Abstract
The purpose of this prospective study was to determine whether the cost and cost-effectiveness of early rehabilitation after stroke are associated with the degree of initial disability. The data for cost calculations were collected by the bottom-up (micro-costing) method alongside the standard inpatient care. The total sample included 87 patients who were transferred from acute care to early rehabilitation unit of three participating stroke centers at the median time poststroke of 11 days (range 4–69 days). The study was pragmatic so that all hospitals followed their standard therapeutic procedures. For each patient, the staff recorded each procedure and the associated time over the hospital stay. The cost and cost-effectiveness were compared between four disability categories. The average cost of the entire hospitalization was CZK 114 489 (EUR 4348) with the daily average of CZK 5103 (EUR 194). The cost was 2.4 times higher for the immobile category (CZK/EU: 167 530/6363) than the self-sufficient category (CZK/EUR: 68 825/2614), and the main driver of the increase was the cost of nursing. The motor status had a much greater influence than cognitive status. We conclude that the cost and cost-effectiveness of early rehabilitation after stroke are positively associated with the degree of the motor but not cognitive disability. To justify the cost of rehabilitation and monitor its effectiveness, it is recommended to systematically record the elements of care provided and perform functional assessments on admission and discharge.
Keywords: community health services, costs and cost analysis, Czech Republic, neurorehabilitation, patient outcome assessment, stroke
Introduction
Stroke treatment has changed remarkably in the last decade with the application of novel neurosurgical and neurological procedures and the establishment of specialized (comprehensive) stroke units that also include early rehabilitation (Hamann et al., 2016; Škoda et al., 2016; Powers et al., 2018; Pross et al., 2018; de Sousa et al., 2019). There is a general agreement that early rehabilitation is beneficial after stroke (Bernhardt et al., 2015b; Hamann et al., 2016; Coleman et al., 2017; Langhorne et al., 2017; Powers et al., 2018). Next to clinical evidence, this is supported by the results of animal experiments indicating that a narrow window of opportunity for reactive neurobiological recovery and repair may exist, and the optimum period for change could be early after stroke (Murphy and Corbett, 2009; Krakauer et al., 2012; Teasell and Hussein, 2016). The high-intensity rehabilitation therapy within the first 90 days is reported to be associated with a lower mortality risk than the low-intensity therapy among patients with mild to moderate stroke severity (Hsieh et al., 2018). However, the percentage of patients referred to early inpatient rehabilitation is still low (Chen et al., 2020).
The definition of ‘early rehabilitation’ differs; however, the 2008 European stroke treatment guidelines consider early rehabilitation when administered 20 or even 30 days after stroke (Hacke et al., 2008; Quinn et al., 2009), but already 6 years later, it decreased to 7 days after stroke (Lynch et al., 2014). Moreover, the A Very Early Rehabilitation Trial after stroke (AVERT) clinical trial investigated the efficacy of a ‘very early mobilization’ within 24 h of stroke onset (Bernhardt et al., 2015b; Langhorne et al., 2017). A comprehensive overview of this problem was published by Bernhardt et al. (2019).
While the clinical aspects of the early rehabilitation have been quite frequently discussed, compare, for example, the reviews (Bernhardt et al., 2015a; Coleman et al., 2017; Langhorne et al., 2017) or the guidelines (Winstein et al., 2016; Küçükdeveci et al., 2018; Powers et al., 2018), little is known about its cost and cost-effectiveness. The limited information was collected mainly during the AVERT trials (Tay-Teo et al., 2008; Sheppard et al., 2016; Gao et al., 2019). Simultaneously, several systematic reviews covering economic evaluations of the rehabilitation after stroke have been published (Brady et al., 2005; Tummers et al., 2012; Chen et al., 2020). Chen et al. (2020) suggest that the rehabilitation ward is cost-effective in comparison with other options (rehabilitation without transfer to the rehabilitation ward, or no rehabilitation). Although Tummers et al. (2012) recommended performing a cost analysis across different severities of stroke almost a decade ago, this information is still missing.
Early rehabilitation after stroke did not exist in the Czech Republic until 2015 when the stroke units were officially established (Ministry of Health of the Czech Republic, 2015). At present, there are 13 comprehensive cerebrovascular centers (consisting of neurosurgical, radiological, neurological and early rehabilitation units) and 32 stroke centers (neurological and early rehabilitation units). Patients are typically transferred to early rehabilitation units between 7 and 14 days after stroke, where they receive 3–4 h of multidisciplinary rehabilitation per day.
Our 2017 tri-center study determined the average costs of early rehabilitation after stroke to be CZK 114 489 (EUR 4348) for the entire hospitalization or CZK 5103 (EUR 194) per day (unpublished to date). To expand on this, the goal of this study is to determine whether the cost and cost-effectiveness of early rehabilitation are associated with the degree of initial disability. Such information is expected to be useful for hospital managers to decide about the content and organization of early rehabilitation after stroke, and for negotiating reimbursement with the regulators. On a broader scale, our approach and results would be informative for international comparisons of cost-effectiveness and organization of early rehabilitation after stroke.
Participants and methods
The data used here come from a national pragmatic study carried out in three hospitals (General University Hospital in Prague, Department of Rehabilitation Medicine; Masaryk Hospital in Ústí nad Labem, Rehabilitation Department; and University Hospital Ostrava, Clinic of Rehabilitation and Physical Medicine) from April to November 2017. The study was approved by the ethics committee of the General University Hospital in Prague. The inclusion criteria were stroke diagnosis (ischemic or hemorrhagic), hospitalization between 4 and 90 days, and no interruption in the early rehabilitation stay unless the treatment for complications occurred in the same hospital. A total of 87 patients were included in this study, and they were admitted to the early rehabilitation unit less than 70 days after stroke. The hospitals listed above contributed 29, 31 and 27 patients, respectively.
Data for cost calculation were collected by the bottom-up (micro-costing) method alongside standard patient care. The study was pragmatic so that all hospitals followed their standard therapeutic procedures. The staff recorded each procedure and the number of therapeutic units or time spent continuously with a patient on 10 treatment forms. The recorded data were transferred to 10 economic forms where each therapeutic unit or time spent was multiplied by the respective cost. Standard statistical analyses were performed using MS Excel and R applications.
Personal and clinical data were recorded on eight clinical forms and captured basic demographics, relevant dates (stroke onset, admission, transfer to the early rehabilitation unit and discharge), physician’s evaluation of functional abilities and categorization (see below), functional tests carried out by trained therapists (starting on day 3 of admission to the rehabilitation unit and then every 2 weeks). In this study, outcomes were assessed by the following functional tests: the Barthel Index (Mahoney and Barthel, 1965), Extended Barthel Index (EBI) (Prosiegel et al., 1996; Katona et al., 2015) and the functional independence measure (FIM) divided into the motor and cognitive subscales (Chumney et al., 2010). The EBI was developed to widen the utility of this scale by adding six cognitive items. Although some authors used the abbreviation EBI when referring to all 16 items together (Maritz et al., 2019), it is recommended to designate with EBI only the six cognitive items (DIMDI, 2018). To avoid any confusion, we use Barthel Index+EBI to denote the combined 16-item scale.
The above-mentioned categorization (hereinafter referred to as disability category) is on the basis of the Czech reimbursement scheme, Section 6 of the Czech Republic Decree No. 134/1998 Coll. (1998), and it applies to all inpatient facilities. The five disability categories are as follows: (1) self-sufficient, (2) partly self-sufficient, (3) requires an enhanced level of supervision, (4) immobile and (5) unconscious. Staff routinely assigns patients to these categories and can be assumed to have rich experience with this classification. The disability category was used as the independent variable to measure the degree of initial disability.
All cost data are given in Czech crowns (CZK) and Euro (EUR) using the 2017 Czech National Bank average exchange rate of EUR 1 = CZK 26.330. The costs were calculated by the micro-costing (bottom-up) method (detailed methodology to be published in a separate article, unpublished to date). The cost-effectiveness ratios were calculated individually for each disability category as the average total cost of the hospitalization in the rehabilitation unit divided by the average incremental change in the outcome (end – beginning). This was done separately for Barthel Index, EBI, Barthel Index+EBI, total FIM, motor FIM and cognitive FIM, yielding six sets of cost-effectiveness values per each disability category.
Results
The age of 87 included patients was between 31 and 95 years (mean 70.5). Sixty-four patients (73.5%) were between 60 and 90 years, almost equally distributed in decades. The laterality in hemiparesis was balanced (left-side in 41 patients, right-side in 41 patients and no or unrecorded in 5 patients). The average length of hospitalization was 22.2 days.
The disability category (see Patients and Methods for the definition) was determined by the physician during the admission interview/examination. No patient was assigned to the unconscious category. Table 1 shows basic baseline data characterizing patient distribution to the categories and the respective initial and final average scores for Barthel Index and FIM. While the age was independent of the categories, both the length of hospitalization in the rehabilitation unit and the number of days between stroke onset and transfer to the rehabilitation unit grew from less to more disabled categories. Also, the scores of Barthel Index and motor FIM were associated with the categories, which was not found for EBI and cognitive FIM (only the patients in the fourth category showed visibly worse results).
Table 1.
Distribution of patients across the four disability categories and their characteristics at the beginning of rehabilitation
| Disability category | 1 | 2 | 3 | 4 | Average |
|---|---|---|---|---|---|
| Number of patients | 15 | 27 | 24 | 21 | 87 |
| Average age (years) | 65.7 (45–88) | 71.5 (31–90) | 71.9 (49–93) | 71.0 (41–91) | 70.5 (31–93) |
| Average length of hospitalization on rehabilitation (days) | 16.4 (9–40) | 19.1 (4–59) | 24.6 (11–50) | 27.5 (4–45) | 22.2 (4–59) |
| Average time from stroke onset to translation to rehabilitation unit (days) | 10.5 (4–22) | 12.6 (4–30) | 14.3 (6–34) | 27.1 (9–69) | 11 (4–69) |
| Barthel Index beginning | 88.7 (65–100) | 65.9 (15–100) | 57.1 (5–90) | 23.8 (0–65) | 57.3 (0–100) |
| Barthel Index end | 96.7 (80–100) | 82.6 (40–100) | 72.9 (20–100) | 44.5 (5–95) | 73.2 (5–100) |
| EBI beginning | 78.0 (40–90) | 72.4 (15–90) | 72.5 (20–90) | 48.8 (20–90) | 67.7 (15–90) |
| EBI end | 83.7 (65–90) | 76.1 (25–90) | 76.3 (15–90) | 57.6 (25–90) | 73 (15–90) |
| Barthel Index + EBI beginning | 166.7 (125–190) | 138.3 (40–185) | 129.6 (40–180) | 72.6 (25–155) | 124.9 (25–190) |
| Barthel Index + EBI end | 180.3 (160–190) | 158.7 (65–190) | 149.2 (60–190) | 102.1 (50–180) | 146.2 (50–190) |
| FIM beginning | 105.3 (83–123) | 86.0 (26–120) | 78.5 (21–114) | 48.3 (20–96) | 78.2 (20–123) |
| FIM end | 113.2 (92–125) | 100.1 (45–126) | 92.3 (28–122) | 61.3 (22–114) | 90.9 (22–126) |
| FIM – Motor beginning | 76.6 (55–91) | 58.7 (20–86) | 50.0 (14–80) | 28.9 (13–72) | 52.2 (13–91) |
| FIM – Motor end | 83.1 (69–91) | 71.5 (29–91) | 62.8 (16–90) | 39.5 (15–85) | 63.4 (15–91) |
| FIM – Cognitive beginning | 28.7 (15–35) | 27.3 (5–35) | 28.5 (7–35) | 19.4 (6–34) | 26 (5–35) |
| FIM – Cognitive end | 30.1 (16–35) | 28.6 (6–35) | 29.5 (10–35) | 21.8 (7–34) | 27.5 (6–35) |
EBI, Extended Barthel Index; FIM, functional independence measure.
Figure 1 illustrates the average improvements in different categories in terms of Barthel Index+EBI and FIM. The same proportional change is representative of the increase in Barthel Index and motor FIM, whereas their cognitive parts did not differentiate between categories 1, 2 and 3). Both the initial and final scores were lower in more disabled categories. Thus, the disability categories as defined by the Czech reimbursement scheme proved valid for grouping patients according to the level of disability, which justifies their use as the independent variable in this study.
Fig. 1.
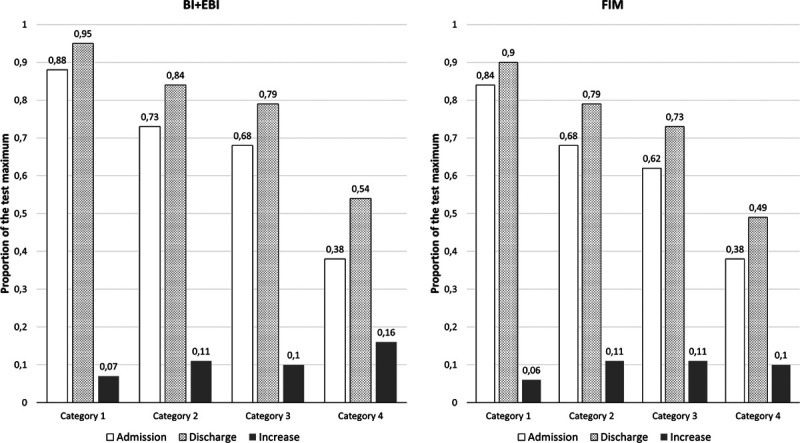
Average scores of Barthel Index+EBI and total FIM in the beginning and at the end of inpatient rehabilitation with the average improvement across the four disability categories. EBI, Extended Barthel Index; FIM, functional independence measure.
For the calculation of cost-effectiveness, we first examined the dependence of the costs and their components on the disability category. The cost data are presented in Table 2.
Table 2.
Average costs across disability categories
| Category | Number of patients | Costs per one patient-CZK (EUR) | Total costs per one patient – index (relation to the average costs in the first category) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | One-day | Total | Personnel | Nursing | Therapeutic | Materials | Devices and aids | Drugs | Complement | ||
| 1 | 15 | 68 825 (2614) | 4283 (162.67) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 2 | 27 | 85 263 (3238) | 4513 (171.40) | 1.239 | 1.100 | 1.313 | 0.943 | 1.817 | 1.123 | 4.942 | 4.010 |
| 3 | 24 | 129 498 (4918) | 5352 (203.27) | 1.882 | 2.441 | 3.993 | 1.302 | 7.269 | 1.278 | 3.797 | 2.064 |
| 4 | 21 | 167 530 (6363) | 6165 (234.14) | 2.434 | 3.514 | 6.178 | 1.559 | 14.732 | 1.399 | 5.163 | 2.237 |
For calculating the cost-effectiveness ratios, we divided the average costs of early rehabilitation hospitalization by the average increase in Barthel Index, EBI, Barthel Index+EBI, total FIM, motor FIM and cognitive FIM from admission to discharge. Thus, the resulting cost-effectiveness ratio can be interpreted as the incremental cost-effectiveness ratio, which provides a standard comparison of the spent money effectiveness over the disability categories. All cost-effectiveness data are presented in Table 3. Since the cognition scores (EBI and FIM-cognitive) had only a small effect on the total results, we focused on the respective motor scores (Barthel Index and motor FIM) and the total scores (Barthel Index+EBI and total FIM). Figure 2 shows the cost-effectiveness ratio dependent on the disability categories for Barthel Index and FIM, indicating the same pattern for FIM and Barthel Index scores. Finally, Fig. 3 provides the breakdown of the cost-effectiveness results across different Barthel Index and FIM scores, expressed as the ratio to the category average.
Table 3.
Cost-effectiveness ratios across disability categories and outcome measures
| Disability category | 1 | 2 | 3 | 4 | ||||
|---|---|---|---|---|---|---|---|---|
| C-Average cost (CZK) | 68 825 | 85 263 | 129 498 | 167 530 | ||||
| E | C/E | E | C/E | E | C/E | E | C/E | |
| Barthel Index | 8.0 | 8603 | 16.7 | 5116 | 15.8 | 8179 | 20.7 | 8088 |
| EBI | 5.7 | 12 075 | 3.7 | 23 044 | 3.8 | 34 078 | 8.8 | 19 038 |
| Barthel Index+EBI | 13.7 | 5024 | 20.4 | 4180 | 19.6 | 6607 | 29.5 | 5679 |
| FIM motor | 6.5 | 10 588 | 12.8 | 6661 | 12.8 | 10 117 | 10.6 | 15 805 |
| FIM cognitive | 1.4 | 49 161 | 1.3 | 65 587 | 1.0 | 129 498 | 2.4 | 69 804 |
| FIM | 7.9 | 8712 | 14.1 | 6047 | 13.8 | 9384 | 13.0 | 12 887 |
C/E values are comparable only in lines, not between different outcomes.
C, average cost for the disability category; C/E, cost-effectiveness ratio for the respective outcome; E, effect, i.e. outcome value; EBI, Extended Barthel Index; FIM, functional independence measure.
Fig. 2.
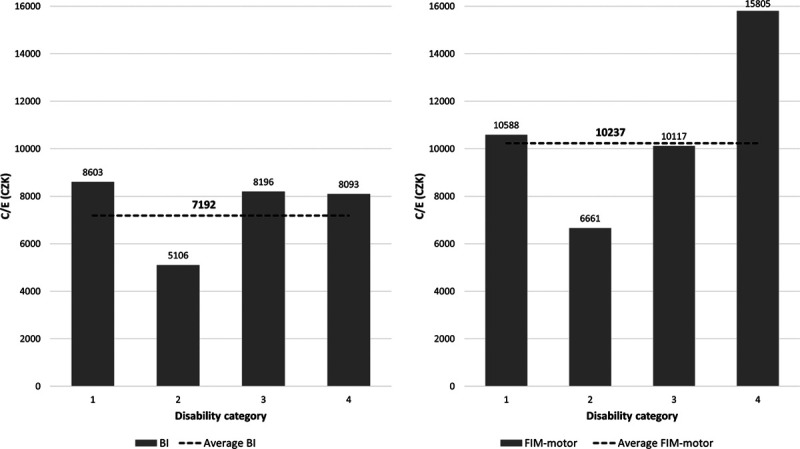
Cost-effectiveness based on the Barthel Index and motor FIM across disability categories. FIM, functional independence measure.
Fig. 3.
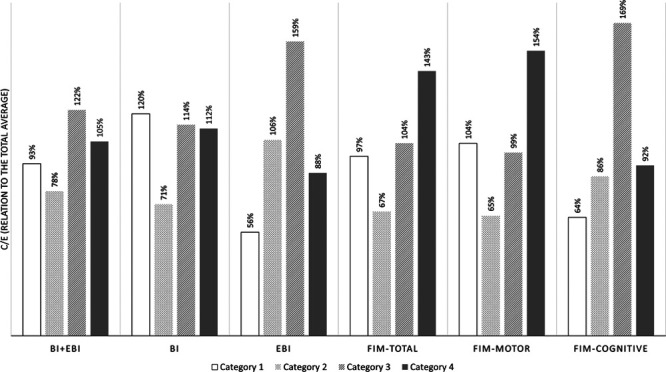
Cost-effectiveness as a percentage of the overall total average cost for different disability categories and outcome measures.
The greatest cost-effectiveness (the smallest expense for achieving a one-point improvement in the functional score) was most often found in category 2 (partly self-sufficient) (i.e. the lowest value across four categories for all outcomes but EBI). The cost-effectiveness based on Barthel Index was similar for categories 1, 3 and 4, while increasing FIM by one point was the most expensive in category 4 (Table 3, last row last column).
Discussion
The idea behind our research was that the costs grow with the degree of disability, which proved true; depending on the disability category, the cost increase was between 56 and 143% (Table 2). This does not fully project into cost-effectiveness figures. The inpatient rehabilitation proved to be most effective for partly self-sufficient patients (disability category 2), although it is closely effective also for self-sufficient patients (category 1) and those that require an enhanced level of supervision (category 3) (Fig. 2).
On the other hand, inpatient rehabilitation appears to be the least effective for the most severely disabled patients (disability category four), who are greatly dependent on others in activities of daily living. Not surprisingly, the worse the initial disability, the longer the time to improve to the point of plateau. They also have a lot of comorbidities and need more medication, more aids and sometimes more therapists and nurses to assist with mobility. Some of them remain severely disabled, showing little to no improvement, and we have no reliable predictive markers to see it early after stroke (Winters et al.,2018). On the other hand, even a small improvement may mean a great difference in the quality of life for this group.
Our results also revealed which disability scales are the most suitable for economic analyses. The scales assessing motor skills (Barthel Index and motor FIM) are much more sensitive than cognitive counterparts (EBI and cognitive FIM). This is not surprising because, after stroke, motor impairments tend to be more common and profound than cognitive impairments, they also recover faster and more, and, by the design, the motor scales cover more functional items and are more sensitive than the cognitive scales. The patient categorization for the Czech reimbursement scheme, based mostly on mobility and motor deficits causing the biggest burden on nursing support and material expenses, appeared to be adequate for both grouping patients into different disability categories and cost-effectiveness analysis.
In our study, the average cost of hospitalization was about one-third of the AVERT costs. The AVERT trial cost was calculated from phase II (Tay-Teo et al., 2008) and phase III (Gao et al., 2019) data. These costs are slightly higher than the Taiwan costs described recently by Chen et al. (2020). According to Gao et al. (2019), their very early mobilization and usual care were associated with comparable costs and outcomes [measured by the modified Rankin Scale and Quality Adjusted Life Years (QALY) gains]. Due to the differences in the purchase power parity (or alternatively, GDP per capita), a direct comparison of costs is not meaningful. Of more interest is the relative cost comparing different cost components or costs under different circumstances.
Limitations and future directions
This study was limited in time (1 year) and research capacity. The main limitations are the relatively small sample from three (although main) Czech hospitals, the sample inhomogeneity (different types and locations of strokes), and no control sample. Finally, this study examined the outcomes of inpatient rehabilitation that do not adequately correspond to the long-time goals and quality of life measures (e.g. QALY gains).
Conclusion
The results indicate that the total and per day costs of early inpatient rehabilitation after stroke increases with increasing disability. On the basis of our disability classification, the difference in the costs between the least costly group (self-sufficient) and the most costly group (immobile) was about 2.4-fold. In absolute values, the main driver of the difference was the personnel costs, above all nursing costs. Monitoring trends in cost and cost-effectiveness of rehabilitation is warranted for the judicious allocation of available resources.
Acknowledgements
The authors would like to express their gratitude to Prof. Olga Švestková, who was the conceptual originator of this research. She motivated us and led us until her death shortly before the project was finished. Olga, you are permanently with us! The research leading to this article was supported by grant No. 410004194 provided by the General Health Insurance Company of the Czech Republic from their Secondary Prevention Fund.
Conflicts of interest
There are no conflicts of interest.
References
- Bernhardt J, Collier JM, Bate PJ, Thuy MN, Bernhardt J. Very early versus delayed mobilization after stroke systematic review and meta-analysis. Stroke. 2019; 50:E178–E179 [Google Scholar]
- Bernhardt J, English C, Johnson L, Cumming TB. Early mobilization after stroke: early adoption but limited evidence. Stroke. 2015a; 46:1141–1146 [DOI] [PubMed] [Google Scholar]
- Bernhardt J, Langhorne P, Lindley RI, Thrift AG, Ellery F, Colliere J, et al. Efficacy and safety of very early mobilisation within 24 h of stroke onset (AVERT): a randomised controlled trial. Lancet. 2015b; 386:46–55 [DOI] [PubMed] [Google Scholar]
- Brady BK, McGahan L, Skidmore B. Systematic review of economic evidence on stroke rehabilitation services. Int J Technol Assess Health Care. 2005; 21:15–21 [DOI] [PubMed] [Google Scholar]
- Chen CM, Yang YH, Lee M, Chen KH, Huang SS. Economic evaluation of transferring first-stroke survivors to rehabilitation wards: a 10-year longitudinal, population-based study. Top Stroke Rehabil. 2020; 27:8–14 [DOI] [PubMed] [Google Scholar]
- Chumney D, Nollinger K, Shesko K, Skop K, Spencer M, Newton RA. Ability of functional independence measure to accurately predict functional outcome of stroke-specific population: systematic review. J Rehabil Res Dev. 2010; 47:17–29 [DOI] [PubMed] [Google Scholar]
- Coleman ER, Moudgal R, Lang K, Hyacinth HI, Awosika OO, Kissela BM, Feng W. Early rehabilitation after stroke: a narrative review. Curr Atheroscler Rep. 2017; 19:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czech Republic. Decree No. 134/1998 Coll., which issues the list of medical procedures with their point values (as amended). Collection of Law. Czech. [cited 2020 May 31]. 1998. https://www.zakonyprolidi.cz/cs/1998-134. [Accessed 24 June 1998]
- DIMDI. ICD-10-GM Version 2018. Erweiterter Barthel-Index. [cited 2020 July 30]. 2018. https://www.dimdi.de/static/de/klassifikationen/icd/icd-10-gm/kode-suche/htmlgm2018/zusatz-07-erwbarthelindex.htm. [Accessed 22 September 2017]
- Gao L, Sheppard L, Wu O, Churilov L, Mohebbi M, Collier J, et al. ; AVERT Trial Collaboration Group. Economic evaluation of a phase III international randomised controlled trial of very early mobilisation after stroke (AVERT). BMJ Open. 2019; 9:e026230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacke W, Ringleb PA, Bousser M-G, et al. Guidelines for management of ischaemic stroke and transient ischaemic attack 2008 – the European Stroke Organisation (ESO) Executive Committee and the ESO Writing Committee. Cerebrovasc Dis. 2008; 25:457–507 [DOI] [PubMed] [Google Scholar]
- Hamann GF, Müller R, Burghard A, Widder B. Treatment in acute stroke – stroke unit is mandatory [Review]. Neurol Psychiatry Brain Res. 2016; 22:105–109 [Google Scholar]
- Hsieh CY, Huang HC, Wu DP, Li CY, Chiu MJ, Sung SF. Effect of rehabilitation intensity on mortality risk after stroke. Arch Phys Med Rehabil. 2018; 99:1042–1048.e6 [DOI] [PubMed] [Google Scholar]
- Katona M, Schmidt R, Schupp W, Graessel E. Predictors of health-related quality of life in stroke patients after neurological inpatient rehabilitation: a prospective study. Health Qual Life Outcomes. 2015; 13:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakauer JW, Carmichael ST, Corbett D, Wittenberg GF. Getting neurorehabilitation right: what can be learned from animal models? Neurorehabil Neural Repair. 2012; 26:923–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Küçükdeveci AA, Stibrant Sunnerhagen K, Golyk V, Delarque A, Ivanova G, Zampolini M, et al. Evidence-based position paper on Physical and Rehabilitation Medicine professional practice for persons with stroke. The European PRM position (UEMS PRM section). Eur J Phys Rehabil Med. 2018; 54:957–970 [DOI] [PubMed] [Google Scholar]
- Langhorne P, Wu O, Rodgers H, Ashburn A, Bernhardt J. A very early rehabilitation trial after stroke (AVERT): a phase III, multicentre, randomised controlled trial. Health Technol Assess. 2017; 21:1–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch E, Hillier S, Cadilhac D. When should physical rehabilitation commence after stroke: a systematic review. Int J Stroke. 2014; 9:468–478 [DOI] [PubMed] [Google Scholar]
- Mahoney FI, Barthel DW. Functional evaluation: the barthel index. Md State Med J. 1965; 14:61–65 [PubMed] [Google Scholar]
- Maritz R, Tennant A, Fellinghauer CS, Stucki G, Pradinger B. The Extended Barthel Index (EBI) can be reported as a unidimensional interval-scaled metric – a psychometric study. Phys Med Rehab Kuror. 2019; 29:224–232 [DOI] [PubMed] [Google Scholar]
- Ministry of Health of the Czech Republic 2015 Seznam center vysoce specializované péče o pacienty s iktem [List of highly specialized centers for the care of patients after acute stroke]. Věstník MZ ČR. No. 11/2015:52-56. Czech. [cited 2020 May 31]. http://www.mzcr.cz/Legislativa/dokumenty/vestnik-c11/2015_10551_3242_11.html. [Accessed 21 July 2015]
- Murphy TH, Corbett D. Plasticity during stroke recovery: from synapse to behaviour. Nat Rev Neurosci. 2009; 10:861–872 [DOI] [PubMed] [Google Scholar]
- Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. ; American Heart Association Stroke Council. 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018; 49:e46–e110 [DOI] [PubMed] [Google Scholar]
- Prosiegel M, Böttger S, Schenk T, Konig N, Marolf M, Vaney C, et al. Der Erweiterte Barthel-Index (EBI) – eine neue Skala zur Erfassung von Fähigkeitsstörungen bei neurologischen Patienten. Neurol Rehabil. 1996; 2:7–13 [Google Scholar]
- Pross C, Berger E, Siegel M, Geissler A, Busse R. Stroke units, certification, and outcomes in German hospitals: a longitudinal study of patient-based 30-day mortality for 2006-2014. BMC Health Serv Res. 2018; 18:880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn TJ, Paolucci S, Sunnerhagen KS, Sivenius J, Walker MF, Toni D, Lees KR; European Stroke Organisation (ESO) Executive Committee; ESO Writing Committee. Evidence-based stroke r-ehabilitation: an expanded guidance document from the European stroke organisation (ESO) guidelines for management of ischaemic stroke and transient ischaemic attack 2008. J Rehabil Med. 2009; 41:99–111 [DOI] [PubMed] [Google Scholar]
- Sheppard L, Dewey H, Bernhardt J, Collier JM, Ellery F, Churilov L, et al. ; AVERT Trial Collaboration Group. Economic evaluation plan (EEP) for a very early rehabilitation trial (AVERT): an international trial to compare the costs and cost-effectiveness of commencing out of bed standing and walking training (very early mobilization) within 24 h of stroke onset with usual stroke unit care. Int J Stroke. 2016; 11:492–494 [DOI] [PubMed] [Google Scholar]
- Škoda O, Herzig R, Mikulík R, Neumann J, Vaclavik D, Bar M, et al. Klinický standard pro diagnostiku a léčbu pacientů s ischemickou cévní mozkovou příhodou a s tranzitorní ischemickou atakou – verze 2016 [Clinical Guideline for the Diagnostics and Treatment of Patients with Ischemic Stroke and Transitory Ischemic Attack – Version 2016]. Cesk Neurol Neurochir. 2016; 79:351–363 [Google Scholar]
- de Sousa DA, von Martial R, Abilleira S, et al. Access to and delivery of acute ischaemic stroke treatments: a survey of national scientific societies and stroke experts in 44 European countries. Eur Stroke J. 2019; 4:13–28 10.1177/2396987318786023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay-Teo K, Moodie M, Bernhardt J, Thrift AG, Collier J, Donnan G, Dewey H. Economic evaluation alongside a phase II, multi-centre, randomised controlled trial of very early rehabilitation after stroke (AVERT). Cerebrovasc Dis. 2008; 26:475–481 [DOI] [PubMed] [Google Scholar]
- Teasell R, Hussein N. Teasell R, Hussein N, Viana R. Brain reorganization, recovery and organized care. Stroke rehabilitation clinician handbook. 2016, London, Ontario, Canada: Department of Physical Medicine and Rehabilitation, Western University [Google Scholar]
- Tummers JFMM, Schrijvers AJP, Visser-Meily JMA. Economic evidence on integrated care for stroke patients; a systematic review. Int J Integr Care. 2012; 12:e193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstein CJ, Stein J, Arena R, Bates B, Cherney LR, Cramer SC, et al. ; American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, and Council on Quality of Care and Outcomes Research. Guidelines for adult stroke rehabilitation and recovery: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2016; 47:e98–e169 [DOI] [PubMed] [Google Scholar]


