Abstract
Aberrant neutrophil (PMN) infiltration of the intestinal mucosa is a hallmark of inflammatory bowel diseases, including Crohn's disease and ulcerative colitis. While the genotoxic function of PMNs and its implications in carcinogenesis have been primarily associated with oxidative stress, recent work by Butin-Israeli and colleagues has defined a novel mechanism where PMN-derived microparticles through the delivery and activity of specific miRNAs promoted formation of double-strand breaks (DSBs), and in parallel, suppressed DSB repair through the downregulation of lamin B1 and Rad51. Respective downregulation of these two proteins compromised the nuclear envelope and high-fidelity repair by homologous recombination, increasing DSB accumulation and aneuploidy. This discovery defined a novel mode of action where PMN-mediated suppression of DSB repair leading to genomic instability in the injured mucosa may facilitate progression toward colorectal cancer.
Keywords: neutrophils, wound healing, microRNAs, inflammation, carcinogenesis, IBD
Introduction
Inflammatory bowel diseases (IBDs), encompassing ulcerative colitis and Crohn's disease, are characterized by recurring episodes of inflammation and tissue injury (Rieder et al., 2007; Matricon et al., 2010). Such relapsing inflammation is associated with dysregulated neutrophil (PMN) infiltration of the intestinal mucosa, and is regarded as a pathological feature and a hallmark of IBDs (Cho, 2008; Wera et al., 2016). Aberrant immune response triggered by PMN accumulation in gut mucosa can adversely affect epithelial barrier integrity, enable translocation of microbes into the interstitium, perpetuate inflammatory response, and promote tissue damage which compromise gut function (Cho, 2008; Sekirov et al., 2010; Weber et al., 2014; Butin-Israeli et al., 2016; Slater et al., 2017). As such, high PMN blood count (Torun et al., 2012; Nishida et al., 2017) and accumulation of PMNs in stool of IBD patients (Silberer et al., 2005; Langhorst et al., 2008) are correlated with active flares and disease severity.
In addition, intestinal inflammation and recurring tissue injury have been shown to predispose IBD patients to gastrointestinal malignancies (Canavan et al., 2006). Supporting this, many correlative studies have documented the increased risk of IBD patients to develop small bowel adenocarcinoma (Bojesen et al., 2017), cholangiocarcinoma (Huai et al., 2014), gastric cancer (Nissen et al., 2016), intestine-associated non-Hodgkin's lymphoma (Farrell et al., 2000), and colorectal cancer (CRC) (Ekbom et al., 1990; Lakatos et al., 2006; Choi et al., 2016). Comparative analyses of different patient cohorts also reveal that the risk of CRC development is increased by sixfold in IBD patients (Herrinton et al., 2012; Ording et al., 2013). Indeed, CRC metastasis accounts for 10–15% of all IBD-related mortalities (Jensen et al., 2006; Ording et al., 2013; Althumairi et al., 2016).
A particular subset of CRC arising on the background of IBD, known as colitis-associated cancer (CAC) (Grivennikov, 2013; Francescone et al., 2015), is strictly associated with PMN accumulation in the gut and is likely driven by PMN-mediated exacerbated inflammation (Shang et al., 2012). A high neutrophil-to-lymphocyte ratio in the systemic circulation of CRC or CAC patients is predictive of poor clinical outcomes and shorter progression-free survival (Shibutani et al., 2013; Ozdemir et al., 2014; Haram et al., 2017; Kim et al., 2019). As a result, there have been tremendous efforts to control aberrant immune activation through immunosuppression and the use of TNFα and interleukin monoclonal antibodies (Zenlea and Peppercorn, 2014; Feagan et al., 2016; Adegbola et al., 2018). Nonetheless, these treatments exert severe side-effects and suppress systemic immune response necessary for the elimination of invading pathogens (Stallmach et al., 2010). To develop effective targeted therapy for IBDs and prevent CRC, it is important to gain a better understanding of mechanisms underlying PMN-driven tissue injury as well as the beneficial functions of PMNs in intestinal homeostasis.
In this review, we summarize our recent discovery of the novel mechanism that links neutrophil infiltration and genomic instability, and will discuss the long-term implications of this biological process in tissue homeostasis and carcinogenesis. Insights into how neutrophil infiltration and genomic instability are mechanistically connected in the context of intestinal inflammation will pave the way for new therapeutic options to alleviate IBD symptoms and prevent progression toward CRC/CAC.
Neutrophils, Reactive Oxygen Species Production, and Induction of Oxidative Stress: A Classical View
PMN-induced exacerbated inflammation is a hallmark of many inflammatory conditions (Wright et al., 2010; Delgado-Rizo et al., 2017), including but not limited to acute lung injury (Zhou et al., 2012), atherosclerosis (Baetta and Corsini, 2010), chronic obstructive pulmonary disease (COPD) (Hoenderdos and Condliffe, 2013), and IBD (Rieder et al., 2007; Cho, 2008; Matricon et al., 2010; Wera et al., 2016). Upon tissue transmigration and detection of invading pathogens, PMNs undergo oxidative burst and degranulation (Nguyen et al., 2017). The tissue-damaging effects of PMNs are primarily attributed to their capacity to generate high levels of reactive oxygen species (ROS). Accumulation of ROS in tissues gradually increases intracellular levels of hydrogen peroxide and superoxides, which oxidize and generate modified DNA bases (Cadet and Wagner, 2013). For example, activated PMNs have been shown to induce ROS-mediated DNA damage in lung and respiratory tract tissue in the context of acute lung injury (Kellner et al., 2017) and COPD (Boukhenouna et al., 2018).
One of the nucleotides that is primarily oxidized and modified by ROS is 8-oxoguanine (8-oxoG), whose presence markedly contributes to G/A nucleotide mismatch and DNA replication errors (Cadet and Wagner, 2013; Cadet et al., 2017). In addition, ROS-mediated base modification or direct alkylation of the DNA sugar backbone generate DNA lesions that stall replication fork and form single-strand breaks (SSBs) (Eccles et al., 2011; Cadet et al., 2017), which are gradually converted to double-strand breaks (DSBs) if not repaired in a timely manner (Mehta and Haber, 2014; Cannan and Pederson, 2016). On this basis, these ROS-dependent genotoxic stressors can contribute to mutagenesis and malignancy transformation.
There is a long-standing doctrine that neutrophils contribute to DNA damage solely through ROS-dependent mechanisms. However, we recently expanded this dogma by identifying a novel, ROS-independent mechanism, whereby PMNs deliver microparticles (MPs) that transport regulatory miRNAs to promote the formation of DSBs in inflamed epithelial cells (Butin-Israeli et al., 2019). We found that coculture of PMNs with cultured colon epithelial cells, HCT116 and Caco2, or with primary patient-derived colonoids induced the formation of γH2AX foci, indicating DSBs, as well as led to activation of downstream DNA damage response. Intriguingly, PMN-MPs did not increase 8-oxoG levels in epithelial cells and were proved to be nonresponsive to ROS inhibition by several well-characterized ROS scavengers. These findings implicate PMNs in causing DSBs through mechanisms distinct from the known induction of ROS-mediated oxidative stress.
PMN-MP-Derived miRNAs Are Novel Mediators of Biological Activities
In addition to releasing soluble mediators, including cytokines and various metalloproteinases, activated PMNs have been recently shown to secrete microvesicles or microparticles (PMN-MPs) as an additional way to exert their effector functions (Dalli et al., 2013; Butin-Israeli et al., 2016; Bui et al., 2018). PMN-MPs are vesicles that include exosomes and larger particles, ranging from 50 to 1000 nm that are generated during PMN activation and migration across endothelial and epithelial barriers (Bui et al., 2018; Finkielsztein et al., 2018). The PMN-MP cargo include proteins, miRNAs, and lipid mediators, all of which are encapsulated and protected by the vesicle lipid bilayer and are efficiently shuttled to surrounding target cells. As such, the exchange of extracellular vesicles or MPs has recently emerged as a novel way of intercellular communication (Hwang, 2013; Pitt et al., 2016). Following uptake, PMN-MP cargo is discharged and functions to modulate many biological activities in recipient cells. A simplified list of PMN effector molecules and their respective biological functions is summarized in Table 1. Importantly, the biological content of PMN-MPs is stimulus dependent and is reflective of the condition, whereby the PMNs were activated. As such, the heterogeneity of MP composition has been shown to drastically change depending on the environmental milieu and tissue condition (Distler et al., 2005; Shai et al., 2012; Alexy et al., 2014). The fact that we and other groups have confirmed rapid induction of miRNAs in activated PMNs, followed by their packaging into PMN-MPs, has redefined the notion that PMNs have low transcriptional activity and operate only through the release of granular stores.
Table 1.
Neutrophil Effector Function in Inflammatory Bowel Disease
| PMN effector function | Contribution to IBD pathology | Association with MPs | References |
|---|---|---|---|
| ROS | Contribute to oxidative stress, base damage/modification, replication errors, and single-strand breaks. | No | Nguyen et al. (2017), Cadet and Wagner (2013), Kellner et al. (2017), Cadet et al. (2017) |
| Myeloperoxidase | Generate ROS, promote cells death, and impede wound healing. | Yes | Slater et al. (2017) |
| Metalloproteinases | Disrupt tissue integrity, increase epithelial and vascular permeability, and immune cell recruitment. | Yes | Butin-Israeli et al. (2016) |
| Cytokines | Immune cells recruitment and inflammatory polarization, impede wound healing. | TBD | Wera et al. (2016), Wright et al. (2010), Wang et al. (2018) |
| miRNAs (miR-23a/-155, miR-9) | Posttranscriptionally regulate signaling pathways. | Yes | Butin-Israeli et al. (2019), Distler et al. (2005) |
The table summarizes the pathological effector functions of PMNs and their association with MPs as it relates to IBD.
IBD, inflammatory bowel disease; MP, microparticles; PMN, neutrophil; ROS, reactive oxygen species; TBD, to be determined.
The Synergistic Action of miR-23a and miR-155 Promotes DSB Accumulation and Paves the Road to Genomic Instability
By examining IBD patient biopsies, we observed that lamin B1 (LB1), a critical nuclear lamin that constitutes the nuclear envelope and protects replication forks (Butin-Israeli et al., 2012, 2015), was significantly reduced. We further found that treatment of cultured colon epithelial cells or patient-derived colonoids with PMN-MPs downregulated LB1 through the action of miRNAs transported by these MPs. We then identified that miR-23a, which has been previously shown to regulate LB1 expression (Lin and Fu, 2009; Dreesen et al., 2013), was highly upregulated in activated PMNs and was enriched in PMN-MPs. The lack of miR-23a primary and precursor transcripts (pri-/pre-miR-23a) in IBD colonic tissues or epithelial cells indicated that the increased level of miR-23a and its inhibitory effect on LB1 was due to PMN-MP deposition, and was not due to endogenous induction during inflammation. LB1 inhibition through miRNA activity compromised nuclear envelope integrity, resulting in replication fork stalling and S-phase arrest (Butin-Israeli et al., 2015). Failure to restart replication precedes replication fork collapse and DSB induction at replication sites, perpetuating cell cycle arrest and affecting cellular fitness (Lopes et al., 2001; Tercero and Diffley, 2001; Zeman and Cimprich, 2014).
Intriguingly, inhibition of miR-23a activity by antagomir, although rescued LB1 expression, only partially reversed DSB accumulation, suggesting contribution of an additional DSB-inducing mechanism. We thus investigated two major DSB repair pathways, including homologous recombination (HR) and nonhomologous end joining (NHEJ) (Mao et al., 2008; Brandsma and Gent, 2012), in the epithelial cells treated with PMN-MPs. While NHEJ activity was not affected by PMN-MP treatment during this short-term coculture experiment (24–48 h), HR was rapidly downregulated. Mechanistically, Rad51, a key HR regulator (Baumann and West, 1998), was found to be significantly downregulated by the specific action of miR-155 (Gasparini et al., 2014), an additional miRNA that was found to be enriched in PMN-MPs (Butin-Israeli et al., 2019). By suppressing Rad51 expression, miR-155 inhibits strand invasion and exchange between the homologous DNA strands on the sister chromatids, a rate-determining step of HR-mediated repair (Baumann and West, 1998; Anand et al., 2017). Disruption of HR machinery and loss of HR activity result in ineffective resolution of DSBs incurred due to replication fork collapse (Costes and Lambert, 2012; Mijic et al., 2017).
Although DSBs are formed at much lower frequency than SSBs, they are the most lethal DNA lesions and can rapidly induce cell death (Ceccaldi et al., 2016). Moreover, DSBs are repaired at a significantly lower rate compared with SSBs, and as a result, impairment of any one of the DSB repair pathways have severe implications to cellular survival and tissue homeostasis (Ceccaldi et al., 2016). In the context of mucosal injury as seen in IBD, DSB accumulation in the inflamed epithelium due to synergistic activity of PMN-MP-derived miRNAs resulted in cell cycle arrest and apoptosis, thus delaying wound resolution. In the mouse model of acute colonic injury, using endoscopy-guided, biopsy-based injury, we showed that administration of antisense oligonucleotides (ASOs) that specifically target miR-23a and miR-155 successfully rescued the expression of LB1 and Rad51, reduced DSB levels, and substantially improved wound healing. These observations affirm the therapeutic potential of miRNA therapy in IBD treatment. Indeed, active flare regions of IBD patients, which are characterized by high PMN infiltration, also have elevated levels of miR-23/miR-155 (Butin-Israeli et al., 2019). Further studies with human cell lines by our group mechanistically showed that enriched miR-23a/miR-155 in epithelial cells can induce DSB accumulation, delay wound healing, and increase aneuploidy, an established marker for onset of carcinoma. Thus, increased miR-23a/miR-155 level coupled with reduced LB1/Rad51 expression have a powerful diagnostic value and can serve as early markers for IBD progression toward CRC/CAC. Patient biopsies can be easily obtained during routine endoscopy, and evaluation of miR-23a/miR-155 enrichment can be effectively performed using commercially available qPCR kits.
Therapeutic Outlook: An Immediate Response for IBD Management and a Long-Term Approach to Prevent Progression Toward CRC/CAC
Although wound healing and durable clinical remission are considered as important clinical endpoints for IBD therapy (Rogler et al., 2013), most current treatments rely on general immunosuppression and provide only temporary respite from the symptomatic disease. Standard therapies include systemic corticosteroids, immunosuppressives (mesalamine, azathioprine, 6-mercaptopurine), or biologics such as TNFα blockers (infliximab, adalimumab, golimumab), and in severe cases, surgical intervention (Hvas et al., 2018). These therapies, although effective in inducing initial remission, have harsh side effects and lose efficacy with disease progression. As has been seen with TNFα blockers, while 30% of patients do not respond to such treatment (Kopylov and Seidman, 2016), others develop antibodies to the biologics and overtime fail to achieve remission (Yanai et al., 2015). Similarly, prolonged use of corticosteroid-based immunosuppression or leukocyte adhesion inhibitors (natalizumab, vedolizumab) leads to leukopenia, bacterial infections, and the life-threatening condition of progressive multifocal leukoencephalopathy (Yousry et al., 2006).
For these reasons, more selective treatments for IBD that specifically target detrimental functions of immune cells need to be developed. Our findings identified one such potential therapeutic approach. As per our observations, targeting miRNAs transported by PMN-MPs can reduce tissue-damaging effects of PMNs without altering their recruitment or antibacterial/proresolving effector functions, highlighting the potential of miR-23a/miR-155 as novel molecular targets for miRNA-based therapy for IBD. In fact, multiple miRNA-targeted therapeutics have reached clinical development (Soroosh et al., 2018), including an RNA mimic of the tumor suppressor miRNA, miR-34, which has reached phase I clinical trials for cancer treatment (Beg et al., 2017), and an antagonist for miR-122, which has reached phase II trials for treating hepatitis (Zeisel and Baumert, 2017). The major challenges of miRNA-based therapy involve the multitude of targets for each miRNA as well as the delivery route of miRNA biologics (Chen et al., 2015). Oral or intravenous delivery will increase off-target effects of therapeutic miRNA mimics/antagonists (Jackson and Linsley, 2010). In an attempt to minimize these issues, a number of studies have attempted to couple drug delivery with routine colonoscopic screening in IBD patients (Philip and Philip, 2010). In fact, colon-targeted drug delivery offers a number of desirable features. Intrarectal administration of ASOs can avoid unnecessary degradation of the biologics in the stomach, intestine, or liver (Philip and Philip, 2010; Ramalingam et al., 2015). In addition, a combination of ASO delivery and colonoscopic screening can facilitate the localized distribution of ASOs at injured tissues and the specific inhibition of PMN-MP-derived miR-23a/miR-155 in these regions. As a result, this strategy enables the efficient combination of routine IBD surveillance and drug administration to increase wound healing and maintain a durable remission.
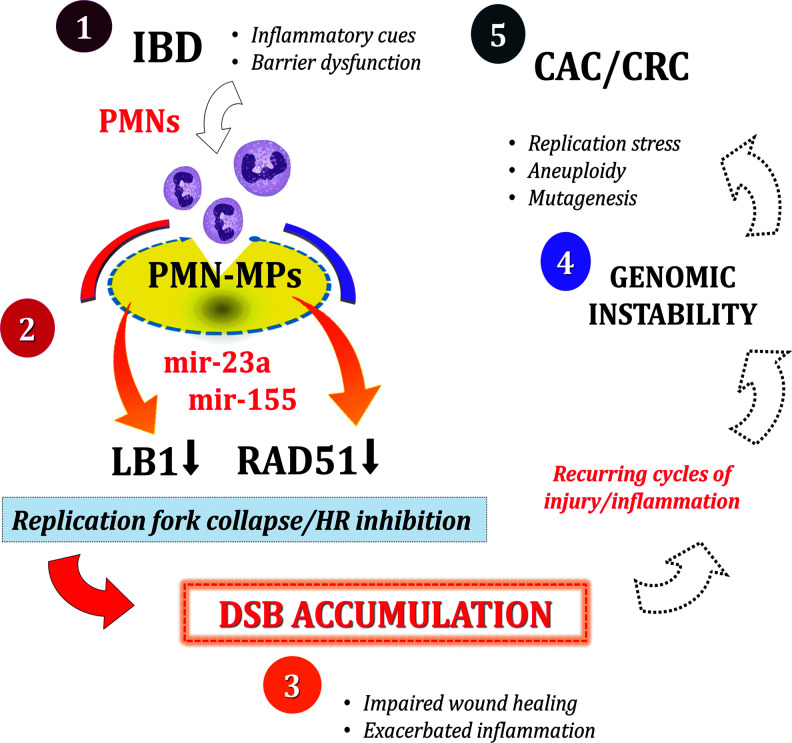
Genomic instability is an emerging hallmark of cancer that increases mutagenesis and chromosomal abnormalities (Negrini et al., 2010). Both features serve as the driving forces for tumorigenesis and tumor progression (Campbell et al., 2017; Levine and Holland, 2018). Genomic instability can be induced due to cell-intrinsic abnormalities (Levine and Holland, 2018) or extrinsically due to inflammatory activity, as seen in CRC (Colotta et al., 2009; Kidane et al., 2014; Li and Chen, 2018). Based on our findings, infiltrating PMNs in IBD patients may promote genomic instability through the induction of DSBs and suppression of HR repair. Genomic instability increases the rate of genetic mutations, accelerates adaptation of precancerous cells, and ensues carcinogenesis (Moon et al., 2019; Raynes and Weinreich, 2019). A diagram schematic of how PMNs contribute to the progression of IBD to CAC/CRC is shown in Figure 1. On this basis, preventing DSB accumulation in the inflamed mucosa of IBD patients by therapeutically targeting miR-23a/miR-155 can preserve the genomic integrity of colon tissues.
FIG. 1.
Schematic diagram showing contribution of PMNs to genomic instability and progression toward CAC/CRC. (1) Recurring disease episodes in IBD patients elicit inflammatory cues and barrier dysfunction that facilitate PMN recruitment to the injured tissues. (2) PMN infiltration precedes deposition of PMN-MPs and delivery of miR-23a/miR-155 onto the intestinal mucosa. miR-23a and miR-155 downregulate LB1 and Rad51, respectively, leading to replication fork collapse and HR inhibition. (3) The synergistic compromise of nuclear envelope integrity and HR-mediated DSB repair results in DSB accumulation, which in turn increases apoptosis, impairs wound healing, and exacerbates inflammation. (4) Recurring cycles of tissue injury further perpetuate unresolved DSB accumulation and likely promote the induction of genomic instability. Genomic instability encompasses a multitude of genotoxic events, including replication stress, mutagenesis, and aneuploidy. (5) As an emerging hallmark of cancer, genomic instability, driven by PMN-mediated inflammation, thereby can drive the progression from IBD to CRC. CAC, colitis-associated cancer; CRC, colorectal cancer; DSB, double-strand break; HR, homologous recombination; IBD, inflammatory bowel disease; LB1, lamin B1; MP, microparticles; PMN, neutrophil.
Finally, PMNs have been shown to mediate both beneficial and detrimental effects in wound healing and cancer (Galdiero et al., 2018; Wang et al., 2018). Although we are still far from completely understanding the mechanisms that underlie these seemingly opposing functions of PMNs, recent insights into the temporal changes of PMN phenotypes and increased survival during disease progression may explain the “good” and the “bad” actions of tissue PMNs (Ng et al., 2019; Yang et al., 2019). As such, one may speculate that during recurring, inflammatory episodes with subsequent waves of recruited PMNs, distinct PMN subsets with specializing activity/function may evolve at injury sites or precancerous lesions. As per our observations, one important feature that may differ in PMN subsets, probably dictating detrimental versus beneficial PMN function, is the content and the ability to generate MPs. This of course has to be investigated in the future. Thus, continued exploration of the PMN biology can offer new therapeutic avenues for IBD therapy and CRC/CAC prevention.
Acknowledgments
The authors thank Hannah Wiesolek for careful review of the article. This work was supported by grants from Digestive Health Foundation, Chicago, and a Research Scholar Grant by the American Cancer Society (RSG-17-235-01-CSM).
Disclosure Statement
No competing financial interests exist.
References
- Adegbola S.O., Sahnan K., Warusavitarne J., Hart A., and Tozer P. (2018). Anti-TNF therapy in Crohn's disease. Int J Mol Sci 19, 2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexy T., Rooney K., Weber M., Gray W.D., and Searles C.D. (2014). TNF-alpha alters the release and transfer of microparticle-encapsulated miRNAs from endothelial cells. Physiol Genomics 46, 833–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Althumairi A.A., Lazarev M.G., and Gearhart S.L. (2016). Inflammatory bowel disease associated neoplasia: a surgeon's perspective. World J Gastroenterol 22, 961–973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand R., Beach A., Li K., and Haber J. (2017). Rad51-mediated double-strand break repair and mismatch correction of divergent substrates. Nature 544, 377–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baetta R., and Corsini A. (2010). Role of polymorphonuclear neutrophils in atherosclerosis: current state and future perspectives. Atherosclerosis 210, 1–13 [DOI] [PubMed] [Google Scholar]
- Baumann P., and West S.C. (1998). Role of the human RAD51 protein in homologous recombination and double-stranded-break repair. Trends Biochem Sci 23, 247–251 [DOI] [PubMed] [Google Scholar]
- Beg M.S., Brenner A.J., Sachdev J., Borad M., Kang Y.K., Stoudemire J., et al. (2017). Phase I study of MRX34, a liposomal miR-34a mimic, administered twice weekly in patients with advanced solid tumors. Invest New Drugs 35, 180–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bojesen R.D., Riis L.B., Hogdall E., Nielsen O.H., and Jess T. (2017). Inflammatory bowel disease and small bowel cancer risk, clinical characteristics, and histopathology: a population-based study. Clin Gastroenterol Hepatol 15, 1900–1907 e2. [DOI] [PubMed] [Google Scholar]
- Boukhenouna S., Wilson M.A., Bahmed K., and Kosmider B. (2018). Reactive oxygen species in chronic obstructive pulmonary disease. Oxid Med Cell Longev 2018, 5730395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandsma I., and Gent D.C. (2012). Pathway choice in DNA double strand break repair: observations of a balancing act. Genome Integr 3, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bui T.M., Mascarenhas L.A., and Sumagin R. (2018). Extracellular vesicles regulate immune responses and cellular function in intestinal inflammation and repair. Tissue Barriers 6, e1431038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butin-Israeli V., Adam S.A., Goldman A.E., and Goldman R.D. (2012). Nuclear lamin functions and disease. Trends Genet 28, 464–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butin-Israeli V., Adam S.A., Jain N., Otte G.L., Neems D., Wiesmuller L., et al. (2015). Role of lamin b1 in chromatin instability. Mol Cell Biol 35, 884–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butin-Israeli V., Bui T.M., Wiesolek H.L., Mascarenhas L., Lee J.J., Mehl L.C., et al. (2019). Neutrophil-induced genomic instability impedes resolution of inflammation and wound healing. J Clin Invest 129, 712–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butin-Israeli V., Houser M.C., Feng M., Thorp E.B., Nusrat A., Parkos C.A., et al. (2016). Deposition of microparticles by neutrophils onto inflamed epithelium: a new mechanism to disrupt epithelial intercellular adhesions and promote transepithelial migration. FASEB J 30, 4007–4020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadet J., Davies K.J.A., Medeiros M.H., Di Mascio P., and Wagner J.R. (2017). Formation and repair of oxidatively generated damage in cellular DNA. Free Radic Biol Med 107, 13–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadet J., and Wagner J.R. (2013). DNA base damage by reactive oxygen species, oxidizing agents, and UV radiation. Cold Spring Harbor Perspect Biol 5, pii: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell B.B., Light N., Fabrizio D., Zatzman M., Fuligni F., de Borja R., et al. (2017). Comprehensive analysis of hypermutation in human cancer. Cell 171, 1042–1056 e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canavan C., Abrams K.R., and Mayberry J. (2006). Meta-analysis: colorectal and small bowel cancer risk in patients with Crohn's disease. Aliment Pharmacol Ther 23, 1097–1104 [DOI] [PubMed] [Google Scholar]
- Cannan W.J., and Pederson D.S. (2016). Mechanisms and consequences of double-strand DNA break formation in chromatin. J Cell Physiol 231, 3–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccaldi R., Rondinelli B., and D'Andrea A.D. (2016). Repair pathway choices and consequences at the double-strand break. Trends Cell Biol 26, 52–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Gao D.Y., and Huang L. (2015). In vivo delivery of miRNAs for cancer therapy: challenges and strategies. Adv Drug Deliv Rev 81, 128–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho J.H. (2008). The genetics and immunopathogenesis of inflammatory bowel disease. Nat Rev Immunol 8, 458–466 [DOI] [PubMed] [Google Scholar]
- Choi J.K., Kim D.W., Shin S.Y., Park E.C., and Kang J.G. (2016). Effect of ulcerative colitis on incidence of colorectal cancer: results from the nationwide population-based cohort study (2003–2013). J Cancer 7, 681–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colotta F., Allavena P., Sica A., Garlanda C., and Mantovani A. (2009). Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis 30, 1073–1081 [DOI] [PubMed] [Google Scholar]
- Costes A., and Lambert S.A. (2012). Homologous recombination as a replication fork escort: fork-protection and recovery. Biomolecules 3, 39–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalli J., Montero-Melendez T., Norling L.V., Yin X., Hinds C., Haskard D., et al. (2013). Heterogeneity in neutrophil microparticles reveals distinct proteome and functional properties. Mol Cell Proteomics 12, 2205–2219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado-Rizo V., Martinez-Guzman M.A., Iniguez-Gutierrez L., Garcia-Orozco A., Alvarado-Navarro A., and Fafutis-Morris M. (2017). Neutrophil extracellular traps and its implications in inflammation: an overview. Front Immunol 8, 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distler J.H., Pisetsky D.S., Huber L.C., Kalden J.R., Gay S., and Distler O. (2005). Microparticles as regulators of inflammation: novel players of cellular crosstalk in the rheumatic diseases. Arthritis Rheum 52, 3337–3348 [DOI] [PubMed] [Google Scholar]
- Dreesen O., Chojnowski A., Ong P.F., Zhao T.Y., Common J.E., Lunny D., et al. (2013). Lamin B1 fluctuations have differential effects on cellular proliferation and senescence. J Cell Biol 200, 605–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles L.J., O'Neill P., and Lomax M.E. (2011). Delayed repair of radiation induced clustered DNA damage: friend or foe? Mutat Res 711, 134–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekbom A., Helmick C., Zack M., and Adami H.O. (1990). Ulcerative colitis and colorectal cancer. A population-based study. N Engl J Med 323, 1228–1233 [DOI] [PubMed] [Google Scholar]
- Farrell R.J., Ang Y., Kileen P., O'Briain D.S., Kelleher D., Keeling P.W., et al. (2000). Increased incidence of non-Hodgkin's lymphoma in inflammatory bowel disease patients on immunosuppressive therapy but overall risk is low. Gut 47, 514–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feagan B.G., Sandborn W.J., Gasink C., Jacobstein D., Lang Y., Friedman J.R., et al. (2016). Ustekinumab as induction and maintenance therapy for Crohn's disease. N Engl J Med 375, 1946–1960 [DOI] [PubMed] [Google Scholar]
- Finkielsztein A., Mascarenhas L., Butin-Israeli V., and Sumagin R. (2018). Isolation and characterization of neutrophil-derived microparticles for functional studies. J Vis Exp 133; DOI: 10.3791/56949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francescone R., Hou V., and Grivennikov S.I. (2015). Cytokines, IBD, and colitis-associated cancer. Inflamm Bowel Dis 21, 409–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galdiero M.R., Varricchi G., Loffredo S., Mantovani A., and Marone G. (2018). Roles of neutrophils in cancer growth and progression. J Leukoc Biol 103, 457–464 [DOI] [PubMed] [Google Scholar]
- Gasparini P., Lovat F., Fassan M., Casadei L., Cascione L., Jacob N.K., et al. (2014). Protective role of miR-155 in breast cancer through RAD51 targeting impairs homologous recombination after irradiation. Proc Natl Acad Sci U S A 111, 4536–4541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grivennikov S.I. (2013). Inflammation and colorectal cancer: colitis-associated neoplasia. Semin Immunopathol 35, 229–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haram A., Boland M.R., Kelly M.E., Bolger J.C., Waldron R.M., and Kerin M.J. (2017). The prognostic value of neutrophil-to-lymphocyte ratio in colorectal cancer: a systematic review. J Surg Oncol 115, 470–479 [DOI] [PubMed] [Google Scholar]
- Herrinton L.J., Liu L., Levin T.R., Allison J.E., Lewis J.D., and Velayos F. (2012). Incidence and mortality of colorectal adenocarcinoma in persons with inflammatory bowel disease from 1998 to 2010. Gastroenterology 143, 382–389 [DOI] [PubMed] [Google Scholar]
- Hoenderdos K., and Condliffe A. (2013). The neutrophil in chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol 48, 531–539 [DOI] [PubMed] [Google Scholar]
- Huai J.P., Ding J., Ye X.H., and Chen Y.P. (2014). Inflammatory bowel disease and risk of cholangiocarcinoma: evidence from a meta-analysis of population-based studies. Asian Pac J Cancer Prev 15, 3477–3482 [DOI] [PubMed] [Google Scholar]
- Hvas C.L., Bendix M., Dige A., Dahlerup J.F., and Agnholt J. (2018). Current, experimental, and future treatments in inflammatory bowel disease: a clinical review. Immunopharmacol Immunotoxicol 40, 446–460 [DOI] [PubMed] [Google Scholar]
- Hwang I. (2013). Cell-cell communication via extracellular membrane vesicles and its role in the immune response. Mol Cells 36, 105–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson A.L., and Linsley P.S. (2010). Recognizing and avoiding siRNA off-target effects for target identification and therapeutic application. Nat Rev Drug Discov 9, 57–67 [DOI] [PubMed] [Google Scholar]
- Jensen A.B., Larsen M., Gislum M., Skriver M.V., Jepsen P., Norgaard B., et al. (2006). Survival after colorectal cancer in patients with ulcerative colitis: a nationwide population-based Danish study. Am J Gastroenterol 101, 1283–1287 [DOI] [PubMed] [Google Scholar]
- Kellner M., Noonepalle S., Lu Q., Srivastava A., Zemskov E., and Black S.M. (2017). ROS signaling in the pathogenesis of acute lung injury (ALI) and acute respiratory distress syndrome (ARDS). Adv Exp Med Biol 967, 105–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidane D., Chae W.J., Czochor J., Eckert K.A., Glazer P.M., Bothwell A.L., et al. (2014). Interplay between DNA repair and inflammation, and the link to cancer. Crit Rev Biochem Mol Biol 49, 116–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H., Jung H.I., Kwon S.H., Bae S.H., Kim H.C., Baek M.J., et al. (2019). Preoperative neutrophil-lymphocyte ratio and CEA is associated with poor prognosis in patients with synchronous colorectal cancer liver metastasis. Ann Surg Treat Res 96, 191–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopylov U., and Seidman E. (2016). Predicting durable response or resistance to antitumor necrosis factor therapy in inflammatory bowel disease. Ther Adv Gastroenterol 9, 513–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakatos L., Mester G., Erdelyi Z., David G., Pandur T., Balogh M., et al. (2006). Risk factors for ulcerative colitis-associated colorectal cancer in a Hungarian cohort of patients with ulcerative colitis: results of a population-based study. Inflamm Bowel Dis 12, 205–211 [DOI] [PubMed] [Google Scholar]
- Langhorst J., Elsenbruch S., Koelzer J., Rueffer A., Michalsen A., and Dobos G.J. (2008). Noninvasive markers in the assessment of intestinal inflammation in inflammatory bowel diseases: performance of fecal lactoferrin, calprotectin, and PMN-elastase, CRP, and clinical indices. Am J Gastroenterol 103, 162–169 [DOI] [PubMed] [Google Scholar]
- Levine M.S., and Holland A.J. (2018). The impact of mitotic errors on cell proliferation and tumorigenesis. Genes Dev 32, 620–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T., and Chen Z.J. (2018). The cGAS-cGAMP-STING pathway connects DNA damage to inflammation, senescence, and cancer. J Exp Med 215, 1287–1299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S.T., and Fu Y.H. (2009). miR-23 regulation of lamin B1 is crucial for oligodendrocyte development and myelination. Dis Model Mech 2, 178–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes M., Cotta-Ramusino C., Pellicioli A., Liberi G., Plevani P., Muzi-Falconi M., et al. (2001). The DNA replication checkpoint response stabilizes stalled replication forks. Nature 412, 557–561 [DOI] [PubMed] [Google Scholar]
- Mao Z., Bozzella M., Seluanov A., and Gorbunova V. (2008). Comparison of nonhomologous end joining and homologous recombination in human cells. DNA Repair 7, 1765–1771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matricon J., Barnich N., and Ardid D. (2010). Immunopathogenesis of inflammatory bowel disease. Self Nonself 1, 299–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta A., and Haber J.E. (2014). Sources of DNA double-strand breaks and models of recombinational DNA repair. Cold Spring Harbor Perspect Biol 6, a016428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mijic S., Zellweger R., Chappidi N., Berti M., Jacobs K., Mutreja K., et al. (2017). Replication fork reversal triggers fork degradation in BRCA2-defective cells. Nat Commun 8, 859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon J.J., Lu A., and Moon C. (2019). Role of genomic instability in human carcinogenesis. Exp Biol Med (Maywood) 244, 227–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negrini S., Gorgoulis V.G., and Halazonetis T.D. (2010). Genomic instability—an evolving hallmark of cancer. Nat Rev Mol Cell Biol 11, 220–228 [DOI] [PubMed] [Google Scholar]
- Ng L.G., Ostuni R., and Hidalgo A. (2019). Heterogeneity of neutrophils. Nat Rev Immunol 19, 255–265 [DOI] [PubMed] [Google Scholar]
- Nguyen G.T., Green E.R., and Mecsas J. (2017). Neutrophils to the ROScue: mechanisms of NADPH oxidase activation and bacterial resistance. Front Cell Infect Microbiol 7, 373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida Y., Hosomi S., Yamagami H., Yukawa T., Otani K., Nagami Y., et al. (2017). Neutrophil-to-lymphocyte ratio for predicting loss of response to infliximab in ulcerative colitis. PLoS One 12, e0169845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissen L.H., Assendorp E.L., van der Post R.S., Derikx L.A., de Jong D.J., Kievit W., et al. (2016). Impaired gastric cancer survival in patients with inflammatory bowel disease. J Gastrointestin Liver Dis 25, 431–440 [DOI] [PubMed] [Google Scholar]
- Ording A.G., Horvath-Puho E., Erichsen R., Long M.D., Baron J.A., Lash T.L., et al. (2013). Five-year mortality in colorectal cancer patients with ulcerative colitis or Crohn's disease: a nationwide population-based cohort study. Inflamm Bowel Dis 19, 800–805 [DOI] [PubMed] [Google Scholar]
- Ozdemir Y., Akin M.L., Sucullu I., Balta A.Z., and Yucel E. (2014). Pretreatment neutrophil/lymphocyte ratio as a prognostic aid in colorectal cancer. Asian Pac J Cancer Prev 15, 2647–2650 [DOI] [PubMed] [Google Scholar]
- Philip A.K., and Philip B. (2010). Colon targeted drug delivery systems: a review on primary and novel approaches. Oman Med J 25, 79–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitt J.M., Kroemer G., and Zitvogel L. (2016). Extracellular vesicles: masters of intercellular communication and potential clinical interventions. J Clin Invest 126, 1139–1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramalingam S., Subramaniam D., and Anant S. (2015). Manipulating miRNA expression: a novel approach for colon cancer prevention and chemotherapy. Curr Pharmacol Rep 1, 141–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raynes Y., and Weinreich D.M. (2019). Genomic clustering of fitness-affecting mutations favors the evolution of chromosomal instability. Evol Appl 12, 301–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder F., Brenmoehl J., Leeb S., Scholmerich J., and Rogler G. (2007). Wound healing and fibrosis in intestinal disease. Gut 56, 130–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogler G., Vavricka S., Schoepfer A., and Lakatos P.L. (2013). Mucosal healing and deep remission: what does it mean? World J Gastroenterol 19, 7552–7560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekirov I., Gill N., Jogova M., Tam N., Robertson M., de Llanos R., et al. (2010). Salmonella SPI-1-mediated neutrophil recruitment during enteric colitis is associated with reduction and alteration in intestinal microbiota. Gut Microbes 1, 30–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shai E., Rosa I., Parguina A.F., Motahedeh S., Varon D., and Garcia A. (2012). Comparative analysis of platelet-derived microparticles reveals differences in their amount and proteome depending on the platelet stimulus. J Proteomics 76 Spec No., 287–296 [DOI] [PubMed] [Google Scholar]
- Shang K., Bai Y.P., Wang C., Wang Z., Gu H.Y., Du X., et al. (2012). Crucial involvement of tumor-associated neutrophils in the regulation of chronic colitis-associated carcinogenesis in mice. PLoS One 7, e51848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibutani M., Maeda K., Nagahara H., Noda E., Ohtani H., Nishiguchi Y., et al. (2013). A high preoperative neutrophil-to-lymphocyte ratio is associated with poor survival in patients with colorectal cancer. Anticancer Res 33, 3291–3294 [PubMed] [Google Scholar]
- Silberer H., Kuppers B., Mickisch O., Baniewicz W., Drescher M., Traber L., et al. (2005). Fecal leukocyte proteins in inflammatory bowel disease and irritable bowel syndrome. Clin Lab 51, 117–126 [PubMed] [Google Scholar]
- Slater T.W., Finkielsztein A., Mascarenhas L.A., Mehl L.C., Butin-Israeli V., and Sumagin R. (2017). Neutrophil microparticles deliver active myeloperoxidase to injured mucosa to inhibit epithelial wound healing. J Immunol 198, 2886–2897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soroosh A., Koutsioumpa M., Pothoulakis C., and Iliopoulos D. (2018). Functional role and therapeutic targeting of microRNAs in inflammatory bowel disease. Am J Physiol Gastrointest Liver Physiol 314, G256–G262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stallmach A., Hagel S., and Bruns T. (2010). Adverse effects of biologics used for treating IBD. Best Pract Res Clin Gastroenterol 24, 167–182 [DOI] [PubMed] [Google Scholar]
- Tercero J.A., and Diffley J.F. (2001). Regulation of DNA replication fork progression through damaged DNA by the Mec1/Rad53 checkpoint. Nature 412, 553–557 [DOI] [PubMed] [Google Scholar]
- Torun S., Tunc B.D., Suvak B., Yildiz H., Tas A., Sayilir A., et al. (2012). Assessment of neutrophil-lymphocyte ratio in ulcerative colitis: a promising marker in predicting disease severity. Clin Res Hepatol Gastroenterol 36, 491–497 [DOI] [PubMed] [Google Scholar]
- Wang X., Qiu L., Li Z., Wang X.Y., and Yi H. (2018). Understanding the multifaceted role of neutrophils in cancer and autoimmune diseases. Front Immunol 9, 2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber D.A., Sumagin R., McCall I.C., Leoni G., Neumann P.A., Andargachew R., et al. (2014). Neutrophil-derived JAML inhibits repair of intestinal epithelial injury during acute inflammation. Mucos Immunol 7, 1221–1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wera O., Lancellotti P., and Oury C. (2016). The dual role of neutrophils in inflammatory bowel diseases. J Clin Med 5, pii: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright H.L., Moots R.J., Bucknall R.C., and Edwards S.W. (2010). Neutrophil function in inflammation and inflammatory diseases. Rheumatology (Oxford) 49, 1618–1631 [DOI] [PubMed] [Google Scholar]
- Yanai H., Lichtenstein L., Assa A., Mazor Y., Weiss B., Levine A., et al. (2015). Levels of drug and antidrug antibodies are associated with outcome of interventions after loss of response to infliximab or adalimumab. Clin Gastroenterol Hepatol 13, 522–530 e2. [DOI] [PubMed] [Google Scholar]
- Yang P., Li Y., Xie Y., and Liu Y. (2019). Different faces for different places: heterogeneity of neutrophil phenotype and function. J Immunol Res 2019, 8016254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousry T.A., Major E.O., Ryschkewitsch C., Fahle G., Fischer S., Hou J., et al. (2006). Evaluation of patients treated with natalizumab for progressive multifocal leukoencephalopathy. N Engl J Med 354, 924–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisel M.B., and Baumert T.F. (2017). Clinical development of hepatitis C virus host-targeting agents. Lancet 389, 674–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeman M.K., and Cimprich K.A. (2014). Causes and consequences of replication stress. Nat Cell Biol 16, 2–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenlea T., and Peppercorn M.A. (2014). Immunosuppressive therapies for inflammatory bowel disease. World J Gastroenterol 20, 3146–3152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X., Dai Q., and Huang X. (2012). Neutrophils in acute lung injury. Front Biosci (Landmark Ed) 17, 2278–2283 [DOI] [PubMed] [Google Scholar]