Abstract
Background:
Whether prostate cancer severity modifies patient-reported functional outcomes after radical prostatectomy (RP) or external beam radiotherapy (EBRT) for localized cancer is unknown.
Objective:
The purpose of this study was to determine whether differences in predicted function over time between RP and EBRT varied by risk group.
Design, setting, and participants:
The Comparative Effectiveness Analysis of Surgery and Radiation (CEASAR) study is a prospective, population-based, observational study that enrolled men with localized prostate cancer in 2011–2012. Among 2117 CEASAR participants who underwent RP or EBRT, 817 had low-risk, 902 intermediate-risk, and 398 high-risk disease.
Outcome measurements and statistical analysis:
Patient-reported, disease-specific function was measured using the 26-item Expanded Prostate Index Composite (at baseline and 6, 12, and 36 mo). Predicted function was estimated using regression models and compared by disease risk.
Results and limitations:
Low-risk EBRT patients reported 3-yr sexual function scores 12 points higher than those of low-risk RP patients (RP, 39 points [95% confidence interval {CI}, 37–42] vs EBRT, 52 points [95% CI, 47–56]; p < 0.001). The difference in 3-yr scores for high-risk patients was not clinically significant (RP, 32 points [95% CI, 28–35] vs EBRT, 38 points [95% CI, 33–42]; p = 0.03). However, when using a commonly used binary definition of sexual function (erections firm enough for intercourse), no major differences were noted between RP and EBRT at 3 yr across low-, intermediate-, and high-risk disease strata. No clinically significant interactive effects between treatment and cancer severity were observed for incontinence, bowel, irritative voiding, and hormone domains. The primary limitation is the lack of firmly established thresholds for clinically significant differences in Expanded Prostate Index Composite domain scores.
Conclusions:
For men with low-risk prostate cancer, EBRT was associated with higher sexual function scores at 3 yr than RP; however, for men with high-risk prostate cancer, no clinically significant difference was noted. Men with high-risk prostate cancer should be counseled that EBRT and RP carry similar sexual function outcomes at 3 yr.
Patient summary:
In this report, we studied the urinary, sexual, bowel, and hormonal functions of patients 3 yr after undergoing prostate cancer surgery or radiation. We found that for patients with high-risk disease, sexual function was similar between surgery and radiation. We conclude that high-risk patients undergoing radiation therapy should be counseled that sexual function may not be as good as low-risk patients undergoing radiation.
Keywords: Comparative effectiveness, Disease risk, Patient-reported function, Prostate cancer, Radiation, Surgery
1. Introduction
Prostate cancer severity is well known to influence oncologic outcomes after treatment for prostate cancer [1]. In the Prostate Cancer Intervention Versus Observation Trial, radical prostatectomy (RP) resulted in significant reductions in all-cause and disease-specific mortality among men with intermediate- and high-risk disease [2]. In the Scandinavian Prostate Cancer Group Study Number-4 trial, men who underwent RP for intermediate-risk disease had improved metastasis-free, cancer-specific, and overall survival than those who did not undergo treatment [3]. While these observations relate primarily to oncologic outcomes, they have nonetheless given rise to an emerging hypothesis that quality of life outcomes after surgery or radiotherapy may be dependent on the severity of the cancer at diagnosis.
Several biologically plausible reasons exist to suspect why the effects of treatment on patient-reported quality of life outcomes would vary by prostate cancer severity. First, the use of androgen-deprivation therapy along with external beam radiotherapy (EBRT) among patients with high-risk disease may lead to substantial decline in hormone and sexual functions, at least in the short term [4]. Second, surgery for high-risk patients is often more radical because surgeons typically avoid nerve-sparing techniques and sacrifice a larger portion of the membranous urethra at the apex [5,6]. As little data evaluating these hypotheses exist, a comparative study was needed to assess how sexual, urinary, bowel, and hormone functions varied by levels of prostate cancer severity after patients were treated for prostate cancer.
In this context, we tested the hypothesis that the effect of treatment on patient-reported urinary, bowel, hormone, and sexual functions would vary by prostate cancer severity according to the D’Amico risk classification system [1]. Since little is known about how the effects of treatment on patient-reported function vary by disease severity, these data will not only fill a substantial knowledge gap in the literature, but will also have important implications for patients and providers as they weigh individualized risks for treatment-related morbidity.
2. Patients and methods
2.1. Study population
The Comparative Effectiveness Analysis of Surgery and Radiation (CEASAR) study is a longitudinal, population-based, prospective observational cohort study designed to measure the effectiveness and harms of contemporary management strategies for men diagnosed with localized prostate cancer (NCT0136286). Patients were accrued from five Surveillance, Epidemiology, and End Results (SEER) registry catchment areas (Louisiana, New Jersey, Utah, Atlanta, and Los Angeles). This dataset was augmented with a sample of men enrolled in Cancer of the Prostate Strategic Urologic Research Endeavor (CaPSURE) [7]. A total of 3709 participants were enrolled in CEASAR between 2011 and 2012. Eligible men were ≤80 yr of age with clinical stage cT1 or cT2 disease, had a prostate-specific antigen (PSA) level of <50 ng/dl, and had been diagnosed within 6 mo of enrollment. Low-risk disease was defined as clinical stage ≤T2a, Gleason score ≤6, and PSA level <10 ng/dl. High-risk disease was defined as T2c or higher, Gleason score ≥8, or PSA >20 ng/dl. Intermediate-risk disease was defined as T2b, Gleason score 7, and PSA level between 10 and 20 ng/dl [1]. The CEASAR methodology has been described previously, including power and sample size calculations [8]. The coordinating site at Vanderbilt, each of the SEER sites, and CaPSURE obtained approvals from the relevant local institutional review board.
2.2. Survey instruments and data abstraction from electronic health records
Patient-reported, disease-specific function was captured using the 26-item Expanded Prostate Index Composite (EPIC) questionnaire. EPIC is a validated survey instrument that evaluates function and bother for sexual, urinary, bowel, and hormone domains as continuous measures on a scale of 0–100, with higher scores indicating better function [9]. To assist in the determination of clinically relevant changes in EPIC domain scores, we used previously published and validated domain score thresholds (clinically relevant point changes: hormone, 4–6; urinary irritative, 5–7; urinary incontinence, 6–9; sexual, 10–12) [10]. Participants were also asked to complete the Total Illness Burden Index for Prostate Cancer, a validated patient-reported 84-item comorbidity assessment of 11 health domains modified for patients with prostate cancer [11,12]. CEASAR also captured patient-reported race, income, age at diagnosis, educational attainment, marital status, employment or retirement status, insurance coverage, general health and function [13], physical function [14], social support, emotional health, cancer-related anxiety, and a depression scale (the Center for Epidemiologic Studies Depression [CES-D] scale) [15].
Tumor characteristics, treatment received, PSA levels, and treatment date were obtained by abstracting data from electronic health records. For patients without health record information, questionnaires and SEER registry data determined treatment received. Patients who underwent both RP and EBRT were categorized on the basis of primary treatment. Patients who received active surveillance, primary androgen-deprivation therapy, or cryoablation were excluded.
2.3. Statistical analysis
Patients’ baseline demographic, socioeconomic, and clinical characteristics were compared across cancer severity categories using Kruskal-Wallis and χ2 tests. To characterize typical changes in patient-reported function over time within each treatment group by the level of risk, we fit longitudinal regression models with EPIC scores as the response variable. The primary covariates were cancer severity, treatment type (RP or EBRT), and months since treatment; second-degree interactions among the three variables were included. To account for the within-subject correlation due to repeated observations, we used generalized estimating equations with an exchangeable working covariance matrix. The following covariates were included in the model: patient age at diagnosis, comorbidity tumor characteristics (PSA level corrected for 5α-reductase inhibitor use, Gleason score [≤6, 3 + 4, 4 + 3, or ≥8], and T stage [T1 or T2]), psychosocial measures (educational attainment, insurance type, employment type, marital status, and Short Form-36) physical function score, social support, CES-D score, participatory decision-making index [16], baseline function, and study site. The relationship between continuous variables and mean function was modeled using restricted cubic splines with three knots at the 10th, 50th, and 90th percentiles. We fit the models after multiple imputation using chained equations [17,18]. Approximately 4%, 6%, 9%, and 15% of patients were missing the primary outcome at baseline and 6, 12, and 36 mo, respectively. The cancer severity–treatment interactions were assessed graphically for each functional domain by plotting the predicted EPIC domain scores from models derived from imputed datasets. Treatment effects are characterized by differences in function score at each time point between treatment groups, and differences in treatment effects among each risk group were characterized by the difference in these treatment effects between risk groups. Thus, our estimate of interest is a difference in differences (DID) accompanied by a 95% confidence interval (CI). All statistical analyses were performed using Stata 14.1 data analysis software (StataCorp. 2015. Release 14. College Station, TX: StataCorp LP) [19].
3. Results
Among the 2117 CEASAR participants in the analytic cohort, 817 (39%) had low-risk disease, 902 (43%) intermediate-risk disease, and the remaining 398 (19%) high-risk disease. Table 1 presents the distributions of selected demographic, socioeconomic, and clinical characteristics by disease-risk strata. In general, high-risk disease occurred more commonly among African-American men and men with lower levels of educational attainment, higher rates of non-Medicare/private insurance, lower levels of social support, lower levels of participatory decision making, and multiple medical comorbidities.
Table 1 –
Demographic, socioeconomic, and clinical characteristics by disease-risk strata
| Characteristic | Groupa | p value | ||
|---|---|---|---|---|
| Low-risk (n = 817) | Intermediate-risk (n = 902) | High-risk (n = 398) | ||
| Age (yr), median (IQR) | 62 (56–67) | 64 (59–70) | 65 (60–71) | <0.001 |
| Race, no. (%) | 0.03 | |||
| White | 598 (74) | 681 (76) | 269 (68) | |
| African American | 108 (13) | 123 (14) | 66 (17) | |
| Other | 108 (13) | 92 (10) | 59 (15) | |
| Body mass index, median (IQR) | 28 (25–31) | 28 (25–30) | 27 (25–31) | 0.7 |
| TIBI-CaP categories, no. (%) | <0.001 | |||
| 0 | 1 (0.1) | 1 (0.1) | 1 (0.3) | |
| 1 | 90 (12) | 68 (8) | 33 (9) | |
| 2 | 169 (22) | 156 (18) | 63 (16) | |
| 3 | 206 (26) | 202 (24) | 83 (22) | |
| 4 | 141 (18) | 155 (18) | 72 (19) | |
| 5 | 73 (9) | 124 (14) | 51 (13) | |
| 6 | 45 (6) | 75 (9) | 24 (6) | |
| 7 | 30 (4) | 37 (4) | 35 (9) | |
| ≥8 | 25 (3) | 42 (5) | 22 (6) | |
| Income, no. (%) | 0.2 | |||
| <$10 000 | 38 (5) | 55 (7) | 26 (7) | |
| $10 000–30 000 | 98 (13) | 118 (15) | 65 (19) | |
| $30 001–50 000 | 137 (19) | 152 (19) | 65 (19) | |
| $50 001–100 000 | 231 (31) | 251 (31) | 110 (31) | |
| >$100 000 | 236 (32) | 226 (28) | 86 (24) | |
| Education, no. (%) | 0.02 | |||
| Grade school or less | 44 (6) | 33 (4) | 24 (6) | |
| Some high school or technical school | 31 (4) | 49 (6) | 35 (9) | |
| High school or technical school graduate | 157 (20) | 189 (22) | 74 (19) | |
| Some college | 165 (21) | 200 (23) | 81 (21) | |
| College graduate | 193 (25) | 188 (22) | 82 (22) | |
| Graduate or professional school after college | 189 (24) | 196 (23) | 85 (22) | |
| Relationship status, no. (%) | 0.8 | |||
| Never married | 33 (4) | 39 (5) | 17 (5) | |
| Married | 632 (81) | 690 (81) | 297 (78) | |
| Separated | 14 (2) | 16 (2) | 9 (2) | |
| Divorced | 77 (10) | 75 (9) | 43 (11) | |
| Widowed | 22 (3) | 34 (4) | 13 (3) | |
| Employment status, no. (%) | <0.001 | |||
| Full time | 439 (54) | 372 (42) | 130 (33) | |
| Retired/part time/unemployed | 371 (46) | 520 (58) | 260 (67) | |
| Insurance status, no. (%) | <0.001 | |||
| Uninsured/VA/Medicaid | 32 (4) | 43 (5) | 39 (10) | |
| Medicare | 279 (34) | 424 (47) | 210 (53) | |
| Private/HMO | 506 (62) | 434 (48) | 148 (37) | |
| Social support, median (IQR) | 95 (70–100) | 95 (75–100) | 95 (65–100) | 0.08 |
| Physical function, median (IQR) | 95 (85–100) | 95 (80–100) | 91 (65–100) | <0.001 |
| Worry, median (IQR) | 29 (14–46) | 32 (18–46) | 32 (18–50) | 0.02 |
| PDM score, median (IQR) | 82 (68–93) | 86 (71–93) | 79 (64–89) | <0.001 |
| Depression score, median (IQR) | 13 (4–30) | 15 (4–30) | 19 (7–37) | 0.09 |
| Surgical approach | 0.06 | |||
| Robotic | 439 (80) | 412 (76) | 156 (70) | |
| Open | 102 (19) | 119 (22) | 63 (29) | |
| Radiation modality | 0.009 | |||
| EBRT | 176 (93) | 297 (97) | 189 (100) | |
| Brachytherapy | 10 (5) | 8 (3) | 0 (0) |
EBRT = external beam radiotherapy; HMO = Health Maintenance Organization; IQR = interquartile range; PDM = participatory decision making; TIBI-CaP = Total Illness Burden Index for Prostate Cancer; VA = Veterans Administration.
Data were missing for a small proportion of patients.
3.1. Sexual domain
Cancer severity modified the effect of treatment on sexual function scores as the differences in predicted function between RP and EBRT varied by risk group over time. Low-risk EBRT patients reported 3-yr sexual function scores that were 12 points higher than those of low-risk RP patients (RP, 39 points [95% CI, 37–42] vs EBRT, 52 points [95% CI, 47–56]; p < 0.001) and intermediate-risk EBRT patients reported scores that were 11 points higher than those of intermediate-risk RP patients (RP, 36 points [95% CI, 33–38] vs EBRT, 47 points [95% CI, 43, 51]; p < 0.001); however, high-risk EBRT patients reported scores that were only 6 points higher than those of high-risk RP patients (RP, 32 points [95% CI, 28–35] vs EBRT, 38 points [95% CI, 33–42]; p = 0.03; Table 2) [16]. Although all these comparisons are statistically significant, only the low- and intermediate-risk patients meet the predefined threshold of clinical significance for the EPIC sexual function domain (10–12 points).
Table 2 –
Adjusted predictions for EPIC sexual domaina
| Risk group | Baseline Estimate (95% CI) | 6 mo Estimate (95% CI) | 12 mo Estimate (95% CI) | 36 mo Estimate (95% CI) |
|---|---|---|---|---|
| Radical prostatectomy | ||||
| Low | 61 (59–64) | 30 (28–33) | 35 (33–38) | 39 (37–42) |
| Intermediate | 62 (59–64) | 26 (24–28) | 33 (31–35) | 36 (33–38) |
| High | 59 (56–63) | 23 (20–26) | 27 (24–30) | 32 (28–35) |
| Differences between low and high risk within RP groupb | 7c (4–12) | 8c (4–12) | 7c (4–12) | |
| External beam RT | ||||
| Low | 63 (59–67) | 52 (47–56) | 52 (48–56) | 52 (47–56) |
| Intermediate | 62 (59–66) | 46 (42–50) | 49 (45–52) | 47 (43–51) |
| High | 55 (50–59) | 37 (33–42) | 37 (33–42) | 38 (33–42) |
| Differences between low and high risk within EBRT groupd | 15c (8–20) | 15c (9–20) | 14c (8–20) |
CES-D = Center for Epidemiologic Studies Depression Scale; CI = confidence interval; EBRT = external beam radiotherapy; EPIC = Expanded Prostate Index Composite; RP = radical prostatectomy; RT = radiotherapy.
Adjusted for patient age at diagnosis, comorbidity tumor characteristics (prostate-specific antigen corrected for 5-reductase inhibitor use, Gleason score [≤6, 3 + 4, 4 + 3, or ≥8], and T-stage [T1 or T2]), psychosocial measures (educational attainment, insurance type, employment type, marital status, Short Form-36 physical function score, social support, CES-D score, and participatory decision-making index [16]), and study site.
Differences within the RP group were calculated by (RPLow RPHigh).
p 0.001.
Differences within the EBRT group were calculated by (EBRTLow EBRTHigh).
To further investigate how the effect of treatment on sexual function relates conditionally to disease severity, we evaluated differences between low-, intermediate-, and high-risk patients within treatment groups (which are summarized for brevity in Fig. 1) [16]. Whereas high-risk RP patients reported sexual function scores that were 7.6 points lower than those of low-risk RP patients (95% CI, 3.5–12; p < 0.001), high-risk EBRT patients reported scores that were 14 points lower than those of low-risk EBRT patients at 3 yr (95% CI, 8.1–20; p < 0.001). This observation indicates that EBRT may lead to comparatively larger declines in sexual function when low- and high-risk patients are compared (DID, 6.4 points [95% CI, 0.02–13]; p = 0.049).
Fig. 1 –
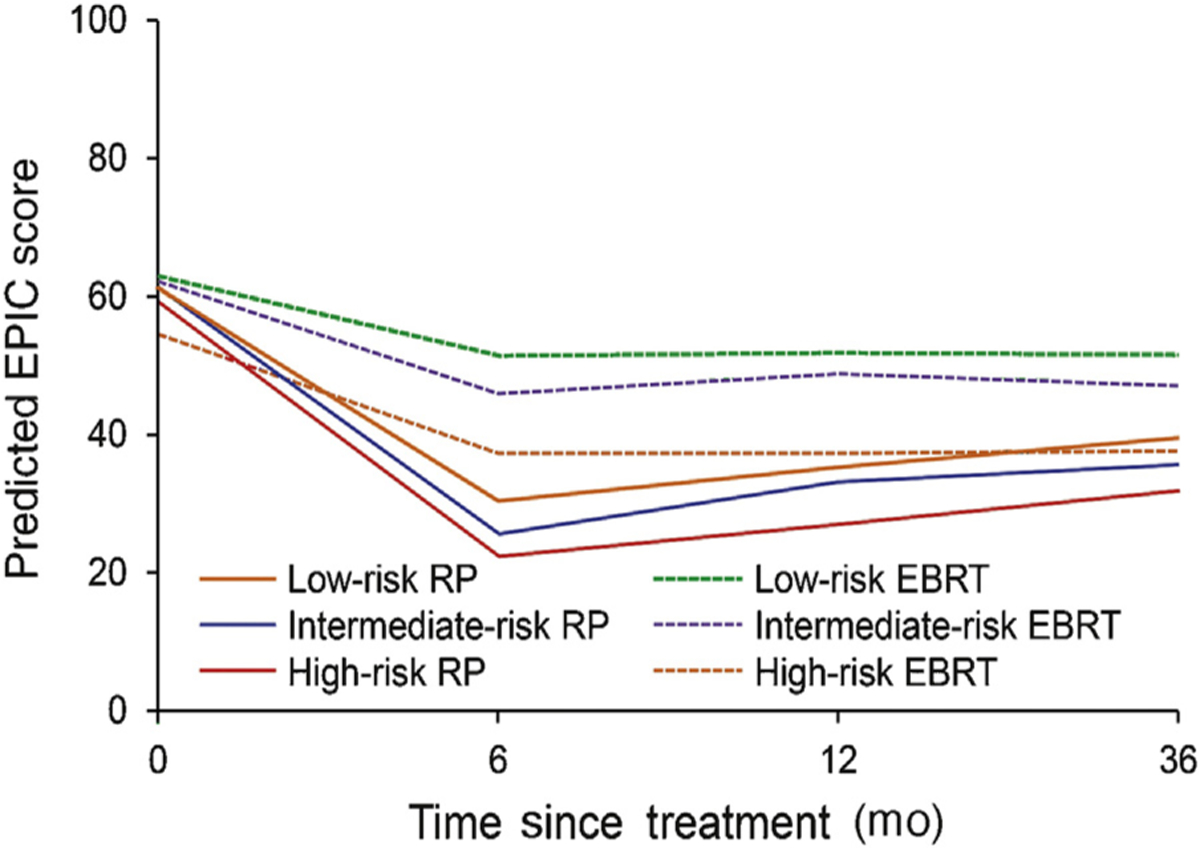
Sexual function by risk and treatment modality. Sexual function trajectories were adjusted for patient age at diagnosis, comorbidity tumor characteristics (PSA corrected for 5α-reductase inhibitor use, Gleason score [≤6, 3 + 4, 4 + 3, or ≥8], and T stage [T1 or T2]), psychosocial measures (educational attainment, insurance type, employment type, marital status, Short Form-36 physical function score, social support, CES-D score, and participatory decision-making index [16]), and study site. CES-D = Center for Epidemiologic Studies Depression Scale; EBRT = external beam radiotherapy; EPIC = expanded prostate index composite; PSA = prostate specific antigen; RP = radical prostatectomy.
In a post hoc analysis of a commonly used definition for erectile function outcomes (ie, erections firm enough for intercourse), there was no significant effect modification of disease severity on outcomes after treatment. Approximately 35% of low-risk RP patients reported erections firm enough for intercourse at 3 yr compared with 33% of low-risk EBRT patients (p = 0.6); for intermediate-risk RP patients, approximately 29% reported erections firm enough for intercourse compared with 32% of EBRT patients (p = 0.4). Last, among high-risk patients, approximately 22% of RP patients reported erections firm enough for intercourse at 3 yr compared with 18% of EBRT patients (p = 0.4).
3.2. Incontinence domain
Unlike sexual function, cancer severity did not modify the effect of treatment on incontinence scores as the differences in predicted function between RP and EBRT between risk groups did not vary over time. At 3 yr, high-risk RP patients reported urinary incontinence scores that were 4 points lower than those of low-risk RP patients (95% CI, 0.8–8; p = 0.02; Table 3) [16]. Although the differences in scores are statistically significant, it is below the threshold of clinical significance for the EPIC incontinence domain (6–9 points) [10]. By contrast, differences between high- and low-risk EBRT patients were both statistically and clinically insignificant (0.6 [95% CI, −3.4 to 4.6]; p = 0.8). This resulted in a DID of 3.8 points over the study period (95% CI, −0.70 to 8.3; p = 0.1). The trajectories for urinary incontinence are shown in Figure 2 [16].
Table 3 –
Adjusted predictions for EPIC incontinence domaina
| Risk group | Baseline Estimate (95% CI) | 6 mo Estimate (95% CI) | 12 mo Estimate (95% CI) | 36 mo Estimate (95% CI) |
|---|---|---|---|---|
| Radical prostatectomy | ||||
| Low | 84 (83–86) | 66 (64–68) | 70 (68–72) | 71 (69–73) |
| Intermediate | 86 (84–87) | 63 (61–65) | 69 (67–71) | 69 (67–71) |
| High | 85 (82–87) | 61 (58–64) | 67 (64–70) | 67 (64–70) |
| Differences between low and high risk within RP groupb | 5c (1–9) | 3 (−0.5 to 7) | 4c (0.8–8) | |
| External beam RT | ||||
| Low | 89 (86–91) | 90 (87–92) | 89 (86–91) | 87 (84–90) |
| Intermediate | 94 (92–96) | 91 (89–93) | 92 (89–94) | 89 (87–91) |
| High | 93 (90–96) | 88 (85–92) | 89 (86–92) | 87 (83–90) |
| Differences between low and high risk within EBRT groupd | 2 (−3 to 5) | −0.7 (−5 to 3) | 0.6 (−3 to 5) |
CES-D = Center for Epidemiologic Studies Depression Scale; CI = confidence interval; EBRT = external beam radiotherapy; EPIC = Expanded Prostate Index Composite; RP = radical prostatectomy; RT = radiotherapy.
Adjusted for patient age at diagnosis, comorbidity tumor characteristics (prostate-specific antigen corrected for 5-reductase inhibitor use, Gleason score [≤6, 3 + 4, 4 + 3, or ≥8], and T-stage [T1 or T2]), psychosocial measures (educational attainment, insurance type, employment type, marital status, Short Form-36 physical function score, social support, CES-D score, and participatory decision-making index [16]), and study site.
Differences within RP group are calculated by (RPLow RPHigh).
p 0.05.
Differences within the EBRT group are calculated by (EBRTLow EBRTHigh).
Fig. 2 –
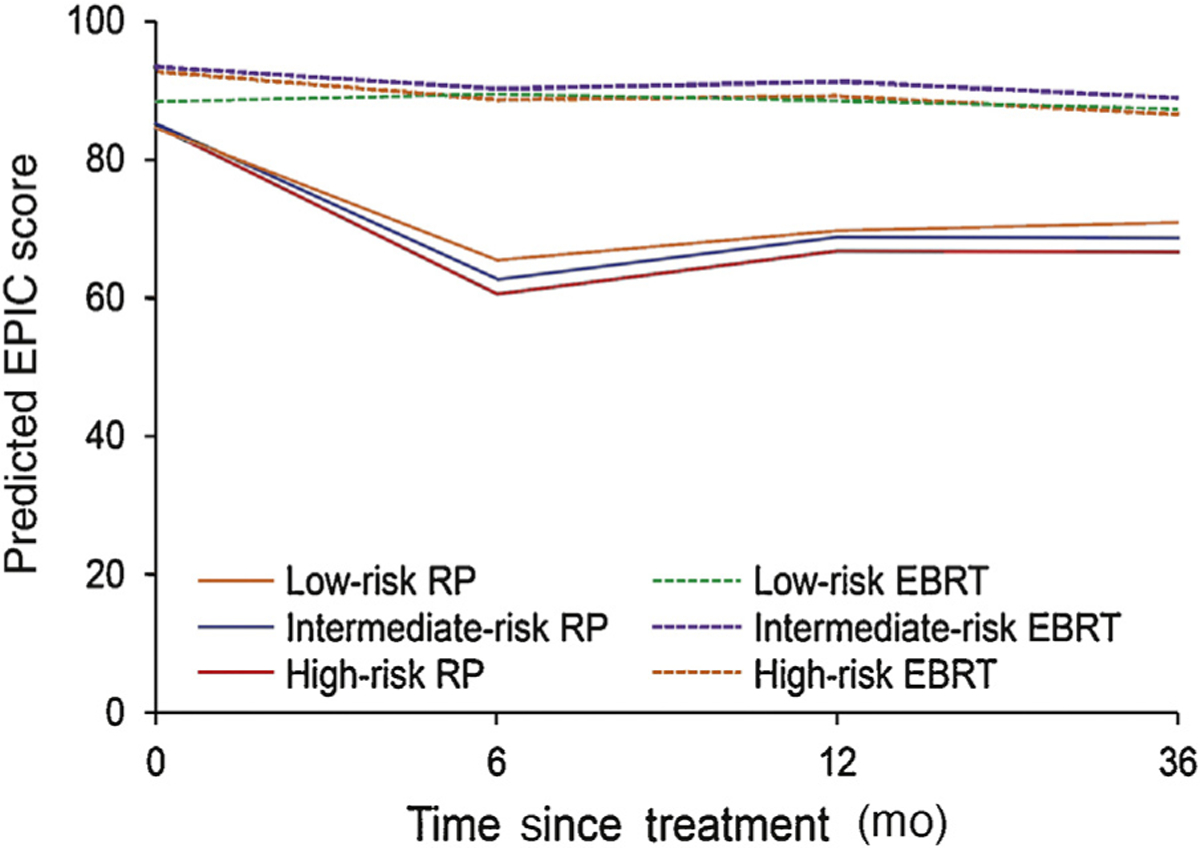
Urinary incontinence by risk and treatment modality. Urinary incontinence trajectories were adjusted for patient age at diagnosis, comorbidity tumor characteristics (PSA corrected for 5α-reductase inhibitor use, Gleason score [≤6, 3 + 4, 4 + 3, or ≥8], and T stage [T1 or T2]), psychosocial measures (educational attainment, insurance type, employment type, marital status, Short Form-36 physical function score, social support, CES-D score, and participatory decision-making index [16]), and study site. CES-D = Center for Epidemiologic Studies Depression Scale; EBRT = external beam radiotherapy; EPIC = expanded prostate index composite; PSA = prostate specific antigen; RP = radical prostatectomy.
In a post hoc analysis of a commonly used definition for continence after treatment (ie, no leakage whatsoever), there was no significant effect modification of disease severity on outcomes after treatment. Approximately 37% of low-risk RP patients reported no leakage whatsoever at 3 yr compared with 53% of low-risk EBRT patients (p = 0.003); approximately 32% of intermediate-risk RP patients reported no leakage whatsoever at 3 yr compared with 53% of EBRT patients (p < 0.001). Last, among high-risk patients, approximately 22% of RP patients reported no leakage whatsoever at 3 yr compared with 53% of EBRT patients (p < 0.001).
3.3. Hormone, bowel, and urinary irritative domains
There were no clinically significant interactive effects between treatment and cancer severity for the bowel (DID, 0.6 points [95% CI, −1.9 to 3.1]; p = 0.7), hormone (DID, 3.0 points [95% CI, 0.5–5.6]; p = 0.02), or irritative domain scores (DID, 2.7 points [95% CI, −0.1 to 5.5]; p = 0.06; Supplementary Fig. 1–3 and Supplementary Table 1).
4. Discussion
In this prospective, longitudinal, population-based study of functional outcomes after contemporary prostate cancer treatment, we observed that the patterns of sexual dysfunction after treatment for prostate cancer differed according to the severity of disease at diagnosis. For men with low- and intermediate-risk prostate cancer, EBRT was associated with better sexual function scores at 3 yr than RP; however, for men with high-risk prostate cancer, sexual function scores at 3 yr were similar between RP and EBRT. However, when using a commonly used binary definition of sexual function (erections firm enough for intercourse), no major differences were noted between RP and EBRT at 3 yr across low-, intermediate-, and high-risk disease strata. This supports the finding that high-risk men should not expect improved erections with EBRT compared with surgery. These data have important clinical implications for men who are considering treatment for high-risk prostate cancer.
Numerous studies have previously reported on the variation in functional outcomes by patient-level factors, such as age [20], race/ethnicity [21], comorbidity [22], and baseline function [23]. However, existing data regarding the variation of functional outcomes by cancer severity are notably lacking. In the Prostate Cancer Outcomes and Satisfaction with Treatment Quality Assessment study, the model predicted probability of having functional erections suitable for intercourse at 2 yr after surgery, and radiotherapy was shown to vary by levels of PSA [24]. However, PSA is only one factor that determines cancer severity, and substantial heterogeneity in cancer severity would be expected above and below the cutoff used in this study (10 ng/dl). Furthermore, the authors did not report how other important outcomes, such as bowel, urinary, and hormone functions, varied by levels of PSA. In a separate study of 62 patients who had RP and 54 patients who had EBRT, Takizawa et al [25] found that high-risk patients undergoing EBRT reported better function in the sexual and urinary domains than high-risk patients undergoing RP. However, their study was relatively small, with limited power and questionable external validity.
The current study offers several important advantages over others in the literature. First, we used longitudinal methods and controlled for many known predictors of function after prostate cancer treatment. Longitudinal cohort studies offer numerous methodological benefits, including the ability to attribute outcomes to specific exposures; define the exposures according to presence, timing, and chronicity; and follow changes in the outcome over time. Second, baseline function was assessed and prespecified in the models. Poorer function at baseline can impact response rates and outcomes, which may produce misleading evidence regarding the effectiveness of treatments under study [26]. Assessing baseline function not only limited the impact of recall bias, but also allowed for the adjustment of pretreatment differences between the surgery and radiotherapy cohorts. Third, we performed a comprehensive evaluation of disease-specific function after treatment for prostate cancer using clearly defined and broadly accepted survey instruments and categories of prostate cancer risk. The EPIC instrument is a psychometrically validated survey instrument designed to assess patient-reported outcomes after treatment for prostate cancer. Likewise, the D’Amico risk classification system is well known and commonly used among clinicians who treat prostate cancer. Fourth, our analysis was based on a large, population-based sample of ethnically diverse men diagnosed in the contemporary era and was appropriately powered for the question under study. Patients were accrued from five SEER registry catchment areas in addition to the CaPSURE database, which allowed for a population-based assessment of patient-reported outcomes. Unlike single institutional cohort studies or randomized trials, population-based studies offer insights into real-world outcomes among patients receiving treatment in the community.
Despite these strengths, several limitations should be acknowledged. First, clinically significant differences in EPIC domain scores are not firmly established, and as a result, we used published thresholds when interpreting these data [10]. For example, several comparisons in this study were statistically significant, but the point estimates for the differences did not always satisfy the criteria for clinical significance. Second, this is an observational study, and unmeasured confounding, such as differential clinician experience, access to high-quality care, differential frame of mind, hospital or provider-level characteristics, radiation techniques, or use of pelvic floor rehabilitation, may give rise to biased effect estimates. To address these concerns, the CEASAR study contains a comprehensive set of patient-level variables, which, in combination with advanced inference model building, should minimize the effects of confounding. Third, radiation techniques are constantly evolving, and many intermediate-risk patients may not need androgen-deprivation therapy under modern dose-escalated radiation protocols. Fourth, many low-risk patients in this cohort received treatment, and this may not be reflective of future practice. Fifth, we acknowledge that the D’Amico classification system comprises relatively crude categories, yet it remains widely used and understood, and has been extensively validated, while each of the alternative systems also has strengths and weaknesses. Finally, although we present the results of several statistical tests, we have not adjusted for multiple comparisons. However, our primary analysis was specified a priori, and we have been careful to interpret the results in the context of clinical relevance in addition to statistical significance [27].
We believe that these findings provide a valuable framework for a more comprehensive understanding of how the effects of treatment on patient-reported functional outcomes relate conditionally to the levels of D’Amico risk. In light of these data, we would advocate for the consideration of cancer severity when men are counseled regarding the pattern of harms after treatment for localized prostate cancer. With longer follow-up, these data could also lay the foundation for decision-support tools that target patients or providers, or both.
5. Conclusions
For men with low- and intermediate-risk prostate cancer, EBRT was associated with higher sexual function scores at 3 yr than RP; however, for men with high-risk prostate cancer, sexual function scores were similar between RP and EBRT. This observation holds even when using a binary definition of sexual function. Men with high-risk prostate cancer should be counseled that EBRT and RP are associated with similar sexual function outcomes at 3 yr.
Supplementary Material
Financial disclosures:
Mark Douglas Tyson II certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: This work was supported by the National Cancer Institute at the National Institutes of Health (5T32CA106183 to M.D.T.), by the American Cancer Society (MSRG-15-103-01-CPHPS to M.J.R.), by the US Agency for Healthcare Research and Quality (1R01HS019356, 1R01HS022640-01), and through a contract from the Patient-Centered Outcomes Research Institute.
Footnotes
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.eururo.2018.02.012.
References
- [1].D’Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA 1998;280:969–74. [DOI] [PubMed] [Google Scholar]
- [2].Wilt TJ, Brawer MK, Jones KM, et al. Radical prostatectomy versus observation for localized prostate cancer. N Engl J Med 2012; 367:203–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bill-Axelson A, Holmberg L, Garmo H, et al. Radical prostatectomy or watchful waiting in early prostate cancer. N Engl J Med 2014;370:932–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Wilke DR, Parker C, Andonowski A, et al. Testosterone and erectile function recovery after radiotherapy and long-term androgen deprivation with luteinizing hormone-releasing hormone agonists. BJU Int 2006;97:963–8. [DOI] [PubMed] [Google Scholar]
- [5].Walsh PC, Marschke P, Ricker D, Burnett AL. Patient-reported urinary continence and sexual function after anatomic radical prostatectomy. Urology 2000;55:58–61. [DOI] [PubMed] [Google Scholar]
- [6].Kundu SD, Roehl KA, Eggener SE, Antenor JA, Han M, Catalona WJ. Potency, continence and complications in 3,477 consecutive radical retropubic prostatectomies. J Urol 2004;172(6 Pt 1): 2227–31. [DOI] [PubMed] [Google Scholar]
- [7].Lubeck DP, Litwin MS, Henning JM, et al. The CaPSURE database: a methodology for clinical practice and research in prostate cancer. CaPSURE Research Panel. Cancer of the Prostate Strategic Urologic Research Endeavor. Urology 1996;48:773–7. [DOI] [PubMed] [Google Scholar]
- [8].Barocas DA, Chen V, Cooperberg M, et al. Using a population-based observational cohort study to address difficult comparative effectiveness research questions: the CEASAR study. J Comp Eff Res 2013;2:445–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Szymanski KM, Wei JT, Dunn RL, Sanda MG. Development and validation of an abbreviated version of the expanded prostate cancer index composite instrument for measuring health-related quality of life among prostate cancer survivors. Urology 2010; 76:1245–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Skolarus TA, Dunn RL, Sanda MG, et al. Minimally important difference for the Expanded Prostate Cancer Index Composite Short Form. Urology 2015;85:101–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Stier DM, Greenfield S, Lubeck DP, et al. Quantifying comorbidity in a disease-specific cohort: adaptation of the total illness burden index to prostate cancer. Urology 1999;54:424–9. [DOI] [PubMed] [Google Scholar]
- [12].Litwin MS, Greenfield S, Elkin EP, Lubeck DP, Broering JM, Kaplan SH. Assessment of prognosis with the total illness burden index for prostate cancer: aiding clinicians in treatment choice. Cancer 2007;109:1777–83. [DOI] [PubMed] [Google Scholar]
- [13].Ware JE Jr, Sherbourne CD, The MOS. 36-item Short-Form Health Survey (SF-36). I. Conceptual framework and item selection. Med Care 1992;30:473–83. [PubMed] [Google Scholar]
- [14].McHorney CA, Ware JE Jr, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care 1993;31:247–63. [DOI] [PubMed] [Google Scholar]
- [15].Andresen EM, Malmgren JA, Carter WB, Patrick DL. Screening for depression in well older adults: evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale). Am J Prev Med 1994;10:77–84. [PubMed] [Google Scholar]
- [16].Kaplan SH, Gandek B, Greenfield S, Rogers W, Ware JE. Patient and visit characteristics related to physicians’ participatory decision-making style. Results from the Medical Outcomes Study. Med Care 1995;33:1176–87. [DOI] [PubMed] [Google Scholar]
- [17].White IR, Royston P, Wood AM. Multiple imputation using chained equations: Issues and guidance for practice. Stat Med 2011;30: 377–99. [DOI] [PubMed] [Google Scholar]
- [18].Royston P, White IR. Multiple Imputation by Chained Equation (MICE): implementation in Stata. J Stat Softw 2011;45. [Google Scholar]
- [19].StataCorp. Stata statistical software: release 14. College Station, TX: StataCorp LP; 2015. [Google Scholar]
- [20].Hampson LA, Cowan JE, Zhao S, Carroll PR, Cooperberg MR. Impact of age on quality-of-life outcomes after treatment for localized prostate cancer. Eur Urol 2015;68:480–6. [DOI] [PubMed] [Google Scholar]
- [21].Tyson MD, Alvarez J, Koyama T, et al. Racial variation in patient-reported outcomes following treatment for localized prostate cancer: results from the CEASAR study. Eur Urol 2017;72: 307–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Reeve BB, Chen RC, Moore DT, et al. Impact of comorbidity on health-related quality of life after prostate cancer treatment: combined analysis of two prospective cohort studies. BJU Int 2014;114:E74–81. [DOI] [PubMed] [Google Scholar]
- [23].Barocas DA, Alvarez J, Resnick MJ, et al. Association between radiation therapy, surgery, or observation for localized prostate cancer and patient-reported outcomes after 3 years. JAMA 2017;317:1126–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Alemozaffar M, Regan MM, Cooperberg MR, et al. Prediction of erectile function following treatment for prostate cancer. JAMA 2011;306:1205–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Takizawa I, Hara N, Nishiyama T, et al. Oncological results, functional outcomes and health-related quality-of-life in men who received a radical prostatectomy or external beam radiation therapy for localized prostate cancer: a study on long-term patient outcome with risk stratification. Asian J Androl 2009;11:283–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Peters M, Crocker H, Dummett S, Jenkinson C, Doll H, Fitzpatrick R. Change in health status in long-term conditions over a one year period: a cohort survey using patient-reported outcome measures. Health Qual Life Outcomes 2014;12:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology 1990;1:43–6. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


