Abstract
Background
Multivalent influenza vaccine products provide protection against influenza A(H1N1)pdm09, A(H3N2), and B lineage viruses. The 2018–2019 influenza season in the United States included prolonged circulation of A(H1N1)pdm09 viruses well-matched to the vaccine strain and A(H3N2) viruses, the majority of which were mismatched to the vaccine. We estimated the number of vaccine-prevented influenza-associated illnesses, medical visits, hospitalizations, and deaths for the season.
Methods
We used a mathematical model and Monte Carlo algorithm to estimate numbers and 95% uncertainty intervals (UIs) of influenza-associated outcomes prevented by vaccination in the United States. The model incorporated age-specific estimates of national 2018–2019 influenza vaccine coverage, influenza virus–specific vaccine effectiveness from the US Influenza Vaccine Effectiveness Network, and disease burden estimated from population-based rates of influenza-associated hospitalizations through the Influenza Hospitalization Surveillance Network.
Results
Influenza vaccination prevented an estimated 4.4 million (95%UI, 3.4 million–7.1 million) illnesses, 2.3 million (95%UI, 1.8 million–3.8 million) medical visits, 58 000 (95%UI, 30 000–156 000) hospitalizations, and 3500 (95%UI, 1000–13 000) deaths due to influenza viruses during the US 2018–2019 influenza season. Vaccination prevented 14% of projected hospitalizations associated with A(H1N1)pdm09 overall and 43% among children aged 6 months–4 years.
Conclusions
Influenza vaccination averted substantial influenza-associated disease including hospitalizations and deaths in the United States, primarily due to effectiveness against A(H1N1)pdm09. Our findings underscore the value of influenza vaccination, highlighting that vaccines measurably decrease illness and associated healthcare utilization even in a season in which a vaccine component does not match to a circulating virus.
Keywords: influenza, vaccination, prevented illnesses, burden
We estimated that influenza vaccination prevented an estimated 4.4 million illnesses, 2.3 million medical visits, 58 000 hospitalizations, and 3500 deaths in the 2018–2019 influenza season.
The 2018–2019 influenza season in the United States included prolonged circulation of influenza A viruses throughout the country. Using preliminary data obtained during the influenza season, the US Centers for Disease Control and Prevention (CDC) estimated that there were between 32.0 and 43.4 million influenza illnesses, between 401 000 and 706 000 hospitalizations, and between 27 300 and 49 000 influenza-associated deaths during 2018–2019 [1]. Influenza A(H1N1)pdm09 viruses predominated early in the season from October to mid-February, but the proportion of influenza cases due to A(H3N2) viruses increased in late February and circulation continued through mid-May; influenza B activity was limited [2].
Influenza vaccination is effective in the prevention of influenza illness and its complications. Recent reports estimate that 63% of children and adolescents and 45% of adults in the United States were vaccinated against influenza during the 2018–2019 season [3]. Vaccine-induced immune responses are specific to the 2 influenza A subtypes and 1 or 2 B lineages included in the vaccine [4]. All 2018–2019 Northern Hemisphere influenza vaccines contained an A/Michigan/45/2015-like A(H1N1)pdm09 virus, an A/Singapore/INFIMH-16–0019/2016-like A(H3N2) clade 3C.2a1 virus, and a B/Colorado/06/2017-like Victoria lineage virus [5]. Quadrivalent vaccines contained an additional B/Phuket/3073/2013-like Yamagata lineage virus. Genetic characterization of viruses indicated that the majority of circulating A(H3N2) viruses in the United States were antigenically distinct, or drifted, from the 2018–2019 Northern Hemisphere vaccine strain, while circulating A(H1N1)pdm09 viruses were well matched to the vaccine [2].
In an influenza season with a mix of virus subtype circulation and antigenic drift in 1 of the circulating viruses, our objective was to quantify the number of influenza-associated illnesses, medical visits, hospitalizations, and deaths prevented by influenza vaccination in the United States.
METHODS
Influenza Vaccine Effectiveness
We used estimates of vaccine effectiveness (VE) against medically attended laboratory-confirmed influenza for the 2018–2019 season published by the US Influenza Vaccine Effectiveness (Flu VE) Network (Supplementary Table 1) [6]. Methods of the Flu VE Network have been described previously [6–8]. Briefly, study staff at each of 5 sites recruited, consented, enrolled, and interviewed patients aged ≥6 months who sought outpatient care for acute respiratory illness with cough within 7 days of symptom onset during periods of local influenza circulation. All patients were tested for influenza and influenza A subtype and B lineage for research purposes using reverse-transcription polymerase chain reaction.
Vaccination status was based on documented receipt of ≥1 dose of 2018–2019 influenza vaccine in electronic immunization records. In addition, at 4 sites, adults aged ≥18 years were considered vaccinated if they reported vaccination timing and location [7]. We calculated VE using a test-negative design comparing vaccination odds among influenza-positive patients and influenza-negative patients from multivariable logistic regression models. VE and 95% confidence intervals (CIs) against influenza virus type or subtype were calculated using separate models.
Vaccine effectiveness in the 2018–2019 influenza season ranged from 9% (95% CI, 0 to 20) against A(H3N2) to 44% (95% CI, 37 to 51) against A(H1N1)pdm09 with variation by age group (Supplementary Table 1). Because of small numbers, VE against influenza B was not stratified by age group.
Influenza Vaccine Coverage
We obtained estimates of influenza vaccination coverage in the United States by month, from August 2018 through April 2019, which were reported at [3]. Vaccine coverage was 73%, 59%, 35%, 47%, and 68% among persons aged 6 month–4 years, 5–17 years, 18–49 years, 50–64 years, and ≥65 years, respectively (Supplementary Table 2).
Estimates of Influenza-associated Outcomes
We obtained estimates of age-specific influenza burden for those aged ≥6 months from the 2018–2019 season, which are available at [9]. The methods for estimating age-specific influenza burden have been detailed elsewhere [10, 11]. Briefly, this method uses mathematical multipliers to calculate symptomatic illnesses, medical visits, and deaths from data on laboratory-confirmed influenza hospitalizations reported through the Influenza Hospitalization Surveillance Network (FluSurv-NET; Supplementary Figure 1). For this analysis, we restricted burden estimates to those aged ≥6 months. To estimate burden by virus type/subtype, we applied type/subtype distributions among cases in the Flu VE Network to rates of illnesses and medical visits and the type/subtype distribution among hospitalized cases to rates of hospitalizations and deaths. Because influenza A subtype was missing for 58% of FluSurv-NET patients with influenza A virus infection, we used multiple imputation (70 imputations using PROC MI with MONOTONE option in SAS, version 9.4) to estimate the rate of hospitalization for each subtype, including patient age (in groups), surveillance site (as state), and admission time period (as October–December, January, February, or March–April) in the imputation model.
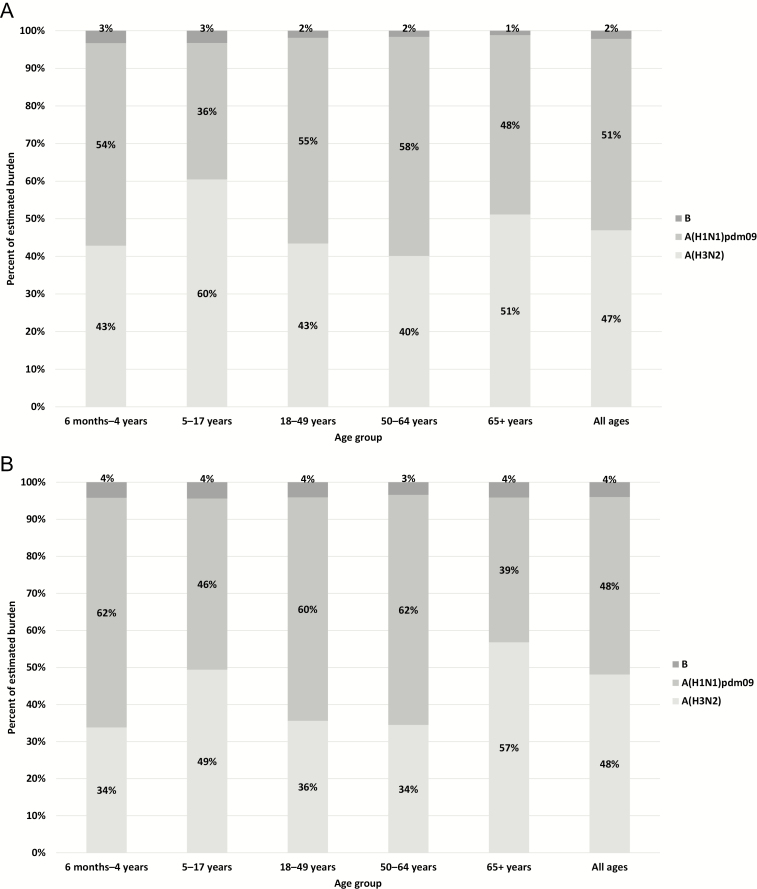
Figure 1.
Proportion of influenza (A) illnesses and (B) hospitalizations due to influenza virus types and subtypes in persons aged ≥6 months—United States, 2018–2019 influenza season.
Influenza-associated Outcomes Prevented by Vaccination
We estimated the effect of seasonal influenza vaccination on disease burden using a mathematical model, stratified by age group as described previously [8, 12]. Briefly, for each month, August to April, the model estimates the population susceptible to influenza by removing the monthly illnesses (as estimating by burden calculations) and those vaccinated and protected (as estimated by vaccination coverage and effectiveness). We calculated the monthly risk of influenza among the susceptible population and projected the number of outcomes that would have occurred in the US population without influenza vaccination. We calculated the prevented outcomes as the difference between outcomes without influenza vaccination and those estimated under observed levels of vaccination (as estimated by the 2018–2019 seasonal burden). Based on evidence from prior seasons when estimates of VE in adult outpatients and inpatients were similar, we assumed that VE estimates from the Flu VE Network applied to all influenza outcomes and were also constant throughout the season [13–15]. Equations for the model are presented by Tokars et al [12].
To obtain an estimate of all influenza-associated outcomes prevented by vaccination, we first used age group–specific VE estimates against A(H1N1)pdm09 and A(H3N2) and the overall VE estimate against influenza B in the model with type/subtype-specific burden. We then combined the number of prevented outcomes for each type/subtype together.
We estimated the number needed to vaccinate (NNV) to prevent 1 influenza A(H1N1)pdm09-associated illness or hospitalization by dividing the number of vaccinated individuals by A(H1N1)pdm09-specific illnesses or hospitalizations prevented by vaccination. When 95% CIs for VE included the null, the undefined value of NNV was indicated as >999 999.
We used a Monte Carlo algorithm with 5000 simulations to estimate 95% uncertainty intervals (UIs). For each simulation, we chose random values from assumed model input distributions (Supplementary Table 3) and estimated prevented outcomes. We truncated values for VE and vaccine coverage at 0.
Sensitivity Analyses
Because VE against A(H3N2) viruses was low overall and for each age group, we conducted a sensitivity analysis where we assumed true VE against A(H3N2) was 0%. We also conducted 8 sensitivity analyses to account for uncertainty in vaccine coverage estimates [3]. Four of these apply various reductions (ie, 5% absolute, 10% absolute, 10% relative, 25% relative) in the estimated vaccine coverage to account for possible overestimation of vaccine coverage by self-report [16–20]. In 2 additional analyses, we assumed those with missing vaccination status in vaccine coverage surveys were all vaccinated or unvaccinated. In 1 analysis, we imputed missing vaccination status. Finally, for 1 analysis, we applied a 5% absolute increase in coverage among adults aged ≥65 to account for possible underestimation of vaccine coverage by the Behavioral Risk Factor Surveillance System [3].
RESULTS
We estimated that influenza was associated with 34.9 million illnesses, 16.1 million medical visits, 480 000 hospitalizations, and 34 000 deaths in 2018–2019 among all persons aged ≥6 months who were eligible for vaccination (Table 1). Influenza A(H3N2) and A(H1N1)pdm09 viruses were associated with approximately equal proportions of the burden of influenza disease, but with important variation by age (Figure 1A, B). Influenza A(H3N2) viruses were associated with an estimated 15.7 million illnesses, 7.4 million medical visits, 230 000 hospitalizations, and 17 000 deaths. Influenza A(H1N1)pdm09 viruses were associated with 17.1 million illnesses, 7.8 million medical visits, 232 000 hospitalizations, and 15 000 deaths. The proportion of influenza illnesses associated with A(H3N2) viruses was highest among children aged 5–17 years and lowest among persons aged 50–64 years, whereas the proportion of influenza hospitalizations associated with A(H3N2) was highest among persons aged ≥65 years (Figure 1A, B). Influenza B virus infections were less common and accounted for 2.1% (735 000) of all influenza-associated illnesses.
Table 1.
Estimated Influenza-Associated Illnesses, Medical Visits, Hospitalizations, and Deaths by Influenza Virus Type and Subtype and Age Group—United States, 2018–2019 Influenza Season
| Illnesses | Medical Visits | Hospitalizations | Deaths | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Influenza (Sub)type | Age Group | Estimate | 95% UIa | Estimate | 95% UI | Estimate | 95% UI | Estimate | 95% UI |
| Influenza A and B | 6 months–4 years | 2 993 292 | (2 064 327–5 813 928) | 2 005 506 | (1 376 651–3 905 571) | 20 868 | (14 391–40 532) | 219 | (53–739) |
| 5–17 years | 7 663 310 | (6 041 811–10 516 276) | 3 984 921 | (3 106 356–5 491 261) | 21 012 | (16 566–28 834) | 211 | (36–656) | |
| 18–49 years | 11 913 203 | (10 087 669–15 785 820) | 4 407 885 | (3 635 026–5 916 066) | 66 869 | (56 622–88 605) | 2450 | (1405–5803) | |
| 50–64 years | 9 238 038 | (6 523 213–16 327 079) | 3 972 356 | (2 788 157–7 021 296) | 97 967 | (69 176–173 144) | 5676 | (2 575–15 577) | |
| ≥65 years | 3 073 227 | (1 990 659–6 157 258) | 1 721 007 | (1 108 265–3 446 930) | 279 384 | (180 969–559 750) | 25 555 | (12 281–59 363) | |
| All ages | 34 881 071 | (30 804 737–44 394 276) | 16 091 676 | (14 130 851–20 561 197) | 486 100 | (384 310–778 363) | 34 110 | (20 753–69 071) | |
| Influenza A(H3N2) | 6 months–4 years | 1 219 060 | (829 889–2 371 609) | 816 770 | (551 737–1 592 697) | 6940 | (4 689–13 608) | 73 | (17–243) |
| 5–17 years | 4 469 055 | (3 536 287–6 111 279) | 2 323 909 | (1 828 242–3 174 928) | 10 278 | (8071–14 076) | 103 | (17–317) | |
| 18–49 years | 4 951 671 | (4 104 164–6 657 477) | 1 832 118 | (1 492 332–2 475 088) | 23 666 | (19 858–31 616) | 867 | (496–2027) | |
| 50–64 years | 3 577 415 | (2 460 247–6 310 877) | 1 538 289 | (1 058 070–2 719 926) | 33 656 | (23 609–59 649) | 1950 | (885–5325) | |
| ≥65 years | 1 520 099 | (974 217–3 100 136) | 851 255 | (543 215–1 733 422) | 157 983 | (102 336–316 414) | 14 450 | (6942–33 420) | |
| All ages | 15 737 300 | (13 895 150–19 857 103) | 7 362 341 | (6 456 292–9 363 997) | 232 523 | (177 682–391 923) | 17 443 | (10 026–36 814) | |
| Influenza A(H1N1)pdm09 | 6 months–4 years | 1 530 699 | (1 036 024–2 969 043) | 1 025 568 | (692 562–1 994 583) | 12 714 | (8 825–24 712) | 134 | (31–441) |
| 5–17 years | 2 681 433 | (2 095 593–3 672 515) | 1 394 345 | (1 087 731–1 917 653) | 9604 | (7555–13 152) | 96 | (16–294) | |
| 18–49 years | 6 235 437 | (5 217 709–8 365 654) | 2 307 112 | (1 887 793–3 112 738) | 40 085 | (33 849–53 304) | 1468 | (841–3441) | |
| 50–64 years | 5 192 363 | (3 656 638–9 215 958) | 2 232 716 | (1 566 427–3 951 761) | 60 584 | (42 749–106 916) | 3510 | (1590–9626) | |
| ≥65 years | 1 417 235 | (893 272–2 856 660) | 793 652 | (498 055–1 584 752) | 108 535 | (70 316–218 063) | 9927 | (4760–22 981) | |
| All ages | 17 057 167 | (14 955 238–22 119 540) | 7 753 393 | (6 763 274–10 115 308) | 231 522 | (188 659–349 338) | 15 136 | (9678–29 640) | |
| Influenza B | 6 months–4 years | 94 129 | (38 740–21 2737) | 63 066 | (26 190–143 306) | 859 | (517–1745) | 9 | (2–30) |
| 5–17 years | 241 037 | (150 833–372 312) | 125 339 | (77 879–192 990) | 916 | (625–1358) | 9 | (1–28) | |
| 18–49 years | 217 659 | (112 960–357 679) | 80 534 | (41 347–132 284) | 2 710 | (2116–3690) | 99 | (55–237) | |
| 50–64 years | 146 304 | (46 017–320 285) | 62 911 | (19 616–138 411) | 3328 | (2247–5900) | 193 | (86–536) | |
| ≥65 years | 35 460 | (0–105 126) | 19 858 | (0–59 293) | 11 524 | (7396–23 360) | 1054 | (507–2440) | |
| All ages | 734 589 | (541 765–1 023 014) | 351 708 | (260 630–493 432) | 19 337 | (15 086–31 583) | 1364 | (816–2806) |
Abbreviation: UI, uncertainty interval.
a95% UI from 5000 Monte Carlo simulations.
Vaccine-prevented Burden
We estimated that influenza vaccination prevented an estimated 4.4 million (95% UI, 3.4 million to 7.1 million) illnesses, 2.3 million (95% UI, 1.8 million to 3.8 million) medical visits, 58 000 (95% UI, 30 000 to 156 000) hospitalizations, and 3500 (95% UI, 1000 to 13 000) deaths associated with influenza viruses (Table 2). In a sensitivity analysis where we conservatively assumed true VE against A(H3N2) was 0%, UIs did not differ from our primary analysis results (data not shown). We estimated that influenza vaccination prevented 3.7 million (95% UI, 2.7 million to 5.6 million) illnesses, 1.9 million (95% UI, 1.4 million to 3.0 million) medical visits, 38 000 (95% UI, 20 000 to 89 000) hospitalizations, and 1900 (95% UI, 500 to 7000) deaths associated with A(H1N1)pdm09 infections (Table 3). These estimates represent 83.5% of total prevented influenza-associated illnesses, 82.7% of medical visits, 65.8% of hospitalizations, and 54.2% of deaths. Because of the modest observed burden associated with influenza B viruses, the remaining prevented outcomes are mainly associated with circulating A(H3N2) viruses (Supplementary Table 4).
Table 2.
Estimates of All Influenza-Associated Illnesses, Medical Visits, Hospitalizations, and Deaths Prevented by Influenza Vaccination—United States, 2018–2019 Influenza Season
| Illnesses | Medical Visits | Hospitalizations | Deaths | |||||
|---|---|---|---|---|---|---|---|---|
| Age Group | Number Prevented | 95% UIa | Number Prevented | 95% UI | Number Prevented | 95% UI | Number Prevented | 95% UI |
| 6 months–4 years | 1 335 840 | (736 978–2 851 485) | 895 012 | (492 938–1 926 307) | 10 569 | (5933–22 379) | 111 | (25–381) |
| 5–17 years | 1 021 821 | (446 661–1 870 063) | 531 347 | (230 928–980 184) | 3269 | (1478–5709) | 33 | (4–108) |
| 18–49 years | 984 698 | (612 071–1 610 237) | 364 338 | (225 540–600 040) | 6239 | (3919–9884) | 229 | (114–588) |
| 50–64 years | 785 710 | (241 045–2 158 024) | 337 855 | (102 597–926 454) | 9250 | (2970–23 951) | 536 | (143–2000) |
| ≥65 years | 300 879 | (3249–1 316 421) | 168 492 | (1844–737 768) | 28 695 | (1533–121 752) | 2625 | (122–12 163) |
| All ages | 4 428 947 | (3 429 414–7 070 624) | 2 297 045 | (1 755 797–3 768 642) | 58 022 | (29 598–156 185) | 3533 | (1016–13 393) |
Abbreviation: UI, uncertainty interval.
a95% UI from 5000 Monte Carlo simulations.
Table 3.
Estimates of Influenza A(H1N1)pdm09-Associated Illnesses, Medical Visits, Hospitalizations, and Deaths Prevented by Influenza Vaccination—United States, 2018–2019 Influenza Season
| Illnesses | Medical Visits | Hospitalizations | Deaths | |||||
|---|---|---|---|---|---|---|---|---|
| Age Group | Number Prevented | 95% UIa | Number Prevented | 95% UIa | Number Prevented | 95% UIa | Number Prevented | 95% UIa |
| 6 months–4 years | 1 176 881 | (633 739– 2 482 324) | 788 510 | (425 367– 1 674 522) | 9555 | (5264–19 769) | 100 | (22–339) |
| 5–17 years | 687 757 | (280 148– 1 181 100) | 357 634 | (145 741– 617 933) | 2420 | (985–4186) | 24 | (3–79) |
| 18–49 years | 915 849 | (541 365– 1 386 802) | 338 864 | (198 438–518 320) | 5742 | (3404–8630) | 210 | (100–522) |
| 50–64 years | 761 047 | (135 579– 1 667 583) | 327 250 | (58 212– 721 722) | 8714 | (1580–19 206) | 505 | (82–1622) |
| ≥65 years | 157 190 | (0– 810 180) | 88 026 | (0– 451 043) | 11 754 | (0–59 698) | 1075 | (0–6011) |
| All ages | 3 698 723 | (2 665 133– 5 594 554) | 1 900 284 | (1 356 641– 2 981 289) | 38 186 | (20 104–89 304) | 1915 | (515–6971) |
Abbreviation: UI, uncertainty interval.
a95% UI from 5000 Monte Carlo simulations.
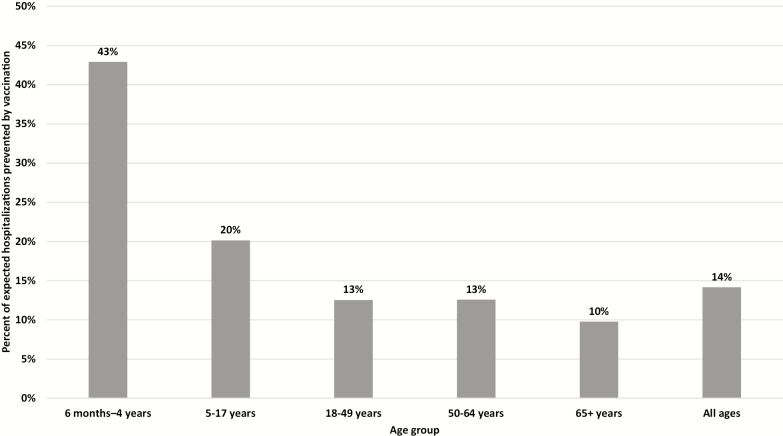
Influenza vaccination prevented 11% of all influenza-associated illnesses that would be projected to occur without influenza vaccination, 11% of projected influenza-associated hospitalizations, and 9% of projected influenza-associated deaths in 2018–2019. The vaccine-prevented fraction specific to A(H1N1)pdm09 was 18% (95% UI, 13% to 23%) for illnesses, 14% (95% UI, 7% to 25%) for hospitalizations, and 11% (95% UI, 3% to 26%) for deaths due to A(H1N1)pdm09. The percent of projected influenza A(H1N1)pdm09-associated hospitalizations prevented by vaccination varied by age group, from 43% (95% UI, 33% to 52%) in children aged 6 months–4 years who had high vaccine coverage and the highest VE against A(H1N1)pdm09 to 10% (95% UI, 0% to 31%) in adults aged ≥65 years (Figure 2).
Figure 2.
Estimated percent of expected influenza A(H1N1)pdm09-associated hospitalizations prevented by vaccination—United States, 2018–2019 influenza season.
Number Needed to Vaccinate
Using the reported vaccine coverage and the estimates of A(H1N1)pdm09-associated illnesses and hospitalizations prevented by vaccination, we estimated that 43 people (95% UI, 28 to 59) needed to be vaccinated for each A(H1N1)pdm09-associated illness prevented and that 4127 people (95% UI, 1774 to 7851) needed to be vaccinated for each hospitalization prevented (Table 4). The NNV to prevent 1 A(H1N1)pdm09-associated illness varied by age group from 11 (95% UI, 5 to 20) among children aged 6 months–4 years to 226 (95% UI, 44 to >99 999) among adults aged ≥65 years.
Table 4.
Number Needed to Vaccinate to Prevent 1 Influenza A(H1N1)pdm09-Associated Illness or Hospitalization—United States, 2018–2019 Influenza Season
| Illness | Hospitalization | |||
|---|---|---|---|---|
| Age Group | NNV | 95% UIa | NNV | 95% UIa |
| 6 months–4 years | 11 | (5 to 20) | 1368 | (658 to 2468) |
| 5–17 years | 46 | (27 to 113) | 12 997 | (7498 to 31 902) |
| 18–49 years | 52 | (35 to 88) | 8326 | (5533 to 13 899) |
| 50–64 years | 39 | (18 to 220) | 3414 | (1554 to 18 889) |
| ≥65 years | 226 | (44 to >99 999) | 3020 | (595 to >99 999) |
| All ages | 43 | (28 to 59) | 4127 | (1774 to 7851) |
Abbreviation: NNV, number needed to vaccinate; UI, uncertainty interval.
a95% UI from 5000 Monte Carlo simulations.
Sensitivity Analyses for Vaccine Coverage
Our estimates of the burden prevented by vaccination were robust and largely unaffected by uncertainty in the vaccine coverage estimates, as all 8 sensitivity analyses around vaccine coverage estimates were within the UIs of our main results using the reported 2018–2019 coverage (Supplementary Figure 2). Among children aged 6 months–4 years, the percent of prevented hospitalizations associated with A(H1N1)pdm09 ranged from 32% to 39% in sensitivity analyses that applied to this age group. The NNV to prevent 1 A(H1N1)pdm09-associated illness ranged from 12 to 13 among children aged 6 months–4 years in sensitivity analyses that applied to this age group.
DISCUSSION
Using mathematical models, we estimated that influenza vaccination prevented an estimated 4.4 million illnesses, 2.3 million medical visits, 58 000 hospitalizations, and 3500 deaths associated with influenza viruses. Currently available influenza vaccines are multivalent vaccines designed to provide protection against A(H1N1)pdm09, A(H3N2), and either 1 or both influenza B lineage viruses. During the 2018–2019 US influenza season, both A(H1N1)pdm09 and A(H3N2) contributed to the substantial observed disease burden [2]. More than 90% of the circulating A(H3N2) viruses were antigenically drifted from the A(H3N2) vaccine component. The vaccine likely had little effect against A(H3N2)-associated disease [2, 6]. However, A(H1N1)pdm09 viruses were antigenically matched, and we estimated that the vaccine prevented 3.7 million A(H1N1)pdm09-associated illnesses and 38 000 hospitalizations [6]. The benefits of vaccination were greatest among children aged 6 months–4 years, with vaccination preventing 43% of all projected A(H1N1)pdm09-associated hospitalizations. Further, we estimated that 1 influenza A(H1N1)pdm09-associated illness episode was prevented for every 11 children vaccinated in this age group. Our results highlight the benefit of annual vaccination with current influenza vaccines that provide protection against more than 1 influenza virus in an influenza season.
The majority of vaccine-prevented outcomes during the 2018–2019 influenza season were attributable to the effectiveness of the A(H1N1)pdm09 vaccine component. The absolute number of hospitalizations associated with A(H1N1)pdm09 viruses during the season was substantial, approximately 231 000 hospitalizations overall, and vaccination prevented 14% of A(H1N1)pdm09-associated hospitalizations. Furthermore, the burden of influenza A(H1N1)pdm09-associated hospitalizations made up roughly half of all influenza-associated hospitalizations in 2018–2019. The relative distribution of influenza virus (sub)types varies each season, and the relative frequency of each virus subtype can vary by age group [21]. On average, A(H1N1)pdm09, A(H3N2), and B lineage viruses accounted for 35%, 45%, and 20% of the circulating viruses, respectively, between the 2009–2010 pandemic and 2016–2017 influenza seasons [21]. With shifting age dynamics of the population, A(H1N1)pdm09 is predicted to predominate more frequently for some age groups and thus could account for a higher burden of severe disease than A(H3N2) in future seasons [21]. Multivalent influenza vaccines prevent influenza illness even during seasons when 1 of the circulating viruses is not well matched to the seasonal influenza vaccine.
The vaccine did not protect against the majority of circulating A(H3N2) viruses in 2018–2019, and the vaccine prevented fewer A(H3N2)-associated outcomes compared with A(H1N1)pdm09. In the Flu VE Network, 7% of genetically characterized A(H3N2) viruses were considered well matched to the A(H3N2) vaccine strain, A/Singapore/INFIMH-16–0019/2016 [6]. Thus, some disease was likely prevented. Influenza A(H3N2) viruses evolve rapidly, and anticipating which genetic group will circulate in an upcoming influenza season is a challenge [22]. It is not yet possible to predict the exact viruses that will circulate in an upcoming influenza season. Due to vaccine manufacturing timelines, influenza virus strains must be selected at least 6 months in advance of the influenza season to ensure timely vaccine availability [23]. For the 2019–2020 Northern Hemisphere influenza season, the A(H3N2) vaccine strain was updated to an A/Kansas/14/2017-like clade 3C.3a virus that more closely represented the predominant A(H3N2) genetic group from the 2018–2019 Northern Hemisphere influenza season [24].
With improvements in influenza VE, particularly against A(H3N2) viruses, and increased rates of influenza vaccination, more illnesses, hospitalizations, and deaths could be prevented. In a recent modeling analysis, improvements to VE would provide the greatest benefit in preventing hospitalizations among those aged ≥65 years, while improvements in vaccine coverage would provide the greatest benefit in preventing illnesses among those aged 18–49 years [25]. Despite low VE against influenza A(H3N2), we estimated that nontrivial numbers of hospitalizations and deaths were prevented in the oldest age group due to high burden of these outcomes and high vaccine coverage in this age group.
Influenza vaccines that provide broader protection against circulating influenza viruses and that do not need to be updated as frequently are among the goals of efforts to develop universal influenza vaccines [26, 27]. One study, assuming an average VE of 44% for existing influenza vaccines and an average uptake of 169 million influenza vaccine doses, estimated that replacing 10% of trivalent and quadrivalent influenza vaccines currently in use with a 75% effective universal influenza vaccine would prevent 5.3 million cases, 81 000 hospitalizations, and 6300 deaths annually [28]. Until newer, more effective or universal vaccines become available, our results demonstrate that vaccination with currently available influenza vaccines prevents millions of illnesses and thousands of hospitalizations and deaths in influenza seasons that are dominated by 1 influenza A subtype as well as during mixed circulation seasons such as 2018–2019 [8].
There are several limitations to our estimates of the benefit of influenza vaccination. First, multipliers used to scale surveillance data to national burden estimates are preliminary at this time; final multipliers often lag by 2 years [1, 8]. While we have used the most conservative multipliers from previous influenza seasons for current estimates, we expect our estimates to change when final data become available. Once data on testing practices from 2018–2019 become available, updated estimates of the vaccine-prevented burden will be posted on the CDC Influenza webpages [29]. Additionally, any changes in care-seeking behavior or patterns of disease severity that occurred during 2018–2019 would not be reflected in our estimates, as multipliers are based on prior seasons’ data. Second, we needed to impute viral subtype-specific hospitalization rates because subtyping is not performed for all cases in FluSurv-NET. Third, our model does not account for possible within-season waning of VE [30–33]. The 2018–2019 season was the longest influenza season in 10 years [2]. It is possible that VE against A(H3N2) virus waned over the influenza season; the interim estimate of VE against A(H3N2) was 44% (95% CI, 13% to 64%) [34], higher than the final estimate (9%) used in our calculations. Any amount of waning of VE would reduce the estimated population benefit. Fourth, vaccination coverage estimates are based on telephone surveys with low response rates and rely on parental or self-reported influenza vaccination status. Estimates may be biased after weighting adjustments designed to mitigate exclusion of households without telephone service, nonresponse, and misclassification of vaccination status [16, 17, 19, 20, 35]. However, results of sensitivity analyses fell within the UIs using the best-estimated reported coverage. Fifth, we assumed that influenza vaccination would not increase the risk of infection and truncated UIs at 0, thus potentially skewing the point estimates in favor of a population benefit. Finally, our model of prevented outcomes may underestimate the population-level benefit of vaccination because it does not account for any possible indirect effects of vaccination (eg, community protection) [36, 37]. Moreover, our estimates do not reflect the economic consequences or postinfection outcomes of these hospitalizations such as cardiovascular disease, escalating frailty, or associated antibiotic usage [38–43].
Our results highlight the large burden of influenza-associated illnesses, medical visits, hospitalizations, and deaths during 2018–2019 and quantify the value of currently available multivalent vaccines to reduce the burden of influenza disease in unpredictable annual influenza epidemics. Even with limited effectiveness against A(H3N2) viruses due to circulation of an antigenically drifted strain, influenza vaccination in the 2018–2019 season prevented substantial numbers of A(H1N1)pdm09-associated severe and nonsevere influenza illness. These data emphasize the importance of annual influenza vaccination in preventing deaths and severe disease even during influenza seasons when a circulating virus is antigenically drifted.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
US Influenza Vaccine Effectiveness Network: Sara S. Kim, Emily T. Martin, Arnold S. Monto, Michael L. Jackson, Lisa A. Jackson, Huong Q. McLean, Edward A. Belongia, Jennifer P. King, Richard K. Zimmerman, Mary Patricia Nowalk, G. K. Balasubramani, Todd M. Bear, Robert Hickey, Jonathan M. Raviotta, Joe Suyama, Alexandra J. Weissman, John V. Williams, Manjusha Gaglani, Chandni Raiyani, Michael Smith, Kempapura Murthy, Lydia Clipper, Michael Reis, Arundhati Rao, Kimberly Walker, Marcus Volz, and Manohar Mutnal.
Influenza Hospitalization Surveillance Network: Charisse N. Cummings, Kimberly Yousey-Hindes, Chelsea McMullen, Shua J. Chai, Evan J. Anderson, Maya L. Monroe, Ilene Risk, Rachel Herlihy, Sue Kim, Nancy Spina, Laurie Billing, William Schaffner, H. Keipp Talbot, Ann Thomas, and Melissa McMahon.
Acknowledgments. The authors acknowledge the great work, support, and contributions of the following: Pengjun Lu, Tammy Santibanez, Anup Srivastav, Yusheng Zhai, James Meek, Amber Maslar, Allen Achkar, Elizabeth Alleman, Trinh Anh Minh, Habeeb Al-Shohatee, Gabriela Augustinaitis, Sarah Bauer, Danielle Carroll, Caroline K. Cheng, Robert Deblander III, Michelle Groesbeck, Emileigh Johnson, Anne Kaniclides, Armanda Kimberly, Jenna Kiryakos, Marym Kuril, Lois E. Lamerato, Ryan E. Malosh, Maria Matta, E. J. McSpadden, Madeleine Mendelow, Joshua G. Petrie, Niharika Rajesh, Bryan Richardson, Stephanie Robinson, Hannah Segaloff, Caleb Sokolowski, Rachael Swanson, Rachel Truscon, Salina Torres, Sarah Khanlian, Lisa Butler, Kathy Angeles, Meaghan Novi, Cory Cline, Kyle Openo, Stepy Thomas, Suzanne Segler, Andrew Martin, Emily Fawcett, Patricia Ryan, Robert Sunkel, Alicia Brooks, Sophia Wozny, Heather Rutz, Cindy Zerrlaut, C. Hallie Phillips, Stacie Wellwood, Erika Kiniry, Suzie Park, Matthew Nguyen, Rachael Burganowski, Nisha Alden, Samie Stephens, Jim Collins, Shannon Johnson, Justin Henderson, Katarina Manzi, Kerianne Engesser, Jessica Shiltz, Nicholas Fisher, Karen Leib, Katie Dyer, Nicole West, Monika Johnson, Alan Aspinall, Heather Eng, David Figucia, Philip Iozzi, Jason Lyons, Krissy K. Moehling, Evelyn C. Reis, Theresa M. Sax, Leonard Urbanski, Ruth Lynfield, Christina Felsen, Maria Gaitan, Nancy Bennett, Debra Blog, and Elizabeth Dufort.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC).
Financial support. This work was supported by the CDC through the following cooperative agreements: Emerging Infections Programs (CDC-RFA-CK17–1701), the Influenza Hospital Surveillance Project (5U38OT000143), the University of Michigan (1U01 IP001034), Kaiser Permanente Washington Research Institute (1U01 IP001037), Marshfield Clinic Research Institute (1U01 IP001038), University of Pittsburgh (1U01 IP001035), and Baylor Scott and White Healthcare (1U01 IP001039). At the University of Pittsburgh, the project was also supported by the National Institutes of Health through grant UL1TR001857.
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
US Influenza Vaccine Effectiveness Network, the Influenza Hospitalization Surveillance Network, and the Assessment Branch, Immunization Services Division, Centers for Disease Control and Prevention:
Sara S Kim, Emily T Martin, Arnold S Monto, Michael L Jackson, Lisa A Jackson, Huong Q McLean, Edward A Belongia, Jennifer P King, Richard K Zimmerman, Mary Patricia Nowalk, G K Balasubramani, Todd M Bear, Robert Hickey, Jonathan M Raviotta, Joe Suyama, Alexandra J Weissman, John V Williams, Manjusha Gaglani, Chandni Raiyani, Michael Smith, Kempapura Murthy, Lydia Clipper, Michael Reis, Arundhati Rao, Kimberly Walker, Marcus Volz, Manohar Mutnal, Charisse N Cummings, Kimberly Yousey-Hindes, Chelsea McMullen, Shua J Chai, Evan J Anderson, Maya L Monroe, Ilene Risk, Rachel Herlihy, Sue Kim, Nancy Spina, Laurie Billing, William Schaffner, H Keipp Talbot, Ann Thomas, and Melissa McMahon
References
- 1. Centers for Disease Control and Prevention. 2018–2019 US flu season: preliminary burden estimates Available at: https://www.cdc.gov/flu/about/burden/preliminary-in-season-estimates.htm. Accessed 15 October 2019.
- 2. Xu X, Blanton L, Elal AIA, et al. . Update: Influenza activity in the United States during the 2018–19 season and composition of the 2019–20 influenza vaccine. Morb Mortal Wkly Rep 2019; 68:544–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Centers for Disease Control and Prevention. Flu vaccination coverage, United States, 2018–19 influenza season Available at: https://www.cdc.gov/flu/fluvaxview/coverage-1819estimates.htm. Accessed 21 October 2019.
- 4. Belongia EA, Simpson MD, King JP, et al. . Variable influenza vaccine effectiveness by subtype: a systematic review and meta-analysis of test-negative design studies. Lancet Infect Dis 2016; 16:942–51. [DOI] [PubMed] [Google Scholar]
- 5. Grohskopf LA, Sokolow LZ, Broder KR, Walter EB, Fry AM, Jernigan DB. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2018–19 influenza season. Morb Mortal Wkly Rep 2018; 67:1– 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Flannery B, Kondor RJG, Chung JR, et al. . Spread of antigenically drifted influenza A(H3N2) viruses and vaccine effectiveness in the United States during the 2018–2019 season. J Infect Dis 2019; 221:8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jackson ML, Chung JR, Jackson LA, et al. . Influenza vaccine effectiveness in the United States during the 2015–2016 season. N Engl J Med 2017; 377:534–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rolfes MA, Flannery B, Chung JR, et al. . Effects of influenza vaccination in the United States during the 2017–2018 influenza season. Clin Infect Dis 2019; 69:1845–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. Estimated influenza illnesses, medical visits, hospitalizations, and deaths in the United States - 2018-2019 influenza season. Available at: https://www.cdc.gov/flu/about/burden/2018-2019.html. Accessed 2020 January 14.
- 10. Reed C, Chaves SS, Daily Kirley P, et al. . Estimating influenza disease burden from population-based surveillance data in the United States. PLoS One 2015; 10:e0118369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rolfes MA, Foppa IM, Garg S, et al. . Annual estimates of the burden of seasonal influenza in the United States: a tool for strengthening influenza surveillance and preparedness. Influenza Other Respir Viruses 2018; 12:132–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tokars JI, Rolfes MA, Foppa IM, Reed C. An evaluation and update of methods for estimating the number of influenza cases averted by vaccination in the United States. Vaccine 2018; 36:7331–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ferdinands JM, Gaglani M, Martin ET, et al. . Prevention of influenza hospitalization among adults in the United States, 2015–2016: results from the US Hospitalized Adult Influenza Vaccine Effectiveness Network (HAIVEN). J Infect Dis 2019; 220:1265–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Feng S, Cowling BJ, Sullivan SG. Influenza vaccine effectiveness by test-negative design—comparison of inpatient and outpatient settings. Vaccine 2016; 34:1672–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Flannery B. Preliminary estimates of 2018–19 seasonal influenza vaccine effectiveness against medically attended influenza from three U.S. networks. In: Atlanta, GA: Advisory Committee on Immunization Practices,2019. [Google Scholar]
- 16. Brown C, Clayton-Boswell H, Chaves SS, et al. ; New Vaccine Surveillance Network Validity of parental report of influenza vaccination in young children seeking medical care. Vaccine 2011; 29:9488–92. [DOI] [PubMed] [Google Scholar]
- 17. Mangtani P, Shah A, Roberts JA. Validation of influenza and pneumococcal vaccine status in adults based on self-report. Epidemiol Infect 2007; 135:139–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Poehling KA, Vannoy L, Light LS, et al. . Assessment of parental report for 2009–2010 seasonal and monovalent H1N1 influenza vaccines among children in the emergency department or hospital. Acad Pediatr 2012; 12:36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Irving SA, Donahue JG, Shay DK, Ellis-Coyle TL, Belongia EA. Evaluation of self-reported and registry-based influenza vaccination status in a Wisconsin cohort. Vaccine 2009; 27:6546–9. [DOI] [PubMed] [Google Scholar]
- 20. King JP, McLean HQ, Belongia EA. Validation of self-reported influenza vaccination in the current and prior season. Influenza Other Respir Viruses 2018; 12:808–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Budd AP, Beacham L, Smith CB, et al. . Birth cohort effects in influenza surveillance data: evidence that first influenza infection affects later influenza-associated illness. J Infect Dis 2019; 220:820–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Monto AS, Petrie JG. Improving influenza vaccine effectiveness: ways to begin solving the problem. Clin Infect Dis 2019; 69:1824–6. [DOI] [PubMed] [Google Scholar]
- 23. Centers for Disease Control and Prevention. Selecting viruses for the seasonal influenza vaccine Available at: https://www.cdc.gov/flu/prevent/vaccine-selection.htm. Accessed 13 November 2019.
- 24. Grohskopf LA, Alyanak E, Broder KR, Walter EB, Fry AM, Jernigan DB. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2019–20 influenza season. Morb Mortal Wkly Rep 2019; 68:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hughes MM, Reed C, Flannery B, et al. . Projected population benefit of increased effectiveness and coverage of influenza vaccination on influenza burden in the United States. Clin Infect Dis 2019:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Erbelding EJ, Post DJ, Stemmy EJ, et al. . A universal influenza vaccine: the strategic plan for the National Institute of Allergy and Infectious Diseases. J Infect Dis 2018; 218:347–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Paules CI, Marston HD, Eisinger RW, Baltimore D, Fauci AS. The pathway to a universal influenza vaccine. Immunity 2017; 47:599–603. [DOI] [PubMed] [Google Scholar]
- 28. Sah P, Alfaro-Murillo JA, Fitzpatrick MC, et al. . Future epidemiological and economic impacts of universal influenza vaccines. Proc Natl Acad Sci U S A 2019; 116:20786–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Centers for Disease Control and Prevention. Past seasons estimated influenza disease burden. Available at: https://www.cdc.gov/flu/vaccines-work/past-burden-averted-est.html. Accessed 14 January 2020.
- 30. Ferdinands JM, Fry AM, Reynolds S, et al. . Intraseason waning of influenza vaccine protection: evidence from the US Influenza Vaccine Effectiveness Network, 2011-12 through 2014-15. Clin Infect Dis 2017; 64:544–50. [DOI] [PubMed] [Google Scholar]
- 31. Belongia EA, Sundaram ME, McClure DL, Meece JK, Ferdinands J, VanWormer JJ. Waning vaccine protection against influenza A (H3N2) illness in children and older adults during a single season. Vaccine 2015; 33:246–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kissling E, Valenciano M, Larrauri A, et al. . Low and decreasing vaccine effectiveness against influenza A(H3) in 2011/12 among vaccination target groups in Europe: results from the I-MOVE multicentre case-control study. Euro Surveill 2013; 18: 33–42. [DOI] [PubMed] [Google Scholar]
- 33. Jimenez-Jorge S, de Mateo S, Delgado-Sanz C, et al. . Effectiveness of influenza vaccine against laboratory-confirmed influenza, in the late 2011–2012 season in Spain, among population targeted for vaccination. BMC Infect Dis 2013; 13:441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Doyle JD, Chung JR, Kim SS, et al. . Interim estimates of 2018–19 seasonal influenza vaccine effectiveness—United States, February 2019. Morb Mortal Wkly Rep 2019; 68: 135–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zimmerman RK, Raymund M, Janosky JE, Nowalk MP, Fine MJ. Sensitivity and specificity of patient self-report of influenza and pneumococcal polysaccharide vaccinations among elderly outpatients in diverse patient care strata. Vaccine 2003; 21:1486–91. [DOI] [PubMed] [Google Scholar]
- 36. Arinaminpathy N, Kim IK, Gargiullo P, et al. . Estimating direct and indirect protective effect of influenza vaccination in the United States. Am J Epidemiol 2017; 186:92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sah P, Medlock J, Fitzpatrick MC, Singer BH, Galvani AP. Optimizing the impact of low-efficacy influenza vaccines. Proc Natl Acad Sci U S A 2018; 115:5151–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kwong JC, Schwartz KL, Campitelli MA, et al. . Acute myocardial infarction after laboratory-confirmed influenza infection. N Engl J Med 2018; 378:345–53. [DOI] [PubMed] [Google Scholar]
- 39. Barker WH, Borisute H, Cox C. A study of the impact of influenza on the functional status of frail older people. Arch Intern Med 1998; 158:645–50. [DOI] [PubMed] [Google Scholar]
- 40. Andrew MK, Gilca V, Waite N, Pereira JA. EXamining the knowledge, Attitudes and experiences of Canadian seniors Towards influenza (the EXACT survey). BMC Geriatr 2019; 19:178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nichol KL, Margolis KL, Wuorenma J, Von Sternberg T. The efficacy and cost effectiveness of vaccination against influenza among elderly persons living in the community. N Engl J Med 1994; 331:778–84. [DOI] [PubMed] [Google Scholar]
- 42. Esposito S, Principi N; European Society of Clinical Microbiology Infectious Diseases Vaccine Study Group Influenza vaccination and prevention of antimicrobial resistance. Expert Rev Vaccines 2018; 17:881–8. [DOI] [PubMed] [Google Scholar]
- 43. Warren-Gash C, Blackburn R, Whitaker H, McMenamin J, Hayward AC. Laboratory-confirmed respiratory infections as triggers for acute myocardial infarction and stroke: a self-controlled case series analysis of national linked datasets from Scotland. Eur Respir J. 2018; 51 : 1701794; doi:10.1183/13993003.01794-2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.