Abstract
Acute myeloid leukemia (AML) in patients over the age of 60 carries a poor prognosis, mainly due to unsatisfactory control of leukemia with chemotherapy alone.
Allogeneic hemopoietic stem cell transplantation (HSCT) would provide significant anti-leukemic effect but is associated with morbidity and mortality, especially in older patients with comorbidities. Reduced-intensity conditioning (RIC) and non-myeloablative (NMA) conditioning regimens have been designed and have led to improved outcomes in this older patient population. New targeted agents, such as Flt3 inhibitors, are currently being used to improve the control of AML further and may be incorporated in a transplant approach. The increasing knowledge of AML in the elderly is currently being associated with a multidimensional approach to identify eligibility and design tailored transplant platforms.
Keywords: Acute myeloid leukemia, Allogeneic stem cell transplantation, Older patients, Reduced-intensity conditioning transplant, Geriatric assessment, Cytogenetic risk
AML
Epidemiology
Acute myeloid leukemia (AML) is a clonal disease of the hematopoietic system with maturation arrest and accumulation of myeloid blasts.1 AML presents in all age groups, but is mainly a disease of the elderly, the median age at diagnosis being 67 years.2 The incidence of AML increases in older patients, with a peak at approximately 80 years of age.3 The yearly incidence of new AML diagnoses is reported to be 17.6/100,000 for people 65 years of age or older.4
According to the National Cancer Institute’s Surveillance, Epidemiology, and End Results Program (SEER), there are 3000 new cases per year of AML in patients aged 70 to 79 years in the United States (US).4 This increased incidence in older adults is attributed to environmental exposures and age-related clonal hematopoiesis.5 Both animal and human models reveal many changes in aging stem cells, such as genome instability, longer frequency in the cycle, and shortening of telomere lengths.6–8
Old Patients with AML
Older age in AML has a significant impact on the disease’s features and is associated with a poor outcome.1,9 A retrospective analysis of five trials, including 968 patients with previously untreated AML, outlined several critical differences between younger and older AML.10 In older patients, AML seems to present with lower white blood cell counts and lower percentages of peripheral blasts, as reported in the United Kingdom Medical Research Council (MRC) trials.11,12
The percentage of patients with favorable cytogenetics drops from 17% in patients younger than age 56 to 4% in patients older than 75 years, while unfavorable cytogenetics increase from 35% to 51% in older patients. This is due to a larger proportion of chromosomal abnormalities involving chromosomes 5,7,17 and complex aberrant karyotypes.13,14 The likelihood of prolonged survival is low for any patient with unfavorable cytogenetics, regardless of age, whereas among patients with intermediate and good risk cytogenetics- t(8;21) and inv(16)-, patients older than 65 seem to do much worse than younger patients.15
A recent multicentric review of 373 cases of core-binding factor confirms that the incidence of drug resistance, as a cause of induction failure and relapse following successful remission induction, increases significantly with patient age.16
Finally, age profoundly affects older patients with a poor ECOG performance status (PS), whose early death rate may be very high.10
Prognosis and Initial Treatment
The median survival for patients over the age of 60 years ranges from 6 to 10 months, with remission rates in the order of 40%, and overall survival (OS) at one year is in the order of 15%.9 This dismal prognosis has been attributed to several factors: high treatment-related mortality, up to 25%, a low complete remission (CR) rate and a high risk of relapse. Several randomized trials have failed to show improvement in standard therapy modifications with newer treatment options.17–18 No additional survival benefit has been shown from more intensive post-remission therapy, adding other agents, or from maintenance therapy. A very recent randomized study has shown a statistically significant survival advantage for Azacytidine over placebo as maintenance therapy after remission induction:19 it should be said that survival at five years was identical in the two arms.
Allogeneic Hemopoietic Stem Cell Transplantation (HSCT)
Allogeneic hematopoietic stem cell transplantation (HSCT), with its graft versus leukemia (GVL) effect, may offer long-term disease-free survival (DFS) in older patients with AML.20 The development of reduced-intensity conditioning (RIC) and non-myeloablative (NMA) regimens, together with improvement in supportive care, has broadened HSCT’s application to include older adults. Currently, 22% of allogeneic HSCT recipients for malignant diseases are older than 60 years,21 and allogeneic HSCT has significantly increased also above the age of 70.21
Nevertheless, despite increasing patients’ age in transplantation, only 6% of AML patients over 60 years undergo an allograft in the USA,22 and also HLA typing is still performed in reduced numbers.23 This indicates that many Hematology Units are unwilling to seriously consider an allogeneic HSCT as a treatment option for older patients with AML.
Conditioning Regimens in Older AML
Myeloablative (MA) conditioning regimens have been traditionally the curative approach for patients with hematologic malignancies and incorporate alkylating agents with or without total body irradiation (TBI) at doses that do not allow autologous stem cell recovery.24 Control of the underlying disease is usually good, but transplant-related mortality (TRM) is high in adults over 60 years of age, in the order of 40%.25
On the other hand, NMA regimens, as well as RIC regimens, preserve GVL with a relatively low TRM.26 One of these NMA regimens combines low dose TBI (2 Gy) and fludarabine and has been widely used in older patients: in a large multicenter study on 372 patients aged 60–75 years, the 5-year cumulative incidences of TRM and relapse were 27% and 41%, and the 5-year OS 35%.27
Reduced dose cyclophosphamide (45 mg/kg instead of the conventional 120 mg/kg) was used in another study, in 56 patients with AML (n = 41) and MDS (n = 15): TRM was 9% and DFS 45%.28 Indeed significantly reduced TRM may favorably affect the OS of older patients with comorbidities.
A large retrospective study from the Center for International Blood and Marrow Transplantation Research (CIBMTR) included 545 AML patients, aged 40–79 in the first remission, who underwent a RIC HSCT:29 there was no impact of age on TRM, DFS, and OS. The 2-year OS in the age groups 60–64 and 65 years or over was 34% and 36%.29 A meta-analysis of 14 studies, including 749 AML patients allografted with RIC regimens, showed a 35% 3-year DFS,30 with a plateau of the relapse curves beyond one year.
Comparing Myeloablative Regimens with NMA or RIC
An extensive retrospective analysis from the European Group for Blood and Marrow Transplantation (EBMT) compared RIC and MAC regimens in older AML patients: RIC was associated with decreased TRM (HR, 0.64), similar relapse risk (HR, 1.34), and similar DFS (HR, 1.04).31 A similar analysis, again only in AML patients, was conducted by the CIBMTR32 and included NMA, RIC, and MAC regimens: NMA resulted in inferior 5 years OS survival (26%), as compared to similar OS of RIC/33%) and MAC (34%), mainly due to an excess of relapse. Therefore, it is possible that one can reduce the intensity of the conditioning to some extent, but a certain degree of tumor-ablation is required for long-term disease-free survival in AML.
Donor Type
Older AML patients are bound to have an older sibling, so, given a choice, who should we choose: an old HLA identical sibling or a young matched unrelated donor.
A retrospective EBMT study was performed in 714 AML patients, aged 55 years and over, grafted from a matched unrelated donor (MUD) (median age 35 years) or an HLA identical matched sibling donor (MSD) (median age 61 years):33 there was no significant difference in 3-year TRM (17% versus 23%; p = 0.17), relapse rate (37% versus 30%; p = 0.12), and OS (49% in both).33 Single Center studies have led to discrepant results, sometimes favoring a young unrelated donor’s choice over an older matched sibling.34 Haploidentical family donors (HAPLO) and unrelated cord blood (CB) units are additional options for patients who lack an HLA related or unrelated donor:35,36 in a study from Seattle, patients with minimal residual disease (MRD) at transplant did better after a UCB transplant as compared to an unrelated transplant, mainly due to a lower risk of relapse.35 In a prospective study from Minnesota, 98 patients with AML aged 55 years or over underwent a RIC HSCT using a matched sibling donor or an unrelated CB unit. There were 26 AML patients in the sibling donor group and 44 in the CB group: there was no difference in OS, relapse rate, and TRM.36 Cord blood HSCT recipients had a lower incidence of chronic GVHD at 2-years post-transplant (61 versus 33%).36
HAPLO donors are being widely used worldwide after the demonstration that post-transplant cyclophosphamide (PT-.CY), combined with a calcineurin inhibitor (CNI) and mycophenolate (MMF), can effectively protect patients from acute and chronic GvHD.37
A CIBMTR study compared HAPLO donors with MUD in AML patients and found no difference in the main outcomes.38 One advantage of selecting a HAPLO donor, compared to an unrelated donor, is the immediate availability of a family member. Therefore family HLA typing should be performed upfront: in the absence of a suitable HLA identical sibling, a matched unrelated donor and family haploidentical donors may be considered. Unrelated cord blood units can also be considered as an alternative stem cell source.
Graft versus Host Disease
Acute and chronic GvHD remain major obstacles to successful HSCT, which is more so in patients over the age of 60. HLA matching is known to influence the risk of GvHD, although in the CIBMTR study in AML, comparing HAPLO and MUD grafts, there was more cGvHD in MUD transplants:38 of note is the fact that HAPLO grafts were protected with PTCY, a CNI, and MMF, whereas MUD grafts received a conventional CNI, methotrexate prophylaxis with or without anti-thymocyte globulin (ATG).38 Therefore, donor type and stem cell source and GvHD prophylaxis must be taken into account. When it comes to age, donors and recipients age may both be relevant for the risk of developing GvHD. We have recently reviewed our GvHD data in patients receiving unmanipulated bone marrow transplants from HAPLO donor, with PTCY, CNI, and MMF as GvHD prophylaxis: older donors and older recipients had significantly increased acute (41% vs. 13%) and increased chronic GvHD (29% vs. 11%).39 Therefore it is probably safe to envisage an effective combination of drugs for GvHD prophylaxis in older patients with AML when an allogeneic transplant is considered. In addition, age may influence not only the risk of developing GvHD but also, as expected, the probability of surviving once GvHD has developed.40
Transplant Related Mortality
GvHD, infections, the toxicity of the conditioning regimen, and the worsening of pre-existing conditions contribute to transplant-related mortality (TRM): this is the argument against an allogeneic HSCT, especially in older patients with AML. TRM has been significantly reduced over the past decades, such that a decrease in the hazards of day 200 TRM by 60% was reported in one study,41 and reduced 2-year cumulative incidence of TRM from 34% to 6% in another study.42 Despite the improvement, older age currently remains a strong predictor of TRM, especially on patients over the age of 60 with GvHD.40 One measure which may be taken into consideration in older AML patients is reducing the number of chemotherapy courses: in a German study in AML, patients receiving one course of chemotherapy followed immediately by an allogeneic HSCT had a remission rate of 91%, with a best survival; on the other hand, repeated courses of chemotherapy only resulted in a higher TRM, with no benefit on leukemia control.43 Indeed more chemotherapy implies prolonged periods of neutropenia and incomplete hematologic recovery, and these patients may come to transplant already infected. A new strategy for older AML patients would be one course of induction chemotherapy followed by an allogeneic HSCT: we are currently testing this hypothesis in a prospective trial (ClinicalTrials.gov Identifier: NCT03902665).
Is Allogeneic HSCT the Best Post-Remission Therapy for Older Patients with AML?
After remission induction, allogeneic HSCT provides an advantage for AML patients compared to other forms of consolidation therapy. This has been clearly shown for patients under the age of 40, in a donor- no donor analysis, but less so in the older age group.44 In a more recent Dutch study, the median OS for 68 older AML patients, consolidated with a RIC allogeneic HSCT, was 65 months, compared to 8 months for 287 patients who did not proceed to transplant.45 Similar results were obtained in the AML15 MRC trial, with a survival advantage for older patients receiving a RIC transplant over chemotherapy: the 5-year survival was 59% for RIC transplants from HLA-matched siblings, 49% from unrelated donors, and 36% for chemotherapy.46 These data, taken together, suggest that allogeneic HSCT remains the most potent anti-leukemic post-remission therapy. The GvL effect is also exerted in older patients, but conditioning regimens must be tailored in order to minimize transplant-related complications; otherwise, the benefit of reduced leukemia relapse is lost. To answer whether this is the best post-remission therapy, one would need a prospective randomized study
Older AML Patients Beyond the First Remission
Beyond first remission (CR1), HSCT is associated with increased TRM, higher incidence of relapse, and worse survival. Recently the presence of a positive minimal residual disease (MRD) at transplant has been shown to be a strong predictor of outcome.1,35 In an EBMT study DFS at 2 years was 65% for patients undergoing transplantation in CR1 (n = 1,410) versus 51% for those undergoing transplantation in second complete remission (CR2) (n = 249; P < .001).47 In the same study, selecting only CR1 patients, the 2-year LFS was 65% for molecular CR1 and 55% for MRD-positive patients, respectively (P = .02). In a study by the Houston group in AML over 65 years of age, survival was excellent (76%) for patients grafted with a negative MRD, whereas poor outcome (8% survival) was seen for patients grafted with overt leukemia or a positive MRD.48 The Seattle data show the same risk of relapse for patients with overt leukemia and patients with MRD+ before an allogeneic HSCT.1
Therefore, the disease phase is a significant predictor of AML patients’ outcome and calls for careful identification of residual disease before the transplant. The choice of an appropriate conditioning regimen, effective GvHD prophylaxis, and donor selection, in other words, the choice of a given transplant platform, may also encourage results in older AML patients with advanced disease, as shown in a recent study.49
Exceeding the Limit of 70 Years of Age
The majority of data available on allografting for older AML patients involves patients 65 years or under, while limited data exist regarding patients transplanted in their eighth decade. Their number has risen every year since 2000: patients ≥ 70 years now represent nearly 4% of allogeneic HSCT recipients.50
The EBMT has compared the outcome of 713 AML patients aged ≥ 70 years to 16161 patients aged 50 to 69 years who underwent HSCT between 2004 and 2014.51 Acute and chronic GvHD were comparable in the two age groups. TRM was higher in the older patients (34% vs. 24%), and overall 2-year survival was lower (38% versus 50%). However, when selecting only active disease patients, the two-year survival was comparable 35% and 33%.51 This study on a large number of patients suggests that 70 years is not an insurmountable barrier, and again selection of patients and selection of the transplant platform needs to be taken into account.
The Role of Cytogenetics
There has been increasing knowledge of the predictive role of cytogenetics in allogeneic HSCT. In a study on 54 older AML patients undergoing an allogeneic HSCT, 46% had adverse cytogenetic risk:52 the 2-year OS rates were significantly higher for patients with favorable/intermediate cytogenetics compared with patients with unfavorable cytogenetics (53% versus 30%, respectively), and 1-year DFS was 60% versus 26%. Intermediate cytogenetic risk AML/MDS had a decreased risk of TRM.52 As expected, the incidence of relapse for AML/MDS patients with favorable/intermediate cytogenetics was significantly lower compared with patients with unfavorable cytogenetics or other diseases (35%versus 68%). Interestingly, age and hematopoietic cell transplantation comorbidity index (HCT-CI) were not significant predictors for those endpoints.
The more unsatisfactory outcome seen in patients with adverse risk cytogenetics is a problem since the conditioning regimen cannot be intensified, and prophylactic cellular therapies may risk GvHD.
Targeted Therapy and HSCT
A different approach would be to use targeted therapy before and/or after transplantation. The German cooperative group has completed a small randomized trial to test whether sorafenib, a Flt3 inhibitor, would be beneficial if given post-transplant in Flt3+ AML:53 with a median follow up of 42 months, the hazard ratio (HR) for relapse or death in the sorafenib-group versus placebo-group was 0.39 (p=0.013), and the 24-months probability of relapse was 47% with placebo versus 15% with sorafenib (HR=0.256, log-rank p=0.002).53 Patients with undetectable MRD prior to HSCT and those with detectable MRD after HSCT had the strongest benefit from sorafenib treatment.
TRM was comparable in the two groups.53 Gilteritinib, a highly selective, oral FLT3 inhibitor with activity against both FLT3 mutation subtypes (ITD and TKD), was tested in patients with relapsed/refractory AML: significantly more patients in the gilteritinib group proceeded to an allogeneic HSCT, as compared to the placebo (25% vs. 15%, p=0.02), and also showed a survival advantage.54 Trials of gilteritinib as part of first-line induction or consolidation therapy and post-HSCT maintenance therapy are underway to assess the best timing for anti-FLT3 intervention to improve treatment outcomes.
These novel treatment strategies, aiming to induce MRD-negativity before HSCT, might synergize with post HSCT maintenance, hopefully even in the older population.
Geriatric Assessment in older AML
Disease status and medical comorbidities, rather than chronological age, appear to predict for outcomes in patients with AML receiving RIC allogeneic transplants.29 Geriatric oncology has been using a comprehensive geriatric assessment (CGA) as a standardized tool to evaluate health status in older patients for more than a decade, and this is now being applied to allogeneic HSCT.55 CGA is a multidisciplinary evaluation that focuses on interventions on the main comorbidities and predicts morbidity and mortality in general oncology practice. This approach appears to be suitable, if not mandatory, when the decision to allograft an older patient with AML is made to make individualization and focus on disease status, comorbidity, donor type, regimen, and center experience.55 Age, performance status, and, more recently, comorbidity have been the most common parameters used by transplantation physicians to verify the suitability for HSCT.56 The Sorror comorbidity index (HCT-CI) has been designed to assess TRM’s risk before an allogeneic HSCT and is based on several comorbidities extracted from the medical history and objective testing.57 This scoring system has been validated in an independent cohort of patients:58 the combination of a high Sorror score (>5) and the age over 60 results in a high risk of TRM.58
The comorbidity score can be integrated with a geriatric assessment in patients over the age of 60, and more so over the age of 70 to better tailor the intensity of the conditioning regimen and the type of GvHD prophylaxis.
Conclusions
Improving survival in AML patients over 60 years of age remains a primary unmet medical need. In the last ten years, these patients’ approaches have gone from avoiding allogeneic transplantation, a “threatening option,” to considering it a fundamental procedure to induce prolonged remissions or cure. It remains the procedure with the most significant anti-leukemia effect, but transplant morbidity and mortality remain a burden. Encouraging results are obtained, especially avoiding repeated courses of chemotherapy before transplantation.
Improvements in the conditioning regimens and the donor’s choice and improvement in supportive care have had a positive impact on the outcome.
Understanding the “biological” age rather than the chronological age is an area of active investigation. Assessment of quality of life endpoints, function, and symptoms in older adult survivors of HSCT is also important.
New protocols are being designed to reduce leukemia relapse after HSCT, a major cause of failure, and include genetically engineered donor lymphocyte infusions, early tapering of immunosuppression, and MRD monitoring before and after HSCT, maintenance therapy post-HSCT, and targeted therapy.
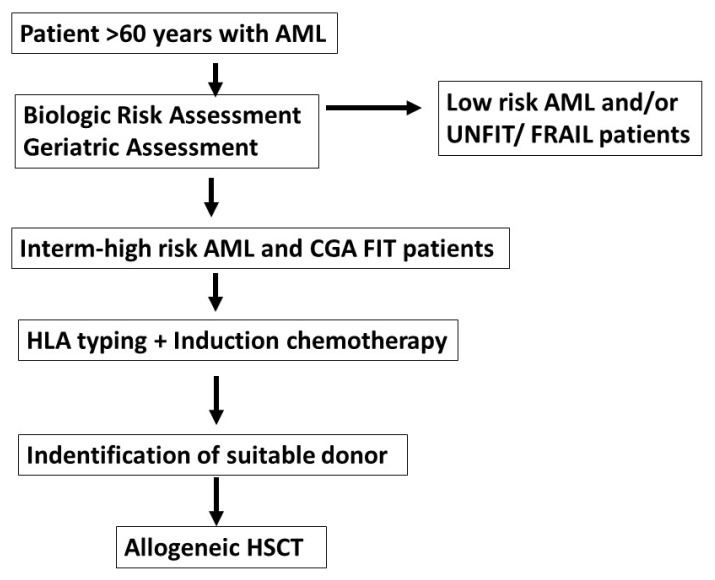
On top of these, possibly early transplantation may represent a simple and effective way of bringing to transplant a larger proportion of patients with AML. Figure 1 outlines a theoretical approach for older patients with AML: risk stratification should identify intermediate and high risk AML, and CGA assessment would then select fit patients; HLA typing and induction therapy would follow, with the possible identification of a suitable donor; an allogeneic HSCT could be performed as early as possible. The advantage of an early transplant would be to avoid prolonged and repeated periods of neutropenia and possibly prevent chemo/radioresistance of the leukemic cells.
Figure 1.
Older patients with AML are assessed for biloligc and geriatric classification; HLA typing and induction is carried out for CGA FIT patients; as soon as a suitable donor is identified, patients should proceed to HSCT:AML=acute myeloid leukemia; Low risk AML=according to ELN2017 guidelines; intermediate high risk AML; CGA comprehensive geriatric assessment; FIT, UNFIT, FRAIL=three categories of CGA; HLA human leukocyte antigen; HSCT=Hemopoietic stem cell transplant; suitable donor=HLA identical sibling; HLA identical unrelated donor; family haploidentical donor; partially mismatched unrelated donor, cord blood unit.
Footnotes
Competing interests: The authors declare no conflict of Interest.
References
- 1.Estey EH. Acute myeloid leukemia: 2016 update on risk stratification and management. Am J Hematol. 2016;91:825–846. doi: 10.1002/ajh.24439. [DOI] [PubMed] [Google Scholar]
- 2.Juliusson G, Lazarevic V, Hörstedt AS, Hagberg O, Höglund M Swedish Acute Leukemia Registry Group. Acute myeloid leukemia in the real world: why population-based registries are needed. Blood. 2012 Apr 26;119(17):3890–9. doi: 10.1182/blood-2011-12-379008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sekeres MA. Treatment of older adults with acute myeloid leukemia: state of the art and current perspectives. Haematologica. 2008;93(12):1769–72. doi: 10.3324/haematol.2008.000497. [DOI] [PubMed] [Google Scholar]
- 4.Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 9 Regs Public-Use, Nov 2004 Sub (19732002), National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch, released April 2005 based on the November 2004 submission. www.seer.cancer.gov
- 5.Warren J, Link DC. Clonal hematopoiesis and risk for hematologic malignancy. Blood. 2020;136:1599–1605. doi: 10.1182/blood.2019000991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McMurray MA, Gottschling DE. An age-induced switch to a hyper-recombinational state. Science. 2003;301:1908–1911. doi: 10.1126/science.1087706. [DOI] [PubMed] [Google Scholar]
- 7.Morrison SJ, Wandycz AM, Akashi K, Globerson A, Weissman IL. The aging of hematopoietic stem cells. NatMed. 1996;2:1011–1016. doi: 10.1038/nm0996-1011. [DOI] [PubMed] [Google Scholar]
- 8.Rufer N, Dragowska W, Thornbury G, Roosnek E, Lansdorp PM. Telomere length dynamics in human lymphocyte subpopulations measured by flow cytometry. Nat Biotechnol. 1998;16:743–747. doi: 10.1038/nbt0898-743. [DOI] [PubMed] [Google Scholar]
- 9.Burnett AK. Treatment of acute myeloid leukemia: are we making progress? Hematology Am Soc Hematol Educ Program. 2012;2012:1–6. doi: 10.1182/asheducation.V2012.1.1.3797038. [DOI] [PubMed] [Google Scholar]
- 10.Appelbaum FR, Gundacker H, Head DR, Slovak ML, Willman CL, Godwin J, et al. Age and acute myeloid leukemia. Blood. 2006 May 1;107(9):3481–5. doi: 10.1182/blood-2005-09-3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldstone AH, Burnett AK, Wheatley K, Smith AG, Hutchinson RM, Clark RE Medical Research Council Adult Leukemia Working Party. Attempts to improve treatment outcomes in acute myeloid leukemia (AML) in older patients: the results of the United Kingdom Medical Research Council AML11 trial. Blood. 2001;98:1302–1311. doi: 10.1182/blood.V98.5.1302. [DOI] [PubMed] [Google Scholar]
- 12.Grimwade D, Walker H, Harrison G, Oliver F, Chatters S, Harrison CJ, et al. Medical Research Council Adult Leukemia Working Party. The predictive value of hierarchical cytogenetic classification in older adults with acute myeloid leukemia (AML); analysis of 1065 patients entered into the United Kingdom Medical Research Council AML11 trial. Blood. 2001;98:1312–1320. doi: 10.1182/blood.V98.5.1312. [DOI] [PubMed] [Google Scholar]
- 13.Moorman AV, Roman E, Willett EV, Dovey GJ, Cartwright RA, Morgan GJ. Karyotype and age in acute myeloid leukemia. Are they linked? Cancer Genet Cytogenet. 2001;126:155–161. doi: 10.1016/S0165-4608(00)00414-3. [DOI] [PubMed] [Google Scholar]
- 14.Fairman J, Wang RY, Liang H, Zhao L, Saltman D, Liang JC, Nagarajan L. Translocations and deletions of 5q13.1 in myelodysplasia and acute myelogenous leukemia: evidence for a novel critical locus. Blood. 1996;88:2259–2266. doi: 10.1182/blood.V88.6.2259.bloodjournal8862259. [DOI] [PubMed] [Google Scholar]
- 15.Farag SS, Archer KJ, Mrózek K, Ruppert AS, Carroll AJ, Vardiman JW, et al. Pretreatment cytogenetics add to other prognostic factors predicting complete remission and long-term outcome in patients 60 years of age or older with acute myeloid leukemia: results from Cancer and Leukemia Group B 8461. Blood. 2006 Jul 1;108(1):63–73. doi: 10.1182/blood-2005-11-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Appelbaum FR, Kopecky KJ, Tallman MS, Slovak ML, Gundacker HM, Kim HT, et al. The clinical spectrum of adult acute myeloid leukaemia associated with core binding factor translocations. Br J Haematol. 2006 Oct;135(2):165–73. doi: 10.1111/j.1365-2141.2006.06276.x. [DOI] [PubMed] [Google Scholar]
- 17.Goldstone AH, Burnett AK, Wheatley K, Smith AG, Hutchinson RM, Clark RE on behalf of the Medical Research Council Adult Leukaemia Working Party. Attempts to improve treatment outcomes in acute myeloid leukemia (AML) in older patients: the results of the United Kingdom Medical Research Council AML11 trial. Blood. 2001;98:1302–1311. doi: 10.1182/blood.V98.5.1302. [DOI] [PubMed] [Google Scholar]
- 18.Anderson JE, Kopecky KJ, Willman CL, Head D, O’Donnell MR, Luthardt FW, et al. Outcome after induction chemotherapy for older patients with acute myeloid leukemia is not improved with mitoxantrone and etoposide compared to cytarabine and daunorubicin: a Southwest Oncology Group study. Blood. 2002;100:3869–3876. doi: 10.1182/blood-2001-12-0354. [DOI] [PubMed] [Google Scholar]
- 19.Wei AH, Dohner H, Pocock C, Montensinos P, Avanasief B, Dombret H, et al. The QUAZAR AML 001 maintenance trial. Results of a phase III international randomized double blind, placebo controlled of CC486 (oral formulation of Azacytidine) in patients with acute myeloid leukemia (AML) in first remission. Blood 2019 (supplement_2) Lba. 3 doi: 10.1182/blood-2019-132405. [DOI] [Google Scholar]
- 20.Storb R. Can reduced-intensity allogeneic transplantation cure older adults with AML? Best Pract Res Clin Haematol. 2007;20:85–90. doi: 10.1016/j.beha.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 21.Muffly L, Pasquini MC, Martens M, Brazauskas R, Zhu X, Adekola K, et al. Increasing use of allogeneic hematopoietic cell transplantation in patients aged 70 years and older in the United States. Blood. 2017 Aug 31;130(9):1156–1164. doi: 10.1182/blood-2017-03-772368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ustun C, Lazarus HM, Weisdorf D. To transplant or not: a dilemma for treatment of elderly AML patients in the twenty-first century. Bone Marrow Transplant. 2013;48:1497–1505. doi: 10.1038/bmt.2013.67. [DOI] [PubMed] [Google Scholar]
- 23.Estey E, de Lima M, Tibes R, Pierce S, Kantarjian H, Champlin R, Giralt S. Prospective feasibility analysis of reduced intensity conditioning (RIC) regimens for hematopoietic stem cell transplantation (HSCT) in elderly patients with acute myeloid leukemia (AML) and high-risk myelodysplastic syndrome (MDS) Blood. 2007;109(4):1395–400. doi: 10.1182/blood-2006-05-021907. [DOI] [PubMed] [Google Scholar]
- 24.Bacigalupo A, Ballen K, Rizzo D, Giralt S, Lazarus H, Ho V, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009;15:1628–1633. doi: 10.1016/j.bbmt.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wallen H, Gooley TA, Deeg HJ, Pagel JM, Press OW, Appelbaum FR, et al. Ablative Allogeneic Hematopoietic Cell Transplantation in Adults 60 Years of Age and Older. J Clin Oncol. 2005;23:3439–3446. doi: 10.1200/JCO.2005.05.694. [DOI] [PubMed] [Google Scholar]
- 26.Goyal G, Gundabolu K, Vallabhajosyula S, Silberstein PT, Bhatt VR. Reduced-intensity conditioning allogeneic hematopoietic-cell transplantation for older patients with acute myeloid leukemia. Ther Adv Hematol. 2016 Jun;7(3):131–41. doi: 10.1177/2040620716643493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sorror ML, Sandmaier BM, Storer BE, Franke GN, Laport GG, Chauncey TR, et al. Long-term outcome among older patients following nonmyeloablative conditioning and allogeneic hematopoietic cell transplantation for advanced hematologic malignancies. JAMA. 2011;306:1874–1883. doi: 10.1001/jama.2011.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davies JK, Taussig D, Oakervee H, Smith M, Agrawal S, Cavenagh JD, et al. Long-term survival with low toxicity after allogeneic transplantation for acute myeloid leukaemia and myelodysplasia using non-myeloablative conditioning without T cell depletion. Br J Haematol. 2013;162:525–529. doi: 10.1111/bjh.12402. [DOI] [PubMed] [Google Scholar]
- 29.McClune BL, Weisdorf DJ, Pedersen TL, Tunes da Silva G, Tallman MS, Sierra J, et al. Effect of age on outcome of reduced-intensity hematopoietic cell transplantation for older patients with acute myeloid leukemia in first complete remission or with myelodysplastic syndrome. J ClinOncol. 2010;28:1878–1887. doi: 10.1200/JCO.2009.25.4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rashidi A, Ebadi M, Colditz GA, DiPersio JF. Outcomes of Allogeneic Stem Cll Transplantation in Elderly Patients with Acute Myeloid Leukemia: A Systematic Review and Meta-analysis. Biol Blood Marrow Transplant. 2016 Apr;22(4):651–657. doi: 10.1016/j.bbmt.2015.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ringdén O, Labopin M, Ehninger G, Niederwieser D, Olsson R, Basara N, et al. Reduced intensity conditioning compared with myeloablative conditioning using unrelated donor transplants in patients with acute myeloid leukemia. J ClinOncol. 2009;27:4570–4577. doi: 10.1200/JCO.2008.20.9692. [DOI] [PubMed] [Google Scholar]
- 32.Luger AM, Ringden O, Zhang M-J, Pe’rez WS, Bishop MR, Bornhauser M, et al. Similar outcomes using myeloablative vs reduced-intensity allogeneic transplant preparative regimens for AML or MDS. Bone Marrow Transplant. 2012;47:203.32. doi: 10.1038/bmt.2011.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peffault de Latour R, Labopin M, Cornelissen J, Vigouroux S, Craddock C, Blaise D, Huyn A, Vindelov L, Maertens J, Chevallier P, Fegueux N, Socié G, Cahn JY, Petersen E, Schouten H, Lioure B, Russell N, Corral LL, Ciceri F, Nagler A, Mohty M. Acute Leukemia Working Party of EBMT. In patients older than 55 years with AML in first CR, should we search for a matched unrelated donor when an old sibling donor is available? Bone Marrow Transplant. 2015;50:1411–1415. doi: 10.1038/bmt.2015.180. [DOI] [PubMed] [Google Scholar]
- 34.Haen SP, Pham M, Faul C, Dörfel D, Vogel W, Kanz L, Bethge WA. Allogeneic hematopoietic cell transplantation in patients ≥ 70 years: which patients may benefit? Blood Cancer Journal. 2016;6:e 443 . doi: 10.1038/bcj.2016.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Milano F, Gooley T, Wood B, Woolfrey A, Flowers E, Doney K, et al. Cord-Blood Transplantation in Patients with Minimal Residual Disease. New Engl J Med. 2016;375:944–953. doi: 10.1056/NEJMoa1602074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Majhail NS, Brunstein CG, Shanley R, Sandhu K, McClune B, Oran B, et al. Reduced-intensity hematopoietic cell transplantation in older patients with AML/MDS: umbilical cord blood is a feasible option for patients without HLA-matched sibling donors. Bone Marrow Transplant. 2012;47:494–498. doi: 10.1038/bmt.2011.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luznik L, O’Donnell PV, Symons HJ, Chen AR, Leffell MS, Zahurak M, Gooley TA, Piantadosi S, Kaup M, Ambinder RF, Huff CA, Matsui W, Bolaños-Meade J, Borrello I, Powell JD, Harrington E, Warnock S, Flowers M, Brodsky RA, Sandmaier BM, Storb RF, Jones RJ, Fuchs EJ. HLA-haploidentical bone marrow ransplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008 Jun;14(6):641–50. doi: 10.1016/j.bbmt.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ciurea SO, Zhang MJ, Bacigalupo AA, Bashey A, Appelbaum FR, Aljitawi OS, et al. Haploidentical transplant with post transplant cyclophosphamide vs matched unrelated donor transplant for acute myeloid leukemia. Blood. 2015 Aug 20;126(8):1033–40. doi: 10.1182/blood-2015-04-639831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bacigalupo A, Raiola AM, Dominietto A, Di Grazia C, Gualandi F, Van Lint MT, et al. Graft versus host disease in unmanipulated haploidentical marrow transplantation with a modified post-transplant cyclophosphamide (PT-CY) regimen: an update on 425 patients. Bone Marrow Transpl. 2019;54:708–712. doi: 10.1038/s41409-019-0594-1. [DOI] [PubMed] [Google Scholar]
- 40.Nakane T, Fukuda T, Kanda J, Taniguchi S, Tetsuia E, Ohashi K, et al. Age influences post-graft-versus-host disease non-relapse mortality in adults with acute graft-versus-host disease of varying severity following allogeneic hematopoietic cell transplant. Leukemia Lymphoma. 2015;56:2392–2397. doi: 10.3109/10428194.2015.1009056. [DOI] [PubMed] [Google Scholar]
- 41.Gooley TA, Chien JW, Pergam SA, Hingorani S, Sorror M, Boechk M, et al. Reduced mortality after allogeneic hemopoietic stem cell transplantation. New Engl J Med. 2010;363:2091–2101. doi: 10.1056/NEJMoa1004383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bacigalupo A, Sormani MP, Lamparelli T, Gualandi F, Occhini D, et al. Reducing transplant related mortality after allogeneic stem cell transplantation. Haematologica. 2004;89:1238–1247. [PubMed] [Google Scholar]
- 43.Schmid C, Schleuning M, Schwerdtfeger R, Hertenstein B, Mischak-Weissinger E, Bunjes D, Harsdorf SV, Scheid C, Holtick U, Greinix H, Keil F, Schneider B, Sandherr M, Bug G, Tischer J, Ledderose G, Hallek M, Hiddemann W, Kolb HJ. Long-term survival in refractory acute myeloid leukemia after sequential allogeneic stem cell transplantation treatment with chemotherapy and reduced-intensity conditioning for allogeneic stem cell transplantation. Blood. 2006;108:1092–1099. doi: 10.1182/blood-2005-10-4165. [DOI] [PubMed] [Google Scholar]
- 44.Cornelissen JJ, van Putten J, Verdonck LF, Theobald M, Jacky E, Daenen SMG, et al. Blood. 2007;109:3658–3666. doi: 10.1182/blood-2006-06-025627. [DOI] [PubMed] [Google Scholar]
- 45.Hilberink J, Hazenberg C, van den Berg E, Mulder A, Schuringa JJ, van der Helm L, et al. Not type of induction therapy but consolidation with allogeneic hematopoietic cell transplantation determines outcome in older AML patients: A single center experience of 355 consecutive patients. Leuk Res. 2019 May;80:33–39. doi: 10.1016/j.leukres.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 46.Russell NH, Kjeldsen L, Craddock C, Pagliuca A, Yin YA, Clark RA, et al. A comparative assessment of the curative potential of reduced intensity allografts in acute myeloid leukaemia. Leukemia. 2015;29:1478–1484. doi: 10.1038/leu.2014.319. [DOI] [PubMed] [Google Scholar]
- 47.Nagler A, Rocha V, Labopin M, Unal A, Othman B, Campos A, et al. Allogeneic Hematopoietic Stem-Cell Transplantation for Acute Myeloid Leukemia in Remission: Comparison of Intravenous Busulfan Plus Cyclophosphamide (Cy) Versus Total-Body Irradiation Plus Cy As Conditioning Regimen-A Report From the Acute Leukemia Working Party of the European Group for Blood and Marrow Transplantation. J Clin:Oncol. 2013;31:3549–3556. doi: 10.1200/JCO.2013.48.8114. [DOI] [PubMed] [Google Scholar]
- 48.VeltriVeltri L, Rezvani K, Oran B, Mehta R, Rondon G, Kebriaei P, et al. Allotransplants for Patients 65 Years or Older with High-Risk Acute Myeloid Leukemia. Biol Blood Marrow Transpl. 2019;25:505–514. doi: 10.1016/j.bbmt.2018.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chiusolo P, Bug G, Olivieri A, Brune M, Mordini N, Alessandrino PE, et al. A Modified Post-Transplant Cyclophosphamide Regimen, for Unmanipulated Haploidentical Marrow Transplantation, in Acute Myeloid Leukemia: A Multicenter Study. Biol Blood Marrow Transplant. 2018 Jun;24(6):1243–1249. doi: 10.1016/j.bbmt.2018.01.031. [DOI] [PubMed] [Google Scholar]
- 50.Rosko A, Artz A. Aging: Treating the Older Patient. Biol Blood Marrow Transplant. 2017 Feb;23(2):193–200. doi: 10.1016/j.bbmt.2016.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ringdén O, Boumendil A, Labopin M, Canaani J, Beelen D, Ehninger G, Niederwieser D, Finke J, Stelljes M, Gerbitz A, Ganser A, Kröger N, Kantz L, Brecht A, Savani B, Sadeghi B, Mohty M, Nagler A. Outcome of Allogeneic Hematopoietic Stem Cell Transplantation in Patients Age >69 Years with Acute Myelogenous Leukemia: On Behalf of the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2019;25:1975–1983. doi: 10.1016/j.bbmt.2019.05.037. [DOI] [PubMed] [Google Scholar]
- 52.Brunner AM, Kim HT, Coughlin E, Alyea EP, 3rd, Armand P, Ballen KK, et al. Outcomes in patients aged 70 or older undergoing allogeneic hematopoietic stem cell transplantation for hematologic malignancies. Biol Blood MarrowTransplant. 2013 Sep;19(9):1374–80. doi: 10.1016/j.bbmt.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 53.Stone RM, Mandrekar SJ, Sanford BL, Laumann K, Geyer S, Bloomfield CD, Thiede C, Prior TW, Döhner K, Marcucci G, Lo-Coco F, Klisovic RB, Wei A, Sierra J, Sanz MA, Brandwein JM, de Witte T, Niederwieser D, Appelbaum FR, Medeiros BC, Tallman MS, Krauter J, Schlenk RF, Ganser A, Serve H, Ehninger G, Amadori S, Larson RA, Döhner H. Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. NEngl J Med. 2017;377:454–64. doi: 10.1056/NEJMoa1614359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Perl Perl AE, Martinelli G, Cortes JE, Neubauer A, Berman E, Paolini S, et al. Gilteritinib or Chemotherapy for Relapsedor Refractory FLT3- Mutated AML N Engl J Med. 2019;381:1728–40. doi: 10.1056/NEJMoa1902688. [DOI] [PubMed] [Google Scholar]
- 55.Artz AS. Comorbidity and beyond: pre-transplant clinical assessment. Bone Marrow Transplant. 2005;36:473–474. doi: 10.1038/sj.bmt.1705042. [DOI] [PubMed] [Google Scholar]
- 56.Pilot Study of Comprehensive Geriatric Assessment (CGA) in Allogeneic Transplant: CGA Captures a High Prevalence of Vulnerabilities in Older Transplant Recipients. Biol Blood Marrow Transplant. 2013;19:429–434. doi: 10.1016/j.bbmt.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 57.Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, Storer B. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–2919. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sorror ML, Logan BR, Zhu X, Rizzo JD, Cooke KR, McCarthy PL, Ho VT, Horowitz MM, Pasquini MC. Prospective validation of the predictive power of the hematopoietic cell transplantation comorbidity index: a center for international blood and marrow transplant research study. Biol Blood Marrow Transplant. 2015;21:1479–1487. doi: 10.1016/j.bbmt.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]