Supplemental digital content is available in the text.
Key Words: Adhansia, FOQUEST, methylphenidate, pharmacokinetics, ADHD
Abstract
Purpose/Background
PRC-063 is a once-daily, extended-release oral formulation of methylphenidate hydrochloride developed to provide early and prolonged symptom improvement in patients with attention-deficit/hyperactivity disorder.
Methods/Procedures
We conducted 3 randomized, open-label crossover studies of the pharmacokinetics of PRC-063 in healthy, nonobese men and women aged 18 to 45 years. PRC-063 (100 mg/d) was compared with immediate-release methylphenidate (20 mg, 3 times daily) when administered on a single day under fasted and fed conditions and at steady state (day 5 of repeat dosing under fasted conditions). The pharmacokinetics of PRC-063 administered as capsule contents sprinkled on apple sauce, yoghurt, or ice cream were also investigated.
Findings/Results
PRC-063 demonstrated biphasic absorption, with 2 distinct peak plasma concentrations. Intake of a high-fat, high-calorie meal did not increase the peak plasma methylphenidate concentration (Cmax) or extent of absorption (area under the curve), however; it resulted in slower uptake versus a fasted state. During repeated dosing, steady state was reached with no further accumulation of methylphenidate from day 3. At steady state, PRC-063 gave higher evening and trough plasma methylphenidate levels than immediate-release methylphenidate (3 times daily). The pharmacokinetics of PRC-063 sprinkled on food were comparable to that of intact capsules. Reported adverse events (AEs) were consistent with the established safety profile of methylphenidate. There were no serious AEs, but 3 subjects discontinued the repeat-dosing study because of AEs assessed as possibly related to study treatment.
Implications/Conclusions
Our data indicate that PRC-063 can be taken with or without food or by sprinkling capsule contents on food.
Attention-deficit/hyperactivity disorder (ADHD) causes impaired functioning, which can manifest as poor school or work performance.1 Attention-deficit/hyperactivity disorder impairments can extend from wakening until bedtime, affecting activities before and after school and work. For this reason, some patients may require a medication that, when taken in the morning, provides rapid and daylong symptom management.2 A rapid onset of action means that patients do not need to wake earlier than usual to take their medication or take a medication the night before to achieve a morning effect,3 whereas a long duration of action means that patients may not need to supplement with short-acting medications later in the day. A rapid onset may also have practical advantages, perhaps helping parents to get their children to school or themselves to work.
Since being established as standard treatments for ADHD, stimulants such as methylphenidate and mixed amphetamine salts have evolved from immediate-release (IR) medications administered as multiple daily doses to longer-acting formulations designed for once-daily dosing.4,5 Multiple dosing is associated with rebound and wear-off affecting the end of the day, quality of life issues relating to privacy and stigmatization, and problems with treatment adherence.6–8 Moreover, although use of IR stimulants permits flexibility of time of administration, this flexibility may contribute to a lifestyle of “disorganization and procrastination.”9 Long-acting stimulants promote a more consistent lifestyle, enhance planning and facilitation of an organized day, and decrease the need for “as needed” IR doses.9 Other potential advantages of longer-acting formulations include less fluctuation in the pharmacokinetic (PK) profile, a simplified treatment regimen, and improved treatment adherence.8 Certain formulations of methylphenidate and mixed amphetamine salts have a sufficient duration of action for once-daily dosing.10–12 However, most currently available long-acting stimulants show evidence of diminished efficacy toward the end of the work or school day.13 If a long-acting stimulant fails to provide a sufficient duration of action, additional IR or long-acting stimulant doses are often needed in the afternoon hours to extend the duration of medication coverage.14–18
To maintain efficacy toward the end of the day, additional, longer-acting stimulant options are needed. PRC-063 (marketed as Adhansia XR [Purdue Pharmaceuticals L.P., Wilson, North Carolina] in the United States and as FOQUEST [Purdue Pharma, Pickering, Ontario] in Canada) is a once-daily, extended-release, racemic d- and l-methylphenidate hydrochloride capsule formulation (with d-methylphenidate being the more active enantiomer). In the United States and Canada, it is used to treat ADHD in patients 6 years and older. PRC-063 capsules contain identical beads, each of which has an IR layer containing approximately 20% of the methylphenidate dose and a polymer-coated controlled-release (CR) layer containing approximately 80% of the methylphenidate dose. Dissolution of the IR layer begins immediately upon ingestion. Release of methylphenidate from the CR layer is delayed and occurs after the beads have passed through the stomach. At a pH of 7 or greater, the delayed-release polymer coating dissolves and methylphenidate in the CR layer diffuses into the gastrointestinal tract and is absorbed. Because of this pH-dependent release, the US Food and Drug Administration–approved full prescribing information for PRC-06319 recommends that patients who are concomitantly taking gastric pH modifiers be monitored for changes in clinical effects.
The safety and efficacy of PRC-063 were previously investigated in a double-blind, randomized, placebo-controlled, crossover workplace environment study in adult patients with ADHD (NCT02225639), conducted at 2 sites in the United States in 2014 to 2015.20 In addition, a randomized, double-blind, parallel-group, placebo-controlled, phase 3 adult laboratory classroom study was completed after approval to evaluate the safety and efficacy in adult patients with ADHD (NCT03618030).21 Patients in both studies showed improved attention, assessed by Permanent Product Measure of Performance total score, averaged from 1 to 16 hours after PRC-063 dosing.20,21 In addition, mean Swanson, Kotkin, Agler, M-Flynn, and Pelham combined score was improved during PRC-063 treatment compared with placebo treatment.20 A similar improvement in the Swanson, Kotkin, Agler, M-Flynn, and Pelham combined score was observed in a 13-hour classroom trial of PRC-063 versus placebo in children aged 6 to 12 years with ADHD (NCT03172481).22
The efficacy and safety of PRC-063 were also evaluated in adults with ADHD in a 4-week, randomized, double-blind, multicenter, placebo-controlled study (NCT02139124). Compared with placebo, PRC-063 demonstrated significantly greater improvements in ADHD Rating Scale 5 total score.23 Similar efficacy results were obtained in children aged 12 to 17 years with ADHD (NCT02139111: unpublished data). In the adult study, similar proportions of PRC-063–treated and placebo-treated adult ADHD patients discontinued treatment because of adverse reactions.23
A series of phase 1 bioavailability studies were conducted to compare the rate and extent of absorption of PRC-063 and 3 times daily (TID) IR methylphenidate (Ritalin) under fasted and fed conditions when administered on a single day (study I) and at steady state on day 5 of repeat dosing under fasted conditions (study II), and to determine whether the PK of PRC-063 is altered when its capsule contents are administered by sprinkling on food rather than as intact capsules swallowed whole (study III). The aim of this article is to describe the PK of PRC-063, which provide the foundation for its clinical efficacy.
MATERIALS AND METHODS
Study Overview
Three independent, phase 1 bioavailability studies were performed at a single clinical facility operated by the contract research organization inVentiv Health Clinical Inc (Québec City, Québec, Canada) between October and November 2013. For each study, the protocol and informed consent form were reviewed and approved by an independent institutional review board (IRB Services, Aurora, Ontario, Canada), and a no objection letter was received from the Canadian authorities before the start of the study. Subjects provided written informed consent before any study procedures were started. All studies were conducted in accordance with International Council for Harmonisation Good Clinical Practices and Good Laboratory Practices, local regulatory requirements, and the most recent version of the Declaration of Helsinki.
Participants
Eligible participants in all studies were healthy men and women aged 18 to 45 years with a body mass index between 18.5 and 30.0 kg/m2. Smokers who smoked 25 or fewer cigarettes per day were eligible (smokers who smoked more than this were excluded because of the risk of cardiovascular and other health complications). To be eligible, women of childbearing potential who were sexually active were required to use an effective form of contraception throughout the study and for 30 days after the last dose of study drug.
Exclusion criteria included the following: any clinically significant abnormality, abnormal laboratory test result, or positive test for hepatitis B, hepatitis C, or HIV at screening; positive drug test result at screening, history of significant alcohol or drug abuse, recent use of recreational drugs, or recent regular use of alcohol; history of allergic reactions to methylphenidate or a related drug; use of any drug known to induce or inhibit hepatic drug metabolism within 30 days before the first dose of study drug; current pregnancy or breastfeeding; any clinically significant electrocardiogram abnormality or vital sign abnormality; use of an investigational drug within 30 days (90 days for biologics) of the first dose of study drug or participation in another investigational study within 30 days; and recent use of any medication other than a topical product without significant systemic absorption or a hormonal contraceptive.
To eliminate typical drug interactions and ensure a consistent release and absorption profile, subjects were required to abstain from the following as indicated until after collection of the last PK blood sample for the dosing period: foods and beverages containing poppy seeds (from 24 hours before each dosing period); energy drinks and foods or beverages containing xanthine derivatives or xanthine-related compounds (from 48 hours before each dosing period); food supplements and herbal supplements (from 7 days before each dosing period); food and beverages containing grapefruit, starfruit, pomegranate, pineapple, or pomelo (from 7 days before each dosing period); recreational drugs; and alcohol (from 24 hours before each dosing period). They were also required not to do vigorous physical activity while at the clinical facility.
Study Design
The 3 studies were randomized, open-label studies that investigated PRC-063 (Purdue Pharma, Ontario, Canada).
Study I was a 4-way crossover study that investigated a single dose of PRC-063 100 mg versus TID IR methylphenidate 60 mg (20 mg at 0, 4, and 8 hours) under fasted and fed conditions to stress the absorption and tolerability of the product. For PRC-063, 100 mg was predicted to be the highest dose based on PK data from pilot studies and is the highest dose available to prescribers in Canada24; for IR methylphenidate, 60 mg is the highest manufacturer-recommended daily dose.25 PRC-063 was administered either after an overnight fast of at least 10 hours, or 30 minutes after subjects started to eat a standard very high-fat, high-calorie breakfast (800–1000 calories, with ~50% of the total caloric content derived from fat) as 45 + 55 mg capsules (the 100-mg capsule was not available at the time the studies were conducted). Subjects were fasted for at least 4 hours after PRC-063 dosing. For IR methylphenidate under fasted conditions, the first daily dose was administered after an overnight fast of at least 10 hours; the second and third daily doses were administered after a fasting period of at least 2 hours; dosing was followed by a fasting period of at least 1.5 hours (first and second doses) or 2 hours (third dose). For IR methylphenidate under fed conditions, the first daily dose was administered 30 minutes after subjects started to eat a standard high-fat, high-calorie breakfast; the second and third daily doses were administered 30 minutes after subjects started to eat lunch (second dose) or dinner (third dose); and dosing was followed by a fasting period of 3.5 hours (first and second doses) or 2 hours (third dose). PRC-063 and IR methylphenidate were each administered on 2 separate days, with a washout period of at least 7 days between doses. During each dosing period, subjects were confined to the clinical facility from at least 11 hours before the start of dosing until after the last blood sample had been obtained. For all treatment groups, subjects were required to abstain from smoking from 2 hours before dosing until 8 hours after the first dosing to prevent any potential interaction with absorption of methylphenidate.
Study II was a 2-way crossover study that investigated PRC-063 100 mg (45 + 55 mg capsules), once daily versus IR methylphenidate 20 mg, TID (at 0, 4, and 8 hours) after an overnight fast of at least 10 hours on 5 consecutive days. For PRC-063, subjects were fasted for at least 1.5 hours after dosing on days 1 to 4 and for at least 4 hours after dosing on day 5. For IR methylphenidate, dosing on all days (including fasting periods) was the same as dosing under fasted conditions in study I. During each dosing period, subjects were confined to the clinical facility from at least 10 hours before the start of dosing until after the last blood sample had been obtained. There was a washout period of 8 days between 5-day dosing periods. Subjects were required to abstain from smoking from 2 hours before dosing on day 5 until 8 hours after the first daily dose.
Study III was a 4-way crossover study that investigated a single dose of PRC-063 100 mg (45 + 55 mg capsules), administered after an overnight fast of at least 10 hours either as intact capsules or as capsule contents sprinkled on 1 tablespoon (15 mL) of unsweetened apple sauce, ice cream, or yoghurt. The food with the sprinkled contents was consumed 10 minutes (±5 minutes) after sprinkling. Subjects were fasted for at least 4 hours after dosing. During each dosing period, subjects were confined to the clinical facility from at least 10 hours before the start of dosing until after the last blood sample had been obtained. There was a washout period of 7 days between administrations. Subjects were required to abstain from smoking from 2 hours before dosing until 6 hours after dosing.
Blood Sampling and Analysis
In study I, blood samples were obtained at the following time points in each dosing period: before the morning PRC-063 or IR methylphenidate dose, every half hour for the first 15 hours after the morning dose, and at 16, 18, and 24 hours after the morning dose. In study II, blood samples were obtained before the morning PRC-063 or IR methylphenidate dose on days 1, 3, 4, and 5. On day 5, additional blood samples were obtained every half hour for the first 15 hours after morning dosing and at 16, 18, 24, 30, and 36 hours after morning dosing. In study III, blood samples were obtained in each dosing period before PRC-063 dosing, every half hour for the first 8 hours after PRC-063 dosing, and at 10, 12, 14, 16, 24, 30, and 36 hours after PRC-063 dosing. In all 3 studies, actual times of blood sampling were recorded and were used in the statistical analyses.
Blood samples (3 mL at each time point) were drawn into K2-EDTA–coated collection tubes, cooled in an ice bath, and (within 2 hours of collection) were centrifuged at 4°C at 3000 rpm (1999g) for 10 minutes. Plasma isolated in this way was transferred to polypropylene tubes containing stabilization buffer (10% w/v Na2-EDTA, 10% w/v citric acid 10%), vortexed for approximately 10 seconds, and stored in a −80°C freezer before measurement of d- and l-methylphenidate at the same clinical facility where the bioavailability studies were performed.
d- and l-methylphenidate levels were measured by high-performance liquid chromatography with detection by tandem mass spectrometry. An internal standard (methylphenidate-d9) was added to each sample. d-methylphenidate, l-methylphenidate, and methylphenidate-d9 were extracted from a 0.2-mL aliquot of acidified plasma by liquid-liquid extraction with ethyl acetate/hexane (after the addition of an alkaline solution to shift the pH to basic) according to a proprietary method. The extracted samples were then injected into a liquid chromatograph equipped with a Chirobiotic V column (150 mm × 4.6 mm, 5 μm; Astec, Oakville, Canada). Mobile phase A was a mixture of methanol, acetic acid, and ammonium hydroxide; mobile phase B was 100% methanol. Isocratic chromatographic chiral separation was performed at room temperature at a flow rate of 1.5 mL/min for pump 1 and of 1 mL/min for pump 2. Detection was performed with an API 5000 tandem mass spectrometer (AB Sciex Concord, Canada) using the following settings: auxiliary gas pressure, 70 psi; nebulizer gas pressure, 60 psi; curtain gas pressure, 50 psi; TurboIonSpray temperature, 350°C; and ion spray voltage, 3000 V. Calibration standards were used to generate a calibration curve from which sample methylphenidate concentrations (normalized to the internal standard) were derived. Blank samples and quality control samples (450, 2250, 15,000, and 22,500 pg/mL for d-methylphenidate; 12, 60, 400, and 600 pg/mL for l-methylphenidate) were also analyzed. The mass transitions quantified were 235.3 to 84.1 amu for d-methylphenidate, 234.2 to 84.1 amu for l-methylphenidate, and 243.3 to 93.1 amu for methylphenidate-d9. The validated quantification range for the assay was 150 to 30,000 pg/mL for d-methylphenidate and 4 to 800 pg/mL for l-methylphenidate. Values below the lower limit of quantification were entered as zero values in the PK analyses. Within-day variability values, measured as the coefficient of variation (CV), were 0.83% to 3.32% for d-methylphenidate and 1.38% to 14.09% for l-methylphenidate. Between-day variability values (CV) were 3.42% to 6.69% for d-methylphenidate and 5.83% to 18.05% for l-methylphenidate.
PK Analyses
In noncompartmental PK analyses, the elimination rate constant (Kel) was calculated as the slope × −1 for regression analyses of natural logarithm (ln)-transformed methylphenidate concentration versus time; the elimination half-life (T½ el) was calculated as ln(2)/Kel; area under the plasma concentration versus time curve from time 0 to the last measured concentration (AUC0–t) and to 24 hours (AUC0–24) was calculated by the trapezoid rule; AUC between time 0 and infinity (AUC0–inf) was calculated as AUC0–t + (Ct/Kel); and residual area (in percent) was calculated as (1 − (AUC0–t/AUC0–inf)) × 100. Maximum plasma concentration (Cmax, Cmax0–4, Cmax8–16) and time to maximum plasma concentration (Tmax, Tmax0–4,Tmax8–16) were also calculated. In study II, Cmin (minimum concentration at steady state) and fluctuation index (peak-trough fluctuation at steady state: 100 × (Cmax − Cmin)/Cav [where Cav = AUC0−τ/τ and τ = 24 hours]) were also calculated.
Presented data are for d- plus l-methylphenidate (total methylphenidate). In all 3 studies, d-methylphenidate accounted for more than 97.5% of the value of AUC0–t for d- plus l-methylphenidate for each treatment/set of treatment conditions (data not shown). Higher oral bioavailability of d- versus l-methylphenidate has been documented previously.26,27
Statistical Analyses
Data from subjects who completed at least 2 dosing periods (studies I and III) or from subjects who completed the study (study II) were used in the PK analyses. In study III, one of the completed dosing periods was required to be dosing with intact capsules. In all studies, subjects included in the PK analyses were required to have an adequately characterized PK profile. Pharmacokinetic and statistical analyses were performed using Pharsight Knowledgebase Server version 4.0.2 and WinNonlin 5.3 or 6.4. SAS version 9.2 was used to calculate number of observations (N), mean, SD, CV (in percent), minimum, maximum, and median for plasma concentrations of d- plus l-methylphenidate for each treatment/set of treatment conditions and time point, and was also used to calculate each of the PK parameters listed previously.
Treatments were compared by analysis of variance (ANOVA) using the general linear models procedure in SAS on ln-transformed data (for Cmax and AUC parameters) or untransformed data (Tmax). The models included sequence, subject within sequence, period, and treatment. Because the daily dose was lower for IR methylphenidate than for PRC-063, additional models were created for studies I and II using data that had been dose normalized to 100 mg. A P value of <0.05 was considered statistically significant.
Based on least-squares (LS) means from ANOVA of ln-transformed data, the ratio of LS means, and corresponding 90% geometric confidence intervals were calculated for Cmax, Cmin, AUC0–t, AUC0–24, and AUC0–inf. Intersubject and intrasubject CVs were also calculated. In study II, steady state was determined using the general linear models procedure in SAS by repeated-measures ANOVA of ln-transformed predose plasma methylphenidate concentrations on days 3, 4, and 5.
Safety
In all 3 studies, analysis of safety was based on all subjects who received at least 1 dose of study medication (the safety population). Throughout each study, subjects were monitored for adverse events (AEs) by being asked about the onset of any new health problems and worsening of existing health problems. Treatment-emergent AEs were coded using MedDRA, version 16.1. Adverse events were assessed for seriousness and relationship to study treatment and were graded according to severity. Study discontinuation due to AEs was recorded.
RESULTS
Baseline Characteristics and Disposition
In all 3 studies, most subjects (83%–100%) were white and more than half were male (Supplemental Table 1 http://links.lww.com/JCP/A690).
In study I, 30 healthy adult subjects were randomized and dosed, of whom 24 completed all study periods. Five subjects permanently discontinued the study because of withdrawal of consent (2 subjects), inability to draw more blood (2 subjects), or a positive drug test (1 subject). One subject temporarily withdrew consent before returning to complete the remainder of the study.
In study II, 21 healthy adult subjects were randomized and dosed. Six subjects failed to complete the study. Reasons for noncompletion were withdrawal of consent (3 subjects) and significant AEs (3 subjects).
Of the 30 healthy adult subjects randomized and dosed in study III, 26 completed all study periods. Three subjects permanently discontinued the study because of withdrawal of consent (2 subjects) or a positive alcohol breath test (1 subject). One subject was temporarily withdrawn from the study because of intake of study medication in a way that contravened the study protocol.
Pharmacokinetics
PRC-063 Versus TID IR Methylphenidate: 1-Day Dosing Under Fed and Fasted Conditions (Study I)
Study I compared the PK of 100 mg of PRC-063 versus TID IR methylphenidate 60 mg after an overnight fast of at least 10 hours or after a high-fat, high-calorie breakfast. Figure 1 shows concentration versus time curves for plasma methylphenidate for PRC-063 and TID IR methylphenidate. There are 3 clear peaks for the 3 IR methylphenidate doses and 2 peaks for the outer and inner methylphenidate layers of PRC-063, indicating a biphasic absorption profile. Median 0- to 4-hour Tmax was 30 minutes sooner for PRC-063 than for the first IR methylphenidate dose under fasting conditions and 1 hour later under fed conditions. Plasma methylphenidate levels were higher with PRC-063 than with TID IR methylphenidate from approximately 13–14 to 24 hours under fed and fasted conditions. The curves for PRC-063 indicate similar overall exposure to methylphenidate in a fed versus fasted state, but slower uptake in a fed state. Median Tmax was 1 hour less when PRC-063 was administered in a fasted versus fed state (Table 1). Mean Cmax was 19% higher for fasted than for fed administration (13.36 vs 11.26 ng/mL). Otherwise, the PK of PRC-063 were generally similar in fed and fasted states. The LS mean ratios for PRC-063 administered in a fed versus fasted state were 97.45% for AUC0–t, 101.87% for AUC0–inf, and 87.49% for Cmax (Supplemental Table 2, http://links.lww.com/JCP/A690 which shows LS mean ratios for plasma methylphenidate for 1-day dosing). For both PRC-063 and IR methylphenidate, levels of methylphenidate uptake varied considerably between individuals (Fig. 1, Table 1).
FIGURE 1.

Concentration versus time curves for plasma d- plus l-methylphenidate after administration of PRC-063 and IR methylphenidate in fed and fasted states (study I). Data are shown as the mean.
TABLE 1.
PK Parameters for Plasma d- Plus l-methylphenidate After Administration of PRC-063 and IR Methylphenidate in Fed and Fasted States (Study I)
| PRC-063 (100 mg) | IR Methylphenidate (20 mg TID) | |||
|---|---|---|---|---|
| Fasted (n = 27) | Fed (n = 27) | Fasted (n = 28) | Fed (n = 28) | |
| AUC0–t*, ng · h/mL, mean ± SD (CV) | 171.63 ± 47.98 (27.95%) | 163.93 ± 41.43 (25.27%) | 133.67 ± 44.62 (33.38%) | 156.65 ± 38.54 (24.60%) |
| AUC0–inf, ng · h/mL, mean ± SD (CV) | 209.20 ± 63.24 (30.23%) | 206.00 ± 59.48 (28.87%) | 137.15 ± 46.57 (33.95%) | 160.73 ± 40.45 (25.17%) |
| Cmax, ng/mL, mean ± SD (CV) | 13.36 ± 4.79 (35.88%) | 11.26 ± 2.82 (25.01%) | 14.21 ± 3.86 (27.18%) | 15.40 ± 3.38 (21.93%) |
| Cmax0–4, ng/mL, mean ± SD (CV) | 9.45 ± 3.28 (34.77%) | 9.35 ± 1.94 (20.79%) | 9.29 ± 3.47 (37.41%) | 11.18 ± 3.48 (31.11%) |
| Cmax8–16, ng/mL, mean ± SD (CV) | 12.91 ± 4.76 (36.90%) | 10.93 ± 3.07 (28.10%) | 13.74 ± 3.75 (27.33%) | 14.29 ± 3.22 (22.54%) |
| Tmax, median (range), h | 11.5 (1.0–16.0) | 12.5 (2.5–24.0) | 9.5 (2.0–11.0) | 6.0 (2.1–10.5) |
| Tmax0–4, median (range), h | 1.5 (1.0–2.5) | 3.0 (1.5–4.0) | 2.0 (1.0–3.0) | 2.0 (1.0–4.0) |
| Tmax8–16, median (range), h | 12.5 (8.5–16.0) | 13.5 (10.5–16.0) | 9.52 (9.0–11.0) | 10.0 (9.0–11.5) |
| T½ el, mean ± SD, h | 6.83 ± 3.19 | 6.94 ± 2.22 | 3.56 ± 0.40 | 3.56 ± 0.45 |
| Residual area†, mean ± SD, % | 17.21 ± 10.75 | 18.41 ± 8.49 | 2.41 ± 0.96 | 2.43 ± 0.91 |
*t = 24 hours.
†Twenty-four hours to infinity.
There were some notable differences in mean values for PK parameters between PRC-063 and IR methylphenidate (Table 1). Mean residual area was higher for PRC-063 than for IR methylphenidate (17.21% vs 2.41% for fasted state, 18.41% vs 2.43% for fed state). Cmax was lower for PRC-063 than for IR methylphenidate.
Ratios for AUC0–t, AUC0–inf, and Cmax for PRC-063 versus IR methylphenidate also showed differences (Supplemental Table 2 http://links.lww.com/JCP/A690). For AUC0–t, AUC0–inf, and Cmax, the dose-normalized ratios for 100 mg PRC-063 versus 60 mg IR methylphenidate were 75.56%, 89.75%, and 53.73%, respectively, for administration in a fasted state and 62.04%, 77.26%, and 42.82%, respectively, for administration in a fed state. The non–dose-normalized ratios for AUC0–t, AUC0–inf, and Cmax for PRC-063 versus IR methylphenidate were 125.94%, 149.58%, and 89.55%, respectively, for administration in a fasted state and 103.40%, 128.77%, and 71.36%, respectively, for administration in a fed state.
PRC-063 Versus IR Methylphenidate: 5-Day Dosing (Study II)
Study II compared the PK of daily doses of 100 mg of PRC-063 versus 60 mg of IR methylphenidate during 5 days of dosing. Figure 2 shows concentration versus time curves for plasma methylphenidate for PRC-063 and IR methylphenidate at steady state. No statistically significant time or time × treatment effect was observed in repeated-measures ANOVA of ln-transformed predose plasma methylphenidate concentrations, indicating that steady state was reached for both PRC-063 and IR methylphenidate. For PRC-063, most subjects reached steady state by day 3 (with no further accumulation of methylphenidate after this point). At steady state, the residual plasma level of methylphenidate 24 hours after dosing was higher for PRC-063 than for IR methylphenidate.
FIGURE 2.
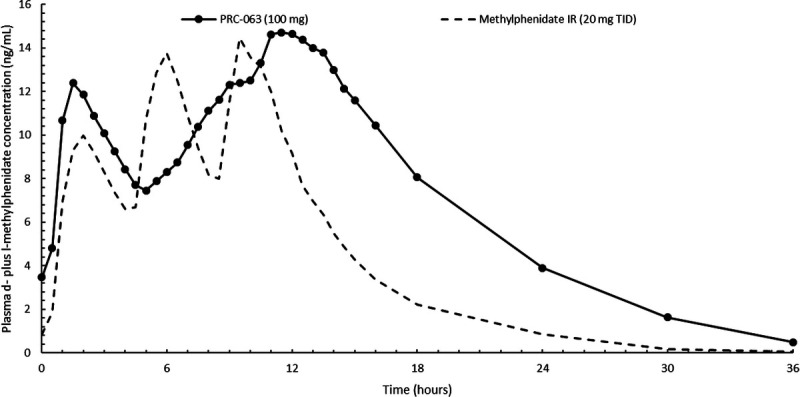
Concentration versus time curves for plasma d- plus l-methylphenidate at steady state (day 5 of a 5-day dosing period) for PRC-063 and IR methylphenidate (study II). Data are shown as the mean.
Table 2 shows calculated PK parameters for PRC-063 and IR methylphenidate on day 5 of the 5-day dosing schedule. Mean values for AUC0–24 and AUC0–t were higher for PRC-063 than for IR methylphenidate. Mean Cmin was higher for PRC-063 than for IR methylphenidate (3.27 vs 0.86 ng/mL). Fluctuation index was lower for PRC-063 than for IR methylphenidate (138.42% vs 244.02%). Cmax was similar for PRC-063 versus IR methylphenidate. However, when doses were normalized, Cmax was lower for PRC-063 than for IR methylphenidate (ratio, 60.25%; Supplemental Table 3, http://links.lww.com/JCP/A690 which shows LS mean ratios for plasma methylphenidate on day 5 of a 5-day dosing schedule).
TABLE 2.
PK Parameters for Plasma d- Plus l-methylphenidate on Day 5 of a 5-day Dosing Schedule: PRC-063 and IR Methylphenidate (Study II)
| PRC-063 (100 mg; n = 15) | IR Methylphenidate (20 mg TID; n = 15) | |
|---|---|---|
| AUC0–24, ng · h/mL, mean ± SD (CV) | 230.86 ± 87.88 (38.07%) | 153.81 ± 54.50 (35.44%) |
| AUC0–t*, ng · h/mL, mean ± SD (CV) | 253.83 ± 105.07 (41.39%) | 156.60 ± 58.13 (37.12%) |
| Cmax, ng/mL, mean ± SD (CV) | 16.14 ± 5.03 (31.15%) | 16.19 ± 5.55 (34.26%) |
| Cmax0–4, ng/mL, mean ± SD (CV) | 12.63 ± 3.81 (30.16%) | 10.35 ± 2.69 (25.99%) |
| Cmax8–16, ng/mL, mean ± SD (CV) | 15.89 ± 5.11 (32.17%) | 15.67 ± 5.69 (36.31%) |
| Cmin, ng/mL, mean ± SD (CV)† | 3.27 ± 2.21 (67.55%) | 0.86 ± 0.62 (71.92%) |
| Tmax, median (range), h | 11.5 (2.0–16.0) | 9.5 (5.0–11.0) |
| Tmax0–4, median (range), h | 1.5 (1.0–2.5) | 2.0 (1.5–2.5) |
| Tmax8–16, median (range), h | 12.0 (8.5–16.0) | 9.5 (9.0–11.0) |
| Fluctuation index, %, mean ± SD (CV)† | 138.42 ± 29.75 (21.49%) | 244.02 ± 33.64 (13.79%) |
*t = 36 hours.
†n = 14 (1 subject was excluded from calculation of summary statistics because no 24-hour sample was collected for the PRC-063 arm).
PRC-063 Administered as Intact Capsules Versus Capsule Contents (Study III)
Study III compared the PK of 100 mg of PRC-063 administered as intact capsules or as capsule contents sprinkled on unsweetened apple sauce, ice cream, or yoghurt. Figure 3 shows concentration versus time curves for plasma methylphenidate. The curves show no notable differences between capsules swallowed whole and capsule contents sprinkled on different foods.
FIGURE 3.

Concentration versus time curves for plasma d- plus l-methylphenidate after administration of PRC-063 as intact capsules or sprinkled on food (study III). Data are shown as the mean.
Table 3 shows calculated PK parameters for intact capsules and capsule contents sprinkled on food. Values for AUC parameters, Cmax, and residual area were similar for intact capsules and capsule contents sprinkled on different foods.
TABLE 3.
PK Parameters for Plasma d- Plus l-methylphenidate After Administration of PRC-063 as Intact Capsules or as Capsule Contents Sprinkled on Different Foods (Study III)
| Intact Capsules (n = 29) | Apple Sauce (n = 28) | Yoghurt (n = 28) | Ice Cream (n = 28) | |
|---|---|---|---|---|
| AUC0–24, ng · h/mL, mean ± SD (CV) | 219.97 ± 81.46 (37.03%) | 225.03 ± 74.45 (33.09%) | 216.61 ± 67.99 (31.39%) | 219.44 ± 66.76 (30.42%) |
| AUC0–t*, ng · h/mL, mean ± SD (CV) | 248.86 ± 99.52 (39.99%) | 249.38 ± 87.66 (35.15%) | 245.64 ± 84.29 (34.31%) | 248.82 ± 88.25 (35.47%) |
| AUC0–inf, ng · h/mL, mean ± SD (CV) | 255.82 ± 104.90 (41.01%) | 254.17 ± 90.81 (35.73%) | 252.74 ± 90.27 (35.72%) | 258.58 ± 105.33 (40.73%) |
| Cmax, ng/mL, mean ± SD (CV) | 15.74 ± 5.59 (35.48) | 17.38 ± 6.07 (34.93%) | 15.69 ± 4.72 (30.08%) | 16.41 ± 4.63 (28.20%) |
| Tmax, median (range), h | 10.0 (1.0–16.0) | 12.0 (1.0–16.0) | 11.0 (0.5–16.0) | 12.0 (1.0–24.0) |
| T½ el, mean ± SD, h | 4.96 ± 1.15 | 4.48 ± 1.06 | 4.99 ± 1.35 | 4.83 ± 1.62 |
| Residual area†, mean ± SD, % | 2.44 ± 2.05 | 1.74 ± 1.38 | 2.41 ± 2.16 | 2.66 ± 4.27 |
*t = 36 hours.
†36 hours to infinity.
For AUC0–t, AUC0–inf, and Cmax, LS mean ratios were calculated for capsule contents sprinkled on apple sauce, yoghurt, or ice cream versus intact capsules (Supplemental Table 4 http://links.lww.com/JCP/A690). For AUC0–t, AUC0–inf, and Cmax, the LS mean ratios ranged from 98% to 108% for apple sauce, 99% to 100% for yoghurt, and 101% to 106% for ice cream, indicating minimal differences in overall PK according to method of administration. The intrasubject CVs were 18.16% for Cmax, 8.15% for AUC0–t, and 8.04% for AUC0–inf. Intersubject CV values indicate substantial between-subject variation in the PK of PRC-063.
Safety
All randomized subjects were evaluated for safety. Across the 3 studies, there were no serious AEs (Supplemental Table 5 http://links.lww.com/JCP/A690). All AEs were mild or moderate. Three AEs in study II led to study withdrawal: dizziness and blood pressure increased during IR methylphenidate treatment, and heart rate increased during PRC-063 treatment (1 subject each). All 3 of these AEs were assessed as possibly related to study medication. No AEs in study I or study III led to study withdrawal.
Across studies, the most common AE with PRC-063 was initial insomnia, reported by 23 subjects (30%). Headache was reported by 18 subjects treated with PRC-063 (23%), dry mouth by 12 subjects (16%), and heart rate increased by 12 subjects (16%). The overall rates of insomnia-related AEs (insomnia plus initial insomnia) were 39% for PRC-063 and 12% for IR methylphenidate.
Other AEs that were frequent with PRC-063 in individual studies included agitation (23% in study III), palpitations (20% in study III), decreased appetite (18% in study I), and insomnia (18% in study I; Supplemental Table 5 http://links.lww.com/JCP/A690).
DISCUSSION
In 3 phase 1 PK studies, PRC-063 was shown to have a PK profile consistent with expectations based on the beaded formulation design.
In study I, the bioavailability of PRC-063 was generally similar when it was taken in a fasted state or after a high-fat, high-calorie meal. However, slower uptake in the fed state suggests that some patients may benefit from taking PRC-063 immediately on waking (rather than after a high-fat breakfast). Whether before or after breakfast, taking PRC-063 in a consistent manner may avoid day-to-day variability in drug uptake and onset of action.
As shown in study I, PRC-063 provides biphasic release of methylphenidate, with the initial plasma methylphenidate peak modeling the peak for the first IR methylphenidate dose. In fact, median 0- to 4-hour Tmax was 30 minutes earlier for PRC-063 than for the first dose of IR methylphenidate under fasted conditions, corroborating that the IR component of the delivery system provides the early onset of clinical effect observed within 1 hour after administration.20,21 The subsequent dip in plasma methylphenidate does not seem to diminish the clinical efficacy of PRC-063, as indicated by continued efficacy in workplace environment/laboratory classroom studies.20,21 It is also important to note that residual area and plasma methylphenidate concentrations at 24 hours in study I were considerably higher after a single dose of PRC-063 than 3 doses (20 mg TID) of IR methylphenidate.
However, repeat dosing in study II showed no further accumulation of methylphenidate after steady state was reached at day 3, and fluctuation index was lower for PRC-063 than for IR methylphenidate (138.42% vs 244.02%). In a separate analysis, PK modeling showed no further accumulation of methylphenidate levels when daily intake of PRC-063 was modeled out to 14 days (unpublished data). In some patients with ADHD who require prolonged symptom improvement, there is a clinical need for long-lasting plasma methylphenidate concentrations and a consistent clinical effect, which the lower fluctuation index of PRC-063 may help to achieve. Our PK findings provide the basis for understanding the clinical effects of PRC-063 that have been demonstrated in other studies in patients with ADHD, including a 1-hour onset of action and a duration of clinical effect of up to 16 hours.20,21 The gradual decline in plasma methylphenidate levels and higher residual methylphenidate levels with PRC-063 versus IR methylphenidate are believed to translate into prolonged symptom improvement throughout the day when other shorter-acting products are wearing off. Indeed, they inform the observed maintenance of clinical response to PRC-063 through evening hours.21,28
In study III, PK profiles were similar when PRC-063 was administered as intact capsules and when capsule contents were sprinkled on food. The present findings indicate that sprinkling capsule contents on certain soft foods does not compromise the PK profile of PRC-063 and is a dosing option that may be useful for patients who have difficulty swallowing capsules. Patients should be reminded to swallow the capsule contents when sprinkled on food, without chewing, immediately or within 10 minutes.
Although the presented data are for d- plus l-methylphenidate (total methylphenidate), it should be noted that d-methylphenidate accounted for more than 97.5% of the value of AUC0–t in all 3 studies. This, together with animal and human studies indicating the relative inactivity of l- versus d-methylphenidate,29 suggests that the clinical effects of PRC-063 result primarily from d-methylphenidate.
No unexpected safety trends were detected: the observed AEs were generally consistent with the established safety profile of methylphenidate. In study II, only 1 subject was withdrawn because of an AE that was possibly linked to PRC-063 compared with 2 subjects for IR methylphenidate. Decreased appetite, headache, dry mouth, and initial insomnia were among the most common AEs with PRC-063. However, the overall rate of insomnia-related AEs was 39% for PRC-063, largely as a result of the high rate of initial insomnia (53%) in study III. This is higher than the rate of 12% for insomnia-related AEs for IR methylphenidate in study II and higher than the rate of 6% and 12% for initial insomnia for PRC-063 in previously reported clinical trials in adults with ADHD.23 It should be noted that participants in this PK study received PRC-063 at a daily dose of 100 mg—the highest available dose—whereas patients are individually titrated to their optimal dose. Moreover, the participants were healthy subjects not previously exposed to stimulant treatment, which may have contributed to their sleep problems. In addition, subjects in all studies required overnight stays and slept in bunk beds in an unfamiliar communal environment, likely contributing to the high rates of insomnia. Insomnia is a common adverse effect of stimulant treatment,30 and previous clinical trials in adult patients with ADHD have reported higher rates of insomnia during treatment with stimulants versus placebo.23,31 However, evidence suggests that multiple daily dosing may have a greater impact on sleep than long-acting stimulant treatment32 and that stimulants may improve sleep parameters in some patients with ADHD.33,34 For these reasons, it is anticipated that persistence of PRC-063–derived methylphenidate in the blood until evening would maintain symptom relief without causing significant sleep problems. Indeed, randomized, double-blind, placebo-controlled studies in adult ADHD patients have shown no significant impact of PRC-063 on overall sleep quality.20,23
Concentration versus time curves for plasma methylphenidate show considerable between-subject variation in the uptake of the same dose of methylphenidate, which is typical of methylphenidate formulations.35,36 This confirms the need for individualization of doses. The large number of available dosage strengths for PRC-063 (25, 35, 45, 55, 70, and 85 mg in the United States; these same dosage strengths plus 100 mg in Canada) facilitates individualization of titration and dosing.
Limitations of the presented studies include the use of a lower daily dose of IR methylphenidate than PRC-063 in studies I and II, which was because the highest approved daily dose for IR methylphenidate is only 60 mg.25 However, this difference was accounted for in the dose-normalized analyses. Inclusion of participants who smoke a maximum of 25 cigarettes a day may have affected the results because smoking may induce enzymes involved in the metabolism of methylphenidate. However, to counteract this possibility, participants were required to refrain from smoking for a period before and after morning dosing on days when blood samples were collected for PK analyses. A further limitation is noncollection of a morning PK sample on day 2 in study II, which limits the calculation of steady state to day 3 or later. Moreover, caution should always be used when interpreting PK data because they do not always correlate with clinical effects. Furthermore, the controlled environment of a PK study in healthy subjects may not reflect real-world conditions for ADHD patients, where variable timing of dosing and the complex interplay between ADHD effects, stimulant effects, and comorbidities can influence treatment effects. An ongoing study collecting real-world data on the use of PRC-063 in standard clinical practice settings (NCT04152629) aims to address this limitation.
CONCLUSIONS
These 3 PK studies provide helpful insights into the clinical effects of PRC-063. Study I demonstrated equivalent plasma methylphenidate levels between PRC-063 and TID IR methylphenidate. It is important to note that the observed higher methylphenidate levels obtained in the evening with PRC-063 versus TID IR methylphenidate point to the extended duration of methylphenidate delivery provided by PRC-063, which is relevant to patients who require a long-acting stimulant. No differences in overall exposure to methylphenidate were seen when PRC-063 was taken with a high-fat meal versus when fasting, confirming that PRC-063 can be taken with or without food. However, slower uptake after a high-fat meal suggests that some patients may benefit from taking PRC-063 before breakfast. Nonetheless, it is prudent to consistently administer PRC-063 with or without food to minimize variability in drug uptake and onset of action. After steady state is reached at day 3, lack of methylphenidate accumulation during repeat dosing and a lower fluctuation index suggest the consistency of this extended-release formulation. Finally, the similar PK for PRC-063 taken as an intact capsule and by sprinkling gives patients who have difficulty swallowing capsules the additional dosing option of sprinkling PRC-063 capsule contents on certain types of soft foods.
Supplementary Material
ACKNOWLEDGMENTS
Medical writing was provided by Stephen Gilliver, PhD (Evidera), and was paid for by Purdue Pharma L.P.
AUTHOR DISCLOSURE INFORMATION
M.K. has received research fees, speaker/advisory board fees, consulting fees, or travel funding from AstraZeneca, Biotics, Boehringer Ingelheim, Bristol-Myers Squibb, Canopy, Eli Lilly, Forrest/Actavis/Allergan, Genuine Health, Janssen, Lundbeck, Merck, Otsuka, Pfizer, Purdue, Shire, Sunovion, and Takeda. He has also received research funding from the Canadian Foundation for Innovation and the Lotte & John Hecht Memorial Foundation. G.M. serves as a speaker for Alkermes, Allergan, Janssen, Lundbeck, Otsuka, Sunovion, and Takeda and as a consultant for Akili, Alkermes, Allergan, Axsome, Ironshore, Intracellular, Janssen, Lundbeck, Otsuka, Neos, Purdue, Rhodes, Sage, Shire, Sunovion, Takeda, and Teva. In addition, he serves as a researcher for Akili, Alkermes, Allergan, Boehringer, Janssen, Medgenics, NLS-1 Pharma AG, Otsuka, Reckitt Benckiser, Roche, Sage, Sunovion, Supernus, and Takeda. L.J.K. has received research grants/consulting fees/speakers bureau/honoraria from the following: Allergan, Bristol-Myers Squibb, CPA, CADDRA, CINP, Janssen, Lundbeck, Otsuka, Pfizer, Purdue, Sunovion, Takeda, and Tilray. M.J.C. is an employee of Purdue Pharma L.P. G.A.E.D. is an employee of Purdue Pharma Canada.
Footnotes
Sources of Support: The studies were funded by Purdue Pharma Canada.
Supplemental digital content is available for this article. Direct URL citation appears in the printed text and is provided in the HTML and PDF versions of this article on the journal’s Web site (www.psychopharmacology.com).
Contributor Information
Martin A. Katzman, Email: MKatzman@startclinic.ca.
Greg Mattingly, Email: greg@mattingly.com.
Larry J. Klassen, Email: larryjklassen@hotmail.com.
Marc J. Cataldo, Email: Marc.Cataldo@pharma.com.
REFERENCES
- 1.Katzman MA Armata RS Fotinos K, et al. Attention deficit hyperactivity disorder and its effects in the Canadian workplace. EC Psychol Psychiatry. 2019;8:278–288. [Google Scholar]
- 2.Mattingly GW, Anderson RH. Optimizing outcomes in ADHD treatment: from clinical targets to novel delivery systems. CNS Spectr. 2016;21(suppl 1):45–59. [DOI] [PubMed] [Google Scholar]
- 3.Jornay PM Evening-dosed methylphenidate for ADHD. Med Lett Drugs Ther. 2019;61:126–128. [PubMed] [Google Scholar]
- 4.Weisler RH, Childress AC. Treating attention-deficit/hyperactivity disorder in adults: focus on once-daily medications. Prim Care Companion CNS Disord. 2011;13:PCC.11r01168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mattingly GW, Wilson J, Rostain AL. A clinician's guide to ADHD treatment options. Postgrad Med. 2017;129:657–666. [DOI] [PubMed] [Google Scholar]
- 6.Steele M Weiss M Swanson J, et al. A randomized, controlled effectiveness trial of OROS-methylphenidate compared to usual care with immediate-release methylphenidate in attention deficit-hyperactivity disorder. Can J Clin Pharmacol. 2006;13:e50–e62. [PubMed] [Google Scholar]
- 7.Dupont RL Coleman JJ Bucher RH, et al. Characteristics and motives of college students who engage in nonmedical use of methylphenidate. Am J Addict. 2008;17:167–171. [DOI] [PubMed] [Google Scholar]
- 8.Feldman M, Bélanger S. Extended-release medications for children and adolescents with attention-deficit hyperactivity disorder. Paediatr Child Health. 2009;14:593–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diller L. Comment on prevalence and correlates of prescription stimulant use, misuse, use disorders, and motivations for misuse among adults in the United States. Am J Psychiatry. 2019;176:77. [DOI] [PubMed] [Google Scholar]
- 10.Weisler RH Greenbaum M Arnold V, et al. Efficacy and safety of SHP465 mixed amphetamine salts in the treatment of attention-deficit/hyperactivity disorder in adults: results of a randomized, double-blind, placebo-controlled, forced-dose clinical study. CNS Drugs. 2017;31:685–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wigal T Childress A Frick G, et al. Effects of SHP465 mixed amphetamine salts in adults with ADHD in a simulated adult workplace environment. Postgrad Med. 2018;130:111–121. [DOI] [PubMed] [Google Scholar]
- 12.Swanson J Gupta S Lam A, et al. Development of a new once-a-day formulation of methylphenidate for the treatment of attention-deficit/hyperactivity disorder: proof-of-concept and proof-of-product studies. Arch Gen Psychiatry. 2003;60:204–211. [DOI] [PubMed] [Google Scholar]
- 13.Sonuga-Barke EJ Swanson JM Coghill D, et al. Efficacy of two once-daily methylphenidate formulations compared across dose levels at different times of the day: preliminary indications from a secondary analysis of the COMACS study data. BMC Psychiatry. 2004;4:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pelham WE Gnagy EM Chronis AM, et al. A comparison of morning-only and morning/late afternoon Adderall to morning-only, twice-daily, and three times-daily methylphenidate in children with attention-deficit/hyperactivity disorder. Pediatrics. 1999;104:1300–1311. [DOI] [PubMed] [Google Scholar]
- 15.Swanson JM. Long-acting stimulants: development and dosing. Can Child Adolesc Psychiatr Rev. 2005;14(suppl 1):4–9. [PMC free article] [PubMed] [Google Scholar]
- 16.Swanson JM, Hechtman L. Using long-acting stimulants: does it change ADHD treatment outcome? Can Child Adolesc Psychiatr Rev. 2005;14(suppl 1):2–3. [PMC free article] [PubMed] [Google Scholar]
- 17.Gormez V, Avery B, Mann H. Switching from immediate release to sustained release methylphenidate in the treatment of children and adolescents with attention deficit/hyperactivity disorder. Eur Rev Med Pharmacol Sci. 2013;17:2345–2349. [PubMed] [Google Scholar]
- 18.Wigal SB Childress A Berry SA, et al. Optimization of methylphenidate extended-release chewable tablet dose in children with ADHD: open-label dose optimization in a laboratory classroom study. J Child Adolesc Psychopharmacol. 2018;28:314–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adhansia XR–methylphenidate hydrochloride capsule, extended release. Full Prescribing Information. July, 2019. Available at: http://app-adlon-prod.s3-website-us-east-1.amazonaws.com/adhansia-xr/fpi/publs.xml. Accessed August 28, 2019.
- 20.Wigal SB Wigal T Childress A, et al. The time course of effect of multilayer-release methylphenidate hydrochloride capsules: a randomized, double-blind study of adults with ADHD in a simulated adult workplace environment. J Atten Disord. 2020;24:373–383. [DOI] [PubMed] [Google Scholar]
- 21.Childress AC, Donnelly G, Bhaskar S. Randomized, double-blind, placebo-controlled, parallel-group, adult laboratory classroom study to evaluate the safety and efficacy of PRC 063 compared with placebo for the treatment of ADHD. Poster presented at: 2020 American Professional Society of ADHD and Related Disorders (APSARD) Annual Meeting; January 17–19, 2020; Washington, DC.
- 22.Childress AC Brams MN Cutler AJ, et al. A randomized, double-blind, placebo-controlled, parallel-group, multi-center laboratory classroom study of the efficacy and safety of PRC-063 (controlled-release methylphenidate) in children with ADHD. Poster presented at: 15th Annual Canadian ADHD resource Alliance (CADDRA) ADHD Conference; October 4–6, 2019; Toronto, Canada.
- 23.Weiss MD, Childress AC, Donnelly GAE. Efficacy and safety of PRC-063, extended-release multilayer methylphenidate in adults with ADHD including 6-month open-label extension. J Atten Disord. 2020;1087054719896853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.FOQUEST® Product Monograph. March 1, 2019. Available at: http://purdue.ca/wp-content/uploads/2019/03/FOQUEST-PM-EN.pdf. Accessed February 27, 2020.
- 25.RITALIN® (methylphenidate hydrochloride) tablets, for oral use, CII RITALIN-SR® (methylphenidate hydrochloride) extended-release tablets, for oral use, CII. Full Prescribing Information. November, 2019. Available at: https://www.pharma.us.novartis.com/sites/www.pharma.us.novartis.com/files/ritalin_ritalin-sr.pdf. Accessed March 8, 2020.
- 26.Srinivas NR Hubbard JW Quinn D, et al. Extensive and enantioselective presystemic metabolism of dl-threo-methylphenidate in humans. Prog Neuropsychopharmacol Biol Psychiatry. 1991;15:213–220. [DOI] [PubMed] [Google Scholar]
- 27.Srinivas NR Hubbard JW Korchinski ED, et al. Enantioselective pharmacokinetics of dl-threo-methylphenidate in humans. Pharm Res. 1993;10:14–21. [DOI] [PubMed] [Google Scholar]
- 28.Weiss M, Donnelly G, Reiz J. Sleep quality outcomes in adults and adolescents with ADHD treated with PRC-063 in two randomized, double-blind, placebo-controlled, multi-center studies with six-month open label extensions. Poster presented at: 12th Annual Canadian ADHD Resource Alliance (CADDRA) ADHD Conference; October 14–16, 2016; Ottawa, Canada.
- 29.Markowitz JS, Patrick KS. Differential pharmacokinetics and pharmacodynamics of methylphenidate enantiomers: does chirality matter? J Clin Psychopharmacol. 2008;28(3 suppl 2):S54–S61. [DOI] [PubMed] [Google Scholar]
- 30.Stein MA, Weiss M, Hlavaty L. ADHD treatments, sleep, and sleep problems: complex associations. Neurotherapeutics. 2012;9:509–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Surman CB, Roth T. Impact of stimulant pharmacotherapy on sleep quality: post hoc analyses of 2 large, double-blind, randomized, placebo-controlled trials. J Clin Psychiatry. 2011;72:903–908. [DOI] [PubMed] [Google Scholar]
- 32.Kidwell KM Van Dyk TR Lundahl A, et al. Stimulant medications and sleep for youth with ADHD: a meta-analysis. Pediatrics. 2015;136:1144–1153. [DOI] [PubMed] [Google Scholar]
- 33.Roth T, Zinsenheim J. Sleep in adults with ADHD and the effects of stimulants. Prim Psychiatry. 2009;16:32–37. [Google Scholar]
- 34.Becker SP, Froehlich TE, Epstein JN. Effects of methylphenidate on sleep functioning in children with attention-deficit/hyperactivity disorder. J Dev Behav Pediatr. 2016;37:395–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chan YP Swanson JM Soldin SS, et al. Methylphenidate hydrochloride given with or before breakfast: II. Effects on plasma concentration of methylphenidate and ritalinic acid. Pediatrics. 1983;72:56–59. [PubMed] [Google Scholar]
- 36.Heal DJ, Pierce DM. Methylphenidate and its isomers: their role in the treatment of attention-deficit hyperactivity disorder using a transdermal delivery system. CNS Drugs. 2006;20:713–738. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


