Supplemental Digital Content is available in the text.
Keywords: cardiovascular disease, diurnal blood pressure variability, heart failure, nighttime blood pressure, nocturnal hypertension
Background:
Ambulatory and home blood pressure (BP) monitoring parameters are better predictors of cardiovascular events than are office BP monitoring parameters, but there is a lack of robust data and little information on heart failure (HF) risk. The JAMP study (Japan Ambulatory Blood Pressure Monitoring Prospective) used the same ambulatory BP monitoring device, measurement schedule, and diary-based approach to data processing across all study centers and determined the association between both nocturnal hypertension and nighttime BP dipping patterns and the occurrence of cardiovascular events, including HF, in patients with hypertension.
Methods:
This practitioner-based, nationwide, multicenter, prospective, observational study included patients with at least 1 cardiovascular risk factor, mostly hypertension, and free of symptomatic cardiovascular disease at baseline. All patients underwent 24-hour ambulatory BP monitoring at baseline. Patients were followed annually to determine the occurrence of primary end point cardiovascular events (atherosclerotic cardiovascular disease and HF).
Results:
A total of 6,359 patients (68.6±11.7 years of age, 48% men) were included in the final analysis. During a mean±SD follow-up of 4.5±2.4 years, there were 306 cardiovascular events (119 stroke, 99 coronary artery disease, 88 HF). Nighttime systolic BP was significantly associated with the risk of atherosclerotic cardiovascular disease and HF (hazard ratio adjusted for demographic and clinical risk factors per 20-mm Hg increase: 1.18 [95% CI, 1.02–1.37], P=0.029; and 1.25 [95% CI, 1.00–1.55], P=0.048, respectively). Disrupted circadian BP rhythm (riser pattern, nighttime BP higher than daytime BP) was significantly associated with higher overall cardiovascular disease risk (1.48 [95% CI, 1.05–2.08]; P=0.024), and especially HF (2.45 [95% CI, 1.34–4.48]; P=0.004) compared with normal circadian rhythm.
Conclusions:
Nighttime BP levels and a riser pattern were independently associated with the total cardiovascular event rate, in particular for HF. These findings suggest the importance of antihypertensive strategies targeting nighttime systolic BP.
Registration:
URL: https://www.umin.ac.jp/ctr/; Unique identifier: UMIN000020377.
Clinical Perspective.
What Is New?
The JAMP study (Japan Ambulatory Blood Pressure Monitoring Prospective) is the largest prospective ambulatory blood pressure (BP) monitoring study to date to use the same device and monitoring protocol across sites.
The results showed that higher nighttime BP and a riser pattern of nocturnal BP were significantly associated with the risk of total cardiovascular disease and heart failure, and that the relationship between a riser pattern and heart failure risk was independent of nighttime BP.
What Are the Clinical Implications?
Higher nighttime systolic BP was found to be associated with greater relative risk for cardiovascular disease events than dipping status.
The risk of coronary artery disease and heart failure was highest in individuals with a riser pattern and higher nighttime systolic BP.
Overall, the study findings highlight the importance of antihypertensive strategies that target nocturnal BP.
Out-of-office blood pressure (BP) measurement using ambulatory BP monitoring (ABPM) or home BP monitoring is recommended for the diagnosis and management of hypertension in the latest guidelines.1–3 Use of ABPM allows determination of daytime, nighttime, and 24-hour BP, and diurnal BP variation.
Ambulatory BP parameters are more strongly associated with target organ damage and cardiovascular disease (CVD) events than are office or clinic BP parameters.4 However, previous studies have many limitations: (1) baseline ambulatory BP monitoring data were collected >30 years ago,4 and therefore, generalizability to current practice may be limited; (2) nonfatal cardiovascular events were not evaluated;5 (3) different monitoring devices were used in each study, meaning that the algorithms used to calculate BP were not necessarily the same; (4) heterogeneous definitions of daytime and nighttime BP were used (clocktime-based or diary-based); and (5) heart failure (HF), an important health outcome in the current aging population, was not assessed.6,7
The importance of nighttime BP as a predictor of cardiovascular risk is increasingly recognized, especially in patients who have initiation and intensification of antihypertensive medication based on office or home BP.8–11 Of the main nocturnal dipping patterns (extreme dipping, dipping, nondipping, and riser), the riser pattern (nighttime-to-daytime systolic BP [SBP] ratio >1.0) is associated with an increased risk of cardiovascular events.12,13 In addition, the extreme dipper pattern (nighttime-to-daytime SBP ratio <0.8) has been linked with silent cerebral infarcts14 and risk of clinical stroke,12 but results are inconsistent.8,15 However, previous studies did not adjust for nighttime BP. Therefore, it remains unclear whether disrupted BP circadian rhythm is associated with adverse health outcomes independent of nighttime BP. A recent meta-analysis showed that the association between extreme dipper status and cardiovascular events was evident only in older patients.16
The JAMP study (Japan Ambulatory Blood Pressure Monitoring Prospective) includes a large outpatient population who had ambulatory BP monitored with the same validated device, measurement schedule, and diary-based definition approach to data processing. We evaluated the association between both nocturnal hypertension and nighttime BP dipping patterns and the occurrence of cardiovascular events, including HF, in patients with hypertension.
Methods
Study Design
The practitioner-based, nationwide, multicenter, prospective, observational study (University Hospital Medical Information Network trials registration: UMIN000020377) was conducted in Japan. Recruitment occurred between 2008 and 2017, with completion of follow-up by December 31, 2022. This analysis includes data from all patients registered between 2008 and 2017 with follow-up information to the end of March 2019. The protocol was approved by the Institutional Review Board of Jichi Medical School, Tochigi, Japan, and the study was conducted according to Declaration of Helsinki principles. All participants provided written informed consent. The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Characteristics of the JAMP Study and Participants
The JAMP study was designed to investigate the prognostic effect of ABPM parameters in general practice. Participants were recruited by general practitioners from 36 prefectures around Japan using the following inclusion and exclusion criteria. Patients with at least 1 of the following cardiovascular risk factors were included: diabetes or glucose intolerance; dyslipidemia; hypertension; current smoking; renal disease; atrial fibrillation; metabolic syndrome; chronic obstructive pulmonary disease;17 or sleep apnea syndrome18 (see Methods in the Data Supplement for full definitions and exclusion criteria).
Outcomes
The primary end point was total cardiovascular events, including atherosclerotic CVD (ASCVD; fatal and nonfatal stroke, fatal and nonfatal coronary artery disease [CAD]) and HF (see Methods in the Data Supplement for full event definitions). Patients were followed up annually (clinic visit or by telephone) to determine vital status and the occurrence of cardiovascular events over the preceding year. All end point events were adjudicated by an independent end point committee who were unaware of the patient’s ambulatory BP profile and clinical characteristics. If events occurred on 2 or more occasions, the first occurrence was included in the analysis. CAD events in patients with HF were classified as CAD.
Assessments
Patient history data were obtained from medical records. Office BP was measured using an upper arm cuff and standard mercury sphygmomanometer or validated oscillometric device. Ambulatory BP was determined using a validated device (TM-2425/2431; A&D Co, Saitama, Japan)19 programmed to take readings every 30 minutes and repeat a reading if SBP (60–280 mm Hg), diastolic BP (40–160 mm Hg), pulse pressure (10–150 mm Hg), or pulse rate (30–200 beats/min) fell outside predefined acceptable ranges. Individuals were instructed to rest or sleep during nighttime and maintain usual daytime activities. Daily activities and sleep and wake times were recorded in a diary.
After wearing monitors for at least 24 hours, participants returned to the clinic, and device data were downloaded. Nighttime readings were defined as those taken from the self-reported time of falling asleep to waking time; all other readings were defined as daytime values. For analysis, ambulatory recordings had to include at least 6 daytime and 3 nighttime readings.20 Patients were classified into 4 groups based on the reduction in SBP while asleep versus awake: extreme dippers (≥20%); dippers (10%–<20%); nondippers (0%–<10%); and risers (any increase).19
Statistical Analysis
Data are presented as mean values with SD, and percentages. Demographic variables and clinical characteristics were compared between the nocturnal BP dipping pattern groups using a t test for the slope in linear regression models (continuous variables) or the Cochran-Armitage test (trends in categorical variables). The cumulative incidence of cardiovascular events in nocturnal dipping pattern subgroups was visualized using Kaplan-Meier curves, adjusted for covariates (age, sex, body mass index, smoking, alcohol intake, diabetes, dyslipidemia, CVD history, antihypertensive drug use, bedtime antihypertensive dosing), and office and 24-hour SBP.
Cox proportional hazards models were used to calculate hazard ratio (HR) and 95% CI values for the risk of cardiovascular events associated with a 20-mm Hg increase in SBP, and a 10% increase in nocturnal SBP dipping (after adjustment for the above covariates); the dipper group was used as the reference in dipping status models. Additional adjustments included office SBP (model 1); office SBP and 24-hour SBP (model 2); office SBP and daytime SBP (model 3); and office SBP and nighttime SBP (model 4). The proportionality assumption for the Cox analyses was confirmed graphically, and comparison of the discriminative ability of each model was conducted using Harrell’s C-statistics (with 95% CI values calculated by bootstrapping),21 net reclassification improvement, and integrated discrimination improvement.22 Heat maps were used to visually represent the relative associations between nocturnal SBP dipping status and nighttime SBP and the occurrence of cardiovascular events.
All statistical analyses were performed with SAS system, v9.4 (SAS Institute, Cary, NC). Two-sided P values <0.05 were defined as statistically significant.
Results
Study Population
A total of 130 doctors from 116 institutions (72 primary practices, 40 hospital-based outpatient clinics, 3 specialized university hospitals, and 1 national center hospital) recruited patients to the study. Of the 6772 participants enrolled, 6359 were included in the final analysis; 6288/6772 participants had 20 daytime and 7 nighttime ABPM recordings. Information about patient withdrawals is provided in the Methods in the Data Supplement (Figure I in the Data Supplement). Almost half of all participants were men (47.6%), 76.7% were taking antihypertensive medication, and mean (±SD) age was 68.6±11.7 years (range, 21–96 years, 67.5% age >65 years) (Table 1). Mean (±SD) follow-up duration was 4.5±2.4 years, during which there were 306 total CVD events: 218 ASCVD events including 119 strokes and 99 CAD events, and 88 episodes of HF (Table I in the Data Supplement).
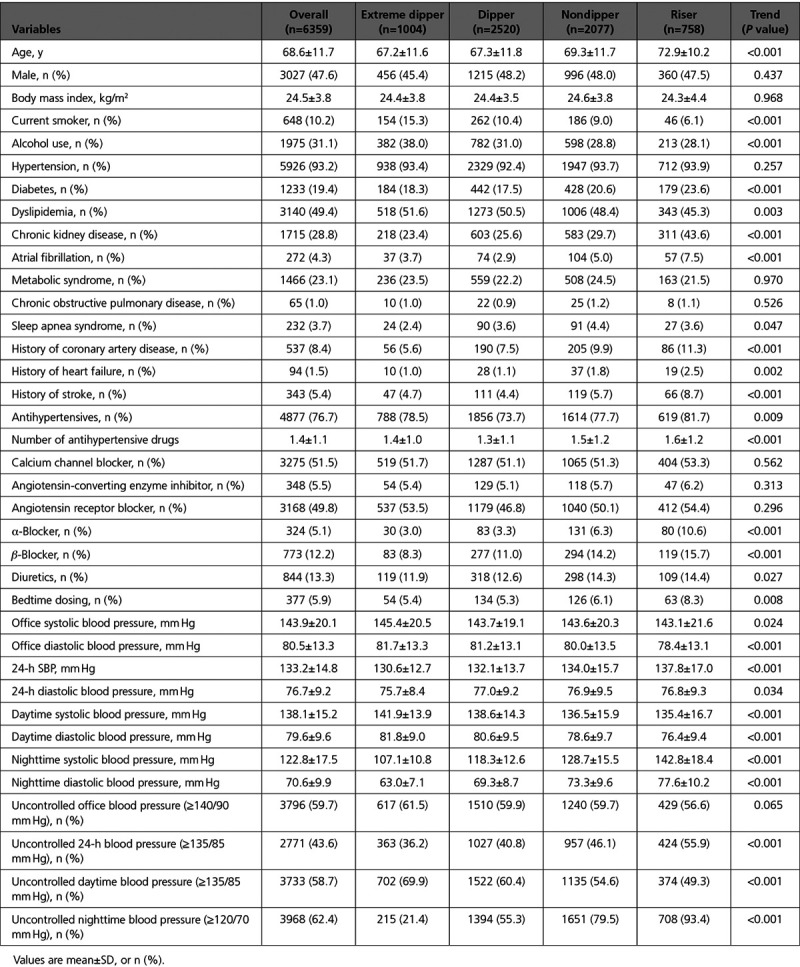
Table 1.
Patient Characteristics at Baseline, Overall, and by Dipping Status
Cardiovascular Events
Adjusted cumulative incidence rates of CVD events by baseline nocturnal SBP dipping status are shown in Figure 1A. The riser versus dipper pattern was associated with a significantly increased risk of total CVD (P=0.024) and HF (P=0.004), independent of office and 24-hour SBP. Adjustment for nighttime rather than 24-hour SBP showed that the riser versus dipper pattern was significantly associated with the occurrence of HF (P=0.027) but not CAD (Figure II in the Data Supplement).
Figure 1.
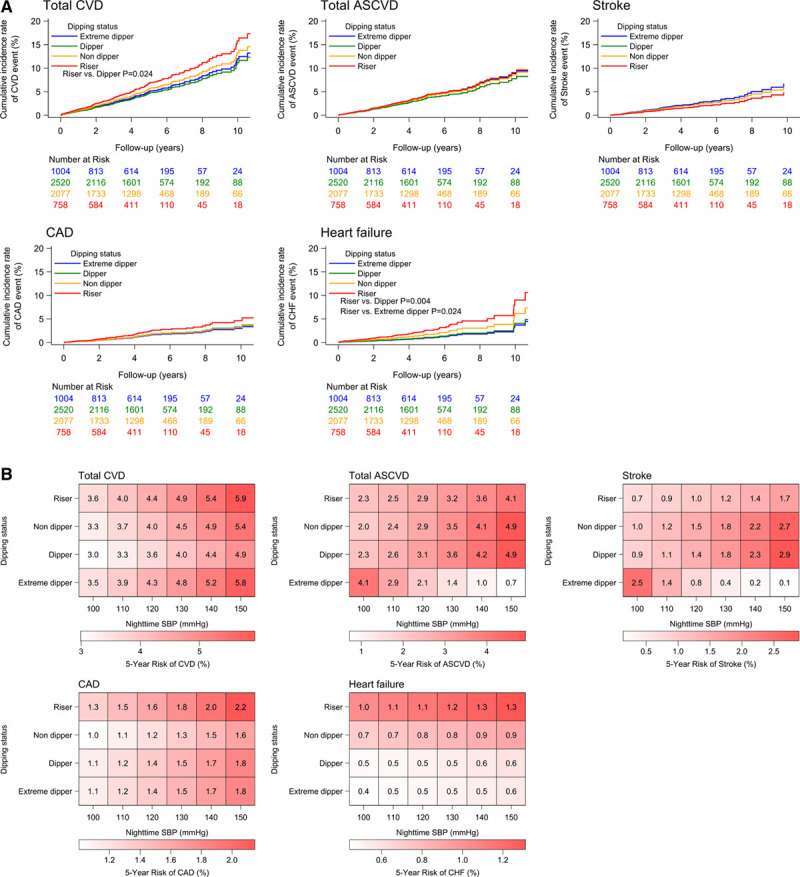
Cardiovascular disease risk. A, Cumulative incidence of different cardiovascular disease events by dipping status (adjusted for age, sex, body mass index, smoking, alcohol use, diabetes, dyslipidemia, history of cardiovascular disease, use of antihypertensive drugs, bedtime antihypertensive dosing, office systolic blood pressure, and 24-hour systolic blood pressure, with dipper status as the reference). B, Heat map showing 5-year risk of cardiovascular disease events by nighttime systolic blood pressure and dipping status (adjusted by age, sex, body mass index, smoking, alcohol use, diabetes, dyslipidemia, history of cardiovascular disease, use of antihypertensive drugs, bedtime antihypertensive dosing, and office systolic blood pressure). ASCVD indicates atherosclerotic cardiovascular disease; CAD, coronary artery disease; CHF, congestive heart failure; CVD, cardiovascular disease; and SBP, systolic blood pressure.
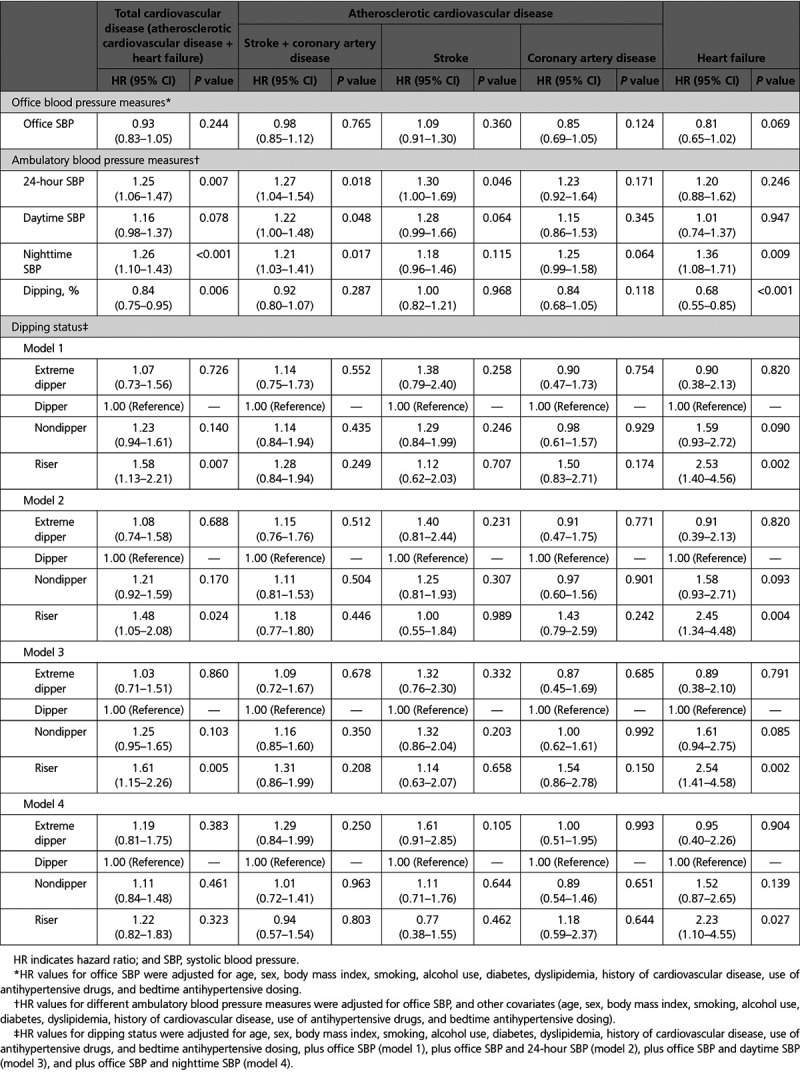
Increasing nighttime, but not office, SBP was also significantly associated with CVD event risk. A 20-mm Hg increase in nighttime SBP was significantly associated with a 21% to 36% increase in CVD event risk, whereas daytime SBP was significantly associated with a 22% increase in stroke risk (Table 2). The riser pattern was also significantly associated with higher total CVD event risk compared with the dipper pattern (58% increase; P=0.007) (Table 2). The risk associated with a riser pattern was greatest for HF (HR, 2.53; P=0.002) and remained significant after adjustment for office SBP and 24-hour SBP (HR, 2.45; P=0.004), for office SBP and daytime SBP (HR, 2.54; P=0.002), and for office SBP and nighttime SBP (HR, 2.23; P=0.027) (Table 2). The risk associated with the riser pattern also persisted after the addition of morning SBP surge to model 1 (total CVD: HR, 1.48, P=0.034; HF: HR, 2.53, P=0.005).
Table 2.
Association Between Different Ambulatory Blood Pressure Measures (per 20-mm Hg Increase in SBP) or Dipping Status of Nighttime Blood Pressure (per 10% Increase in Nocturnal SBP Dipping) and Risk of Cardiovascular Disease
Heat maps for the 5-year risk of CVD showed that higher nighttime SBP was associated with a greater relative risk for CVD events than dipping status (Figure 1B). The risk of both ASCVD and stroke increased as nighttime SBP decreased in extreme dippers but increased in parallel with increasing nighttime SBP in the other dipping status groups (Figure 1B). The risk of CAD and HF was highest in individuals with a riser pattern and higher nighttime SBP (Figure 1B). There was a significantly higher risk of stroke in patients with well-controlled 24-hour SBP and an extreme dipper pattern (HR, 2.30 [95% CI, 1.22–4.35]; P=0.010), and a significantly higher risk of HF in those with well-controlled SBP and a riser pattern (HR, 3.78 [95% CI, 1.61–8.89]; P=0.002) (Figure 2).
Figure 2.
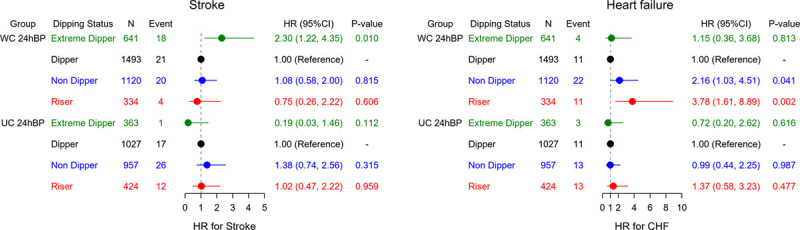
Risk of stroke and heart failure by nocturnal blood pressure dipping status and 24-hour systolic blood pressure control status. Values are adjusted for age, sex, body mass index, smoking, alcohol intake, diabetes, dyslipidemia, prevalent cardiovascular disease, use of antihypertensive drugs, bedtime dosing, and office and 24-hour systolic blood pressure. CHF indicates congestive heart failure; HR, hazard ratio; UC 24hBP, uncontrolled 24-hour blood pressure (24-hour systolic blood pressure >130 mm Hg); and WC 24hBP, well-controlled 24-hour systolic blood pressure (24-hour systolic blood pressure ≤130 mm Hg).
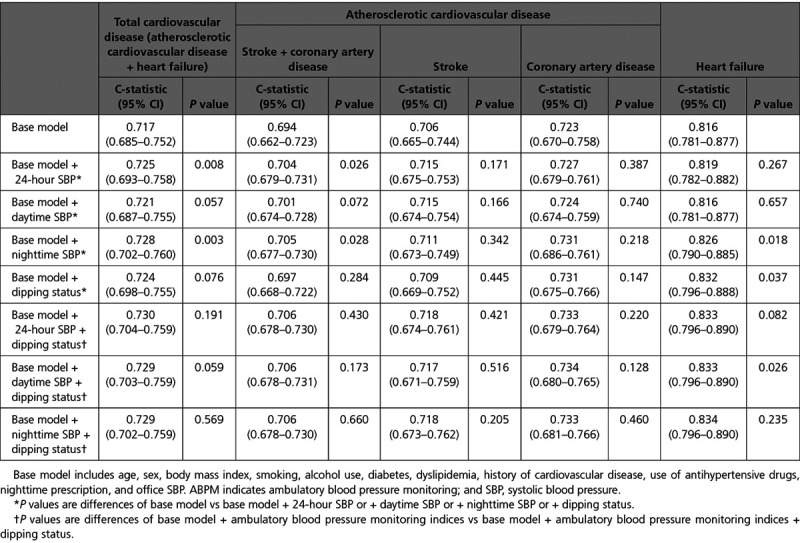
The base model yielded a C-statistic value of 0.717 (95% CI, 0.685–0.752) for total CVD, 0.694 (95% CI, 0.662–0.723) for ASCVD, and 0.816 (95% CI, 0.781–0.877) for HF (Table 3). Adding nighttime SBP to the base model resulted in small but statistically significant increments in C-statistic values (P=0.003, P=0.028, and P=0.018, respectively); a similarly small but significant increase in the C-statistic was also seen for HF when dipping status was added to the base model (P=0.037) (Table 3). In addition, increments in net reclassification improvement for total CVD risk after the addition of nighttime SBP and dipping status to the base model were 0.150 (95% CI, 0.026–0.275; P=0.011) and 0.200 (95% CI, 0.059–0.324; P=0.003), respectively, whereas the increment in net reclassification improvement for HF risk when dipping status was added was 0.413 (P<0.001) (Table II in the Data Supplement). Increments in integrated discrimination improvement for the risk of total CVD, ASCVD, and HF when nighttime SBP and dipping status were added to the base model were all statistically significant (Table III in the Data Supplement). However, model performance to predict the risk of total CVD, ASCVD, and HF was not significantly improved by adding daytime SBP when looking at the C-statistic (Table 3) or net reclassification improvement (Table II in the Data Supplement). Adding daytime SBP to the model increased integrated discrimination improvement values for total CVD and ASCVD risk but not HF risk (Table III in the Data Supplement).
Table 3.
Improvements in Model Performance (C-Statistic)
Discussion
This is the largest prospective ABPM study to date to use the same device and monitoring protocol across sites. The results showed that higher nighttime BP and a riser pattern of nocturnal BP were significantly associated with the risk of total CVD and HF and that the relationship between a riser pattern and HF risk was independent of nighttime BP. Daytime SBP was a significant risk factor for ASCVD only (not for HF). Furthermore, in patients with well-controlled 24-hour SBP, those at one end of the extreme of disrupted diurnal BP variation (ie, extreme dipper) had a significantly greater relative risk of stroke, whereas those at the other extreme (ie, riser) had the greatest relative risk of developing HF.
Nighttime BP Versus Daytime BP
Our findings in a population of mostly treated patients with hypertension suggest that each 20-mm Hg increase in nighttime SBP was associated with increased risk of ASCVD (21% increase, P=0.017) and HF (36% increase, P=0.009). Each 20-mm Hg increase in daytime SBP was associated with a significantly increased relative risk of ASCVD (22% increase, P=0.048), but not total CVD (16% increase, P=0.078). The model performance for discrimination of total CVD, ASCVD, and HF risk was slightly but significantly improved by the addition of nighttime BP and dipping status, whereas adding daytime BP slightly improved model performance for ASCVD risk only. Therefore, higher nighttime BP and dipping status appear to be more important contributors to cardiovascular risk than daytime BP, especially for HF.
The clinical impact of elevated nighttime BP versus daytime BP might depend on the stage of hypertension and be affected by antihypertensive drug therapy. In early-stage hypertension where patients have high-normal BP or mild hypertension, daytime BP may be a stronger determinant of left ventricular hypertrophy. Conversely, the prognostic impact of nighttime BP increases in patients with treated hypertension. In the current study, in which 77% of participants were receiving antihypertensive therapy, office SBP was not significantly associated with CVD risk. Physicians often use office BP to guide the initiation and intensification of antihypertensive medication. Thus, during the follow-up period, both office BP and daytime BP might be controlled by antihypertensive medication. However, assessment of only office and daytime BP may leave a significant proportion of medicated patients with undetected nocturnal hypertension, and therefore at high risk for cardiovascular events.
ABPM is the standard approach to determine nighttime BP, but this is not widely implicated in routine clinical practice. Home BP monitoring devices with the ability to monitor nocturnal BP have recently become available10,23 but are not yet in widespread clinical use. In the J-HOP study (Japan Morning Surge-Home Blood Pressure), we suggested that nocturnal BP measurements using a home device were a significant predictor of ASCVD events, independent of office, morning, and evening home BP.11
Riser Pattern of Nocturnal BP
In this study, the riser pattern of nighttime BP was significantly associated with the risk of both total CVD events (HR, 1.58; P=0.007) and HF (HR, 2.53; P=0.002). The riser pattern is the extreme end of the continuum of nondipping nocturnal BP and disrupted circadian variation, and has been associated with organ damage and poor cardiovascular prognosis.12,14 We found that the association between the riser pattern and total CVD events remained significant even after adjusting for 24-hour and daytime SBP, but the association disappeared after adjustment for nighttime SBP. This suggests that higher nighttime BP is more important than the riser patter as a risk factor for total CVD. This is consistent with the recent findings from the International Database of Ambulatory Blood Pressure in relation to Cardiovascular Outcome,15 suggesting that the risk associated with a riser pattern is at least partly mediated by high nighttime BP. Similarly, the increased HF risk associated with the riser pattern decreased slightly after controlling for nighttime SBP in our study, but the risk remained at least 2 times higher (HR, 2.23; P=0.027) in the riser group, indicating that a riser patter might be more important than nighttime BP as a risk factor for HF. Only 1 previous study has evaluated the link between ambulatory BP and HF.13 It showed that a riser pattern was an independent predictor of HF risk, even when cases of myocardial infarction were excluded. The same nonischemic definition of HF events was used in our study.
Heart Failure
In our study, CAD risk was not significantly associated with either higher nighttime SBP or a riser pattern of nocturnal BP. Nevertheless, CAD is an important cause of HF, as shown in a recent study of older patients from Japan.24 In the current study, when acute HF occurred with acute coronary syndrome, it was classified as CAD. Thus, the observed association between nighttime SBP and risk of HF is likely to be independent of a possible overlap between HF and CAD.
Left ventricular hypertrophy is the form of target organ damage most likely to precede nonischemic HF. Other studies have shown that nighttime BP and the nocturnal fall in BP are stronger correlates of left ventricular hypertrophy than daytime or average 24-hour BP.25 In addition, nighttime BP was a risk factor for the development of left ventricular hypertrophy and increased natriuretic peptide levels in the J-HOP study.26 Data from a large general population in Italy were similar, showing that nighttime BP was a reliable independent predictor of the development of left ventricular hypertrophy in subjects with normal left ventricular mass.27 Furthermore, a recent comprehensive 2-dimensional echocardiographic examination including multilayer strain analysis demonstrated an association between the riser pattern and left ventricular mechanical dysfunction in patients with hypertension.28
The association between a riser pattern and HF, independent of 24-hour, daytime and nighttime BP, is indicative of a “beyond BP” pathophysiological mechanism. There are 3 possible mechanisms to explain this. The first is vascular factors, with the riser pattern being associated with advanced vascular disease such as endothelial dysfunction and increased arterial stiffness. The second is circulating volume. Nondipping of nocturnal BP, including a riser pattern, is known to be associated with increased circulating volume, which is predominantly determined by salt sensitivity and salt intake. Decreased salt intake and treatment with diuretics reduces nighttime BP to a greater extent than daytime BP, causing a shift from a nondipper to a dipper pattern.29,30 The third is sympathetic nerve activity. The riser pattern is characterized by high muscle sympathetic nerve traffic,31 and recent trial data showed that renal denervation significantly reduced 24-hour BP, including nighttime BP.32 All these factors increase preload or afterload, or directly impact on the left ventricle, contributing to development of HF.
Extreme Dipper
Our findings suggest that excessive reduction of BP during sleep may also be detrimental. Extreme dipper patients with well-controlled 24-hour SBP showed a significantly increased risk of stroke, and stroke risk increased as nighttime SBP decreased. These associations need to be investigated in prospective studies. Given that the 2 extreme diurnal BP patterns were significantly associated with cardiovascular event risk in patients with well-controlled 24-hour SBP (stroke in extreme dippers and HF for those with a riser pattern), there appears to be a need for pathophysiology-based antihypertensive strategies that can reduce these residual risks.
Strengths and Limitations
One of the strengths of this study is the use of consistent technology, methodology, and definitions across centers. In addition, ambulatory BP parameters were defined based on individual awake-sleep behavior, which is the gold standard approach for defining nighttime BP and accurately assessing diurnal BP variation.19 However, some limitations need to be taken into account when interpreting our findings. First, ambulatory BP data were obtained once at baseline, and we do not have information on the contribution of subsequent changes in ambulatory BP to prognosis. Nevertheless, use of effective antihypertensive therapy is likely to weaken, rather than strengthen, associations between baseline ambulatory BP and cardiovascular outcomes. This analysis focused on systolic, rather than diastolic, BP because of the older age of our study population. Furthermore, study evaluations did not include echocardiography, precluding differentiation between HF with preserved versus reduced ejection fraction. The study population was Japanese, limiting external validity.
Conclusions
This ABPM study showed that nighttime BP and a riser pattern of nighttime BP were significantly and independently associated with the risk of ASCVD and HF events. This highlights the potential for, and importance of, antihypertensive medication strategies targeting nocturnal BP.
Acknowledgments
The authors thank all nurses and physicians of the participating centers for their excellent cooperation and help. The authors also thank Ms Noriko Harada and Ms Tomoko Morimoto for providing study management; Ms Kimiyo Saito, Ms Tomoko Shiga, and Ms Chiharu Saito for their assistance with study coordination and data management; and Mr Nobuhiko Yasui and Mr Toshiyuki Kiuchi for their valuable advice. Medical writing support was provided by Nicola Ryan, independent medical writer, funded by Jichi Medical University.
Sources of Funding
This study was financially supported in part by a grant from the Foundation for the Development of the Community (Tochigi).
Disclosures
K.K. has received research funding from Omron Healthcare Co, Fukuda Denshi, and A&D Co. The other authors report no conflicts.
Supplemental Materials
JAMP Study Group
Data Supplement Methods
Data Supplement Tables I–III
Data Supplement Figures I and II
Supplementary Material
Footnotes
Sources of Funding, see page 1819
The Data Supplement, podcast, and transcript are available with this article at https://www.ahajournals.org/doi/suppl/10.1161/CIRCULATIONAHA.120.049730.
Contributor Information
Satoshi Hoshide, Email: hoshide@jichi.ac.jp.
Hiroyuki Mizuno, Email: m01085_hm@yahoo.co.jp.
Tomoyuki Kabutoya, Email: kabu@jichi.ac.jp.
Masafumi Nishizawa, Email: nishi@jichi.ac.jp.
Tetsuro Yoshida, Email: tetsuro-moet@nifty.com.
Hideyasu Abe, Email: hiiragi@mtg.biglobe.ne.jp.
Tomohiro Katsuya, Email: tkatsuya@iris.eonet.ne.jp.
Yumiko Fujita, Email: dr.yumi0102@gmail.com.
Osamu Okazaki, Email: ookazaki@hosp.ncgm.go.jp.
Yuichiro Yano, Email: yyano@jichi.jp.
Naoko Tomitani, Email: tomitani.n@jichi.ac.jp.
Hiroshi Kanegae, Email: kanegae@genkiplaza.or.jp.
References
- 1.Umemura S, Arima H, Arima S, Asayama K, Dohi Y, Hirooka Y, Horio T, Hoshide S, Ikeda S, Ishimitsu T, et al. The Japanese Society of Hypertension guidelines for the management of hypertension (JSH 2019). Hypertens Res. 2019;42:1235–1481. doi: 10.1038/s41440-019-0284-9 [DOI] [PubMed] [Google Scholar]
- 2.Whelton PK, Carey RM, Aronow WS, Casey DE, Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2018;138:e426–e483. doi: 10.1161/CIR.0000000000000597 [DOI] [PubMed] [Google Scholar]
- 3.Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, et al. ; ESC Scientific Document Group. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018;39:3021–3104. doi: 10.1093/eurheartj/ehy339 [DOI] [PubMed] [Google Scholar]
- 4.Kario K, Thijs L, Staessen JA. Blood pressure measurement and treatment decisions. Circ Res. 2019;124:990–1008. doi: 10.1161/CIRCRESAHA.118.313219 [DOI] [PubMed] [Google Scholar]
- 5.Ruilope LM, Ruiz-Hurtado G, Barderas MG, de la Cruz JJ, Lucia A, de la Sierra A, Gorostidi M, Vinyoles E, Segura J, Solís J, et al. Frequency and prognosis of treated hypertensive patients according to prior and new blood pressure goals. Hypertension. 2019;74:130–136. doi: 10.1161/HYPERTENSIONAHA.119.12921 [DOI] [PubMed] [Google Scholar]
- 6.Wright JT, Jr., Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, Lewis CE, et al. A randomized trial of intensive versus standard blood-pressure Control. N Engl J Med. 2015;373:2103–2116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, et al. ; EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720 [DOI] [PubMed] [Google Scholar]
- 8.Boggia J, Li Y, Thijs L, Hansen TW, Kikuya M, Björklund-Bodegård K, Richart T, Ohkubo T, Kuznetsova T, Torp-Pedersen C, et al. ; International Database on Ambulatory B. lood P. ressure M. onitoring in R. elation to Cardiovascular Outcomes (IDACO) I. nvestigators. Prognostic accuracy of day versus night ambulatory blood pressure: a cohort study. Lancet. 2007;370:1219–1229. doi: 10.1016/S0140-6736(07)61538-4 [DOI] [PubMed] [Google Scholar]
- 9.Hansen TW, Li Y, Boggia J, Thijs L, Richart T, Staessen JA. Predictive role of the nighttime blood pressure. Hypertension. 2011;57:3–10. doi: 10.1161/HYPERTENSIONAHA.109.133900 [DOI] [PubMed] [Google Scholar]
- 10.Kario K. Nocturnal hypertension: new technology and evidence. Hypertension. 2018;71:997–1009. doi: 10.1161/HYPERTENSIONAHA.118.10971 [DOI] [PubMed] [Google Scholar]
- 11.Kario K, Kanegae H, Tomitani N, Okawara Y, Fujiwara T, Yano Y, Hoshide S. Nighttime blood pressure measured by home blood pressure monitoring as an independent predictor of cardiovascular events in general practice. Hypertension. 2019;73:1240–1248. doi: 10.1161/HYPERTENSIONAHA.118.12740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kario K, Pickering TG, Matsuo T, Hoshide S, Schwartz JE, Shimada K. Stroke prognosis and abnormal nocturnal blood pressure falls in older hypertensives. Hypertension. 2001;38:852–857. doi: 10.1161/hy1001.092640 [DOI] [PubMed] [Google Scholar]
- 13.Ingelsson E, Björklund-Bodegård K, Lind L, Arnlöv J, Sundström J. Diurnal blood pressure pattern and risk of congestive heart failure. JAMA. 2006;295:2859–2866. doi: 10.1001/jama.295.24.2859 [DOI] [PubMed] [Google Scholar]
- 14.Kario K, Matsuo T, Kobayashi H, Imiya M, Matsuo M, Shimada K. Nocturnal fall of blood pressure and silent cerebrovascular damage in elderly hypertensive patients. Advanced silent cerebrovascular damage in extreme dippers. Hypertension. 1996;27:130–135. doi: 10.1161/01.hyp.27.1.130 [DOI] [PubMed] [Google Scholar]
- 15.Yang WY, Melgarejo JD, Thijs L, Zhang ZY, Boggia J, Wei FF, Hansen TW, Asayama K, Ohkubo T, Jeppesen J, et al. ; International Database on Ambulatory Blood Pressure in Relation to Cardiovascular Outcomes (IDACO) Investigators. Association of office and ambulatory blood pressure with mortality and cardiovascular outcomes. JAMA. 2019;322:409–420. doi: 10.1001/jama.2019.9811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palatini P, Verdecchia P, Beilin LJ, Eguchi K, Imai Y, Kario K, Ohkubo T, Pierdomenico SD, Saladini F, Schwartz JE, et al. Association of extreme nocturnal dipping with cardiovascular events strongly depends on age. Hypertension. 2020;75:324–330. doi: 10.1161/HYPERTENSIONAHA.119.14085 [DOI] [PubMed] [Google Scholar]
- 17.Müllerova H, Agusti A, Erqou S, Mapel DW. Cardiovascular comorbidity in COPD: systematic literature review. Chest. 2013;144:1163–1178. doi: 10.1378/chest.12-2847 [DOI] [PubMed] [Google Scholar]
- 18.Monahan K, Redline S. Role of obstructive sleep apnea in cardiovascular disease. Curr Opin Cardiol. 2011;26:541–547. doi: 10.1097/HCO.0b013e32834b806a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kario K, Shin J, Chen CH, Buranakitjaroen P, Chia YC, Divinagracia R, Nailes J, Hoshide S, Siddique S, Sison J, et al. Expert panel consensus recommendations for ambulatory blood pressure monitoring in Asia: the HOPE Asia Network. J Clin Hypertens (Greenwich). 2019;21:1250–1283. doi: 10.1111/jch.13652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang WY, Thijs L, Zhang ZY, Asayama K, Boggia J, Hansen TW, Ohkubo T, Jeppesen J, Stolarz-Skrzypek K, Malyutina S, et al. ; International Database; on Ambulatory B. lood P. ressure in R. elation to Cardiovascular Outcomes (IDACO) Investigators. Evidence-based proposal for the number of ambulatory readings required for assessing blood pressure level in research settings: an analysis of the IDACO database. Blood Press. 2018;27:341–350. doi: 10.1080/08037051.2018.1476057 [DOI] [PubMed] [Google Scholar]
- 21.Pencina MJ, D’Agostino RB. Overall C as a measure of discrimination in survival analysis: model specific population value and confidence interval estimation. Stat Med. 2004;23:2109–2123. doi: 10.1002/sim.1802 [DOI] [PubMed] [Google Scholar]
- 22.Pencina MJ, D’Agostino RB, Sr, D’Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–72. discussion 207. doi: 10.1002/sim.2929 [DOI] [PubMed] [Google Scholar]
- 23.Asayama K, Fujiwara T, Hoshide S, Ohkubo T, Kario K, Stergiou GS, Parati G, White WB, Weber MA, Imai Y; International Expert Group of Nocturnal Home Blood Pressure. Nocturnal blood pressure measured by home devices: evidence and perspective for clinical application. J Hypertens. 2019;37:905–916. doi: 10.1097/HJH.0000000000001987 [DOI] [PubMed] [Google Scholar]
- 24.Kitai T, Miyakoshi C, Morimoto T, Yaku H, Murai R, Kaji S, Furukawa Y, Inuzuka Y, Nagao K, Tamaki Y, et al. Mode of death among Japanese adults with heart failure with preserved, midrange, and reduced ejection fraction. JAMA Netw Open. 2020;3:e204296 doi: 10.1001/jamanetworkopen.2020.4296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Henskens LH, Kroon AA, van Oostenbrugge RJ, Haest RJ, Lodder J, de Leeuw PW. Different classifications of nocturnal blood pressure dipping affect the prevalence of dippers and nondippers and the relation with target-organ damage. J Hypertens. 2008;26:691–698. doi: 10.1097/HJH.0b013e3282f4225f [DOI] [PubMed] [Google Scholar]
- 26.Ishikawa J, Hoshide S, Eguchi K, Ishikawa S, Shimada K, Kario K; Japan Morning Surge-Home Blood Pressure Study Investigators Group. Nighttime home blood pressure and the risk of hypertensive target organ damage. Hypertension. 2012;60:921–928. doi: 10.1161/HYPERTENSIONAHA.112.198101 [DOI] [PubMed] [Google Scholar]
- 27.Cuspidi C, Facchetti R, Bombelli M, Sala C, Negri F, Grassi G, Mancia G. Nighttime blood pressure and new-onset left ventricular hypertrophy: findings from the Pamela population. Hypertension. 2013;62:78–84. doi: 10.1161/HYPERTENSIONAHA.111.00682 [DOI] [PubMed] [Google Scholar]
- 28.Tadic M, Cuspidi C, Majstorovic A, Pencic B, Mancia G, Bombelli M, Grassi G, Kocijancic V, Djukic V, Celic V. The association between 24-h blood pressure patterns and left ventricular mechanics. J Hypertens. 2020;38:282–288. doi: 10.1097/HJH.0000000000002241 [DOI] [PubMed] [Google Scholar]
- 29.Uzu T, Ishikawa K, Fujii T, Nakamura S, Inenaga T, Kimura G. Sodium restriction shifts circadian rhythm of blood pressure from nondipper to dipper in essential hypertension. Circulation. 1997;96:1859–1862. doi: 10.1161/01.cir.96.6.1859 [DOI] [PubMed] [Google Scholar]
- 30.Uzu T, Kimura G. Diuretics shift circadian rhythm of blood pressure from nondipper to dipper in essential hypertension. Circulation. 1999;100:1635–1638. doi: 10.1161/01.cir.100.15.1635 [DOI] [PubMed] [Google Scholar]
- 31.Grassi G, Seravalle G, Quarti-Trevano F, Dell’Oro R, Bombelli M, Cuspidi C, Facchetti R, Bolla G, Mancia G. Adrenergic, metabolic, and reflex abnormalities in reverse and extreme dipper hypertensives. Hypertension. 2008;52:925–931. doi: 10.1161/HYPERTENSIONAHA.108.116368 [DOI] [PubMed] [Google Scholar]
- 32.Bohm M, Kario K, Kandzari DE, Mahfoud F, Weber MA, Schmieder RE, Tsioufis K, Pocock S, Konstantinidis D, Choi JW, et al. Efficacy of catheter-based renal denervation in the absence of antihypertensive medications (SPYRAL HTN-OFF MED Pivotal): a multicentre, randomised, sham-controlled trial. Lancet. 2020;395:1444–1451. doi: 10.1016/S0140-6736(20)30554-7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


