Abstract
Background
Water signal contamination is a major challenge for direct ultrashort echo time (UTE) imaging of myelin in vivo because water contributes most of the signals detected in white matter.
Purpose
To validate a new short repetition time (TR) adiabatic inversion recovery (STAIR) prepared UTE (STAIR-UTE) sequence designed to suppress water signals and to allow imaging of ultrashort T2 protons of myelin in white matter using a clinical 3-T scanner.
Materials and Methods
In this prospective study, an optimization framework was used to obtain the optimal inversion time for nulling water signals using STAIR-UTE imaging at different TRs. Numeric simulation and phantom studies were performed. Healthy volunteers and participants with multiple sclerosis (MS) underwent MRI between November 2018 and October 2019 to compare STAIR-UTE and a clinical T2-weighted fluid-attenuated inversion recovery sequence for assessment of MS lesions. UTE measures of myelin were also performed to allow comparison of signals in lesions and with those in normal-appearing white matter (NAWM) in patients with MS and in normal white matter (NWM) in healthy volunteers.
Results
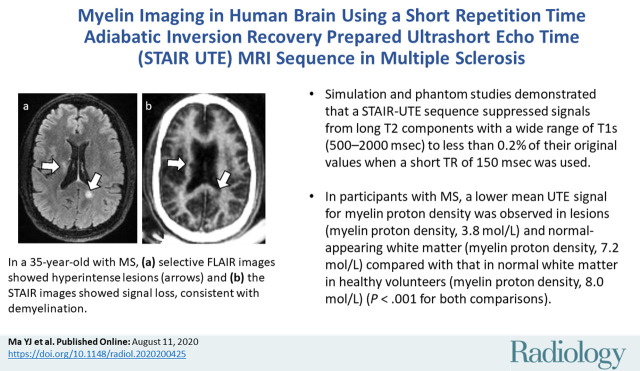
Simulation and phantom studies both suggest that the proposed STAIR-UTE technique can effectively suppress long T2 tissues with a broad range of T1s. Ten healthy volunteers (mean age, 33 years ± 8 [standard deviation]; six women) and 10 patients with MS (mean age, 51 years ± 16; seven women) were evaluated. The three-dimensional STAIR-UTE sequence effectively suppressed water components in white matter and selectively imaged myelin, which had a measured T2* value of 0.21 msec ± 0.04 in the volunteer study. A much lower mean UTE measure of myelin proton density was found in MS lesions (3.8 mol/L ± 1.5), and a slightly lower mean UTE measure was found in NAWM (7.2 mol/L ± 0.8) compared with that in NWM (8.0 mol/L ± 0.8) in the healthy volunteers (P < .001 for both comparisons).
Conclusion
The short repetition time adiabatic inversion recovery–prepared ultrashort echo time sequence provided efficient water signal suppression for volumetric imaging of myelin in the brain and showed excellent myelin signal contrast as well as marked ultrashort echo time signal reduction in multiple sclerosis lesions and a smaller reduction in normal-appearing white matter compared with normal white matter in volunteers.
© RSNA, 2020
Online supplemental material is available for this article.
See also the editorial by Messina and Port in this issue.
Summary
A new three-dimensional short repetition time adiabatic inversion recovery prepared ultrashort echo time sequence provided selective imaging of brain myelin with water signals efficiently suppressed as confirmed by numeric simulation, phantom studies, and studies of patients with multiple sclerosis.
Key Results
■ Simulation and phantom studies demonstrated that the proposed short repetition time adiabatic inversion recovery prepared ultrashort echo time (UTE) sequence can suppress signals from long T2 components with a wide range of T1s (500–2000 msec) to less than 0.2% of their original values when a short repetition time of 150 msec is used.
■ In participants with multiple sclerosis, a lower mean UTE signal for myelin proton density was observed in lesions (myelin proton density, 3.8 mol/L) and normal-appearing white matter (myelin proton density, 7.2 mol/L) compared with that in normal white matter in healthy volunteers (myelin proton density, 8.0 mol/L) (P < .001 for both comparisons).
Introduction
Demyelination is the hallmark of many inflammatory and neurodegenerative disorders, such as multiple sclerosis (MS) (1). Demyelinating lesions in this disease are typically hyperintense on clinical T2-weighted MRI scans (2). However, the lesions seen with clinical MRI sequences are not specific for MS. Substantial overlap exists in the appearances of abnormalities observed in other demyelinating diseases and other brain abnormalities (eg, with edema or gliosis without demyelination) (3). More specific MRI techniques, such as myelin water imaging (4–7), have been developed as potential markers for the presence of myelin. These provide indirect information about myelin content because signals from myelin protons are not detectable with conventional clinical MRI sequences due to their ultrashort T2s (<1 msec) (8,9). In heterogeneous pathologic changes such as those seen in MS, direct MRI of myelin may be more specific and more informative than available indirect methods of myelin imaging and quantification (4,8–14).
Ultrashort echo time (UTE) sequences with echo times less than 50 µsec can help directly detect signals from myelin protons in the brain. However, even in myelin-rich white matter, more than 90% of the detected UTE signal originates from long T2 water protons (15). Efficient suppression of this signal is crucial for direct specific imaging of myelin. A common way of achieving this is to use a relatively long adiabatic inversion recovery (IR) pulse to invert and null the longitudinal magnetizations of long T2 tissues while leaving their short T2 components largely saturated because of their substantial transverse relaxation during the long adiabatic inversion process (11,15,16). This is followed by a UTE data acquisition at the inversion time (TI) appropriate for the inverted long T2 magnetization of the tissue to reach its nulling point and to allow selective detection of signals from ultrashort T2 tissues such as myelin (11,15,16). This can be combined with dual-echo acquisition and subtraction in the form of an adiabatic IR–prepared UTE (IR-UTE) sequence, which shows myelin in the white matter of the brain with high contrast (10,17). However, long T2 signal suppression is sensitive to the value of TI used for long T2 signal suppression using currently available IR-UTE techniques (15,17,18). A slight TI offset from the optimum may cause significant long T2 signal contamination, with the residual long T2 signal higher than that from myelin. The T1 of long T2 tissue components may change with age and disease in the brain (19,20). This makes determination of the correct TI for successful water nulling challenging. Furthermore, a single TI may not be sufficient to null all long T2 signals in the brain in the same person because of normal variation in T1 in different white matter regions of the brain (5,21,22).
To address these problems, we propose a new short repetition time (TR) adiabatic IR (STAIR) prepared UTE (STAIR-UTE) technique to provide more efficient long T2 signal suppression regardless of T1 variations in white matter. This sequence has four key components. First, an adiabatic full passage (AFP) pulse with low sensitivity to B1 and B0 inhomogeneities is used to invert the longitudinal magnetizations of long T2 components (23). Second, a short TR (eg, 130–200 msec, as allowed by specific absorption ratio [SAR] limitations for brain imaging at 3 T) is used. Third, a three-dimensional UTE data acquisition with multiple spokes after each AFP pulse is employed to reduce the scanning time (24–26). Fourth, the TI necessary for the long T2 signals to be nulled is determined by an optimization framework. Our hypothesis is that the efficient signal suppression of long T2 components with a broad range of T1s necessary for selective myelin imaging in white matter with an IR-UTE sequence can be achieved using a short TR with a single optimized value of TI. In this study, we aimed to establish the feasibility of using the STAIR-UTE sequence for imaging of ultrashort T2 myelin protons in white matter with a clinical 3-T scanner using numeric simulation, as well as studies of phantoms, healthy volunteers, and patients with MS, and to demonstrate its clinical potential by comparing UTE measures of myelin content in healthy volunteers and in participants with MS.
Materials and Methods
Local institutional review board approval was received for this prospective study, and written informed consent was obtained from all participants.
STAIR-UTE Sequence
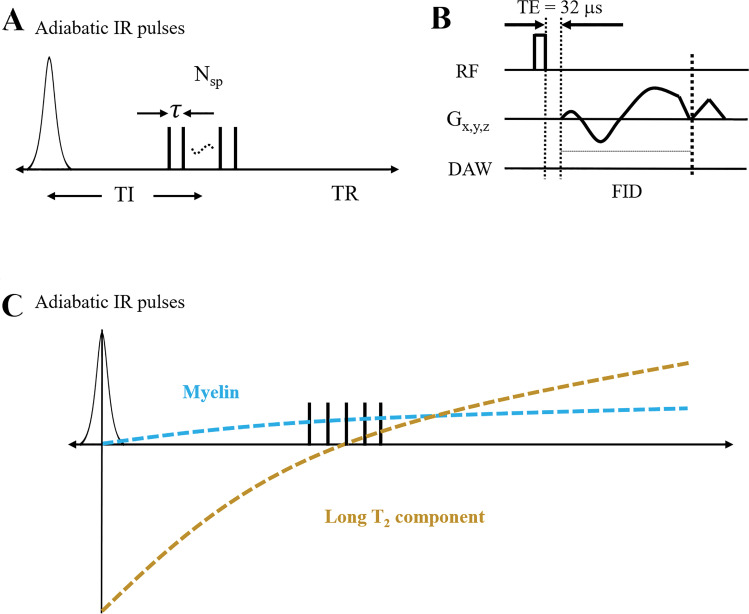
Figure 1 shows the key features of the three-dimensional STAIR-UTE sequence used in this study (25,27). Following an AFP pulse, multiple k-space spokes separated by an identical time interval τ are used for fast data acquisition. TI is defined as the time from the center of the AFP pulse to the center of the multispoke acquisition (Fig 1, A). A short rectangular pulse with a duration of 20–120 μsec is used for signal excitation. This is followed by spiral trajectories with three-dimensional conical view ordering (Fig 1, B) (27). After radiofrequency (RF) excitation with a minimal nominal echo time (TE) of 32 µsec, data sampling starts from the center of k-space. Figure 1, C, shows the contrast mechanism for myelin imaging. The longitudinal magnetization of the long T2 components of white matter is inverted by the AFP pulse and begins to recover immediately afterward. The longitudinal magnetization of the myelin protons is not inverted by the AFP pulse but is largely saturated because of its fast transverse relaxation during the relatively long pulse (16,28,29). Its longitudinal magnetization recovers from zero at the end of the AFP pulse. Pure ultrashort T2 myelin signals can be acquired at the specific TI when the long T2 components are nulled. The detailed signal equation and optimization for the STAIR-UTE sequence accommodating the variable degree of inversion and/or saturation produced by the AFP pulse as well as the use of the multispoke data acquisition is included in Appendix E1 (online).
Figure 1:
A, Diagram shows how three-dimensional short repetition time (TR) adiabatic inversion recovery (IR) (STAIR) prepared ultrashort echo time (STAIR-UTE) sequence employs an adiabatic full passage (AFP) pulse to invert the longitudinal magnetization of long T2 components, followed by equally separated (with a time interval of τ) multispoke three-dimensional UTE-cones data acquisitions. Nsp = number of spokes. B, Diagram shows how basic three-dimensional UTE-cones sequence employs a short rectangular pulse for signal excitation and three-dimensional spiral sampling following conical view ordering. DAW = data acquisition window, FID = free induction decay, Gx,y,z = gradients in x, y, and z axes, RF = radiofrequency, TE = echo time. C, Diagram shows how longitudinal magnetization (y-axis) of the long T2 component is inverted by the AFP pulse, whereas magnetization of the ultrashort T2 myelin component is not inverted but largely saturated because of its fast transverse magnetization decay during the relatively long AFP pulse. This leads to selective imaging of the myelin component around a specific inversion time where long T2 signals are very largely nulled.
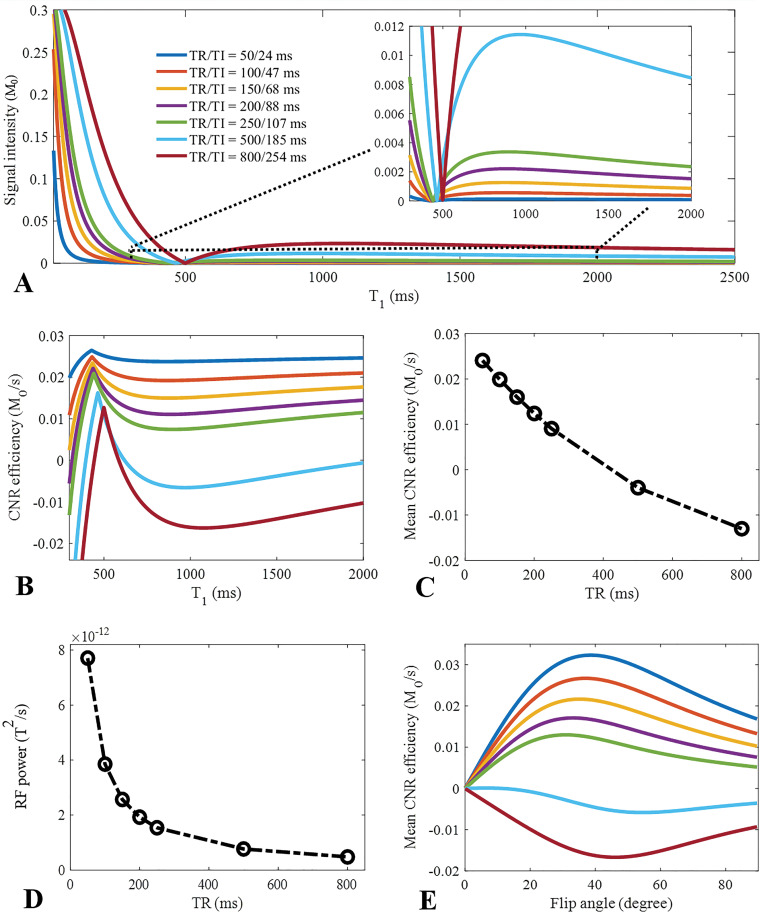
Numeric Simulation
Numeric simulation was performed to investigate the efficiency of long T2 signal suppression for the STAIR preparation with different TRs (ie, 50, 100, 150, 200, 250, 500, and 800 msec). The simulated T1s of the long T2 components ranged from 20 to 2500 msec, which covers T1 values for both white and gray matter. Flip angle α, time interval, and number of spokes were set to 20°, 5 msec, and five, respectively (25,30). Myelin water is trapped between the layers of the myelin sheath, leading to both a short T1 (ie, mean T1 around 500 msec at 3 T) and a short T2 and/or T2* (ie, mean T2* around 10 msec at 3 T) (5–7,31). In this simulation, TIs were determined by using Equation (E10) in Appendix E1 (online) to minimize the average signals from both short and long T2 water components, with T1s ranging from 250 to 1500 msec.
To evaluate the contrast and efficiency of the STAIR-UTE sequence with different TRs, contrast-to-noise ratio (CNR) efficiency, defined as the signal intensity difference between short and long T2 components normalized to TR, was used. The mean CNR efficiency was calculated by averaging the CNR efficiency for T1s between 300 and 1500 msec. The T1 of short T2 protons was set to 350 msec (32), and the proton density was assumed to be 6.5% of that of the long T2 components (12,33). To assess the SAR of the STAIR-UTE sequence, RF power was calculated by adding the squares of all RF waveforms used in the sequence and by normalizing this with the TR (34). We also investigated the CNR efficiency of myelin imaging using the STAIR-UTE sequence with excitation flip angles ranging from 0° to 90°.
Phantom Study
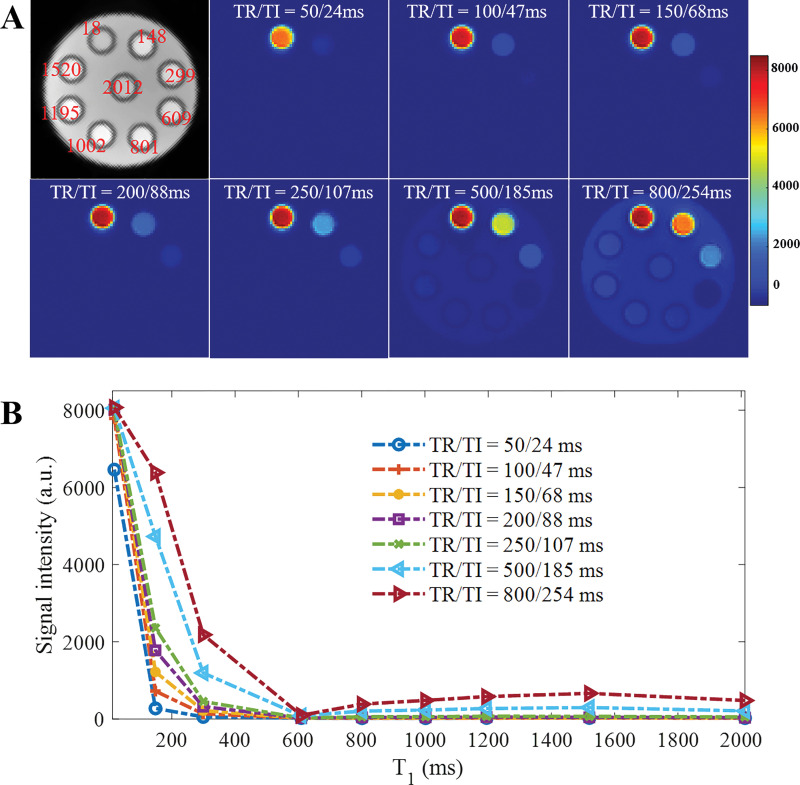
The three-dimensional STAIR-UTE sequence (Fig 1) was implemented with a 3-T clinical scanner (MR750; GE Healthcare, Milwaukee, Wis). The AFP pulse employed a hyperbolic secant function, with a duration of 8.64 msec, a spectral bandwidth of 1.15 kHz, and a maximum B1 amplitude of 12 µT (35). A hard pulse with a duration of 100 μsec was used for signal excitation. An eight-channel transmit-receive knee coil was used for both RF excitation and signal reception. The knee coil was used rather than the head coil because of its higher excitation efficiency and the consequent lower SAR. This allowed us to investigate shorter values of TR than with the head coil and to establish the efficiency of the STAIR-UTE sequence for long T2 signal suppression over a wider range of operating conditions.
To demonstrate the ability of the STAIR technique to suppress long T2 components, a series of water phantoms with different T1s was prepared. Nine 5-mL water phantoms with MnCl2˙4H2O concentrations of 0.0055, 0.01, 0.015, 0.0195, 0.0265, 0.0375, 0.085, 0.18, and 1.4828 g/L were produced. The phantoms were placed in parallel in a cylindrical container filled with 1% agarose. The T1 values of the nine phantoms with different concentrations of MnCl2∙4H2O were 2012, 1520, 1195, 1002, 801, 609, 299, 148, and 18 msec, respectively, as measured with a recently developed three-dimensional UTE variable TR method combined with actual flip angle imaging (36).
The phantoms were next scanned using the three-dimensional STAIR-UTE sequence with TRs of 50, 100, 150, 200, 250, 500, and 800 msec. Similar to the numeric simulation, the TIs necessary to minimize the average signal from long T2 components with T1 values ranging from 250 to 1500 msec were determined with Equation (E10) in Appendix E1 (online). The detailed sequence parameters of the STAIR-UTE sequence are included in the Table.
Sequence Parameters for Water Phantom, Myelin-D2O Phantom, and in Vivo Brain Studies
A myelin-D2O phantom was made by compounding myelin lipid powder (type 1 bovine brain lipid extract, B1502; Sigma-Aldrich, St Louis, Mo) with D2O in 1-mL syringes. A total of six myelin-D2O tubes with myelin concentrations of 0%, 4%, 8%, 12%, 16%, and 20% weight per volume were prepared. The myelin phantoms were imaged simultaneously in a 30-mL custom-made transmit-receive birdcage coil using the STAIR- UTE sequence. The T2* of the phantoms was assessed using data acquired with a three-dimensional UTE sequence as described in the Table.
In Vivo Brain Study
Healthy volunteers and patients with MS underwent 3-T MRI between November 2018 and October 2019. A 12-channel receive-only head coil was used for signal reception. A rubber phantom with an ultrashort T2 of around 0.3 msec was placed between each participant’s head and the head coil to serve as a reference to calibrate the proton density of ultrashort T2 components (11). A UTE measure of myelin proton density, ρm, was calculated using the following equation:
 |
where Im and Iref are the signal intensities of myelin and rubber, respectively, and Mz,m and Mz,ref are the magnetizations of myelin and rubber determined from Equation (E8) in Appendix E1 (online). To measure the proton density of the rubber phantom, ρref, the rubber and a MnCl2˙4H2O-doped H2O-D2O phantom (with a proton fraction of 20% and T2 of approximately 0.3 msec) were scanned together using a STAIR -UTE protocol. The proton density of the rubber calculated from Equation (1) was around 30 mol/L (12).
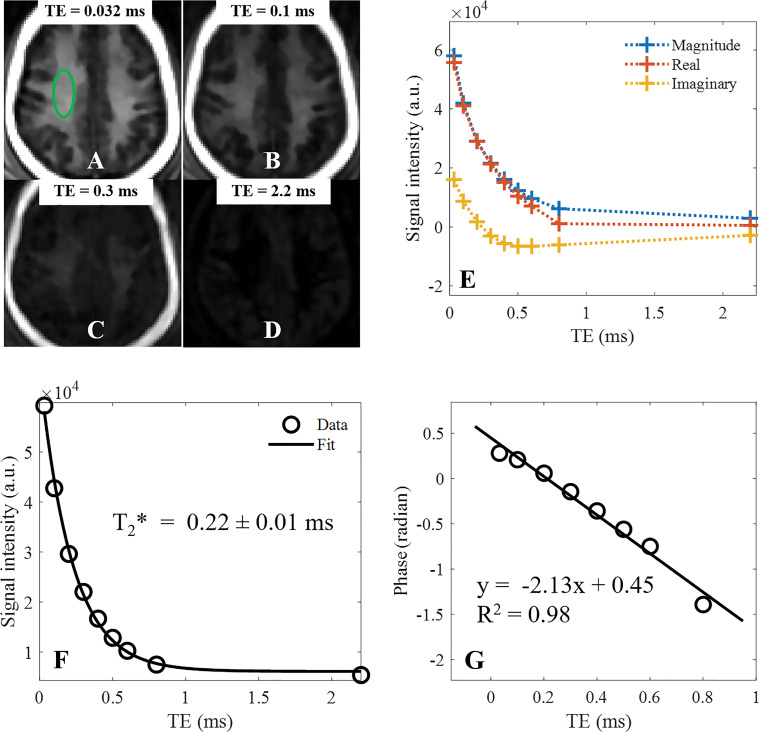
Both STAIR-UTE and clinical T2-weighted fluid-attenuated inversion recovery (T2-FLAIR) sequences were performed for all the participants. To optimize the sequence parameters for the STAIR-UTE sequence, trade-offs between spatial resolution, scanning time, SAR, and signal-to-noise ratio (SNR) were considered. The shortest TR within SAR limitations was used for STAIR-UTE imaging. The spatial resolution was chosen to achieve an image SNR of at least 30 with a scanning time of 10 minutes or less. In addition, eight separate STAIR-UTE scans with TEs of 0.032/2.2, 0.1, 0.2, 0.3, 0.4, 0.5, 0.6, and 0.8 msec were employed to measure T2* and the chemical shift of the myelin signal in one of the volunteer examinations. The total scanning time for this was 56 minutes. The measured T2* was used to indirectly evaluate the efficiency of long T2 suppression with the three-dimensional STAIR-UTE sequence. Detailed parameters of these sequences are given in the Table. The same volunteer was also scanned with a regular dual-echo UTE sequence without an inversion pulse preparation at the same examination to generate a B0 field map with a TE of 0.032/2.2 msec. This was to correct the background field for the myelin chemical shift measurement.
Data Analysis
Nonlinear minimization of Equation (E10) in Appendix E1 (online) was performed using a trust region reflective algorithm (37). To assess the efficiency of long T2 signal suppression with the STAIR-UTE sequence, T2* values in whole-brain white matter and in eight brain regions within white matter (ie, left and right centrum semiovale, subcortical white matter and periventricular regions, and splenium and genu of the corpus callosum) were calculated using data from a healthy volunteer. T2* values were estimated using a single exponential fitting. Least-squares regression was performed on the myelin phantom data to determine the relationship between STAIR-UTE signal intensity and myelin concentration.
Both the STAIR-UTE and T2-FLAIR signals were normalized with respect to their receiver gains during image reconstruction. Before in vivo data measurement, background noise was estimated then subtracted from the STAIR-UTE signals (38). The standard deviation of background noise was estimated from a carefully selected 5 × 5 × 3 noise cube (avoiding regions with artifacts) on the three-dimensional STAIR-UTE images of each participant. Two experienced radiologists (Z.C. and Y.X.), both with more than 10 years of experience in MRI diagnosis, independently measured the MS lesion signal intensities on both the STAIR-UTE and the T2-FLAIR images. Additionally, signals in both normal-appearing white matter (NAWM) in patients with MS and normal white matter (NWM) in volunteers (eight regions, including the left and right centrum semiovale, subcortical white matter and periventricular regions, and splenium and genu of the corpus callosum) were measured.
Statistical Analysis
A two-way random-effects model was used to calculate the intraclass correlation between the two radiologists to evaluate the reproducibility of their measurements (39). The relationship between the STAIR-UTE and T2-FLAIR signals in each structure was examined separately. For each structure, correlations between the STAIR-UTE and T2-FLAIR signals were calculated using a nonparametric bootstrap technique with resampling by participant to adjust for within-participant dependence. Bootstrap-based bias-corrected accelerated confidence intervals and bootstrap-based P values were computed for each correlation coefficient. Analysis of variance was performed to evaluate the signal difference between lesions and relatively NWM (including NAWM in participants with MS and NWM in healthy volunteers), as well as between NAWM in participants with MS and NWM in healthy volunteers. Bonferroni correction was performed after the analysis of variance test. All analysis algorithms were written with Matlab software (version 2018b; MathWorks, Natick, Mass) and executed offline.
Results
Simulation Results
Numeric simulation results of STAIR-UTE imaging with different TRs (ie, 50, 100, 150, 200, 250, 500, and 800 msec) are shown in Figure 2. As seen in Figure 2, A, the calculated signal intensity takes the form of a notch T1 tissue property filter with a linear x-axis (40). A broad range of signals with different T1s was effectively suppressed when a TR of less than 250 msec was used. Better signal suppression (ie, lower signal intensities over a broader range of T1s) was achieved with shorter TRs. Higher CNR efficiency over a broad range of T1s (ie, from 300 to 1500 msec) was achieved when shorter TRs were used (Fig 2, B). With a relatively long TR (eg, 800 msec), reasonable signal suppression or high CNR efficiency was only achieved over a limited range of T1s. In this situation, the signal suppression for different T1s was highly dependent on the value of TI. As seen in Figure 2, C, signal suppression increased with shorter TRs. However, RF power substantially increased when shorter TRs were used (Fig 2, D), constraining use of the STAIR-UTE sequence. Mean CNR efficiency also changed with excitation flip angle, as demonstrated in Figure 2, E. Higher CNR efficiency was achieved using flip angles between 20° and 40° with a TR less than 250 msec. Furthermore, to investigate the signal suppression of the myelin water component when using STAIR-UTE, we assumed that myelin water has a T1 of 500 msec and that nonwater myelin protons have half the proton density of myelin water. The simulation demonstrated that the measured signal ratios between the nonwater myelin protons and myelin water were decreased from 154 to 29 when TRs were increased from 50 to 250 msec with STAIR-UTE. Thus, myelin water signals were significantly suppressed with the STAIR-UTE sequence.
Figure 2:
Graphs show numeric simulation used to investigate the effect of repetition time (TR) and flip angle on suppression of, A, long T2 signals; B, C, and E, contrast-to-noise (CNR) efficiency; and, D, specific absorption ratio performance in short TR adiabatic inversion recovery (STAIR) prepared ultrashort echo time (STAIR-UTE) imaging. For the investigation of the signal suppression performance with different TRs (ie, 50, 100, 150, 200, 250, 500, and 800 msec), a wide range of T1s (ie, 20–2500 msec) are shown imaged with STAIR-UTE (A). Better visual comparison is shown in the zoomed curves in A, with T1s ranging from 300 to 2000 msec. CNR efficiency is defined by signal intensity difference between short and long T2 components normalized to TR. Higher CNR efficiency and mean CNR efficiency (calculated by averaging CNR efficiency of T1s between 300 and 1500 msec) are achieved when a shorter TR is used with STAIR-UTE sequence (B and C). Radiofrequency (RF) power is calculated by the sum of the squares of RF waveforms used in the sequence normalized to TR. This increases significantly with a shorter TR (D). Mean CNR efficiency also changes with excitation flip angle (E). Higher mean CNR efficiency is achieved when a flip angle between 20° and 40° are used with a TR less than 250 msec. B and E use the same color coding for TR-T1 combinations as in A.
Phantom Results
The mean T2* of the six myelin-D2O phantoms was 0.25 msec ± 0.03 (standard deviation). Figure E2 (online) shows the strong correlation between STAIR-UTE signal intensity and myelin concentration for these six phantoms (R2 = 0.98).
Figure 3 shows the results of the STAIR-UTE imaging with different TRs (ie, 50, 100, 150, 200, 250, 500, and 800 msec) in the phantom study. Similar to the simulation results shown in Figure 2, A, when the TR was less than 250 msec, signals in phantoms with T1s greater than 299 msec were almost completely suppressed. When a long TR of 800 msec was used, signals in most phantoms and the agarose gel appeared bright on STAIR-UTE images. Figure 3, B, shows signal intensities for all phantoms with different TRs. Better signal suppression was achieved with shorter TRs, which was similar to results from the simulation study (Fig 2, A).
Figure 3:
Results of phantom study to investigate signal suppression of long T2 signal with three-dimensional short repetition time (TR) adiabatic inversion recovery–prepared ultrashort echo time imaging using different TRs (ie, 50, 100, 150, 200, 250, 500 and 800 msec). A, Nine 5-mL water phantoms were prepared with MnCl2˙4H2O concentrations of 0.0055, 0.01, 0.015, 0.0195, 0.0265, 0.0375, 0.085, 0.18, and 1.4828 g/L. Phantoms were then placed in parallel in a cylinder container filled with 1% agarose. Corresponding T1s of these nine phantoms were 2012, 1520, 1195, 1002, 801, 609, 299, 148, and 18 msec, respectively. B, Graph shows signal intensity curves measured in all phantoms with different TR and inversion time (TI) combinations. a.u. = arbitrary units.
In Vivo Brain Results
Ten healthy volunteers (mean age, 33 years ± 8; six women) and 10 participants with MS (mean age, 51 years ± 16; seven women) were evaluated.
Figure 4 shows STAIR-UTE images of the brain of a healthy volunteer (29-year-old woman). The STAIR-UTE image with a TE of 32 μsec shows high signal intensity in white matter. The signal intensity drops quickly as TE is increased. The background B0 field inhomogeneity was removed by phase subtraction of the B0 field map generated from the dual-echo UTE data obtained from the STAIR-UTE image. Figure 4, E, shows the magnitude, real, and imaginary components of the mean signal intensities inside a green elliptical region of interest at different TEs. Exponential fitting of the magnitude signal intensities obtained with the different TEs showed a short T2* of 0.22 ± 0.01 msec in this region (Fig 4, F). In addition, the mean T2* of whole brain white matter was 0.21 msec ± 0.04. Mean T2* values in eight white matter regions (left and right centrum semiovale, subcortical white matter, periventricular regions, and splenium and genu of the corpus callosum) were 0.22 msec ± 0.02, 0.24 msec ± 0.03, 0.23 msec ± 0.03, 0.21 msec ± 0.03, 0.23 msec ± 0.03, 0.24 msec ± 0.02, 0.23 msec ± 0.02, and 0.25 msec ± 0.02, respectively. All T2* values were ultrashort (around 0.2–0.25 msec), demonstrating the efficiency of long T2 signal suppression with the STAIR-UTE sequence. Long T2 water signal contamination would have increased the T2* values. Figure 4, G, shows linear fitting of the phase signal intensities with R2 = 0.98. The phase data at a TE of 2.2 msec was not included in this fitting because of complete loss of signal from the ultrashort T2 myelin components. The frequency shift of the detected myelin signals relative to the long T2 water components was calculated from the slope of the fitting line, which gave a shift of approximately –340 Hz or –2.7 ppm. Representative whole-brain myelin images acquired with the three-dimensional STAIR-UTE sequence from the same volunteer are shown in Figure 5. The pads around the head both show high signal intensity on the STAIR-UTE images, demonstrating detection of short or ultrashort T2 material using the STAIR-UTE sequence. Signal intensity is not seen from these pads with conventional sequences.
Figure 4:
Representative short repetition time (TR) adiabatic inversion recovery–prepared ultrashort echo time brain images (TR = 140 msec) in a healthy volunteer (29-year-old woman) with echo times (TEs) of, A, 0.032 msec, B, 0.1 msec, C, 0.3 msec, and, D, 2.2 msec. The magnitude and real and imaginary parts of mean signal intensity with different TEs of a region of interest (green oval in A) are plotted in E. F, Exponential fitting of magnitude signals at different TEs shows a short T2* of 0.22 ± 0.01 msec in this region of white matter. G, Linear fitting of phase signals with TE (R2 = 0.98) clearly demonstrates a chemical shift of detected myelin signals. a.u. = arbitrary units.
Figure 5:
Representative three-dimensional short repetition time (TR) adiabatic inversion recovery prepared ultrashort echo time images in same volunteer as in Figure 4 with TR of 140 msec and inversion time of 61 msec demonstrate volumetric myelin imaging of whole brain.
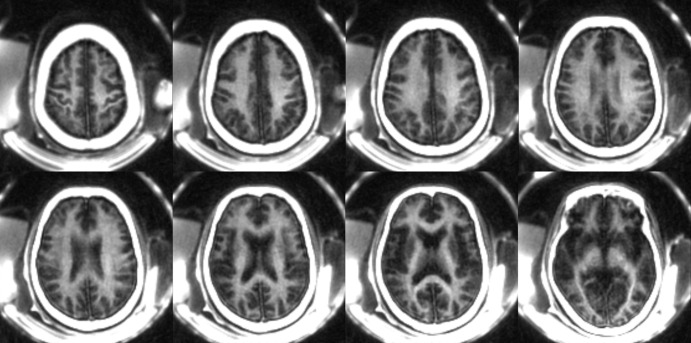
Figure 6 shows comparisons of STAIR-UTE and clinical T2-FLAIR images in three representative participants with MS. Hyperintense lesions detected on T2-FLAIR images show signal intensity loss on the corresponding STAIR-UTE images, attributable to demyelination in the latter case.
Figure 6:
A–E, Selective T2-weighted fluid-attenuated inversion recovery (FLAIR) (repetition time [TR] msec/echo time msec, 7600/117), and, F–J, short TR adiabatic inversion recovery (STAIR) prepared ultrashort echo time (UTE) (TR = 140 msec) images in three representative participants with multiple sclerosis (MS). A, B, F, and G were obtained in a 35-year-old man; C, D, H, and I were obtained in a 69-year-old woman; E and J were obtained in a 48-year-old man. Arrows indicate MS lesions. Hyperintense lesions detected on T2-FLAIR images show signal loss on corresponding STAIR-UTE images, consistent with demyelination.
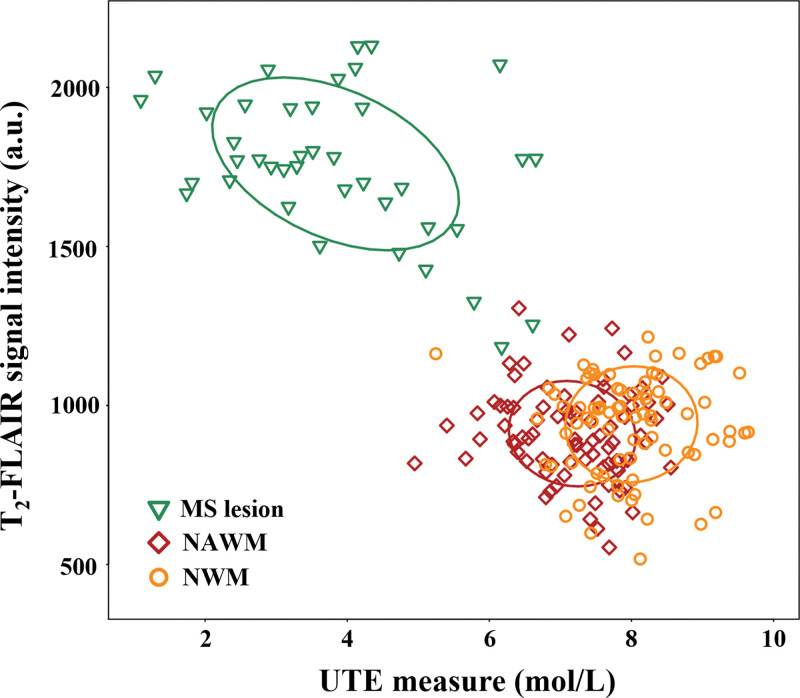
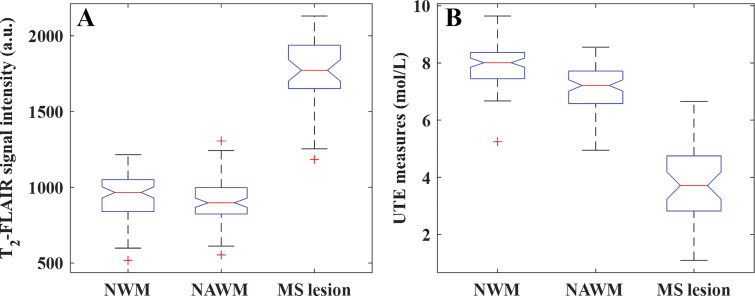
Figure E3 (online) shows morphologic images with corresponding STAIR-UTE signal measure maps (calculated from Equation [1]) in a healthy volunteer (36-year-old man). Both the T2-FLAIR and STAIR-UTE measurements showed very good reproducibility between the two radiologists, with intraclass correlation coefficients of 0.98 and 0.96, respectively. As seen in Figure 7, a moderate correlation existed between STAIR-UTE and T2-FLAIR measurements in the MS lesions (r = –0.43, P = .04). The correlations between the two measurements found with the two sequences for NAWM and NWM were low and did not achieve a statistically significant difference (NAWM: r = –0.09, P = .14; NWM: r = 0.04, P = .84). Both STAIR-UTE and T2-FLAIR sequences showed significant differences between lesions (STAIR-UTE–measured ρm: 3.8 mol/L ± 1.5; T2-FLAIR signal intensity: 1760 ± 231) and NAWM (STAIR-UTE–measured ρm: 7.2 mol/L ± 0.8; T2-FLAIR signal intensity: 911 ± 141) in participants with MS (P < .001 in each comparison) (Fig 8). Significant differences were also observed between the lesions in participants with MS and NWM in healthy volunteers (STAIR-UTE–measured ρm: 8.0 mol/L ± 0.8; T2-FLAIR signal intensity: 941 ± 155) (P < .001 for each comparison). This demonstrated the clinical potential of the STAIR-UTE sequences for helping detect and assess MS lesions quantitatively. In addition, the STAIR-UTE–measured ρm showed a significant difference between NWM in healthy volunteers (mean, 8.0 mol/L ± 0.8) and NAWM in participants with MS (mean, 7.2 mol/L ± 0.8; P < .001) (Fig 8, B). No significant difference was found among measured T2-FLAIR signal intensities between these two groups (NWM: 941 ± 155, NAWM: 911 ± 141; P = .82) (Fig 8, A).
Figure 7:
Image shows correlation of T2 fluid-attenuated inversion recovery (T2-FLAIR) and short repetition time adiabatic inversion recovery–prepared ultrashort echo time (UTE) measurements in multiple sclerosis (MS) lesions and normal-appearing white matter (NAWM) in 10 participants with MS and signal intensities in normal white matter (NWM) in 10 healthy volunteers. A moderate correlation exists between T2-FLAIR and UTE measurements in the MS lesions (r = –0.43; P = .04). Low correlation exists between the two measurements for NAWM and NWM. These did not reach a statistically significant difference (NAWM: r = –0.09, P = .14; NWM: r = 0.04, P = .84). a.u. = arbitrary units.
Figure 8:
Boxplots show results of statistical analysis for measurements of normal white matter (NWM), normal-appearing white matter (NAWM), and multiple sclerosis (MS) lesions with, A, T2-weighted fluid-attenuated inversion recovery (T2-FLAIR) and, B, short repetition time adiabatic inversion recovery (STAIR) prepared ultrashort echo time (STAIR-UTE) measures. Both the T2-FLAIR and STAIR-UTE sequences show signal intensity differences in MS lesions compared with NWM in healthy volunteers and NAWM in participants with MS (P < .001). The STAIR-UTE measurements show a significant difference between NWM in healthy volunteers and NAWM in participants with MS (P < .001), which is not observed in corresponding T2-FLAIR data (P = .82). The red line in each boxplot indicates the median. The bottom and top edges of each box indicate the 25th and 75th percentiles, respectively. + = outliers. a.u. = arbitrary units.
Discussion
In this study, we demonstrated that the short repetition time (TR) adiabatic inversion recovery (STAIR) prepared ultrashort echo time (UTE) sequence can help effectively suppress signals from long T2 components and thereby allow selective imaging of myelin in white matter. Numeric simulation showed that signal suppression for long T2 tissues with different T1s using a conventional inversion recovery scheme with a relatively long TR (eg, TR = 800 msec) is sensitive to the selection of inversion time (TI). With this approach, regional or disease-related variation in the T1s of long T2 components may lead to significant water signal contamination, with loss of myelin contrast and inaccurate quantitation. In comparison, the STAIR preparation effectively suppressed long T2 signals from tissues with a broad range of T1s using a single TR-TI combination. The phantom study also demonstrated the ability of the sequence to efficiently suppress long T2 signals with a wide range of T1s. The volunteer study demonstrated the efficiency of the three-dimensional STAIR-UTE sequence for suppressing long T2 signals in white matter and for allowing selective imaging of ultrashort T2 protons, as confirmed by their measured T2* of 0.21 msec ± 0.04 in whole-brain white matter, which is very close to the T2* of approximately 0.25 msec found in lyophilized purified myelin-D2O phantoms. Furthermore, participants with multiple sclerosis (MS) had lower STAIR-UTE–measured ρm in MS lesions (3.8 mol/L ± 1.5) compared with those in normal white matter (NWM) of healthy volunteers (8.0 mol/L ± 0.8). To our knowledge, this is the first study to show myelin proton density differences between NWM of healthy volunteers (8.0 mol/L ± 0.8) and normal-appearing white matter of participants with MS (7.2 mol/L ± 0.8) using UTE-based sequences when these were not apparent with conventional T2 fluid-attenuated inversion recovery sequences.
Most myelin-targeted MRI techniques are based on indirect assessment of myelin protons through detection of signal from water (4–6,41). The IR-UTE sequence enables images of myelin (11,12,17,24). At the optimal TI, it suppresses the long T2 water components in white matter. With UTE data acquired at this nulling point combined with dual-echo subtraction, myelin in white matter shows high contrast (12,17). However, the optimal TI depends on the T1 of the long T2 components, making the IR-UTE technique difficult for clinical use since T1 values may vary in different patients with MS (19,20). A patient-specific TI would therefore be necessary for effective long T2 suppression and would require robust T1 measurement, which is technically challenging and time consuming. To make the situation worse, significant T1 variations exist in white matter within the normal brain (5,21,22). Therefore, a single TI may not suppress all long T2 components. The STAIR-UTE sequence with a short TR-TI combination provides efficient suppression of long T2 components over a wide range of T1s and yields a high CNR efficiency. However, as shown in the simulation study, SAR is significantly increased when short TRs are used. In our in vivo study, a TR of 140 msec was used to balance effectiveness of long T2 suppression with SAR limitations. A relatively high flip angle of 32° with the shortest RF duration (limited by the peak power of the RF amplifier) was used for signal excitation to improve SNR performance, as suggested by the simulation. Oversampling (ie, acquiring more densely distributed cones than is necessary for the targeted resolution) of k-space data by a factor of 2.3 also helped improve SNR and reduce potential signal contamination from tissues outside of the imaging field of view due to the use of a short rectangular RF pulse for signal excitation.
Studies have shown that multiple water compartments exist in white matter with different MRI properties (4,5). T2 signal suppression must include the relatively short T1 myelin water (mean T1 around 500 msec at 3 T). The TI calculated from Equation (E10) in Appendix E1 (online) suppresses T2 components with T1s ranging from 250 msec to 1500 msec when applied to the in vivo study (5–7). Because myelin water has a relatively short T2* of around 10 msec, the inversion efficiency factor Q may not reach –1 (6,31). To compensate for this imperfect inversion and to achieve sufficient suppression of myelin water, a TI 1–2 msec shorter than that derived from the optimization was used for our in vivo study. Our results suggest that a TI of 61 msec with a TR of 140 msec provides effective long T2 signal suppression in white matter of the brain, as evidenced by the measured short T2* of 0.22 ± 0.01 msec for myelin. Very little residual gray matter signal is shown in Figure 4, D, consistent with effective long T2 signal suppression in a tissue with a longer T1 than white matter.
Long T2 signal contamination is a major source of error in quantitative UTE imaging (29). Because the STAIR-UTE sequence provides selective imaging of myelin protons, it could also be used for quantitative evaluation of myelin proton density as well as its T1 and T2* (32). In our study, the STAIR-UTE acquisition, together with a reference rubber phantom, provided volumetric UTE assessment of myelin proton density, which is likely to be a useful marker for evaluating demyelination and remyelination. We found reduced UTE measurements of NAWM in participants with MS compared with those of NWM in healthy volunteers. Other researchers also found a reduced magnetization transfer ratio in NAWM compared with that of NWM (42,43), suggesting loss of myelin in NAWM. A more recent quantitative synthetic MRI study also demonstrated myelin loss in NAWM in patients with cognitive impairment (44). A comparison between STAIR-UTE imaging and other quantitative MRI measurements may provide useful information for understanding the specific contribution to these differences arising from myelin loss.
In our study, the frequency shift of myelin signals relative to long T2 water components was around –2.7 ppm, which corresponds to an absolute chemical shift of around –2 ppm. This shift compares with previously reported measurements of between –2.5 ppm and –3.4 ppm (33). These chemical shifts are near those of methylene protons of alkyl chains, which are the main components of myelin (11). As a result of the chemical shift, STAIR-UTE images undergo off-resonance excitation for myelin species, which induces blurring. This blurring could be eliminated by shifting the excitation frequency to the on-resonance frequency of myelin species or by compensating for the chemical shift in the image reconstruction.
Recently, a double-echo sliding inversion recovery (DESIRE) UTE (DESIRE-UTE) sequence has been developed to suppress long T2 water components and to selectively image myelin (14). Both the proposed STAIR-UTE and DESIRE-UTE techniques are based on IR-UTE sequences. Using different mechanisms, they both suppress signal from long T2 components with a wide range of T1s in the brain. The DESIRE-UTE sequence uses a much longer TR (eg, TR = 1000 msec) than the STAIR-UTE sequence (eg, TR = 140 msec). As a result, finetuning of TI is necessary with DESIRE-UTE imaging to effectively suppress the signal from water components. To achieve this, the DESIRE-UTE technique uses a sliding window method to provide a wide range of TIs and to allow choice of particular TIs to null signal from the long T2 components with different T1s. Compared with the STAIR-UTE sequence, the DESIRE-UTE sequence is more flexible in selection of imaging parameters such as TR and image resolution, and the SNR is expected to be higher because a longer TR is used. A major advantage of the STAIR-UTE sequence is its simplicity. The sequence parameters are determined by means of numeric optimization and can be used in all patients. In addition, the STAIR-UTE sequence does not need complicated off-line image reconstruction as the DESIRE-UTE sequence does. As a result, the STAIR-UTE sequence is easier to translate into clinical practice, such as for multicenter multivendor studies. The echo subtraction method used in DESIRE-UTE imaging can also be implemented in STAIR-UTE imaging to improve water suppression, especially in gray matter where residual water signals may exist due to the limitation in TR reduction with STAIR-UTE imaging arising from SAR considerations. A second echo image also provides a method for monitoring the effectiveness of the long T2 signal suppression. This should be zero with effective long T2 signal suppression coupled with rapid decay of the ultrashort T2 myelin signal.
Although the STAIR-UTE technique can provide efficient water signal suppression and selective myelin imaging, generating high spatial resolution myelin images using current clinical MRI systems has technical limitations. First, myelin proton density is low, leading to a low myelin signal (eg, <10% of the total UTE signal in the brain). To address this, a relatively low spatial resolution (voxel size = 1.57 × 1.57 × 5 mm3, cuboid shape) was used in our study to improve the image SNR. Second, the fast T2 decay of myelin signal and the limited gradient performance of clinical MRI systems lead to image blurring. The full width at half maximum of the point spread function of the sequence used in our study is approximately 3, which means that the true image resolution is decreased to one-third of the nominal spatial resolution. The low spatial resolution together with substantial spatial blurring lead to a loss of fine detail in white matter of the brain. A dedicated RF coil with a higher B1 (thus more efficiency in exciting myelin with its extremely short T2), and parallel transmission (more localized excitation and lower SAR), together with higher coil sensitivity, are likely to help STAIR-UTE imaging of myelin and to lead to higher spatial resolution and SNR. A high-performance gradient system with a stronger gradient amplitude and a higher slew rate could also improve spatial resolution by shortening the sampling window and thus reducing blurring.
Our study had another limitation. The 9.5-minute scanning time for the in vivo study is relatively long for clinical use. Advanced fast imaging techniques, such as compressed sensing or deep learning, could be used to accelerate the scan (45,46). More spokes in each TR would also help reduce the scanning time. However, imaging with a larger number of spokes may lead to artifacts due to signal variation among the different spokes during a single TR. A design with a variable flip angle scheme could be used to reduce this signal variation and to reduce the scanning time without introducing substantial artifacts. The total number of spokes and the variable flip angle scheme are also constrained by the need to minimize water signal contamination. In our study, we kept the number of spokes low (ie, 5) to generate relatively high-quality images with negligible artifact or water signal contamination.
In conclusion, the new three-dimensional short repetition time adiabatic inversion recovery (STAIR) prepared ultrashort echo time (UTE) sequence with a single short TR–inversion time combination provides efficient suppression of long T2 components in white matter over a wide range of T1s and allows selective in vivo volumetric imaging of myelin in white matter of the brain. STAIR-UTE measures of myelin content may be a useful marker for evaluating demyelination and remyelination, including that occurring during remyelination-enhancing therapy in patients with multiple sclerosis.
APPENDIX
SUPPLEMENTAL FIGURES
Supported by the National Institutes of Health (1R01 NS092650 and 1R21 AR075851), VA Clinical Science and Rehabilitation Research and Development Service (Merit Awards I01CX001388 and I01RX002604), and GE Healthcare.
Disclosures of Conflicts of Interest: Y.M. Activities related to the present article: institution received a grant from the National Institutes of Health. Activities not related to the present article: disclosed no relevant relationships. Other relationships: disclosed no relevant relationships. H.J. disclosed no relevant relationships. Z.W. disclosed no relevant relationships. Z.C. disclosed no relevant relationships. Y.X. disclosed no relevant relationships. R.L.R. disclosed no relevant relationships. E.Y.C. disclosed no relevant relationships. G.M.B. disclosed no relevant relationships. J.C.B. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: has received payment for lectures, including service on speakers bureaus, from Teva Pharmaceuticals and Serono. Other relationships: disclosed no relevant relationships. J.D. Activities related to the present article: institution received a grant from the National Institutes of Health. Activities not related to the present article: disclosed no relevant relationships. Other relationships: disclosed no relevant relationships.
Abbreviations:
- AFP
- adiabatic full passage
- CNR
- contrast-to-noise ratio
- FLAIR
- fluid-attenuated IR
- IR
- inversion recovery
- MS
- multiple sclerosis
- NAWM
- normal-appearing white matter
- NWM
- normal white matter
- RF
- radiofrequency
- SAR
- specific absorption ratio
- SNR
- signal-to-noise ratio
- STAIR
- short repetition time adiabatic inversion recovery
- TE
- echo time
- TI
- inversion time
- TR
- repetition time
- UTE
- ultrashort TE
References
- 1.Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG. Multiple sclerosis. N Engl J Med 2000;343(13):938–952. [DOI] [PubMed] [Google Scholar]
- 2.Filippi M, Rocca MA. MR imaging of multiple sclerosis. Radiology 2011;259(3):659–681. [DOI] [PubMed] [Google Scholar]
- 3.Brück W, Bitsch A, Kolenda H, Brück Y, Stiefel M, Lassmann H. Inflammatory central nervous system demyelination: correlation of magnetic resonance imaging findings with lesion pathology. Ann Neurol 1997;42(5):783–793. [DOI] [PubMed] [Google Scholar]
- 4.Alonso-Ortiz E, Levesque IR, Pike GB. MRI-based myelin water imaging: A technical review. Magn Reson Med 2015;73(1):70–81. [DOI] [PubMed] [Google Scholar]
- 5.Deoni SCL, Rutt BK, Arun T, Pierpaoli C, Jones DK. Gleaning multicomponent T1 and T2 information from steady-state imaging data. Magn Reson Med 2008;60(6):1372–1387. [DOI] [PubMed] [Google Scholar]
- 6.Oh SH, Bilello M, Schindler M, Markowitz CE, Detre JA, Lee J. Direct visualization of short transverse relaxation time component (ViSTa). Neuroimage 2013;83:485–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bouhrara M, Spencer RG. Rapid simultaneous high-resolution mapping of myelin water fraction and relaxation times in human brain using BMC-mcDESPOT. Neuroimage 2017;147:800–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horch RA, Gore JC, Does MD. Origins of the ultrashort-T2 1H NMR signals in myelinated nerve: a direct measure of myelin content? Magn Reson Med 2011;66(1):24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robson MD, Gatehouse PD, Bydder M, Bydder GM. Magnetic resonance: an introduction to ultrashort TE (UTE) imaging. J Comput Assist Tomogr 2003;27(6):825–846. [DOI] [PubMed] [Google Scholar]
- 10.Waldman A, Rees JH, Brock CS, Robson MD, Gatehouse PD, Bydder GM. MRI of the brain with ultra-short echo-time pulse sequences. Neuroradiology 2003;45(12):887–892. [DOI] [PubMed] [Google Scholar]
- 11.Wilhelm MJ, Ong HH, Wehrli SL, et al. Direct magnetic resonance detection of myelin and prospects for quantitative imaging of myelin density. Proc Natl Acad Sci U S A 2012;109(24):9605–9610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Du J, Ma G, Li S, et al. Ultrashort echo time (UTE) magnetic resonance imaging of the short T2 components in white matter of the brain using a clinical 3T scanner. Neuroimage 2014;87(Supplement C):32–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fan SJ, Ma Y, Chang EY, Bydder GM, Du J. Inversion recovery ultrashort echo time imaging of ultrashort T2 tissue components in ovine brain at 3 T: a sequential D2 O exchange study. NMR Biomed 2017;30(10):e3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma YJ, Searleman AC, Jang H, et al. Whole-Brain Myelin Imaging Using 3D Double-Echo Sliding Inversion Recovery Ultrashort Echo Time (DESIRE UTE) MRI. Radiology 2020;294(2):362–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fan SJ, Ma Y, Zhu Y, et al. Yet more evidence that myelin protons can be directly imaged with UTE sequences on a clinical 3T scanner: Bicomponent T2* analysis of native and deuterated ovine brain specimens. Magn Reson Med 2018;80(2):538–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Larson PEZ, Conolly SM, Pauly JM, Nishimura DG. Using adiabatic inversion pulses for long-T2 suppression in ultrashort echo time (UTE) imaging. Magn Reson Med 2007;58(5):952–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sheth V, Shao H, Chen J, et al. Magnetic resonance imaging of myelin using ultrashort Echo time (UTE) pulse sequences: Phantom, specimen, volunteer and multiple sclerosis patient studies. Neuroimage 2016;136:37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li S, Ma L, Chang EY, et al. Effects of inversion time on inversion recovery prepared ultrashort echo time (IR-UTE) imaging of bound and pore water in cortical bone. NMR Biomed 2015;28(1):70–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suzuki S, Sakai O, Jara H. Combined volumetric T1, T2 and secular-T2 quantitative MRI of the brain: age-related global changes (preliminary results). Magn Reson Imaging 2006;24(7):877–887. [DOI] [PubMed] [Google Scholar]
- 20.Cheng HL, Stikov N, Ghugre NR, Wright GA. Practical medical applications of quantitative MR relaxometry. J Magn Reson Imaging 2012;36(4):805–824. [DOI] [PubMed] [Google Scholar]
- 21.Stikov N, Boudreau M, Levesque IR, Tardif CL, Barral JK, Pike GB. On the accuracy of T1 mapping: searching for common ground. Magn Reson Med 2015;73(2):514–522. [DOI] [PubMed] [Google Scholar]
- 22.Rooney WD, Johnson G, Li X, et al. Magnetic field and tissue dependencies of human brain longitudinal 1H2O relaxation in vivo. Magn Reson Med 2007;57(2):308–318. [DOI] [PubMed] [Google Scholar]
- 23.Tannús A, Garwood M. Adiabatic pulses. NMR Biomed 1997;10(8):423–434. [DOI] [PubMed] [Google Scholar]
- 24.Seifert AC, Li C, Wilhelm MJ, Wehrli SL, Wehrli FW. Towards quantification of myelin by solid-state MRI of the lipid matrix protons. Neuroimage 2017;163:358–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carl M, Bydder GM, Du J. UTE imaging with simultaneous water and fat signal suppression using a time-efficient multispoke inversion recovery pulse sequence. Magn Reson Med 2016;76(2):577–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma YJ, Zhu Y, Lu X, Carl M, Chang EY, Du J. Short T2 imaging using a 3D double adiabatic inversion recovery prepared ultrashort echo time cones (3D DIR-UTE-Cones) sequence. Magn Reson Med 2018;79(5):2555–2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gurney PT, Hargreaves BA, Nishimura DG. Design and analysis of a practical 3D cones trajectory. Magn Reson Med 2006;55(3):575–582. [DOI] [PubMed] [Google Scholar]
- 28.Horch RA, Gochberg DF, Nyman JS, Does MD. Clinically compatible MRI strategies for discriminating bound and pore water in cortical bone. Magn Reson Med 2012;68(6):1774–1784 10.1002/mrm.24186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Du J, Bydder GM. Qualitative and quantitative ultrashort-TE MRI of cortical bone. NMR Biomed 2013;26(5):489–506 10.1002/nbm.2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ma YJ, Chen Y, Li L, et al. Trabecular bone imaging using a 3D adiabatic inversion recovery prepared ultrashort TE Cones sequence at 3T. Magn Reson Med 2020;83(5):1640–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hwang D, Kim DH, Du YP. In vivo multi-slice mapping of myelin water content using T2* decay. Neuroimage 2010;52(1):198–204. [DOI] [PubMed] [Google Scholar]
- 32.Du J, Sheth V, He Q, et al. Measurement of T1 of the ultrashort T2* components in white matter of the brain at 3T. PLoS One 2014;9(8):e103296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boucneau T, Cao P, Tang S, et al. In vivo characterization of brain ultrashort-T2 components. Magn Reson Med 2018;80(2):726–735 10.1002/mrm.27037.Pubm [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eichner C, Wald LL, Setsompop K. A low power radiofrequency pulse for simultaneous multislice excitation and refocusing. Magn Reson Med 2014;72(4):949–958. [DOI] [PubMed] [Google Scholar]
- 35.Silver MS, Joseph RI, Hoult DI. Highly selective π2 and π pulse generation. J Magn Reason (1969) 1984;59(2):347–351. [Google Scholar]
- 36.Ma YJ, Lu X, Carl M, et al. Accurate T1 mapping of short T2 tissues using a three-dimensional ultrashort echo time cones actual flip angle imaging-variable repetition time (3D UTE-Cones AFI-VTR) method. Magn Reson Med 2018;80(2):598–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coleman T, Li Y. An Interior Trust Region Approach for Nonlinear Minimization Subject to Bounds. SIAM J Optim 1996;6(2):418–445. [Google Scholar]
- 38.Miller AJ, Joseph PM. The use of power images to perform quantitative analysis on low SNR MR images. Magn Reson Imaging 1993;11(7):1051–1056. [DOI] [PubMed] [Google Scholar]
- 39.McGraw KO, Wong SP. Forming inferences about some intraclass correlation coefficients. Psychol Methods 1996;1(1):30–46. [Google Scholar]
- 40.Young IR, Szeverenyi NM, Du J, Bydder GM. Pulse sequences as tissue property filters (TP-filters): a way of understanding the signal, contrast and weighting of magnetic resonance images. Quant Imaging Med Surg 2020;10(5):1080–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sled JG, Pike GB. Quantitative imaging of magnetization transfer exchange and relaxation properties in vivo using MRI. Magn Reson Med 2001;46(5):923–931. [DOI] [PubMed] [Google Scholar]
- 42.Filippi M, Campi A, Dousset V, et al. A magnetization transfer imaging study of normal-appearing white matter in multiple sclerosis. Neurology 1995;45(3 Pt 1):478–482. [DOI] [PubMed] [Google Scholar]
- 43.Ostuni JL, Richert ND, Lewis BK, Frank JA. Characterization of differences between multiple sclerosis and normal brain: a global magnetization transfer application. AJNR Am J Neuroradiol 1999;20(3):501–507. [PMC free article] [PubMed] [Google Scholar]
- 44.Park M, Moon Y, Han SH, Kim HK, Moon WJ. Myelin loss in white matter hyperintensities and normal-appearing white matter of cognitively impaired patients: a quantitative synthetic magnetic resonance imaging study. Eur Radiol 2019;29(9):4914–4921. [DOI] [PubMed] [Google Scholar]
- 45.Lustig M, Donoho D, Pauly JM. Sparse MRI: The application of compressed sensing for rapid MR imaging. Magn Reson Med 2007;58(6):1182–1195. [DOI] [PubMed] [Google Scholar]
- 46.Zhu B, Liu JZ, Cauley SF, Rosen BR, Rosen MS. Image reconstruction by domain-transform manifold learning. Nature 2018;555(7697):487–492. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




![A–E, Selective T2-weighted fluid-attenuated inversion recovery (FLAIR) (repetition time [TR] msec/echo time msec, 7600/117), and, F–J, short TR adiabatic inversion recovery (STAIR) prepared ultrashort echo time (UTE) (TR = 140 msec) images in three representative participants with multiple sclerosis (MS). A, B, F, and G were obtained in a 35-year-old man; C, D, H, and I were obtained in a 69-year-old woman; E and J were obtained in a 48-year-old man. Arrows indicate MS lesions. Hyperintense lesions detected on T2-FLAIR images show signal loss on corresponding STAIR-UTE images, consistent with demyelination.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/ee04/7643815/8c6dc17e8770/radiol.2020200425.fig6.jpg)