Abstract
The transmission of direct-acting antiviral resistance-associated substitutions (RAS) could hamper hepatitis C virus (HCV) cure rates and elimination efforts. A phylogenetic analysis of 87 men who have sex with men recently infected with HCV genotype 1a placed one-third (28/87) in a large cluster, in which 96% harbored NS5A M28V RAS.
Keywords: hepatitis C virus, resistant associated substitutions, phylogenetic analysis, the WHO 2030 elimination goals, HIV co-infected MSM
In this study we analyzed a population of men who have sex with men (MSM) who were mostly also living with human immunodeficiency virus (HIV), were diagnosed with a recently acquired hepatitis C virus (HCV) genotype 1a infection, and participated in 2 trials studying the effectiveness of direct-acting antiviral (DAA) therapy. A recent HCV infection is considered to be an infection less than 6 months old using criteria that have been described elsewhere [1]. In both trials, patients with a recently acquired HCV infection were enrolled at 11 HIV treatment sites in the Netherlands and Belgium [1, 2]. A total of 44 individuals received boceprevir, pegylated interferon, and ribavirin (2013–2014, Dutch Acute HCV in HIV Study [DAHHS] 1 study), while 43 were treated with grazoprevir and elbasvir (2016–2018, DAHHS 2 study) [1, 2]. We generated whole-genome sequences based on the Illumina sequencing platform from pre-therapy samples in the DAHHS 1 study and NS5A and NS5B partial gene sequences using Sanger sequencing in DAHHS 2 (for methods, see the Supplementary Data). The analysis for resistance-associated substitutions (RAS) in the baseline samples showed a very high prevalence of the NS5A M28V RAS, which was present in 31 patients (35.6%; 95% confidence interval, 26.4–46.1). This prevalence is substantially higher than the 0–6% prevalence described previously in other studies, including a study among MSM from Poland living with both HCV and HIV [3–5].
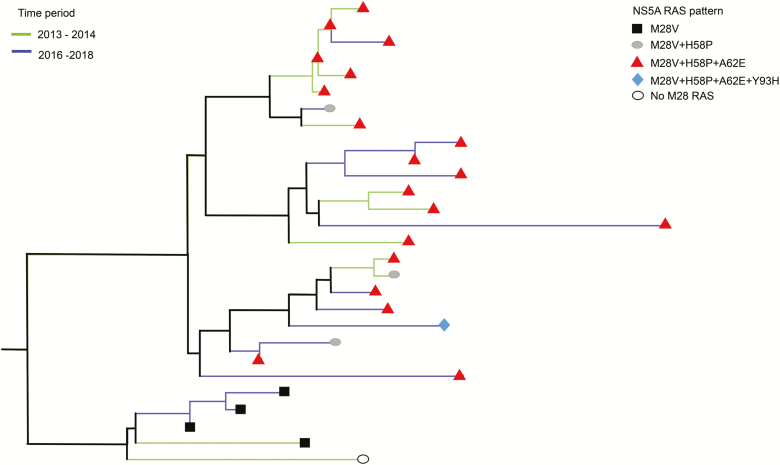
A phylogenetic analysis showed that 28 of the 31 sequences containing a M28V substitution belonged to a single, large cluster with a small genetic distance threshold (<3%), representing approximately one-third of all newly diagnosed infections (for methods, see Supplementary Data; Figure 1). This high M28V prevalence can be explained by a founder effect (ie, the occurrence of an amino acid substitution in high frequency in a particular population). Our M28V cluster could be subdivided into 2 sub-clusters, 1 of which contained just the M28V variant. In the other sub-cluster, in addition to the M28V substitution, several other NS5A RAS were observed: for example, NS5A H58P, A62E, and even Y93H (Figure 1). In 16 of the 28 M28V-containing sequences, the M28V RAS occurred as a pattern with variants H58P and A62E. Although these RAS are not considered to be clinically relevant in HCV genotype 1a, the high prevalence of the combination supports a founder effect. The M28V RAS was already present in samples obtained in 2013–2014, which was before the widespread use of DAA therapy in the Netherlands, strongly suggesting that this RAS occurred and spread naturally in HCV 1a viruses rather than as the result of selective pressure of DAA therapy. We could not identify any association towards a RAS pattern and geographic location, or with patient demographics, such as HIV status.
Figure 1.
Phylogenetic analysis of a concatenated NS5A and NS5B alignment showed clusters including 28 MSM (n = 27 living with HIV; n = 1 living without HIV). Of the individuals in this cluster, 96% harbored an NS5A M28V–containing variant. All patients in this cluster treated with an NS5A-inhibitor (grazoprevir and elbasvir for 8 weeks) obtained a sustained virologic response. The sequences depicted with green were collected prior to the massive direct-acting antiviral therapy uptake in 2015, and the sequences depicted with blue were collected in the years thereafter. The cluster had a bootstrap value and Shimodaira Hasegawaa–like approximate likelihood ratio of >90 and a genetic distance threshold of <3%. For methods, see Supplementary Materials. Abbreviations: HIV, human immunodeficiency virus; MSM, men who have sex with men; RAS, resistance-associated substitutions.
In vitro data previously showed that the M28V substitution lowers the susceptibility of HCV genotype 1a to the NS5A inhibitors ombitasvir (58-fold change), daclatasvir, and pibrentasvir (<2.5-fold change) [6]. In addition, there is no indication that the M28V variant decreases the susceptibility of velpatasvir. Furthermore, the M28V substitution proved to decrease the cure rates of chronic HCV genotype 1a when treated with grazoprevir and elbasvir [1, 6–8]. Luckily, the M28V substitution or a combination of RAS including the M28V substitution did not affect the treatment outcome among patients with a recently acquired HCV infection who were treated with a shortened 8-week regimen of grazoprevir and elbasvir [2]. Currently, there are no other reports that discuss the clinical implication of the RAS pattern M28V+H58P+A62E (+Y93H). Nevertheless, the Y93H substitution as a singleton confers high-level (>100 fold-change) resistance to NS5A inhibitors, such as daclatasvir, elbasvir, ledipasvir, and velpatasvir, and low (<10 fold-change) resistance to pibrentasvir in vitro [9].
The transmission of RAS-carrying viruses has been described previously, although the transmission of an NS5A RAS in such a large cluster has not been reported before. Previous transmission of the M28V RAS was described among 5 MSM in France [10]. Additionally, the NS3 RAS V36M and, in particular, the Q80K were reported to be more prevalent among MSM living with both HCV and HIV, in whom the Q80K occurred in several clusters [5, 11, 12]. The presence of the Q80K had large clinical implications, since the susceptibility to the NS3 protease inhibitor simeprevir was lowered in genotype 1a patients. Therefore, clinicians were advised to analyze the presence of Q80K prior to simeprevir treatment among genotype 1a–infected patients. Although pibrentasvir- and velpatasvir-containing DAA regimens are also highly effective against viruses with RAS, caution remains required. Firstly, the availability of these DAA regimens may differ geographically. Secondly, outbreaks of HCV infections and transmission to sex or needle-sharing partners continue to occur. Our observation illustrates that the transmission of DAA-resistant variants can continue to occur over a prolonged period, irrespective of the broad rollout of DAA therapy. This may be partly explained by the persistence of highly fit, NS5A-resistant viruses in patients.
Currently, only 5 million of the total 71 million HCV-infected individuals have been treated [13]. The spread of RAS-containing viruses may, in case they affect local cure rates, jeopardize global HCV elimination efforts. With a further rollout of DAA therapy on a global scale, surveillance for the spread of RAS is highly recommended [14]. In cases of significant transmission rates of clinically relevant RAS-containing viruses, targeted precautions can then be taken.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. S. P., B. R., and D. vdV. designed the study. S. P., R. V., L. C., B. R., and D. vdV. analyzed the data. S. P., L. C., C. B., A. V., B. R., and D. vdV. interpreted the results. S. P. wrote the first draft of the paper. All authors critically revised and approved the final version of the manuscript. The Dutch Acute HCV in HIV Study investigators are Fanny Lauw, Dirk Posthouwer, Sebastiaan Hullegie, Wouter Bierman, Anthonius Dofferhof, Gert Jan Kootstra, Eliane Leyten, Jan den Hollander, Marjo van Kasteren, Robin Soutekouw, Heidi Ammerlaan, and Eric Florence.
Financial support. This work was supported by Health Holland (grant number LSHM15024), Gilead Sciences (grant number NL-2018-000171), the no co-infection grant provided by Gilead Sciences (grant number IN-NL-987–4558), the Fonds voor Wetenschappelijk Onderzoek Vlaanderen (project G0B2317N), and Merck Sharp & Dohme (MSD) via support for the DAHHS1 and 2 studies.
Potential conflicts of interest. S. P. has received funding outside the submitted work from Merck Sharp and Dohme (MSD), ViiV, and Janssen Pharmaceutica. J. E. A. has received advisory board fees paid to his institution from Gilead, Janssen, ViiV, and MSD, outside the submitted work. C. B. received honoraria from ViiV, outside of the submitted work. B. R. has received research grants from Gilead and MSD and honoraria from Janssen-Cilag, Bristol-Myers Squibb, Pfizer, and ViiV. D. vdV. has received grants from Gilead Sciences, MSD, ViiV, and Janssen Pharmaceutica. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
for the Dutch Acute HCV in HIV Study Investigators:
Fanny Lauw, Dirk Posthouwer, Sebastiaan Hullegie, Wouter Bierman, Anthonius Dofferhof, Gert Jan Kootstra, Eliane Leyten, Jan den Hollander, Marjo van Kasteren, Robin Soutekouw, Heidi Ammerlaan, and Eric Florence
References
- 1. Boerekamps A, De Weggheleire A, van den Berk GE, et al. Treatment of acute hepatitis C genotypes 1 and 4 with 8 weeks of grazoprevir plus elbasvir (DAHHS2): an open-label, multicentre, single-arm, Phase 3b trial. Lancet Gastroenterol Hepatol 2019; 4:269–77. [DOI] [PubMed] [Google Scholar]
- 2. Hullegie SJ, Claassen MA, van den Berk GE, et al. Boceprevir, peginterferon and ribavirin for acute hepatitis C in HIV infected patients. J Hepatol 2016; 64:807–12. [DOI] [PubMed] [Google Scholar]
- 3. Liu Z, Mao X, Wu J, et al. World-wide prevalence of substitutions in HCV genome associated with resistance to direct-acting antiviral agents. Clin Gastroenterol Hepatol 2019. pii: S1542-3565(19)31248-0. Epub ahead of print. doi: 10.1016/j.cgh.2019.10.046 [DOI] [PubMed] [Google Scholar]
- 4. Zeuzem S, Mizokami M, Pianko S, et al. NS5A resistance-associated substitutions in patients with genotype 1 hepatitis C virus: prevalence and effect on treatment outcome. J Hepatol 2017; 66:910–8. [DOI] [PubMed] [Google Scholar]
- 5. Parczewski M, Cielniak I, Kordek J, et al. Transmission networks of HCV genotype 1a enriched with pre-existing polymorphism Q80K among HIV-infected patients with acute hepatitis C in Poland. J Acquir Immune Defic Syndr 2018; 77:514–22. [DOI] [PubMed] [Google Scholar]
- 6. RESEARCH FCFDEA. FDA glecaprevir/pibrentasvir NDA microbiology virology reviews_209394Orig1s000micror FDA- Center for Drug Evaluation and Research application number: 209394Orgi1s000. Clinical Microbiology/virology review(s). Available at: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2017/209394Orig1s000MicroR.pdf
- 7. Krishnan P, Beyer J, Mistry N, et al. In vitro and in vivo antiviral activity and resistance profile of ombitasvir, an inhibitor of hepatitis C virus NS5A. Antimicrob Agents Chemother 2015; 59:979–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. European Medical Agency. Maviret glecaprevir/pibrentasvir assessment report United Kingdom: European Medicines Agency (EMA), Assessment report Maviret (glecaprevir/pibrentasvir), 2017. EMEA/H/C/004430/0000. Available at: https://www.ema.europa.eu/en/documents/assessment-report/maviret-epar- public-assessment-report_en.pdf
- 9. Wyles DL, Luetkemeyer AF. Understanding hepatitis C virus drug resistance: clinical implications for current and future regimens. Top Antivir Med 2017; 25:103–9. [PMC free article] [PubMed] [Google Scholar]
- 10. Abravanel F, Métivier S, Chauveau M, Péron J-M, Izopet J. Transmission of HCV NS5A inhibitor–resistant variants among HIV-infected men who have sex with men. Clin Infect Dis 2016; 63:1271–2. [DOI] [PubMed] [Google Scholar]
- 11. Newsum AM, Ho CK, Lieveld FI, et al. The hepatitis C virus nonstructural protein 3 Q80K polymorphism is frequently detected and transmitted among HIV-infected MSM in the Netherlands. AIDS 2017; 31:105–12. [DOI] [PubMed] [Google Scholar]
- 12. Franco S, Tural C, Nevot M, et al. Detection of a sexually transmitted hepatitis C virus protease inhibitor-resistance variant in a human immunodeficiency virus-infected homosexual man. Gastroenterology 2014; 147:599–601.e1. [DOI] [PubMed] [Google Scholar]
- 13. World Health Organization. Progress report on access to hepatitis C treatment. Geneva, Switzerland: World Health Organization, 2018. [Google Scholar]
- 14. Popping S, Cento V, García F, et al. The need for a European hepatitis C programme monitoring resistance to direct-acting antiviral agents in real life to eliminate hepatitis C. J Virus Erad 2018; 4:179–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.