 | … | … | … | Southeast US | … |
| PNUSAC007906 | C. jejuni | … | … | … | … | … | … | … |
Colored rows differentiate genomic clusters.
Abbreviations: AZM, azithromycin; F, female; FQ, fluoroquinolone; GEN, gentamicin; HIV, human immunodeficiency virus; M, male; MSM, men who have sex with men; N, no; NA, not available; NCBI, National Center for Biotechnology Information; TET, tetracycline; US, United States; Y, yes.
In addition to resistance to fluoroquinolones and macrolides, 2 C. coli isolates from Washington State and 2 from Québec were resistant to gentamicin (Table 2). All C. coli isolates were also resistant to tetracycline.
Table 2.
Antimicrobial Resistance Patterns of the Campylobacter coli and Campylobacter jejuni Isolates
| Isolate | Minimum Inhibitory Concentration, μg/mL | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| AMCa | CIPb,c | ERYb,c | FOS | GENd | MERa | TETb,c | RIF | AZMd,e | CLId | CHLd | |
| 42478 | 4 (S) | >32 (R) | >256 (R) | 16 (–) | >256 (R) | 0.064 (S) | >256 (R) | >32 (–) | >256 (R) | 32 (R) | 8 (S) |
| 43371 | 2 (S) | >32 (R) | >256 (R) | 16 (–) | >256 (R) | 0.32 (S) | >256 (R) | >32 (–) | >256 (R) | 32 (R) | 8 (S) |
| 48777 | 4 (S) | >32 (R) | 128 (R) | 64 (–) | 0.25 (S) | 0.125 (S) | >256 (R) | >32 (–) | 32 (R) | 256 (R) | 32 (R) |
| 76331 | 4 (S) | 16 (R) | >256 (R) | 32 (–) | 0.5 (S) | 0.032 (S) | >256 (R) | >32 (–) | >256 (R) | 8 (R) | 16 (S) |
| 138449 | 4 (S) | >32 (R) | >256 (R) | 32 (–) | 0.25 (S) | 0.5 (S) | >256 (R) | >32 (–) | >256 (R) | 8 (R) | 8 (S) |
| 143854 | 4 (S) | >32 (R) | >256 (R) | 32 (–) | 0.125 (S) | 0.5 (S) | >256 (R) | >32 (–) | >256 (R) | 8 (R) | 8 (S) |
| 143970 | 8 (R) | >32 (R) | >256 (R) | 64 (–) | 0.125 (S) | 0.5 (S) | >256 (R) | >32 (–) | >256 (R) | 16 (R) | 8 (S) |
| 148558 | 4 (S) | >32 (R) | >256 (R) | 32 (–) | 0.125 (S) | 0.125 (S) | >256 (R) | >32 (–) | >256 (R) | 8 (R) | 8 (S) |
| 158403 | 8 (R) | >32 (R) | >256 (R) | 16 (–) | 0.5 (S) | 0.5 (S) | >256 (R) | >32 (–) | >256 (R) | 16 (R) | 16 (S) |
| S871 | 16 (R) | >32 (R) | >256 (R) | 64 (–) | 0.25 (S) | 1.0 (S) | >256 (R) | >32 (–) | … | … | … |
| SP15-082 | 8 (S) | >32 (R) | >256 (R) | >1024 (–) | 0.25 (S) | 0.5 (S) | >256 (R) | >32 (–) | >256 (R) | 16 (R) | 32 (R) |
| SP16-070 | 8 (S) | >32 (R) | >256 (R) | 32 (–) | 0.5 (S) | 0.5 (S) | >256 (R) | >32 (–) | >256 (R) | 32 (R) | 16 (S) |
| SP18-054 | 4 (S) | >32 (R) | >256 (R) | 16 (–) | >256 (R) | 0.064 (S) | >256 (R) | >32 (–) | >256 (R) | 64 (R) | 8 (S) |
| SP18-090 | 2 (S) | >32 (R) | >256 (R) | 16 (–) | >256 (R) | 0.032 (S) | >256 (R) | >32 (–) | >256 (R) | 64 (R) | 16 (S) |
| SP18-232 | 4 (S) | >32 (R) | 128 (R) | 64 (–) | 0.125 (S) | 0.5 (S) | >256 (R) | >32 (–) | >256 (R) | 4 (R) | 4 (S) |
| 18–164 | 4 (S) | >32 (R) | >256 (R) | 32 (–) | 0.25 (S) | 0.008 (S) | 0.5 (S) | >32 (–) | >256 (R) | 128 (R) | 4 (S) |
| HMC314 | 4 (S) | 8 (R) | >256 (R) | 32 (–) | 0.25 (S) | 0.016 (S) | 0.25 (S) | >32 (–) | >256 (R) | 16 (R) | 4 (S) |
| SP17-196 | 4 (S) | >32 (R) | >256 (R) | 32 (–) | 0.25 (S) | 0.5 (S) | >256 (R) | >32 (–) | 32 (R) | 256 (R) | 256 (R) |
Susceptibility interpretations are from the Clinical and Laboratory Standards Institute (CLSI) and/or the European Committee on Antimicrobial Susceptibility Testing (EUCAST). (–) Indicates no CLSI or EUCAST interpretation.
Abbreviations: AMC, amoxicillin–clavulanic acid; AZM, azithromycin; CHL, chloramphenicol; CIP, ciprofloxacin; CLI, clindamycin; ERY, erythromycin; FOS, fosfomycin; GEN, gentamicin; MER, meropenem; R, resistant; RIF, rifampin; S, susceptible; TET, tetracycline.
aEUCAST pharmacokinetic/pharmacodynamic (non–species related) breakpoints version 9.0.
bCLSI M45 3rd edition: 2016 breakpoints.
cEUCAST breakpoints version 9.0.
dEUCAST epidemiologic cutoff value (accessed 6 August 2019).
eEUCAST breakpoints version 9.0; note that ERY susceptibility can be used to determine AZM susceptibility.
Two Clusters of C. coli From Seattle and Montréal With Near Identity
Multilocus sequencing typing using the combined C. jejuni and C. coli typing scheme for our 18 Campylobacter isolates yielded 12 different sequence types (STs) (Supplementary Table 1) [25]. Only 2 of the STs contained >1 isolate. Notably, we identified novel glyA, pgm, tkt, and uncA alleles from isolate HMC314. In addition, we identified 6 new Campylobacter STs from our isolates, which have been deposited in PubMLST [21].
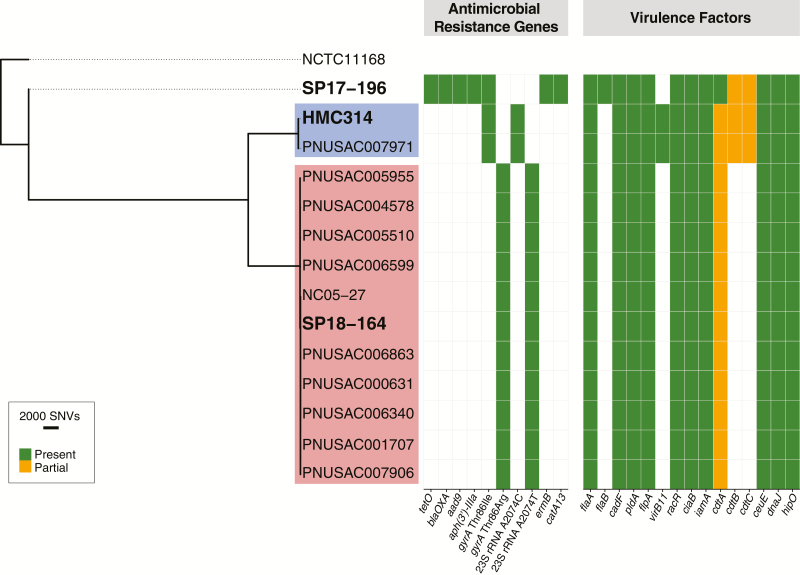
Next, we performed core genome SNP phylogenies to assess the genetic relatedness of the 3 C. jejuni and 15 C. coli isolates. No significant clustering was seen among the 3 C. jejuni isolates from Seattle, with 14 697–34 182 pairwise SNPs seen between each strain (Figure 1; Supplementary Table 2).
Figure 1.
Core genome single-nucleotide polymorphism (SNP) phylogenetic tree for multidrug-resistant Campylobacter jejuni isolates. Isolates sequenced in this study (in bold) are shown along with closely related isolates identified by the National Center for Biotechnology Information’s Pathogen Detection database (individual SNP clusters are highlighted by color). Isolates in the blue cluster differed by 7 SNPs while isolates in the red cluster differed by 0–55 SNPs. Antimicrobial resistance genes and virulence factors are denoted for each isolate. Of note, the cdtA-C toxin locus was interrupted by frameshifts in every C. jejuni isolate in this study. Abbreviations: rRNA, ribosomal RNA; SNV, single nucleotide variant.
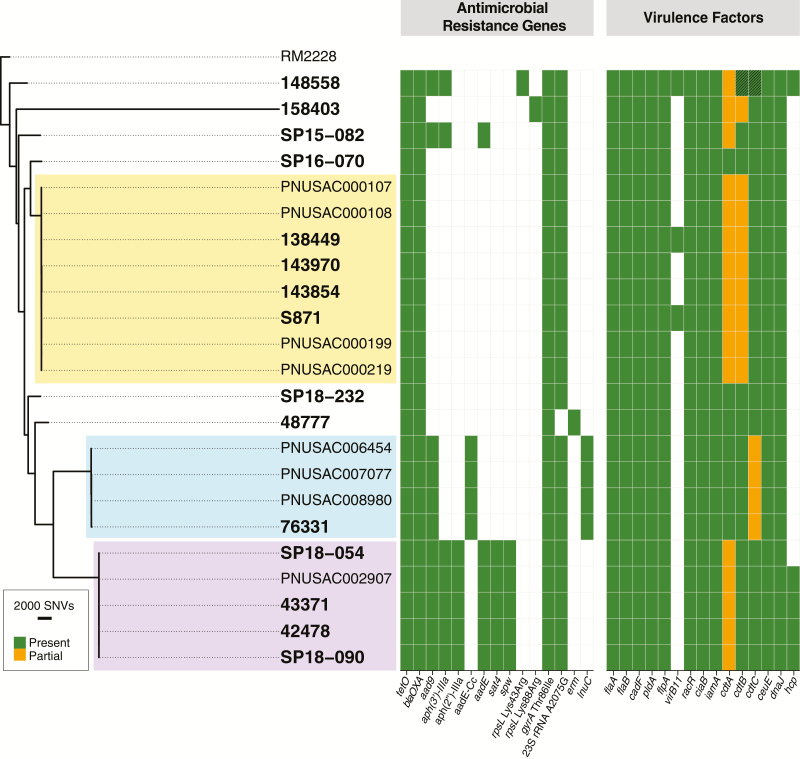
The C. coli isolates differed from each other by an average of 11 132 pairwise core genome SNPs (range, 0–29 321). Two distinct clusters, each containing 4 C. coli isolates, were identified from our analysis. The first cluster contained 3 Montréal isolates and 1 Seattle isolate. The isolates in this cluster differed from each other by an average of 29 (range, 1–43) pairwise core genome SNPs (Figure 2; Supplementary Table 3). Of note, the Seattle isolate S871 and the Montréal isolate 138449 in this cluster differed by only 6 pairwise SNPs. The second cluster contained 2 Montréal isolates and 2 Seattle isolates. This cluster showed minimal genomic variability with an average of 2.5 (range, 0–5) pairwise SNPs separating the 4 isolates.
Figure 2.
Core genome single-nucleotide polymorphism (SNP) phylogenetic tree for multidrug-resistant Campylobacter coli isolates. Isolates sequenced in this study (in bold) are shown along with closely related isolates identified by the National Center for Biotechnology Information’s (NCBI) Pathogen Detection database (individual SNP clusters are highlighted by color). By NCBI Pathogen Detection, the yellow cluster differed by 1–11 SNPs, the turquoise cluster differed by 3–10 SNPs, and the purple cluster differed by 1–7 SNPs. Antimicrobial resistance genes and virulence factors are denoted for each isolate. Of note, the cdtA gene was interrupted by frameshifts in almost every C. jejuni isolate in this study. The cdtA-C toxin locus was also interrupted by frameshifts in almost every C. coli isolate in this study. Abbreviations: rRNA, ribosomal RNA; SNV, single nucleotide variant.
Interrogation of the NCBI Pathogen Detection database queried >31 000 C. jejuni/C. coli isolate genomes for relatedness and confirmed the Seattle–Montréal clusters identified above. We further identified 11 additional C. jejuni isolates and 8 additional C. coli isolates that met the NCBI Pathogen Detection definition of an SNP cluster (maximum 50 SNPs by whole genome MLST [wgMLST]) with our 18 sequenced isolates (Figures 1 and 2). Core genome analysis confirmed the tight evolutionary relationship of the clusters detected by wgMLST analysis (Supplementary Tables 2 and 3). Of note, each of the Campylobacter isolates sequenced from MSM in Washington State yielded additional cluster isolates, whereas isolates not associated with MSM did not yield any additional isolates by SNP cluster. Sample metadata were available for 11 of the 19 newly identified isolates and demonstrated that 9 of the isolates came from 20- to 39-year-olds, with 8 of them deriving from Midwestern states in the United States (US Department of Health and Human Services region 5; Table 1). This age distribution was significantly different from that of 5039 Campylobacter isolates in the NCBI Pathogen Detection database collected between 2014 and 2018 for which host age range information was available (Fisher exact test, P = .006; Supplementary Table 6). Antimicrobial resistance genes were identical within clusters, indicating that these additional isolates were also likely multidrug resistant, despite the lack of phenotypic resistance data.
Antimicrobial Resistance Genes Readily Predict Antimicrobial Susceptibility Data
Consistent with a previous report, WGS analysis was highly accurate in predicting the ciprofloxacin resistance phenotype of the Campylobacter isolates [26]. In 17 of 18 isolates, the well-described fluoroquinolone resistance Thr86Ile gyrA mutation was identified (Figures 1 and 2; Supplementary Table 4) [27]. The less frequently encountered fluoroquinolone resistance–associated Thr86Arg gyrA mutation was identified in the remaining isolate, SP18-164 [28].
Tetracycline resistance was also accurately predicted from the sequencing analysis, as all 16 tetracycline-resistant isolates contained the tetO gene and the 2 tetracycline-susceptible isolates did not contain tetO [29]. In the single isolate with a significantly elevated MIC to chloramphenicol, we were able to identify the cat chloramphenicol resistant determinant, which was not present in the other 17 strains [30].
The 4 isolates displaying resistance to gentamicin each contained 5 aminoglycoside resistance determinants: aad9, aadE, aph(3’)-IIIa, aph(2″)-IIIa, and spw [31]. This combination of aminoglycoside resistance genes was unique to the gentamicin-resistant strains. Three of these determinants, aad9, aadE, and aph(3’)-IIIa, as well as the aadE-Cc determinant [31] and the aminoglycoside resistance–associated mutations in rpsL (Lys43Arg and Lys88Arg), were identified in 6 gentamicin-susceptible strains [32]. It is unclear if these genes and resistance-associated mutations confer resistance to other aminoglycosides.
We also identified the lincosamide resistance gene lnuC in 1 Campylobacter strain, the streptothricin resistance determinant sat4 in 4 isolates, and genes encoding OXA-61 family β-lactamases in 16 isolates. However, the impact of these genes on phenotypic resistance is unclear.
We were further able to determine the genetic basis of erythromycin for all 18 Campylobacter isolates by screening for point mutations in the 23S ribosomal RNA (rRNA) and the acquired erm erythromycin resistance determinants. We identified 23S rRNA mutations (A2074G, A2074C, or A2075T) associated with macrolide resistance in 16 of the strains [33]. The 1 C. jejuni isolate without a macrolide resistance–associated mutation in 23S rRNA carried the resistance determinant ermB.
Identification of a Novel erm Resistance Gene in the CRISPR Array
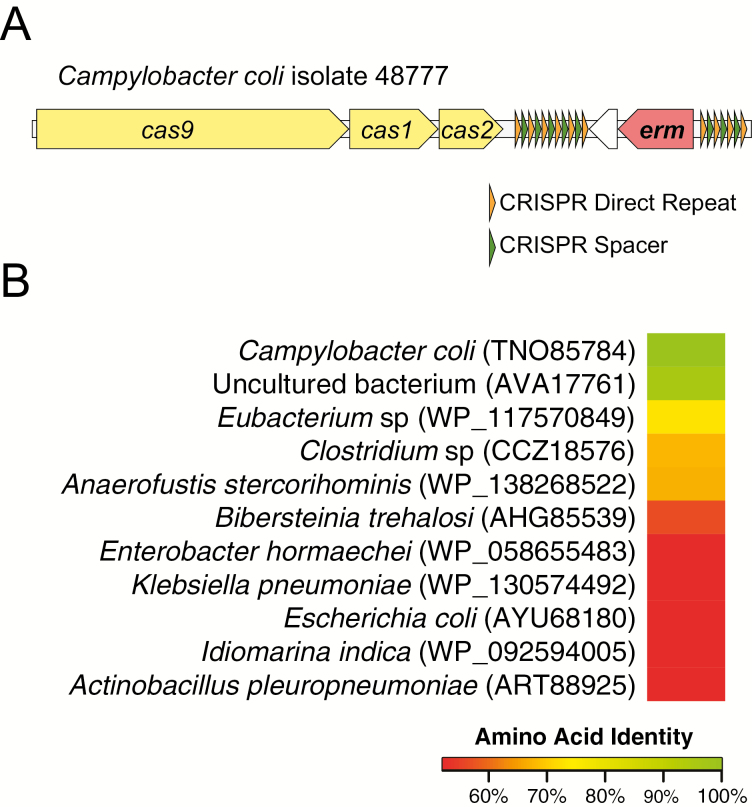
The remaining C. coli strain contained a novel erm gene (TNO85784.1) (Figure 3A). The encoded Erm protein was highly similar (96% identity by amino acid) to a 23S methyltransferase from an uncultured bacterium (AVA17761.1) that demonstrated high levels of resistance to erythromycin but not azithromycin when characterized in vitro [34] (Figure 3B). Whereas this Erm-containing isolate was resistant to both erythromycin and azithromycin, it had lower MICs to both of these drugs compared to other isolates. The next closest hit to the novel Erm protein by BLASTP shared 73% identity by amino acid to a 23S methyltransferase from a Eubacterium species (WP_117570849.1). No significant homology of the sequence could be found when querying the Campylobacter NCBI WGS database or BIGSI [35].
Figure 3.
Novel erythromycin resistance gene detected in the clustered regularly interspaced short palindromic repeats (CRISPR) repeat array locus in Campylobacter coli isolate 48777. A, The erm gene was located downstream of the cas9-cas1-cas2 locus between 6 and 4 CRISPR direct repeats and a neighboring hypothetical protein. The translated amino acid sequence of this gene most closely aligned with an Erm protein from an uncultured bacterium (B). The next-highest hits by BLASTP were <75%, suggesting that these 2 erm genes form a novel erythromycin resistance gene family. Abbreviations: CRISPR, clustered regularly interspaced short palindromic repeats.
Analysis of locus surrounding the erm gene in isolate 48777 revealed that the Cas9-Cas1-Cas2 locus was located 770 bp downstream from the erm gene. Searching of this locus with the clustered regularly interspaced short palindromic repeats (CRISPR) CasFinder tool revealed 6 CRISPR direct repeats with 5 spacers downstream of the erm gene and 4 direct repeats and 3 spacers upstream of the erm gene [36] (Figure 3A). Sanger sequencing confirmed the genomic organization of the CRIPSR locus inferred from short-read sequencing. These data suggest that the novel erm may have been acquired by this C. coli isolate via recombination between repetitive sequences contained in a CRISPR array, making it the first such acquisition of erm in Campylobacter and the first detection of an antimicrobial resistance gene within a CRISPR array in any clinical isolate detected to date [37].
DISCUSSION
The sexual transmission of enteric pathogens in MSM has long been recognized [8, 9, 12]. The emergence of multidrug resistance in Shigella spp associated with MSM is now known to result in large part from the global spread of specific clades [2, 3]. Here we show that multidrug-resistant lineages of C. coli are also spreading through sexual transmission in MSM across international boundaries. Outside of the MSM population, multidrug-resistant Campylobacter spp were also isolated from individuals in Seattle who recently returned from travel to Asia, suggesting 2 potential independent risk factors for acquisition of multidrug-resistant Campylobacter.
MSM are at increased risk for the acquisition of multidrug-resistant enteric pathogens from sexual practices resulting in fecal-oral transmission and from frequent exposure to antimicrobial agents for the treatment of sexually transmitted infections [38, 39]. The availability of preexposure prophylaxis to prevent HIV transmission may be promoting the spread of other sexually transmitted pathogens as a result of risk compensation [40].
Broad epidemiological data suggest that sexually transmitted Campylobacter infections in MSM may be underrecognized. Our data combined with increasing genomic surveillance of clinical Campylobacter isolates suggests that there are indeed clusters associated with transmission among MSM. We note that of the Washington State isolates, only Campylobacter sequenced from MSM yielded additional cluster isolates when interrogating NCBI databases. Although gender was not available for these additional isolates identified via genomic epidemiology, a skewed gender distribution of enteric infections has been suggested as a possible sign of enteric outbreaks among MSM in major metropolitan areas in the United Kingdom [41]. In addition to Shigella, a male skew for non-travel-associated enteric infections was found for Campylobacter [41]. Surveillance of MSM undergoing testing for rectal Chlamydia infections in the United Kingdom found that 1.8% of specimens contained Campylobacter spp, half of which were asymptomatic [42]. In the absence of specific treatment, asymptomatic carriage of enteric pathogens may persist for weeks after the clinical resolution of acute gastroenteritis, allowing ongoing transmission.
Conventional treatment of Campylobacter enteritis has relied on macrolides and fluoroquinolones. Due to increasing fluoroquinolone resistance, macrolides are considered the drugs of choice, but macrolide resistance due to 23S rRNA mutations is increasing, particularly in C. coli [33]. The optimal management of infections caused by macrolide- and fluoroquinolone-resistant strains is not established. Fosfomycin has been suggested as a possible alternative therapeutic agent [43].
The detection of a novel macrolide resistance determinant flanked by CRISPR direct repeats is intriguing. Although CRISPR-Cas systems are often viewed as a barrier to horizontal gene transfer, recent evidence suggests that they may also promote horizontal gene transfer [44]. Further investigation will be required to determine whether the association between erm and CRISPR-Cas in C. coli represents an unusual exception or a novel mechanism of antimicrobial resistance gene acquisition.
This study was chiefly limited by the small sample size and limited metadata associated with the isolates in the NCBI Pathogen Detection database. Nonetheless, it is remarkable that, in sampling cases from only 2 different cities, we found clonally related isolates to be associated with transmission among MSM. As isolates continue to be sequenced, we would hypothesize that the clusters identified here would grow in both number and scale.
The global emergence of multidrug-resistant enteric pathogens in MSM poses an urgent public health challenge that may require new approaches for surveillance and prevention. As Campylobacter isolates are not routinely submitted to the Washington State Public Health Laboratory, the relatedness of C. coli isolates from Seattle and Montréal was not detected from routine surveillance activities. Foodborne and sexually transmitted infections are traditionally the purview of separate branches of the public health hierarchy, but an integrated effort may be required to address this important problem. Rapid advances in the genomic epidemiology of enteric infections may provide the means to integrate these efforts.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Financial support. This work was supported by departmental funds from the Department of Laboratory Medicine, University of Washington School of Medicine, Seattle.
Potential conflicts of interest. F. C. F. has received grants, personal fees, and nonfinancial support from BioFire and Cepheid; personal fees from the Infectious Diseases Society of America; and grants and nonfinancial support from EliTech, outside the submitted work. A. L. G. has received personal fees from Abbott Molecular, outside the submitted work. All other authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Dritz SK, Back AF. Letter: Shigella enteritis venereally transmitted. N Engl J Med 1974; 291:1194. [PubMed] [Google Scholar]
- 2. Baker KS, Dallman TJ, Ashton PM, et al. Intercontinental dissemination of azithromycin-resistant shigellosis through sexual transmission: a cross-sectional study. Lancet Infect Dis 2015; 15:913–21. [DOI] [PubMed] [Google Scholar]
- 3. Ingle DJ, Easton M, Valcanis M, et al. Co-circulation of multidrug-resistant Shigella among men who have sex with men in Australia. Clin Infect Dis 2019; 69:1535–44. [DOI] [PubMed] [Google Scholar]
- 4. Chiou C-S, Izumiya H, Kawamura M, et al. The worldwide spread of ciprofloxacin-resistant Shigella sonnei among HIV-infected men who have sex with men, Taiwan. Clin Microbiol Infect 2016; 22:383.e11–16. [DOI] [PubMed] [Google Scholar]
- 5. Gaudreau C, Ratnayake R, Pilon PA, Gagnon S, Roger M, Lévesque S. Ciprofloxacin-resistant Shigella sonnei among men who have sex with men, Canada, 2010. Emerg Infect Dis 2011; 17:1747–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kozyreva VK, Jospin G, Greninger AL, Watt JP, Eisen JA, Chaturvedi V. Recent outbreaks of shigellosis in California caused by two distinct populations of Shigella sonnei with either increased virulence or fluoroquinolone resistance. mSphere 2016; 1. doi:10.1128/mSphere.00344-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Simmons PD, Tabaqchali S. Campylobacter species in male homosexuals. Br J Vener Dis 1979; 55:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Quinn TC, Stamm WE, Goodell SE, et al. The polymicrobial origin of intestinal infections in homosexual men. N Engl J Med 1983; 309:576–82. [DOI] [PubMed] [Google Scholar]
- 9. Mitchell H, Hughes G. Recent epidemiology of sexually transmissible enteric infections in men who have sex with men. Curr Opin Infect Dis 2018; 31:50–6. [DOI] [PubMed] [Google Scholar]
- 10. Geissler AL, Bustos Carrillo F, Swanson K, et al. Increasing Campylobacter infections, outbreaks, and antimicrobial resistance in the United States, 2004–2012. Clin Infect Dis 2017; 65:1624–31. [DOI] [PubMed] [Google Scholar]
- 11. Molina J, Casin I, Hausfater P, et al. Campylobacter infections in HIV-infected patients: clinical and bacteriological features. AIDS 1995; 9:881–5. [DOI] [PubMed] [Google Scholar]
- 12. Gaudreau C, Michaud S. Cluster of erythromycin- and ciprofloxacin-resistant Campylobacter jejuni subsp. jejuni from 1999 to 2001 in men who have sex with men, Québec, Canada. Clin Infect Dis 2003; 37:131–6. [DOI] [PubMed] [Google Scholar]
- 13. Gaudreau C, Rodrigues-Coutlée S, Pilon PA, Coutlée F, Bekal S. Long-lasting outbreak of erythromycin- and ciprofloxacin-resistant Campylobacter jejuni subspecies jejuni from 2003 to 2013 in men who have sex with men, Quebec, Canada. Clin Infect Dis 2015; 61:1549–52. [DOI] [PubMed] [Google Scholar]
- 14. Gaudreau C, Helferty M, Sylvestre J-L, et al. Campylobacter coli outbreak in men who have sex with men, Quebec, Canada, 2010–2011. Emerg Infect Dis 2013; 19:764–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gaudreau C, Pilon PA, Sylvestre J-L, Boucher F, Bekal S. Multidrug-resistant Campylobacter coli in men who have sex with men, Quebec, Canada, 2015. Emerg Infect Dis 2016; 22:1661–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Marchand-Senécal X, Bekal S, Pilon PA, Sylvestre J-L, Gaudreau C. Campylobacter fetus cluster among men who have sex with men, Montreal, Quebec, Canada, 2014–2016. Clin Infect Dis 2017; 65:1751–3. [DOI] [PubMed] [Google Scholar]
- 17. Bankevich A, Nurk S, Antipov D, et al. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol 2012; 19:455–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Seemann T. Prokka: rapid prokaryotic genome annotation. Bioinformatics 2014; 30:2068–9. [DOI] [PubMed] [Google Scholar]
- 19. Feldgarden M, Brover V, Haft DH, et al. Validating the NCBI AMRFinder tool and resistance gene database using antimicrobial resistance genotype-phenotype correlations in a collection of NARMS isolates. Antimicrob Agents Chemother 2019; 63. doi:10.1128/AAC.00483-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu B, Zheng D, Jin Q, Chen L, Yang J. VFDB 2019: a comparative pathogenomic platform with an interactive web interface. Nucleic Acids Res 2019; 47:D687–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jolley KA, Bray JE, Maiden MCJ. Open-access bacterial population genomics: BIGSdb software, the PubMLST.org website and their applications. Wellcome Open Res 2018; 3:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kozyreva VK, Truong CL, Greninger AL, Crandall J, Mukhopadhyay R, Chaturvedi V. Validation and implementation of clinical laboratory improvements act-compliant whole-genome sequencing in the public health microbiology laboratory. J Clin Microbiol 2017; 55:2502–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Parkhill J, Wren BW, Mungall K, et al. The genome sequence of the food-borne pathogen Campylobacter jejuni reveals hypervariable sequences. Nature 2000; 403:665–8. [DOI] [PubMed] [Google Scholar]
- 24. Fouts DE, Mongodin EF, Mandrell RE, et al. Major structural differences and novel potential virulence mechanisms from the genomes of multiple Campylobacter species. PLoS Biol 2005; 3:e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dingle KE, Colles FM, Falush D, Maiden MC. Sequence typing and comparison of population biology of Campylobacter coli and Campylobacter jejuni. J Clin Microbiol 2005; 43:340–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhao S, Tyson GH, Chen Y, et al. Whole-genome sequencing analysis accurately predicts antimicrobial resistance phenotypes in Campylobacter spp. Appl Environ Microbiol 2016; 82:459–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hakanen A, Jalava J, Kotilainen P, Jousimies-Somer H, Siitonen A, Huovinen P. gyrA polymorphism in Campylobacter jejuni: detection of gyrA mutations in 162 C. jejuni isolates by single-strand conformation polymorphism and DNA sequencing. Antimicrob Agents Chemother 2002; 46:2644–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cha W, Mosci R, Wengert SL, et al. Antimicrobial susceptibility profiles of human Campylobacter jejuni isolates and association with phylogenetic lineages. Front Microbiol 2016; 7:589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Avrain L, Vernozy-Rozand C, Kempf I. Evidence for natural horizontal transfer of tetO gene between Campylobacter jejuni strains in chickens. J Appl Microbiol 2004; 97:134–40. [DOI] [PubMed] [Google Scholar]
- 30. Wang Y, Taylor DE. Chloramphenicol resistance in Campylobacter coli: nucleotide sequence, expression, and cloning vector construction. Gene 1990; 94:23–8. [DOI] [PubMed] [Google Scholar]
- 31. Fabre A, Oleastro M, Nunes A, et al. Whole-genome sequence analysis of multidrug-resistant Campylobacter isolates: a focus on aminoglycoside resistance determinants. J Clin Microbiol 2018; 56. doi:10.1128/JCM.00390-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Olkkola S, Juntunen P, Heiska H, Hyytiäinen H, Hänninen ML. Mutations in the rpsL gene are involved in streptomycin resistance in Campylobacter coli. Microb Drug Resist 2010; 16:105–10. [DOI] [PubMed] [Google Scholar]
- 33. Bolinger H, Kathariou S. The current state of macrolide resistance in Campylobacter spp.: trends and impacts of resistance mechanisms. Appl Environ Microbiol 2017; 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. González-Plaza JJ, Šimatović A, Milaković M, Bielen A, Wichmann F, Udiković-Kolić N. Functional repertoire of antibiotic resistance genes in antibiotic manufacturing effluents and receiving freshwater sediments. Front Microbiol 2017; 8:2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bradley P, den Bakker HC, Rocha EPC, McVean G, Iqbal Z. Ultrafast search of all deposited bacterial and viral genomic data. Nat Biotechnol 2019; 37:152–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Couvin D, Bernheim A, Toffano-Nioche C, et al. CRISPRCasFinder, an update of CRISRFinder, includes a portable version, enhanced performance and integrates search for Cas proteins. Nucleic Acids Res 2018; 46:W246–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Varble A, Meaden S, Barrangou R, Westra ER, Marraffini LA. Recombination between phages and CRISPR-cas loci facilitates horizontal gene transfer in staphylococci. Nat Microbiol 2019; 4:956–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Murray K, Reddy V, Kornblum JS, et al. Increasing antibiotic resistance in Shigella spp. from infected New York City residents, New York, USA. Emerg Infect Dis 2017; 23:332–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bowen A, Grass J, Bicknese A, Campbell D, Hurd J, Kirkcaldy RD. Elevated risk for antimicrobial drug-resistant Shigella infection among men who have sex with men, United States, 2011–2015. Emerg Infect Dis 2016; 22:1613–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Barreiro P. Hot news: sexually transmitted infections on the rise in PrEP users. AIDS Rev 2018; 20:71. [PubMed] [Google Scholar]
- 41. Mook P, Gardiner D, Kanagarajah S, et al. Use of gender distribution in routine surveillance data to detect potential transmission of gastrointestinal infections among men who have sex with men in England. Epidemiol Infect 2018; 146:1468–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hughes G, Silalang P, Were J, et al. Prevalence and characteristics of gastrointestinal infections in men who have sex with men diagnosed with rectal chlamydia infection in the UK: an ‘unlinked anonymous’ cross-sectional study. Sex Transm Infect 2018; 94:518–21. [DOI] [PubMed] [Google Scholar]
- 43. Aguilar-Company J, Los-Arcos I, Pigrau C, et al. Potential use of fosfomycin-tromethamine for treatment of recurrent Campylobacter species enteritis. Antimicrob Agents Chemother 2016; 60:4398–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Watson BNJ, Staals RHJ, Fineran PC. CRISPR-Cas-mediated phage resistance enhances horizontal gene transfer by transduction. MBio 2018; 9. doi:10.1128/mBio.02406-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.