Abstract
Background
Remission in schizophrenia is difficult to achieve. Antipsychotic drugs are critical in the treatment of schizophrenia. International guidelines for the pharmacological treatment of schizophrenia recommend a 3-step algorithm with clozapine being the third-line antipsychotic agent. This study investigated the 1-year outcome and the application of the guidelines for the pharmacological treatment of nonremitted first-episode schizophrenia (FES) patients during the first year of follow-up.
Methods
A sample of 78 FES patients from the Norwegian TIPS (Early Treatment and Intervention in Psychosis) 2 study was assessed at the end of the first year of follow-up. The symptom remission criteria were those defined by the Remission in Schizophrenia Working Group. The adherence to the pharmacological guidelines was assessed by reading the medical files and by a digital search of the words “clozapine,” “klozapin,” and “Leponex” in the hospital electronic data system.
Results
The majority (n = 53, 67.9%) of the patients included were nonremitted at the 1-year follow-up. The majority of the nonremitted patients received either none (7.5%), one (56.6%), or 2 types (15.1%) of antipsychotic drugs during the first year of follow-up. Only 2 (3.8%) received treatment with clozapine, and 3 (5.7%) in total were offered it.
Conclusions
For our FES sample, there was a low 1-year remission rate and a poor adherence to the pharmacological guidelines. Higher adherence to treatment guidelines with a more intensified antipsychotic treatment, which in some cases will include clozapine, will enhance the quality of treatment and may enhance the rates of remission for schizophrenia.
Key Words: schizophrenia, first episode, remission, guidelines, antipsychotics
Schizophrenia is a severe mental illness with serious consequences for the majority of patients and their families, and for society at large, even though many patients have a favorable course.1–3 Despite the establishment of early detection and intervention services and the development of new psychosocial interventions, 40% to 50% of patients with first-episode schizophrenia (FES) have symptoms at 10 years of follow-up.4,5 Studies examining the 1-year outcome in FES, show highly variable remission rates ranging from 17% to 81%.6,7
Antipsychotic medication remains the most effective treatment for positive symptoms in schizophrenia.8 Clozapine has been shown to be the most efficacious drug in this drug class.9 Moreover, clozapine is effective in about one third of patients not responding to 2 trials with nonclozapine antipsychotics, hereafter termed the treatment-resistant patients.10 The antipsychotic treatment algorithms incorporated in the guidelines for the treatment of schizophrenia are outlined in Figure 1.11–16 The algorithms generally recommend 2 trials with different antipsychotic drugs of adequate dosing and duration before clozapine is indicated. However, the initiation of clozapine may not match the time frame given in guidelines. The profile of adverse effects for clozapine may increase this delay in the treatment of FES patients, who are generally more vulnerable to antipsychotic adverse effects such as weight gain and the metabolic syndrome.17 However, clozapine has achieved favorable results in the treatment of FES patients. One study examined the use of clozapine in a cohort of consecutive FES patients with a 2-year follow-up.18 Although patients who received clozapine had more positive and negative symptoms at baseline, they managed to reach rates of remission and recovery similar to those who had never received clozapine, indicating additional effects of clozapine for FES. Another study showed a robust response to clozapine for FES patients who had not responded well to initial trials of second-generation antipsychotic drugs.19 The use of clozapine as a second-line antipsychotic for FES-spectrum disorders has also achieved favorable results.20
FIGURE 1.
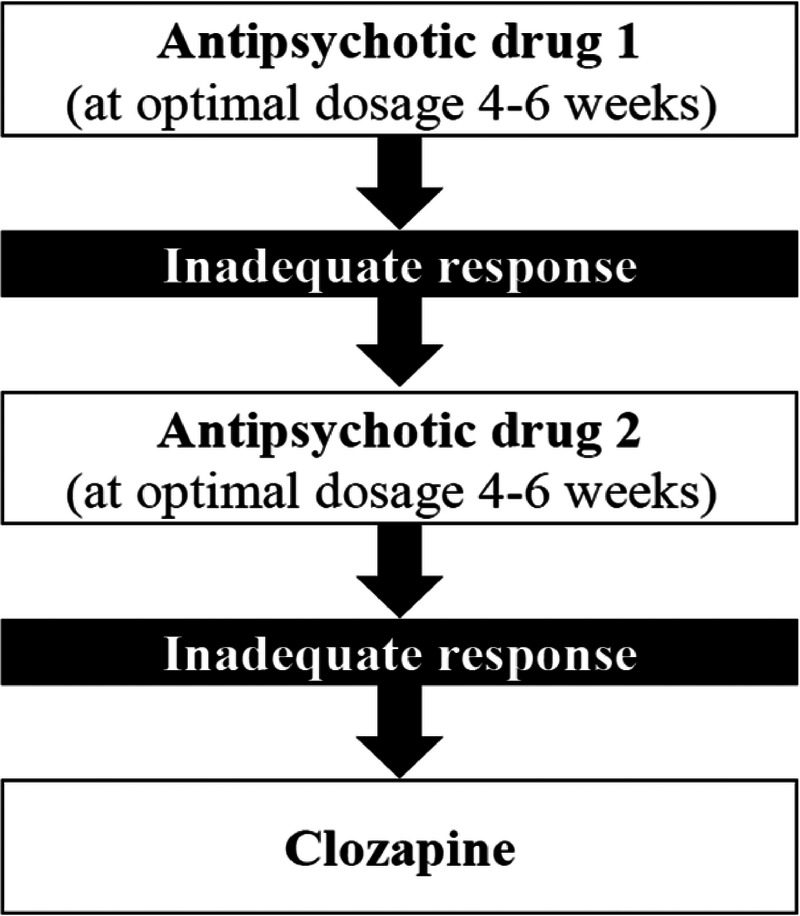
The algorithm for the pharmacological treatment of FES.
Given the serious long-term sequences of nonremission in FES,21 optimization of the antipsychotic treatment with thorough consideration of clozapine and skillful long-term specialist follow-up are of outmost importance in this patient group.
We aimed to study the 1-year remission rate and the antipsychotic treatment for a cohort of consecutively recruited FES patients. The prescribed antipsychotic treatment was compared with treatment guideline recommendations.
METHODS
The sample is drawn from the TIPS (Early Treatment and Intervention in Psychosis) 2 cohort study that included all first-episode psychosis patients admitted to either outpatient clinics or hospital.22–25 The study was carried out within the publicly funded specialist psychiatric catchment area services in Rogaland County, Norway, with a total of 370,000 inhabitants. The study comprises data from the time of inclusion until 1-year follow-up. All patients were assessed within a week of contact with the psychiatric services and assigned to the standard treatment program consisting of an antipsychotic medication algorithm, multifamily group work, and supportive psychotherapy. All patients entering the study gave written informed consent. The project received ethical approval from the Regional Committee for Medical Research Ethics Health Region West, Norway (015.03).
Participants
This study included all eligible participants with FES recruited to the TIPS cohort study during the period January 2002 to August 2013. The inclusion criteria were the following: living in the catchment area in Rogaland County, Norway; age 15 to 65 years; meeting the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) (DSM-IV; American Psychiatric Association, 1994) criteria for schizophrenia, schizoaffective disorder, or schizophreniform disorder, described further on as core schizophrenia; being actively psychotic, as measured by the Positive and Negative Syndrome Scale (PANSS)26 score of 4 or more on at least 1 of the Positive subscale items: 1 (delusions), 3 (hallucinatory behavior), 5 (grandiosity), 6 (suspiciousness/persecution), or General subscale item 9 (unusual thought content); not receiving previous adequate treatment for psychosis (defined as antipsychotic medication of more than 3.5 haloperidol equivalents for 12 weeks or until remission of the psychotic symptoms); having no neurological or endocrine disorders with relationship to the psychosis; having no contraindications to antipsychotic medication; understanding and/or speaking a Scandinavian language; having an IQ score of higher than 70.
From the total cohort, 122 had a core-schizophrenia diagnosis (FES). From this group of 122 FES patients, 78 completed the 1-year follow-up, as shown in Figure 2.
FIGURE 2.
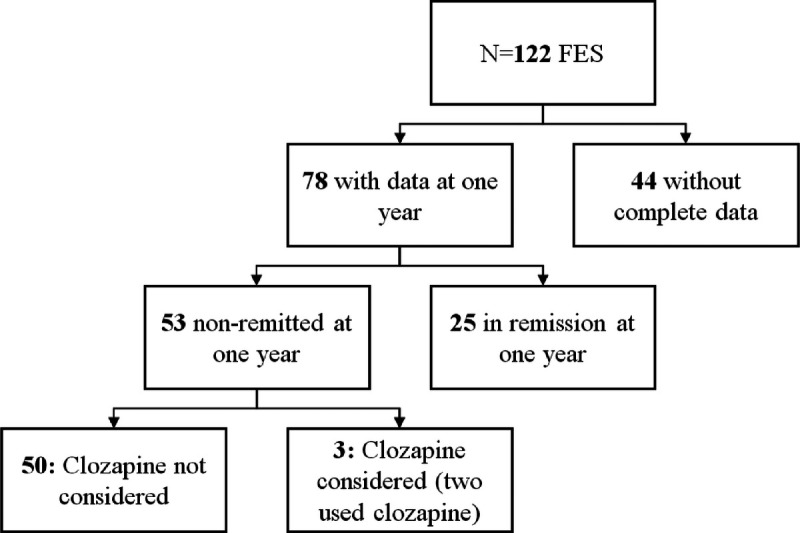
Flow chart with studied population. Remission was defined in accordance with the Remission in Schizophrenia Working Group standardized symptom remission criteria.27
Design
The study was an observational cohort design with a 1-year follow-up. Participants were categorized as remitted or nonremitted according to their remission status, as per the Remission in Schizophrenia Working Group criteria27 (see hereinafer), at the 1-year follow-up. Participant characteristics at baseline and 1-year follow-up were compared, and the pharmacological treatment was assessed in detail. The algorithm used in TIPS was recommended as a standard hospital policy and was a modification of the Norwegian treatment guidelines.15
Assessments
For the TIPS cohort, patients were assessed at baseline, 3 months, and 12 months. For this study, only the baseline and 12 month assessments were used. Assessment teams were clinically experienced and trained research personnel. Raters were trained by rating preprepared case notes and audio/videotapes. Good interrater reliability was achieved on major parameters in the research group.25 The Structured Clinical Interview for DSM-IV Axis I disorders was used for diagnostic purposes.28 Symptom levels were measured by the PANSS.26 For the PANSS, we calculated the total and the positive, negative, excitative, depressive, and cognitive component scores. Other characteristics were also assessed, such as suicidality and duration of untreated psychosis (DUP). Presence of suicidality was defined as having suicidal thoughts, plans, or attempts. The DUP was measured as the time from onset of psychosis until the start of adequate treatment. Onset of psychosis was defined as the first week with positive psychotic symptoms corresponding to a PANSS score of 4 or more on Positive subscale items 1, 3, 5, or 6 or on General subscale item 9. In cases with long DUP, all available data sources were used to ascertain the length of this period to the best achievable level, including semistructured personal interviews with patients and relatives. Adequate treatment was defined as the start of structured treatment with antipsychotic medication, start of hospitalization, or the start of outpatient clinic psychotherapy designed to manage psychotic symptoms.25 Global functioning was measured by the Global Assessment of Functioning scale with scores calculated for symptom and function subscales.29 Misuse or dependence of alcohol and drugs was measured by the Clinicians Rating Scale.30
Remission
Remission was defined in accordance with the Remission in Schizophrenia Working Group standardized symptom remission criteria.27 The Remission in Schizophrenia Working Group is a panel of experts from the United States, who developed in 2004 a consensus definition of remission applied to schizophrenia. The proposed criteria were also supported by international field experts and were published in 2005. These criteria consist of no score of 4 or higher for the past 6 months on any of the following PANSS items: P1 (delusions), P2 (disorganized thought), P3 (hallucinatory behavior), N1 (affective flattening), N4 (passive social withdrawal), N6 (lack of spontaneity), G5 (bizarre posture), or G9 (unusual thought content). Patients were categorized as nonremitted if they reported any relapse, defined as deterioration of symptoms with score of >4 on the relevant PANSS subscales during the previous 6 months. Remission status at the last available observation was based on the assessment at the 1-year follow-up.
Pharmacological Treatment
At 1-year follow-up, we examined the pharmacological strategies applied for patients by assessing adherence to the algorithm using patient files. The algorithm described 3 drug alternatives with clozapine being the third drug of choice, as shown in Figure 1. The first author (P.D.) read all the patient files and clinical descriptions (Structured Clinical Interview for DSM-IV and PANSS) to ascertain whether participants had remitted or relapsed during the first year of follow-up. Clinical descriptions were scrutinized and assessed for indications for a switch to clozapine. Pharmacological treatment was assessed in detail. The total duration of antipsychotic treatment, as well as the number of periods with antipsychotic treatment, was calculated using the patient files. The different antipsychotics used as first, second, third, or fourth choice were also assessed. In addition, the first author performed a digital search in all the medical files of the patients in the hospital data system, with index “clozapine,” “klozapin” (the Norwegian term for clozapine), and “Leponex” (the brand name for clozapine in Norway). Unclear cases were discussed with one of the coauthors (T.K.L.). Whenever possible, we sought to identify the reasons for clozapine not being considered or offered to patients.
Data Analysis
Analyses were performed using the SPSS Statistical Program Package version 20.0 (IBM, Armonk, NY). Participants were classified according to remission status (yes/no) at the 1-year follow-up. Categorical variables were presented in cross tables and analyzed using χ2 or Fisher exact test as appropriate. All group comparisons of continuous and ordinal data were analyzed using the nonparametric Mann-Whitney U test because of nonnormality as assessed with visual inspection of histograms.
RESULTS
Sample Characteristics and 1-Year Remission
Characteristics of the remitted and nonremitted groups are outlined in Table 1. A total of 78 patients were included in the sample, and the majority (68%) were nonremitted at the 1-year follow-up. All of the nonremitted patients (n = 53) scored 4 or more on at least 1 of the positive symptoms included in the remission criteria, whereas this was the case for 43 patients (81% of the nonremitted) regarding the negative and general symptoms. Both groups comprised mostly young men (mean age of 27 and 26 years, respectively, for the remitted and nonremitted group; 64% and 72%, respectively, were men).
TABLE 1.
Characteristics of 78 Patients With First-Episode Core Schizophrenia, Divided by Remission Status at 1-Year Follow-up
| Baseline | 1 y | |||
|---|---|---|---|---|
| Remitted (n = 25) (32%) | Nonremitted (n = 53) (68%) | Nonremitted (n = 53) | P | |
| Male, n (%) | 16 (64) | 38 (72) | 0.492 | |
| Age, mean (SD) | 27.4 (9.6) | 26.1 (7.6) | 0.776 | |
| Years of education, mean (SD) | 12.6 (4.1) | 12 (2.1) | 0.089 | |
| GAF, symptom, mean (SD) | 31.5 (7.7) | 30.8 (6.3) | 36.3 (8.5)* | 0.410 |
| GAF, function, mean (SD) | 41.2 (9.3) | 38.7 (9.5) | 41.7 (10.3)* | 0.211 |
| PANSS, mean (SD) | ||||
| Positive | 3.3 (0.72) | 3.3 (0.9) | 2.7 (0.89) | 0.759 |
| Negative | 2.1 (0.86) | 2.4 (1.15) | 2.3 (0.84) | 0.259 |
| Excitative | 1.5 (0.54) | 1.6 (0.78) | 1.3 (0.65) | 0.822 |
| Depressive | 3.2 (1.05) | 3 (1.18) | 2.5 (1.2) | 0.461 |
| Cognitive | 1.8 (0.85) | 2.3 (1.27) | 1.8 (1) | 0.188 |
| Suicidality, n (%) | 16 (66.6) | 34 (65.4) | 16 (39)† | 0.999 |
| DUP | ||||
| Median (SD), wk | 16 (176.2) | 40 (304)* | 0.054 | |
| Interquartile range | 41.5 | 144 | ||
| Alcohol misuse/dependence, n (%) | 2 (8.3) | 9 (17) | 7 (14.3)‡ | 0.486 |
| Drugs misuse/dependence, n (%) | 3 (12.5) | 15 (28.3) | 14 (28.6)‡ | 0.156 |
All P values are based on baseline comparisons.
*n = 51.
†n = 41.
‡n = 49.
GAF indicates Global Assessment of Functioning scale.
At baseline, there were no significant differences between the remitted and nonremitted group for sex, age, years of education, symptom, and function profile (Global Assessment of Functioning scale, PANSS), misuse or dependence of alcohol and drugs, and suicidality. We found a trend for DUP being longer in the nonremitted group, but this was not statistically significant (median of 40 weeks compared with 16 weeks, P = 0.054).
Antipsychotic Treatment in the Remitted and the Nonremitted Groups
The average number of periods of treatment with any antipsychotic drugs and the total duration of treatment during the first year were not significantly different between the 2 groups, as shown in Table 2.
TABLE 2.
Use of Medication During First Year of Treatment for Patients With First-Episode Core Schizophrenia, Divided by Remission Status at 1-Year Follow-up
| Remitted (n = 25) | Nonremitted (n = 53) | P | |
|---|---|---|---|
| Total duration of antipsychotic treatment, mean (SD), wk | n = 22 | n = 51 | 0.196 |
| 41.5 (15.7) | 33.9 (20.2) | ||
| No. periods with antipsychotic treatment, n (SD) | n = 25 | n = 53 | 0.481 |
| 1.32 (0.8) | 1.5 (1.05) | ||
| No. antipsychotics used, n (%) | n = 25 | n = 53 | |
| 1 | 17 (68) | 30 (56.6) | |
| 2 | 4 (16) | 8 (15.1) | |
| 3 | 2 (8) | 9 (17) | |
| 4 | 0 (0) | 2 (3.8) | |
| No use | 2 (8) | 4 (7.5) | 0.255* |
| First antipsychotic of choice, n (%) | n = 23 | n = 49 | |
| Olanzapine | 11 (47.8) | 22 (44.9) | |
| Risperidone | 4 (17.4) | 8 (16.3) | |
| Quetiapine | 3 (13) | 8 (16.3) | |
| Aripiprazole | 3 (13) | 2 (4.1) | |
| Ziprazidone | 2 (8.7) | 4 (8.2) | |
| Perphenazine | 0 | 2 (4.1) | |
| Haloperidol | 0 | 1 (2.0) | |
| Chlorpromazine | 0 | 1 (2.0) | |
| Amisulpride | 0 | 1 (2.0) | |
| Second antipsychotic of choice, n | n = 6 | n = 19 | |
| Risperidone | 1 | 5 | |
| Aripiprazole | 2 | 5 | |
| Olanzapine | 3 | 2 | |
| Amisulpride | 0 | 2 | |
| Haloperidol | 0 | 2 | |
| Ziprazidone | 0 | 1 | |
| Quetiapine | 0 | 1 | |
| Clozapine | 0 | 1 | |
| Third antipsychotic of choice, n | n = 2 | n = 11 | |
| Quetiapine | 0 | 3 | |
| Risperidone | 0 | 2 | |
| Olanzapine | 0 | 1 | |
| Ziprazidone | 1 | 2 | |
| Aripiprazole | 1 | 1 | |
| Perphenazine | 0 | 1 | |
| Levomepromazine | 0 | 1 | |
| Fourth antipsychotic of choice, n | n = 0 | n = 2 | |
| Risperidone | 0 | 1 | |
| Clozapine | 0 | 1 |
*χ2 calculated from no use, use of 1 antipsychotic, and use of more than 1 antipsychotic.
Two patients (8%) in the remitted group and 4 patients (7%) in the nonremitted group had no trials of antipsychotic drugs during the study period. The majority in both groups, 17 patients (68%) of the remitted and 30 patients (57%) of the nonremitted, received 1 type of antipsychotic drug during the first year. Four remitted patients (16%) and 8 nonremitted patients (15%) were treated with 2 different antipsychotic drugs. There were no significant group differences for no use of antipsychotic drugs, use of 1 antipsychotic drug, or use of 2 or more antipsychotic drugs.
Regarding the first-choice antipsychotic drug, there were no significant group differences. The most frequently prescribed antipsychotic drugs were olanzapine, risperidone, and quetiapine. Olanzapine was the first used antipsychotic drug for 48% of the remitted patients and 45% of the nonremitted (remitted, 11; nonremitted, 22 patients).
Three (6%) of the nonremitted patients were offered, and 2 (4%) received treatment with clozapine. For 1 patient, this was a second-choice drug, and for the other one, this was a fourth choice. There was no information that indicated an evaluation of clozapine for any of the other patients in the sample.
The vast majority of patients were treated with second-generation antipsychotic drugs. None of the remitted patients was treated with first-generation antipsychotic drugs. Of the nonremitted patients, 8% were treated with a first-generation antipsychotic as a first-choice drug. Three of the 4 patients were switched to second-generation antipsychotic drugs only. The fourth patient was switched first to olanzapine and then to levomepromazine, which is a first-generation antipsychotic drug. Two more patients were treated with a first-generation antipsychotic as a second-choice drug. The first one switched from risperidone to haloperidol and had no further switches during the first year. The second patient switched from olanzapine to haloperidol, then switched from haloperidol to perphenazine (another first-generation antipsychotic drug), and finally from perphenazine to risperidone.
Psychiatric Treatment
The majority of patients were in contact with the health care system during the study period, with around two thirds in each group having at least 1 inpatient admission at the psychiatric department. There were no significant group differences in the duration of inpatient admissions, as shown in Table 3.
TABLE 3.
Characteristics of Admissions During First Year of Treatment for Patients With First-Episode Core Schizophrenia, Divided by Remission Status at 1-Year Follow-up
| Remitted (n = 25) | Nonremitted (n = 53) | P | |
|---|---|---|---|
| No. admissions, n (%) | Total, n = 25 | Total, n = 50 | 0.294 |
| • None | 10 (40.0) | 14 (28.0) | |
| • One or more | 15 (60.0) | 36 (72.0) | |
| Duration of all admissions, mean (SD), wk | n = 25 | n = 49 | 0.157 |
| 9.66 (14.9) | 13.16 (17.4) |
DISCUSSION
A major finding in this study is the 1-year remission rate of 32% for FES patients. Results from other studies for this patient group vary considerably. In a review of remission in schizophrenia with 27 studies included,6 the rate of remission in FES ranged from 17% to 78%, whereas another study showed a remission rate of 81%.7 The variability of the reported remission rates may be a result of the heterogeneity among the studies. Contributing factors to the heterogeneity include selection bias and low representativeness of the studies, the definition of remission, and the frequency and duration of follow-up. The stringent criteria used in this study, such as a core-schizophrenia diagnosis, FES, and both dimensions of the proposed remission criteria27 —symptom reduction and duration, are likely to have contributed to the relatively low remission rate. However, treatment factors vary between studies and are likely to influence remission rates. We found that all of the nonremitted patients had at least 1 positive symptom during follow-up. These results indicate the use of antipsychotics in all of the nonremitted patients in our study, given that antipsychotics are most effective in the treatment of positive symptoms of schizophrenia. It is possible that these patients may have remitted, had they received a more intensified treatment with antipsychotics according to the guidelines. A proportion of these patients should probably be evaluated for treatment with clozapine. These results also underline, however, that even with optimal antipsychotic effect on positive symptoms, there would be a proportion of patients still not achieving remission, because of the presence of negative symptoms.
The second major finding of our study is a low exploitation of treatment options of antipsychotic treatment in the nonremitted group. For this group, 7% did not use any antipsychotic drug during the 1-year follow-up, and a further 57% were treated with one antipsychotic drug only. Contrary to the guidelines, the majority of the nonremitted FES patients (64%) did not commence the second step of the algorithm. Fifteen percent of the nonremitted patients received 2 antipsychotic drugs, 1 of these was prescribed clozapine, and the others were not further switched to clozapine despite nonremission and therefore did not pass the second step of the guideline algorithm. Altogether, only 2 patients were offered clozapine, one as a second choice and the other as a fourth choice. In both cases, the recommended guidelines were not followed.
The 7% nonuse together with 57% of patients receiving 1 antipsychotic drug only in a cohort of nonremitted patients with schizophrenia must be regarded as nonadherence to well-known national and international treatment guidelines. The nonuse of antipsychotics is a major concern. We have not explored other reasons for not taking any antipsychotic, such as patients' unwillingness, and therefore, it is not possible to conclude that the clinicians did not follow the guidelines for schizophrenia treatment in all of these cases. There are many factors shown to influence nonadherence to consensus-based guidelines by treating clinicians. The applicability of clinical practice guidelines in psychiatry has been studied during recent years, including trying to identify barriers to guideline adherence.31–34 Some proposed barriers are physician related, such as lack of awareness or familiarity, lack of agreement and concerns about control over professional practice. There are also patient-related barriers such as nonadherence and reluctance, clarity and complexity of guidelines, and environmentally related barriers such as resources and facilities.31,35
However, clinicians in our study performed good clinical practice in prescribing mainly second-generation antipsychotic drugs, and first-generation antipsychotic drugs were used in only a few cases. For example, the United Kingdom NICE guidelines recommend treatment with at least 1 nonclozapine second-generation antipsychotic drug for treatment-resistant patients.11 The Texas Medication Algorithm Project algorithm for schizophrenia provides some recommendations for treatment of FES.36 In the 2003 version, clinicians could choose either a first-generation antipsychotic drug or clozapine after 2 inadequate trials with second-generation antipsychotics. In the 2007 update, first-generation antipsychotics were an option at the second step of the algorithm and after a trial of a second-generation antipsychotic at the first step. The third step included the use of clozapine only. By contrast, the Norwegian guidelines do not differentiate the 2 types of antipsychotic drugs in terms of timing of treatment.15 In general, the wide use of second-generation antipsychotic drugs by clinicians in our study does not come in conflict with the contemporary national and international guidelines for treatment of FES.
The finding of similar rates of nonuse of antipsychotics between the remitted and the nonremitted groups may be indicative of the heterogeneity of schizophrenia. It would be valuable to follow the course of these patients with respect to the course of illness, diagnosis, and future antipsychotic treatment. Our finding that 2 patients remitted without using antipsychotics may challenge the general recommendation for continuous antipsychotic treatment in the first year of FES.
Regarding clozapine treatment and its limited use, various barriers have been investigated.37–40 Studies show that most practitioners are familiar with the guidelines and the effectiveness of clozapine38 but are reluctant to prescribe the drug because of the risk of fatal adverse reactions such as agranulocytosis.37,39 Lack of knowledge of the current evidence for clozapine may be contributing to this finding because very few patients on clozapine progress from neutropenia to agranulocytosis,41 and other antipsychotics also pose a risk of agranulocytosis.42 This small risk of agranulocytosis is, in our opinion, outweighed by a reduced mortality associated with clozapine treatment.43
For the proportion of nonremitted patients with at least 2 antipsychotics trials (19/53), several reasons could explain the possible low use of clozapine. First, we are not sure if this sample can be regarded as treatment resistant because we did not explore if the previous treatment periods were of adequate duration and if the antipsychotics were given in adequate doses. This is crucial to define treatment resistance, and clozapine is only recommended once treatment resistance is demonstrated. Second, patients may have been offered but refused the suggested treatment because of the comprehensive blood-monitoring associated with clozapine use, or refused for other reasons. Third, medical contraindications for the use of clozapine may apply. However, we found no written evaluation of indications of clozapine in the medical files.
Adherence to guidelines could be enhanced through regular training sessions for physicians on the implementation of the guidelines and including information on the current evidence base for the management of treatment with clozapine. Concerns by patients about clozapine could be addressed by providing them with information on the advantages of clozapine and the adoption of shared decision-making methods by clinicians. In addition, the facilities and infrastructure of the psychiatric institutions should be improved to allow an easier and more user-friendly follow-up for those receiving clozapine but also other antipsychotic drugs. Some clozapine clinics have been established, and it is possible that these facilities increase the quality of antipsychotic treatment and the remission rate in schizophrenia.44
Future research is needed on how to optimize implementation of evidence-based guidelines for the treatment of schizophrenia in the early stages because this may be imperative for improving outcome for these patients.
Strengths and Limitations
The strengths of our study include a robust design, whereby all the consecutive FES patients in a catchment area were assessed and followed up for 1 year. The study had access to the medical files of all patients and thus information on treatment over the 1-year follow-up. The stringent inclusion criteria ensured homogeneity in terms of diagnosis. Our study, however, was limited by the lack of assessment of the attitudes or behavior of patients toward treatment by medication. We are unable to determine whether nonadherence to guidelines is underpinned by patients refusing to receive antipsychotics including clozapine. Second, the attitudes of clinicians toward clozapine were not explored in our study. Given the lack of written considerations of clozapine use in the medical files, we cannot exclude that clinicians may have considered clozapine and refused prescribing for acceptable medical reasons.
CONCLUSIONS
Our study is one of the first to examine the adherence to guidelines for the pharmacological treatment and the 1-year remission rate for FES patients using stringent criteria. The quality of care given to patients is often correlated to the degree to which the clinicians follow the guidelines.45 Guidelines suggest a clinical practice that influences good patient outcomes. Therefore, adherence to guidelines is crucial for providing effective and evidence-based treatment. Given the finding of low adherence to the guidelines and insufficient pharmacological treatment for the FES patients in our study, a more evidence-based practice could be achieved by increasing guideline awareness and training in their implementation among the clinicians.
ACKNOWLEDGMENTS
The authors thank the staff of the detection team in TIPS, Stavanger, for their valuable contribution to the detection, inclusion, and follow-up of the patients.
AUTHOR DISCLOSURE INFORMATION
The authors declare no conflicts of interest. This study was supported by a research grant from Helse Vest RHF (the Western Norway Regional Health Authority) (project number, 912140).
Contributor Information
Kolbjørn Brønnick, Email: bronnick@gmail.com.
Inge Joa, Email: inge.joa@sus.no.
Jan Olav Johannessen, Email: jan.olav.johannessen@sus.no.
Erik Johnsen, Email: erik.johnsen@helse-bergen.no.
Rune Andreas Kroken, Email: rune.andreas.kroken@helse-bergen.no.
Helen Joy Stain, Email: helenstain3@gmail.com.
Wenche Ten Velden Hegelstad, Email: wenche.ten.velden.hegelstad@sus.no.
Tor Ketil Larsen, Email: tkmaclarsen@mac.com.
REFERENCES
- 1.Vos T Flaxman AD Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2163–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knapp M. Schizophrenia costs and treatment cost-effectiveness. Acta Psychiatr Scand Suppl. 2000;102:15–18. [DOI] [PubMed] [Google Scholar]
- 3.Tajima-Pozo K de Castro Oller MJ Lewczuk A, et al. Understanding the direct and indirect costs of patients with schizophrenia. F1000Res. 2015;4:182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hegelstad WT Larsen TK Auestad B, et al. Long-term follow-up of the TIPS early detection in psychosis study: effects on 10-year outcome. Am J Psychiatry. 2012;169:374–380. [DOI] [PubMed] [Google Scholar]
- 5.Lally J Ajnakina O Stubbs B, et al. Remission and recovery from first-episode psychosis in adults: systematic review and meta-analysis of long-term outcome studies. Br J Psychiatry. 2017;211:350–358. [DOI] [PubMed] [Google Scholar]
- 6.AlAqeel B, Margolese HC. Remission in schizophrenia: critical and systematic review. Harv Rev Psychiatry. 2012;20:281–297. [DOI] [PubMed] [Google Scholar]
- 7.Zhang HX Shen XL Zhou H, et al. Predictors of response to second generation antipsychotics in drug naïve patients with schizophrenia: a 1 year follow-up study in Shanghai. Psychiatry Res. 2014;215:20–25. [DOI] [PubMed] [Google Scholar]
- 8.Tandon R, Nasrallah HA, Keshavan MS. Schizophrenia, “just the facts” 5. Treatment and prevention. Past, present, and future. Schizophr Res. 2010;122:1–23. [DOI] [PubMed] [Google Scholar]
- 9.Leucht S Cipriani A Spineli L, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;382:951–962. [DOI] [PubMed] [Google Scholar]
- 10.Hasan A Falkai P Wobrock T, et al. World Federation of Societies of Biological Psychiatry (WFSBP) Guidelines for Biological Treatment of Schizophrenia, part 1: update 2012 on the acute treatment of schizophrenia and the management of treatment resistance. World J Biol Psychiatry. 2012;13:318–378. [DOI] [PubMed] [Google Scholar]
- 11.NICE Psychosis and schizophrenia in adults: prevention and management. Available at: https://www.nice.org.uk/guidance/cg178. Accessed November 2, 2020.
- 12.International Early Psychosis Association Writing Group International clinical practice guidelines for early psychosis. Br J Psychiatry Suppl. 2005;48:s120–s124. [DOI] [PubMed] [Google Scholar]
- 13.Taylor D, Paton C, Kapur S. The Maudsley Prescribing Guidelines in Psychiatry. 12th ed West Sussex, United Kingdom: Wiley Blackwell; 2015. [Google Scholar]
- 14.Helsedirektoratet Nasjonal faglig retningslinje for utredning, behandling og oppfølging av personer med psykoselidelser (National guideline for assessment, treatment and follow-up of persons with psychotic disorders). Available at: https://helsedirektoratet.no/retningslinjer/nasjonal-faglig-retningslinje-for-utredning-behandling-og-oppfolging-av-personer-med-psykoselidelser. Accessed November 2, 2020.
- 15.Statens Helsetilsyn Schizofreni. Kliniske retningslinjer for utredning og behandling. Available at: http://folk.ntnu.no/flovig/Rundskriv%20og%20behandlingsveiledninger/Schizofreni%202726.pdf. Accessed November 2, 2020.
- 16.Keating D McWilliams S Schneider I, et al. Pharmacological guidelines for schizophrenia: a systematic review and comparison of recommendations for the first episode. BMJ Open. 2017;7:e013881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patel JK Buckley PF Woolson S, et al. Metabolic profiles of second-generation antipsychotics in early psychosis: findings from the CAFE study. Schizophr Res. 2009;111:9–16. [DOI] [PubMed] [Google Scholar]
- 18.Tang C Subramaniam M Ng BT, et al. Clozapine use in first-episode psychosis: the Singapore Early Psychosis Intervention Programme (EPIP) perspective. J Clin Psychiatry. 2016;77:e1447–e1453. [DOI] [PubMed] [Google Scholar]
- 19.Agid O Remington G Kapur S, et al. Early use of clozapine for poorly responding first-episode psychosis. J Clin Psychopharmacol. 2007;27:369–373. [DOI] [PubMed] [Google Scholar]
- 20.Kahn RS Winter van Rossum I Leucht S, et al. Amisulpride and olanzapine followed by open-label treatment with clozapine in first-episode schizophrenia and schizophreniform disorder (OPTiMiSE): a three-phase switching study. Lancet Psychiatry. 2018;5:797–807. [DOI] [PubMed] [Google Scholar]
- 21.Ten Velden Hegelstad W Haahr U Larsen TK, et al. Early detection, early symptom progression and symptomatic remission after ten years in a first episode of psychosis study. Schizophr Res. 2013;143(2–3):337–343. [DOI] [PubMed] [Google Scholar]
- 22.Larsen TK Melle I Auestad B, et al. Early detection of first-episode psychosis: the effect on 1-year outcome. Schizophr Bull. 2006;32:758–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Larsen TK Melle I Friis S, et al. One-year effect of changing duration of untreated psychosis in a single catchment area. Br J Psychiatry Suppl. 2007;51:s128–s132. [DOI] [PubMed] [Google Scholar]
- 24.Johannessen JO Friis S Joa I, et al. First-episode psychosis patients recruited into treatment via early detection teams versus ordinary pathways: course, outcome and health service use during first 2 years. Early Interv Psychiatry. 2007;1:40–48. [DOI] [PubMed] [Google Scholar]
- 25.Joa I Johannessen JO Auestad B, et al. The key to reducing duration of untreated first psychosis: information campaigns. Schizophr Bull. 2008;34:466–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. [DOI] [PubMed] [Google Scholar]
- 27.Andreasen NC Carpenter WT Jr. Kane JM, et al. Remission in schizophrenia: proposed criteria and rationale for consensus. Am J Psychiatry. 2005;162:441–449. [DOI] [PubMed] [Google Scholar]
- 28.First M, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV Axis I Disorders. Patient Edition SCID I/P, Version 2.0. New York, NY: New York State Psychiatric Institute, Biometrics Research Department; 1995. [Google Scholar]
- 29.Karterud S Pedersen G Løvdahl H, et al. Global Assessment of Functioning—Split Version. Oslo, Norway: Oslo Universitessykehus; 1998. [Google Scholar]
- 30.Drake RE Osher FC Noordsy DL, et al. Diagnosis of alcohol use disorders in schizophrenia. Schizophr Bull. 1990;16:57–67. [DOI] [PubMed] [Google Scholar]
- 31.Cabana MD Rand CS Powe NR, et al. Why don't physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282:1458–1465. [DOI] [PubMed] [Google Scholar]
- 32.Forsner T Hansson J Brommels M, et al. Implementing clinical guidelines in psychiatry: a qualitative study of perceived facilitators and barriers. BMC Psychiatry. 2010;10:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saddichha S, Chaturvedi SK. Clinical practice guidelines in psychiatry: more confusion than clarity? A critical review and recommendation of a unified guideline. ISRN Psychiatry. 2014;2014:828917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yeisen RA Joa I Johannessen JO, et al. Use of medication algorithms in first episode psychosis: a naturalistic observational study. Early Interv Psychiatry. 2016;10:503–510. [DOI] [PubMed] [Google Scholar]
- 35.Hayward RS. Clinical practice guidelines on trial. CMAJ. 1997;156:1725–1727. [PMC free article] [PubMed] [Google Scholar]
- 36.Moore TA Buchanan RW Buckley PF, et al. The Texas Medication Algorithm Project antipsychotic algorithm for schizophrenia: 2006 update. J Clin Psychiatry. 2007;68:1751–1762. [DOI] [PubMed] [Google Scholar]
- 37.Verdoux H Quiles C Bachmann CJ, et al. Prescriber and institutional barriers and facilitators of clozapine use: a systematic review. Schizophr Res. 2018;201:10–19. [DOI] [PubMed] [Google Scholar]
- 38.Gee S Vergunst F Howes O, et al. Practitioner attitudes to clozapine initiation. Acta Psychiatr Scand. 2014;130:16–24. [DOI] [PubMed] [Google Scholar]
- 39.Warnez S, Alessi-Severini S. Clozapine: a review of clinical practice guidelines and prescribing trends. BMC Psychiatry. 2014;14:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gee SH, Shergill SS, Taylor DM. Patient attitudes to clozapine initiation. Int Clin Psychopharmacol. 2017;32:337–342. [DOI] [PubMed] [Google Scholar]
- 41.Ingimarsson O MacCabe JH Haraldsson M, et al. Neutropenia and agranulocytosis during treatment of schizophrenia with clozapine versus other antipsychotics: an observational study in Iceland. BMC Psychiatry. 2016;16:441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Agid O Foussias G Singh S, et al. Where to position clozapine: re-examining the evidence. Can J Psychiatry. 2010;55:677–684. [DOI] [PubMed] [Google Scholar]
- 43.Tiihonen J Lonnqvist J Wahlbeck K, et al. 11-year follow-up of mortality in patients with schizophrenia: a population-based cohort study (FIN11 study). Lancet. 2009;374:620–627. [DOI] [PubMed] [Google Scholar]
- 44.Williams T, Purvis TL. Development of an outpatient pharmacist-managed clozapine clinic. Am J Health Syst Pharm. 2012;69:1192–1195. [DOI] [PubMed] [Google Scholar]
- 45.Milchak JL Carter BL James PA, et al. Measuring adherence to practice guidelines for the management of hypertension: an evaluation of the literature. Hypertension. 2004;44:602–608. [DOI] [PMC free article] [PubMed] [Google Scholar]


