Morbidity and mortality associated with Coronavirus disease 2019 (COVID-19) is substantial, and underlying cardiovascular disease (CVD) is a risk factor for severe disease.1,2 Early retrospective case series of hospitalized patients suggested that biomarkers reflecting CVD and inflammation were strongly associated with poor prognosis.1–4 These studies identified C-reactive protein, lactate dehydrogenase, ferritin, cardiac troponins, N-terminal pro-B-type natriuretic peptide, and D-dimer as markers of increased risk. Retrospective studies carry significant risk of selection bias, because the indication for measurements is at the discretion of the treating physician. We aimed to overcome such limitations by prospectively investigating associations between CVD and inflammatory biomarkers and COVID-19.
COVID-MECH (COVID-19 Mechanisms Study; NCT04314232) was a prospective, observational study enrolling consecutive adult patients hospitalized with laboratory-confirmed COVID-19 at Akershus University Hospital (Norway) March 18 to May 4, 2020. Study-specific consent forms were signed by participants or next-of-kin for patients unable to consent. The study was approved by local regulatory authorities. Study data are not publicly available because of Norwegian General Data Protection Regulation.
The primary end point in COVID-MECH was the composite of hospital mortality or admission to the intensive care unit prompted by need for mechanical ventilation and lasting >24 hours. Follow-up to 30 days or discharge or death was complete. Clinical information was extracted from electronic medical records by the investigators. Blood samples were drawn by venipuncture at admission in the emergency department and analyzed immediately at the central laboratory. The association between log-transformed admission biomarker concentrations and the primary end point was examined in univariable and multivariable logistic regression models adjusting for age, sex, and race (model 1). Model 2 additionally adjusted for CVD, body mass index, estimated glomerular filtration rate, and symptom duration. Model 3 additionally adjusted for National Early Warning Scores (NEWS), calculated from admission respiratory rate, oxygen saturation, supplemental oxygen, systolic blood pressure, pulse rate, temperature, and level of consciousness.5 Discrimination for the primary end point was assessed using area under the receiver operating curve. Reclassification was calculated using the Net Reclassification Index.
In total, 131 of 135 patients included in COVID MECH had blood samples available for this analysis. Mean (SD) age was 59.6±14.1 (range 25–86) years; 80 (61%) patients were men; and 79 (60%) had ≥1 comorbidity: 39 (30%) hypertension, 37 (29%) obesity, 17 (13%) CVD, 22 (17%) diabetes, 8 (6%) chronic kidney disease, and 6 (5%) chronic obstructive pulmonary disease. Seventy (53%) patients were White; these were older (mean 65.4±13.4 versus 53.1±11.8 years; P<0.001) and had a comparable comorbidity burden (66% versus 54%; P=0.18) to non-Whites. Time from symptom start to hospitalization was 9.4±4.5 days; 106 (81%) patients had a fever, 106 (81%) had cough, and 94 (72%) had dyspnea. Admission body temperature was 38.0±0.9 °C, respiratory rate was 27±9/min, systolic blood pressure was 132±18 mm Hg, and oxygen saturation was 93±6%.
Forty patients (31%) reached the primary end point, of whom 36 were admitted to the intensive care unit (34 were treated with invasive mechanical ventilation) and 8 died. NEWS score at admission, white blood cell count, C-reactive protein, ferritin, lactate dehydrogenase, D-dimer, N-terminal pro-B-type natriuretic peptide, and high-sensitivity cardiac troponin T were higher among patients reaching the primary end point (Table). However, after adjusting for demographics (model 1), this association was attenuated for the cardiovascular biomarkers D-dimer, N-terminal pro-B-type natriuretic peptide, and high-sensitivity cardiac troponin T. In models additionally adjusting for CVD, body mass index, estimated glomerular filtration rate, and symptom duration (model 2) and NEWS score (model 3), only ferritin and lactate dehydrogenase remained associated with the primary end point. The areas under the receiver operating curve of all biomarkers for the primary end point were inferior to the NEWS score. No biomarker improved prognostic reclassification of patients if added to the NEWS score. Similar results were found when assessing in-hospital mortality: unadjusted associations between outcome and D-dimer (P=0.01), N-terminal pro-B-type natriuretic peptide (P=0.016), and high-sensitivity cardiac troponin T (P<0.001) were attenuated when adjusting for demographics (P=0.11, P=0.28, and P=0.57, respectively).
Table.
Cardiovascular and Inflammatory Biomarkers Measured at Hospital Admission and Their Associations With Hospital Mortality or Admission to the Intensive Care Unit in Unselected Patients Hospitalized for Coronavirus Disease 2019
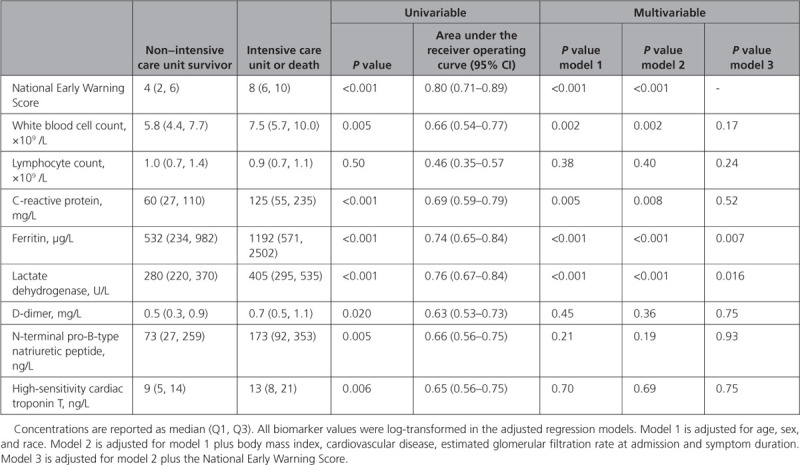
In our prospective study of unselected patients hospitalized with COVID-19, measurements of established cardiovascular biomarkers at admission did not provide prognostic information beyond that obtained from clinical characteristics and NEWS. Higher concentrations of ferritin and lactate dehydrogenase were associated with poor outcome, but were inferior to NEWS as discriminators for the primary end point. Although cardiovascular biomarker levels were higher among patients in the intensive care unit and nonsurvivors, this difference did not persist when accounting for clinical characteristics. These findings are in contrast with retrospective reports from China.1–4 The discrepancies may relate to differences in trial design. If baseline risk influences the indication for measuring biomarkers, selection bias is introduced. In addition, we measured troponin by a high-sensitivity assay as a continuous variable, which allowed precise quantification of concentrations within the normal range. This provides a more comprehensive assessment of myocardial injury than conventional assays and the use of arbitrary cutoffs. Our study had limited sample size, it might suffer from type 2 errors, and we did not adjust for multiple testing. We only evaluated admission biomarker levels and did not assess their diagnostic value. In conclusion, our findings do not support routine measurements of cardiovascular and inflammatory biomarkers on admission for prognostic purposes in patients hospitalized for COVID-19.
Acknowledgments
We are grateful for the invaluable contributions by study biochemists S. Navaruban and A. Meklif, and study nurses J. Dokken and A. Abueg. We also thank H. Husby and the Unit of Data Analysis at Akershus University Hospital, Lørenskog, Norway, for help with clinical data acquisition from the data warehouse at Akershus University Hospital.
Sources of Funding
Dr Myhre is supported by grants from the South-Eastern Norway Regional Health Authority.
Disclosures
Dr Omland has served on advisory boards for Abbott Diagnostics, Roche Diagnostics, and Bayer, and has received research support from Abbott Diagnostics, Novartis, Roche Diagnostics, Singulex, and SomaLogic via Akershus University Hospital, and speaker’s or consulting honoraria from Roche Diagnostics, Siemens Healthineers, and CardiNor. Dr Røsjø has received personal fees from Novartis and Thermo Fischer BRAMS, CardiNor, and SpinChip Diagnostics. Dr Myhre has served on advisory boards for Novartis and Novo Nordisk, and has received consulting honoraria from Novartis, AmGen, and Novo Nordisk. All other authors report no relevant disclosures.
Footnotes
This manuscript was sent to Prof Allan S. Jaffe, Guest Editor, for review by expert referees, editorial decision, and final disposition.
Contributor Information
Christian Prebensen, Email: christian.prebensen@gmail.com.
Ragnhild Røysland, Email: ragnhiro@medisin.uio.no.
Signe Søvik, Email: signe.sovik@medisin.uio.no.
Vibecke Sørensen, Email: Vibecke.Sorensen@ahus.no.
Helge Røsjø, Email: helge.rosjo@medisin.uio.no.
My Svensson, Email: m.h.s.svensson@medisin.uio.no.
Jan Erik Berdal, Email: jan-erik.berdal@hotmail.com.
Peder L. Myhre, Email: p.l.myhre@medisin.uio.no.
References
- 1.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, et al. ; China Medical Treatment Expert Group for Covid-19. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, Gong W, Liu X, Liang J, Zhao Q, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5:802–810. doi: 10.1001/jamacardio.2020.0950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ni W, Yang X, Liu J, Bao J, Li R, Xu Y, Guo W, Hu Y, Gao Z. Acute myocardial injury at hospital admission is associated with all-cause mortality in COVID-19. J Am Coll Cardiol. 2020;76:124–125. doi: 10.1016/j.jacc.2020.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Royal College of Physicians. National Early Warning Score (NEWS) 2. December 19, 2017Accessed August 6, 2020. https://www.rcplondon.ac.uk/projects/outputs/national-early-warning-score-news-2


