Abstract
Background
The 2-drug regimen dolutegravir (DTG) + lamivudine (3TC) is indicated for treatment-naive adults with human immunodeficiency virus type 1 (HIV-1). We present efficacy and safety of switching to DTG/3TC in virologically suppressed individuals.
Methods
TANGO is an open-label, multicenter, phase 3 study that randomized adults (1:1, stratified by baseline third agent class) with HIV-1 RNA <50 copies/mL to switch to once-daily fixed-dose DTG/3TC or remain on a tenofovir alafenamide (TAF)–based regimen. The primary end point was proportion of participants with HIV-1 RNA ≥50 copies/mL at week 48 (US Food and Drug Administration Snapshot algorithm) in the intention-to-treat–exposed population (4% noninferiority margin).
Results
743 adults were enrolled; 741 received ≥1 dose of study drug (DTG/3TC, N = 369; TAF-based regimen, N = 372). At week 48, proportion of participants with HIV-1 RNA ≥50 copies/mL receiving DTG/3TC was 0.3% (1/369) vs 0.5% (2/372) with a TAF-based regimen (adjusted treatment difference [95% confidence interval], −0.3 [−1.2 to .7]), meeting noninferiority criteria. No participants receiving DTG/3TC and 1 receiving a TAF-based regimen met confirmed virologic withdrawal criteria, with no emergent resistance at failure. Drug-related grade ≥2 adverse events and withdrawals due to adverse events occurred in 17 (4.6%) and 13 (3.5%) participants with DTG/3TC and 3 (0.8%) and 2 (0.5%) with a TAF-based regimen, respectively.
Conclusions
DTG/3TC was noninferior in maintaining virologic suppression vs a TAF-based regimen at week 48, with no virologic failure or emergent resistance reported with DTG/3TC, supporting it as a simplification strategy for virologically suppressed people with HIV-1.
Clinical Trials Registration
Keywords: 2-drug regimen, integrase strand transfer inhibitor, nucleoside reverse transcriptase inhibitor, simplification, virologic suppression
Switching to dolutegravir/lamivudine was noninferior in maintaining virologic suppression vs remaining on a tenofovir alafenamide–based regimen through 48 weeks, with no virologic failure or emergent resistance in the dolutegravir/lamivudine group, supporting its use in virologically suppressed individuals living with human immunodeficiency virus type 1.
The success of 3-drug regimens (3DRs) in people living with human immunodeficiency virus (HIV; PLWH) has decreased mortality and improved life expectancies near to those of the general population in the majority of treated patients [1]. Consequently, PLWH will likely remain on antiviral therapy (ART) for decades. Although current regimens tend to have fewer toxicities than older regimens, concerns remain over the cumulative effects of long-term ART [2, 3]. Two-drug regimens (2DRs) have been investigated as a means for reducing the number of antiretroviral agents (ARVs) taken by individuals who need lifelong ART [2].
Currently, there are two 2DRs that have received marketing authorization for treatment of individuals living with HIV-1. Dolutegravir (DTG) in combination with rilpivirine (RPV) is indicated as a complete regimen for virologically suppressed adults based on results from the SWORD-1 and SWORD-2 trials [4, 5]. The fixed-dose combination (FDC) of DTG/lamivudine (3TC) is indicated for treatment-naive and treatment-experienced adults with no known or suspected resistance to the individual components [6, 7]. This is based on results from the identically designed, phase 3 GEMINI-1 and GEMINI-2 trials, which demonstrated noninferiority of DTG + 3TC vs DTG + tenofovir disoproxil fumarate/emtricitabine (TDF/FTC) in treatment-naive PLWH [8].
Dolutegravir is a strong candidate for inclusion in 2DRs because of its high potency and barrier to resistance [2, 3, 9]. In the GEMINI trials, DTG + 3TC and DTG + TDF/FTC showed similar rapid declines in plasma viral load, regardless of baseline viral load, and no resistance was reported for up to 96 weeks [8, 10]. The efficacy and safety of switching to DTG + 3TC in adults suppressed on 3DRs have been demonstrated in smaller studies [11, 12]. Here, we present the week 48 primary end-point analysis of the phase 3 TANGO study in which the efficacy and safety of a switch to DTG/3TC FDC in adults living with HIV-1 with virologic suppression on a 3- or 4-drug tenofovir alafenamide (TAF)–based regimen was evaluated.
METHODS
Study Design and Participants
The TANGO trial is an ongoing, phase 3, randomized, open-label, noninferiority study evaluating the efficacy and safety of switching to DTG/3TC vs remaining on a TAF-based regimen in virologically suppressed adults living with HIV-1. A summarized version of the TANGO protocol can be found at [13]. The study was designed in accordance with the International Conference on Harmonisation Good Clinical Practice, following the principles of the Declaration of Helsinki, with protocol approvals and informed consent obtained before participant screening.
Adults living with living HIV-1 with virologic suppression (HIV-1 RNA <50 copies/mL) for >6 months and taking a stable, first-line 3- or 4-drug TAF-based regimen (TDF to TAF switch ≥3 months before screening was allowed) were eligible. Screening regimens included TAF/FTC plus a protease inhibitor (PI), integrase strand transfer inhibitor (INSTI), or nonnucleoside reverse transcriptase inhibitor (NNRTI). Participants who switched between the pharmacokinetic enhancers ritonavir and cobicistat were also eligible. Female participants were eligible if they were confirmed not to be pregnant or lactating and were using approved contraception.
Key exclusion criteria included a history of any major nucleoside reverse transcriptase inhibitor (NRTI) or INSTI resistance-associated mutations [14], Centers for Disease Control and Prevention stage 3 disease (except cutaneous Kaposi’s sarcoma not requiring systemic therapy and CD4+ cell count <200 cells/mm3), severe hepatic impairment (Child-Pugh class C), and hepatitis B virus infection. Participants with any plasma HIV-1 RNA measurement ≥50 copies/mL within 6 months of screening; ≥2 measurements ≥50 copies/mL or any measurement >200 copies/mL within 6 and 12 months of screening; or a prior regimen switch due to virologic failure (plasma HIV-1 RNA ≥ 400 copies/mL) were ineligible.
Procedures
Eligible participants were randomized 1:1 to switch to once-daily DTG 50 mg/3TC 300 mg FDC tablet or to remain on their current TAF-based regimen through 144 weeks of therapy. Randomization was stratified by baseline third agent class. No regimen modifications were allowed, except for switches between ritonavir and cobicistat or for multiple-component ART to the same multi-ingredient FDC.
Study visits were planned for baseline, every 4 weeks through week 12, and every 12 weeks thereafter. Plasma for HIV-1 RNA quantification was collected at each visit and upon study withdrawal and analyzed using the Abbott RealTime HIV-1 assay (lower detection limit of 40 copies/mL). Confirmed virologic withdrawal (CVW) was defined as HIV-1 RNA ≥50 copies/mL followed by a second consecutive HIV-1 RNA assessment ≥200 copies/mL. If CVW criteria were met, the participant was withdrawn from the study, and a plasma sample from the initial elevated viral load at virologic failure was tested for HIV-1 protease, reverse transcriptase, and integrase genotype and phenotype (Monogram Biosciences), with corresponding analyses of stored baseline whole blood samples.
Safety, including adverse events (AEs) and serious AEs (SAEs), was assessed at each study visit. Events were graded according to the Division of AIDS Table for Grading the Severity of Adult and Pediatric Adverse Events, version 2.1 [15]. All SAEs and AEs of special interest were followed until resolution, stabilization, the participant was lost to follow-up, or the event was otherwise explained. Clinical chemistry values, hematology values, and lymphocyte subsets were assessed at all study visits. Measurements of renal biomarkers, homeostasis model of assessment–insulin resistance (HOMA-IR; fasting serum glucose and insulin), fasting lipids, and urinalysis were conducted at baseline and weeks 24 and 48.
Outcomes
The primary, preplanned analysis of the study was to assess noninferior antiviral activity of switching to once-daily DTG/3TC FDC compared with continuation of a TAF-based regimen over 48 weeks. The primary end point was the proportion of participants with HIV-1 RNA ≥50 copies/mL (US Food and Drug Administration [FDA] Snapshot algorithm) at week 48 in the intention-to-treat–exposed (ITT-E) population. Secondary virologic end points included the proportion of participants with HIV-1 RNA <50 copies/mL at week 48 (FDA Snapshot; ITT-E population) and incidence of observed genotypic/phenotypic resistance in participants with CVW. Safety and tolerability were secondary objectives assessed through the incidence and severity of AEs and laboratory abnormalities, and discontinuations due to AEs. Change from baseline in CD4+ cell count, CD4+/CD8+ cell count ratio, renal biomarkers, fasting lipids, and health status using the EuroQol–5 Dimensions–5 Levels (EQ-5D-5L) utility score and visual analog scale (VAS) were additional secondary end points. Changes from baseline to week 48 in HOMA-IR, weight, and inflammation biomarkers were exploratory end points.
Statistical Analyses
Assuming a 2% virologic failure rate, a 4% noninferiority margin, and a 2.5% 1-sided significance level, 275 participants were required per treatment group to provide 92% power to demonstrate noninferiority. With an assumed screen failure rate of 30%, it was estimated that 800 PLWH would need to be screened to achieve target enrollment. Because of an unexpected surge in enrollment in the last week of screening, 919 PLWH were screened and 743 were randomized, resulting in 97.3% power for noninferiority.
Efficacy end points were evaluated based on the ITT-E population, which included all randomized participants who received ≥1 dose of study medication. Participants were assessed according to their randomized treatment, regardless of the treatment received. The per-protocol population excluded participants with protocol violations that had the potential to impact antiviral activity and was used for sensitivity analysis of week 48 virologic outcomes. The safety population included participants who received ≥1 dose of study medication, analyzed according to treatment received.
The primary analysis was the adjusted treatment difference (DTG/3TC − TAF-based regimen) in the proportion of participants with HIV-1 RNA ≥50 copies/mL, based on Cochran-Mantel-Haenszel stratified analysis, adjusting for baseline third agent class (PI, INSTI, or NNRTI). The noninferiority margin for the upper bound of a 2-sided 95% confidence interval (CI) was 4% for the primary end point and −8% for the key secondary end point of HIV-1 RNA <50 copies/mL. Incidence and severity of AEs were summarized descriptively. Changes in renal, lipid (post hoc analysis), and inflammatory biomarkers; weight; body mass index (BMI); and insulin resistance were estimated as mean change from baseline at week 48 (or geometric mean ratios of week 48 to baseline for log-transformed end points) in each group calculated using mixed model repeated measures, adjusting for all relevant covariates as detailed in the tables and figures. A post hoc logistic regression model was used to compare the proportion of patients with HOMA-IR ≥2 at week 48 between treatment groups, adjusting for baseline variables associated with HOMA-IR response (using a stepwise selection approach). An independent data monitoring committee was retained with reviews to be triggered by a predefined number of observed CVWs through week 24; none were triggered.
RESULTS
Study Participants
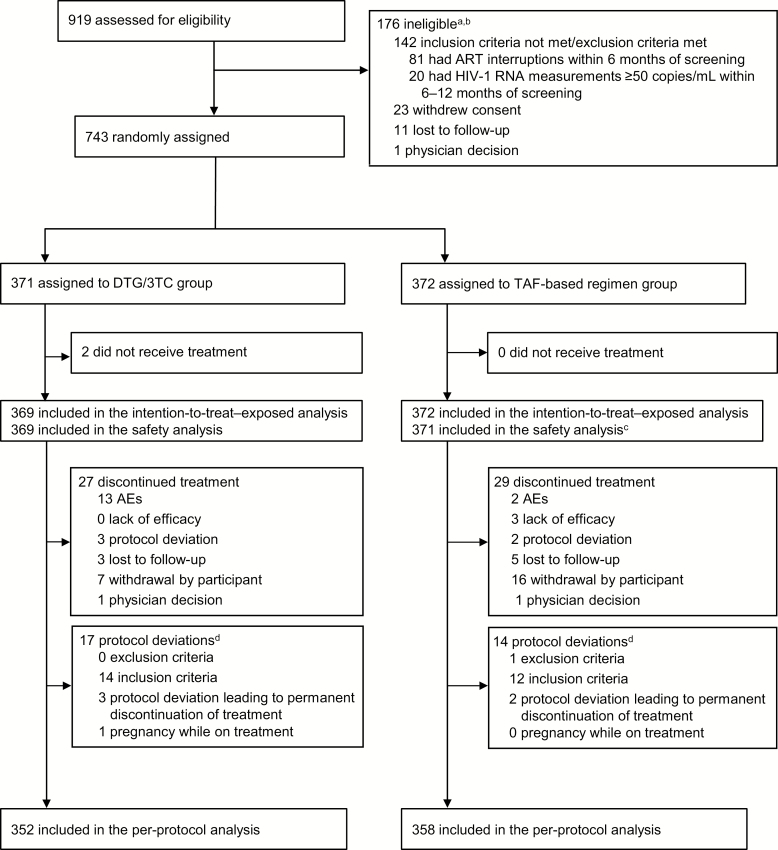
Between 18 January 2018 and 18 May 2018, 919 individuals were screened for eligibility, with 743 randomized to switch to DTG/3TC (N = 371) or remain on a TAF-based regimen (N = 372; Figure 1).
Figure 1.
Trial profile. aParticipants could have multiple reasons for ineligibility. bThe most common reasons for not meeting inclusion criteria or meeting exclusion criteria are listed. All other reasons occurred in <1% of participants. cOne participant in the TAF–based regimen group was found to be taking a tenofovir disoproxil fumarate–based regimen at baseline and therefore was excluded from the safety population. dProtocol deviations leading to exclusion from the per-protocol population; participants could have had more than 1 reason. Abbreviations: AE, adverse event; ART, antiretroviral therapy; DTG, dolutegravir; HIV-1, human immunodeficiency virus type 1; 3TC, lamivudine; TAF, tenofovir alafenamide.
In the ITT-E population, baseline and disease characteristics were well balanced between treatment groups (Table 1). Most participants were male (DTG/3TC, 93.2%; TAF-based regimen, 91.1%) and white (DTG/3TC, 80.5%; TAF-based regimen, 77.7%). The most common baseline third agent class was INSTI (DTG/3TC, 78.3%; TAF-based regimen, 79.6%), most frequently elvitegravir/cobicistat.
Table 1.
Baseline Demographics and Clinical Characteristics in the Intention-to-Treat–Exposed Population
| Demographic/Characteristic | Dolutegravir/ Lamivudine (N = 369) | Tenofovir Alafenamide– Based Regimen (N = 372) |
|---|---|---|
| Age, y | ||
| Median (range) | 40 (20–74) | 39 (18–73) |
| Age ≥50, n (%) | 79 (21.4) | 92 (24.7) |
| Sex, n (%) | ||
| Female | 25 (6.8) | 33 (8.9) |
| Male | 344 (93.2) | 339 (91.1) |
| Race, n (%) | ||
| African American/African heritage | 50 (13.6) | 58 (15.6) |
| Asian | 13 (3.5) | 13 (3.5) |
| White | 297 (80.5) | 289 (77.7) |
| Other | 9 (2.4) | 12 (3.2) |
| Ethnicity, n (%) | ||
| Hispanic or Latino | 69 (18.7) | 66 (17.7) |
| Not Hispanic or Latino | 300 (81.3) | 306 (82.3) |
| Median CD4+ cell count, cells/mm3 (range) | 682 (133–1904) | 720 (119–1810) |
| CD4+ cell count, cells/mm3, n (%) | ||
| <500 | 98 (26.6) | 74 (19.9) |
| ≥500 | 271 (73.4) | 298 (80.1) |
| Baseline third agent class | ||
| Integrase strand transfer inhibitor | 289 (78.3) | 296 (79.6) |
| Elvitegravir/cobicistat | 243 (65.9) | 249 (66.9) |
| Nonnucleoside reverse transcriptase inhibitor | 51 (13.8) | 48 (12.9) |
| Rilpivirine | 43 (11.7) | 45 (12.1) |
| Protease inhibitor | 29 (7.9) | 28 (7.5) |
| Boosted darunavir | 25 (6.8) | 27 (7.3) |
| Duration of antiretroviral therapy before day 1, median (range), months | 33.8 (7.1–201.2) | 35.1 (7.0–160.8) |
| Duration of tenofovir alafenamide–based regimen before day 1, median (range), months | 17.7 (3.6–73.7) | 18.2 (3.9–71.2) |
Efficacy
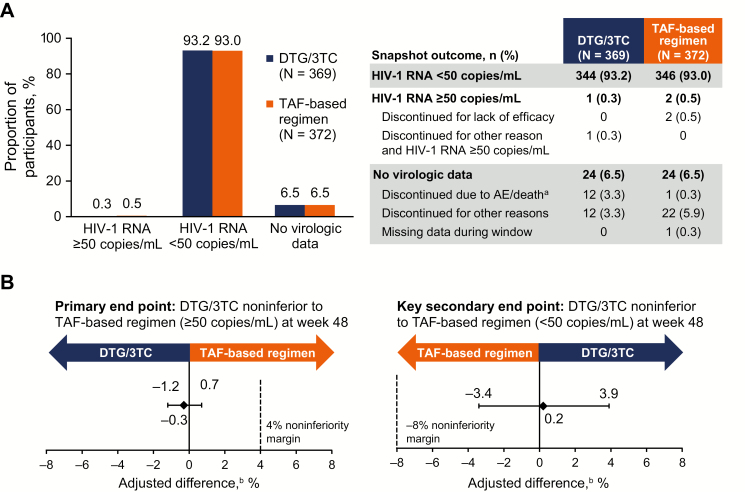
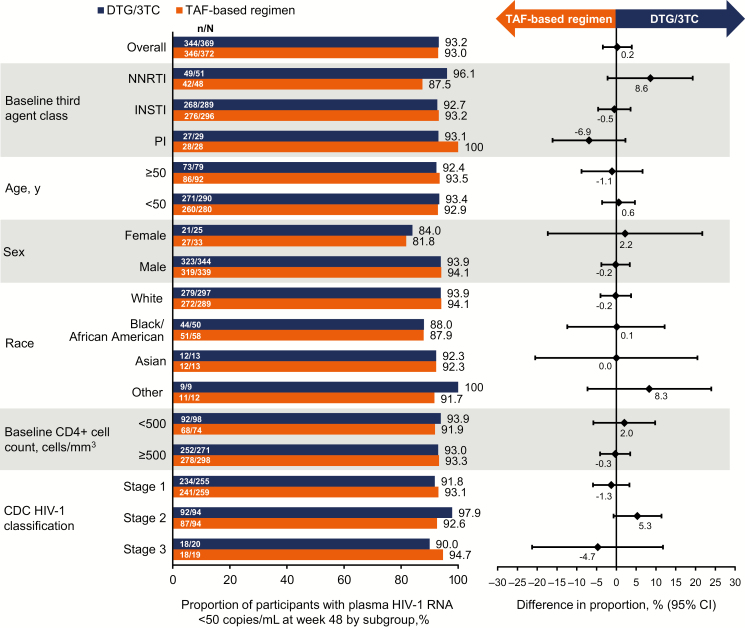
At week 48, 1 participant in the DTG/3TC group (0.3%) and 2 participants in the TAF-based regimen group (0.5%) had HIV-1 RNA ≥50 copies/mL. This demonstrated noninferiority of DTG/3TC compared with a TAF-based regimen, with an adjusted treatment difference (95% CI) of −.3 (−1.2 to .7; Figure 2). In the per-protocol population, 0 of 352 participants in the DTG/3TC group and 2 of 358 (0.6%) in the TAF-based regimen group had HIV-1 RNA ≥50 copies/mL at week 48 (adjusted treatment difference [95% CI], −.6 [−1.3 to .2]). Results across subgroups were generally consistent with those of the overall population (Figure 3).
Figure 2.
A, Virologic outcomes at week 48 in the intention-to-treat–exposed population by US Food and Drug Administration Snapshot algorithm. B, Adjusted treatment differences. aOne fatal adverse event unrelated to study treatment occurred (homicide). bBased on Cochran-Mantel-Haenszel stratified analysis adjusting for baseline third agent class. Abbreviations: 3TC, lamivudine; AE, adverse event; DTG, dolutegravir; HIV-1, human immunodeficiency virus type 1; TAF, tenofovir alafenamide.
Figure 3.
Proportion of participants with HIV-1 RNA <50 copies/mL at week 48 by subgroup in the intention-to-treat–exposed study population (US Food and Drug Administration Snapshot algorithm). Abbreviations: 3TC, lamivudine; CDC, Centers for Disease Control and Prevention; CI, confidence interval; DTG, dolutegravir; HIV-1, human immunodeficiency virus type 1; INSTI, integrase strand transfer inhibitor; NNRTI, nonnucleoside reverse transcriptase inhibitor; PI, protease inhibitor; TAF, tenofovir alafenamide.
There were 344 participants in the DTG/3TC group (93.2%) and 346 in the TAF-based regimen group (93.0%) with HIV-1 RNA <50 copies/mL at week 48 using Snapshot criteria (adjusted treatment difference [95% CI], .2 [−3.4 to 3.9]), demonstrating noninferiority.
Median CD4+ cell counts increased from 682.0 cells/mm3 at baseline by 22.5 cells/mm3 in the DTG/3TC group and from 720.0 cells/mm3 at baseline by 11.0 cells/mm3 in the TAF-based regimen group at week 48. At baseline, the median CD4+/CD8+ cell count ratio was 0.95 for the DTG/3TC group and 0.96 for the TAF-based regimen group, with median changes at week 48 of 0.03 and 0.05, respectively.
Zero participants in the DTG/3TC group and 1 in the TAF-based regimen group met CVW criteria. No resistance mutations were observed at virologic failure.
In a post hoc analysis, proviral DNA genotyping was conducted retrospectively on baseline whole blood samples (Monogram Biosciences using GenoSure Archive). Preexisting archived M184V/I (all detected as mixtures with wild-type) was observed in 4/322 participants in the DTG/3TC group and 3/321 in the TAF-based regimen group. All 7 participants maintained HIV-1 RNA <50 copies/mL at all on-treatment time points through week 48.
Safety
Up to the week 48 analysis cutoff date, ≥1 AE was reported for 79.9% (n = 295) of participants after switching to DTG/3TC compared with 78.7% (n = 292) who remained on a TAF-based regimen (Table 2). The most common AEs overall were nasopharyngitis, upper respiratory tract infection, and diarrhea. Incidences of specific AEs were mostly similar between treatment groups. There was a higher proportion of participants who withdrew because of AEs in the DTG/3TC group (n = 13 [3.5%] vs n = 2 [0.5%]), including 1 fatal AE unrelated to study treatment (homicide). In the DTG/3TC group, AEs that led to withdrawal in ≥2 participants were anxiety (n = 3; 0.8%), insomnia (n = 3; 0.8%), weight increase (n = 2; 0.5%), and fatigue (n = 2; 0.5%). No drug-related SAEs were reported in either treatment group.
Table 2.
Summary of Adverse Events in the Safety Population
| AE | Dolutegravir/Lamivudine (N = 369), n (%) | Tenofovir Alafenamide –Based Regimen (N = 371), n (%) |
|---|---|---|
| Any AE | 295 (79.9) | 292 (78.7) |
| AEs occurring in ≥5% of participants in either group | ||
| Nasopharyngitis | 43 (11.7) | 41 (11.1) |
| Upper respiratory tract infection | 31 (8.4) | 32 (8.6) |
| Diarrhea | 30 (8.1) | 26 (7.0) |
| Headache | 24 (6.5) | 17 (4.6) |
| Syphilis | 24 (6.5) | 13 (3.5) |
| Back pain | 21 (5.7) | 28 (7.5) |
| Fatigue | 20 (5.4) | 3 (0.8) |
| Bronchitis | 8 (2.2) | 20 (5.4) |
| Drug-related AEsa | 45 (12.2) | 5 (1.3) |
| Drug-related grade 2–5 AEs | 17 (4.6) | 3 (0.8) |
| Drug-related grade 2–5 AEs occurring in ≥0.5% of participants in either group | ||
| Insomnia | 4 (1.1) | 0 |
| Constipation | 2 (0.5) | 1 (0.3) |
| Flatulence | 2 (0.5) | 0 |
| Headache | 2 (0.5) | 0 |
| AEs leading to withdrawal from the studyb | 13 (3.5)c | 2 (0.5) |
| Anxiety | 3 (0.8) | 0 |
| Insomnia | 3 (0.8) | 0 |
| Weight increased | 2 (0.5) | 1 (0.3) |
| Fatigue | 2 (0.5) | 0 |
| Any serious AEsc | 21 (5.7)c | 16 (4.3) |
Abbreviation: AE, adverse event.
aAll drug-related AEs were grade 2 or less.
bParticipants may have had more than 1 AE leading to withdrawal. AEs of interest are listed. Other AEs that led to withdrawal from the study in the dolutegravir/lamivudine group were abdominal discomfort, gastroesophageal reflux disease, hypoesthesia oral, nausea, paraesthesia oral, drug hypersensitivity, gunshot wound (not treatment related), diffuse large B-cell lymphoma (not treatment related), lung adenocarcinoma (not treatment related), disturbance in attention, hypoesthesia, paraesthesia, irritability, suicidal ideation (not treatment related), genital hypoesthesia, genital paraesthesia, and pruritus. Those in the tenofovir alafenamide–based regimen group were depression and suicide attempt (not treatment related).
cNo serious AEs were drug related. One fatal AE occurred (homicide).
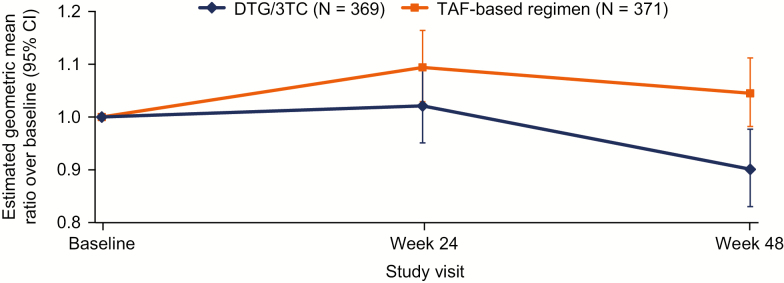
An AE of increased weight was reported for 3 (0.8%) participants in the DTG/3TC group and 6 (1.6%) in the TAF-based regimen group (regimens included cobicistat-boosted elvitegravir [n = 2], RPV [n = 2], DTG [n = 1], and raltegravir [n = 1]). All increased weight AEs were grade 1/2. The adjusted mean weight increase from baseline to week 48 was 0.8 kg in both groups (P = .863), and the adjusted mean increase in BMI was 0.25 kg/m2 in the DTG/3TC group and 0.26 kg/m2 in the TAF-based regimen group (P = .932). There was a mean 9.7% decrease in adjusted geometric mean HOMA-IR in those who switched to DTG/3TC (baseline median HOMA-IR, 2.80) and a mean 4.5% increase in those who remained on a TAF-based regimen (baseline median HOMA-IR, 2.60; Figure 4), with a statistically significant difference between groups (0.864; P = .001). At baseline, 69% and 68% of participants in the DTG/3TC and TAF-based regimen groups, respectively, had HOMA-IR ≥2. At week 48, 65% of participants in the DTG/3TC group and 74% in the TAF-based regimen group had insulin resistance defined as HOMA-IR ≥2 (odds ratio, 0.59; 95% CI, .40 to .87; P = .008; Supplementary Table 1).
Figure 4.
Change from baseline in homeostasis model assessment–insulin resistance (HOMA-IR) in the safety population. Geometric mean ratio and 95% confidence interval for postbaseline values based on a loge transformation. Change from baseline was calculated using a repeated measures model adjusting for treatment, visit, baseline third agent class, CD4+ cell count (continuous), age (continuous), sex, race, body mass index (continuous), presence of hypertension, loge-transformed baseline HOMA-IR, treatment-by-visit interaction, and baseline value-by-visit interaction, with visit as the repeated factor. Abbreviations: 3TC, lamivudine; CI, confidence interval; DTG, dolutegravir; TAF, tenofovir alafenamide.
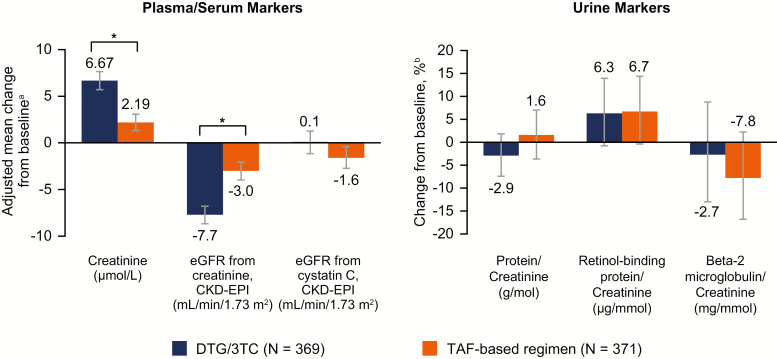
Effects on inflammation biomarkers were small and inconclusive, with significant differences observed in differing directions for DTG/3TC and TAF-based regimens (Table 3). Changes in renal biomarkers were minimal and similar between treatment groups (Figure 5).
Table 3.
Change From Baseline to Week 48 in Inflammation Biomarkers
| Parameter | Treatment | n/N | Baseline Geometric Mean (95% CI)a | Visit to Baseline Ratio (95% CI)b | Treatment Ratio (95% CI)c | P Valued |
|---|---|---|---|---|---|---|
| Blood D-dimer, nmol/L FEU | DTG/3TC | 334/369 | 1.69 (1.59, 1.79) | 0.968 (.920, 1.019) | 0.973 (.907, 1.044) | 0.440 |
| TAF-based regimen | 334/371 | 1.66 (1.58, 1.76) | 0.995 (.948, 1.044) | |||
| Serum hs-CRP, mg/L | DTG/3TC | 342/369 | 1.37 (1.23, 1.53) | 1.012 (.911, 1.124) | 0.934 (.811, 1.075) | 0.341 |
| TAF-based regimen | 342/371 | 1.30 (1.16, 1.46) | 1.083 (.986, 1.190) | |||
| Serum IL-6, ng/L | DTG/3TC | 343/369 | 1.64 (1.52, 1.78) | 0.990 (.909, 1.078) | 1.163 (1.045, 1.293) | 0.006 |
| TAF-based regimen | 340/371 | 1.67 (1.54, 1.80) | 0.852 (.800, .907) | |||
| Serum sCD14, ng/L | DTG/3TC | 343/369 | 1606.5 (1573.1, 1640.6) | 0.953 (.933, .973) | 0.971 (.942, 1.000) | 0.048 |
| TAF-based regimen | 343/371 | 1578.6 (1546.4, 1611.4) | 0.982 (.962, 1.002) | |||
| Serum sCD163, µg/L | DTG/3TC | 342/369 | 660.9 (630.5, 692.7) | 0.916 (.889, .943) | 1.013 (.974, 1.054) | 0.508 |
| TAF-based regimen | 342/371 | 642.0 (615.3, 670.0) | 0.904 (.881, .927) |
Abbreviations: 3TC, lamivudine; DTG, dolutegravir; FEU, fibrinogen equivalent unit; hs-CRP, high-sensitivity C-reactive protein; IL-6, interleukin-6; MMRM, mixed model repeated measures; sCD14, soluble CD14; sCD163, soluble CD163; TAF, tenofovir alafenamide.
aGeometric mean is calculated by exponentiating the mean of loge-transformed baselines values.
bRatio is the estimated adjusted ratio (Week 48 to baseline) in each group calculated using MMRM applied to change from baseline in loge-transformed data adjusting for the following: treatment, visit, baseline third agent class, CD4+ cell count (continuous), age (continuous), sex, race, body mass index (continuous), smoking status, hepatitis C virus coinfection status, loge-transformed baseline biomarker (continuous), treatment-by-visit interaction, and baseline value-by-visit interaction, with visit as the repeated factor.
cTreatment ratio is DTG/3TC to TAF-based regimen.
d P value for treatment comparison.
Figure 5.
Change from baseline at week 48 in renal biomarkers. aEstimated mean change from baseline at week 48 in each group calculated from mixed model repeated measures adjusting for treatment, visit, baseline third agent class, CD4+ cell count (continuous), age (continuous), sex, race, body mass index (continuous), presence of diabetes mellitus, presence of hypertension, baseline biomarker (continuous), treatment-by-visit interaction, and baseline value-by-visit interaction, with visit as the repeated factor. bBased on estimated geometric means ratio of week 48 vs baseline. Based on the same model as plasma/serum markers except adjusting for loge-transformed baseline biomarker (continuous). *P < .001. Abbreviations: 3TC, lamivudine; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration equation; DTG, dolutegravir; eGFR, estimated glomerular filtration rate; TAF, tenofovir alafenamide.
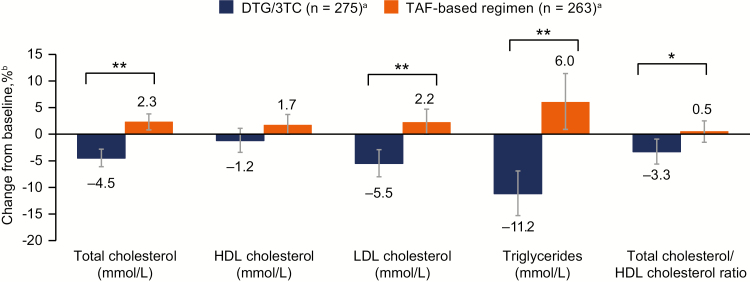
Mean baseline values were similar in the DTG/3TC and TAF-based regimen groups for total cholesterol (5.0 mmol/L and 4.9 mmol/L, respectively), high-density lipoprotein (HDL) cholesterol (1.4 mmol/L and 1.4 mmol/L, respectively), low-density lipoprotein (LDL) cholesterol (2.9 mmol/L and 2.9 mmol/L, respectively), triglycerides (1.6 mmol/L and 1.5 mmol/L, respectively), and total cholesterol to HDL cholesterol ratio (3.9 and 3.9, respectively). Greater decreases from baseline were observed with DTG/3TC vs TAF-based regimen for percentage changes in total cholesterol, LDL cholesterol, and triglycerides (all P < .001) and total cholesterol to HDL cholesterol ratio (P = .017; Figure 6). HDL cholesterol decreased from baseline in the DTG/3TC group and increased from baseline in the TAF-based regimen group; however, the difference was not statistically significant (P = .059).
Figure 6.
Change from baseline in serum or plasma lipids at week 48. an = number of participants with nonmissing fasting lipid data at baseline and week 48, removing those with lipid-modifying agent administered at baseline (lipid data collected after initiation of a lipid-modifying agent were censored and a last observation carried forward method was applied so that the last available fasted, on-treatment lipid value before initiation of a lipid-modifying agent was used). bPercent change from baseline based on adjusted ratio (week 48 to baseline) in each group calculated from a repeated measures model applied to change from baseline in loge-transformed data adjusting for the following: treatment, visit, baseline third agent class, CD4+ cell count (continuous), loge-transformed baseline value (continuous), treatment-by-visit interaction, and baseline value-by-visit interaction, with visit as the repeated factor. *P = .017. **P < .001. Abbreviations: 3TC, lamivudine; DTG, dolutegravir; HDL, high-density lipoprotein; LDL, low-density lipoprotein; TAF, tenofovir alafenamide.
No significant differences between the DTG/3TC and TAF-based regimen groups were observed in mean change from baseline in the EQ-5D-5L health state utility scale or VAS (Supplementary Table 2).
DISCUSSION
The TANGO study represents the largest trial to evaluate switching patients who are virologically suppressed on a 3- or 4-drug regimen to a 2DR of DTG/3TC. This study showed that switching to DTG/3TC was noninferior to remaining on a TAF-based regimen, whether this is assessed through Snapshot virologic failure or virologic success at week 48. Importantly, no participants who switched to DTG/3TC met CVW criteria or developed resistance through 48 weeks.
These results confirm observations from earlier smaller studies that showed the effective maintenance of viral suppression after a switch to DTG + 3TC in individuals on a first-line ART regimen that contained 3 or more ARVs [11, 12]. In the single-arm ANRS 167 LAMIDOL trial (n = 104), 97% maintained virologic suppression at 48 weeks after switching to DTG + 3TC [11]. In the randomized ASPIRE trial that compared a switch to DTG + 3TC with continuation of current 3DR ART in participants with virologic suppression (N = 89), 91% and 89% of participants, respectively, maintained HIV-1 RNA <50 copies/mL at week 48 [12]. Among the 2 participants on DTG + 3TC in the ANRS 167 LAMIDOL and ASPIRE trials (1 per trial) with virologic failure, no major resistance mutations were reported [11, 12].
Proviral DNA genotyping in the TANGO study detected archived M184V/I at baseline in approximately 1% of participants, as would be expected from the study population [16]. Although the numbers are small, it is reassuring that all participants who harbored baseline provirus with M184V/I maintained plasma HIV-1 RNA <50 copies/mL at all on-treatment study visits through week 48.
The safety profile of DTG/3TC in this study was consistent with the safety profile of DTG + 3TC in ART-naive patients [8]. Although the overall rate of AEs was similar between treatment groups, there were higher proportions of participants with drug-related AEs and AEs that led to discontinuation in the DTG/3TC switch group compared with those who remained on a stable TAF-based regimen. This difference is expected because the TAF-based regimen group tolerated their current regimen for an extended period (median time of 18.2 months). The vast majority (approximately 90%) of participants in the switch group were exposed to 2 new ARVs at switch. This observation is consistent with those from other switch trials [5, 17, 18].
Shifts in lipids at week 48 were broadly favorable after switching to DTG/3TC. In addition, insulin resistance, as measured by HOMA-IR, improved significantly after switching to DTG/3TC. Results from analyses of bone (data not shown), renal, and inflammation biomarkers were inconclusive. Longer-term data from TANGO are planned to determine the significance of early shifts in these parameters.
This study enrolled a predominantly white male population, which is not representative of the global population of PLWH and limits the generalizability of the results. Similarly, the number of participants living with advanced HIV was low but expected with a cohort of virologically suppressed PLWH with no evidence of prior treatment failure. Another limitation of the study is the open-label design, which could potentially introduce bias from physicians or participants. However, the open-label design was necessary because of a number of different dosing requirements for the various TAF-based regimens (logistical barrier to blinding). A protocol amendment that delayed the timing of the switch from TAF-based regimens to DTG/3TC from 48 to 144 weeks led to 4 participants voluntarily withdrawing from the study in the TAF-based regimen group (none withdrew in the DTG/3TC group). However, the results of a preplanned “withdrawal bias” sensitivity analysis to censor participants who did not provide an on-treatment week 48 HIV-1 RNA viral load (n = 2) confirmed the noninferiority results (data not shown). Last, in this analysis, we are currently unable to determine the risk of virologic failure and resistance emergence beyond 1 year. Data from the GEMINI trial showed that, through week 96, there was no treatment-emergent resistance in ART-naive participants who received DTG + 3TC [10]. The TANGO study is ongoing with planned analyses after 96 and 144 weeks to further assess the durability of DTG/3TC.
In conclusion, the primary results from this large, randomized, phase 3, multicenter study demonstrate that switching to DTG/3TC FDC was noninferior to remaining on a TAF-based regimen through week 48 in virologically suppressed adults with no prior history of virologic failure or known major resistance mutations to NRTIs or INSTIs. These findings support the use of DTG/3TC as a switch option for PLWH with viral suppression on a 3- or 4-drug regimen who wish to receive fewer ARTs, including due to treatment complexity, avoidance of potential drug-associated toxicity, risk of drug–drug interactions, or cost. On the basis of the results from the GEMINI and TANGO studies, the 2DR of DTG/3TC FDC is now a recommended preferred regimen in the US Department of Health and Human Services and European AIDS Clinical Society guidelines for ART-naive and ART-experienced virologically suppressed PLWH [19, 20].
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank the study participants and their families and caregivers; the investigators and site staff who participated in the study; and the ViiV Healthcare, GlaxoSmithKline (GSK), Pharmaceutical Product Development, and Phastar study team members. Editorial assistance was provided under the direction of the authors by Jonathan Morgan, PhD, CMPP, and Jennifer Rossi, MA, ELS, MedThink SciCom, and was funded by ViiV Healthcare.
Financial support. This work was supported by ViiV Healthcare.
Potential conflicts of interest. J. v W., M. A.-K., M. C. N., K. A. P., R. W., A. R. T., B. W., M. A., M. J. G., and K. Y. S. are employees of ViiV Healthcare and own stock in GSK. F. B. has received honoraria from Gilead and ViiV Healthcare and travel grants from Gilead to attend scientific meetings. S. D. W. has received grants from ViiV Healthcare, Gilead, Merck Sharpe & Dohme (MSD), and Janssen. J. P. S. has received grants and nonfinancial support from ViiV Healthcare, Gilead, and Janssen and has received personal fees from ViiV Healthcare, Gilead, Janssen, MSD, and AbbVie. J.-P. R. has received grants from ViiV Healthcare. C. W. has received personal fees from AbbVie, Bristol-Myers Squibb, Gilead, Hexal, Janssen, and MSD for speaking at educational events or participating in advisory boards. J. W. is an employee of GSK and owns stock in GSK. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Trickey A, May MT, Vehreschild J-J, et al. Survival of HIV-positive patients starting antiretroviral therapy between 1996 and 2013: a collaborative analysis of cohort studies. Lancet HIV 2017; 4: e349–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Back D. 2-Drug regimens in HIV treatment: pharmacological considerations. Germs 2017; 7:113–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kelly SG, Nyaku AN, Taiwo BO. Two-drug treatment approaches in HIV: finally getting somewhere? Drugs 2016; 76:523–31. [DOI] [PubMed] [Google Scholar]
- 4. Juluca [package insert]. Research Triangle Park, NC: ViiV Healthcare; 2019. [Google Scholar]
- 5. Llibre JM, Hung CC, Brinson C, et al. Efficacy, safety, and tolerability of dolutegravir-rilpivirine for the maintenance of virological suppression in adults with HIV-1: phase 3, randomised, non-inferiority SWORD-1 and SWORD-2 studies. Lancet 2018; 391:839–49. [DOI] [PubMed] [Google Scholar]
- 6. Dovato [package insert]. Research Triangle Park, NC: ViiV Healthcare; 2019. [Google Scholar]
- 7. Dovato [summary of product characteristics]. Uxbridge, UK: ViiV Healthcare; 2019. [Google Scholar]
- 8. Cahn P, Madero JS, Arribas JR, et al. ; GEMINI Study Team Dolutegravir plus lamivudine versus dolutegravir plus tenofovir disoproxil fumarate and emtricitabine in antiretroviral-naive adults with HIV-1 infection (GEMINI-1 and GEMINI-2): week 48 results from two multicentre, double-blind, randomised, non-inferiority, phase 3 trials. Lancet 2019; 393:143–55. [DOI] [PubMed] [Google Scholar]
- 9. Brenner BG, Wainberg MA. Clinical benefit of dolutegravir in HIV-1 management related to the high genetic barrier to drug resistance. Virus Res 2017; 239:1–9. [DOI] [PubMed] [Google Scholar]
- 10. Cahn P, Madero JS, Arribas JR, et al. Durable efficacy of dolutegravir plus lamivudine in antiretroviral treatment-naive adults with HIV-1 infection: 96-week results from the GEMINI-1 and GEMINI-2 randomized clinical trials. J Acquir Immune Defic Syndr 2019. [Epub ahead of print]. doi: 10.1097/QAI.0000000000002275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Joly V, Burdet C, Landman R, et al. Dolutegravir and lamivudine maintenance therapy in HIV-1 virologically suppressed patients: results of the ANRS 167 trial (LAMIDOL). J Antimicrob Chemother 2018; 74: 739–745. [DOI] [PubMed] [Google Scholar]
- 12. Taiwo BO, Marconi VC, Berzins B, et al. Dolutegravir plus lamivudine maintains human immunodeficiency virus-1 suppression through week 48 in a pilot randomized trial. Clin Infect Dis 2018; 66:1794–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. ViiV Healthcare. ViiV study register. 204862-Switch study to evaluate dolutegravir plus lamivudine in virologically suppressed human immunodeficiency virus type 1 positive adults (TANGO). Available at: https://www.viiv-studyregister.com/study/19609. Accessed 24 January 2020.
- 14. Wensing AM, Calvez V, Günthard HF, et al. 2017 update of the drug resistance mutations in HIV-1. Top Antivir Med 2017; 24: 132–3. [PMC free article] [PubMed] [Google Scholar]
- 15. Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health, US Department of Health and Human Services. Division of AIDS (DAIDS) table for grading the severity of adult and pediatric adverse events, corrected version 2.1 Available at: https://rsc.niaid.nih.gov/sites/default/files/daidsgradingcorrectedv21.pdf. Accessed 14 August 2019.
- 16. Vannappagari V, Ragone L, Henegar C, et al. Prevalence of pretreatment and acquired HIV-1 mutations associated with resistance to lamivudine or rilpivirine: a systematic review. Antivir Ther 2019; 24:393–404. [DOI] [PubMed] [Google Scholar]
- 17. Trottier B, Lake JE, Logue K, et al. Dolutegravir/abacavir/lamivudine versus current ART in virally suppressed patients (STRIIVING): a 48-week, randomized, non-inferiority, open-label, phase IIIb study. Antivir Ther 2017; 22:295–305. [DOI] [PubMed] [Google Scholar]
- 18. Palella FJ Jr, Fisher M, Tebas P, et al. Simplification to rilpivirine/emtricitabine/tenofovir disoproxil fumarate from ritonavir-boosted protease inhibitor antiretroviral therapy in a randomized trial of HIV-1 RNA-suppressed participants. AIDS 2014; 28:335–44. [DOI] [PubMed] [Google Scholar]
- 19. US Department of Health and Human Services. AIDSinfo. Guidelines for the use of antiretroviral agents in adults and adolescents with HIV Updated December 18, 2019. Available at: https://aidsinfo.nih.gov/guidelines/html/1/adult-and-adolescent-arv/0. Accessed 22 December 2019.
- 20. European AIDS Clinical Society. European AIDS Clinical Society Guidelines Version 10.0 Updated November 2019. Available at: https://www.eacsociety.org/files/2019_guidelines-10.0_final.pdf. Accessed 22 December 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.