Abstract
Achieving a sufficient level of functional ambulation remains to be a challenge to most stroke survivors. Different modes of transcranial direct current stimulation (tDCS) have been applied for improving various aspects of walking and mobility following stroke. However, systematic reviews before 2017 provided only general effects of tDCS on limited walking outcomes. Therefore, the aims of this study were to update the evidence of tDCS for improving walking and mobility after stroke with emphasis on individual outcomes and to delineate the effects of different modes of tDCS in subgroup analysis. The systematic search of PubMed, Medline, PEDro, Scopus, and Cochrane databases for studies published up to January 2019 identified 14 eligible reports. The PEDro scale indicated a good methodological quality of the included studies (score 6.8). The meta-analysis of primary outcomes revealed that active tDCS had no better effect than sham on walking speed [n = 7, standardized mean difference (SMD) = 0.189, P = 0.252] and 6-minute walking distance (n = 3, SMD = 0.209, P = 0.453). Among the secondary outcomes, significant positive effects were found on functional ambulation category (FAC) (n = 5, SMD = 0.542, P = 0.008), Rivermead Mobility Index (n = 3, SMD = 0.699, P = 0.008), and timed up and go test (TUG) (n = 5, SMD = 0.676, P = 0.001), whereas non-significant positive effects were found on Tinetti test (n = 3, SMD = 0.441, P = 0.062) and Berg Balance Scale (n = 2, SMD = 0.408, P = 0.177). In subgroup analyses, anodal tDCS had significant positive effects on FAC (n = 4, SMD = 0.611, P = 0.005) and dual-hemispheric tDCS on TUG (n = 2, SMD = 1.090, P = 0.000). The results provide up-to-date evidence of variable effects of tDCS on walking and functional mobility after stroke.
Keywords: ambulation, meta-analysis, rehabilitation, stroke, transcranial direct current stimulation
Introduction
Stroke is a leading cause of disability and morbidity associated with substantial economic costs for post-stroke care (Rajsic et al., 2019). More than 50% of patients with chronic stroke live with motor dysfunctions (Charvet et al., 2015). Among them, ambulation difficulty has been identified as one of the major functional deficits in stroke survivors (Winstein et al., 2016). Furthermore, walking with optimal velocity and endurance to support functional ambulation remains to be a challenge to most of the stroke survivors and rehabilitation personnel (Eng and Tang, 2007).
Transcranial direct current stimulation (tDCS), a noninvasive electrical stimulation technique has been extensively investigated for its effects on stroke recovery (Bastani and Jaberzadeh, 2012; Elsner et al., 2016; Li et al., 2018). The low-intensity current of tDCS is able to modulate the membrane potential of cortical neurons and to induce long-term potentiation-like plasticity in motor cortex (Paulus et al., 2012; Filmer et al., 2014). With a single pair of electrodes, tDCS elicits different physiological effects depending on its configurations over lesioned or non-lesioned sides of the brain (Nitsche and Paulus, 2000). To improve motor recovery after stroke, tDCS is expected to balance the abnormal interhemispheric interaction, decrease the maladaptive plasticity of the affected brain (Fregni and Pascual-Leone, 2007), and enhance motor learning during rehabilitation (Kang et al., 2016). At large, the effects of anodal and cathodal tDCS on motor recovery of the upper extremity for stroke patients have been revealed by many systematic reviews and meta-analyses (Bastani and Jaberzadeh, 2012; Tedesco Triccas et al., 2016; Chhatbar et al., 2016). However, compared to the evidences available for effects of tDCS on the function of upper extremity, evidences on the recovery of lower extremity and ambulation ability are relatively scarce.
A recent meta-analysis allocating randomized controlled trials published before 2017 revealed a significant effect of tDCS on improving general mobility but not on walking speed and endurance (Li et al., 2018). However, the positive effect on general mobility was based on analysis of pooled outcome measures [timed up and go (TUG) test, Tinetti test, and functional ambulatory category (FAC)] with diverse measurement properties among studies. Moreover, it was not clear which outcome was selected as an index of mobility for certain trials with multiple outcomes. Furthermore, owing to limited number of trials available for analysis, some of the effects of tDCS were mixed with the effects of transcutaneous spinal direct stimulation (tsDCS) (Picelli et al., 2015). Therefore, evidences to the effect of tDCS on ambulation ability after stroke remain inconclusive and require update. In addition, clinicians have adopted dual-hemispheric or bihemispheric tDCS more frequently to enhance motor recovery after stroke (Lindenberg et al., 2010; Mahmoudi et al., 2011). Therefore, the effects of different modes of tDCS should be delineated when evidences become available. For these reasons, the aims of this study were to investigate the effects of tDCS for improving ambulation ability with outcome representing different aspects of walking ability and to perform subgroup analysis to delineate the effects of different modes of tDCS on improving ambulation ability following stroke.
Methods
Literature search strategy
The Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) was used to guide this systematic review and meta-analysis (Moher et al., 2009). To allocate eligible studies published until January 2019, a literature search was performed using the following databases: PubMed, Medline, PEDro, Scopus, and Cochrane. The key search terms were: (‘transcranial direct current stimulation’ or ‘tDCS’) AND (‘stroke[Mesh]’) AND (‘gait’ or ‘ambulation’ or ‘locomotion’). Two reviewers independently identified the relevant studies according to the inclusion and exclusion criteria and progressively retrieved the suitable studies.
Selection criteria
The published articles matched the following criteria would be included: (1) application of tDCS in patients with stroke who were over 18 years of age; (2) outcome assessments including gait parameters, walking speed and endurance, functional mobility test or questionnaire for walking ability and balance; (3) pre-post and randomized controlled clinical study design; (4) active tDCS versus sham tDCS and could combine other rehabilitation treatments in two groups; (5) published in English or Chinese language. Studies were excluded if: (1) patients had other types of neurological or musculoskeletal diseases or subjects were non-human subjects; (2) treatment combined other types of stimulation; (3) the articles were non-clinical trials including review, case report, editorial comment, and meta-analysis. Two reviewers independently screened the studies by reading titles and abstracts of the extracted studies. If the abstracts were ambiguous and had no sufficient details, reviewers would read the full text to make the final decision. Different decisions between reviewers were resolved by consensus.
Quality assessment
Quality assessment was conducted by using the Physiotherapy Evidence Database (PEDro) scale to evaluate the methodological quality of the eligible studies (Moseley et al., 2002). The PEDro scale consists of 10 ratings to assess the methodological quality of a clinical study. Total PEDro score ranges from 0 to 10. Scores ranging from 10-9, 8-6, and 5-4 on the PEDro scale have been considered as ‘excellent’, ‘good’, and ‘fair’ quality. Studies scoring below four are considered as ‘poor’ (Foley et al., 2003). Furthermore, the strength of evidence for the therapeutic measure was assessed according to Guidelines for Management of Ischemic, Transient Ischemic and Intracranial Hemorrhage from The European Stroke Organization (ESO) (European Stroke Organisation (ESO) Executive Committee, ESO Writing Committee, 2008). Two reviewers independently graded the PEDro score of the individual studies. Different scores between reviewers were resolved by consensus or by discussing with the third independent reviewer.
Outcome measures
The effects of tDCS for ambulation were involved in extracting the primary and secondary outcomes from eligible studies. The primary outcomes in this meta-analysis were defined as walking speed and endurance. The walking speed was derived from 10-Meter Walking Test or by other quantitative gait analysis and the walking endurance was represented by 6-Minute Walking Test (6MWT). The secondary outcomes were related to functional mobility and balance assessed by functional ambulation category (FAC), Tinetti test [Performance Oriented Mobility Assessment (POMA)], Rivermead Mobility Index (RMI), TUG test, and Berg Balance Scale (BBS) (Salter et al., 2013; Canbek et al., 2013). However, if the required data of the outcome measures were unavailable even after contacting the corresponding author, we would extract the results but not include the data into the meta-analysis.
Data extraction
After two reviewers screened the studies according to the inclusion/exclusion criteria, the following data and descriptive information relevant to the aims of this study were extracted: (1) study design; (2) characteristics of the study, including the number and age of subjects, stroke type, affected side and stroke duration; (3) parameters of tDCS and treatment protocols including the mode of application, size of electrode, the placement of electrode, current intensity and density; (4) outcomes for both pre- and post-treatment in active and sham tDCS groups; (5) harm or adverse effects.
Statistical analysis
The meta-analysis was conducted by using comprehensive meta-analysis (ver. 3.0; Biostat, Englewood, New Jersey, USA). The standardized mean differences (SMDs) derived from changing scores of post- and pre-treatment between active and sham tDCS groups were adopted as the measure of effect size. The correlation coefficient between pre- and post-treatment was inputted (r = 0.7) as a conservative estimate according to the recommendation by Rosenthal (1991). For the cross-over study, we extracted the data from the first period only (Elbourne et al., 2002). In addition, the heterogeneity in outcome was tested by the Q statistic and the I2 test. When P values in Cochrane’s Q test were less than 0.10 and I2 values were greater than 50%, it showed significant heterogeneity and a random effect model was adopted to adjust for variance (Higgins and Thompson, 2002; Higgins and Green, 2011). Otherwise, the fixed-effect model was used. In the subgroup analysis, the effects of different stimulation modes were investigated for the possible post-hoc subgroup effect. Studies were grouped into anodal, cathodal, and dual-hemispheric tDCS for further analyses. P < 0.05 was considered a statistical significance of each meta-analysis.
Results
Identification and selection of studies
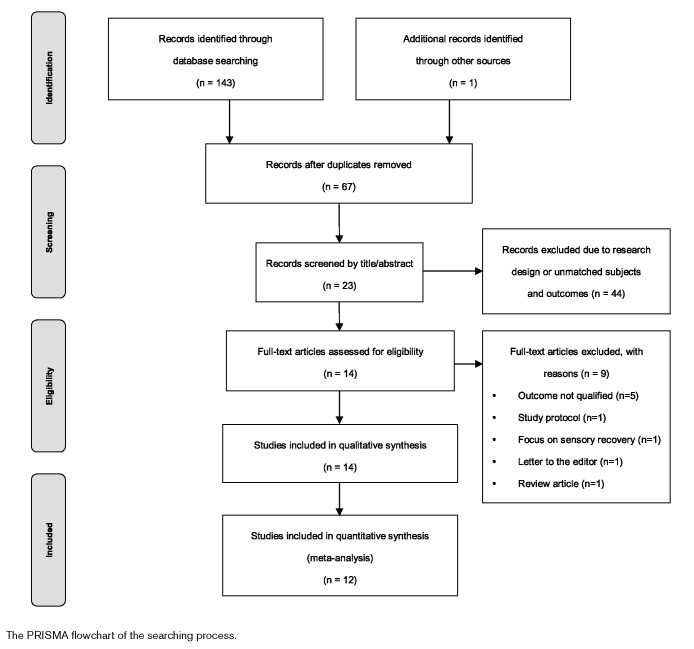
After removing the duplicated studies, screening the titles, abstracts, and full-text reviewing the 143 studies identified through database searching, 14 studies were extracted for the final analysis. The PRISMA flowchart (Fig. 1) showed the searching and extracting process with results. Among the 14 studies included in the final analysis, 13 of them were randomized controlled trials. Five studies adopted cross-over design (Table 2) (van Asseldonk and Boonstra, 2016; Saeys et al., 2015; Klomjai et al., 2018; Manji et al., 2018; Utarapichat and Kitisomprayoonkul, 2018). However, two of the 14 studies (Danzl et al., 2013; van Asseldonk and Boonstra, 2016) did not reply upon the data request. The data reported by Danzl et al. (2013) were marked in figures only and had been extracted by a plot digitizer program (Plot Digitizer, 2015) in a meta-analysis (Li et al., 2018). However, with overlapped means and SDs on the figures, we were not confident to extract data directly from those figures without confirmations from authors (Danzl et al., 2013). Therefore, the results of those two studies were excluded from the meta-analysis.
Fig. 1.
The PRISMA flowchart of the searching process.
Quality assessment of the studies
The PEDro score of the included 14 studies ranged from 5 to 9 with a mean score of 6.8, which indicated a good methodological quality of the included studies (Foley et al., 2003). Besides, six of those studies were ranked as the highest level of evidence (class I) and all other studies were ranked as class II by the classification system of ESO (European Stroke Organisation (ESO) Executive Committee, ESO Writing Committee, 2008). The rating of PEDro scale and level of evidence for each study were presented in Table 1.
Table 1.
PEDro scale for quality assessment and level of evidence by the European Stroke Organization in the included studies
| Study | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | Total | Quality | LoE |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Geroin et al. (2011) | √ | √ | √ | √ | √ | √ | √ | 6 | Good | Class I | ||||
| Danzl et al. (2013) | √ | √ | √ | √ | √ | √ | 5 | Fair | Class II | |||||
| Cha et al. (2014) | √ | √ | √ | √ | √ | √ | 5 | Fair | Class II | |||||
| Fusco et al. (2014) | √ | √ | √ | √ | √ | √ | √ | 6 | Good | Class II | ||||
| Tahtis et al. (2014) | √ | √ | √ | √ | √ | √ | √ | √ | √ | 8 | Good | Class I | ||
| Chang et al. (2015) | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | 9 | Excellent | Class I | |
| Park et al. (2015) | √ | √ | √ | √ | √ | √ | 5 | Fair | Class II | |||||
| Saeys et al. (2015) | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | 9 | Excellent | Class I | |
| van Asseldonk and Boonstra (2016) | √ | √ | √ | √ | √ | √ | √ | 6 | Good | Class II | ||||
| Leon et al. (2017) | √ | √ | √ | √ | √ | √ | √ | 6 | Good | Class II | ||||
| Seo et al. (2017) | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | 9 | Excellent | Class I | |
| Klomjai et al. (2018) | √ | √ | √ | √ | √ | √ | √ | √ | √ | 8 | Good | Class I | ||
| Manji et al. (2018) | √ | √ | √ | √ | √ | √ | √ | √ | 7 | Good | Class II | |||
| Utarapichat and Kitisomprayoonkul (2018) | √ | √ | √ | √ | √ | √ | √ | 6 | Good | Class II |
ESO, European Stroke Organization; LoE, level of evidence; PEDro scale, Physiotherapy Evidence Database scale.
Participants in the included studies
The 14 studies extracted in this review included a total of 266 patients with stroke. The stroke duration ranged from 16 days to 152.5 months which comprised patients from acute to chronic stage of recovery. The major cause of stroke was an ischemic type including 199 patients and 47 patients were the hemorrhagic type. One of the studies (n = 20) did not provide the information about the type of stroke patients (Cha et al., 2014). Overall, a total of 248 patients were allocated in the final meta-analysis. The characteristics of participants for each study were illustrated in Table 2.
Table 2.
Characteristics of included studies
| Study ID | Study design | Total, N (tDCS/Sham) | Age (tDCS/Sham) | Stroke duration (tDCS/Sham) | Stroke type, tDCS(I/H) | Stroke type, sham(I/H) | Affected hemisphere (tDCS/Sham) | Additional treatment | Adverse effect | Follow up |
|---|---|---|---|---|---|---|---|---|---|---|
| Geroin et al. (2011) | RCT | 20 (10/10) | 63.6 ± 6.7/63.3 ± 6.4 | 25.7 ± 6.0/26.7 ± 5.1 (m/o) | 10/0 | 10/0 | – | RAGT | No harm | After 2 weeks |
| Danzl et al. (2013) | RCT | 8 (4/4) | 64.8 ± 14.9/70.8 ± 11.1 | 4.8 ± 4.5/3.2 ± 2.7 (m/o) | 2/2 | 4/0 | 4L/4L | RGO | No harm | After one month |
| Cha et al. (2014) | RCT | 20 (10/10) | 59.8 ± 11.4/57.8 ± 9.9 | 13.8 ± 4.6/14.5 ± 3.6 (m/o) | – | – | 4R 6L/5R 5L | – | – | – |
| Fusco et al. (2014) | RCT | 11 (5/6) | 56.4 ± 17.2/60.0 ± 8.1 | 19.01 ± 8.0 (days) | 11/0 | 3R 2L/2R 4L | – | – | After 10 days (1 month and discharge | |
| Tahtis et al. (2014) | RCT | 14 (7/7) | 67.3 ± 11.8/56.4 ± 12.3 | 19.7 ± 5.2/25.3 ± 10.9 (days) | 7/0 | 7/0 | 4R 3L/4R 3L | – | No harm | – |
| Chang et al. (2015) | RCT | 24 (12/12) | 59.9 ± 10.2/65.8 ± 10.6 | 16.0 ± 6.2/16.6 ± 5.2 (days) | 12/0 | 12/0 | 6R 6L/7R 5L | – | – | – |
| Park et al. (2015) | RCT | 16 (8/8) | 59.0 ± 6.0/57.7 ± 10.0 | 23.8 ± 16.2/22.5 ± 14.5 (m/o) | 4/4 | 5/3 | 3R 5L/4R 4L | TRT | – | – |
| Saeys et al. (2015) | RCT cross-over design | 31 (16/15) | 62.00 ± 9.61/64.53 ± 7.23 | 45.5 ± 21.8/38.4 ± 15.1 (days) | 26/5 | 14R 17L | – | No harm | – | |
| van Asseldonk and Boonstra (2016) | RCT cross-over design | 10 | 58.0 ± 11.7 | 44.7 ± 37.7 (m/o) | 8/2 | 4R 6L | – | – | After 15 and 45 mins | |
| Leon et al. (2017) | Active-control | 32 (9/23) | 49 ± 9/49 ± 11 | 53 ± 25/64 ± 33 (days) | 6/3 | 13/10 | 33.3%R/52.2%R | RAGT | +2 | – |
| Seo et al. (2017) | RCT | 21 (11/10) | 62.9 ± 8.9/61.1 ± 8.9 | 152.5 ± 122.8 /75.5 ± 83.4 (m/o) | 7/3 | 9/2 | 8R 2L/5R 6L | RAGT | – | After 4 weeks |
| Klomjai et al. (2018) | RCT cross-over design | 19 (10/9) | 57.2 ± 2.8 | 3.2 ± 0.4 (m/o) | 19/0 | 7R 12L | – | +1 | – | |
| Manji et al. (2018) | RCT cross-over design | 30 (15/15) | 62.2 ± 10.1/63.7 ± 11.0 | 134.5 ± 55.7 /149.7 ± 24.2 (days) | 9/6 | 8/7 | – | BWSTT | – | – |
| Utarapichat and Kitisomprayoonkul (2018) | RCT cross-over design | 10 (5/5) | 57.1 ± 12.2 | 34.1 ± 18.9 (m/o) | 10/0 | 5R 5L | – | No harm | – |
–, not reported; +1, Mild headache after tDCS; +2, Mild headache during and after tDCS, slight itching; BWSTT, body weight-supported treadmill; H, hemorrhage; I, ischemic; L, left; m/o, month after onset; mins, minutes; R, right; RAGT, robot-assisted gait training; RCT, randomized controlled trial; RGO, robotic gait orthosis; TRT, task-related training.
Parameters of transcranial direct current stimulation in the included studies
The mode of tDCS application was determined by arrangement of the position and polarity of the electrodes over ipsilesional or/and contralesional side of brain. In the included studies, nine studies used anodal tDCS and one study used cathodal tDCS, while three studies used dual-hemispheric stimulation by putting anode on ipsilesional and cathode on contralesional side of brain. The placement of electrode in anodal mode was to place the anode overlying the motor cortex of the leg area which centered on a short distance lateral to Cz or on C3/C4. The reference electrode was put on the contralateral supraorbital region near Fp1/Fp2 in most of the studies.
Among the included studies, nine used a current intensity of 2 mA, three used 1.5 mA, and two used 1 mA for treatment. As for the size of electrode, most of the trials used a 35 cm2 sponge electrode as an active electrode (Paulus et al., 2012). The current density of tDCS applications ranged from 0.029 to 0.08 mA/cm2 while 0.057 mA/cm2 was the most commonly used current density. Eight of the included studies did not provide the fade-in and fade-out settings of the tDCS. Other studies faded in and faded out the current gradually from 5 to 30 seconds. The parameters of tDCS application were listed in Table 3.
Table 3.
Treatment protocol of transcranial direct current stimulation
| Study ID | UNI/DUAL | iH | cH | Anode | Cathode | Intensity (mA) | Size (cm2) | Duration (min) | Session | Current density (mA/cm2) | Fade-in/fade-out |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Geroin et al. (2011) | UNI | A | – | (leg area)a | CSRa | 1.5 | 35 | 7 | 10 | 0.043 | NA |
| Danzl et al. (2013) | UNI | A | – | Motor area (leg area) a | CSRa | 2 | A:25/C:35 | 20 | 12 | 0.08/0.057 | Fade in and out over first 75 seconds (sham tDCS) |
| Cha et al. (2014) | UNI | A | – | PMC (C3 or C4) | Foreheada | 1 | 35 | 20 | 20 | 0.029 | NA |
| Fusco et al. (2014) | UNI | – | C | Noncephalic sideb | PMC (C3’ or C4’) | 1.5 | 35 | 10 | 10 | 0.043 | Gradually increased and progressively reduced |
| Tahtis et al. (2014) | DUAL | A | C | Bilateral MC over 5 mm lateral to Cz (leg area) | 2 | 25 | 15 | 1 | 0.08 | Fade in less than 30 seconds (sham tDCS) | |
| Chang et al. (2015) | UNI | A | – | TA area of the PGa | CSRa | 2 | A:7.07 C:28.26 | 10 | 10 | 0.283/0.071 | NA |
| Park et al. (2015) | UNI | A | – | Cz area of the left PL | Right upper orbita | 2 | – | 15 | 12 | – | NA |
| Saeys et al. (2015) | DUAL | A | C | MC (C3 or C4) | 1.5 | 35 | 20 | 16 | 0.043 | Fade in for 5 seconds/Fade out last 5 seconds (both groups) | |
| van Asseldonk and Boonstra (2016) | UNI | A | – | Hotspot of TA by TMSa | CSRa | 2 | 35 | 10 | 1 | 0.057 | Fade in for 30 seconds (sham tDCS) |
| DUAL | A | C | Hotspot of TA shifted laterally 1 cma | 2 | 35 | 10 | 1 | 0.057 | |||
| Leon et al. (2017) | UNI | A | – | Leg: vertex (Cz) | CSRa | 2 | 35 | 20 | 20 | 0.057 | NA |
| Seo et al. (2017) | UNI | A | – | Lateral to Cz | CSRa | 2 | 35 | 20 | 10 | 0.057 | NA |
| Klomjai et al. (2018) | DUAL | A | C | Bilateral PMC placed 5 mm lateral from vertex (Cz) | 2 | 35 | 20 | 1 | 0.057 | NA | |
| Manji et al. (2018) | UNI | A | – | 3.5 cm anterior to Cz (SMA) | Iniona | 1 | 25 | 20 | 7 | 0.04 | NA |
| Utarapichat and Kitisomprayoonkul (2018) | UNI | A | – | 1 cm posterior and lateral to Cz | CSRa | 2 | 10 | 10 | 1 | 0.2 | Fade in for 10 seconds/fade out for 10 seconds (both groups) |
A, anodal tDCS; C, cathodal tDCS; cH, contralesional hemisphere; CSR, contralateral supraorbital region; DUAL, dual-hemispheric tDCS; iH, ipsilesional hemisphere; MC, motor cortex; NA, not available; PG, precentral gyrus; PL, parietal lobe; PMC, primary motor cortex; SMA, supplementary motor area; TA, tibialis anterior; TMS, transcranial magnetic stimulation; UNI, uni-hemispheric tDCS.
No description according to 10/20 system.
Noncephalic side is located above the right shoulder, contralateral to the electric circuit of the heart.
Treatment programs along with transcranial direct current stimulation
In addition to active and sham tDCS, there were six studies providing additional treatment with tDCS related to ambulation training (Table 2). Among them, three studies used robot-assisted gait training as rehabilitation programs (Geroin et al., 2011; Leon et al., 2017; Seo et al., 2017). One study used robotic gait orthosis (RGO) for locomotion training (Danzl et al., 2013) and the other studies used either task-related training (Park et al., 2015) or body weight-supported treadmill training (Manji et al., 2018) to treat gait disturbance after stroke. Besides, four studies provided tDCS simultaneously during the treatment program (Geroin et al., 2011; Park et al., 2015; Leon et al., 2017; Manji et al., 2018) while two studies applied tDCS before interventions (Danzl et al., 2013; Seo et al., 2017).
Adverse events
Among 14 studies, two studies reported some of the adverse events associated with tDCS (Leon et al., 2017; Klomjai et al., 2018). Among them, with patients in both studies reported transitory itching and tingling during tDCS. In addition, one subject experienced mild headache after tDCS and resolved without any treatment within 24 h (Klomjai et al., 2018). Furthermore, one patient was excluded due to mild headache during and after stimulation (Leon et al., 2017). According to the Common Terminology Criteria for Adverse Events, the above results suggested tDCS may cause mild to moderate adverse events (grades 1 and 2) which involved mild symptoms with or without medical treatment (Antal et al., 2017). However, the reports of adverse events were inconsistent in the included studies. Five of them reported no adverse effect associated with tDCS (Geroin et al., 2011; Danzl et al., 2013; Tahtis et al., 2014; Saeys et al., 2015; Utarapichat and Kitisomprayoonkul, 2018) while seven studies did not provide such information (Cha et al., 2014; Fusco et al., 2014; Chang et al., 2015; Park et al., 2015; van Asseldonk and Boonstra, 2016; Seo et al., 2017; Manji et al., 2018).
Quantitative data synthesis
Among all of the meta-analysis, the only marginal heterogeneity was found on the effect of dual-hemispheric tDCS on Tinetti test in subgroup analysis (I2 = 52.065%). Therefore, only that effect was analyzed by the random effect model and the rest of analyses adopted the fixed effect model.
In the analyses of the primary outcomes, active tDCS did not improve walking speed [n = 7; SMD: 0.189, 95% confidence interval (CI) −0.135 to 0.513, P = 0.252] and 6MWT (n = 3; SMD: 0.209, 95% CI −0.338 to 0.756, P = 0.453) better than the sham tDCS (Fig. 2).
Fig. 2.

Forest plots for primary and secondary outcomes with subgroup analyses of mode application (a) gait speed, (b) six-minute walking test, (c) functional ambulation category, (d) Tinetti test, (e) Rivermead Mobility Index, (f) timed up and go test, (g) Berg Balance Scale. CI, confidence interval; Std diff in means, standard difference in means. *Anode tDCS used the fixed-effect model while dual-hemispheric tDCS used the random effect model. #Data without subgroup analysis.
In the analyses of the secondary outcomes, significant and beneficial effects of active tDCS were found on FAC (n = 5; SMD = 0.542, 95% CI 0.142–0.942, P = 0.008), RMI (n = 3; SMD = 0.699, 95% CI 0.180–1.219, P = 0.008), and TUG (n = 5; SMD = 0.676, 95% CI 0.293–1.058, P = 0.001). However, the effects on Tinetti test (n = 3; SMD = 0.441, 95% CI −0.022 to 0.904, P = 0.062) and BBS (n = 2; SMD = 0.408, 95% CI −0.184 to 0.999, P = 0.177) were both insignificant but in favor of the active tDCS than the sham tDCS (Fig. 2).
When the effects of tDCS were further analyzed according to the mode of application, significant effects of anodal tDCS on FAC (n = 4; SMD = 0.611, 95% CI 0.186–1.036, P = 0.005) and dual-hemispheric tDCS on TUG (n = 2; SMD = 1.090, 95% CI 0.507–1.672, P = 0.000) were extracted from subgroup analyses (Fig. 2).
Discussion
In this study, we evaluated the effects of tDCS on the recovery of ambulation ability in patients with stroke. Meta-analysis on studies with proper design and methodological quality revealed positive effects of active tDCS in half of the outcomes measuring walking ability (3/6). Essentially, FAC, RMI, and TUG improved significantly following active tDCS (effect sizes: 0.542–0.687). However, tDCS had non-significant effects on walking speed, walking endurance (6MWT), and Tinetti test. Yet, all these effects were in favor of the active tDCS rather than sham tDCS. Similarly, active tDCS could not effectively improve BBS. Furthermore, subgroup analyses revealed that, anodal tDCS had significant effect on FAC (n = 4; effect size = 0.611) while dual-hemispheric tDCS improved TUG (n = 2; effect size = 1.090) significantly (Fig. 2f).
Results of this meta-analysis provide up-to-date evidence that tDCS has the beneficial effects to restore walking ability and functional mobility following stroke. After stroke, decreased excitability of the motor cortex owing to lesion of the affected brain or unbalanced transcallosal inhibition or both have been documented for decades (McDonnell and Stinear, 2017). Thus, tDCS was expected to balance the excitability between two hemispheres after brain lesion (Fregni and Pascual-Leone, 2007; Gomez Palacio Schjetnan et al., 2013). The study has revealed that unilateral anodal tDCS applied on the leg area of the primary motor cortex of the affected hemisphere during walking could increase excitability of the motor cortex while simultaneously decrease excitability of the unaffected side (Jayaram and Stinear, 2009). It is feasible to stimulate the leg area of the primary motor cortex which is located in the edge of the hemisphere and the mesial surface in the median longitudinal fissure (Penfield and Boldrey, 1937). In addition, meta-analysis showed that tDCS improved muscle strength of lower limb in stroke (Li et al., 2018) and increased the activity of motor cortex involved in learning (Madhavan and Shah, 2012). Thus, tDCS was expected to be able to improve the walking ability following stroke.
Compared to the most recent meta-analysis on the effects of tDCS on walking after stroke (Li et al., 2018) which allocated 10 studies (n = 194) published between 2011 and 2016 into analysis, four of these studies were excluded from our analysis owing to that the effect of tDCS might be confounded by tsDCS (Picelli et al., 2015), outcomes did not measure walking ability or mobility (Khedr et al., 2013; Montenegro et al., 2016) and lack of precise data for analysis (Danzl et al., 2013). In contrast, current analysis included five additional studies published between 2017 and 2018 and provided the most up-to-date synthesis of the evidence (12 studies, n = 248). Besides, instead of pooling all related measures (TUG, Tinetti test, and FAC) into a common category of mobility (Li et al., 2018), the current study extracted and examined the effects of each mobility measurements. Essentially, analyses revealed that the effect size for Tinetti test (SMD = 0.441), walking speed (SMD = 0.195), and walking endurance (SMD = 0.209) were all relatively small and non-significant (Cohen, 1988).
The reason accounting for the small effect size on Tinetti test may be that the scale reflects more on balance ability than gait or walking performance. Tinetti test comprises of a balance subscale (POMA-B) and a gait subscale (POMA-G). The balance score of Tinetti test (16/28) takes more weight than the gait score (12/28) (Canbek et al., 2013). Therefore, Tinetti test may reflect a patient’s balance ability better than patient’s gait or walking ability. Furthermore, according to the previous meta-analyses and our result, tDCS seemed to be less effective on improving balance function after stroke (Li et al., 2018; Kang et al., 2020). Therefore, we speculated that balance ability which depends on the integrated actions of multiple systems may be difficult to be improved by tDCS alone. The same rationale might account for the non-significant findings on gait speed and walking endurance (6MWT) as well. Balance ability as measured by BBS has been identified as the strongest predictor for both 10 m and 6 minutes walking in stroke patients (Patterson et al., 2007). Therefore, when the tDCS could not effectively improve balance ability, the capability to improve walking speed and walking endurance may also be limited.
In subgroup analyses, we found positive effects of the unilateral anodal tDCS on FAC (trials = 4) and dual-hemispheric tDCS on TUG (trials = 2). To the best of our knowledge, these positive effects were revealed for the first time in the literature. From the analysis on FAC, when the effect of cathodal tDCS was removed, the effect of anodal tDCS remained significant with larger effect size (Fig. 2c). Notably, dual-hemispheric tDCS not only exerted a larger effect size than unilateral anodal tDCS but also was the only significant finding in the subgroup analyses on TUG (Fig. 2f). Previous study has demonstrated that dual-hemispheric tDCS could increase excitability in the ipsilesional hemisphere, reduce cortical excitability in the contralesional hemisphere and reduce the transcallosal inhibition from the contralesional hemisphere simultaneously in stroke patients (Bolognini et al., 2011). Therefore, dual-hemispheric tDCS has been expected to improve gait or motor performance of lower extremity in stroke patients better than unilateral tDCS (van Asseldonk and Boonstra, 2016). From our review and subgroup analyses, four studies have examined the effects of dual-hemispheric tDCS on gait-related outcomes after stroke. However, due to unavailable of research data (van Asseldonk and Boonstra, 2016) and limited outcomes adopted by studies, only the effects on TUG and Tinetti test could be extracted. In contrast, dual-hemispheric tDCS not only significantly improved TUG but also exerted larger effect size (SMD = 1.090) than anodal tDCS. It is of interest to know that Klomjai et al. (2018) did not find a significant effect of dual-hemispheric-tDCS on TUG (P = 0.883). However, with statistical synthesis of two studies, the effect of dual-hemispheric tDCS on TUG became evident (Tahtis et al., 2014; Klomjai et al., 2018). In addition, a strong negative association had been identified between TUG score and the maximal torque generated by gastrocnemius (r = −0.86) in people with chronic stroke (Ng and Hui-Chan, 2005). Thus, when tDCS was found to effectively improve muscle strength of lower limb in stroke patients (Li et al., 2018), performance of TUG may be enhanced by tDCS as well. Based on these findings, current results support in part that dual-hemispheric tDCS may have its unique contribution in promoting walking ability in stroke patients. Finally, in the included studies, only Fusco et al. (2014) have examined the effect of cathodal tDCS on gait and walking-related performances after stroke. Therefore, none of effects could be extracted for cathodal tDCS from our analysis.
This systematic review and meta-analysis provides up-to-date evidences on the effects of tDCS. However, the results should be interpreted with caution under following limitations. First, studies published in languages other than English and Chinese were not included. Second, two of the included studies did not provide the data for quantitative evidence synthesis. Third, to extract the effects for each outcome related to walking ability and different modes of tDCS, the subgroup analyses were limited by the small trial number. Fourth, some of the included studies explored the immediate effects of tDCS with only one session of treatment (Tahtis et al., 2014; van Asseldonk and Boonstra, 2016; Klomjai et al., 2018; Utarapichat and Kitisomprayoonkul, 2018), effects revealed by current study may be contributed by single as well as multiple sessions of tDCS. Finally, the publication bias should be considered as a positive result and future research should be more representative.
Conclusion
In conclusion, this meta-analysis suggests that tDCS improves walking ability with an exception of walking speed and endurance in patients with stroke. Both anodal and dual-hemispheric tDCS exert positive effects on promoting walking-related performances after stroke. However, difficulty in improving balance performance by tDCS may limit the effects of tDCS on walking speed and/or walking endurance.
Acknowledgements
The authors would like to thank the hard work of all the authors contributing to this study and sincerely thank Alexis Lin and Jacob Lin for proofreading the manuscript of this article.
Conflicts of interest
There are no conflicts of interest.
References
- Antal A, Alekseichuk I, Bikson M, Brockmöller J, Brunoni AR, Chen R, et al. Low intensity transcranial electric stimulation: Safety, ethical, legal regulatory and application guidelines. Clin Neurophysiol. 2017; 128:1774–1809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastani A, Jaberzadeh S. Does anodal transcranial direct current stimulation enhance excitability of the motor cortex and motor function in healthy individuals and subjects with stroke: a systematic review and meta-analysis. Clin Neurophysiol. 2012; 123:644–657 [DOI] [PubMed] [Google Scholar]
- Bolognini N, Vallar G, Casati C, Latif LA, El-Nazer R, Williams J, et al. Neurophysiological and behavioral effects of tDCS combined with constraint-induced movement therapy in poststroke patients. Neurorehabil Neural Repair. 2011; 25:819–829 [DOI] [PubMed] [Google Scholar]
- Canbek J, Fulk G, Nof L, Echternach J. Test-retest reliability and construct validity of the Tinetti performance-oriented mobility assessment in people with stroke. J Neurol Phys Ther. 2013; 37:14–19 [DOI] [PubMed] [Google Scholar]
- Cha HK, Ji SG, Kim MK, Chang JS. Effect of transcranial direct current stimulation of function in patients with stroke. J Phys Ther Sci. 2014; 26:363–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang MC, Kim DY, Park DH. Enhancement of Cortical Excitability and Lower Limb Motor Function in Patients With Stroke by Transcranial Direct Current Stimulation. Brain Stimul. 2015; 8:561–566 [DOI] [PubMed] [Google Scholar]
- Charvet LE, Kasschau M, Datta A, Knotkova H, Stevens MC, Alonzo A, et al. Remotely-supervised transcranial direct current stimulation (tDCS) for clinical trials: guidelines for technology and protocols. Front Syst Neurosci. 2015; 9:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhatbar PY, Ramakrishnan V, Kautz S, George MS, Adams RJ, Feng W. Transcranial Direct Current Stimulation Post-Stroke Upper Extremity Motor Recovery Studies Exhibit a Dose-Response Relationship. Brain Stimul. 2016; 9:16–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. 19882nd ed, Hillsdale, NJ: Erlbaum [Google Scholar]
- Danzl MM, Chelette KC, Lee K, Lykins D, Sawaki L. Brain stimulation paired with novel locomotor training with robotic gait orthosis in chronic stroke: a feasibility study. Neurorehabilitation. 2013; 33:67–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta-analyses involving cross-over trials: methodological issues. Int J Epidemiol. 2002; 31:140–149 [DOI] [PubMed] [Google Scholar]
- Elsner B, Kugler J, Pohl M, Mehrholz J. Transcranial direct current stimulation (tDCS) for improving activities of daily living, and physical and cognitive functioning, in people after stroke. Cochrane Database Syst Rev. 2016; 3:CD009645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng JJ, Tang PF. Gait training strategies to optimize walking ability in people with stroke: a synthesis of the evidence. Expert Rev Neurother. 2007; 7:1417–1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Stroke Organisation (ESO) Executive Committee, ESO Writing Committee Guidelines for management of ischaemic stroke and transient ischaemic attack 2008. Cerebrovasc Dis. 2008; 25:457–507 [DOI] [PubMed] [Google Scholar]
- Filmer HL, Dux PE, Mattingley JB. Applications of transcranial direct current stimulation for understanding brain function. Trends Neurosci. 2014; 37:742–753 [DOI] [PubMed] [Google Scholar]
- Foley NC, Teasell RW, Bhogal SK, Doherty T, Speechley MR. The efficacy of stroke rehabilitation: a qualitative review. Top Stroke Rehabil. 2003; 10:1–18 [DOI] [PubMed] [Google Scholar]
- Fregni F, Pascual-Leone A. Technology insight: noninvasive brain stimulation in neurology-perspectives on the therapeutic potential of rTMS and tDCS. Nat Clin Pract Neurol. 2007; 3:383–393 [DOI] [PubMed] [Google Scholar]
- Fusco A, Assenza F, Iosa M, Izzo S, Altavilla R, Paolucci S, Vernieri F. The ineffective role of cathodal tDCS in enhancing the functional motor outcomes in early phase of stroke rehabilitation: an experimental trial. Biomed Res Int. 2014; 2014:547290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geroin C, Picelli A, Munari D, Waldner A, Tomelleri C, Smania N. Combined transcranial direct current stimulation and robot-assisted gait training in patients with chronic stroke: a preliminary comparison. Clin Rehabil. 2011; 25:537–548 [DOI] [PubMed] [Google Scholar]
- Gomez Palacio Schjetnan A, Faraji J, Metz GA, Tatsuno M, Luczak A. Transcranial direct current stimulation in stroke rehabilitation: a review of recent advancements. Stroke Res Treat. 2013; 2013:170256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions. 2011 Version 5.1.0 [Online]. Available at: http://handbook-5-1.cochrane.org/ [Accessed:15 April 2019]
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002; 21:1539–1558 [DOI] [PubMed] [Google Scholar]
- Jayaram G, Stinear JW. The effects of transcranial stimulation on paretic lower limb motor excitability during walking. J Clin Neurophysiol. 2009; 26:272–279 [DOI] [PubMed] [Google Scholar]
- Kang N, Lee RD, Lee JH, Hwang MH. Functional Balance and Postural Control Improvements in Patients With Stroke After Noninvasive Brain Stimulation: A Meta-analysis. Arch Phys Med Rehabil. 2020; 101:141–153 [DOI] [PubMed] [Google Scholar]
- Kang N, Summers JJ, Cauraugh JH. Transcranial direct current stimulation facilitates motor learning post-stroke: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2016; 87:345–355 [DOI] [PubMed] [Google Scholar]
- Khedr EM, Shawky OA, El-Hammady DH, Rothwell JC, Darwish ES, Mostafa OM, Tohamy AM. Effect of anodal versus cathodal transcranial direct current stimulation on stroke rehabilitation: a pilot randomized controlled trial. Neurorehabil Neural Repair. 2013; 27:592–601 [DOI] [PubMed] [Google Scholar]
- Klomjai W, Aneksan B, Pheungphrarattanatrai A, Chantanachai T, Choowong N, Bunleukhet S, et al. Effect of single-session dual-tDCS before physical therapy on lower-limb performance in sub-acute stroke patients: a randomized sham-controlled crossover study. Ann Phys Rehabil Med. 2018; 61:286–291 [DOI] [PubMed] [Google Scholar]
- Leon D, Cortes M, Elder J, Kumru H, Laxe S, Edwards DJ, et al. tDCS does not enhance the effects of robot-assisted gait training in patients with subacute stroke. Restor Neurol Neurosci. 2017; 35:377–384 [DOI] [PubMed] [Google Scholar]
- Li Y, Fan J, Yang J, He C, Li S. Effects of transcranial direct current stimulation on walking ability after stroke: a systematic review and meta-analysis. Restor Neurol Neurosci. 2018; 36:59–71 [DOI] [PubMed] [Google Scholar]
- Lindenberg R, Renga V, Zhu LL, Nair D, Schlaug G. Bihemispheric brain stimulation facilitates motor recovery in chronic stroke patients. Neurology. 2010; 75:2176–2184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhavan S, Shah B. Enhancing motor skill learning with transcranial direct current stimulation - a concise review with applications to stroke. Front Psychiatry. 2012; 3:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoudi H, Borhani Haghighi A, Petramfar P, Jahanshahi S, Salehi Z, Fregni F. Transcranial direct current stimulation: electrode montage in stroke. Disabil Rehabil. 2011; 33:1383–1388 [DOI] [PubMed] [Google Scholar]
- Manji A, Amimoto K, Matsuda T, Wada Y, Inaba A, Ko S. Effects of transcranial direct current stimulation over the supplementary motor area body weight-supported treadmill gait training in hemiparetic patients after stroke. Neurosci Lett. 2018; 662:302–305 [DOI] [PubMed] [Google Scholar]
- McDonnell MN, Stinear CM. TMS measures of motor cortex function after stroke: A meta-analysis. Brain Stimul. 2017; 10:721–734 [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Plos Med. 2009; 6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montenegro RA, Midgley A, Massaferri R, Bernardes W, Okano AH, Farinatti P. Bihemispheric motor cortex transcranial direct current stimulation improves force steadiness in post-stroke hemiparetic patients: a randomized crossover controlled trial. Front Hum Neurosci. 2016; 10:426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley AM, Herbert RD, Sherrington C, Maher CG. Evidence for physiotherapy practice: a survey of the Physiotherapy Evidence Database (PEDro). Aust J Physiother. 2002; 48:43–49 [DOI] [PubMed] [Google Scholar]
- Ng SS, Hui-Chan CW. The timed up & go test: its reliability and association with lower-limb impairments and locomotor capacities in people with chronic stroke. Arch Phys Med Rehabil. 2005; 86:1641–1647 [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. 2000; 527Pt 3633–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SD, Kim JY, Song HS. Effect of application of transcranial direct current stimulation during task-related training on gait ability of patients with stroke. J Phys Ther Sci. 2015; 27:623–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson SL, Forrester LW, Rodgers MM, Ryan AS, Ivey FM, Sorkin JD, Macko RF. Determinants of walking function after stroke: differences by deficit severity. Arch Phys Med Rehabil. 2007; 88:115–119 [DOI] [PubMed] [Google Scholar]
- Paulus W, Antal A, Nitsche MA. Chapter 4. Physiological basis and methodological aspects of transcranial electric stimulation (tDCS, tACS and tRNS). In:. Transcranial brain stimulation. 2012, Florida: CRC Press; 93–111 [Google Scholar]
- Penfield W, Boldrey E. Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain. 1937; 60:389–443 [Google Scholar]
- Picelli A, Chemello E, Castellazzi P, Roncari L, Waldner A, Saltuari L, Smania N. Combined effects of transcranial direct current stimulation (tDCS) and transcutaneous spinal direct current stimulation (tsDCS) on robot-assisted gait training in patients with chronic stroke: a pilot, double blind, randomized controlled trial. Restor Neurol Neurosci. 2015; 33:357–368 [DOI] [PubMed] [Google Scholar]
- Plot Digitizer Plot Digitizer. 2015 http://plotdigitizer.sourceforge.net. [Accessed 20 October 2019]
- Rajsic S, Gothe H, Borba HH, Sroczynski G, Vujicic J, Toell T, Siebert U. Economic burden of stroke: a systematic review on post-stroke care. Eur J Health Econ. 2019; 20:107–134 [DOI] [PubMed] [Google Scholar]
- Rosenthal R. Meta-Analytic Procedures for Social Research. 1991, Newbury Park: Sage Publications [Google Scholar]
- Saeys W, Vereeck L, Lafosse C, Truijen S, Wuyts FL, Van De Heyning P. Transcranial direct current stimulation in the recovery of postural control after stroke: a pilot study. Disabil Rehabil. 2015; 37:1857–1863 [DOI] [PubMed] [Google Scholar]
- Salter K, Jutai JW, Teasell R, Foley NC, Bitensky J, Bayley M. Issues for selection of outcome measures in stroke rehabilitation: ICF activity. Disabil Rehabil. 2005; 27:315–340 [DOI] [PubMed] [Google Scholar]
- Seo HG, Lee WH, Lee SH, Yi Y, Kim KD, Oh BM. Robotic-assisted gait training combined with transcranial direct current stimulation in chronic stroke patients: a pilot double-blind, randomized controlled trial. Restor Neurol Neurosci. 2017; 35:527–536 [DOI] [PubMed] [Google Scholar]
- Tahtis V, Kaski D, Seemungal BM. The effect of single session bi-cephalic transcranial direct current stimulation on gait performance in sub-acute stroke: A pilot study. Restor Neurol Neurosci. 2014; 32:527–532 [DOI] [PubMed] [Google Scholar]
- Tedesco Triccas L, Burridge JH, Hughes AM, Pickering RM, Desikan M, Rothwell JC, Verheyden G. Multiple sessions of transcranial direct current stimulation and upper extremity rehabilitation in stroke: a review and meta-analysis. Clin Neurophysiol. 2016; 127:946–955 [DOI] [PubMed] [Google Scholar]
- Utarapichat S, Kitisomprayoonkul W. Effects of transcranial direct current stimulation on motor activity of lower limb muscles in chronic stroke. J Med Assoc Thai. 2018; 101:131–136 [Google Scholar]
- van Asseldonk EH, Boonstra TA. Transcranial Direct Current Stimulation of the Leg Motor Cortex Enhances Coordinated Motor Output During Walking With a Large Inter-Individual Variability. Brain Stimul. 2016; 9:182–190 [DOI] [PubMed] [Google Scholar]
- Winstein CJ, Stein J, Arena R, Bates B, Cherney LR, Cramer SC, et al. ; American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, and Council on Quality of Care and Outcomes Research. Guidelines for adult stroke rehabilitation and recovery: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2016; 47:e98–e169 [DOI] [PubMed] [Google Scholar]