INTRODUCTION
In the pediatric population, hemorrhagic symptoms such as bruising and epistaxis are among the key clinical features that suggest the presence of a hereditary bleeding disorder. Nevertheless, making an accurate assessment of bleeding symptom severity can be more challenging in pediatric patients compared to their adult counterparts. Easy bruising and epistaxis are reported in a high percentage (24-39%) of healthy children as well as those with bleeding disorders, making it difficult to distinguish between pathologic and normal bleeding.1 Furthermore, young children generally have had fewer exposures to events such as surgery, menarche, and childbirth which would provide opportunities for symptoms to manifest. Thus, there is a need for a sensitive diagnostic tool that will facilitate the standardized evaluation of hemorrhagic symptoms in children.
Recently, a number of bleeding assessment tools (BATs) have been developed to allow the conversion of a broad range of bleeding symptoms into one total, quantitative bleeding score (BS). The Pediatric Bleeding Questionnaire (PBQ) was generated and validated in 2009 by Bowman et al. as a screening tool for von Willebrand Disease (VWD).2 Bleeding categories, including epistaxis, cutaneous bleeding, and post-operative bleeding, are assigned a score based on the type and severity of episodes, and the total BS is used to assess whether an individual has abnormal bleeding. When administered by experts to children presenting with bleeding symptoms, the PBQ had a sensitivity of 83% and specificity of 79% for VWD, as well as a negative predictive value of 0.99.2 The International Society on Thrombosis and Hemostasis Bleeding Assessment Tool (ISTH-BAT), quite similar to the PBQ, was also recently developed and validated for reporting bleeding symptoms in both adult and pediatric populations.3
A disadvantage of the PBQ, ISTH-BAT, and previous BATs is that they all must be expert-administered. This requirement can be a barrier for use in a busy clinic with limited healthcare personnel and resources.4 To address this, Deforest et al. modified the ISTH-BAT to generate a self-administered bleeding assessment tool (Self-BAT) that can be completed by patients without assistance.5
Although the Self-BAT was validated for adult patients only, Casey et al. subsequently undertook the validation of a self-administered bleeding questionnaire for use in a pediatric population. The self-administered PBQ (Self-PBQ) was generated by combining the expert-administered PBQ and the ISTH-BAT, then translating the questionnaire into terms that can be understood by children and their parents.6 A prospective multicentre study was carried out to validate the Self-PBQ as a screening tool for VWD in children presenting to the hematology clinic for investigation of a bleeding disorder. The Self-PBQ demonstrated a sensitivity of 78%, a specificity of 37%, a positive predictive value of 0.18, and a negative predictive value of 0.91 for VWD. Furthermore, the Self-PBQ had a high level of agreement with the expert-administered PBQ, with an intraclass correlation (ICC) of 0.917.6 However, only children above the age of 12 completed the Self-PBQ by themselves while those under 12 had a parent fill out the questionnaire on their behalf.
There is abundant literature indicating that children as young as 7 years old are able to self-complete patient-reported outcome questionnaires with sufficient validity and reliability.7,8 The purpose of this study was to adapt Casey et al.’s Self-PBQ into a version that can be completed by younger patients aged 8-12 years with minimal adult assistance. Our objectives were to determine whether a modified School Age Self-PBQ can be completed effectively by 8-12 year old children, and to evaluate its validity as a surrogate for the expert-administered version.
METHODS
The present study is sub-study of Casey et al.’s multicenter study, conducted in two phases. The first phase, which was limited to Children’s Hospital of Eastern Ontario (CHEO), involved cognitive debriefing of the original Self-PBQ with 8-12 year-olds diagnosed with a bleeding disorder followed by optimization of the tool to make a School Age Self-PBQ. The second phase involved prospective validation of the School Age Self-PBQ in a new group of children with hereditary bleeding disorders. This phase was undertaken at 5 participating treatment centres across Canada: CHEO, Ottawa; Queen’s University, Kingston; The Hospital for Sick Children, Toronto; Alberta Children’s Hospital, Calgary; and British Columbia Children’s Hospital, Vancouver. Each institution obtained approval from their respective research ethics boards prior to the start of the study. Informed consent or assent was obtained from all participating children and parents/caregivers.
Phase 1: Cognitive debriefing and generation of the School Age Self-PBQ
Cognitive debriefing of the Self-PBQ was undertaken in a cohort of children below 12 years of age. Two populations were recruited from CHEO: 1) children with a confirmed diagnosis of type 1 VWD and 2) normal children without bleeding disorders. Patients with type 1 VWD were defined as those with a VWF antigen (VWF:Ag) and VWF ristocetin cofactor/activity (VWF:RCo/Act) between 0.05 and 0.50 U/mL on two occasions, a RCo:Ag/Act ratio >0.60, and normal VWF multimers, based on laboratory criteria from previous studies.2,6 Normal controls were either unaffected siblings of the patients with normal VWF levels or children with unrelated disorders.
Both the affected and control groups completed the original Self-PBQ. Following completion of the Self-PBQ, subjects underwent cognitive debriefing using the method described by Jobe et al. to identify any problems in the questionnaire; responses were recorded and analyzed.9 Based on respondent feedback, questions were rephrased and item reduction was performed to simplify the questionnaire to be understandable for younger children. The cognitive debriefing process was then repeated with new affected and control subjects for the revised Self-PBQ until saturation occurred. This process generated a version optimized for school age children.
Phase 2: Prospective validation of the School Age Self-PBQ
The modified School Age Self-PBQ was prospectively administered at all 5 participating institutions to determine if pre-teen children could effectively fill out the questionnaire with minimal assistance. Eligibility criteria included all pediatric patients aged 8-12 years with a known diagnosis of an inherited bleeding disorder, including VWD, coagulation factor deficiencies, and platelet disorders. Basic demographic information was extracted from the medical records of all subjects, including diagnosis, month and year of birth, language spoken at home, grade in school, and gender. Hemostatic test results were also obtained, including ABO blood group and tests for VWD (VWF:Ag, VWF:RCo, and factor VIII:C).
Children completed as much of the School Age Self-PBQ without assistance to the best of their ability and only received help from their parents if necessary. The length of time and the amount of help that each child required to complete the questionnaire was recorded. Parents filled out the questionnaire independently to serve as a comparison. The School Age Self-PBQ was scored using the ISTH-BAT scoring key (Supplementary Figure 1), where scores range from 0 (no bleeding) to +4 (severe bleeding requiring medical intervention) and a BS of ≥3 is the cut-off for abnormal bleeding in children below 18 years of age.10
BSs derived from the child-completed Self-PBQ were then compared with those of the parent proxy. The parent-completed Self-PBQ was chosen as a comparison for the child self-report because Casey et al. have already shown that the parent report is a valid and reliable surrogate for the expert-administered PBQ.6 A single measure ICC based on absolute agreement was used to determine the degree of agreement between the child and parent reports. An ICC ≥ 0.70 was chosen as the minimum standard for reliability based on existing quality criteria for health-related questionnaires.11 Agreement was classified based on Landis and Koch’s guidelines, with an ICC of 0.0 to 0.2 indicating slight agreement, 0.21 to 0.40 indicating fair agreement, 0.41 to 0.60 indicating moderate agreement, 0.61 to 0.80 indicating substantial agreement, and 0.81 to 1.0 indicating almost perfect agreement.12
Two subgroup analyses established a priori were performed to examine whether the agreement differed in younger age groups (8-9 years) versus older age groups (10-12 years), and whether it differed based on the severity of the disease. For the severity analysis, type 1 VWD was considered a less severe bleeding phenotype than other inherited bleeding disorders such as Glanzmann thrombasthenia and other VWD types.13 In addition, due to the fact that many of the subjects required moderate to significant assistance from their parents, a third subgroup analysis was performed to assess the effect of parental assistance on agreement. For this analysis, the ICC from children requiring significant help (>5 questions) was compared with the ICC of those requiring moderate or minimal help (≤5 questions).
The definition of VWD used in this study was based on published diagnostic criteria.2 Type 1 VWD was defined as in Phase 1. Type 2A was defined as a VWF:Ag and/or VWF:RCo/Act between 0.05 and 0.50 IU/mL on at least two occasions and an abnormal multimer profile, and Type 2M was defined as VWF:Ag and/or VWF:RCo/Act between 0.05 and 0.50 IU/mL on at least two occasions, RCo:Ag/Act ratio < 0.60, and a normal multimer profile. Type 2B VWD had the same criteria as Type 2A VWD with the addition of a RIPA with increased sensitivity at low dose ristocetin. Type 2N was defined as FVIII < VWF:Ag. Type 3 VWD was defined as VWF:Ag and/or VWF:RCo/Act < 0.05 IU/mL and FVIII:C < 0.10 IU/mL.2
Platelet function disorders were defined by abnormal platelet aggregation testing, with diagnostic criteria outlined by the Rare Inherited Bleeding Disorders Committee of the Association of Hemophilia Clinic Directors of Canada.14 A coagulation factor deficiency, including factor I, II, V, VII, VIII, X, XI, or XIII, was defined as having a low measured factor level (0.5 IU/ml) upon two occasions.
RESULTS
Phase 1: Cognitive debriefing and generation of the School Age Self-PBQ
Seven subjects, 5 with a confirmed diagnosis of type 1 VWD and 2 controls, participated in the cognitive debriefing and generation of the School Age Self-PBQ. The ages of the children ranged from 8-11 years. Three subjects completed the original version of the Self-PBQ and 4 subjects completed the first modified version. Cognitive debriefing interviews took place upon completion of the questionnaire and lasted an average of 40.8 minutes (n = 4). The average number of problems encountered by respondents was 15.3 (n = 7), while the average number of items with a problem for respondents was 11.1 (n = 7). The Phase 1 patient characteristics are shown in Table 1.
Table 1:
Phase 1 patient characteristics
| Subject | Age (years) | Sex | Diagnosis | Interview duration (min) | No. of problems | No. of items with a problem | Self-PBQ version |
|---|---|---|---|---|---|---|---|
| 1 | 8 | F | Type 1 VWD | Unknown | 13 | 9 | Original |
| 2 | 9 | F | Type 1 VWD | 57 | 25 | 12 | Original |
| 3 | 9 | F | Type 1 VWD | 32 | 26 | 17 | Original |
| 4 | 11 | M | Type 1 VWD | 36 | 15 | 13 | 1st modification |
| 5 | 10 | F | Type 1 VWD | 38 | 9 | 8 | 1st modification |
| 6 | 11 | M | Control | Unknown | 12 | 13 | 1st modification |
| 7 | 9 | M | Control | Unknown | 7 | 6 | 1st modification |
The primary changes made to the Self-PBQ included: 1) removal of items that were identified to be redundant, and 2) modifications to the question wording and response options. The major change throughout the document was a substitution of “you” or “your” for the term “research participant” in order to directly address participants, since the respondents had trouble understanding that the term “research participant” referred to themselves. Questions regarding referrals to specialists and laboratory testing were removed as the terms created confusion in the respondents and did not impact on the scoring. Some fo the respondents were not familiar with the symbols > and < so they were spelled out as “greater than” and “lesser than”.
The revised questionnaire, known as the School Age Self-PBQ, was used for Phase 2 of the study. The School Age Self-PBQ has 13 yes/no Section questions (such as “Have you ever had nosebleeds”) that are required to be completed by all participants. Only those who respond “Yes” to a Section question would be required to complete the items in a given section. Each section has between 4 and 10 questions. Section 14 must be completed by all respondents and has 12 questions, so the minimum number of questions that need to be completed is 25, with the maximum being 112.
Phase 2: Prospective validation of the School Age Self-PBQ
Between May 2016 and May 2017, 31 patients with inherited bleeding disorders were enrolled at 5 treatment centres across Canada. All 31 child-parent proxy pairs filled out the Self-PBQ; however, 2 pairs were excluded from the analysis because their BSs could not be calculated. In both cases, both the child and parent had missing responses and no BS for at least one Self-PBQ category.
The mean age of the patient sample was 9.9 years (range 8-12 years). Type 1 VWD was the most common diagnosis, seen in 48% of patients; other diagnoses included type 2 VWD subtypes (2A, 2B, and 2M) (17%), hemophilia A (14%), and other rare inherited bleeding disorders (21%). The average time to completion of the School Age Self-PBQ among patients was 27 min (n = 27, range 5-55 min). The average time to completion among parents was 21 min (n = 26, range = 5-60 min). The Phase 2 patient demographic characteristics by subgroup are summarized in Table 2. Total BSs from a final sample of 29 child-parent pairs, scored using the ISTH-BAT scoring key, were used for analysis. Child and parent proxy scores were compared and demonstrated a high level of agreement, with an ICC of 0.83. The Figure is a difference plot that depicts the agreement between child and parent BSs.
Table 2:
Phase 2 patient demographic data, laboratory values, and BSs
| Subgroup: Age | Subgroup: Disease severity | Total | |||
|---|---|---|---|---|---|
| 8-9 years | 10-12 years | Type 1 VWD | Other disorder | ||
| Number of patients | 12 | 17 | 14 | 15† | 29 |
| Male gender (%) | 7 (58%) | 7 (41%) | 7 (50%) | 7 (47%) | 14 (48%) |
| Mean age years (range) | 8.42 (8-9) | 10.94 (10-12) | 10.14 (8-12) | 9.67 (8-12) | 9.9 (8-12) |
| Median Self-PBQ BS (range) | 7.5 (2-19) | 5 (0-20) | 5.5 (0-19) | 8 (2-20) | 6 (0-20) |
| Mean VWF:Ag, IU/mL (range) | 0.59 (0.17-1.3) | 0.43 (0.07-0.98) | 0.41 (0.25-0.63) | 0.58 (0.07-1.3) | 0.48 (0.07-1.30)* |
| Mean VWF:RCo, IU/mL (range) | 0.42 (0.14-1) | 0.36 (0.07-1) | 0.30 (0.14-0.41) | 0.48 (0.07-1) | 0.38 (0.07-1.0)* |
| Mean FVIII, IU/mL (range) | 0.47 (0.11-0.87) | 0.50 (0.26-0.89) | 0.62 (0.31-0.89) | 0.31 (0.11-0.44) | 0.49 (0.11-0.89)* |
Seven patients were missing hemostatic laboratory values (including VWF:Ag, VWF:RCo, and factor VIII:C). Study population means for these categories were calculated using a sample size of 22.
Other bleeding disorders included type 2B VWD (n = 2), type 2M VWD (n = 2), type 2A VWD (n = 1), hemophilia A (n = 4), platelet dysfunction disorders (n = 2), hypofibrinogenemia (n = 1), factor XIII deficiency (n = 1), storage pool deficiencies (n = 1), and Glanzmann’s thrombasthenia (n = 1).
Figure:
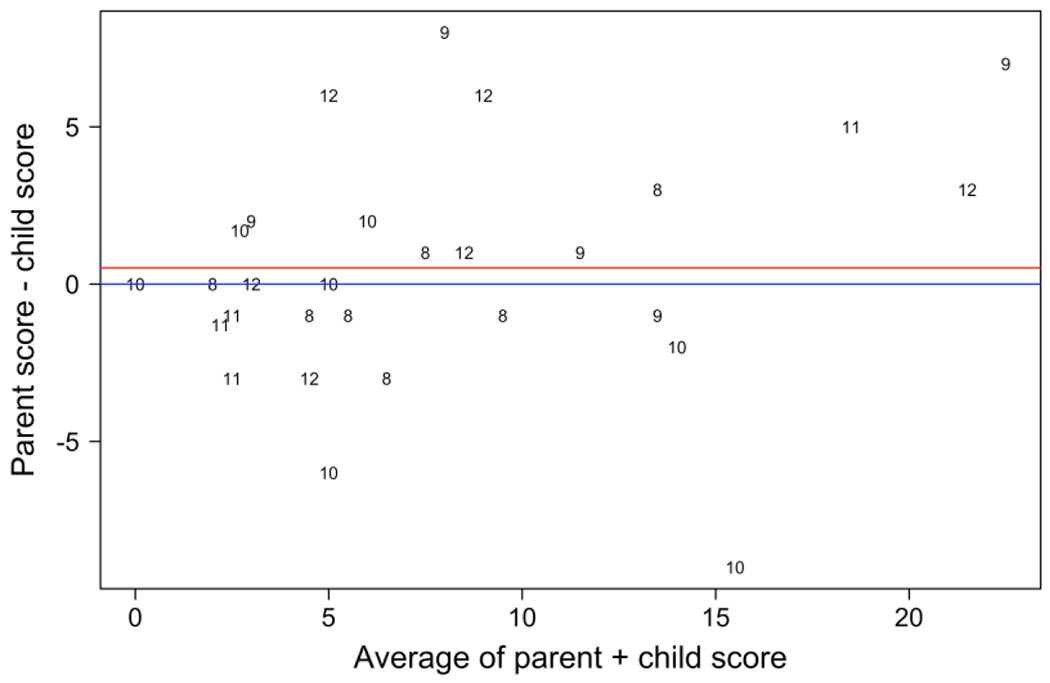
Agreement between child and parent (proxy) total reported bleeding scores. The difference between child and parent-derived bleeding scores is plotted against the mean of the two measurements. The average of the differences is shown by the red line. The numbers plotted reflect ages of the children (range 8-12 years).
The age subgroup analysis was performed on 12 patients in the 8-9 year-old age group and 17 patients in the 10-12 year-old age group. The ICC was ≥0.80 for both groups, indicating that the Self-PBQ is equally valid when completed by younger children (Table 3). Likewise, the disease severity subgroup analysis was carried out on 14 patients with type 1 VWD and 15 patients with other, more severe bleeding disorders. The ICC was again ≥0.80 in both groups, demonstrating no significant effect of disease severity on Self-PBQ scores (Table 3). The ICCs and 95% CIs for all subgroup analyses are summarized in Table 3.
Table 3:
ICC for age, disease severity, and help needed subgroups
| Number of patients | ICC | 95% CI | |
|---|---|---|---|
| Subgroup: Age | |||
| Age 8-9 years | 12 | 0.83 | 0.54-0.95 |
| Age 10-12 years | 17 | 0.82 | 0.58-0.93 |
| Subgroup: Disease severity | |||
| Type 1 VWD | 14 | 0.83 | 0.56-0.94 |
| Other bleeding disorders | 15 | 0.80 | 0.51-0.93 |
| Subgroup: Help needed | |||
| Significant/all (>5 questions) | 17 | 0.85 | 0.64-0.94 |
| Moderate/minimal/none (<5 questions ) | 12 | 0.75 | 0.34-0.92 |
The Self-PBQ is meant to be completed by children with no or minimal assistance from their parents. In the study population of 29 patients, the majority of children (n = 17) needed significant help (>5 questions) when filling out the PBQ. Within the 8-9 year-old age group, 75% of children needed help with more than 5 questions, compared to 47% of children in the 10-12 year-old age group (Table 4). In the subgroup analysis based on degree of help needed, subjects who needed help with >5 questions demonstrated almost perfect agreement with the parent questionnaire, while those who needed help with ≤5 questions demonstrated substantial agreement (Table 3). Thus, parental assistance had some effect on the reported agreement.
Table 4:
Amount of help needed by each age group
| Age group | Number of children requiring help (% of age group) | |||
|---|---|---|---|---|
| Significant/all help (>5 questions) | Moderate help (3-5 questions) | Minimal/no help (<3 questions) | Total | |
| 8-9 y/o | 9 (75%) | 1 (8%) | 2 (17%) | 12 |
| 10-12 y/o | 8 (47%) | 5 (29%) | 4 (24%) | 17 |
DISCUSSION
An essential step in the diagnosis of an inherited bleeding disorder is assessing abnormal bleeding symptoms based on the patient’s bleeding history. This initial task is crucial in directing further investigations and laboratory testing. In the last several years, BATs have offered a standardized method of describing bleeding symptoms and evaluating disease severity. Pediatric BATs are particularly useful for screening children for bleeding disorders because hemorrhagic symptoms, especially bruising and epistaxis, commonly overlap between healthy individuals and those with pathologies.
Although BATs can be time-consuming to apply in a busy clinical practice,15 a recent effort has been made to improve efficiency through the use of self-administered questionnaires instead of those requiring expert administration. In 2017, the Self-PBQ developed by Casey et al. was shown to be a sensitive and reliable tool for identifying VWD in children referred to the hematology clinic for investigation of a bleeding disorder.6 Of importance to the present study, the Self-PBQ has not yet been validated when completed by patients under 12 years of age.
Our study reports the development of the School Age Self-PBQ for pediatric patients with inherited bleeding disorders. We assessed the effectiveness of the questionnaire when completed by 8-12 year old children with minimal adult assistance. Total BSs derived from the child self-report were found to have excellent agreement with those from the parent proxy report, with an ICC of 0.83. Furthermore, there was no correlation between age or disease severity and the degree of agreement. When separated by age, the agreement was maintained in younger children aged 8-9 years compared to older children aged 10-12 years. Similarly, patients with type 1 VWD demonstrated a comparable level of agreement with the parent proxy compared to those with other disorders. These results suggest that the Self-PBQ is equally valid in younger children and in those with more severe diseases.
One concern we had centered around the degree of assistance that children required from their parents to complete the Self-PBQ. Twenty-three children, the majority of the study population (79%), required significant or moderate help with the questionnaire. Three children were able to complete the questionnaire with minimal help, and 3 required no assistance. Given that this study used agreement with the parent questionnaire to assess the effectiveness of the Self-PBQ, subjects receiving substantial help may produce misleading results. However, based on our subgroup analysis, children who received more help only had a slightly higher ICC than those who received less help (ICC = 0.85 and 0.75 respectively), and those needing less help still met the minimum standard for reliability.11 Moreover, unlike quality of life instruments that are designed to capture patient experiences, BATs are used as diagnostic screening tools. The Self-PBQ elicits objective responses about the presence or absence of symptoms; thus, neither the parent nor the child can be considered the “gold standard” over the other. Rather, the child-completed Self-PBQ may yield additional information not gathered from the parent version, and vice versa. Allowing the child and parent work together on the questionnaire may produce the most accurate results as one respondent can provide information not known by the other when necessary.
A limitation of this study was that the School Age Self-PBQ was only tested in children with a previously established diagnosis of an inherited bleeding disorder. In the future, we would like to validate the Self-PBQ as a screening tool in children presenting to a hematologist for the first time for evaluation of bleeding symptoms.
The Self-PBQ is the first self-administered BAT that has been developed for pediatric patients presenting with bleeding disorders, and our modified version, the School Age Self-PBQ, is the only BAT validated for the 8-12 year-old age group. Our study demonstrates that the School Age Self-PBQ can be completed effectively by pre-teen children with moderate to significant assistance from their parents. Child and parent-reported BSs were found to have excellent agreement, and this agreement was maintained in younger children aged 8-9 years and in children on the more severe spectrum of bleeding disorders. Our results indicate that the School Age Self-PBQ may be suitable to be incorporated into routine clinical care for the 8-12 year-old population.
Supplementary Material
ACKNOWLEDGEMENTS
Special thanks to Tracy Jackson, Melissa Morrison, and all participating institutions. This study was conducted with support from the University of Ottawa Faculty of Medicine Summer Studentship Program.
Abbreviations Key:
- BAT
Bleeding assessment tool
- BS
Bleeding score
- PBQ
Pediatric Bleeding Questionnaire
- VWD
Von Willebrand disease
- ISTH-BAT
International Society on Thrombosis and Hemostasis Bleeding Assessment Tool
- Self-BAT
Self-administered Bleeding Assessment Tool
- Self-PBQ
Self-administered Pediatric Bleeding Questionnaire
- ICC
Intraclass correlation
- VWF:Ag
Von Willebrand factor antigen
- VWF:RCo
Von Willebrand factor ristocetin cofactor activity
- FVIII
Factor VIII
- FVIII:C
Factor VIII coagulant activity
- RIPA
Ristocetin-induced platelet agglutination
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors declare that there is no conflict of interest.
Contributor Information
Jessica Bui, Department of Medicine, University of Ottawa, Ottawa, Canada.
David Martyres, University of Toronto, Toronto, Canada.
Paula D. James, Department of Medicine, Queen’s University, Kingston, Canada
Julie Grabell, Queen’s University, Kingston, Canada.
John Wu, Division of Hematology/Oncology, British Columbia Children’s Hospital, Vancouver, Canada.
MacGregor Steele, Section of Pediatric Hematology, Alberta Children’s Hospital, Calgary, Canada.
Mariana Silva, Kingston General Hospital, Kingston, Canada.
Margaret L. Rand, Division of Hematology/Oncology and Translational Medicine, The Hospital for Sick Children; Departments of Laboratory Medicine & Pathobiology, Biochemistry, and Pediatrics, University of Toronto, Toronto, Canada
Victor S. Blanchette, Division of Hematology/Oncology, Department of Pediatrics, University of Toronto, Hospital for Sick Children, Toronto, Canada
Nick Barrowman, Department of Pediatrics, University of Ottawa, Ottawa, Canada.
Robert J. Klaassen, Department of Pediatrics, Division of Hematology/Oncology, Children’s Hospital of Eastern Ontario, Ottawa, Canada
REFERENCES
- 1.Nosek-Cenkowska B, Cheang MS, Pizzi NJ, Israels ED, Gerrard JM. Bleeding/bruising symptomatology in children with and without bleeding disorders. Thromb Haemost. 1991;65(3):237–241. http://www.ncbi.nlm.nih.gov/pubmed/2048048 [PubMed] [Google Scholar]
- 2.Bowman M, Riddel J, Rand ML, Tosetto A, Silva M, James PD. Evaluation of the diagnostic utility for von Willebrand disease of a pediatric bleeding questionnaire. J Thromb Haemost. 2009;7(8):1418–1421. 10.1111/j.1538-7836.2009.03499.x [DOI] [PubMed] [Google Scholar]
- 3.Rodeghiero F, Tosetto A, Abshire T, et al. ISTH/SSC bleeding assessment tool: a standardized questionnaire and a proposal for a new bleeding score for inherited bleeding disorders. J Thromb Haemost. 2010;8(9):2063–2065. 10.1111/j.1538-7836.2010.03975.x [DOI] [PubMed] [Google Scholar]
- 4.Rydz N, James PD. The evolution and value of bleeding assessment tools. J Thromb Haemost. 2012;10(11):2223–2229. 10.1111/j.1538-7836.2012.04923.xs [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deforest M, Grabell J, Albert S, et al. Generation and optimization of the self-administered bleeding assessment tool and its validation as a screening test for von Willebrand disease. Haemophilia. 2015;21(5):e384–e388. 10.1111/hae.12747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casey LJ, Tuttle A, Grabell J, et al. Generation and optimization of the self-administered pediatric bleeding questionnaire and its validation as a screening tool for von Willebrand disease. Pediatr Blood Cancer. 2017;64:e26588 10.1002/pbc.26588 [DOI] [PubMed] [Google Scholar]
- 7.Varni JW, Burwinkle TM, Seid M, Skarr D. The PedsQL™* 4.0 as a pediatric population health measure: feasibility, reliability, and validity. Ambulatory Pediatrics. 2003. December 31;3(6):329–41. [DOI] [PubMed] [Google Scholar]
- 8.Varni JW, Limbers CA, Burwinkle TM. How young can children reliably and validly self-report their health-related quality of life?: An analysis of 8,591 children across age subgroups with the PedsQL™ 4.0 Generic Core Scales. Health and quality of life outcomes. 2007. January 3;5(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jobe JB. Cognitive psychology and self-reports: models and methods. Qual Life Res 2003;12:219–27. [DOI] [PubMed] [Google Scholar]
- 10.Elbatarny M, Mollah S, Grabell J, et al. Normal range of bleeding scores for the ISTH-BAT: adult and pediatric data from the merging project. Haemophilia. 2014;20(6):831–835. 10.1111/hae.12503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Terwee C, Bot S, de Boer M et al. Quality criteria were proposed for measurement properties of health status questionnaires. J Clin Epidemiol. 2007;60(1):34–42. doi: 10.1016/j.jclinepi.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 12.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33:159–74. [PubMed] [Google Scholar]
- 13.Castaman G, Linari S. Diagnosis and Treatment of von Willebrand Disease and Rare Bleeding Disorders. J Clin Med. 2017;6(4):45. doi: 10.3390/jcm6040045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diagnostic Criteria For Inherited Platelet Function Disorders For The Canadian Rare Inherited Bleeding Disorders Registry (RIBDR). Toronto: Inherited Bleeding Disorders Subcommittee of the Association of Hemophilia Clinic Directors of Canada; 2013. Available at: https://www.ahcdc.ca/storage/files/diagnostic-criteria-2013.pdf. [Google Scholar]
- 15.Bidlingmaier C, Grote V, Budde U, Olivieri M, Kurnik K. Prospective evaluation of a pediatric bleeding questionnaire and the ISTH bleeding assessment tool in children and parents in routine clinical practice. J Thromb Haemost. 2012;10(7):1335–1341. 10.1111/j.1538-7836.2012.04775.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


