Study Design:
This was a single-center, retrospective study.
Objective:
The objective of this study was to assess the risk factors for deformity progression after scoliosis correction surgery in spinal muscular atrophy (SMA) patients.
Summary of Background Data:
Moderate residual postoperative scoliosis curve is common in SMA populations; however, the acceptable postoperative scoliosis curve for preventing deformity progression remains uncertain.
Materials and Methods:
Twenty-nine SMA patients undergoing scoliosis correction surgery were included. Scoliosis progression was defined as an increase of 10 degrees in the major curve of Cobb angle (MCCA); pelvic obliquity (PO) or concave-side hip progression was arbitrarily defined as an increase of ≥1 grade after surgery. Risk factors for deformity progression were examined using Cox proportional hazard models. The cumulative incidence rate of deformity progression was performed by the Kaplan-Meier survival analysis
Results:
The mean age at surgery was 13.3 years (range: 8–25 y) and the mean follow-up time was 7 years (range: 2–22.9 y). The mean MCCA was corrected from 69 to 34.6 degrees at initial follow-up and 42.2 degrees at the final follow-up. Postoperative MCCA (P=0.002) and PO (P=0.004) at initial follow-up were the risk factors for scoliosis progression. Postoperative MCCA at initial follow-up (P=0.007) and age at the time of surgery (P=0.017) were the risk factors for PO progression. Different cutoff points of postoperative MCCA at initial follow-up were compared for predicting deformity progression. We found the patient with postoperative MCCA of <30 degrees at initial follow-up had a significantly less cumulative incidence rate of progression than their counterparts for scoliosis (P=0.005), PO (P=0.023), and concave-side hip progressions (P=0.008).
Conclusions:
We recommended that MCCA should be corrected to <30 degrees to prevent postoperative scoliosis, PO, and concave-side femoral head coverage percentage progressions. Patients receiving surgery earlier had less postoperative MCCA at initial follow-up but with no increase in the risk of postoperative scoliosis progression.
Key Words: spinal muscular atrophy, scoliosis, pelvic obliquity
Spinal muscular atrophy (SMA) is an inherited autosomal recessive neuromuscular disease that is caused by mutations in the survival of motor neuron gene 1 (SMN1). SMA typically occurs in infancy or early childhood and the syndrome is progressive proximal muscle weakness resulting in respiratory compromise and hypotonic immobility.1,2 Scoliosis, pelvic obliquity (PO), and hip subluxation or dislocation occur frequently in nonambulatory SMA patients.3–5 Scoliosis is typically progressive in nature and increases 5–15 degrees annually, resulting in severe deformity. Surgical correction is performed for patients with scoliosis, which can improve sitting balance and quality of life, maintain pulmonary function, and reduce the frequency of respiratory infections.6–8
The mean correction percentage for patients with scoliosis, reviewed by Fujak et al5 was 49% (range: 37%–65%). Even with the advances in pedicle screw instrumentation design, complete scoliosis correction remains difficult.5,9 The major curve of Cobb angle (MCCA) of postoperative residual scoliosis deformity ranging from 18 to 69 degrees has been reported in SMA patients (Table 1).5,8–18,20–24 Postoperative deformity progression may be a potential complication for SMA patients.20 To our best knowledge, the relationship between residual deformity and deformity progression has not been well investigated in SMA patients.
TABLE 1.
Result of Surgical Treatment of Scoliosis in SMA: Review of the Relevant Literature
| Major Curve of Cobb Angle (deg.) | ||||||
|---|---|---|---|---|---|---|
| References | Patient Number | Mean Age at Surgery | Length of Follow-up (y) | Preoperative | Postoperative | Final Follow-up |
| Evans et al10 | 11 | 12 | 6.3 (2–12) | 87.8±21.0 | 44.6±20.3 | 54.9±22.2 |
| Aprin et al8 | 22 | 12 | 5.7 (0.5–14.7) | 89.1±25.7 | 47.91±20.0 | 52.4±23.6 |
| Riddick et al11 | 16 | 16.3 | 2.6 (0–7) | 95.8±31.7 | 61.9±23.0 | — |
| Daher et al12 | 15 | 13.7 | 2.6 (1–4) | 92.5±28.22 | 46.9±21.0 | 54.1±23.6 |
| Broom et al13 | 40 | 12.0 | 8.5 (2–19) | 61.2±20.2 | 33.2±16.4 | 37.3±17.3 |
| Piasecki et al14 | 19 | 15.5 | 5.6 (0.2–13.9) | 110.7±21.7 | 69.6±28.1 | 83.4±31.4 |
| Granata et al15 | 15 | 16 | 5.3 (3–10.2) | 105.8±30.3 | 53.6±18.7 | 58.7±18.7 |
| Robinson et al16 | 16 | 13.6 | 3.7 (0–9.5) | 87.1±25.4 | 52.9±20.8 | 61.8±21.6 |
| Bentley et al9 | 33 | — | — | 92 (50–150) | 45 | — |
| Chong et al17 | 8 | 9.6 | — | 65.4±18 | 22.6±9.5 | — |
| Modi et al18 | 9 | 15.2 | 2.6 | 86.8±30.3 | 30.5±20.9 | 33.1±21.6 |
| Chong et al19 | 11 | 12.3 | 1.9±1.0 | 80.7±22.5 | 39.0±19.6 | 41.7±18.7 |
| Zebala et al20 | 22 | 8.4 | 8.2 (5.1–12.8) | 76.5±21.6 | 29.8±15.7 | 39.3±15.9 |
| Chandran et al21 | 11 | 6 | 3.6 (2–6.3) | 51.5 (38–76) | 21.6 (2–34) | 18.7 (5–34) |
| Fujak et al5 | ||||||
| Group A* | 24 | 12.3 | 8.6 (3–12.2) | 83±17 | 39±18 | — |
| Group B* | 17 | 6.7 | 6.1 (2.9–10.2) | 62±16 | 18±10 | — |
| Lenhart et al22 | 16 | 5.8 | 4.7 (2.7–9.5) | 70.7±24.6 | 27.2±8.9 | 23.4±11.9 |
| Holt et al23 | 16 | 9.8 | 10.1 (3.1–26) | 78±20 | 25±20 | 27±24 |
*Groups A and B were stabilized with multisegmental instrumentation and telescopic rod, respectively.
SMA indicates spinal muscular atrophy.
The purpose of the present study was (1) to investigate the predictors for postoperative deformity progression in SMA patients, and (2) to suggest a tolerant postoperative residual deformity for preventing further deformity progression after surgery.
MATERIALS AND METHODS
Participants
This study was approved by the Institutional Review Board of Kaohsiung Medical University Chung-Ho Memorial Hospital (IRB-KMUH-20140280). The SMA patients who underwent scoliosis correction surgery at our institute between 1993 and 2016 were retrospectively reviewed after IRB approval. The disease of SMA was diagnosed by pediatric neurologists according to SMN1 gene deletion or mutation and/or neuropathic changes in a muscle biopsy or electromyogram. The indication of scoliosis correction surgery included MCCA ≥40 degrees, forced vital capacity ≥40%, age at the time of surgery 25 years or younger, and SMA with types II and III. These patients after surgery were followed up at a regular interval of 6 months. Patients with <2 years of follow-up, incomplete clinical or radiographic data were excluded. Data were collected from preoperative, initial follow-up (within 6 wk after surgery), and each follow-up visit.
Surgery
SMA patients subsequently underwent surgery using segmental spinal instrumentation with pedicle screws or sublaminar wires and the Galveston pelvic fixation technique (Fig. 1). The fusion level extended from T2 or T3 to the pelvic. The pedicle screws were inserted as far as possible to correct the rotational deformity. However, the number of screws inserted may have been limited by a small pedicle size or distorted vertebral anatomy. To achieve successful bone fusion, the patients were asked to wear a Boston brace for at least 3 months.
FIGURE 1.
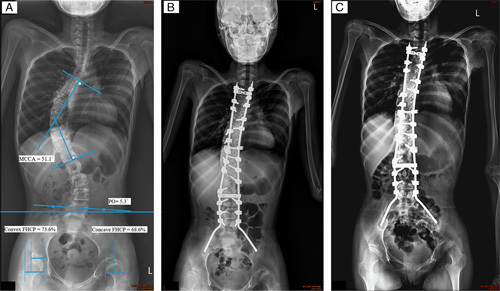
All the posterior-anterior radiography was made with the patient in supine position with the head, trunk, and lower extremities as anatomically straight as possible. A, Preoperative radiography of a 12-year-old girl with closed triradiate cartilage and a 51.1 scoliosis. The major curve of Cobb angle (MCCA), pelvic obliquity (PO), and femoral head coverage percentage (FHCP) were measured as previously described.4,15,16 B, The patient had received segmental spinal instrumentation with pedicle screws and the Galveston pelvic fixation technique. The fusion level was from T2 to the pelvis. Postoperative radiography showed that the residual MCCA was 21.1 degrees and the residual PO was 2 degrees. C, Postoperative radiography at 24 months, with no obvious change in MCCA or PO. “Windshield wiper” sign (iliac bone osteolysis) was observed but this did not cause fusion defects or decrease the PO correction.
Radiography Assessment and Grading for Deformity
A comprehensive radiographic assessment of long cassette posteroanterior radiographs was performed by 2 independent authors. The patients were carefully positioned in a supine position on the radiographic table with the head, trunk, and lower extremities as anatomically straight as possible. The major curve of scoliosis was measured using the Cobb method.25 We followed the previous reported method to measure the PO angle.4,26 An oblique line connected the most superior parts of the 2 iliac crests, and a transverse line was parallel to the lowermost exposure line of the radiograph. The angle formed between these 2 lines was defined as the PO angle (Fig. 1). The femoral head coverage percentage (FHCP) was defined as the percentage of the ossified femoral head covered by the ossified acetabular roof, and this was used to evaluate the position of the hip joints. PO and concave-side hip FHCP were converted to a scale of 1–5 as previously described.4 A patient with a PO of 0–1.9 degrees was defined as normal (grade 1), 2–4.9 degrees was mild (grade 2), 5–8.9 degrees was moderate (grade 3), 9–11.9 degrees was severe (grade 4), and ≥12 degrees was very severe (grade 5). A patient with an FHCP ≥67% was defined as normal (grade 1), 51%–66.9% was mild (grade 2), 33%–50.9% was moderate (grade 3), <33% was severe (grade 4), and no coverage was classified as complete dislocation (grade 5). Deformity progression was identified by the changes in MCCA, PO, and concave-side hip FHCP between initial and the other follow-ups. Scoliosis progression was defined as an increase of 10 degrees in MCCA.20 PO or concave-side hip progression was arbitrarily defined as an increase of ≥1 grade in PO or concave-side hip FHCP.
Statistical Analysis
All analyses were performed using the Statistical Package for the Social Sciences (Version 19.0; SPSS Inc., Chicago, IL). The intraclass correlation coefficient 2-way random model on the absolute agreement was used to analyze the interobserver reliability of the measurements. Patient demographic and radiographic data between progression and stable groups were examined using the Student t test or Fisher exact test. Radiographic data between 2 timepoints were examined using the paired t test, while the Pearson correlation was used for analysis between variables. The Cox proportional hazards regression analysis was used to assess risk factors for progression during the follow-up period. To analyze time-to-event data (an event defined as the incidence of deformity progression), we used the Kaplan-Meier survival analysis to estimate the nonincidence rate of each deformity progression after surgery. The log-rank test was used to compare the nonincidence distributions between 2 groups. A P-value <0.05 was considered significant.
RESULTS
Demographic Data and Radiographic Measurements
This study included 29 patients (13 males and 16 females) with a mean age of 13.3 years (range: 8–25 y) at the surgery and a mean follow-up time of 7 years (range: 2–22.9 y). Patients with SMA type II or with closed triradiate cartilage were dominant (type II: 24, type III: 5; open: 7, closed: 22). The interobserver reliability of angular and coverage percentage measurements was high (intraclass correlation coefficient value range: 0.959–0.996). All patients exhibited successful posterolateral spinal fusion during the follow-up period. Postoperative neurological deficits, wound infections, and/or screw or rod breakage was not observed in these patients.
Radiographic measurements at different timepoints are shown in Table 2. The mean MCCA was corrected from 69 to 34.6 degrees (P<0.001) at initial follow-up and 42.2 degrees (P<0.001) at the final follow-up. The mean PO was corrected from 12.1 to 8 degrees at initial follow-up (P=0.004) and 10.7 degrees at the final follow-up (P=0.209). The mean concave-side hip FHCP and convex-side hip FHCP were not significantly affected by surgery at initial (concave-side: P=0.100; convex-side: P=0.961) and final (concave-side: P=0.605; convex-side: P=0.872) follow-ups.
TABLE 2.
Radiographic Measurements for All SMA Patients
| Timepoint | P | ||||
|---|---|---|---|---|---|
| Radiographic Measurement | Preoperative | Initial Follow-up | Final Follow-up | Preoperative vs. Initial Follow-up | Preoperative vs. Final Follow-up |
| MCCA (deg.) | 69.0±20.9 | 34.6±15.8 | 42.2±23.0 | <0.001 | <0.001 |
| Pelvic obliquity (deg.) | 12.1±9.3 | 8.0±6.6 | 10.7±8.2 | 0.004 | 0.209 |
| Concave-side hip FHCP (%) | 45.2±27.7 | 49.9±28.2 | 43.2±33.5 | 0.100 | 0.605 |
| Convex-side hip FHCP (%) | 80.9±16.9 | 80.8±15.2 | 81.2±21.0 | 0.961 | 0.872 |
Bolded values denote statistical significance to P<0.05.
FHCP indicates femoral head coverage percentage; MCCA, major curve of Cobb angle; SMA, spinal muscular atrophy.
Deformity Progression and Stable Groups
Radiographic measurements for the progression and stable groups at different follow-up timepoints are shown in Table 3. When progression and stable groups were separated based on MCCA change, preoperative mean MCCA was significantly larger for the progression group than for the stable group (P=0.027). Similar results also occurred at initial (P=0.017) and final follow-ups (P<0.001). The progression group lost 20.6 degrees of MCCA (P<0.001) and the stable group lost 0.7 degrees of MCCA (P=0.463) between initial and final follow-ups.
TABLE 3.
Radiographic Measurements of Progression and Stable Patients
| Timepoint | P | ||||
|---|---|---|---|---|---|
| Radiographic Measurements | Group | Preoperative | Initial Follow-up | Final Follow-up | Initial Follow-up vs. Final Follow-up |
| Scoliosis progression group: postoperative MCCA increase >10 degrees | |||||
| MCCA (deg.) | Progression | 80.7±23.9 | 44.1±15.1 | 64.7±18.8 | <0.001 |
| Stable | 62.9±16.75 | 29.7±14.1 | 30.4±14.9 | 0.463 | |
| P | 0.027 | 0.017 | <0.001 | ||
| Pelvic obliquity progression group: postoperative pelvic obliquity increase ≥1 grade | |||||
| Pelvic obliquity (deg.) | Progression | 14.1±10.9 | 5.9±3.1 | 15.0±8.2 | 0.001 |
| Stable | 8.6±6.9 | 5.6±4.0 | 5.2±3.0 | 0.417 | |
| P | 0.165 | 0.840 | 0.004 | ||
| Concave-side hip progression group: concave-side hip increase ≥1 grade | |||||
| Concave-side hip FHCP (%) | Progression | 38.9±15.9 | 45.9±21.2 | 24.8±24.0 | 0.001 |
| Stable | 58.9±26.2 | 62.8±23.4 | 65.3±25.2 | 0.541 | |
| P | 0.035 | 0.071 | <0.001 | ||
Bolded values denote statistical significance to P<0.05.
FHCP indicates femoral head coverage percentage; MCCA, major curve of Cobb angle.
When the progression and stable groups were separated based on PO, the progression group had significantly larger PO than did the stable group only at the final follow-up (P=0.004). The progression group lost 9.1 degrees of PO (P=0.001), but no loss correction of PO (P=0.417) was found in the stable group between initial and final follow-ups.
When the progression and stable groups were separated based on concave-side hip FHCP, the progression group had significantly lesser concave-side hip FHCP than did the stable group only at preoperative (P=0.035) and final follow-up (P<0.001). Progression group decreased 21.1% (P=0.001) and stable group increased 2.5% (P=0.541) concave-side hip FHCP between initial and final follow-ups.
Association Between Age and Radiographic Measurements
Age was positively correlated with PO at preoperative (r=0.425, P=0.011), MCCA at initial follow-up (r=0.390, P=0.018), and PO at initial follow-up (r=0.337, P=0.037), but not correlated with MCCA at preoperative (r=0.217, P=0.129), MCCA at final follow-up (r=0.243, P=0.102), and PO at final follow-up (r=0.282, P=0.069). Age was not correlated with change (between initial and final follow-ups) of MCCA (r=−0.050, P=0.799) and PO (r=0.009, P=0.962). The change in MCCA was positively correlated with that in PO (r=0.524, P=0.004).
Risk Factors for Deformity Progression
Cox regression analyses revealed that MCCA at initial follow-up was positively associated with postoperative progression in MCCA and PO even after adjusting for confounding factors (including age, sex, SMA type, and body mass index) (Tables 4, 5). Preoperative PO was positively associated with the progression in MCCA and PO even after adjusting for the confounding factors. PO at initial follow-up was positively associated with progression in MCCA and concave-side FHCP even after adjusting for the confounding factors. After adjusting for the confounding factors, the open triradiate cartilage (P=0.043) was negatively associated with postoperative PO progression.
TABLE 4.
Cox Regression Analyses of Postoperative Deformity Progression in SMA Patients
| Scoliosis | PO | Concave-side Hip | ||||
|---|---|---|---|---|---|---|
| Progression (n=10) vs. Stable (n=19) | Progression (n=10) vs. Stable (n=14) | Progression (n=11) vs. Stable (n=15) | ||||
| Variables | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P |
| Age at surgery (y) | 1.03 (0.858–1.229) | 0.776 | 1.23 (1.036–1.448) | 0.017 | 0.89 (0.718–1.100) | 0.277 |
| Sex (male vs. female) | 0.24 (0.054–1.080) | 0.063 | 0.53 (0.130–2.183) | 0.382 | 0.41 (0.115–1.462) | 0.169 |
| BMI (kg/m2) | 0.98 (0.851–1.118) | 0.724 | 0.85 (0.707–1.025) | 0.089 | 0.97 (0.851–1.098) | 0.602 |
| SMA type (III vs. II) | 1.38 (0.285–6.709) | 0.687 | 4.73 (0.665–33.560) | 0.120 | 2.90 (0.692–12.180) | 0.145 |
| Pedicle screw use (yes vs. no) | 1.73 (0.428–7.017) | 0.441 | 1.78 (0.432–7.301) | 0.426 | 1.52 (0.444–5.228) | 0.503 |
| Triradiate cartilage (open vs. closed) | 1.32 (0.263–6.580) | 0.738 | 0.36 (0.044–2.996) | 0.348 | 0.98 (0.257–3.704) | 0.972 |
| Initial follow-up | ||||||
| MCCA (deg.) | 1.107 (1.039–1.178) | 0.002 | 1.083 (1.022–1.148) | 0.007 | 1.045 (0.999–1.093) | 0.053 |
| PO (deg.) | 1.177 (1.054–1.314) | 0.004 | 1.099 (0.908–1.329) | 0.332 | 1.213 (1.027–1.432) | 0.023 |
| Concave-side FHCP (%) | 0.982 (0.958–1.006) | 0.145 | 0.982 (0.956–1.008) | 0.178 | 0.986 (0.963–1.010) | 0.248 |
| Convex-side FHCP (%) | 1.046 (0.980–1.116) | 0.174 | 1.036 (0.971–1.106) | 0.280 | 1.021 (0.975–1.068) | 0.383 |
| Cutoff points of postoperative MCCA at initial follow-up | ||||||
| MCCA ≥20 vs. <20 degrees | 32.91 (0.023–46801) | 0.346 | 35.13 (0.055–22304) | 0.280 | 2.51 (0.317–19.817) | 0.384 |
| MCCA ≥25 vs. <25 degrees | 46.15 (0.133–16043) | 0.199 | 1.99 (0.417–9.516) | 0.388 | 5.21 (0.665–40.841) | 0.116 |
| MCCA ≥30 vs. <30 degrees | 12.02 (1.448–99.727) | 0.021 | 5.20 (1.070–25.232) | 0.041 | 6.42 (1.356–30.381) | 0.019 |
| MCCA≥35 vs. <35 degrees | 6.67 (1.330–33.460) | 0.021 | 3.90 (0.963–15.765) | 0.057 | 4.59 (1.187–17.709) | 0.027 |
Bolded values denote statistical significance to P<0.05.
BMI indicates body mass index; CI, confidence interval; FHCP, femoral head coverage percentage; HR, hazard ratio; MCCA, major curve of Cobb angle; PO, pelvic obliquity; SMA, spinal muscular atrophy.
TABLE 5.
Cox Regression Analyses of Postoperative Deformity Progression in SMA Patients After Adjusting for Age, Sex, SMA Type, and BMI
| Scoliosis | PO | Concave-side Hip | ||||
|---|---|---|---|---|---|---|
| Progression (n=10) vs. Stable (n=19) | Progression (n=10) vs. Stable (n=14) | Progression (n=11) vs. Stable (n=15) | ||||
| Variables | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P |
| Pedicle screw use (yes vs. no) | 1.60 (0.367–7.016) | 0.530 | 1.71 (0.359–8.159) | 0.500 | 4.29 (0.753–24.414) | 0.101 |
| Triradiate cartilage (open vs. closed) | 0.10 (0.006–1.712) | 0.112 | 0.01 (0.00–0.866) | 0.043 | 0.12 (0.013–1.126) | 0.064 |
| Initial follow-up | ||||||
| MCCA (deg.) | 1.217 (1.051–1.410) | 0.009 | 1.132 (1.017–1.261) | 0.024 | 1.042 (0.988–1.099) | 0.130 |
| PO (deg.) | 1.188 (1.031–1.370) | 0.017 | 1.129 (0.888–1.436) | 0.323 | 1.181 (1.007–1.384) | 0.041 |
| Concave-side FHCP (%) | 0.982 (0.957–1.009) | 0.193 | 0.972 (0.940–1.005) | 0.094 | 0.980 (0.951–1.010) | 0.185 |
| Convex-side FHCP (%) | 1.041 (0.969–1.118) | 0.271 | 1.007 (0.948–1.070) | 0.815 | 1.022 (0.971–1.076) | 0.396 |
| Cutoff points of postoperative MCCA at initial follow-up | ||||||
| MCCA ≥20 vs. <20 degrees | * | * | * | * | 3.29 (0.378–28.623) | 0.280 |
| MCCA ≥25 vs. <25 degrees | * | * | 6.12 (0.519–72.124) | 0.150 | 4.98 (0.616–40.216) | 0.132 |
| MCCA ≥30 vs. <30 degrees | 17.75 (1.539–204) | 0.021 | 6.75 (0.947–48.184) | 0.057 | 7.08 (1.224–40.920) | 0.029 |
| MCCA≥35 vs. <35 degrees | 9.77 (1.161–82.151) | 0.036 | 3.41 (0.612–18.979) | 0.162 | 5.18 (1.128–23.785) | 0.034 |
Bolded values denote statistical significance to P<0.05.
*Estimated cannot be computed.
BMI indicates body mass index; CI, confidence interval; FHCP, femoral head coverage percentage; HR, hazard ratio; MCCA, major curve of Cobb angle; PO, pelvic obliquity; SMA, spinal muscular atrophy.
Cutoff Points of Postoperative MCCA at Initial Follow-up for Predicting Deformity Progression
Cox regression analyses revealed that MCCA of >30 degrees at initial follow-up had the highest risk of progression in MCCA and concave-side hip even after adjusting for the confounding factors (Tables 4, 5). In addition, MCCA of >30 degrees at initial follow-up had the highest risk of progression in PO.
Survival Analysis of Deformity Progression
When high-risk and low-risk groups were separated using MCCA=30 degrees at initial follow-up, the Kaplan-Meier survival curve analysis for cumulative nonincidence rate of progression between groups illustrates the difference as shown in Figure 2. The high-risk group (MCCA ≥30 degrees) had a significantly higher cumulative incidence rate of progression than did the low-risk group (MCCA <30 degrees) in scoliosis (P=0.005), PO (P=0.023), and concave-side hip (P=0.008) progressions.
FIGURE 2.
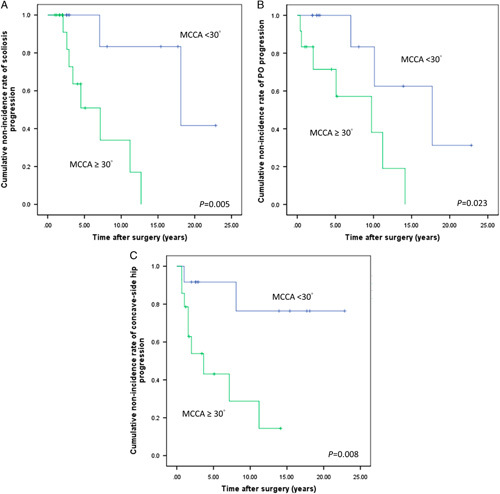
Kaplan-Meier survival analyses of large and low MCCA groups for scoliosis progression (A), pelvic obliquity progression (B), and concave-side hip progression (C). MCCA indicates major curve of Cobb angle.
DISCUSSION
Management of large scoliosis curve and PO in the SMA population is challenging and usually requires a long operative time to achieve an acceptable outcome. The longer operative time would increase the risk of blood loss and duration of admission. To prevent these events, it is crucial to suggest a tolerable residual deformity for SMA patients after scoliosis correction surgery. This study reported the radiographic measurements of SMA patients treated with surgical scoliosis correction at our institution. Overall, MCCA and PO were significantly improved at initial follow-up compared with preoperative measures. MCCA and PO at initial follow-up were the significant risk factors for postoperative scoliosis progression. Our results showed that when MCCA was corrected to <30 degrees, the risk for postoperative deformity progression in MCCA, PO, and concave-side hip would be significantly reduced.
To our best knowledge, the sample size of 29 patients with a long mean follow-up of 7 years (range: 2.0–22.9 y) in this study is one of the largest SMA cohorts reported in the literature. Bentley et al9 have investigated a slightly larger sample size of 33 surgical SMA patients with the average follow-up of 6.8 years. Zebala et al20 have reported surgical results of a smaller cohort of 22 surgical SMA patients, but its minimum follow-up time was 5 years. Aprin et al8 have also reported a smaller sample size of 22 SMA patients regarding comparable surgical outcomes but with a slightly short mean follow-up (5.7 y). A very small sample size of 11 surgical SMA patients in terms of radiologic, pulmonary and functional outcomes has been reported by Chong et al.19 Thus, our radiographic measurements using such a sample size could provide valuable information in the surgical SMA cohort.
To identify scoliosis progression in idiopathic, congenital, and neuromuscular scoliosis, an increase of 10 degrees in MCCA between initial and postoperative follow-ups has been widely adopted.20,27 An increase of 10 degrees has been reported to represent a true MCCA change with 95% confidence interval.28,29 Our results showed that the scoliosis progression group significantly lost correction at the final follow-up, indicating that an increase of 10 degrees in MCCA could be used to identify scoliosis progression. Otherwise, PO progression was investigated in this study, and at least 1-grade increase in PO was arbitrarily used to identify this progression. The results showed PO progression group significantly lost correction of PO at final follow-up, indicating a 1-grade increase in PO may be used to identify PO progression. Similar results were observed in the concave-side hip progression group. It implied that at least a 1-grade increase in PO and concave-side hip FHCP might be representative of deformity progression.
SMA types II and III showed different progressive trends in scoliosis for the patients who were not treated.30 SMA types II and III had 8 and 3 degrees increase annually, respectively. But SMA type was not associated with postoperative scoliosis progression in this study. This should be further confirmed using a larger multicenter database. Skeletal immaturity at surgery is generally considered to increase the risk for postoperative scoliosis progression. Previous studies have revealed that 23%–36% of SMA patients had crankshaft, defined as increments of MCCA of ≥10 degrees.8,20,31 Skeletal immaturity resulting in scoliosis progression has been reported in SMA patients.20 In adolescent idiopathic scoliosis, patients with open triradiate cartilage at surgery had a higher risk for scoliosis progression.32 In the present study, we observed that open triradiate cartilage or younger age at the surgery would not increase the risk of crankshaft phenomenon or other postoperative deformity progression in SMA patients. This observation may imply that other factors may contribute to postoperative scoliosis progression. For example, progressive osteoporosis, motor weakness and muscle atrophy, which are characteristics of SMA, may play a role in postoperative deformity progression.
We found MCCA at initial follow-up could be one of the most important risk factors for deformity progression in SMA patients. With regard to the acceptable MCCA at initial follow-up for preventing postoperative scoliosis progression, the previous studies have suggested that it should be <∼35 degrees following sublaminar spinal instrumentation.32,33 Although their suggestion was not specific for SMA patients, the suggestion is similar to our result (MCCA <30 degrees), indicating that our suggested angle in MCCA may be reliable. The factors affecting spinal correction in SMA population may include the osteoporotic bone, rigid deformity, and severe distorted anatomy. There is still no consensus with regard to the indication and effectiveness of aggressive surgical procedure (such as vertebral column resection) in SMA patients. Our results showed age was positively correlated with MCCA at initial follow-up, indicating that younger SMA patients might have less MCCA at initial follow-up. Thus, we suggested that SMA patients with progressive scoliosis should receive the scoliosis correction surgery as young as when their lungs become mature, which could reduce the MCCA at initial follow-up and thus reduce the risk of postoperative deformity progression.
There were several limitations in this study. First, it was a retrospective study, which contains original bias. Second, a small sample size of 29 patients would have lower power of statistical testing. Third, the effect of deformity flexibility on deformity correction surgery and postoperative progression was not considered in this study. Finally, we did not investigate the relationship between clinical outcomes (such as patient satisfaction and daily activity functions) and these radiographic measurements. To confirm our findings, these results should be validated using other SMA cohorts.
CONCLUSIONS
Deformity progression can occur in SMA patients after scoliosis correction surgery. Our results indicate that residual MCCA and PO at initial follow-up were the risk factors of deformity progression in MCCA for SMA patients after surgery. When MCCA was corrected to <30 degrees, the risk of deformity progression for MCCA, PO, and concave-side hip would be significantly reduced. SMA patients receiving surgery earlier tend to have smaller scoliosis deformity at initial follow-up and may have a lower risk for developing postoperative deformity progression.
ACKNOWLEDGMENTS
The authors thank Yi-Chun Hung for assistance with statistical analysis and Shu-Hua Ko for intraoperative neuromonitoring in scoliosis surgery.
Footnotes
The authors declare no conflict of interest.
REFERENCES
- 1.Lefebvre S, Burglen L, Reboullet S, et al. Identification and characterization of a spinal muscular atrophy-determining gene. Cell. 1995;80:155–165. [DOI] [PubMed] [Google Scholar]
- 2.Wirth B. An update of the mutation spectrum of the survival motor neuron gene (SMN1) in autosomal recessive spinal muscular atrophy (SMA). Hum Mutat. 2000;15:228–237. [DOI] [PubMed] [Google Scholar]
- 3.Fujak A, Raab W, Schuh A, et al. Natural course of scoliosis in proximal spinal muscular atrophy type II and IIIa: descriptive clinical study with retrospective data collection of 126 patients. BMC Musculoskelet Dis. 2013;14:283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel J, Shapiro F. Simultaneous progression patterns of scoliosis, pelvic obliquity, and hip subluxation/dislocation in non-ambulatory neuromuscular patients: an approach to deformity documentation. J Child Orthop. 2015;9:345–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fujak A, Raab W, Schuh A, et al. Operative treatment of scoliosis in proximal spinal muscular atrophy: results of 41 patients. Arch Orthop Trauma Surg. 2012;132:1697–1706. [DOI] [PubMed] [Google Scholar]
- 6.Phillips DP, Roye DP, Farcy JPC, et al. Surgical-treatment of scoliosis in a spinal muscular-atrophy population. Spine. 1990;15:942–945. [DOI] [PubMed] [Google Scholar]
- 7.Chou SH, Lin GT, Shen PC, et al. The effect of scoliosis surgery on pulmonary function in spinal muscular atrophy type II patients. Eur Spine J. 2017;26:1721–1731. [DOI] [PubMed] [Google Scholar]
- 8.Aprin H, Bowen JR, MacEwen GD, et al. Spine fusion in patients with spinal muscular atrophy. J Bone Joint Surg Am. 1982;64:1179–1187. [PubMed] [Google Scholar]
- 9.Bentley G, Haddad F, Bull TM, et al. The treatment of scoliosis in muscular dystrophy using modified Luque and Harrington-Luque instrumentation. J Bone Joint Surg Br. 2001;83b:22–28. [DOI] [PubMed] [Google Scholar]
- 10.Evans GA, Drennan JC, Russman BS. Functional classification and orthopaedic management of spinal muscular atrophy. J Bone Joint Surg Br. 1981;63B:516–522. [DOI] [PubMed] [Google Scholar]
- 11.Riddick MF, Winter RB, Lutter LD. Spinal deformities in patients with spinal muscle atrophy: a review of 36 patients. Spine (Phila Pa 1976). 1982;7:476–483. [DOI] [PubMed] [Google Scholar]
- 12.Daher YH, Lonstein JE, Winter RB, et al. Spinal surgery in spinal muscular atrophy. J Pediatr Orthop. 1985;5:391–395. [DOI] [PubMed] [Google Scholar]
- 13.Broom MJ, Banta JV, Renshaw TS. Spinal fusion augmented by luque-rod segmental instrumentation for neuromuscular scoliosis. J Bone Joint Surg Am. 1989;71:32–44. [PubMed] [Google Scholar]
- 14.Piasecki JO, Mahinpour S, Levine DB. Long-term follow-up of spinal fusion in spinal muscular atrophy. Clin Orthop Relat Res. 1986;207:44–54. [PubMed] [Google Scholar]
- 15.Granata C, Cervellati S, Ballestrazzi A, et al. Spine surgery in spinal muscular atrophy: long-term results. Neuromuscul Disord. 1993;3:207–215. [DOI] [PubMed] [Google Scholar]
- 16.Robinson D, Galasko CS, Delaney C, et al. Scoliosis and lung function in spinal muscular atrophy. Eur Spine J. 1995;4:268–273. [DOI] [PubMed] [Google Scholar]
- 17.Chng SY, Wong YQ, Hui JH, et al. Pulmonary function and scoliosis in children with spinal muscular atrophy types II and III. J Paediatr Child Health. 2003;39:673–676. [DOI] [PubMed] [Google Scholar]
- 18.Modi HN, Suh SW, Hong JY, et al. Treatment and complications in flaccid neuromuscular scoliosis (Duchenne muscular dystrophy and spinal muscular atrophy) with posterior-only pedicle screw instrumentation. Eur Spine J. 2010;19:384–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chong HS, Moon ES, Kim HS, et al. Comparison between operated muscular dystrophy and spinal muscular atrophy patients in terms of radiological, pulmonary and functional outcomes. Asian Spine J. 2010;4:82–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zebala LP, Bridwell KH, Baldus C, et al. Minimum 5-year radiographic results of long scoliosis fusion in juvenile spinal muscular atrophy patients: major curve progression after instrumented fusion. J Pediatr Orthop. 2011;31:480–488. [DOI] [PubMed] [Google Scholar]
- 21.Chandran S, McCarthy J, Noonan K, et al. Early treatment of scoliosis with growing rods in children with severe spinal muscular atrophy: a preliminary report. J Pediatr Orthop. 2011;31:450–454. [DOI] [PubMed] [Google Scholar]
- 22.Lenhart RL, Youlo S, Schroth MK, et al. Radiographic and respiratory effects of growing rods in children with spinal muscular atrophy. J Pediatr Orthop. 2017;37:e500–e504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holt JB, Dolan LA, Weinstein SL. Outcomes of primary posterior spinal fusion for scoliosis in spinal muscular atrophy: clinical, radiographic, and pulmonary outcomes and complications. J Pediatr Orthop. 2017;37:e505–e511. [DOI] [PubMed] [Google Scholar]
- 24.Brown JC, Zeller JL, Swank SM, et al. Surgical and functional results of spine fusion in spinal muscular atrophy. Spine (Phila Pa 1976). 1989;14:763–770. [DOI] [PubMed] [Google Scholar]
- 25.Diab KM, Sevastik JA, Hedlund R, et al. Accuracy and applicability of measurement of the scoliotic angle at the frontal plane by Cobb’s method, by Ferguson’s method and by a new method. Eur Spine J. 1995;4:291–295. [DOI] [PubMed] [Google Scholar]
- 26.Osebold WR, Mayfield JK, Winter RB, et al. Surgical-treatment of paralytic scoliosis associated with myelomeningocele. J Bone Joint Surg Am. 1982;64:841–856. [PubMed] [Google Scholar]
- 27.Hamill CL, Bridwell KH, Lenke LG, et al. Posterior arthrodesis in the skeletally immature patient—assessing the risk for crankshaft: is an open triradiate cartilage the answer? Spine. 1997;22:1343–1351. [DOI] [PubMed] [Google Scholar]
- 28.Carman DL, Browne RH, Birch JG. Measurement of scoliosis and kyphosis radiographs—intraobserver and interobserver variation. J Bone Joint Surg Am. 1990;72a:328–333. [PubMed] [Google Scholar]
- 29.Mannherz RE, Betz RR, Clancy M, et al. Juvenile idiopathic scoliosis followed to skeletal maturity. Spine. 1988;13:1087–1090. [DOI] [PubMed] [Google Scholar]
- 30.Granata C, Merlini L, Magni E, et al. Spinal muscular atrophy: natural history and orthopaedic treatment of scoliosis. Spine (Phila Pa 1976). 1989;14:760–762. [DOI] [PubMed] [Google Scholar]
- 31.Schwentker EP, Gibson DA. The orthopaedic aspects of spinal muscular atrophy. J Bone Joint Surg Am. 1976;58:32–38. [PubMed] [Google Scholar]
- 32.Sanders JO, Herring JA, Browne RH. Posterior arthrodesis and instrumentation in the immature (Risser-Grade-0) spine in idiopathic scoliosis. J Bone Joint Surg Am. 1995;77a:39–45. [DOI] [PubMed] [Google Scholar]
- 33.Smucker JD, Miller F. Crankshaft effect after posterior spinal fusion and unit rod instrumentation in children with cerebral palsy. J Pediatr Orthop. 2001;21:108–112. [DOI] [PubMed] [Google Scholar]


