Abstract
Interest in spectral CT for diagnostics and therapy evaluation has been growing. Acquisitions of data from distinct energy spectra provide, among other advantages, quantitative density estimations for multiple materials. We introduce a novel spectral CT concept that includes a fine-pitch grating for prefiltration of the x-ray beam. The attenuation behavior of this grating changes significantly if x-rays are slightly angled in relation to the grating structures. To apply such an angle (i.e. switch between the different filtrations) we propose a fast, controllable, and precise solution by moving the focal spot of the x-ray tube. In this work, we performed preliminary evaluations with a grating prototype on a CT test bench. Our results include x-ray spectrometer measurements that reveal diverse and controllable spectral shaping between 4° and 6° for a specific grating design. Additional experiments with a contrast agent phantom illustrated the capability to decompose clinically relevant iodine concentrations (5, 10, 20, and 50mg/mL) – demonstrating the feasibility of the grating-based approach. Ongoing and future studies will investigate the potential of this novel concept as a relatively simple upgrade to standard energy-integrating CT.
Keywords: Spectral CT, dual energy CT, material decomposition, quantitative CT, adaptive imaging
I. Introduction
Spectral CT is finding increased use in both research and clinical settings. Obtaining projection data across two or more spectral channels enables material decomposition and quantitative material density estimation. This drives multiple clinical diagnostic tests including quantitation of exogenous contrast agents, virtual non-contrast images, and endogenous material quantification (e.g. bone mineral density estimation [1]). Similarly, spectral CT can be used to reduce energy-dependent effects like beam hardening. There are multiple strategies for obtaining multi-channel spectral data. For example, x-ray detectors with energy discrimination including multi-layer [2] and photon counting detectors [3], [4] have permitted data acquisition with multiple spectral bins. On the source side, kV-switching [5] and multiple x-ray tubes [6] can provide two (or more) channels by changing the peak energy of the x-ray beam. Other techniques include various beam filtration methods where the x-ray spectrum is shaped using, for example, k-edge filters. Such strategies include split filters[7], rotating filters[8], and moving tiled filters [9]. All of these techniques have various advantages and disadvantages. In particular, many options involve new and expensive hardware, motion systems that can be complicated to integrate within the CT gantry, or limited control over the spectral response. In this work, we present a new source-side filtration method that allows dynamic spectral beam shaping without moving the filter.
II. Methods
A. Overview of Grating-based Filtration
The essential idea explored in this work is to use a periodic grating (much like an anti-scatter grid) to filter the x-ray beam wherein the grating is composed of, for example, a transition metal with a k-edge in the diagnostic range. Consider a regular grating like that shown in Fig. 1 with no material in-between the metal grating structure. Additionally, consider that the grating period is on the order of the focal spot size or smaller, so that the grating structure itself cannot be resolved (again, like a grid). When x-rays traverse the filter at 0°, a portion of the beam is attenuated by a relatively long length of metal (effectively blocking the beam). The remaining portion of the beam is essentially unfiltered through the air. Thus, the net effect is to not change the beam spectrum very much at all, but there is a decrease in the overall fluence by a proportion based on the relative blocker and gap widths. Next, consider the case when the incident beam is angled slightly. More of the beam is filtered by the metal, and the thickness through the metal is decreased. Thus, the overall beam spectrum has a smaller contribution of unfiltered x-rays and the x-ray filtration is less extreme, yielding a weighted average of the unfiltered beam and the k-edge filter. Similar weighted averaging of spectra occurs for increased angles wherein the beam traverses two or more periods. Thus, by changing the angulation of the beam, one can control the x-ray spectrum. While this illustration relies on parallel x-rays, one could also design a focused grating to achieve the same effect with a diverging beam.
Fig. 1.

Illustration of the spectral grating filter concept showing lamellae in blue. As the incident x-ray beam angle changes the amount of beam filtration can be controlled. Grating specifications for the particular grating used in this work are specified. Similarly, two critical angles are shown: 1) The angle at which all rays pass through exactly one lamella of the grating − 4.7°; and 2) the angle where all x-rays pass through exactly two lamellae − 5.7°.
Note that this kind of spectral shaping does require angulation of the x-ray beam. While this could be achieved through source or grating motion, the grating can be designed to be sensitive to small angular changes. With proper design of the grating to achieve a significant spectral change over a small enough angle, x-ray motion can be achieved through electromagnetic deflection of the focal spot (of the same kind used in modern CT systems to achieve improved sampling [10], [11]).
In the following subsections we illustrate the design and fabrication of a grating prototype for spectral shaping, present x-ray spectrometer measurements through this grating as a function of x-ray angle, and integrate the grating prototype into CT test-bench and conduct a simple material decomposition of clinically relevant iodine concentrations.
B. Grating Design
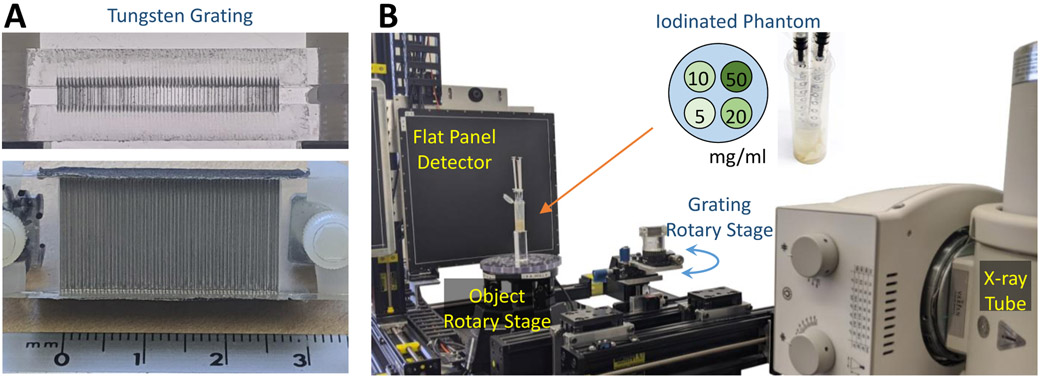
There are various options for grating design. In this work, we adopt a fabrication approach based on the creation of a notched form in which thin rectangles of metal foil are placed to form the grating. The notched form is created from deep etched acrylic using a laser cutter/etcher. This manufacturing method permits the use of a wide range of filtration materials with no interstitial material. It is limited by the resolution of the laser cutter and depth control, and the need for metal used for the grating to be self-supporting.
The design chosen for this work is illustrated in Fig. 1 with 50 μm wide tungsten lamellae 3 mm thick. The center-to-center spacing of the metal foil is 300 μm yielding a 250 μm air-gap. The acrylic form is deep etched to about 300 μm. The face of the fabricated grating is 2.5 cm wide x 1.5 cm tall. (The results of this fabrication can be seen in Fig. 3A.) This design results in two “critical points” at 4.7° and 5.7° - namely the first angle where all x-rays pass through one grating lamella; and where all x-rays pass through two grating lamellae (as illustrated in Fig. 1). We expect that the greatest amount of spectral shaping occurs between these two critical points based on the thickness of tungsten traversed.
Fig. 3.
A) Illustration of the manufactured grating showing the top view and the surface perpendicular to the x-ray path. B) The grating-based spectral CT test-bench including a small phantom with four concentrations of iodine (5, 10, 20, and 50 mg/mL of iodine - Omnipaque).
C. X-ray Spectrometer Measurements
While we envision that spectral grating will ideally be implemented using focal spot deflection, we investigate the grating structure using a computer-controlled rotation stage. In a first set of experiments, we use an Amptek XR-100T CdTe x-ray spectrometer (Bedford, MA) to characterize the filtered x-ray beam. Due to count rate limitations, the spectrometer is collimated using a Fluke 10 μm pinhole (part number 07-633) placed in a 13 mm thick lead sheet. The experimental setup is shown in Figure 2. All measurements were taken at 100 kVp and 0.8 mAs/pulse, and averaged over 300 pulses. The pinhole was carefully aligned by maximizing count rate. Spectra were obtained over 0°-7° with 0.25° increments.
Fig. 2.
Illustration of the test bench used for x-ray spectroscopy including a manual rotary stage for the grating, pinhole collimation and an x-ray spectrometer.
D. Grating-based Spectral CT Experiment
A spectral CT experiment was conducted using the test-bench shown in Fig. 3B. The test-bench consists of an object rotation stage, a Varex 4343CB (Salt Lake City, UT) flat-panel detector, and a Varex Rad-94 Rad/Fluoro tube. A small 25 mm diameter iodine-water phantom was prepared with four different concentrations of Omnipaque – 5, 10, 20, and 50 mg/mL of iodine. The phantom is intentionally small because the spectral grating was constructed with parallel lamellae – a relatively small phantom limits differential filtration due to diverging x-rays by constraining the projection data to a smaller angular range. Similarly, a relatively long baseline geometry was used to limit the effects of divergent x-rays. Specifically, the source-to-detector distance was 1600 mm, the source-to-axis distance was 1100 mm, and source-to-grating distance was 640 mm. Projection data were obtained using the 0.8 mm focal spot, 100 kVp, and 2 mAs/frame with 360 frames evenly spaced over 360°. Data were obtained at grating angles of 4° and 6°. These two angles were chosen based on the results of the x-ray spectrum estimates.
Since some banding artifacts appeared in projection data with simple gain correction (likely because the grating period was not small enough compared to the focal spot size, and because of focal spot instability and small shifts), we applied two projection-domain correction strategies: 1) a focal spot shift estimation and application to the gain scan [12]; and 2) a radial filtering operation to suppress residual bands. Data were reconstructed using filtered-backprojection and additional image-domain ring-artifact suppression routines were applied. Both 4° and 6° grating projection data were processed. Iodine and water maps were formed using a simple empirically selected image-domain weighted sum of the two spectral channels.
III. Results
A. X-ray Spectra as a Function of Grating Angle
The results of the x-ray spectrometry experiment are shown in Fig 4. Fig. 4A shows the raw photon count measurements for grating angulations from 0° to 7°. While some beam shaping is evident, the more noticeable feature is the overall reduction in fluence with the changing angle. To highlight the spectral shaping, we show normalized spectra in Fig. 4B. In this case, the spectra are divided by the total number of counts. Note the obvious shaping of the beam and the dramatic reduction of higher energy photons (above the k-edge of tungsten at 69.5 keV). We see the most significant change in spectral shape between 4° and 6° where the angulation crosses the previously identified “critical points.” To better illustrate the transition spectra in this range, a third plot in Fig. 4C shows a finer sampling of spectra between 4.5° and 6°. Note that 0.25° increments over this range result in significant changes to the x-ray spectrum.
Fig. 4.
Measured x-ray spectra as a function of grating tilt angle. A) Raw absolute measurements from 0° to 7° in 1° increments. B) The same measurements normalized by the total number of photon counts better illustrating the change in spectral shape from 0° to 7°. C) A finer sampling of spectra using 0.25° increments over 4.5° to 6.0° to illustrate additional intermediate spectra. Note the prominent change in spectral shape about 69.5 keV – the k-edge of tungsten.
B. Grating-based Spectral CT
Based on the spectral estimation results from the previous subsection we chose to collect CT projection data at 4° and 6° rotations of the grating. Reconstructions from these two datasets are shown in Fig. 5A. Note the contrast differences (particularly noticeable in the highest concentration of iodine) between the inserts and background due to the differing incident x-ray spectrum. Image-domain decomposition was applied using a weighted sum of the two reconstructions. Approximate iodine and water basis channels are shown in Fig. 5B. We see a good representation of the four concentration levels of iodine in the iodine basis channel, and a relatively uniform water basis channel for both the inserts and the water background.
Fig. 5.

Reconstructions from the angled grating system. A) Image volumes generated from the 4° and 6° grating rotations show differential enhancement for each angulation due to spectral shaping. B) Water-iodine decomposition shows good visualization of the iodinated inserts and the water background in the phantom.
IV. Discussion
The presented results reflect a proof-of-principle demonstration of a novel spectral CT concept that is a relatively simple upgrade to standard energy-integrating CT. Our concept entails a fine-pitch grating that controls the spectral shaping through slight rotations. We demonstrated in a preliminary experiment that spectral CT imaging with a grating prototype is feasible and that material decomposition of iodine in clinical relative concentration is possible. For a detailed analysis of sensitivity and robustness, studies with increased field-of-view, enabled focal-spot movements, and optimized grating design are required. To this end, our results illustrate that gratings with the required specifications can be manufactured without need of highly expensive processes. For future studies, we will investigate different fabrication processes, including lithography, mechanical folding, and 3D laser cutting, with the goal to drive grating designs with high aspect ratios to translate this concept from bench to clinical CT systems.
For implementation into a clinical CT gantry, we propose to move the focal spot of the x-ray tube through electromagnetic steering, to switch between the filtration scenarios (i.e. applying the necessary angle). Focal spot movement is a precise (sub-pixel) and commonly used technique in most modern x-ray tubes and is rapidly performed from projection to projection image [11]. Utilizing focal spot movements will allow: (i) the generation of spectral data without mechanical motions (which are challenging to coordinate within a fast-rotating gantry), and (ii) extension of our concept to multi-energy CT. For example, one can combine two gratings – each manufactured from a different k-edge material (e.g., tantalum and gold) in series. With orthogonally oriented lamella, two-dimensional angulations achieved via electromagnetically deflection of the focal spot in two directions would allow an even more diverse continuum of emitted x-ray spectra. This extension to multi-energy CT will drive the clinical introduction and translation of novel diagnostic applications like dual-contrast protocols in oncology or cardiovascular imaging [13]-[18].
Future studies will investigate comparisons between this and other spectral CT methods. For example, kV-switching can achieve different spectra without mechanical motion; however, the effected spectra may have decreased separation versus the grating based approach. Moreover, combinations of spectral methods – e.g. kV-switching and grating – may be combined for increased sensitivity and material decomposition performance.
V. Conclusion
The translation and introduction of spectral CT into the clinical arena is ongoing and becoming increasingly popular. Our concept of spectral CT imaging with gratings adds an attractive and relatively simple alternative solution to existing implementation, which may enable multi-energy CT imaging in the future.
VI. Acknowledgment
We thank Roland Proksa and Kevin Brown for fruitful discussions.
This work was supported in part by NIH grant R21EB026849.
References
- [1].Mei K et al. , “Bone mineral density measurements in vertebral specimens and phantoms using dual-layer spectral computed tomography,” Sci. Rep, vol. 7, no. 1, pp. 1–10, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Canni R, Naveh G, and Altman A, “Material Separation with Dual-Layer CT,” in IEEE Nuclear Science Symposium Conference Record, 2005, vol. 4. pp. 1876–1878. [Google Scholar]
- [3].Schlomka J-P et al. , “Experimental feasibility of multi-energy photon-counting K-edge imaging in pre-clinical computed tomography,” Phys. Med. Biol, vol. 53, no. 15, pp. 4031–4047, Aug. 2008. [DOI] [PubMed] [Google Scholar]
- [4].Roessl E and Proksa R, “K-edge imaging in x-ray computed tomography using multi-bin photon counting detectors,” Phys. Med. Biol, vol. 52, no. 15, pp. 4679–4696, Aug. 2007. [DOI] [PubMed] [Google Scholar]
- [5].Xu D et al. , “Dual energy CT via fast kVp switching spectrum estimation,” in SPIE Medical Imaging, 2009, p. 72583T. [Google Scholar]
- [6].Yu L, Primak AN, Liu X, and McCollough CH, “Image quality optimization and evaluation of linearly mixed images in dual-source, dual-energy CT,” Med. Phys, vol. 36, no. 3, pp. 1019–1024, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Rutt B and Fenster A, “Split-filter computed tomography: a simple technique for dual energy scanning.,” J. Comput. Assist. Tomogr, vol. 4, no. 4, pp. 501–9, Aug. 1980. [DOI] [PubMed] [Google Scholar]
- [8].Hauck R, Gray F, and Brandt R, “Spinning filter for x-ray,” US Patent Number: 4,399,550.
- [9].Tivnan M, Wang W, Tilley S, Siewerdsen JH, and Stayman JW, “Optimized spatial-spectral CT for multi-material decomposition,” in 15th International Meeting on Fully Three-Dimensional Image Reconstruction in Radiology and Nuclear Medicine, 2019, p. 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Flohr TG, Stierstorfer K, Ulzheimer S, Bruder H, Primak AN, and McCollough CH, “Image reconstruction and image quality evaluation for a 64-slice CT scanner with z-flying focal spot,” Med. Phys, vol. 32, no. 8, pp. 2536–2547, Jul. 2005. [DOI] [PubMed] [Google Scholar]
- [11].Behling R, Modern diagnostic x-ray sources : technology, manufacturing, reliability. CRC Press, 2015. [Google Scholar]
- [12].Gang GJ et al. , “Dynamic fluence field modulation in computed tomography using multiple aperture devices,” Phys. Med. Biol, vol. 64, no. 10, May 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Connode DP et al. , “Multicolor spectral photon-counting computed tomography: in vivo dual contrast imaging with a high count rate scanner,” Sci. Rep, vol. 7, no. 1, p. 4784, Dec. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Dangelmaier J et al. , “Experimental feasibility of spectral photon-counting computed tomography with two contrast agents for the detection of endoleaks following endovascular aortic repair,” Eur. Radiol, pp. 1–8, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Muenzel D et al. , “Spectral Photon-counting CT: Initial Experience with Dual–Contrast Agent K-Edge Colonography,” Radiology, vol. 283, no. 3, pp. 723–728, 2017. [DOI] [PubMed] [Google Scholar]
- [16].Si-Mohamed S et al. , “Spectral Photon-Counting Computed Tomography (SPCCT): in-vivo single-acquisition multi-phase liver imaging with a dual contrast agent protocol,” Sci. Rep, vol. 9, no. 1, p. 8458, Dec. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Symons R et al. , “Dual-contrast agent photon-counting computed tomography of the heart: initial experience,” Ira. J. Cardiovasc. Imaging, vol. 33, no. 8, pp. 1253–1261, Aug. 2017. [DOI] [PubMed] [Google Scholar]
- [18].Tao S, Rajendran K, McCollough CH, and Leng S, “Feasibility of multi-contrast imaging on dual-source photon counting detector (PCD) CT: An initial phantom study,” Med. Phys, vol. 46, no. 9, pp. 4105–4115, Sep. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]