Abstract
The severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2) viral genome is an RNA virus consisting of approximately 30,000 bases. As part of testing efforts, whole genome sequencing of human isolates has resulted in over 1,600 complete genomes publicly available from GenBank. We have performed a comparative analysis of the sequences, in order to detect common mutations within the population. Analysis of variants occurring within the assembled genomes yields 417 variants occurring in at least 1% of the completed genomes, including 229 within the 5’ untranslated region (UTR), 152 within the 3’UTR, 2 within intergenic regions and 34 within coding sequences.
Introduction
SARS-CoV-2, formerly known as the “Wuhan seafood market pneumonia virus,” is a novel coronavirus that first appeared at the seafood and wildlife wholesale market in Wuhan, Hubei Provence, China during late November/early December, 2019 [1]. Due to its high human-to-human transmission rate [2], longer than normal latent period [3] and mortality rates in vulnerable populations, a global pandemic was declared by the World Health Organization (WHO) on March 11, 2020 for the associated COVID-19 disease [4]. As of May 10, 2020, a total of 4,006,257 cases resulting in 278,892 deaths in 215 countries have been confirmed [5].
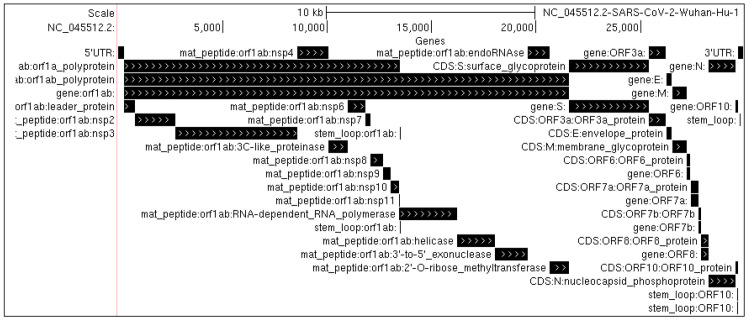
The reference genome of the SARS-Cov-2 RNA virus (GenBank accession NC_045512) consists of 29,903 bases. Among its features are a 265 base 5’ untranslated region (UTR) and a 3’ UTR composed of 229 bases. Its coding regions consist of 10 open reading frames (ORFs) coding for 26 genes [3], including 13,218 bases coding for the ORF1ab polyproteins, whose transcription includes a 1 bp ribosomal slippage event [6]; 3,822 bases coding for a spike surface glycoprotein (S), 228 bases coding a small envelope protein (E), 669 bases coding for a membrane glycoprotein protein (M) and 1,260 bases coding for a nucleocapsid protein (N) along with five additional ORFs (ORF3a, ORF6, ORF7a/ORF7b, ORF8, and ORF10) (Fig 1).
Fig 1. Genome structure of SARS-CoV-2.
Shown are the locations of the 10 major open reading frames, as well as specific peptides and structural elements produced within them.
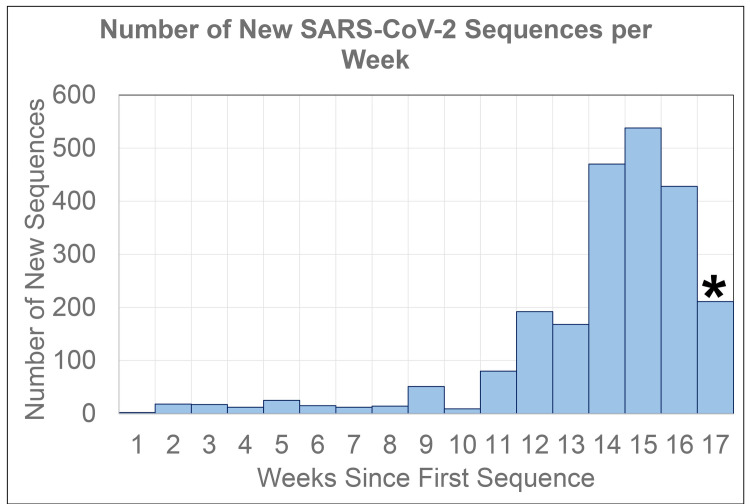
Since the public release of the first reference sequence (MN908947; NC_045512) from NCBI’s GenBank [7] on January 12, 2020, the number of sequences available has increased exponentially, at a current rate of approximately 70 new sequences per day (Fig 2). Isolates have been sequenced from 27 countries (Table 1), as well as 34 states, Washington DC, and passengers from a cruise (Table 2).
Fig 2. Number of new SARS-CoV-2 sequences deposited in GenBank on a weekly basis through May 7, 2020.
*Week 17 is only a partial week.
Table 1. Isolate groups and geographic locations with identical SARS-CoV-2 genomic sequences.
| Name | Num Seqs | Location | Other Locations |
|---|---|---|---|
| MULTIPLE_31 | 18 | USA-WA | -- |
| MULTIPLE_25 | 16 | USA-WA | USA-NY,USA-NC,USA-UT |
| MULTIPLE_9 | 14 | CHN-Zhejiang | -- |
| MULTIPLE_8 | 13 | USA-MA | USA-KS,USA-NC,USA-PA |
| MULTIPLE_21 | 12 | USA-NY | -- |
| MULTIPLE_11 | 9 | USA-CruiseA | USA-IL |
| MULTIPLE_16 | 7 | USA-GA | USA-SC |
| MULTIPLE_26 | 7 | USA-NY | -- |
| MULTIPLE_35 | 5 | USA-NY | -- |
| MULTIPLE_6 | 4 | USA-MA | -- |
| MULTIPLE_12 | 4 | USA-IA | -- |
| MULTIPLE_45 | 4 | USA-NY | USA-RI,USA-OH |
| MULTIPLE_2 | 3 | USA-MI | -- |
| MULTIPLE_3 | 3 | USA-NY | -- |
| MULTIPLE_4 | 3 | HKG | -- |
| MULTIPLE_10 | 3 | KOR | -- |
| MULTIPLE_18 | 3 | USA-NY | -- |
| MULTIPLE_23 | 3 | USA-NY | USA-NJ |
| MULTIPLE_24 | 3 | USA-OH | USA-PA |
| MULTIPLE_28 | 3 | CZE | -- |
| MULTIPLE_29 | 3 | HKG | -- |
| MULTIPLE_33 | 3 | USA-NH | -- |
| MULTIPLE_37 | 3 | USA-NY | -- |
| MULTIPLE_40 | 3 | USA-WA | -- |
| MULTIPLE_43 | 3 | CHN-Beijing | -- |
| MULTIPLE_1 | 2 | USA | USA-CA,USA-WA |
| MULTIPLE_5 | 2 | USA-NY | -- |
| MULTIPLE_7 | 2 | CHN | -- |
| MULTIPLE_13 | 2 | USA-CA | -- |
| MULTIPLE_14 | 2 | -- | USA-CA,TWN |
| MULTIPLE_15 | 2 | USA-IN | -- |
| MULTIPLE_17 | 2 | USA-IA | -- |
| MULTIPLE_19 | 2 | USA-WA | -- |
| MULTIPLE_20 | 2 | CHN | -- |
| MULTIPLE_22 | 2 | TWN | -- |
| MULTIPLE_27 | 2 | USA-NY | -- |
| MULTIPLE_30 | 2 | USA-NY | -- |
| MULTIPLE_32 | 2 | USA-FL | -- |
| MULTIPLE_34 | 2 | ESP | -- |
| MULTIPLE_36 | 2 | USA-NE | -- |
| MULTIPLE_38 | 2 | USA-CA | -- |
| MULTIPLE_39 | 2 | USA-MI | -- |
| MULTIPLE_41 | 2 | USA-WA | -- |
| MULTIPLE_42 | 2 | USA | -- |
| MULTIPLE_44 | 2 | USA-CA | -- |
| MULTIPLE_46 | 2 | -- | USA-PA,USA-VA |
| MULTIPLE_47 | 2 | USA-NY | -- |
Table 2. Number of SARS-CoV-2 isolate sequences extracted from GenBank by country.
| Country | Count | Country | Count |
|---|---|---|---|
| USA | 1025 | MYS | 2 |
| CHN | 64 | PAK | 2 |
| TWN | 21 | VNM | 2 |
| HKG | 19 | AUS | 1 |
| IND | 10 | FRA | 1 |
| ESP | 7 | IRN | 1 |
| PRI | 5 | ITA | 1 |
| GRC | 4 | NPL | 1 |
| KAZ | 4 | PER | 1 |
| KOR | 4 | SWE | 1 |
| LKA | 4 | TUR | 1 |
| BRA | 2 | ZAF | 1 |
| ISR | 2 |
SARS-CoV-2 is postulated to have originated from zoonotic transfer of a pangolin betacoronavirus based on a phylogenetic analysis of coronavirus sequences, due to a common insertion of 12 nucleotides within the receptor binding domain of the S protein region that optimizes binding to the human ACE2 receptor, although the most similar betacoronavirus is the bat RaTG13 [8]. RNA viruses are characterized by a high mutation rate [9] driven by RNA dependent RNA polymerase (RdRp) that results in viral evolution [10]. Given the importance of the genome sequence with host transmission (in particular the S protein), it is important to understand common mutations within the population in order to have a better handle on viral load, virus spread, virus evolution, and disease severity.
A number of previous studies have examined variants within SARS-CoV-2 isolates. One prior study looking at the genome diversity of SARS-CoV-2 have identified 93 mutations occurring in at least one isolate from a set of 86 complete genomes [11]. A second report from a set of 220 complete genomes identified eight novel recurring mutations, with specific prevalence within Asian, North American, and European populations [10]. This study by Pachetti, et. al, also showed the occurrence of mutations over time based on sequence sampling dates. A report by Yin [12] identified fifteen high-frequency single nucleotide polymorphisms when comparing a set of 558 SARS-CoV-2 strains. This study found four of these mutations, 241C > T, 3037C > T, 14408C > T, and 23403A > G to be more prevalent in European strains, where the COVID-19 is typically more severe. Wang et. al [13] detected 13 variation sites among 95 full-length genomic sequences (variants occurring in at least 3 isolates), with two at positions 8,782 and 28,144 showing a high mutation rate around 30%. Khailany et. al [14] looked at mutations within 95 complete genome sequences, and found 116 mutations occurring in at least one isolate, with the most common being 8762C > T, 28144T > C, and 29095C > T. Tang et. al [15] studied mutations across 103 strains, and determined there were mutations in 149 sites, including six nonsynonymous mutations occurring at least twice. Additionally, this study identified two different mutations (with near complete linkage between 8782T > C and 28144C > T) that separated the virus into two groups labeled L and S. Using deep sequencing reads, Tang et. al also identified 18 locations showing intrahost variants, demonstrating heterogeneity of the virus within a specific host. Wang et. al [16] identified ten high frequency mutations within a set of 108 isolates, which they say can be used to classify SARS-CoV-2 into five main groups. The mutations published include 28144T > C,8782C > T, 23403A > G, 3037C > T, 11083G > T, 26144G > T, 2261G > T.
Forster et. al [17] used phylogenetic network analysis to find three central variants, A, B, and C, distinguishing East Asian isolates from European and American isolates. In their study, variants found defined clusters and/or subclusters by synonymous mutations 29095T > C (subclustering A), synonymous mutation 8782T > C and nonsynonymous mutation 28144C > T (separation of clusters A and B). In addition, Shen et. al [18] observed a median of 1–4 intrahost variants, ranging from 0–51, from a set of eight patients infected with SARS-CoV-2. Included in the Shen study were two patients from the same household, one of which was likely to have infected the other. Interestingly, only 7/25 variants detected in these two individuals were shared, illustrating the high viral mutation rate.
The SARS-CoV-2 spike (S) protein is of particular interest, due to its interaction with the human ACE2 protein that helps to mediate infection of host cells. Korber, et. al [19] have set up a workflow for measuring the dynamics of nonsynonymous mutations within the S protein coding region. This study has uncovered a number of amino acid mutations in the S protein, including D614G (23403G > A), which is thought to increase transmissibility due to its rapid expansion in global samples. This mutation was shown to have a high association with two other mutations, 3037C > T and 14409C > T.
Materials and methods
All available SARS-CoV-2 nucleotide sequences and associated annotations (including locality) were downloaded from NCBI (https://www.ncbi.nlm.nih.gov/genbank/sars-cov-2-seqs/) on 5/7/2020, resulting in 2,262 sequences. Geographical location information was reduced to the corresponding ISO 3166–1 alpha-3 country code (S1 Table), and the two-letter abbreviated state code. Sequences were then filtered to include only those listed as complete with an isolation source of “Homo sapiens”, which left 1,695 sequences listed as complete genomes isolated from human samples. A total of 538 sequences from this set containing gaps in their assembly (defined by the character “N”) were removed from further analysis, leaving 1,157 complete, ungapped genomes. One-hundred and fifty-nine (159) isolate sequences were represented two or more times, resulting in 45 unique genomes from the set of 159. The isolates that were 100% matches across their entire length were merged into a single representative sequence (Table 3). A final total of 1,043 unique complete genomic sequences without gaps were used in the final analysis, including three from Kentucky that we previously analyzed [20]. These 1,043 sequences were then compared against each other, and a distance matrix was constructed based on the number of gaps and mismatches, as calculated by NCBI blastn (v2.10.0) [21]. A multiple sequence alignment was performed using kalign (v3.2.5) [22] which resulted in a.aln alignment file which was used for further detection and analysis of variants. The results from kalign were used as input into a custom perl script, findVariants.pl, which determined all minor allele frequencies, their genomic positions, overlap with gene annotations, and effect on codons and amino acids. The alignment file as well as other supporting data and scripts are available on our github site: https://github.com/UofLBioinformatics/SARS_CoV_2_Variants.
Table 3. Number of SARS-CoV-2 isolate sequences extracted from GenBank by state/region.
| State/Region | Count | State/Region | Count |
|---|---|---|---|
| USA-WA | 334 | USA-SC | 7 |
| USA-NY | 185 | USA-AZ | 6 |
| USA-CA | 115 | USA-TX | 5 |
| USA-MI | 44 | USA-IN | 4 |
| USA-VA | 37 | USA-LA | 4 |
| USA-CT | 28 | USA-NH | 4 |
| USA-CruiseA | 25 | USA-OR | 4 |
| USA-ID | 22 | USA-RI | 4 |
| USA-MA | 19 | USA-KY | 3 |
| USA-GA | 15 | USA-NJ | 3 |
| USA-FL | 10 | USA-OH | 3 |
| USA-UT | 10 | USA-HI | 2 |
| USA-WI | 10 | USA-MD | 2 |
| USA-PA | 9 | USA-NE | 2 |
| USA-IA | 7 | USA-NV | 2 |
| USA-IL | 7 | USA-DC | 1 |
| USA-MN | 7 | USA-KS | 1 |
| USA-NC | 7 | USA-MO | 1 |
Variants were analyzed for their linkage using Haploview (v4.2) which generates a logarithm of odds (LOD) score to statistically describe whether two variants are likely to be inherited together based on their covariance and chromosomal position. In the case of a small genome, such as SARS-CoV-2, the covariance described by the r2 correlation coefficient is likely to provide the most informative information relating two variants.
Results
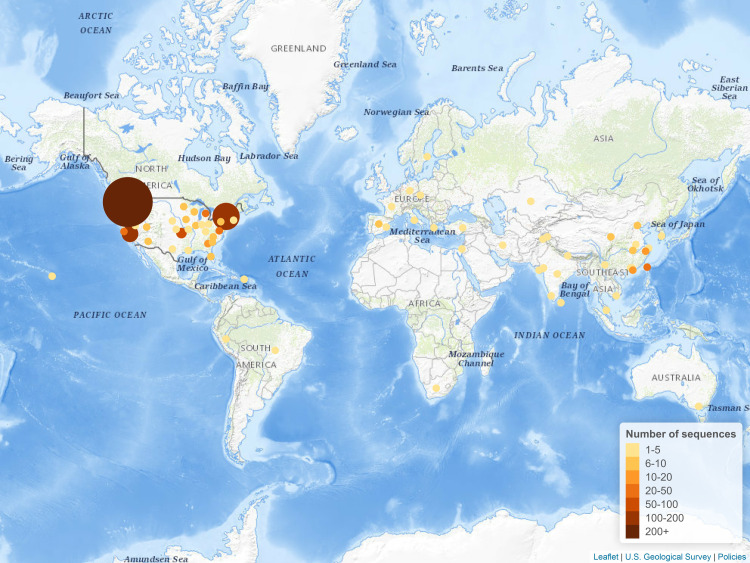
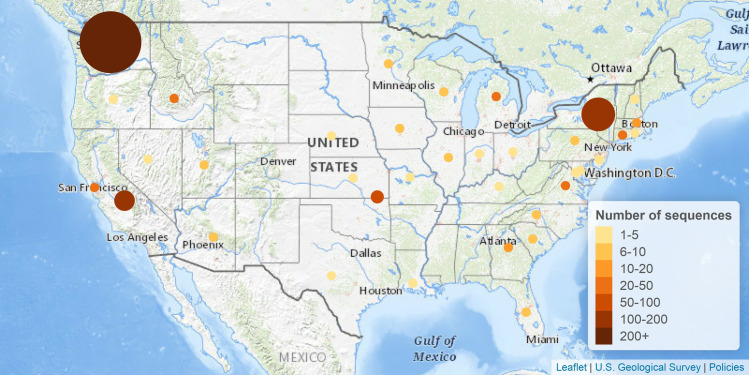
Our analysis focused on a set of 1,043 filtered sequences, including 1,040 publicly available in GenBank, as well as three new isolates sequenced by our group in Kentucky (GenBank accessions MT365025, MT365026, and MT365027). From the non-filtered group of complete and ungapped SARS-CoV-2 genomic sequences, we found 47 groups of sequences that had at least two isolates that were 100% identical, including one group with 18 isolates all from Washington state; a second group with 16 isolates primarily from Washington state, and one group of 14 isolates all from Zhejiang, China (Table 1). These clustered groups of sequences are not surprising, particularly when examining the geographical similarities, suggesting clusters of similar transmission in both time and viral strains. The majority of the sequences were from the United States (Table 2, Fig 3), with California, New York, and Washington comprising the majority of the sequences (Table 3, Fig 4).
Fig 3. Distribution of the number of SARS-CoV-2 sequences in GenBank worldwide.
Figure created using Leaflet (leafletjs.com) with tiles from The National Map. Map services and data available from U.S. Geological Survey, National Geospatial Program.
Fig 4. Distribution of the number of SARS-CoV-2 sequences in the United States available in GenBank.
Figure created using Leaflet (leafletjs.com) with tiles from The National Map. Map services and data available from U.S. Geological Survey, National Geospatial Program.
Based on a variant threshold of minor allele frequencies of 1% or more, we detected a total of 417 locations where isolate genomes express an alternate allele than the reference, NC_045512, including 229 in the 5’UTR, 21 in ORF1ab, 2 in S, 3 in ORF3a, 1 in M, 3 in ORF8, 4 in N, 2 intergenic, and 152 in the 3’UTR. The vast majority of the detected variants lie within the 5’UTR and 3’UTR, and represent deletion events. Since these may be due to a number of factors such as sequencing preparation (i.e. selection of amplicon primers) and difficulty in multiple sequence alignment construction that contribute to less reliability, we only retained seven of the UTR variants for further consideration. These seven UTR variants represent non-indel events which are unlikely to be sequencing artifacts. Overall, our filtering led to a total of 44 variants (Table 4). Of these, twenty have previously been reported (S2 Table), including the pair at locations 8782/28144 which was previously demonstrated to have a high linkage [15] and the tuples at locations 8782/18060/28144, 241/3037/23403/28144, and 241/3037/14408/23403 which were shown to have a high number of descendants [12]. We uncovered 24 novel variants not previously described to our knowledge, which may be a result of more recent mutation events and/or fixation of a mutation within the population. Surprisingly, two of these, 1059C > T in nsp2 region of ORF1ab and 25563G > T in ORF3a are found at a high frequency within the population, at a rate of 43% and 49%, respectively.
Table 4. Common variants (> 1%) detected within SARS-CoV-2 isolates.
| Reference | Allele Counts | Nucleotide | Codon | Amino Acid | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Position | Region | MAF | Cons | Alt | Ref | Cons | Alt | Ref | Cons | Alt | Ref | |
| 50 | 5’UTR | C:811;-:220;T:10;A:2 | 0.010 | C | T | C | NA | NA | NA | NA | NA | NA |
| 241 | 5’UTR | T:599;C:436;-:7;Y:1 | 0.418 | T | C | C | NA | NA | NA | NA | NA | NA |
| 490 | ORF1ab1 | T:1022;A:20;W:1 | 0.192 | T | A | T | GAT | GAA | GAT | Asp | Glu | Asp |
| 833* | ORF1ab1 | T:1011;C:32 | 0.031 | T | C | T | TTC | CTC | TTC | Phe | Leu | Phe |
| 1059 | ORF1ab1 | C:593;T:449;Y:1 | 0.430 | C | T | C | ACC | ATC | ACC | Thr | Ile | Thr |
| 1397 | ORF1ab1 | G:1030;A:13 | 0.013 | G | A | G | GTA | ATA | GTA | Val | Ile | Val |
| 2416 | ORF1ab1 | C:1023;T:20 | 0.089 | C | T | C | TAC | TAT | TAC | Tyr | Tyr | Tyr |
| 3037 | ORF1ab1 | T:586;C:457 | 0.438 | T | C | C | TTT | TTC | TTC | Phe | Phe | Phe |
| 3177 | ORF1ab1 | C:1022;T:20;Y:1 | 0.192 | C | T | C | CCT | CTT | CCT | Pro | Leu | Pro |
| 6040 | ORF1ab1 | C:1028;T:15 | 0.014 | C | T | C | TTC | TTT | TTC | Phe | Phe | Phe |
| 8782 | ORF1ab1 | C:791;T:251;Y:1 | 0.241 | C | T | C | AGC | AGT | AGC | Ser | Ser | Ser |
| 11083 | ORF1ab1 | G:973;T:69;C:1 | 0.066 | G | T | G | TTG | TTT | TTG | Leu | Phe | Leu |
| 11916* | ORF1ab1 | C:1008;T:35 | 0.036 | C | T | C | TCA | TTA | TCA | Ser | Leu | Ser |
| 14408 | ORF1ab2 | T:603;C:440 | 0.422 | T | C | C | CTT | CCT | CCT | Leu | Pro | Pro |
| 14805 | ORF1ab2 | C:1003;T:40 | 0.038 | C | T | C | TAC | TAT | TAC | Tyr | Tyr | Tyr |
| 17247 | ORF1ab2 | T:1018;C:25 | 0.024 | T | C | T | CGT | CGC | CGT | Arg | Arg | Arg |
| 17747 | ORF1ab2 | C:850;T:189;Y:4 | 0.181 | C | T | C | CCT | CTT | CCT | Pro | Leu | Pro |
| 17858 | ORF1ab2 | A:851;G:192 | 0.184 | A | G | A | TAT | TGT | TAT | Tyr | Cys | Tyr |
| 18060 | ORF1ab2 | C:849;T:194 | 0.186 | C | T | C | CTC | CTT | CTC | Leu | Leu | Leu |
| 18736* | ORF1ab2 | T:1024;C:19 | 0.018 | T | C | T | TTT | CTT | TTT | Phe | Leu | Phe |
| 18877 | ORF1ab2 | C:1004;T:39 | 0.037 | C | T | C | CTA | TTA | CTA | Leu | Leu | Leu |
| 18998* | ORF1ab2 | C:1017;T:26 | 0.025 | C | T | C | GCA | GTA | GCA | Ala | Val | Ala |
| 20268 | ORF1ab2 | A:1031;G:12 | 0.012 | A | G | A | TTA | TTG | TTA | Leu | Leu | Leu |
| 23403 | S | G:602;A:439;R:2 | 0.421 | G | A | A | GGT | GAT | GAT | Gly | Asp | Asp |
| 24034 | S | C:1017;T:25;Y:1 | 0.024 | C | T | C | AAC | AAT | AAC | Asn | Asn | Asn |
| 25563* | ORF3a | G:536;T:507 | 0.486 | G | T | G | CAG | CAT | CAG | Gln | His | Gln |
| 25692 | ORF3a | C:1022;T:21 | 0.020 | C | T | C | GGC | GGT | GGC | Gly | Gly | Gly |
| 26144 | ORF3a | G:999;T:44 | 0.042 | G | T | G | GGT | GTT | GGT | Gly | Val | Gly |
| 26729 | M | T:1020;C:22;Y:1 | 0.021 | T | C | T | GCT | GCC | GCT | Ala | Ala | Ala |
| 27964 | ORF8 | C:990;T:52;-:1 | 0.050 | C | T | C | TCA | TTA | TCA | Ser | Leu | Ser |
| 28077 | ORF8 | G:1017;C:22;T:2;-:1;S:1 | 0.021 | G | C | G | GTG | CTG | GTG | Val | Leu | Val |
| 28144 | ORF8 | T:791;C:250;-:1;Y:1 | 0.240 | T | C | T | TTA | TCA | TTA | Leu | Ser | Leu |
| 28688 | N | T:1032;C:11 | 0.011 | T | C | T | TTG | CTG | TTG | Leu | Leu | Leu |
| 28881 | N | G:990;A:53 | 0.051 | G | A | G | AGG | AAG | AGG | Arg | Lys | Arg |
| 28882 | N | G:991;A:52 | 0.050 | G | A | G | AGG | AGA | AGG | Arg | Arg | Arg |
| 28883 | N | G:991;C:52 | 0.050 | G | C | G | GGA | CGA | GGA | Gly | Arg | Gly |
| 29540 | INTERGENIC | G:1017;A:26 | 0.025 | G | A | G | NA | NA | NA | NA | NA | NA |
| 29553 | INTERGENIC | G:939;A:104 | 0.100 | G | A | G | NA | NA | NA | NA | NA | NA |
| 29700 | 3’UTR | A:1023;G:20 | 0.019 | A | G | A | NA | NA | NA | NA | NA | NA |
| 29742 | 3’UTR | G:1025;T:11;A:4;-:3 | 0.011 | G | T | G | NA | NA | NA | NA | NA | NA |
| 29864 | 3’UTR | G:746;-:275;A:19;T:1;R:1;V:1 | 0.018 | G | A | G | NA | NA | NA | NA | NA | NA |
| 29867 | 3’UTR | T:645;-:361;A:37 | 0.035 | T | A | T | NA | NA | NA | NA | NA | NA |
| 29868 | 3’UTR | G:625;-:367;A:39;C:8;R:3;D:1 | 0.051 | G | A | G | NA | NA | NA | NA | NA | NA |
| 29870 | 3’UTR | C:608;-:378;A:50;M:5;H:1;T:1 | 0.048 | C | A | C | NA | NA | NA | NA | NA | NA |
Cons: alignment consensus; Alt: alignment alternative; Ref: NC_045512 reference sequence. Note that the ORF1ab open reading frame is split into two parts, ORF1ab1 which occurs from bases 266…13468 in the reference sequence, and ORF1ab2 which occurs from bases 13468…21555 as result of a polymerase slippage event. MAF: Minor allele frequency
*Previously unreported nonsynonymous mutation.
One of the variants found, 29742G > T, occurs within the 3’ UTR stem loop II-like motif (s2m) which plays a role in viral replication and recruitment of host transcriptional machinery [23]. This mutation therefore may affect the folding of the s2m motif. None of the other non-coding mutations are found within known structural elements.
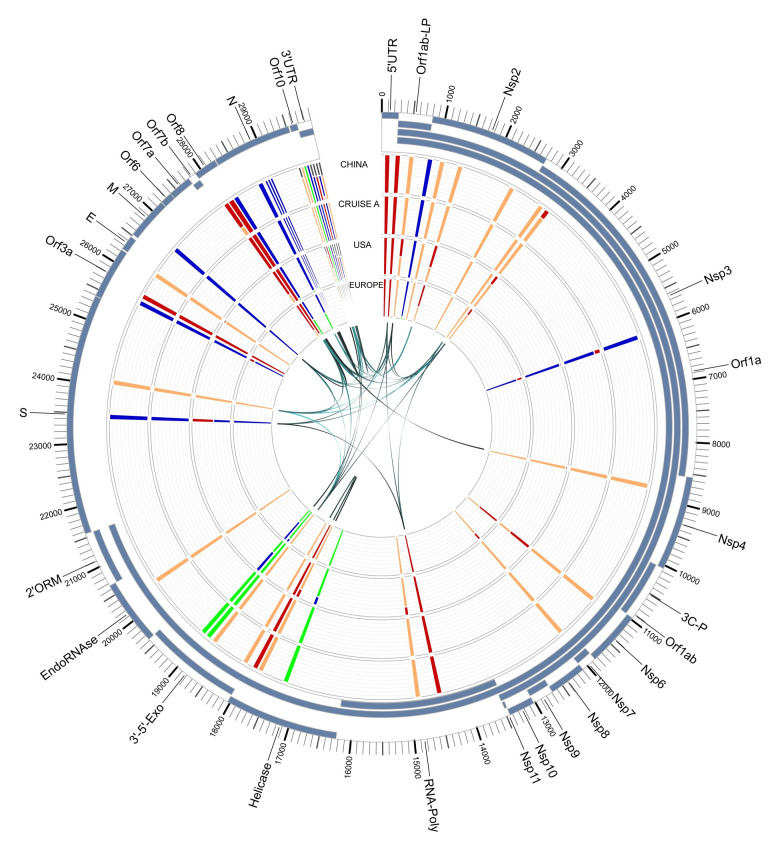
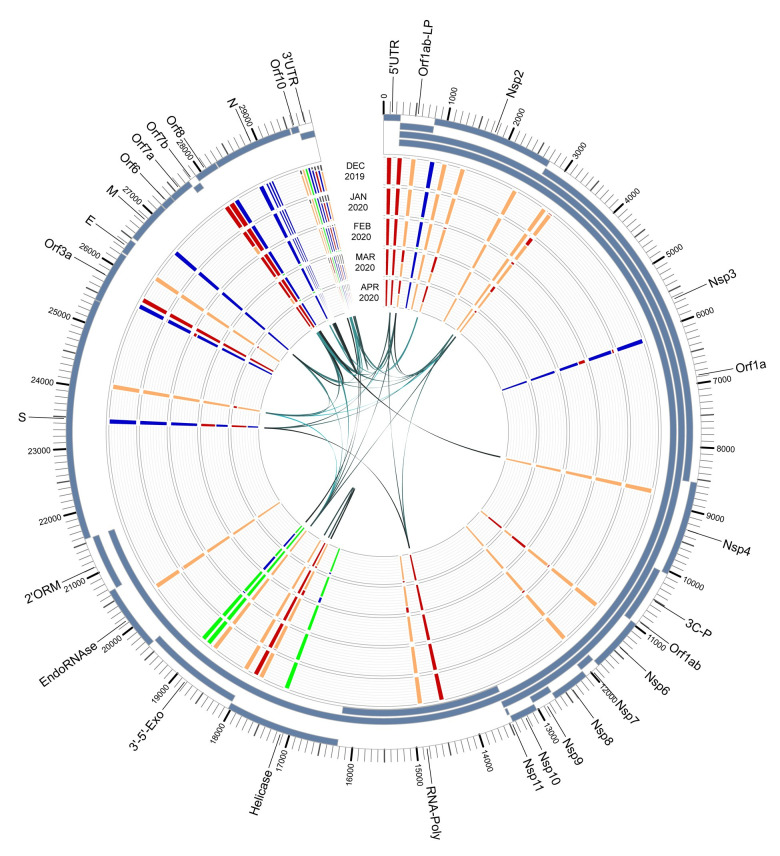
To understand if the variant alleles were linked to each other, we analyzed the linkage between the detected variants using Haploview (v4.2) [24] (Figs 5 and 6). A total of 38 associations with an r2 value > = 0.8 were detected (Table 5). In order to further examine these associations for viral evolution, we looked at their frequencies within geographic groupings, including China, East Asia, Hong Kong and Taiwan (Fig 7); China, India, and West Asia (Fig 8); and China, CruiseA, USA, and Europe (Fig 9), as well as by sampling date (Fig 10). Intriguingly, the time analysis shows nine variants that are shifting frequency in samples more recently procured during March-April 2020, including 490T> A, 1397G> A, 23403A> G, and 29540G> A. Eight of these variants are in coding regions, with six producing non-synonymous mutations in the amino acid sequence. One of these, 23403A> G has been recently reported as a mutation in the Spike protein resulting in a more transmissible form of SARS-CoV-2 [19].
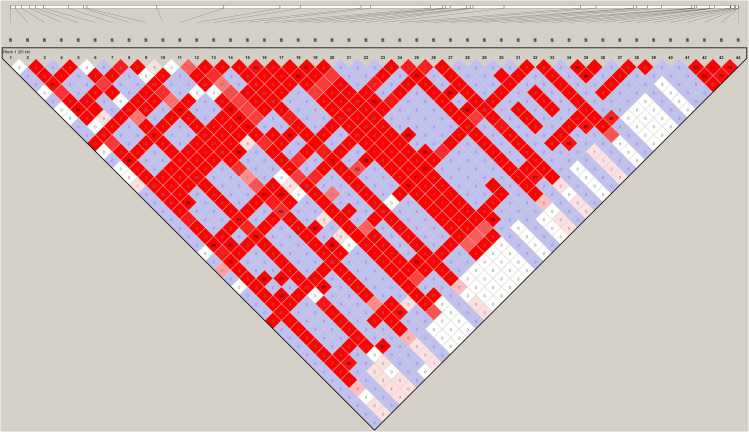
Fig 5. Linkage disequilibrium between SARS-CoV-2 variants.
Shown is the Haploview pairwise association between variants. The colors are determined based on the default D’/LOD score. The value shown in each cell is the r2 value.
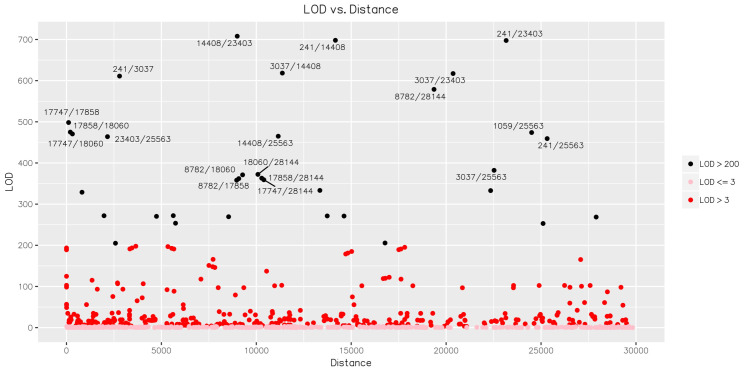
Fig 6. LOD of each variant pair vs. their distance.
The points in black represent variant pairs with a LOD score > 200. The twenty pairs with the highest LOD scores are labeled based on their positions within the reference genome.
Table 5. Associations found with r2 > 0.8 in Haploview.
| Locations | r2 | Locations | r2 |
|---|---|---|---|
| 490/3177 | 1 | 3177/26729 | 0.927 |
| 8782/28144 | 1 | 3177/28077 | 0.927 |
| 14408/23403 | 1 | 490/29700 | 0.923 |
| 17747/17858 | 1 | 3177/29700 | 0.923 |
| 18998/29540 | 1 | 3037/14408 | 0.916 |
| 26729/28077 | 1 | 3037/23403 | 0.915 |
| 28882/28883 | 1 | 241/3037 | 0.912 |
| 241/14408 | 0.997 | 24034/26729 | 0.901 |
| 241/23403 | 0.997 | 24034/28077 | 0.901 |
| 28881/28882 | 0.981 | 18736/26729 | 0.891 |
| 28881/28883 | 0.981 | 18736/28077 | 0.891 |
| 29867/29868 | 0.972 | 1397/28688 | 0.874 |
| 17858/18060 | 0.968 | 1397/29742 | 0.874 |
| 17747/18060 | 0.967 | 28688/29742 | 0.861 |
| 490/18736 | 0.961 | 26729/29700 | 0.855 |
| 3177/18736 | 0.961 | 28077/29700 | 0.855 |
| 18736/29700 | 0.961 | 490/24034 | 0.835 |
| 490/26729 | 0.927 | 3177/24034 | 0.835 |
| 490/28077 | 0.927 | 18736/24034 | 0.802 |
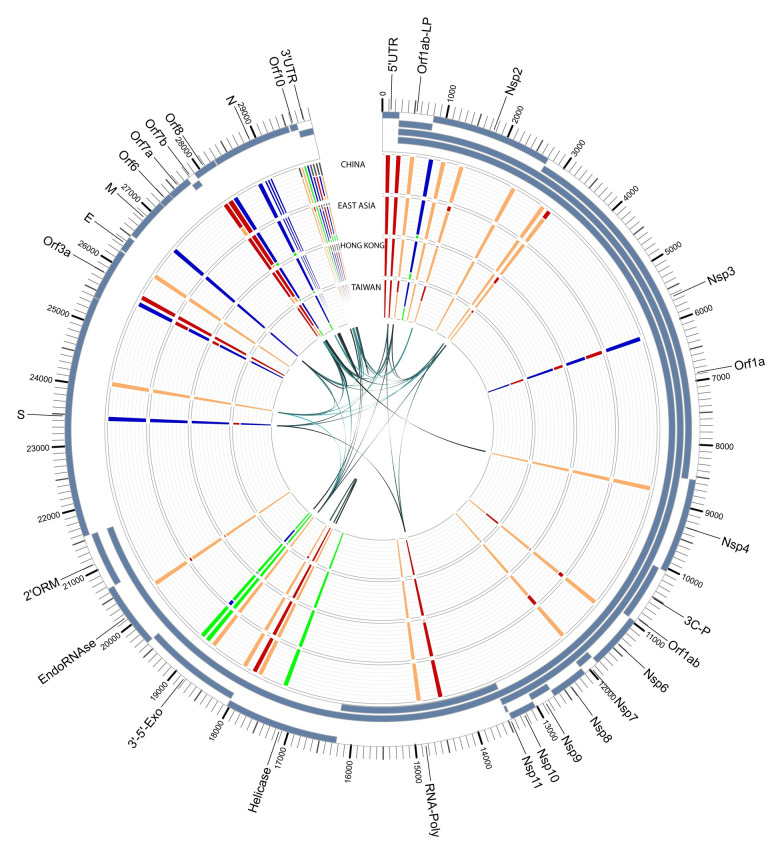
Fig 7. SARS-CoV-2 genome variants for China, East Asia, Hong Kong, and Taiwan.
The outer track shows the gene/protein coding regions displayed in blue. The four inner tracks display nucleotide frequencies (green (A), orange (C), blue (G), and red (T)) at 44 locations with an alternate allele frequency > 1%. The inner-most track of arcs shows variant locations having a high linkage with r2 values ranging from 0.8 (narrow, light) to 1 (wide, dark). Abbreviations: leader protein (LP), 3C-like-proteinase (3C-P), RNA-dependent-RNA-polymerase (RNA-Poly), 3′-to-5′-exonuclease (3′-5′-Exo), 2′-O-ribose-methyltransferase (2′ORM).
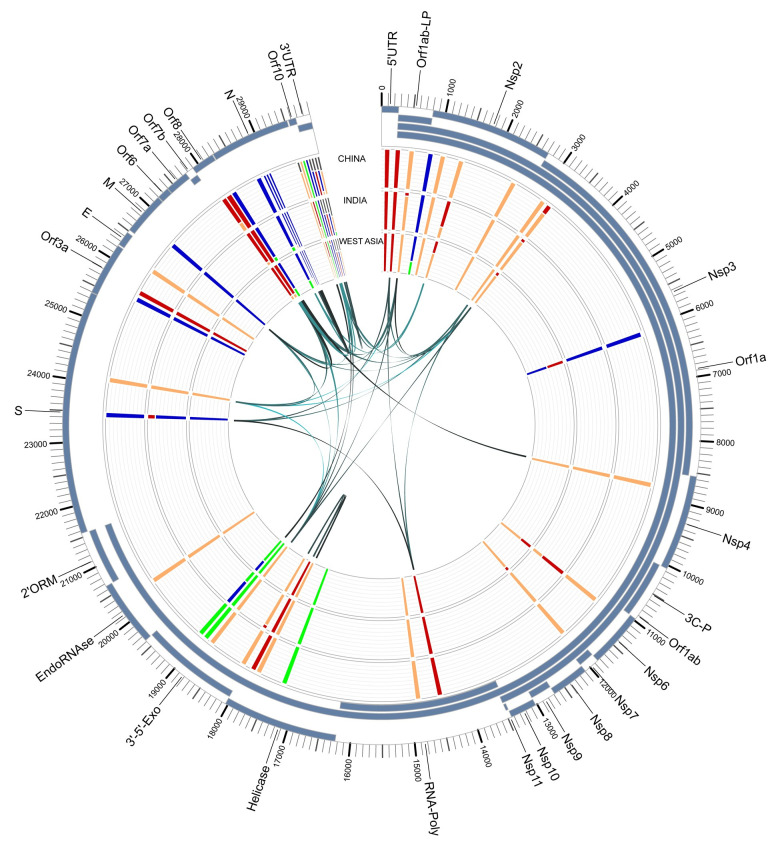
Fig 8. SARS-CoV-2 genome variants for China, India, and West Asia.
The outer track shows the gene/protein coding regions displayed in blue. The four inner tracks display nucleotide frequencies (green (A), orange (C), blue (G), and red (T)) at 44 locations with an alternate allele frequency > 1%. The inner-most track of arcs shows variant locations having a high linkage with r2 values ranging from 0.8 (narrow, light) to 1 (wide, dark). Abbreviations: leader protein (LP), 3C-like-proteinase (3C-P), RNA-dependent-RNA-polymerase (RNA-Poly), 3'-to-5'-exonuclease (3’-5’-Exo), 2'-O-ribose-methyltransferase (2’ORM).
Fig 9. SARS-CoV-2 genome variants for China, CruiseA (Diamond Princess cruise docked in Oakland, CA), USA, and Europe.
The outer track shows the gene/protein coding regions displayed in blue. The four inner tracks display nucleotide frequencies (green (A), orange (C), blue (G), and red (T)) at 44 locations with an alternate allele frequency > 1%. The inner-most track of arcs shows variant locations having a high linkage with r2 values ranging from 0.8 (narrow, light) to 1 (wide, dark). Abbreviations: leader protein (LP), 3C-like-proteinase (3C-P), RNA-dependent-RNA-polymerase (RNA-Poly), 3'-to-5'-exonuclease (3’-5’-Exo), 2'-O-ribose-methyltransferase (2’ORM).
Fig 10. SARS-CoV-2 genome variants across time.
Shown are the variants based on sampling date, ranging from December, 2019 to April, 2020. The outer track shows the gene/protein coding regions displayed in blue. The four inner tracks display nucleotide frequencies (green (A), orange (C), blue (G), and red (T)) at 44 locations with an alternate allele frequency > 1%. The inner-most track of arcs shows variant locations having a high linkage with r2 values ranging from 0.8 (narrow, light) to 1 (wide, dark). Abbreviations: leader protein (LP), 3C-like-proteinase (3C-P), RNA-dependent-RNA-polymerase (RNA-Poly), 3'-to-5'-exonuclease (3’-5’-Exo), 2'-O-ribose-methyltransferase (2’ORM).
Discussion
Of the 44 common variants we found, 20 result in nonsynonymous mutations which may have functional relevance. Understanding these specific variants is critical to being able to react to the evolution of SARS-CoV-2, in particular, in developing an effective vaccine. Among previously reported variants, a few proposed functional consequences have been reported. 14408C > T within the RNA-dependent RNA polymerase gene (RdRp) may potentially affect proofreading or binding with other cofactors, thus affecting viral mutation rates [10]. The variant 23403G > A within the S protein has been hypothesized to increase transmissibility due to its expansion in global samples. This is thought to occur via one of two mechanisms, the first by diminishing interactions between the S1 and S2 promoters of the spike protein based on structural changes, and the second by affecting immunological response due to its location within an immune-dominant epitope [19].
Our analysis uncovered six previously unreported nonsynonymous variants, including 833T > C, 1059C > T, 11916 C > T, 18736T > C, 18998C > T, and 25563G > T. Five of these transition events occur within ORF1ab, and one occurs within ORF3a. Of these, two (833T > C and 1059C > T) are within the nsp2 region, which is postulated to play a role in the host cell survival pathway via interactions with prohibitin (PHB) and prohbitin 2 (PHB2); one (11916 C> T) is within the nsp7 region that may act as a primase and therefore be involved in viral replication; two (18376T > C and 18998C > T) are within the 3’ to 5’ exonuclease, which functions in proofreading; and one (25563G > T) in ORF3a which forms viroporin ion channels and may modulate virus release [25].
While the SARS-CoV-2 virus is the product of a single-stranded RNA genome and our analysis of variants having a high association may be more applicable to eukaryotic genomes, it is known that recombination between strains is a key contributor to coronavirus evolution [26–28]. Korber, et. al [19] demonstrate that one particular mutation in the S protein (S943P) is likely a result of recombination between strains, due to the fact that at the time of reporting, it was found only in Belgium, but in multiple lineages, suggesting it was not a result of a founder sequence. Many associations have been identified [10, 12, 15, 19], including the 38 we found with r2 > 0.8. Further analysis of these is necessary, in order to rule out founder effect. A follow-up study using the reverse-genetic approach would be needed to understand the biological effects of the variant nucleotides and the nature of the high association between the variant sequences (e.g. compensatory mutations) [29, 30].
Conclusion
Our analysis resulted in 44 common variants within SARS-CoV-2, 24 of which had not been previously described. From these variants, a total of 38 pairwise associations had an r2 value > 0.8, indicating a high correlation. Analysis over time shows a shifting frequency of nine variants. One of these, 23403A > G, causes the D614G amino acid change in the spike protein. Recent studies have shown an increase of D614G genotypes in viral isolates (from 10% on March 1, 2020 to 78% on May 29, 2020), which is associated with lower viral loads [31]. Since COVID-19 is an emerging disease, a lot of current research efforts are focused on understanding the origin and mutation of the SARS-CoV-2 virus and isolates. As a result, the amount of sequence information is increasing on a daily basis. In this study, we constructed a framework that will allow us to update our analysis rather efficiently with little intervention. We hope to expand our analysis to include more recent submissions to the NCBI GenBank database, as well as the GISAID EpiFlu database [32]. As more data becomes available, with additional isolates resulting from community-acquired transmission, a more complete picture of the evolution of SARS-CoV-2 will be possible.
Supporting information
(DOCX)
“X” represents allele found in isolate genome while “*” represents an alternate allele detected using deep sequencing.
(DOCX)
Acknowledgments
The authors wish to thank Jae Hwang and Juw Won Park for helpful discussions.
Data Availability
All data used in this study is available from the Genbank database (https://www.ncbi.nlm.nih.gov/genbank/sars-cov-2-seqs/). The alignment file as well as other supporting data and scripts are available on our github site: https://github.com/UofLBioinformatics/SARS_CoV_2_Variants.
Funding Statement
This work was supported by the National Institutes of Health (NIH) grant P20GM103436 (ECR, JHC) and the University of Louisville Center for Predictive Medicine for Biodefense and Emerging Infectious Diseases (DC). Its contents are solely the responsibility of the authors and do not represent the official views of the funding organizations. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–33. 10.1056/NEJMoa2001017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li R, Pei S, Chen B, Song Y, Zhang T, Yang W, et al. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV-2). Science. 2020;368(6490):489–93. 10.1126/science.abb3221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bar-On YM, Flamholz AI, Phillips R, Milo R. SARS-CoV-2 (COVID-19) by the numbers. arXiv preprint arXiv:200312886. 2020. [DOI] [PMC free article] [PubMed]
- 4.Cohen J, Kupferschmidt K. Countries test tactics in ‘war’against COVID-19. Science. 2020;367(6484):1287–8. 10.1126/science.367.6484.1287 [DOI] [PubMed] [Google Scholar]
- 5.Organization WH. Coronavirus Disease (COVID-19) Pandemic 2020 [cited 2020 05/11/2020]. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019.
- 6.Atkins JF, Loughran G, Bhatt PR, Firth AE, Baranov PV. Ribosomal frameshifting and transcriptional slippage: from genetic steganography and cryptography to adventitious use. Nucleic Acids Res. 2016;44(15):7007–78. 10.1093/nar/gkw530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sayers EW, Cavanaugh M, Clark K, Ostell J, Pruitt KD, Karsch-Mizrachi I. GenBank. Nucleic Acids Res. 2019;47(D1):D94–D9. 10.1093/nar/gky989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andersen KG, Rambaut A, Lipkin WI, Holmes EC, Garry RF. The proximal origin of SARS-CoV-2. Nat Med. 2020;26(4):450–2. 10.1038/s41591-020-0820-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duffy S. Why are RNA virus mutation rates so damn high? PLoS Biol. 2018;16(8):e3000003 10.1371/journal.pbio.3000003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pachetti M, Marini B, Benedetti F, Giudici F, Mauro E, Storici P, et al. Emerging SARS-CoV-2 mutation hot spots include a novel RNA-dependent-RNA polymerase variant. J Trans Med. 2020;18(1):1–9. 10.1186/s12967-020-02344-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Phan T. Genetic diversity and evolution of SARS-CoV-2. Infect Genet Evol. 2020;81:104260 10.1016/j.meegid.2020.104260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yin C. Genotyping coronavirus SARS-CoV-2: methods and implications. Genomics. 2020;112(5):3588–96. 10.1016/j.ygeno.2020.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang C, Liu Z, Chen Z, Huang X, Xu M, He T, et al. The establishment of reference sequence for SARS‐CoV‐2 and variation analysis. J Medical Virol. 2020;92(6):667–74. 10.1002/jmv.25762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khailany RA, Safdar M, Ozaslan M. Genomic characterization of a novel SARS-CoV-2. Gene Reports. 2020:100682 10.1016/j.genrep.2020.100682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang X, Wu C, Li X, Song Y, Yao X, Wu X, et al. On the origin and continuing evolution of SARS-CoV-2. Natl Sci Rev. 2020; 7(6):1012–23. 10.1093/nsr/nwaa036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang M, Li M, Ren R, Brave A, van der Werf S, Chen E-Q, et al. International expansion of a novel SARS-CoV-2 mutant. medRxiv. 2020. 10.1128/JVI.00567-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Forster P, Forster L, Renfrew C, Forster M. Phylogenetic network analysis of SARS-CoV-2 genomes. National Academy of Sciences (US). 2020;117(17):9241–3. 10.1073/pnas.2004999117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shen Z, Xiao Y, Kang L, Ma W, Shi L, Zhang L, et al. Genomic diversity of severe acute respiratory syndrome—coronavirus 2 in patients with coronavirus disease. Clin Infect Dis. 2020. 71(15):713–20. 10.1093/cid/ciaa203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Korber B, Fischer W, Gnanakaran SG, Yoon H, Theiler J, Abfalterer W, et al. Spike mutation pipeline reveals the emergence of a more transmissible form of SARS-CoV-2. bioRxiv. 2020. 10.1101/2020.04.29.069054 [DOI] [Google Scholar]
- 20.Chung D, Rouchka EC, Ramirez J, Palmer KE, Lasnik AB, Carrico R, et al. Characterizaton of a SARS-CoV-2 nasopharyngeal swab sample with an extremely high RNA load. 2020. [Google Scholar]
- 21.Johnson M, Zaretskaya I, Raytselis Y, Merezhuk Y, McGinnis S, Madden TL. NCBI BLAST: a better web interface. Nucleic Acids Res. 2008;36(suppl_2):W5–W9. 10.1093/nar/gkn201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lassmann T. Kalign 3: multiple sequence alignment of large datasets. Bioinformatics. 2020;36(6):1928–9. 10.1093/bioinformatics/btz795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Islam MR, Hoque MN, Rahman MS, Alam ARU, Akther M, Puspo JA, et al. Genome-wide analysis of SARS-CoV-2 virus strains circulating worldwide implicates heterogeneity. Sci Rep. 2020;10(1):1–9. 10.1038/s41598-019-56847-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21(2):263–5. 10.1093/bioinformatics/bth457 [DOI] [PubMed] [Google Scholar]
- 25.SwissProt. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) 2020 [cited 2020 5/19/2020]. Available from: https://swissmodel.expasy.org/repository/species/2697049.
- 26.Graham RL, Baric RS. Recombination, reservoirs, and the modular spike: mechanisms of coronavirus cross-species transmission. J Virol. 2010;84(7):3134–46. 10.1128/JVI.01394-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li X, Giorgi EE, Marichann MH, Foley B, Xiao C, Kong X-P, et al. Emergence of SARS-CoV-2 through Recombination and Strong Purifying Selection. bioRxiv. 2020. 10.1101/2020.03.20.000885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rehman Su, Shafique L, Ihsan A, Liu Q. Evolutionary trajectory for the emergence of novel coronavirus SARS-CoV-2. Pathogens. 2020;9(3):240 10.3390/pathogens9030240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dinnon KH, Leist SR, Schafer A, Edwards CE, Martinez DR, Montgomery SA, et al. A mouse-adapted SARS-CoV-2 model for the evaluation of COVID-19 medical countermeasures. bioRxiv. 2020. 10.1101/2020.05.06.081497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xie X, Muruato A, Lokugamage KG, Narayanan K, Zhang X, Zou J, et al. An Infectious cDNA Clone of SARS-CoV-2. Cell Host & Microbe. 2020;27:841–8. 10.1016/j.chom.2020.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Korber B, Fischer WM, Gnanakaran S, Yoon H, Theiler J, Abfalterer W, et al. Tracking changes in SARS-CoV-2 Spike: evidence that D614G increases infectivity of the COVID-19 virus. Cell. 2020;182(4):812–27. e19. 10.1016/j.cell.2020.06.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elbe S, Buckland‐Merrett G. Data, disease and diplomacy: GISAID's innovative contribution to global health. Global Challenges. 2017;1(1):33–46. 10.1002/gch2.1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
“X” represents allele found in isolate genome while “*” represents an alternate allele detected using deep sequencing.
(DOCX)
Data Availability Statement
All data used in this study is available from the Genbank database (https://www.ncbi.nlm.nih.gov/genbank/sars-cov-2-seqs/). The alignment file as well as other supporting data and scripts are available on our github site: https://github.com/UofLBioinformatics/SARS_CoV_2_Variants.