Abstract
Background
Isoniazid preventive therapy (IPT) is widely used to protect against tuberculosis (TB) in people living with human immunodeficiency virus (HIV). Data on the safety and efficacy of IPT in pregnant women living with HIV (PWLHIV) are mixed. We used an individual-level, population-wide health database to examine associations between antenatal IPT exposure and adverse pregnancy outcomes, maternal TB, all-cause mortality, and liver injury during pregnancy through 12 months postpartum.
Methods
We used linked routine electronic health data generated in the public sector of the Western Cape, South Africa, to define a cohort of PWLHIV on antiretroviral therapy. Pregnancy outcomes were assessed using logistic regression; for maternal outcomes we applied a proportional hazards model with time-updated IPT exposure.
Results
Of 43 971 PWLHIV, 16.6% received IPT. Women who received IPT were less likely to experience poor pregnancy outcomes (adjusted odds ratio [aOR], 0.83 [95% confidence interval {CI}, .78–.87]); this association strengthened with IPT started after the first trimester compared with none (aOR, 0.71 [95% CI, .65–.79]) or with first-trimester exposure (aOR, 0.64 [95% CI, .55–.75]). IPT reduced the risk of TB by approximately 30% (aHR, 0.71 [95% CI, .63–.81]; absolute risk difference, 1518/100 000 women). The effect was modified by CD4 cell count with protection conferred if CD4 count was ≤350 cells/μL (aHR, 0.51 [95% CI, .41–.63]) vs 0.93 [95% CI, .76–1.13] for CD4 count >350 cells/µL).
Conclusions
This analysis of programmatic data is reassuring regarding the safety of antenatal IPT, with the greatest benefits against TB disease observed in women with CD4 count ≤350 cells/μL.
Keywords: isoniazid preventive therapy, pregnancy, HIV
Analysis of individual-level population-wide data is reassuring regarding the safety and efficacy of isoniazid preventive therapy to prevent tuberculosis disease in a cohort of >43 000 pregnant women living with HIV on antiretroviral therapy in South Africa.
Tuberculosis disease (TB) during pregnancy is a major cause of nonobstetric maternal death in high-burden settings and is associated with maternal complications and poor pregnancy outcomes [1–3]. While TB infection and disease in pregnancy are not routinely reported by national programs, the prevalence of TB in high-burden countries was estimated to be between 0.69% and 11% and 0.07% and 0.53% in pregnant and postpartum women living with and without human immunodeficiency virus (HIV), respectively [3]. The incidence of TB in pregnancy in the African region has been calculated as 360/100 000 pregnant women per annum (95% confidence interval [CI], 300–450) and almost 3-fold higher in South Africa (1030/100 000 [95% CI, 540–1760]) [1]. In a cohort of South African peripartum women living with HIV in 2013–2014, TB incidence was high: 2466/100 000 (95% CI, 1863–3202) in the 18 months preconception, 1127/100 000 (95% CI, 600–1928) during pregnancy, and 1447/100 000 (95% CI, 694–2661) in the first 6 months postpartum [4]. The risk of presenting with clinical TB is greater postpartum than during pregnancy [4–6], probably due to pregnancy-related immunological changes [7].
Isoniazid preventive therapy (IPT) has been shown to provide protection against TB in people living with HIV independent of treatment with antiretroviral therapy (ART) [8, 9]. The World Health Organization (WHO) strongly recommends at least 6 months of isoniazid for people living with HIV regardless of ART status, tuberculin skin test positivity, or prior TB treatment [8, 10, 11]. Since 2011, WHO guidelines have explicitly included pregnant women in this recommendation [11, 12]. Isoniazid exposure during pregnancy has been considered safe [13, 14], and analysis of inadvertent exposures during randomized controlled trials (RCTs) of IPT regimens found no association with adverse pregnancy outcomes [15, 16]. In South Africa, IPT has been routinely offered to pregnant women living with HIV (PWLHIV) since 2010 as the benefits of preventive therapy were felt to outweigh the risks [17]. However more recently, a noninferiority RCT (International Maternal Pediatric Adolescent AIDS Clinical Trials Network P1078 TB Ante vs Postpartum Prevention with INH in HIV Seropositive Mothers and Their Exposed Infants), the only RCT of isoniazid in pregnancy, demonstrated that antenatal vs postnatal IPT was associated with no significant difference in the prevention of TB but an increased risk of combined adverse pregnancy outcomes in women with antenatal exposure [18, 19].
Given this mixed evidence, we assessed the impact and risks of IPT in PWLHIV in a programmatic setting using routinely collected electronic data in the Western Cape province, South Africa. We aimed to determine if IPT during pregnancy was safe and effective in reducing incident TB. Among women receiving IPT, we investigated whether there were any differences if IPT was given during or after the first trimester.
METHODS
We defined a retrospective cohort of PWLHIV, initiating ART either before or during an index pregnancy beginning between 1 January 2015 and 31 December 2017 within the Western Cape Provincial Health Data Centre (PHDC). The PHDC consolidates administrative, laboratory, and pharmacy data from routine electronic clinical information systems used in all public sector health facilities in the province [20, 21]. Moderate-high confidence evidences of pregnancy in the PHDC (see Supplementary Materials) were used to define the cohort. Women were categorized as having (1) no exposure to IPT or (2) exposure to IPT antenatally (ie, IPT initiated between pregnancy start and pregnancy end dates.) Women were excluded if they had evidence of treatment for TB at pregnancy start, or evidence of laboratory investigations for TB within 30 days of first presentation for antenatal care in the absence of IPT (ie, IPT may have been withheld due to suspicion and investigation of active TB; the WHO TB symptom screen is standard of care at the first antenatal visit). The women were followed up until 12 months postpregnancy outcome. We assessed pregnancy outcome, IPT dispensing, laboratory evidence of maternal liver injury, incident TB, and all-cause maternal mortality.
The South African HIV treatment guidelines at the time recommended 6–12 months of isoniazid 5 mg/kg/day (maximum 300 mg/day) for all adults and adolescents living with HIV on ART (including pregnant women) regardless of CD4 cell count [17, 22]. PWLHIV were eligible for lifelong ART (WHO Option B+). The majority of women were receiving fixed-dose combination ART comprising tenofovir, emtricitabine, and efavirenz. South Africa is a country with a high TB burden, and the incidence of TB disease in the Western Cape province was 681/100 000 population in 2015 [23] with IPT coverage 53% in people living with HIV nationally [24].
Definitions
Pregnancy in the PHDC was defined as any conception regardless of outcome and identified by combining electronic evidences of pregnancy from various data sources including laboratory, pharmacy and facility visit data. These multiple sources contributed to the confidence of ascertaining a pregnancy (see Supplementary Materials) [21, 25]. Pregnancy end date was defined as the date of recorded pregnancy outcome. Pregnancy start date was back-calculated from gestational age at outcome where clinical data were available; otherwise, it was calculated using standard algorithms [26, 27]. Pregnancies that ended in elective termination were excluded. If there was insufficient information to determine the start/end date of a pregnancy, it was excluded.
TB was defined by any of the following: (1) laboratory evidence of infection with Mycobacterium tuberculosis from any anatomical site (Xpert RIF/MTB, microscopy, culture); (2) registration on the Electronic TB Register or Electronic Drug Resistant TB Register; (3) pharmacy record of combination treatment for TB; (4) admission to a specialized TB hospital. We did not distinguish between pulmonary and extrapulmonary disease. TB was defined as present if the first electronic evidence date of TB was during pregnancy or within 12 months of the pregnancy outcome date.
IPT was determined from electronic dispensing data and classified as any dispensing of isoniazid after the estimated pregnancy start date and before the pregnancy outcome date. Duration of treatment was based on the quantity dispensed and defined as the period from first IPT dispensing date to last IPT-in-hand date. Liver injury was defined as elevation of alanine aminotransferase ≥5 times the upper limit of normal (Division of AIDS grade 3 or higher) [28]. We were unable to confirm the clinical indication for these investigations. Adverse pregnancy outcome included any of the following: miscarriage (loss of the products of conception before 27 weeks’ gestation); stillbirth (delivery of a fetus with no signs of life after 27 completed weeks’ gestation); neonatal death (death of an infant within 28 days of birth); or low birth weight (<2500 g). Since gestational age was estimated in some cases, we did not include prematurity as an outcome. Women and liveborn or stillborn infants were linked via entry into electronic medical records systems at the time of delivery as per provincial protocols.
We included the CD4 cell count performed during pregnancy or within 2 weeks of pregnancy end date. If no such result was available, the CD4 count closest to and within 12 months of pregnancy start date was used. CD4 was categorized as <200 cells/μL, 200–350 cells/μL, 351–500 cells/μL, >500 cells/μL, or missing. Viral load (VL) results were similarly determined and the variable expressed as virally suppressed (VL<50 copies/mL), not suppressed or missing.
Data Analysis
Data were analyzed using Stata version 15.0 software (StataCorp, College Station, Texas). Continuous variables were summarized using mean and standard deviation (SD) or 95% CIs, or median and interquartile range (IQR) for normally and nonnormally distributed variables, respectively. Categorical variables were described using proportions and compared using frequency tables. Significance was tested using a 2‐sample t test or Wilcoxon rank‐sum test depending on the distribution for numerical data, and the χ 2 test or Fisher exact test for categorical data. Risk differences for TB, hepatotoxicity, maternal mortality, and poor pregnancy outcomes were calculated per 100 000 women.
We fitted a Cox proportional hazards model to determine the association between IPT exposure and risk of TB (and liver injury and death) between pregnancy start and 12 months postpregnancy end date. IPT exposure was included as a time-varying ever-exposed variable. Covariables were determined a priori and the models were adjusted for age, CD4 cell count, HIV viral suppression status, ART status prior to pregnancy, history of TB, site of first antenatal visit and delivery (ie, primary care or hospital), and whether this was a first recorded pregnancy. Associations between poor pregnancy outcomes and covariables were estimated using logistic regression. We performed sensitivity analyses restricting IPT start to between 14 and 34 weeks’ gestation and further categorizing time on IPT (none, 0–5.9 months, 6–12 months, >12 months).
The study was approved by University of Cape Town Human Research Ethics Committee and the Provincial Government of the Western Cape Department of Health Research Committee. A waiver of informed consent was granted as the data were collected as part of routine care and fell within the formal protection policies of the Western Cape Government. All data were anonymized before being accessed by the researchers.
RESULTS
A total of 56 454 PWLHIV and receiving ART antenatally were identified by the PHDC over the study period (Supplementary Figure 1). Of these, 7291 (12.9%) were excluded as we were unable to accurately determine pregnancy start and end dates from the records. A further 2538 pregnancies ended in elective termination and were excluded. Two hundred twenty-one women were being treated for TB at pregnancy start date and 556 had evidence of TB workup within 30 days of presenting for antenatal care. IPT was initiated after pregnancy outcome in 1877 women who were excluded. After these exclusions, the cohort comprised 43 971 PWLHIV who received ART prior to and/or during the index pregnancy.
Mean age was 29.8 years (SD, 5.8 years) and did not differ across IPT category. IPT was dispensed to 16.6% of women at some point after pregnancy start and before pregnancy outcome. Median duration of IPT was 168 days (IQR, 84–285 days); median duration of antenatal exposure was 124 days (IQR, 63–223 days). Median duration of ART at delivery was 17.2 months (IQR, 4.9–48.0 months) with no IPT and 19.0 months (IQR, 5.7–47.1 months) with antenatal IPT (Table 1). There was no evidence of increased liver injury in the IPT group; maternal mortality crudely appeared lower, noting the small number of events. TB to the 12-month postpregnancy outcome was less frequent in women who received antenatal IPT, as were all adverse pregnancy outcomes (Table 2).
Table 1.
Characteristics at Antenatal Presentation by Isoniazid Preventive Therapy Category
| Characteristic | Total (N = 43 971) | No IPT (n = 36 661 [83.4%]) | ANC IPT (n = 7310 [16.6%]) | P Value |
|---|---|---|---|---|
| Age, y, mean (SD) | 29.8 (5.8) | 29.7 (5.8) | 30.1 (5.5) | |
| ART prior to pregnancy | 33 779 (76.8) | 28 547 (71.6) | 5232 (77.9) | < .001 |
| Duration ART prior to delivery, mo, median (IQR) | 17.5 (5.1–47.8) | 17.2 (4.9–48.0) | 19.0 (5.7–47.1) | |
| CD4 cell count | ||||
| No. | 32 454a | 26 877 | 5577 | |
| Median, cells/µL (IQR) | 422 (280–584.6) | 432 (287–589) | 420 (279–583) | |
| CD4 category, cells/μL | < .001 | |||
| < 200 | 4269 (9.7) | 3576 (9.8) | 693 (9.5) | |
| 200–350 | 7798 (17.7) | 6497 (17.7) | 1301 (17.8) | |
| 351–500 | 8511 (19.4) | 7056 (19.3) | 1455 (19.9) | |
| > 500 | 11 876 (27.0) | 9748 (26.6) | 2128 (29.1) | |
| CD4 count missing | 11 517 (26.2) | 9784 (26.7) | 1733 (23.7) | |
| HIV VL | ||||
| No. | 39 280a | 32 272 | 7008 | |
| VL suppressed | 25 224 (57.4) | 20 573 (56.1) | 4671 (63.9) | < .001 |
| VL missing | 4691 (10.7) | 4389 (12.0) | 4691 (4.1) | |
| First ANC visit in primary carea (n = 43 594b) | 26 510 (60.8) | 21 639 (59.5) | 4871 (67.3) | < .001 |
| Delivered primary carec (n = 43 798)b | 10 527 (24.0) | 8720 (23.9) | 1807 (24.8) | .08 |
| First recorded pregnancy | 20 205 (54.6) | 20 260 (55.3) | 3765 (51.5) | < .001 |
| History of TB disease | 5535 (12.6) | 4764 (13.0) | 771 (10.6) | < .001 |
Data are presented as no. (%) unless otherwise indicated.
Abbreviations: ANC, antenatal care; ART, antiretroviral therapy; HIV, human immunodeficiency virus; IPT, isoniazid preventive therapy; IQR, interquartile range; SD, standard deviation; TB, tuberculosis disease; VL, viral load.
aFirst antenatal visit at a primary healthcare facility (midwife obstetric unit as opposed to hospital).
bNumber of cases for which data were available.
cDelivery in a primary healthcare facility (midwife obstetric unit as opposed to hospital).
Table 2.
Maternal and Infant Outcomes by Isoniazid Preventive Therapy Category
| Characteristic | Total (N = 43 971) | No IPT (n = 36 661) | ANC IPT (n = 7310) | Risk Difference (95% CI) per 100 000 Women | P Value |
|---|---|---|---|---|---|
| Maternal TB | 1002 (2.3) | 928 (2.5) | 74 (1.0) | −1518 (−1799 to −1238) | < .001 |
| Maternal death | 64 (0.15) | 59 (0.16) | 5 (0.07) | −92 (−165 to −19) | .06a |
| ALT high (n = 6564b) | 127 (2.1) | 104 (2.0) | 23 (2.4) | 422 (−624 to 1468) | .4a |
| Pregnancy outcome | |||||
| Miscarriage | 1416 (3.22) | 1272 (3.47) | 144 (1.97) | 1497 (−1869 to −1130) | < .001 |
| Stillbirth | 720 (1.64) | 630 (1.72) | 90 (1.23) | −487 (−773 to −201) | .003 |
| Neonatal death | 356 (0.81) | 302 (0.82) | 54 (0.74) | −85 (−302 to 131) | .459 |
| Birth weight, No. (live births only) | 36 994b | 30 758 | 6236 | ||
| Median, g (IQR) | 3080 (2760–3400) | 3070 (2700–3400) | 3106 (2760–3440) | −2916 (−3853 to −1979) | |
| LBW | 5806 (13.9) | 4976 (14.3) | 827 (11.7) | … | < .001 |
| LBW missing | 4841 (11.6) | 4001 (11.5) | 840 (11.9) | … | |
| Poor outcome compositec | 8046 (18.3) | 6966 (19.0) | 1080 (14.8) | −4226 (−5134 to −3320) | < .001 |
| Delivered primary care (n = 43 798b) | 10 527 (24.0) | 8720 (23.9) | 1807 (24.8) | … | .08 |
Data are presented as no. (%) unless otherwise indicated.
Abbreviations: ALT, alanine aminotransferase; ANC, antenatal; CI, confidence interval; IPT, isoniazid preventive therapy; IQR, interquartile range; LBW, low birth weight; TB, tuberculosis disease.
aStatistical test: Fisher exact test; otherwise χ 2 test used to assess the difference between the proportions.
bNo. of cases for which data were available.
cCombination of miscarriage, stillbirth, LBW, and neonatal death.
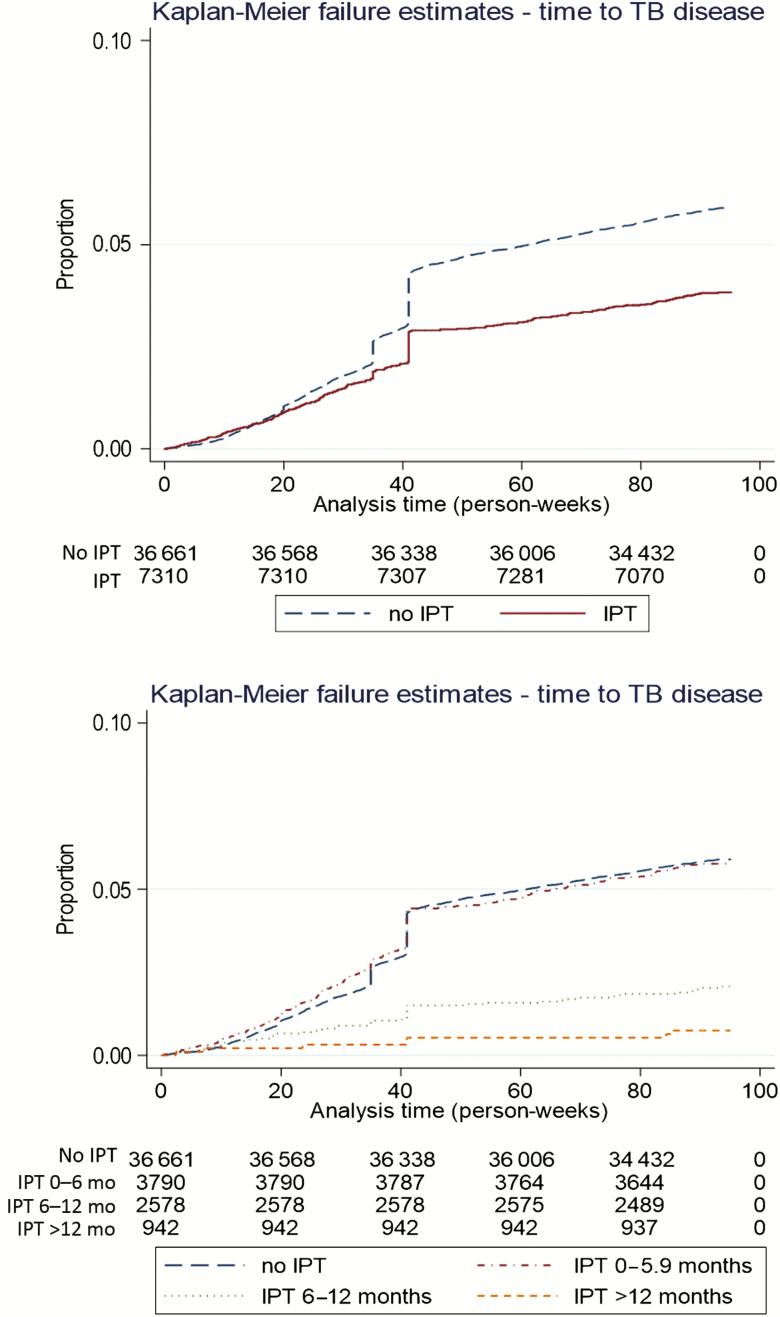
IPT was protective against incident TB to the 12-month postpregnancy outcome, with an absolute risk difference of 1518 TB cases per 100 000 women (95% CI, 1238–1799/100 000). The effect increased with increasing duration of IPT (Table 3; Figure 1). The adjusted hazard ratio (aHR) for TB in the presence of IPT was 0.71 (95% CI, .63–.81). A history of TB increased risk, as did low CD4 count relative to >500 cells/μL (Table 3). When stratified by CD4 count, antenatal IPT conferred protection with CD4 count ≤350 cells/μL but not at higher CD4 counts (Table 4).
Table 3.
Cox Proportional Hazard Model for Risk of Tuberculosis Disease
| Variable | Unadjusted | Receipt of Any IPT | IPT Categorized by Duration |
|---|---|---|---|
| HR (95% CI) | aHR (95% CI) | aHR (95% CI) | |
| Age (per 10-y increase) | 0.92 (.86–.98) | 0.87 (.80–.93) | 0.87 (.81–.93) |
| First recorded pregnancy | 0.92 (.85–1.0) | 0.91 (.84–1.00) | 0.92 (.85–1.00) |
| ART prior to pregnancy | 1.07 (.98–1.18) | 1.00 (.90–1.25) | 1.02 (.92–1.14) |
| Duration ART prior to delivery, mo | 1.00 (.99–1.00) | … | … |
| History of TB disease | 2.68 (2.45–2.93) | 2.49 (2.27–2.73) | 2.49 (2.27–2.73) |
| CD4 count, cells/μL | |||
| < 200 | 5.68 (5.0–6.46) | 4.51 (3.94–5.15) | 4.44 (3.89–5.08) |
| 200–350 | 2.07 (1.81–2.38) | 1.88 (1.63–2.16) | 1.87 (1.63–2.15) |
| 351–500 | 1.54 (1.34–1.78) | 1.47 (1.28–1.71) | 1.47 (1.27–1.70) |
| > 500 | Ref | Ref | Ref |
| Missing | 1.62 (1.41–1.85) | 1.47 (1.28–1.68) | 1.47 (1.28–1.69) |
| HIV VL | |||
| ≥ 50 copies/mL | Ref | Ref | Ref |
| < 50 copies/mL | 0.49 (.45–.53) | 0.68 (.62–1.75) | 0.69 (.63–.76) |
| Missing | 0.92 (.82–1.04) | 1.07 (.95–1.22) | 1.07 (.94–1.21) |
| First ANC visit in primary carea | 0.90 (.83–.98) | 0.98 (.89–1.07) | 0.97 (.89–1.07) |
| Delivered in primary careb | 0.82 (.74–.90) | 0.81(.73–.90) | 0.82 (.74–.91) |
| IPT | |||
| Any IPT | 0.64 (.57–.73) | 0.71 (.63–.81) | … |
| Duration of IPT | |||
| No IPT | Ref | … | Ref |
| 0–5.9 mo (8.6%) | 1.0 (.85–1.12) | … | 1.02 (.89–1.17) |
| 6–12 mo (5.9%) | 0.34 (.30–.45) | … | 0.40 (.30–.52) |
| > 12 mo (2.1%) | 0.12 (.06–.23) | … | 0.16 (.08–.34) |
Abbreviations: aHR, adjusted hazard ratio; ANC, antenatal care; ART, antiretroviral therapy; CI, confidence interval; HIV, human immunodeficiency virus; HR, hazard ratio; IPT, isoniazid preventive therapy; TB, tuberculosis; VL, viral load.
aFirst antenatal visit at a primary healthcare facility (midwife obstetric unit as opposed to hospital).
bDelivery in a primary healthcare facility (midwife obstetric unit as opposed to hospital).
Figure 1.
Kaplan-Meier estimates for risk of tuberculosis disease by isoniazid preventive therapy exposure group. Abbreviations: IPT, isoniazid preventive therapy; TB, tuberculosis.
Table 4.
Cox Proportional Hazard Model for Risk of Tuberculosis Disease, Stratified by CD4 Cell Count
| Variable | CD4 ≤350 cells/µL (27.6%) | CD4 >350 cells/µL (46.9%) | CD4 Missing (25.6%) |
|---|---|---|---|
| Age (per 10-y increase) | 0.97 (.87–1.08) | 0.79 (.69–.90) | 0.85 (.73–.98) |
| First recorded pregnancy | 0.81 (.72–.91) | 1.00 (.86–1.16) | 1.09 (.92–1.30) |
| ART in prior pregnancy | 0.98 (.85–1.13) | 0.93 (.77–1.11) | 1.45 (1.01–2.10) |
| History of TB disease | 2.56 (2.23–2.93) | 3.10 (2.61–3.69) | 2.16 (1.78–2.61) |
| HIV VL | |||
| ≥ 50 copies/mL | Ref | Ref | Ref |
| < 50 copies/mL | 0.59 (.52–.68) | 0.76 (.65–.87) | 0.50 (.41–.91) |
| Missing | 1.15 (.97–1.37) | 0.81 (.61–1.09) | 0.91 (.71–1.15) |
| First ANC visit in primary carea | 1.07 (.93–1.23) | 0.93 (.79–1.10) | 0.92 (.75–1.11) |
| Delivered in primary careb | 0.83 (.72–.97) | 0.80 (.67–.98) | 0.69 (.53–.89) |
| Any IPT | 0.51 (.41–.63) | 0.93 (.76–1.13) | 0.83 (.63–1.08) |
Data are presented as adjusted hazard ratio (95% confidence interval).
Abbreviations: ANC, antenatal care; ART, antiretroviral therapy; HIV, human immunodeficiency virus; IPT, isoniazid preventive therapy; TB, tuberculosis; VL, viral load.
aFirst antenatal visit at a primary healthcare facility (midwife obstetric unit as opposed to hospital).
bDelivery in a primary healthcare facility (midwife obstetric unit as opposed to hospital).
Most TB (75.7% [759/1002]) presented around or after the pregnancy outcome; within this group of women, 35.6% (270/759) occurred within the first 3 months of pregnancy outcome.
IPT was not associated with maternal mortality in adjusted survival analyses (aHR, 0.75 [95% CI, .46–1.22]. There was an increased association with severe liver injury (aHR, 1.51 [95% CI, 1.18–1.93]), although the overall number of events was low (n = 127) (Supplementary Tables 1 and 2).
IPT was associated with a reduced risk of adverse pregnancy outcomes combined (adjusted odds ratio [aOR], 0.83 [95% CI, .78–.87]) compared with no IPT, and the effect increased with increasing duration (Table 5). When disaggregated by individual outcome, reductions in miscarriage and stillbirth seemed to be driving the effect (Supplementary Table 3). Within the group of women who received IPT, we compared outcomes in those with first-trimester exposures (ie, IPT started before 14 weeks’ gestation (n = 2647 [36.2%]) with those who received IPT after 14 weeks (n = 4663 [63.8%]). The risk of poor pregnancy outcome was reduced in women who received IPT after 14 weeks compared with no IPT (aOR, 0.71 [95% CI, .65–.79]) and with those with first-trimester exposure (aOR, 0.64 [95% CI, .55–.75]). In this case, miscarriage had the largest impact (Supplementary Tables 4 and 5).
Table 5.
Univariable and Multivariable Analyses for Adverse Pregnancy Outcomes
| Poor Outcome Compositea | Unadjusted | Receipt of Any IPT | IPT Categorized by Duration |
|---|---|---|---|
| OR (95% CI) | aOR (95% CI) | aOR (95% CI) | |
| Age (per 10-y increase) | 1.17 (1.12–1.22) | 1.17 (1.12–1.22) | 0.17 (1.12–1.22) |
| First recorded pregnancy | 1.18 (1.13–1.24) | 1.16 (1.10–1.21) | 1.16 (1.10–1.22) |
| ART prior pregnancy | 1.20 (1.13–1.28) | 1.02 (.96–1.09) | 1.03 (.96–1.10) |
| History of TB disease | 1.13 (1.29–1.48) | 1.34 (1.25–1.44) | 1.34 (1.25–1.44) |
| CD4 count, cells/μL | |||
| < 200 | 1.40 (1.28–1.53) | 1.22 (1.11–1.33) | 1.21 (1.10–1.33) |
| 200–350 | 1.09 (1.01–1.18) | 1.03 (.95–1.12) | 1.03 (.95–1.12) |
| 351–500 | 1.03 (.95–11) | 1.01 (.93–1.09) | 1.01 (.93–1.09) |
| > 500 (normal) | Ref | Ref | Ref |
| Missing | 1.42 (1.33–1.52) | 1.12 (1.13–1.30) | 1.21 (.13–1.30) |
| HIV VL | |||
| ≥ 50 copies/mL | Ref | Ref | Ref |
| < 50 copies/mL | 0.82 (.78–.87) | 0.82 (.78–.87) | 0.82 (.78–.87) |
| Missing | 2.18 (2.02–2.35) | 2.17 (2.01–2.35) | 2.17 (2.01–2.35) |
| First ANC visit in primary careb | 0.60 (.57–.63) | 0.84 (.79–.89) | 0.84 (.78–.87) |
| Delivered in primary carec | 0.42 (.39–.45) | 0.46 (.42–.49) | 0.46 (.42–.49) |
| Any IPT | 0.74 (.69–.79) | 0.83 (.78–.87) | … |
| Duration of IPT | |||
| No IPT | Ref | … | Ref |
| 0–5.9 mo (8.6%) | 0.78 (.72–.86) | … | 0.88 (.80–.97) |
| 6–12 mo (5.9%) | 0.72 (.65–.81) | … | 0.83 (.74–.93) |
| > 12 mo (2.1%) | 0.60 (.50–.73) | … | 0.65 (.53–.79) |
Abbreviations: ANC, antenatal care; aOR, adjusted odds ratio; ART, antiretroviral therapy; CI, confidence interval; HIV, human immunodeficiency virus; IPT, isoniazid preventive therapy; OR, odds ratio; TB, tuberculosis; VL, viral load.
aCombination of miscarriage, stillbirth, low birth weight, and neonatal death.
bFirst antenatal visit at a primary healthcare facility (midwife obstetric unit as opposed to hospital).
cDelivery in a primary healthcare facility (midwife obstetric unit as opposed to hospital).
There were no differences in the risk of maternal mortality and severe liver injury by timing of IPT, and the reduced risk of TB persisted when IPT was started after 14 weeks’ gestation (aHR, 0.63 [95% CI, .54–.74] vs no IPT).
DISCUSSION
This analysis of population-wide data suggests that antenatal IPT was protective against TB to the 12-month postpregnancy outcome, reducing the incidence by 30% in comparison with no IPT. This is comparable to the 33%–44% reduction in incident TB in nonpregnant HIV-infected populations receiving IPT reported in meta-analyses [8]. The effect was strongest with longer duration of IPT. The differences between these results and those observed in the antenatal arm of the TB APPRISE RCT may be due to the higher median CD4 count in the trial compared with our cohort (493 vs 422 cells/μL) [18]. Although the CD4 count was ≥350 cells/μL in the majority of our cohort the protective effect was greatest in the presence of immunocompromise (ie, CD4 <350 cells/μL). In addition, the number of TB events in the APPRISE study was low (n = 6).
ART alone confers protection against TB in people living with HIV, and further benefit is gained from the addition of IPT [29]. In this analysis, the group receiving IPT had been on ART for longer and protection against incident TB may reflect the cumulative value of ART and IPT. This group of women may have been more consistently engaged in care. However, adjusting for ART exposure variables did not impact the size of the effect of IPT.
In addition, our analysis suggests that antenatal IPT was protective against poor pregnancy outcomes with lower proportions of miscarriage, stillbirth, low birth weight, and neonatal death. A recent subanalysis of PWLHIV in the Tshepiso cohort (a prospective study of pregnant women with and without TB in South Africa) similarly found IPT to be safe and protective against adverse pregnancy outcomes, although numbers were small [30]. An analysis of women who conceived on IPT during the Isoniazid Preventive Therapy Trial in Botswana (median IPT, 341 days before pregnancy) also found IPT to be protective against adverse outcomes (aOR, 0.6 [95% CI, .3–1.1]) [15]. Better health and health-seeking behavior in women who received antenatal IPT may account for some of this difference, especially in those who accessed IPT in the first trimester of pregnancy.
There were several limitations to this study. The use of a health information exchange infrastructure (PHDC) enabled us to assemble a large cohort of PWLHIV to address clinically relevant questions regarding the safety of IPT in pregnancy. Although the definitions used for the cohort, exposure, and outcomes were comprehensive, it is possible that some participants have been misclassified. Data were limited to information that was entered into any of the electronic medical records systems used in the government sector in the Western Cape. We had no control over the quality of data entered and cannot account for missing data. However, 96% of women deliver at healthcare facilities in South Africa [23], so the majority of pregnancy outcomes within the state-funded health systems were included. Similarly, all laboratory tests are conducted by a single provider and logged electronically, as are all ART and TB prescriptions (the majority of HIV and TB treatment occurs via state-funded mechanisms). TB disease as an outcome was ascertained using several sources; however, it is possible that some cases were not included. Of all PWLHIV and receiving ART antenatally identified over the study period, 12.9% were excluded as we were unable to determine pregnancy start and end dates, which may have resulted in bias. All the women included in the analysis had pregnancy start and end dates and thus ascertainment of pregnancy outcomes within the cohort was complete. The data on IPT reflected dispensing of isoniazid as captured electronically, and missing evidence of IPT prescribing could have diluted the effects described in this study. While women received the medication, there was further no guarantee that it was taken as prescribed or of adherence. Median duration of IPT was 168 days (6 months), suggesting that many women collected their prescriptions for the recommended period. We did not include data on individual ART regimens; specific risk factors and/or drug combinations may have influenced the decision to prescribe IPT. Most women were receiving fixed-dose combination ART.
This was an observational cohort using data that were not collected for these specific analyses and we were unable to account for unmeasured confounders, including history of adverse pregnancy outcomes and exposures to tobacco, alcohol and illicit drugs, and over-the-counter and traditional medicines, which are not captured on provincial electronic systems. CD4 and VL results were not available for all women and the indications for the liver function assays were unknown. Assessment of liver function during pregnancy is not routine in South Africa and tests are ordered only when clinically indicated.
Accurate gestational age was not available for all participants and, where necessary, was calculated using standard algorithms [26, 27]. We felt these systems were insufficiently robust to include premature birth as a variable in the pregnancy outcome analyses, although this outcome was colinear with low birth weight which we did include. Similarly, data on congenital anomalies were not included as an outcome. We performed numerous sensitivity analyses varying the timing of IPT, which did not change the reported associations.
CONCLUSIONS
This analysis of operational population-wide, patient-level data has been reassuring in terms of maternal and fetal adverse outcomes in pregnancies exposed to IPT. Antenatal IPT protected against incident TB during pregnancy and in the 12-month postpregnancy outcome in this cohort, especially in women with a CD4 count <350 cells/μL, and the size of the effect increased with increasing duration of IPT. Adverse pregnancy outcomes were less likely in women receiving antenatal IPT, and the risk was further reduced if IPT was deferred to after 14 weeks’ gestation.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors acknowledge Florence Phelanyane at the Western Cape Provincial Health Data Centre and Polite Nduru for analytic advice.
Financial support. This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (grant number R01 HD080465 to A. B.) and by the University of Cape Town’s Research Committee (E. K.).
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Sugarman J, Colvin C, Moran AC, Oxlade O. Tuberculosis in pregnancy: an estimate of the global burden of disease. Lancet Glob Health 2014; 2:e710–6. [DOI] [PubMed] [Google Scholar]
- 2. Hoffmann CJ, Variava E, Rakgokong M, et al. High prevalence of pulmonary tuberculosis but low sensitivity of symptom screening among HIV-infected pregnant women in South Africa. PLoS One 2013; 8:e62211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mathad JS, Gupta A. Tuberculosis in pregnant and postpartum women: epidemiology, management, and research gaps. Clin Infect Dis 2012; 55:1532–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Odayar J, Rangaka MX, Zerbe A, et al. Burden of tuberculosis in HIV-positive pregnant women in Cape Town, South Africa. Int J Tuberc Lung Dis 2018; 22:760–5. [DOI] [PubMed] [Google Scholar]
- 5. Zenner D, Beer N, Harris RJ, Lipman MC, Stagg HR, van der Werf MJ. Treatment of latent tuberculosis infection: an updated network meta-analysis. Ann Intern Med 2017; 167:248–55. [DOI] [PubMed] [Google Scholar]
- 6. Malhamé I, Cormier M, Sugarman J, Schwartzman K. Latent tuberculosis in pregnancy: a systematic review. PLoS One 2016; 11:e0154825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bates M, Ahmed Y, Kapata N, Maeurer M, Mwaba P, Zumla A. Perspectives on tuberculosis in pregnancy. Int J Infect Dis 2015; 32:124–7. [DOI] [PubMed] [Google Scholar]
- 8. Briggs MA, Emerson C, Modi S, Taylor NK, Date A. Use of isoniazid preventive therapy for tuberculosis prophylaxis among people living with HIV/AIDS: a review of the literature. J Acquir Immune Defic Syndr 2015; 68(Suppl 3):S297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rangaka MX, Wilkinson RJ, Boulle A, et al. Isoniazid plus antiretroviral therapy to prevent tuberculosis: a randomised double-blind, placebo-controlled trial. Lancet 2014; 384:682–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. World Health Organization. Preventive therapy against tuberculosis in people living with HIV. Wkly Epidemiol Rec 1999; 74:385–98. [PubMed] [Google Scholar]
- 11. World Health Organization. Guidelines for intensified tuberculosis case-finding and isoniazid preventive therapy for people living with HIV in resource-constrained settings. 2011. Available at: https://www.who.int/hiv/pub/tb/9789241500708/en/. Accessed 10 October 2019.
- 12. World Health Organization. Latent tuberculosis infection: updated and consolidated guidelines for programmatic management. 2018. Available at: https://www.who.int/tb/publications/2018/latent-tuberculosis-infection/en/. Accessed 10 October 2019.
- 13. Czeizel AE, Rockenbauer M, Olsen J, Sørensen HT. A population-based case-control study of the safety of oral anti-tuberculosis drug treatment during pregnancy. Int J Tuberc Lung Dis 2001; 5:564–8. [PubMed] [Google Scholar]
- 14. Scheinhorn DJ, Angelillo VA. Antituberculous therapy in pregnancy. Risks to the fetus. West J Med 1977; 127:195–8. [PMC free article] [PubMed] [Google Scholar]
- 15. Taylor AW, Mosimaneotsile B, Mathebula U, et al. Pregnancy outcomes in HIV-infected women receiving long-term isoniazid prophylaxis for tuberculosis and antiretroviral therapy. Infect Dis Obstet Gynecol 2013; 2013:195637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Moro RN, Scott NA, Vernon A, et al. Exposure to latent tuberculosis treatment during pregnancy. The PREVENT TB and the iAdhere trials. Ann Am Thorac Soc 2018; 15:570–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Republic of South Africa Department of Health. Guidelines for tuberculosis preventive therapy among HIV infected individuals in South Africa. Pretoria, South Africa: Department of Health, 2010. [Google Scholar]
- 18. Gupta A, Montepiedra G, Aaron L, et al. IMPAACT P1078 TB APPRISE Study Team Isoniazid preventive therapy in HIV-infected pregnant and postpartum women. N Engl J Med 2019; 381:1333–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gupta A, Montepiedra G, Aaron L, et al. Randomized trial of safety of isoniazid preventive therapy during or after pregnancy. In: Conference on Retroviruses and Opportunistic Infections, Boston, MA, 4–7 March 2018. [Google Scholar]
- 20. Boulle A, Heekes A, Tiffin N, et al. Data centre profile: the Provincial Health Data Centre of the Western Cape Province, South Africa. Int J Pop Data Sci 2019; 4:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Heekes A, Tiffin N, Dane P, et al. Self-enrolment antenatal health promotion data as an adjunct to maternal clinical information systems in the Western Cape Province of South Africa. BMJ Glob Health 2018; 3:e000565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Provincial Government of the Western Cape. The Western Cape consolidated guidelines for HIV treatment: prevention of mother-to- child transmission of HIV (PMTCT), children, adolescents and adults (amended version 2018). Cape Town, South Africa: Department of Health,2018. [Google Scholar]
- 23. Day C, Gray A. Health and related indicators. South African Health Review 2017; 2017:248. [Google Scholar]
- 24. World Health Organization. Global tuberculosis report 2018. Available at: https://www.who.int/tb/publications/global_report/en/. Accessed 10 October 2019.
- 25. Johnson LF, Mutemaringa T, Heekes A, Boulle A. The effect of HIV and antiretroviral treatment on pregnancy rates in the Western Cape province of South Africa [manuscript published online ahead of print 23 July 2019]. J Infect Dis 2019. doi:10.1093/infdis/jiz362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Eberg M, Platt RW, Filion KB. The estimation of gestational age at birth in database studies. Epidemiology 2017; 28:854–62. [DOI] [PubMed] [Google Scholar]
- 27. Matcho A, Ryan P, Fife D, Gifkins D, Knoll C, Friedman A. Inferring pregnancy episodes and outcomes within a network of observational databases. PLoS One 2018; 13:e0192033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. National Institute of Allergy and Infectious Diseases, Division of AIDS. Table for grading the severity of adult and pediatric adverse events, corrected version 2.1.2017. Available at: https://rsc.niaid.nih.gov/sites/default/files/daidsgradingcorrectedv21.pdf. Accessed 10 October 2019.
- 29. Samandari T, Agizew TB, Nyirenda S, et al. 6-month versus 36-month isoniazid preventive treatment for tuberculosis in adults with HIV infection in Botswana: a randomised, double-blind, placebo-controlled trial. Lancet 2011; 377:1588–98. [DOI] [PubMed] [Google Scholar]
- 30. Salazr-Austin N, Lala S, Waja Z, et al. IPT and pregnancy outcomes in HIV-positive women: the Tshepiso Cohort. In: Conference on Retroviruses and Opportunistic Infections, Seattle, WA, 4–7 March 2019. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.