Abstract
Sepsis is the leading cause of death in hospitalized patients and beyond the hospital stay and these long-term sequelae are due in part to unresolved inflammation. Metabolic shift from oxidative phosphorylation to aerobic glycolysis links metabolism to inflammation and such a shift is commonly observed in sepsis under normoxic conditions. By shifting the metabolic state from aerobic glycolysis to oxidative phosphorylation, we hypothesized it would reverse unresolved inflammation and subsequently improve outcome. We propose a shift from aerobic glycolysis to oxidative phosphorylation as a sepsis therapy by targeting the pathways involved in the conversion of pyruvate into acetyl-CoA via pyruvate dehydrogenase (PDH). Chemical manipulation of PDH using dichloroacetic acid (DCA) will promote oxidative phosphorylation over glycolysis and decrease inflammation. We tested our hypothesis in a Drosophila melanogaster model of surviving sepsis infected with Staphylococcus aureus. Drosophila were divided into 3 groups: unmanipulated, sham and sepsis survivors, all treated with linezolid; each group was either treated or not with DCA for one week following sepsis. We followed lifespan, measured gene expression of Toll, defensin, cecropin A, and drosomycin, and levels of lactate, pyruvate, acetyl-CoA as well as TCA metabolites. In our model, metabolic effects of sepsis are modified by DCA with normalized lactate, TCA metabolites, and was associated with improved lifespan of sepsis survivors, yet had no lifespan effects on unmanipulated and sham flies. While Drosomycin and cecropin A expression increased in sepsis survivors, DCA treatment decreased both and selectively increased defensin.
Introduction
Advances in diagnostic modalities, prevention of complications, and care bundles improve sepsis-associated short-term mortality; however, sepsis remains a leading cause of death in hospitalized patients and beyond [1]. In addition to high mortality rates, sepsis survivors experience long-term complications, such as accelerated cardiovascular and neuro-cognitive decline, new infections, cancer, and metabolic disturbances [2, 3]. Importantly, late deaths among sepsis survivors are not solely explained by health status before sepsis, implying some feature of the sepsis itself contributes to these late sequelae [4–7].
During sepsis, immune cells shift their metabolic balance towards aerobic glycolysis over oxidative phosphorylation producing excessive amounts of lactate, a marker for sepsis severity, even under normoxic conditions [8–11]. The metabolic changes occurring during sepsis in turn lead to further immune cell activation and unresolved inflammation [12, 13]. Clinical [14] and laboratory [13, 15–17] work suggests a link between unresolved inflammation and long-term sequelae making the aerobic glycolysis a major component of this complex regulatory network. Lactate, the signature molecule of aerobic glycolysis, is a pro-inflammatory metabolite regulating cytokines and macrophage polarization [13, 18]. Lactate is generated from pyruvate by the enzyme lactate dehydrogenase (LDH). The availability of pyruvate for lactate production is regulated by pyruvate dehydrogenase (PDH), a key enzyme in the tricarboxylic acid cycle (TCA) transforming pyruvate into acetyl-CoA and subsequent mitochondrial respiration [10, 18, 19]. Interestingly, PDH quantity and activity are decreased during sepsis with resultant accumulation of pyruvate [9]. Because lactate dehydrogenase (LDH) is an equilibrium enzyme, increased lactate production during sepsis would be due to a mass-action effect exerted by an increased availability of pyruvate [20]. Consequently, a decrease in PDH activity during sepsis will result in increased lactate production and decreased mitochondrial oxidative phosphorylation [13, 21].
The PDH complex is at a key control point of energy metabolism and subject to regulation by multiple mechanisms, including succinylation of PKM2, posttranslational phosphorylation and subsequent inactivation by pyruvate dehydrogenase kinase (PDK) [22]. Dichloroacetate (DCA), a classic PDK inhibitor, has been successfully used to decrease levels of lactate and shifts metabolism towards oxidative phosphorylation in patients with congenital hyperlactatemia by directly decreasing the PDK activity with subsequent increase in the downstream enzyme, PDH, activity [23].
In the current study, we hypothesized that increased aerobic glycolysis leading to unresolved inflammation contributes to long-term complications of sepsis, including shortened lifespan [5]. We tested our hypothesis in a D. melanogaster model of surviving sepsis by manipulating PDH activity with DCA to modify the increased glycolysis, to normalize antimicrobial peptide expression, and improve lifespan [24, 25].
Materials and methods
Drosophila melanogaster strain and maintenance
The flies were raised on standard cornmeal-molasses agar medium at 22–25°C, 60% humidity, and on a 12-h light/dark cycle. The vials were changed every 3 days. We selected male flies 2–3 days after eclosion for experiments. Wild-type (WT) Canton S flies were obtained from Bloomington stock (Bloomington Drosophila Stock Center, Indiana University).
Experimental design: Fly infection and treatment
We used our previously established model of percutaneous infection in D. melanogaster with subsequent antibiotic treatment to mimic the clinical course and treatment of human sepsis [26]. The flies were divided into 3 groups: unmanipulated, sham, and sepsis survivors. To study the impact of DCA in diet, each experimental group was further divided into 2 groups depending on DCA treatment.
We prepared Staphylococcus aureus suspension in Luria-Bertani (LB) broth and grew the bacteria to an optical density (OD) of 1.0 at 600 nm. At OD of 1.0, there were ~1.67 × 106 CFU of S. aureus. We anesthetized flies with CO2 and pricked with a tungsten needle (0.01 mm at the tip and 0.25 mm across the needle body) into their thorax. The sham group was pricked with a sterile needle while the sepsis survivor group had the needle dipped into the bacterial solution. We pricked the sham flies first to coat the tungsten needle with hemolymph to achieve consistent bacterial coating with the infection group.
We transferred unmanipulated, sham, and sepsis survivors to vials containing antibiotic (linezolid) with DCA [DCA(+)] or without DCA [DCA(-)] after recovery from anesthesia and kept flies in these vials for 18 h before transferring the flies back to antibiotic-free vials. All flies were treated with linezolid (0.5 mg/mL) in the diet as described. DCA treatment continued for one week after the initial infection and it was incorporated into the diet (DCA, 0.5 mg/mL, Sigma Aldrich, St. Louis, USA) [27]. Immune and metabolic outcomes and lifespan were followed over a 7-day course.
Fly survival and lifespan
After infection and treatment with antibiotics, fly survival was assessed by visual inspection of living flies. The flies that died within 6 h after the initial inoculation were excluded from the survival analysis, because death within the first 6 hours was considered to be secondary to trauma from inoculation rather than infection. During lifespan observations, the fly media was changed and survival assessed every 3 days. The infections were performed around the same time of the day (Fig 1).
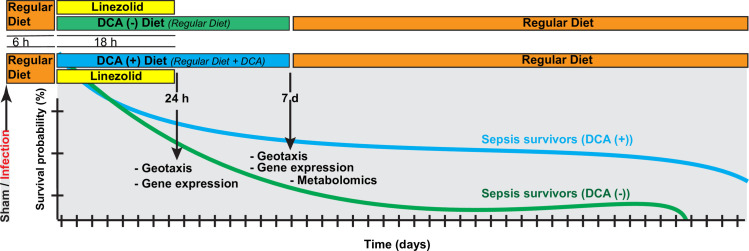
Fig 1. Experimental design.
Conceptual depiction of the experimental design. The flies underwent either sterile needle injury (“sham”) or injury with a bacteria-laden needle (“sepsis”). Following a 6-hour recovery period in vials with regular diet to assure that the needle injury did not lead to death, flies were transferred into vials containing linezolid +/- dichloroacetate (DCA). We followed the lifespan of the flies and in a subset, we measured the gene expression, geotaxis, and metabolomic changes.
Rapid Iterative Negative Geotaxis (RING)
We performed geotaxis assays to evaluate the fitness-related locomotion traits following sepsis. We joined two empty polystyrene vials by tape vertically facing each other forming an 18.5-cm-long tube. We transferred groups of 20 flies into the vials and allowed to acclimatize to the new setting for 5 min before conducting the assay. Flies were gently tapped down to the bottom of the vial for 10 s with the same interval and strength by the same operator throughout the whole experiment. Pictures of the flies were taken with a digital camera at 5 s. We repeated each geotaxis experiment six times, allowing for 1-min rest periods between each trial and pictures were analyzed by counting the number of flies that climb above the 10-cm mark in 5 s after the tap. We calculated the average of the number of flies crossing the 10-cm threshold and expressed the results as percentage of the total number of flies in the tube (% climbing index). Each geotaxis experiment was performed 1 h before the needle pricking (0 h baseline), 1 day, and 7 days after needle pricking. The data are presented as percent of the baseline at time 0 h.
Patterns of host response gene expression
We determined pathogen recognition receptor Toll and antimicrobial peptide (AMP) expression with quantitative real-time polymerase chain reaction (qRT-PCR) at 24 h and 1 week after inoculation with bacteria. AMP expression levels provide an indication of the degree to which the immune system is activated, a surrogate for inflammation. Gene expression of Toll (FBgn0262473), defensin (FBgn0010385), drosomycin (FBgn0283461), and cecropin A (FBgn0000276) were normalized to actin5C (FBgn0000042); all data were normalized to the unmanipulated group. We used 20 flies per group in triplicate. TaqMan® Gene Expression Assay primers included the following: toll, Dm02151201_g1; defensin, Dm01818074_s1; drosomycin, Dm01822006_s1; cecropin A, Dm02609400_sH; and actin5C, Dm02361909_s1. Assay details are provided as (S1 Table).
Metabolomic analysis
LC-MS sample preparation
Each sample containing 30 flies was homogenized in MeOH:ACN:H2O (2:2:1), snap frozen and sonicated. To precipitate the proteins, samples were centrifuged at 1500 g for 10 min at 4˚C and the supernatant was transferred to a new vial and dried under N2. The sample was re-suspended in 500 μL CHCl3:MeOH:H2O (2:1:1) and centrifuged for 5 min at 4°C at 1500 g to separate the upper polar phase from the lower organic phase. A similar extraction was also performed for 5 mg 13C algal cells, whose supernatant was used as an internal standard.
Targeted analysis of organic acids
Organic acids were analyzed by derivatizing them to their corresponding 3-nitrophenylhydrazones [28]. Briefly 100 μL of the supernatant along with 10 μL of internal standard was heated at 50°C with 50 μL of 200 mM 3-nitrophenylhydrazine in 50% aqueous acetonitrile and 50 μL of 120 mM N-(3-dimethylaminopropyl)-N-ethylcarbodiimide HCl in a 6% pyridine solution in the same solvent for 20 min. From this reaction, 100 μL was diluted to 500 μL using 50% aqueous acetonitrile (ACN). The reconstituted sample, 10 μL was injected into the Ultimate 3000 RSLC system (Thermo Fisher Scientific, Waltham, MA) connected to a Thermo Fisher Scientific Q Exactive mass spectrometer. Chromatographic separation was conducted using a reversed phase Phenomenex (Torrence, CA) Luna C18 column (2.1 mm × 150 mm, 5 μm) column using gradient elution with a mobile phase consisting of H2O + 0.1% formic acid (A) and acetonitrile + 0.1% formic acid (B), delivered at a flow rate of 0.2 mL/min. The gradient program was 3% B (0–3 min), from 3% B to 95% B (3–50 min), 95% B (50–55 min), from 95% B to 3% B (55–57 min) and 3% B (57–60 min). The mass spectrometer was equipped with an ESI source and was operated in negative ion mode using a full scan range of 150 m/z to 900 m/z. Analyte identification was confirmed by high resolution accurate mass and compared to the 13C internal standard spectra and retention time [29] (S2 Table).
Untargeted analysis of primary metabolites
The primary metabolites in the polar phase were separated using ion-pairing reversed phase chromatography on the LC-MS system described above [30]. The mobile phase consisted of 5mM hexylamine in H2O (A) acetonitrile (B), delivered at a flow rate of 0.15 mL/min with a post column addition of 0.1 mL/min acetonitrile before entering the mass spectrometer. The gradient was held at 3% B for the first 3 min and increased to 30% B (3–30 min) following an additional increase to 95% B (30–55 min), a 5 min wash at 95% B (55–60 min), 95% B-100% B (60–61 min), 100% B (61–66 min), and return to initial conditions for a 3 min equilibration. The mass spectrometer was operated in negative ion mode using a full scan range of 100 m/z to 900 m/z. Analyte identification was confirmed by high resolution accurate mass and compared to the 13C internal standard. Principal component analysis (PCA) was applied to visualize grouping patterns using unsupervised multivariate data analysis.
Statistical analysis
Kaplan-Meyer survival analysis was performed using Stata 15 (StataCorp. LLC, College Station, TX). Log-rank test was performed with the Kaplan-Meier survival curves for the groups adjusting for clusters, where each vial was treated as a cluster. Statistical analysis between different groups was accomplished with ANOVA using Graph-Pad Prism software version 8.0 (GraphPad Software Inc., La Jolla, CA).
SIMCA 14.1 (SIMCA, Umetrics, Umeå, Sweden) was used for LC-MS and Principal component analysis (PCA). Data obtained from LC-MS analysis were imported into SIMCA for multivariate analysis. PCA were carried out to discriminate the metabolic patterns between groups according to common variability within groups (unmanipulated vs. sham vs. sepsis survivors as well as diet effects among sepsis survivors) after mean centering and unit variance scaling. In our PCA analyses, R2X represents percentage variability of the X data (metabolites assessment by LC-MS raw values) explained by each principal component. The results are given as score plots of the first two principal components with their R2X values.
Results
DCA improves lifespan after surviving sepsis
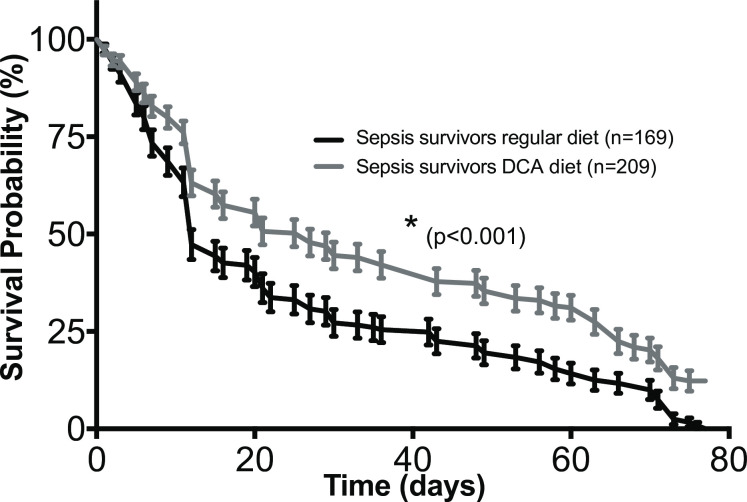
The lifespan of flies was followed every day until death occurred in all the experimental groups of flies. The DCA treatment did not affect lifespan in unmanipulated and sham groups (S1 Fig). Interestingly, one week of DCA treatment in the sepsis survivor group led to significantly longer lifespan when compared to flies surviving sepsis not treated with DCA (p<0.001) (Fig 2). In sepsis survivors on regular diet, median lifespan was 12 days (IQR: 7–35 days) compared to sepsis survivors on DCA diet with a median survival of 20 days (IQR: 11–58 days). The sepsis survivors on DCA diet showed better survival throughout of the study period, especially after day 12.
Fig 2. Drosophila lifespan after surviving sepsis is prolonged after a 1-week exposure to DCA.
The flies were divided into 3 experimental groups: unmanipulated, sham, and sepsis survivors. To study the impact of DCA in diet, each experimental group was further divided into 2 groups depending on DCA treatment [DCA (-) and DCA (+)]. Survival of Drosophila melanogaster after septic injury with Staphylococcus aureus was assessed after the initial 4–6 hours to exclude trauma-associated mortality. All flies received oral linezolid (0.5 mg/mL) for 18 h. Lifespan analysis was performed using the Kaplan-Meyer survival analysis. In the DCA (+) groups, flies were fed DCA (0.5 mg/mL) only for 1 week following sepsis and then switched back to regular diet. The DCA (+) sepsis survivors had improved lifespan compared to flies that were fed regular diet (*p < 0.001).
Geotaxis among sepsis survivors is not improved by DCA
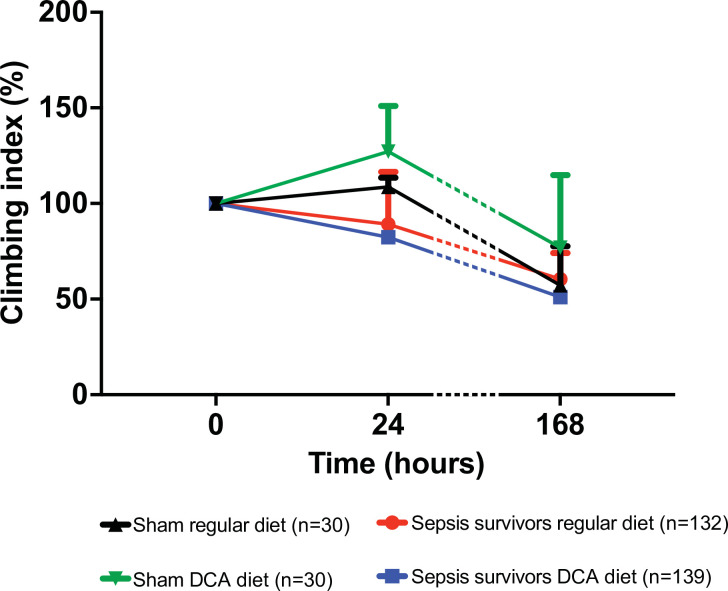
The geotaxis was assessed 24-hour and 7-days after surviving sepsis. While we observed a significant lifespan benefit with DCA, there was no early separation of lifespan by day 7. There was also no improvement in geotaxis in sepsis survivors exposed to DCA compared to regular diet (Fig 3).
Fig 3. Geotaxis among sepsis survivors is not improved by DCA.
Despite lifespan advantage provided by the 1-week exposure to DCA, geotaxis–a composite index of locomotor performance- did not improve negative geotaxis among survivors of sepsis.
Flies treated with DCA had decreased levels of antimicrobial peptides
Gram (+) bacterial Lys-type peptidoglycan, a characteristics of Gr (+) bacteria, strongly stimulate the Toll system with downstream activation of pathways leading to AMP expression. AMP expression levels provide a surrogate marker of immune system activation [26].
We assessed the Toll receptor (FBgn0262473) along with three effector AMPs; defensin (FBgn0010385), drosomycin (FBgn0283461), and cecropin A (FBgn0000276), all are most strongly regulated by the Toll pathway. In a recent model of Drosophila surviving sepsis, AMPs increased and remained elevated following clearance of bacterial pathogen [26]. The values reported in Fig 4 are relative values to the one measured in the unmanipulated groups [DCA (-) and DCA (+)] at 24 hr and 1-week post sepsis (Fig 4).
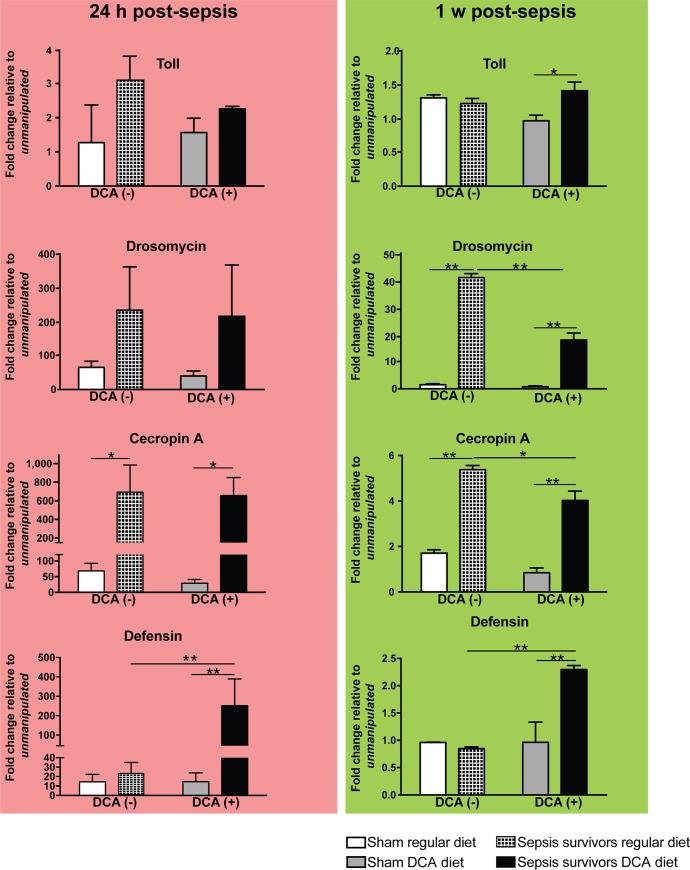
Fig 4. DCA selectively affects early and late AMP expression patterns in Drosophila surviving sepsis.
Flies were infected with Staphylococcus aureus and then treated with orally available linezolid (0.5 mg/mL) in the diet for 18 hours. DCA treatment continued for one week after the initial infection and it was also incorporated into the diet (DCA, 0.5 mg/mL). AMP transcription was assessed after 24 h and 1 week surviving sepsis by quantitative PCR and normalized to unmanipulated flies in each respective diet group. Significant different expression values across treatment within each antimicrobial peptide transcript were determined by one-way ANOVA and Tukey’s multiple comparison tests (*<0.05 and **<0.01). After 24 h surviving sepsis, cecropin A expression was significantly elevated regardless of the diet exposure. However, only the DCA exposed flies had significantly elevated defensin expression levels. After 1 week surviving sepsis, addition of DCA significantly decreased the expression of cecropin A and drosomycin while increasing defensin expression. The number of biological repeats for qPCR was n = 6.
In the early post-sepsis phase (24 h), the cecropin A (FBgn0000276) expression was significantly higher among flies surviving sepsis on both regular (10.2-fold) and DCA (22.6-fold) diet. The expression of defensin (FBgn0010385) was significantly increased among sepsis survivors exposed to DCA 24 h after septic injury compared to sham flies fed DCA (17.9-fold). Defensin (FBgn0010385) was also significantly elevated in the DCA (+) sepsis survivors compared to DCA (-) sepsis survivors (11.4-fold). The transmembrane protein Toll (FBgn0262473) expression was not affected by the DCA treatment.
When the same set of genes were examined 1 week after the injury, expression of drosomycin (FBgn0283461) (29-fold in DCA (-) and 25.4-fold in DCA (+)) and cecropin A (FBgn0000276) (3.11-fold in DCA (-) and 4.8-fold in DCA (+)) were significantly increased in sepsis groups when compared to sham groups regardless of the diet. However, DCA treatment significantly decreased the expression of drosomycin (FBgn0283461) (0.43-fold) and cecropin A (FBgn0000276) (0.75-fold) in flies surviving sepsis.
While DCA treatment significantly diminished drosomycin (FBgn0283461) and cecropin A (FBgn0000276) expression, the expression of defensin (FBgn0010385) was significantly higher in DCA-treated flies after sepsis compared to sepsis survivors on regular diet (2.4-fold). In summary, DCA treatment for 1 week facilitated a focused and sustained expression of defensin (FBgn0010385), the AMP against gram-positive bacteria, whereas DCA treatment reduced expression of drosomycin (FBgn0283461) and cecropin A (FBgn0000276).
Treatment of Drosophila surviving sepsis with DCA demonstrates metabolomic reprogramming
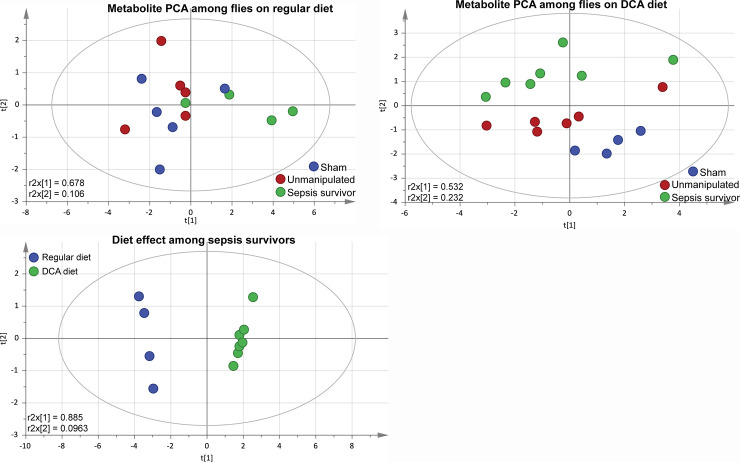
We then studied changes in metabolite levels in sham and sepsis survivor groups with or without DCA treatment. We used multivariate analysis with an unsupervised PCA model to study metabolites. PCA results were calculated by reducing the number of dimension while preserving the initial information (metabolites variability) (S2 Table) [31]. The two principal components of the score plot are considered as a combination of the initial variables, each of them having a different “weight” in the calculation of the principal component (PC). The weights of each variable on the PC1 and PC2 describe the metabolites variation responsible for the discrimination of the clusters in the PCA score-plots. The multivariate analysis with the unsupervised PCA model showed that flies on regular diet could not be separated according to their group (Fig 5A). On the contrary, when flies were treated with DCA, the PCA score plot shows that the 2 first components could discriminate sham from unmanipulated and from sepsis survivors (Fig 5B). When comparing the DCA effect among the sepsis survivors, the PCA model could clearly discriminate two clusters along the first component (R2X = 0.885) (Fig 5C). The metabolites having the highest weight on the first component are α-ketoglutarate, fumarate, and pyruvate. These PCA models confirm the metabolic consequences of the DCA treatment. For the further comparison of metabolic assessment, we used the unmanipulated flies to normalize metabolite concentrations in sham and sepsis survivor groups.
Fig 5. Multivariate analysis of metabolomic LC-MS data show DCA-dependent changes among sepsis survivors.
We studied changes in metabolite levels in sham and sepsis survivors with or without DCA treatment. We used the unmanipulated flies to normalize metabolite concentrations in sham and sepsis survivor groups. (A) Multivariate analysis with unsupervised PCA model showed that flies on regular diet could not be separated according to their group (r2x = 0.678) (Fig 5A). (B) However, when flies were treated with DCA, PCA score plot shows that the 2 first components could discriminate sham from unmanipulated and sepsis survivors (r2x = 0.532) (Fig 5B). (C) Finally, when we compared the effects of DCA among the sepsis survivors, the PCA model could clearly discriminate two clusters along the first component (r2x = 0.885) (Fig 5C). The metabolites having the highest weight on the first component were α-ketoglutarate, fumarate, and pyruvate. The number of biological repeats for LC-MS was n = 5.
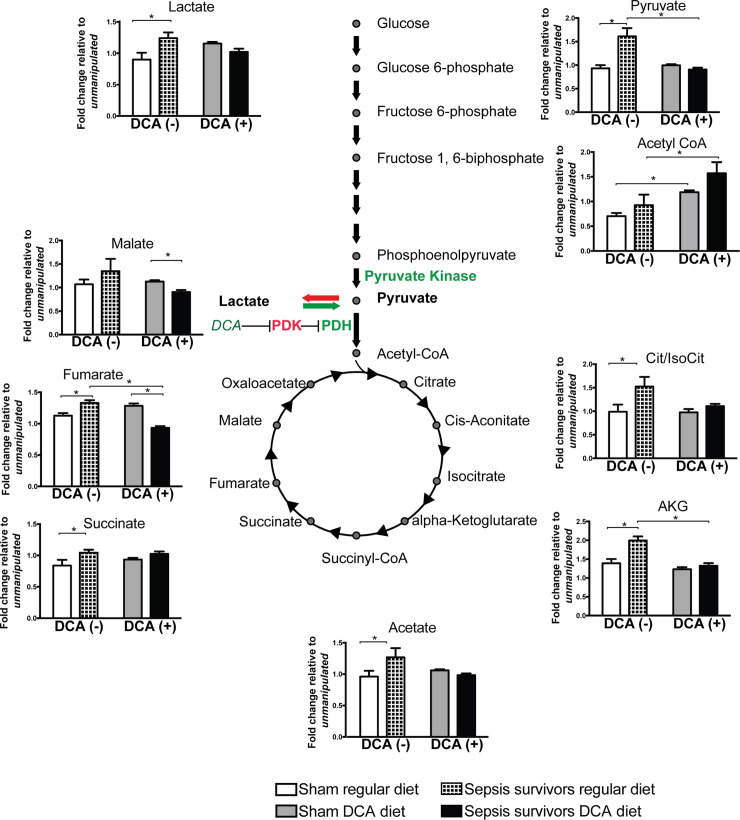
From the results shown in Fig 6, two metabolomic effects became obvious. The first observed difference was the effect of infection. We observed aerobic glycolysis with acetate, lactate, and TCA metabolite accumulation such as pyruvate, citrate/isocitrate, α-ketoglutarate, succinate, and fumarate among sepsis survivors on regular diet compared to sham groups on regular diet. The second observation was the diet effect. DCA treatment has no effect on sham flies as none of the metabolites measured were significantly modified with the introduction of DCA. Once we introduced DCA into the diet, the infection effect was partially reversed. DCA (+) resulted in a decrease in lactate levels among sepsis survivors, with a return to levels similar to that in the sham group. Only malate and fumarate were significantly lower in sepsis survivors compared to sham group while on DCA. In addition, DCA diet led to decreased fumarate, α-ketoglutarate and pyruvate levels in comparison to sepsis surviving flies on regular diet. Among sepsis survivors, levels of pyruvate, citrate, α-ketoglutarate, acetate, and succinate were normalized to the levels of sham in the DCA group (Fig 6).
Fig 6. DCA promotes a shift from aerobic glycolysis to oxidative phosphorylation in flies surviving sepsis.
We studied changes in metabolite levels in sham and sepsis survivors with or without DCA treatment. We used the unmanipulated flies to normalize metabolite concentrations in sham and sepsis survivor groups. Sepsis led to acetate, lactate, and TCA metabolite accumulation such as pyruvate, citrate/isocitrate, α-ketoglutarate, succinate, and fumarate among sepsis survivors on regular diet compared to sham groups on regular diet. When flies were fed DCA, infection effect was partially reversed with a decrease in lactate among sepsis survivors returning to levels similar to that in the sham group. Only malate and fumarate were significantly lower in sepsis survivors compared to sham group while on DCA. In addition, DCA diet led to decreased fumarate, α-ketoglutarate and pyruvate levels in comparison to sepsis surviving flies on regular diet. Once exposed to DCA, the TCA metabolites were either brought back to baseline–like succinate- or decreased lower than sham in sepsis survivors. In summary, DCA reversed the systemic metabolic signatures of aerobic glycolysis among survivors of sepsis. The number of biological repeats for TCA analysis was n = 5.
Levels of acetyl-CoA in DCA (+) flies were significantly higher when compared to flies on regular diet for each corresponding group (p<0.05). However, neither in regular diet, nor in the DCA diet groups, there were no differences between the sham and sepsis survivor groups within each respective group (Fig 6).
Discussion
Sepsis was recently defined by the SEPSIS 3 consortium as a ‘life-threatening organ dysfunction caused by a dysregulated host response to infection’ [24]. Acute inflammation during early phases of sepsis is necessary to survive infection, yet sustained inflammation is deleterious [26, 32, 33]. Activated immune cells during early phases of sepsis undergo rapid activation to generate ATP and biosynthetic intermediates utilizing aerobic glycolysis and lactate accumulation, which in turn propagates further antibacterial defenses and inflammation [13, 18, 34]. In sepsis survivors, such an excessive acute immune response could transition to dysfunctional immunity with long-term poor outcomes [18, 19]. As such, the new paradigm in the management of sepsis is towards improving potentially modifiable variables of late morbidity and mortality [35–37]. However, causality between sepsis and “post-acute mortality” in sepsis survivors is not well established [38, 39]. Metabolic profiling of rodents and humans show a shift from oxidative phosphorylation to aerobic glycolysis with increased lactate production during early phases of sepsis [10, 40, 41]. While metabolic changes during an acute immune response are not novel, recent work conceptualized that acute inflammatory responses in immune cells are both supported and regulated by metabolic shifts leading to “trained immunity” in innate immune cells [18, 42]. In the current work, we used the Drosophila sepsis survival model to understand the association between innate immunity and metabolomic changes during long-term sepsis sequelae. Drosophila provides the advantage that it only has the innate immunity, yet survives infections and maintains an immune memory (trained immunity) as a whole organism [43, 44]. Trained immune memory confers a long-term protection against secondary infections, yet may consume resources for the organism to live and survive on the long run, a pragmatic trade-off [7, 45–50]. This becomes especially relevant in organisms that lack adaptive immune systems, such as the Drosophila. Key metabolic enzymes of glucose oxidation and citric acid cycle are studied within the context of trained immunity [19, 51]. One of the upstream enzymes in glucose oxidation, pyruvate dehydrogenase (PDH), is a key regulatory enzyme determining the fate of pyruvate into the TCA cycle and its activity is decreased in sepsis [9, 52].
While acknowledging the limitations of pre-clinical models, others and we defined “sepsis” in Drosophila, where the flies were infected with Staphylococcus and then treated with orally available antibiotics [25]. The antibiotic exposure eliminated the bacterial burden, however inflammation (“dysregulated host response”) persisted. In our original work, we showed decrease and subsequent elimination of bacterial burden with antibiotic treatment.
We tested our hypothesis that manipulating PDH activity with a small molecular PDH activator, DCA, will reverse the increased glycolysis in sepsis, normalize AMP expression, and improve lifespan. Pyruvate dehydrogenase kinase 1 (PDK1) is upstream of PDH, phosphorylates and inhibits PDH preventing conversion of pyruvate to acetyl-CoA and shifting cell metabolism towards aerobic glycolysis highlighted by increased lactate production. DCA, by inhibiting PDK1, activates PDH and promotes the entry of pyruvate into the TCA cycle [53]. We here showed that 1-week exposure to DCA improves lifespan of Drosophila surviving sepsis over a course of almost 12 weeks, regulates inflammatory AMP, and promotes conversion of pyruvate into acetyl-CoA facilitating oxidative phosphorylation over aerobic glycolysis. DCA is reported to promote lifespan extension in C. elegans and D. melanogaster upon continuous exposure, yet we did not observe such an effect in the control flies exposed to DCA as we exposed flies for only 1 week and subsequently reared them on regular diet [54, 55]. The relatively brief exposure to DCA could explain the lack of survival advantage in non-infected control flies. Nevertheless, DCA effect on lifespan among flies surviving sepsis was profound and did last beyond the 1-week exposure. DCA has ben used to manipulate metabolic and inflammatory changes in other infection models. Work by Yamane et al did show in a murine model of influenza that both DCA as well as a DCA analog (diisopropylamine dichloroacetate) improved survival associated with inhibition of pyruvate dehydrogenase kinase 4 [56]. While we observed increased lifespan, we did not observe a functional recovery among sepsis survivors as evaluated with geotaxis [56, 57].
Fungi or gram-positive bacteria activate the Toll pathway with subsequent activation of the NF-κΒ factor Dif, Relish, or Dorsal and production of AMPs such as drosomycin, cecropin A, and defensin [58–61]. We studied AMP’s as a surrogate for inflammation based on our earlier research [26]. In our prior work using the same model, we explored the components of the canonical transcription factor NF-κB (dorsal, Dif, Relish) and a wider panel of receptors, signaling molecules, and antimicrobial peptides (PGRP-SD, Toll, Metchnikowin, Cecropin A, JNK, Drosomycin, Defensin, InR, IRS, PTEN, Akt1, Foxo, mTORC1, and ratio of p-Akt/Akt) as surrogates for sustained inflammation among flies surviving sepsis. We observed persistent elevation of NF-κB gene expression as well as activation and AMP’s in the absence of any obvious infection as shown by culturing flies surviving sepsis. This enabled us to establish the basis for the “sepsis survivor” phenotype with the goal of mimicking patients surviving sepsis, yet having ongoing inflammation even at hospital discharge.
In our experiments, among flies not treated with DCA, the drosomycin and cecropin A expression was elevated 1 week after surviving sepsis despite clearance of bacterial burden suggesting a sustained expression and activation of AMPs. Activation of AMPs, markers of inflammation, could contribute to lifespan reduction. Support for this idea comes from the observations by the Ganetzky laboratory that overexpression of AMPs, such as drosocin, attacin, defensin and drosomycin, in young flies induces neurodegeneration in mature flies and shortens lifespan [32]. Similarly, AMP overexpression in flies deficient in Methuselah-like receptor-10 (Mthl10) did limit lifespan in Drosophila [50].
The metabolite patterns of flies surviving sepsis reared on regular diet showed characteristics of the aerobic glycolysis with lactate and TCA cycle metabolites accumulating. Lactate has been used as a marker for poor clinical outcome and, in our experience, high lactate level in Drosophila is associated with a shortened lifespan [19, 26, 62]. In addition to lactate accumulation among sepsis survivors, pyruvate, citrate/isocitrate, α-ketoglutarate, acetate, succinate, and fumarate were significantly elevated compared to sham group. However, following DCA treatment, lactate and pyruvate levels came to baseline, suggesting a shift of pyruvate into the TCA cycle away from the lactate production. Among TCA cycle metabolites, citrate/isocitrate, α-ketoglutarate, acetate and succinate also came to baseline levels with DCA treatment. Fumarate and malate levels decreased significantly in the DCA group. The decrease in fumarate and malate may also indicate a redirection of flux from pyruvate carboxylase to PDH entry of pyruvate, yet this requires flux studies [52]. We used unsupervised PCA method to analyze the metabolomic changes. When we studied the metabolic impact of DCA treatment in the sham group, DCA diet has no metabolic effects compared to regular diet other than an increase in acetyl-CoA. However, in the sepsis survivor group, DCA diet effects on metabolite contents are in good agreement with the DCA mechanism of action and the expected reversal of aerobic glycolysis. Interestingly, when compared to the sham group on regular diet, the DCA-treated sepsis-surviving group, none of the metabolites differed between these two groups. This result may support our hypothesis that metabolomic effects of sepsis have been reversed by DCA. When regular diet- and DCA- survivors were compared, we observed a clear clustering in the PCA loading plots.
How the relatively short course of DCA affects the sustained levels of anti-Gr(+) defensin expression is of interest as it could link metabolomic changes to inflammation. Pyruvate can be converted to acetyl-CoA in the nucleus by the nuclear PDH, providing a source of acetyl for histone acetylation and DCA could act in a similar fashion promoting histone acetylation and thus linking metabolic changes with epigenetic control of AMP expression [63–66].
We studied geotaxis up to day 7 to avoid any survivor bias effect on later time points and found no difference between the regular and DCA diet groups on day 1 and day 7 after sepsis. Our goal of using a functional outcome was to mimic the human experience surviving sepsis [57].
As for the limitations of our current work, the DCA effect on the life span is not mechanistically shown and requires cellular level genetically modified flies to test our hypothesis further along with 13C-based metabolomic flux studies.
In summary, our results suggest an association between DCA-induced metabolic changes in Drosophila surviving sepsis and their lifespan. This sepsis survival model in an organism with only innate immunity lends itself for further mechanistic exploration of various spatial and temporal interventions towards lifespan and healthspan outcomes.
Supporting information
To study the impact of DCA in diet, sterile needle injured (“sham”) flies were divided to either receive regular or DCA diet. Survival of Drosophila melanogaster after sterile injury was assessed following the initial 4–6 hours to exclude trauma-associated mortality. All flies received oral linezolid (0.5 mg/mL) for 18 h. Lifespan analysis was performed using the Kaplan-Meyer survival analysis. In the DCA diet group, sham flies were fed DCA (0.5 mg/mL) only for 1 week following needle injury and then switched back to regular diet. There was no lifespan difference between regular and DCA diet receiving sham flies (p > 0.05).
(TIF)
This folder contains all of the data for the manuscript and the data files are titled with their corresponding figure panel.
(ZIP)
(DOCX)
(DOCX)
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
Dr. Kaynar received research grant support from NIH (HL126711). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Dr. Kaynar received salary support from NIH (HL126711).
References
- 1.Zimmerman JE, Kramer AA, Knaus WA. Changes in hospital mortality for United States intensive care unit admissions from 1988 to 2012. Critical care. 2013;17(2):R81 10.1186/cc12695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leibovici L, Samra Z, Konigsberger H, Drucker M, Ashkenazi S, Pitlik SD. Long-term survival following bacteremia or fungemia. JAMA: the journal of the American Medical Association. 1995;274(10):807–12. [PubMed] [Google Scholar]
- 3.Linder A, Guh D, Boyd JH, Walley KR, Anis AH, Russell JA. Long-term (10-year) mortality of younger previously healthy patients with severe sepsis/septic shock is worse than that of patients with nonseptic critical illness and of the general population. Critical care medicine. 2014;42(10):2211–8. 10.1097/CCM.0000000000000503 [DOI] [PubMed] [Google Scholar]
- 4.Yende S, Angus DC. Long-term outcomes from sepsis. Current infectious disease reports. 2007;9(5):382–6. 10.1007/s11908-007-0059-3 [DOI] [PubMed] [Google Scholar]
- 5.Prescott HC, Osterholzer JJ, Langa KM, Angus DC, Iwashyna TJ. Late mortality after sepsis: propensity matched cohort study. BMJ. 2016;353:i2375 10.1136/bmj.i2375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shankar-Hari M, Rubenfeld GD. Understanding Long-Term Outcomes Following Sepsis: Implications and Challenges. Current infectious disease reports. 2016;18(11):37 10.1007/s11908-016-0544-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shirai T, Nazarewicz RR, Wallis BB, Yanes RE, Watanabe R, Hilhorst M, et al. The glycolytic enzyme PKM2 bridges metabolic and inflammatory dysfunction in coronary artery disease. J Exp Med. 2016;213(3):337–54. 10.1084/jem.20150900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shapiro NI, Howell MD, Talmor D, Nathanson LA, Lisbon A, Wolfe RE, et al. Serum lactate as a predictor of mortality in emergency department patients with infection. Annals of emergency medicine. 2005;45(5):524–8. 10.1016/j.annemergmed.2004.12.006 [DOI] [PubMed] [Google Scholar]
- 9.Nuzzo E, Berg KM, Andersen LW, Balkema J, Montissol S, Cocchi MN, et al. Pyruvate Dehydrogenase Activity Is Decreased in the Peripheral Blood Mononuclear Cells of Patients with Sepsis. A Prospective Observational Trial. Ann Am Thorac Soc. 2015;12(11):1662–6. 10.1513/AnnalsATS.201505-267BC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tannahill GM, Curtis AM, Adamik J, Palsson-McDermott EM, McGettrick AF, Goel G, et al. Succinate is an inflammatory signal that induces IL-1beta through HIF-1alpha. Nature. 2013;496(7444):238–42. 10.1038/nature11986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brooks GA. The Science and Translation of Lactate Shuttle Theory. Cell metabolism. 2018;27(4):757–85. 10.1016/j.cmet.2018.03.008 [DOI] [PubMed] [Google Scholar]
- 12.Tan Z, Xie N, Cui H, Moellering DR, Abraham E, Thannickal VJ, et al. Pyruvate dehydrogenase kinase 1 participates in macrophage polarization via regulating glucose metabolism. Journal of immunology. 2015;194(12):6082–9. 10.4049/jimmunol.1402469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krejcova G, Danielova A, Nedbalova P, Kazek M, Strych L, Chawla G, et al. Drosophila macrophages switch to aerobic glycolysis to mount effective antibacterial defense. Elife. 2019;8 10.7554/eLife.50414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yende S, D'Angelo G, Kellum JA, Weissfeld L, Fine J, Welch RD, et al. Inflammatory markers at hospital discharge predict subsequent mortality after pneumonia and sepsis. American journal of respiratory and critical care medicine. 2008;177(11):1242–7. 10.1164/rccm.200712-1777OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Santa F, Totaro MG, Prosperini E, Notarbartolo S, Testa G, Natoli G. The histone H3 lysine-27 demethylase Jmjd3 links inflammation to inhibition of polycomb-mediated gene silencing. Cell. 2007;130(6):1083–94. 10.1016/j.cell.2007.08.019 [DOI] [PubMed] [Google Scholar]
- 16.Okin D, Medzhitov R. The Effect of Sustained Inflammation on Hepatic Mevalonate Pathway Results in Hyperglycemia. Cell. 2016;165(2):343–56. 10.1016/j.cell.2016.02.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo H, Diao N, Yuan R, Chen K, Geng S, Li M, et al. Subclinical-Dose Endotoxin Sustains Low-Grade Inflammation and Exacerbates Steatohepatitis in High-Fat Diet-Fed Mice. Journal of immunology. 2016;196(5):2300–8. 10.4049/jimmunol.1500130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Colegio OR, Chu NQ, Szabo AL, Chu T, Rhebergen AM, Jairam V, et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. 2014;513(7519):559–63. 10.1038/nature13490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng SC, Quintin J, Cramer RA, Shepardson KM, Saeed S, Kumar V, et al. mTOR- and HIF-1alpha-mediated aerobic glycolysis as metabolic basis for trained immunity. Science. 2014;345(6204):1250684 10.1126/science.1250684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lack JB, Lange JD, Tang AD, Corbett-Detig RB, Pool JE. A Thousand Fly Genomes: An Expanded Drosophila Genome Nexus. Molecular biology and evolution. 2016;33(12):3308–13. 10.1093/molbev/msw195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heigenhauser GJ, Parolin ML. Role of pyruvate dehydrogenase in lactate production in exercising human skeletal muscle. Advances in experimental medicine and biology. 1999;474:205–18. 10.1007/978-1-4615-4711-2_17 [DOI] [PubMed] [Google Scholar]
- 22.Wang F, Wang K, Xu W, Zhao S, Ye D, Wang Y, et al. SIRT5 Desuccinylates and Activates Pyruvate Kinase M2 to Block Macrophage IL-1beta Production and to Prevent DSS-Induced Colitis in Mice. Cell reports. 2017;19(11):2331–44. 10.1016/j.celrep.2017.05.065 [DOI] [PubMed] [Google Scholar]
- 23.Zhou ZH, McCarthy DB, O'Connor CM, Reed LJ, Stoops JK. The remarkable structural and functional organization of the eukaryotic pyruvate dehydrogenase complexes. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(26):14802–7. 10.1073/pnas.011597698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA: the journal of the American Medical Association. 2016;315(8):801–10. 10.1001/jama.2016.0287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Broderick KE, Feala J, McCulloch A, Paternostro G, Sharma VS, Pilz RB, et al. The nitric oxide scavenger cobinamide profoundly improves survival in a Drosophila melanogaster model of bacterial sepsis. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2006;20(11):1865–73. [DOI] [PubMed] [Google Scholar]
- 26.Kaynar AM, Bakalov V, Laverde SM, Cambriel AI, Lee BH, Towheed A, et al. Cost of surviving sepsis: a novel model of recovery from sepsis in Drosophila melanogaster. Intensive Care Med Exp. 2016;4(1):4 10.1186/s40635-016-0075-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abdelmalak M, Lew A, Ramezani R, Shroads AL, Coats BS, Langaee T, et al. Long-term safety of dichloroacetate in congenital lactic acidosis. Mol Genet Metab. 2013;109(2):139–43. 10.1016/j.ymgme.2013.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han J, Lin K, Sequeira C, Borchers CH. An isotope-labeled chemical derivatization method for the quantitation of short-chain fatty acids in human feces by liquid chromatography-tandem mass spectrometry. Anal Chim Acta. 2015;854:86–94. 10.1016/j.aca.2014.11.015 [DOI] [PubMed] [Google Scholar]
- 29.Han J, Gagnon S, Eckle T, Borchers CH. Metabolomic analysis of key central carbon metabolism carboxylic acids as their 3-nitrophenylhydrazones by UPLC/ESI-MS. Electrophoresis. 2013;34(19):2891–900. 10.1002/elps.201200601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo K, Peng J, Zhou R, Li L. Ion-pairing reversed-phase liquid chromatography fractionation in combination with isotope labeling reversed-phase liquid chromatography-mass spectrometry for comprehensive metabolome profiling. Journal of chromatography A. 2011;1218(23):3689–94. 10.1016/j.chroma.2011.04.024 [DOI] [PubMed] [Google Scholar]
- 31.Saccenti E, Hoefsloot HCJ, Smilde AK. Reflections on univariate and multivariate analysis of metabolomics data. Metabolomics. 2013;10:361–74. [Google Scholar]
- 32.Cao Y, Chtarbanova S, Petersen AJ, Ganetzky B. Dnr1 mutations cause neurodegeneration in Drosophila by activating the innate immune response in the brain. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(19):E1752–60. 10.1073/pnas.1306220110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamamoto-Hino M, Muraoka M, Kondo S, Ueda R, Okano H, Goto S. Dynamic regulation of innate immune responses in Drosophila by Senju-mediated glycosylation. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(18):5809–14. 10.1073/pnas.1424514112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weber GF, Chousterman BG, He S, Fenn AM, Nairz M, Anzai A, et al. Interleukin-3 amplifies acute inflammation and is a potential therapeutic target in sepsis. Science. 2015;347(6227):1260–5. 10.1126/science.aaa4268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boomer JS, To K, Chang KC, Takasu O, Osborne DF, Walton AH, et al. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA: the journal of the American Medical Association. 2011;306(23):2594–605. 10.1001/jama.2011.1829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Honselmann KC, Buthut F, Heuwer B, Karadag S, Sayk F, Kurowski V, et al. Long-term mortality and quality of life in intensive care patients treated for pneumonia and/or sepsis: Predictors of mortality and quality of life in patients with sepsis/pneumonia. Journal of critical care. 2015;30(4):721–6. 10.1016/j.jcrc.2015.03.009 [DOI] [PubMed] [Google Scholar]
- 37.Quartin AA, Schein RM, Kett DH, Peduzzi PN. Magnitude and duration of the effect of sepsis on survival. Department of Veterans Affairs Systemic Sepsis Cooperative Studies Group. JAMA: the journal of the American Medical Association. 1997;277(13):1058–63. [PubMed] [Google Scholar]
- 38.Dick A, Liu H, Zwanziger J, Perencevich E, Furuya EY, Larson E, et al. Long-term survival and healthcare utilization outcomes attributable to sepsis and pneumonia. BMC Health Serv Res. 2012;12:432 10.1186/1472-6963-12-432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mayr FB, Talisa VB, Balakumar V, Chang CH, Fine M, Yende S. Proportion and Cost of Unplanned 30-Day Readmissions After Sepsis Compared With Other Medical Conditions. JAMA: the journal of the American Medical Association. 2017;317(5):530–1. 10.1001/jama.2016.20468 [DOI] [PubMed] [Google Scholar]
- 40.Meiser J, Kramer L, Sapcariu SC, Battello N, Ghelfi J, D'Herouel AF, et al. Pro-inflammatory Macrophages Sustain Pyruvate Oxidation through Pyruvate Dehydrogenase for the Synthesis of Itaconate and to Enable Cytokine Expression. The Journal of biological chemistry. 2016;291(8):3932–46. 10.1074/jbc.M115.676817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cambiaghi A, Pinto BB, Brunelli L, Falcetta F, Aletti F, Bendjelid K, et al. Characterization of a metabolomic profile associated with responsiveness to therapy in the acute phase of septic shock. Scientific reports. 2017;7(1):9748 10.1038/s41598-017-09619-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arts RJ, Novakovic B, Ter Horst R, Carvalho A, Bekkering S, Lachmandas E, et al. Glutaminolysis and Fumarate Accumulation Integrate Immunometabolic and Epigenetic Programs in Trained Immunity. Cell metabolism. 2016;24(6):807–19. 10.1016/j.cmet.2016.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pham LN, Dionne MS, Shirasu-Hiza M, Schneider DS. A specific primed immune response in Drosophila is dependent on phagocytes. PLoS pathogens. 2007;3(3):e26 10.1371/journal.ppat.0030026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dolezal T, Krejcova G, Bajgar A, Nedbalova P, Strasser P. Molecular regulations of metabolism during immune response in insects. Insect biochemistry and molecular biology. 2019;109:31–42. 10.1016/j.ibmb.2019.04.005 [DOI] [PubMed] [Google Scholar]
- 45.Newsholme P, Curi R, Gordon S, Newsholme EA. Metabolism of glucose, glutamine, long-chain fatty acids and ketone bodies by murine macrophages. The Biochemical journal. 1986;239(1):121–5. 10.1042/bj2390121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Masui K, Tanaka K, Akhavan D, Babic I, Gini B, Matsutani T, et al. mTOR complex 2 controls glycolytic metabolism in glioblastoma through FoxO acetylation and upregulation of c-Myc. Cell metabolism. 2013;18(5):726–39. 10.1016/j.cmet.2013.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Palsson-McDermott EM, Curtis AM, Goel G, Lauterbach MA, Sheedy FJ, Gleeson LE, et al. Pyruvate kinase M2 regulates Hif-1alpha activity and IL-1beta induction and is a critical determinant of the warburg effect in LPS-activated macrophages. Cell metabolism. 2015;21(1):65–80. 10.1016/j.cmet.2014.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang L, Xie M, Yang M, Yu Y, Zhu S, Hou W, et al. PKM2 regulates the Warburg effect and promotes HMGB1 release in sepsis. Nature communications. 2014;5:4436 10.1038/ncomms5436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Everts B, Amiel E, Huang SC, Smith AM, Chang CH, Lam WY, et al. TLR-driven early glycolytic reprogramming via the kinases TBK1-IKKvarepsilon supports the anabolic demands of dendritic cell activation. Nature immunology. 2014;15(4):323–32. 10.1038/ni.2833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sung EJ, Ryuda M, Matsumoto H, Uryu O, Ochiai M, Cook ME, et al. Cytokine signaling through Drosophila Mthl10 ties lifespan to environmental stress. Proceedings of the National Academy of Sciences of the United States of America. 2017;114(52):13786–91. 10.1073/pnas.1712453115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cheng SC, Scicluna BP, Arts RJ, Gresnigt MS, Lachmandas E, Giamarellos-Bourboulis EJ, et al. Broad defects in the energy metabolism of leukocytes underlie immunoparalysis in sepsis. Nature immunology. 2016;17(4):406–13. 10.1038/ni.3398 [DOI] [PubMed] [Google Scholar]
- 52.Wang H, Lu J, Kulkarni S, Zhang W, Gorka JE, Mandel JA, et al. Metabolic and oncogenic adaptations to pyruvate dehydrogenase inactivation in fibroblasts. The Journal of biological chemistry. 2019;294(14):5466–86. 10.1074/jbc.RA118.005200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Han ZS, Ip YT. Interaction and specificity of Rel-related proteins in regulating Drosophila immunity gene expression. The Journal of biological chemistry. 1999;274(30):21355–61. 10.1074/jbc.274.30.21355 [DOI] [PubMed] [Google Scholar]
- 54.Schaffer S, Gruber J, Ng LF, Fong S, Wong YT, Tang SY, et al. The effect of dichloroacetate on health- and lifespan in C. elegans. Biogerontology. 2011;12(3):195–209. 10.1007/s10522-010-9310-7 [DOI] [PubMed] [Google Scholar]
- 55.Pandey A, Vimal D, Chandra S, Saini S, Narayan G, Kar Chowdhuri D. Long-term dietary exposure to low concentration of dichloroacetic acid promoted longevity and attenuated cellular and functional declines in aged Drosophila melanogaster. Age (Dordr). 2014;36(3):9628 10.1007/s11357-014-9628-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yamane K, Indalao IL, Chida J, Yamamoto Y, Hanawa M, Kido H. Diisopropylamine dichloroacetate, a novel pyruvate dehydrogenase kinase 4 inhibitor, as a potential therapeutic agent for metabolic disorders and multiorgan failure in severe influenza. PloS one. 2014;9(5):e98032 10.1371/journal.pone.0098032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Borges RC, Carvalho CR, Colombo AS, da Silva Borges MP, Soriano FG. Physical activity, muscle strength, and exercise capacity 3 months after severe sepsis and septic shock. Intensive care medicine. 2015;41(8):1433–44. 10.1007/s00134-015-3914-y [DOI] [PubMed] [Google Scholar]
- 58.Tzou P, Reichhart JM, Lemaitre B. Constitutive expression of a single antimicrobial peptide can restore wild-type resistance to infection in immunodeficient Drosophila mutants. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(4):2152–7. 10.1073/pnas.042411999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Petersen UM, Bjorklund G, Ip YT, Engstrom Y. The dorsal-related immunity factor, Dif, is a sequence-specific trans-activator of Drosophila Cecropin gene expression. The EMBO journal. 1995;14(13):3146–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hedengren M, Asling B, Dushay MS, Ando I, Ekengren S, Wihlborg M, et al. Relish, a central factor in the control of humoral but not cellular immunity in Drosophila. Molecular cell. 1999;4(5):827–37. 10.1016/s1097-2765(00)80392-5 [DOI] [PubMed] [Google Scholar]
- 61.Chowdhury M, Zhang J, Xu XX, He Z, Lu Y, Liu XS, et al. An in vitro study of NF-kappaB factors cooperatively in regulation of Drosophila melanogaster antimicrobial peptide genes. Dev Comp Immunol. 2019;95:50–8. 10.1016/j.dci.2019.01.017 [DOI] [PubMed] [Google Scholar]
- 62.van Vught LA, Klein Klouwenberg PM, Spitoni C, Scicluna BP, Wiewel MA, Horn J, et al. Incidence, Risk Factors, and Attributable Mortality of Secondary Infections in the Intensive Care Unit After Admission for Sepsis. JAMA: the journal of the American Medical Association. 2016. 10.1001/jama.2016.2691 [DOI] [PubMed] [Google Scholar]
- 63.Gibala MJ, Saltin B. PDH activation by dichloroacetate reduces TCA cycle intermediates at rest but not during exercise in humans. The American journal of physiology. 1999;277(1 Pt 1):E33–8. [DOI] [PubMed] [Google Scholar]
- 64.Mukherjee K, Fischer R, Vilcinskas A. Histone acetylation mediates epigenetic regulation of transcriptional reprogramming in insects during metamorphosis, wounding and infection. Front Zool. 2012;9(1):25 10.1186/1742-9994-9-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hu YT, Wu TC, Yang EC, Wu PC, Lin PT, Wu YL. Regulation of genes related to immune signaling and detoxification in Apis mellifera by an inhibitor of histone deacetylation. Scientific reports. 2017;7:41255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Anastasiou D, Yu Y, Israelsen WJ, Jiang JK, Boxer MB, Hong BS, et al. Pyruvate kinase M2 activators promote tetramer formation and suppress tumorigenesis. Nature chemical biology. 2012;8(10):839–47. 10.1038/nchembio.1060 [DOI] [PMC free article] [PubMed] [Google Scholar]