Abstract
In most modern bony vertebrates, a considerable portion of the chondrocranium remains cartilaginous only during a relatively small window of embryonic development, making it difficult to study this complex structure. Yet, the transient nature of some chondrocranial elements is precisely why it is so intriguing. Since the chondrocranium has never been lost in any vertebrate, its function is critical to craniofacial development, disease, and evolution. Experimental evidence for the various roles of the chondrocranium is limited, and though snapshots of chondrocranial development in various species at isolated time points are valuable and informative, these cannot provide the data needed to determine the functions of the chondrocranium, or its relationship to the dermatocranium in evolution, in development, or in disease. Observations of the spatiotemporal associations of chondrocranial cartilage, cartilage bone, and dermal bone over early developmental time are available for many vertebrate species and these observations represent the data from which we can build hypotheses. The testing of those hypotheses requires precise control of specific variables like developmental time and molecular signaling that can only be accomplished in a laboratory setting. Here, we employ recent advances in contrast-enhanced micro computed tomography to provide novel 3D reconstructions of the embryonic chondrocranium in relation to forming dermal and cartilage bones in laboratory mice across three embryonic days (E13.5, E14.5, and E15.5). Our observations provide support for the established hypothesis that the vertebrate dermal (exo-) skeleton and endoskeleton evolved as distinct structures and remain distinct. Additionally, we identify spatiotemporal patterning in the development of the lateral wall, roof, and braincase floor of the chondrocranium and the initial mineralization and growth of the bones associated with these cartilages that provides support for the hypothesis that the chondrocranium serves as a scaffold for developing dermatocranial bones. The experimental protocols described and data presented provide tools for further experimental work on chondrocranial development.
Keywords: Cartilage, cartilage bone, dermal bone, skull evolution
Introduction
The chondrocranium is a complex structure that appears during embryonic development of the head in all crown vertebrates. Together with the pharyngeal skeleton, it comprises the cranial endoskeleton (endocranium). In agnathans and chondrichthyans, the chondrocranium composes the cartilaginous skull throughout life, and among osteichthyans, a large portion of the cartilaginous chondrocranium persists in the adult skull in some clades, including sturgeons (Hilton & Bemis, 1999) and lungfish (Kemp, 1999). Still in other bony vertebrates, significant portions of the chondrocranium are present only during a relatively small window of embryonic development, and as development progresses, chondrocranial elements either dissolve as cranial dermal bones ossify intramembranously to form the cranial dermal skeleton (dermatocranium), or they ossify into cartilage bone. The pattern of specific elements undergoing perichondral ossification followed by endochondral ossification is often described as bony elements “replacing” the cartilaginous elements. Less is known of the relationship between specific chondrocranial and dermatocranial elements.
The transient nature and complex structural composition of the chondrocranium in some vertebrates has made the association of the chondrocranium and dermatocranium difficult to study across vertebrate species. Because the chondrocranium and the dermatocranium evolved separately (Patterson, 1977; Hirasawa & Kuratani, 2015) and form separately in the embryo, it is common for investigations to maintain their separation as research foci. For example, current research in biological anthropology rests heavily on the analysis of the mineralized skeleton, especially the skull, yet the chondrocranium is rarely investigated or its role in evolution or development mentioned (discussed by KawasaKi & Richtsmeier, 2017). Foundational research on the chondrocranium (e.g., Gaupp, 1906; Goodrich, 1930; de Beer, 1937; Gregory, 1933) coupled with modern approaches using histology and optically cleared embryos with stained cartilage (e.g., McBratney-Owen et al., 2008; SáncHez-Villagra & Forasiepi, 2017; Hüppi et al., 2018; Werneburg & Yaryhin, 2019) have established chondrocranial anatomy in various vertebrate species, but we lack a thorough understanding of the three-dimensional changes of the chondrocranium during embryonic development and its relationship to developing cranial bones. Recent advances in technologies used to visualize the soft tissues of small embryonic specimens using contrast-enhanced micro computed tomography (μCT) (e.g., Metscher, 2009a, 2009b) make it possible to visualize and analyze the three-dimensional structure of the developing chondrocranium and assess its relationship to the formation of cranial bones at different stages of development in laboratory mice (Kaucka et al., 2017; Lesciotto et al., 2020; Gabner et al., 2020).
The laboratory mouse is currently the most widely used experimental model for studying human development and disease. The basic structure of the chondrocranium has been documented for several rodent species (e.g., Fawcett, 1917, 1923; Eloff, 1948), and specific topics relevant to chondrocranial development have been presented for different rodents (e.g., Youssef, 1966, 1969; Kadam, 1976). Depew’s comprehensive analysis (Depew et al., 2002), McBratney-Owen et al.’s (2008) investigation of the developing cranial base, and Kaucka et al.’s (2017) study of how oriented cell behavior and molecular signals control cartilage growth and shaping of the nasal capsule all focus on mice and offer details of mouse chondrocranium anatomy, but embryonic development of the chondrocranium has yet to be systematically described for the laboratory mouse. Though there are many differences between the mouse and human skull, knowledge of the 3D structure of the chondrocranium, the timing of its appearance, and details of its relationship to the dermatocranium can provide important information for the study of cranial development and evolution of other vertebrates, including humans.
Critical information is gained by studying the chondrocranium across vertebrate species, but there are important advantages to studying chondrocranial development in the laboratory mouse, not the least of which is tight temporal control. Harvesting age measures the time elapsed between conception and collection of an embryo based on timed matings and is routinely used in experimental work because it is a simple metric that is easy to apply in practice. Timed matings provide the opportunity to follow chondrocranial development from its first appearance near embryonic day 12.5 (E12.5) and its relationship to endochondrally and intramembranously forming bone beginning at E14.5 and continuing through adulthood. A single harvesting age is recorded for all embryos in a litter but differences in developmental progress exist among littermates (Miyake et al., 1995; Wanek et al., 1989). Variation introduced by the use of harvesting age can affect our understanding of the timing and sequence of important developmental processes and events (e.g., initiation of cell differentiation, migration, or death; expression of a particular marker of a developmental process) creating an obscured confounding factor in understanding morphogenesis (Musy et al., 2018). Developmental staging describes the morphological maturity of an embryo through estimates of the amount of progress that an individual embryo has made along its ontogenetic trajectory based on phenotypic characters (Musy, et al., 2018). There are several staging systems available for the analysis of development in mice (e.g., Theiler, 1989; Kaufman, 1992; Musy et al., 2018; Hall & Miyake, 1995; Boehm et al., 2011), each with different degrees of temporal resolution that are appropriate for specific research questions. To understand process and determine mechanism about the interaction between the forming chondrocranium and dermatocranium, staging systems capable of assessing fine-scale and short-duration gene and tissue interactions in developing mice can add precision to observations.
Perhaps most critically, laboratory mice provide the opportunity for experimentation and direct testing of hypotheses. One way to advance understanding of a complex biological system like the interaction between chondrocranial and dermatocranial development is to disrupt the system through experimental design. In laboratory mice, this is commonly done by disturbing the function of a gene. Mouse models carrying mutations that are proposed to disproportionately affect cartilage development have been created but have not been used to investigate the question of chondrocranial-dermatocranial integration. Analysis of mouse models carrying mutations in the Col2a1 gene encoding type-II collagen (the most abundant extracellular matrix protein of cartilage) focus primarily on long bones of the appendicular skeleton and cartilage bones that compose the adult cranial base (Rintala et al., 1993, 1997; Savontaus et al., 1996; Eyre, 2001; Gaiser et al., 2002; Cionni et al., 2014). Li et al. (1995) created Col2a1-inactivated transgenic mice, which produced cartilage composed of highly disorganized chondrocytes with a complete lack of extracellular collagen fibrils. The authors’ conclusion, that well-organized cartilage matrix is required as a primary tissue for development of some, but not other components of the vertebrate skeleton is based primarily on their observations of a lack of cartilage bone and epiphyseal growth plates in long bones (Li et al., 1995). However, the study also reveals that heterozygote and homozygote mice had palatal clefts or a complete lack of palatal shelves, bulging foreheads, and shorter snouts (Li et al., 1995) suggesting that Col2a1 deficient mice also experienced changes in the development of dermal bone. Mouse cartilage matrix deficiency (cmd/cmd) is an autosomal recessive lethal mutation associated with a major reduction in aggrecan in the cartilage matrix and with defects of murine cartilage tissues. Analysis of cmd mice relative to normal littermates, revealed these mice to have deformities indicative of effects on cartilage matrix and cartilage bone, but cmd mice also had cleft palate and short snout (Watanabe et al., 1994) indicating that errors in cartilage matrix production may affect the formation of dermal bone. Experiments like these demonstrate the potential value of the laboratory mouse for investigating the interaction of the chondrocranium and dermatocranium in development, disease, and evolution.
Using data obtained from laboratory mice harvested at three embryonic days (E13.5, E14.5, and E15.5) and staged to provide a more precise developmental age for each specimen (Musy et al., 2018), we provide 3D microCT reconstructions and a detailed description of the cartilages of the braincase floor, the roof of the occipital region, the lateral wall and the roof of the preoccipital region, and information about their temporospatial association with the forming dermatocranium and additional cranial bones. Formation of the nasal and otic capsules is not considered here. Data from mice are used to consider the evolutionary and developmental significance of these regions of the chondrocranium to the establishment of early forming cranial bones that mineralize perichondrally/endochondrally and intramembranously. Understanding the temporal and spatial association of chondrocranial cartilage, chondrocranial cartilage bone, and dermatocranial dermal bone in development provides information from which we can build hypotheses and design experiments regarding the evolutionary, developmental, and functional significance of the chondrocranium.
Building a modern skull
Initial formation and growth of the chondrocranium and cranial bones
The modern vertebrate skull is primarily composed of cartilage and bone. Cartilage provides an excellent material for the support of swiftly developing cranial soft tissues and organs that continuously change shape as they expand in size. This is because as cartilage forms in the organic matrix produced by chondrocytes, it can grow interstitially and appositionally. Interstitial growth occurs when chondrocytes proliferate within the cartilage matrix resulting in an increase in cell number followed by subsequent secretion of cartilage matrix and rapid growth. Appositional growth occurs through the recruitment of new chondrocytes along the outer surface of cartilage (perichondrium), resulting in the formation of new cartilage peripherally. Most cranial bones of the endoskeleton (the endocranium) form by either perichondral ossification or perichondral and subsequent endochondral ossification where mesenchymal cells differentiate into chondrocytes that form cartilage that is later replaced by bone (cartilage bone). Cranial bones of the dermal skeleton form by intramembranous ossification where aggregated mesenchymal cells directly differentiate into osteoblasts (Komori et al., 1997; Hartmann, 2009). Mesenchymal cells that form these aggregations are a loosely defined class of osteogenic cells, referred to collectively as osteoblast lineage cells (OLCs). OLCs form condensations that serve as templates for future dermal elements, proliferate, and move through sequential stages of differentiation until they are identifiable as osteoblasts (Hall & Miyake, 2000; Long, 2012). Osteoblasts secrete organic extracellular matrix (Kawasaki et al., 2009) that undergoes mineralization as calcium and phosphate that are concentrated by osteoblasts (Mahamid et al., 2011). Cells trapped within the mineralized matrix take on new functions and are referred to as osteocytes (Franz-Odendaal et al., 2006). Osteocytes, the most abundant cells in bone, are mechanosensory, allow for inter-osteocyte exchange of information via elongated cytoplasmic extensions, and most likely enable adaptive responses of the skeleton to environmental changes by coordinating the function of osteoblasts and osteoclasts (Bellido et al., 2014).
It is well known that select cranial bones form via perichondral/endochondral ossification using specific chondrocranial elements as templates (Table 1A). The developmental relationship between these cartilages and the bones that mineralize endochondrally replacing their cartilage template is clear but the relative timing of ossification of these cartilages requires further investigation. Less clear is the relationship between aspects of the chondrocranium that do not mineralize endochondrally or perichondrally and dermal bones that form through intramembranous ossification (Table 1B). It has been hypothesized that the chondrocranium serves as a scaffold for the later development of dermal bones in the mouse dermatocranium (Kawasaki & Richtsmeier, 2017), a relationship that is accepted for the lower jaw and Meckel’s cartilage, an element of the pharyngeal skeleton.
Table 1.
Relationship between chondrocranial and bony elements of the skull. A. Examples of chondrocranial elements of the mouse crania that ossify via perichondral and endochondral ossification; and B. examples of bones of the mouse crania formed via intramembranous ossification and associated chondrocranial elements.
| A. | |
|---|---|
| Cranial bones formed through peri/endochondral ossification | Associated chondrocranial cartilages |
| basioccipital | parachordal |
| basisphenoid | hypophyseal |
| presphenoid | trabecular |
| orbitosphenoid | hypochiasmatic, orbital |
| exoccipital | occipital arch |
| supraoccipital | tectum posterius |
| petrous temporal | pars cochlearis, pars canalicularis |
| ethmoid | septum nasi, cribriform plate, ethmoturbinals, lateral plate |
| B. | |
| Cranial bones formed through intramembranous ossification | Associated chondrocranial cartilages |
| frontal | ala orbitalis, sphenethmoid commissure, |
| parietal | tectum transversum, orbitoparietal commissure, parietal plate |
| maxillae | pars intermedia, septum nasi, paraseptal |
| lacrimal | pars intermedia, paranasal process |
| premaxillae | pars anterior, paraseptal |
| vomer | paraseptal, septum nasi, lamina transversalis posterior |
| palatine | pila metoptica, cupula nasi posterior, presphenoid |
| pterygoid | hypophyseal, alicochlear commissure |
| interparietal | parietal plate, tectum posterius |
| squamous temporal | orbitoparietal commissure, tegmen tympani |
| nasal | tectum nasi, pars anterior, cribriform plate |
The distinction between the chondrocranium and the dermatocranium lies in their dissimilar modes of ossification and their relative position, both reflecting their unique evolutionary origins and trajectories. Ancestral vertebrates had two distinct skulls: an outer dermatocranium which persists as parts of the modern dermal skeleton, and an inner endocranium, composed of the chondrocranium and the pharyngeal skeleton. Originally, the chondrocranium formed deep within the head to support the brain and other sense organs (Werneburg 2019), while dermal bones of the dermatocranium developed superficially, in contact with the epithelial cell layer. In sum, the ancient dermatocranium encased the endocranium (Kardong, 2018). During evolution, events occurred that caused the topological distinctions of the dermatocranium and the chondrocranium to become less obvious, especially in the adult, and this has caused confusion (Patterson, 1977). Reviews of the literature of mouse models carrying genetic variants reveals considerable reference to “ectopic” cartilages forming in the crania of embryos. Though ectopic cartilages certainly form, in some of those cases the cartilages may not be ectopic, but instead malformations of the chondrocranium. Sorting ectopic cartilages from typically developing or dysmorphic chondrocranial elements requires a thorough understanding of chondrocranial development. Consideration of the distinction between the chondrocranium and the dermatocranium, as well as their associations during development can help us understand the significance of the diverse developmental relationships between aspects of the chondrocranium that undergoes perichondral/endochondral ossification, as well those that either remain as cartilage in the adult or exist only transiently in the embryo, but have a spatiotemporal relationship with intramembranously forming dermal bones.
Evolutionary significance of cartilage and the development of the vertebrate head
Of the two principal skeletal tissues that comprise the vertebrate skull, cartilage and bone, it is likely that cartilage evolved first (Northcutt & Gans, 1983; Smith & Hall, 1990; Cervantes-Diaz et al., 2017). Interestingly, while cartilages are also found in some protostome taxa, their cartilages likely evolved independently from those in deuterostomes, including vertebrates, even though cartilages in both deuterostomes and protostomes employ similar gene regulatory networks (Tarazona et al., 2016). In the vertebrate lineage, the earliest evidence of cartilage is found in the stacked, disc-like formation of the branchial bars of the fossil Haikouella, dated to 530 Mya (Fig. 1) (Chen et al., 1999; Mallatt & Chen, 2003). Haikouella fossils show evidence of eyes and olfactory organs but no evidence of the chondrocranium (Mallat & Chen, 2003). These findings support the idea that the cranial endoskeleton arose first as the pharyngeal skeleton before the origin of the chondrocranium.
Fig. 1.
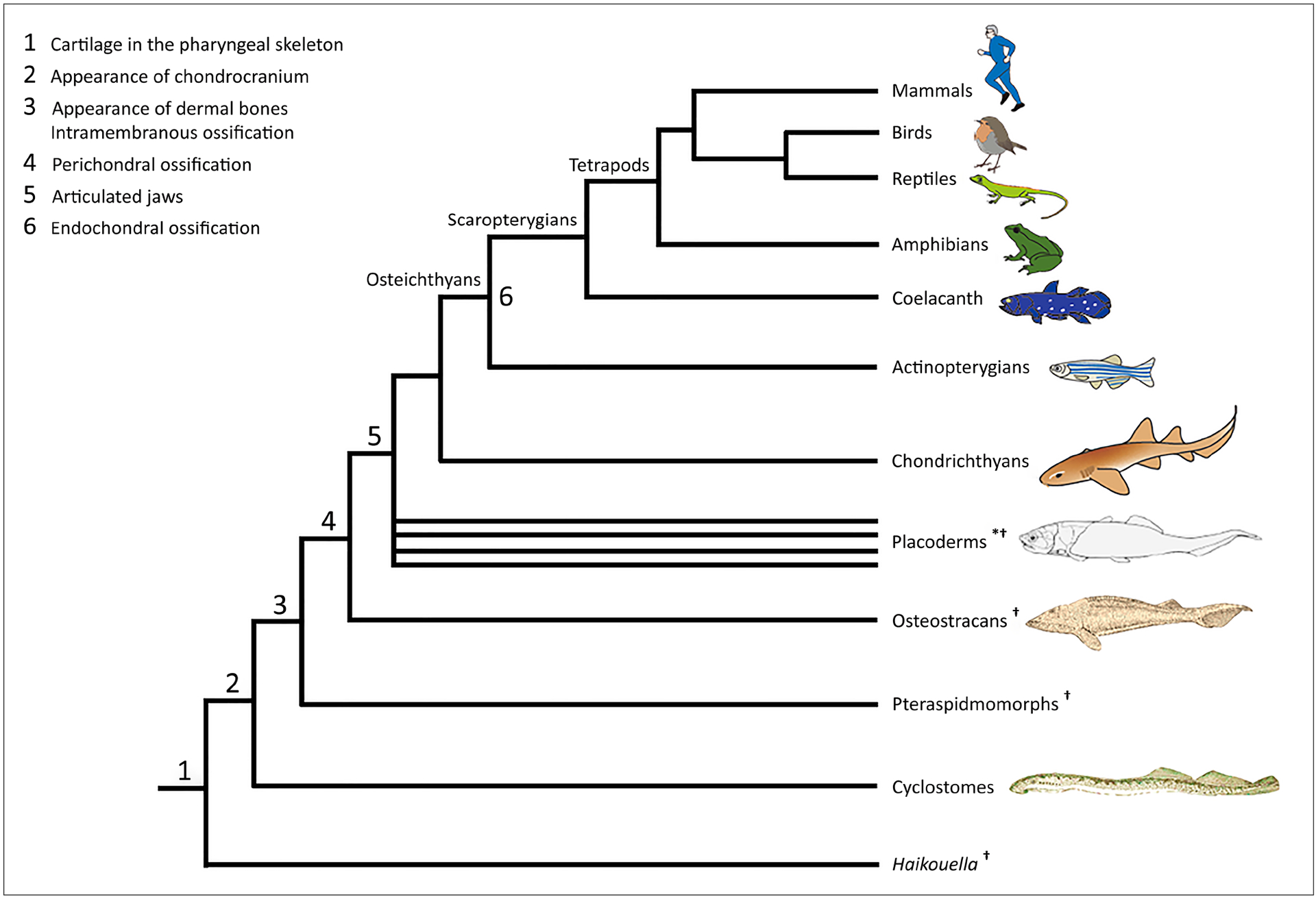
Major events in the evolution of cranial cartilage and bone. The phylogeny of these vertebrate clades is based on Janvier (2015) and Brazeau & Friedman (2015). Figures of cyclostomes, osteostracans, and placoderms are adapted from Romer (1959). * indicates a paraphyletic group; † denotes extinct groups.
The earliest indispuTable bone is found in Ordovician agnathans, classified as pteraspidomorphs (Fig. 1), evidenced by an extensive dermal skeleton, including a large head shield and shoulder girdles, ornamented with dermal denticles (Janvier, 2015; Keating et al., 2018). Because dermal denticles develop through epithelial-mesenchymal interactions, the dermal skeleton of Ordovician agnathans formed in contact with the epidermis (Patterson, 1977). In addition to a dermal skeleton, a perichondrally ossified endoskeleton is found in Silurian and Devonian agnathans. Osteostracans and galeaspids are among these agnathans and are phylogenetically more closely related to gnathostomes (Fig. 1) (Donoghue, 2006). These agnathans developed an endoskeletal head shield composed of a single mass of cartilage lined with a thin layer of perichondral bone (Janvier, 2006) with no distinct divisions among the parts that cover the brain or different sense organs (Donoghue, 2002). Mineralized cartilages have been found in some early agnathans before the emergence of perichondral ossification, but these cartilages are thought to have evolved independently from perichondrally ossified cartilages found in osteostracans, galeaspids, and gnathostomes (Janvier, 2006; Janvier & Arsenault, 2002).
Similar to osteostracans, early extinct gnathostomes (stem gnathostomes, collectively known as placoderms; Fig. 1) developed a single cartilaginous endoskeletal unit, protecting the orbito-temporal, otic, and occipital regions (Schulze, 1993). With some exceptions in placoderms (Miles & Young, 1977), divisions in the chondrocranium are characteristic for acanthodians (paraphyletic stem chondrichthyans) and osteichthyans (Fig. 1). The chondrocranium of acanthodians and early osteichthyans is partially divided along the rostro-caudal axis into the occipital, otic, and orbito-temporal regions by two fissures: the otico-occipital fissure and the ventral fissure. In addition, the ethmoid region occupies a location rostral to the orbito-temporal region (Goodrich, 1930; Schultze, 1993). The ventral fissure reflects the border between the two principal elements of the embryonic braincase floor, the trabecular and the parachordal cartilages (Forey, 1998). In early sarcopterygians and the extant coelacanth, the ventral fissure extends dorsally and splits the chondrocranium into two portions, which are joined by the intracranial joint (Forey, 1998). These divisions, along with perichondral ossification, were secondarily lost in modern chondrichthyans (Schultze, 1993). Unlike the chondrichthyan lineage noted for loss of perichondral ossification, the osteichthyan lineage is marked by the generation of novelty with the appearance of endochondral ossification (Fig. 1). Endochondral ossification, thus, postdates perichondral ossification. Like the order of their evolutionary appearance, various parts of the osteichthyan chondrocranium undergo perichondral ossification first, followed by endochondral ossification. In osteichthyans, the increased complexity in morphology and mineralization pattern of the chondrocranium contributes to its complex spatiotemporal relationship to the dermatocranium.
There is a demonstrated pattern of loss and/or fusion of dermal bones during the evolution of most vertebrate taxa including mammals (Sidor, 2001; Schoch, 2006), making the identification of homologues difficult. For example, among the dermal bones in the mouse, the premaxilla incorporates the prevomer, the pterygoid is a composite of the early pterygoid and the ectopterygoid, the vomer is derived from part of the parasphenoid (de Beer, 1937; Moore, 1981), and the interparietal arose by the paired tabulars and the paired postparietals (Koyabu et al., 2012). More relevant to our study, evolutionary changes have obscured the archetypal distinction between dermatocranial and chondrocranial elements. In early osteichthyans as in extant agnathans, dermal bones are covered with dermal denticles that almost entirely cover the head and jaws, and the distinction between superficial dermal bones and bones formed by mineralization of the cranial endoskeletal cartilages is relatively clear. With evolution, dermal bones of some taxa began to form without dermal denticle ornament in relatively deep anatomical locations proximate to the brain and other sense organs, and peripheral additions of some cartilage bones began to extend toward the more superficial dermatocranium. As indicated by Patterson (1977) and Moore (1981), fusion of some dermal bones with cartilage bones (e.g., in mouse intramembranously ossified squamosal fuses with endochondrally ossified auditory capsule) and direct articulation of bones of the chondrocranium and dermatocranium at sutures on the surface of the cranium (e.g., in mouse endochondrally ossified occipital articulates with intramembranously ossified interparietal and parietal) contribute to the blurred distinction of chondrocranium and dermatocranium. These associations of chondrocranium and dermatocranium begin early in embryogenesis, prior to endochondral ossification and imply a fundamental and perhaps essential interaction between the cranial endo and dermal skeletons.
To understand the significance of the association between the cartilaginous chondrocranium, and development and mineralization of the bony cranium, temporal and spatial relationships between chondrocranial cartilage and developing skull bones need further experimental study. Here, we begin this process with the laboratory mouse, the most widely used experimental animal that we can investigate at important points in embryonic development.
Methods
C57BL/6J mice were used for this study and were cared for following standard IACUC protocols at Pennsylvania State University (IACUC# 46558). To visualize the 3D structure of the chondrocranium, mice of various embryonic ages (E13.5, E14.5, E15.5) were stained with phosphotungstic acid (PTA) and scanned using contrast-enhanced μCT following protocols described in Lesciotto et al. (2020). High-resolution μCT images with voxel size ranging from 0.005 to 0.006 mm were acquired by the Center for Quantitative X-Ray Imaging at the Pennsylvania State University (www.cqi.psu.edu) using the General Electric v|tom|x L300 nano/microCT system. Cranial cartilage was manually segmented from the PTA-enhanced μCT images using avizo 9.4 (Thermo Fisher Scientific) to create 3D surface renderings of the chondrocranium. Bone was segmented automatically using a minimum threshold of 70–100 mg/cm3 partial density hydroxyapatite (HA) (based on HA phantoms imaged with specimens) to reconstruct bony isosurfaces in avizo 9.4. To determine developmental age, these mice were staged using the Embryonic Mouse Ontogenetic Staging System, EMOSS (Musy et al. (2018) (https://limbstaging.embl.es/).
Additional C57BL/6J mouse embryos were stained using alcian blue, with or without alizarin red, and then optically cleared using glycerol (Behringer et al., 2014). These mice were used to assess and validate initial formation of the chondrocranium at E12.5, as well as at E13.5 prior to mineralization of the frontal, parietal, exoccipital, and basioccipital bones (Fig. 2). Further development of the frontal, parietal, exoccipital, and basioccipital bones were assessed using μCT for specimens at E15.5 described above. Our immunohistochemistry analysis was based on a standard method (Behringer et al., 2014) using an anti-RUNX2 antibody (sc-8655, Santa Cruz) and an anti-COL2A1 antibody (sc-7764, Santa Cruz).
Fig. 2.

Mouse embryos aged chronologically at E12.5 (A) and E13.5 (B, C) stained using alcian blue (glycosaminoglycans in cartilage) and alizarin red (calcium in bone) and then optically cleared showing the braincase floor of the early chondrocranium at E12.5 (A) and the lack of staining of cranial bones at E13.5 (B & C). Inferior view (B) and lateral views (A & C) with identification of chondrocranial features. The globe of the eye blocks most of the ala orbitalis in the lateral view at E13.5 (C). Abbreviations: AO, ala orbitalis; AR, acrochordal cartilage; COP, orbitoparietal commissure; CSE, sphenethmoid commissure; H, hypophyseal cartilage; OA, occipital arch; P, parachordal cartilage; PCA, pars canalicularis; PN, paries nasi; PP, parietal plate; SN, septum nasi; T, trabecular cartilage; TT, tectum transversum; Y, hypochiasmatic cartilage.
Results
1. The braincase floor and the roof of the occipital region
The braincase floor of the chondrocranium is comprised of four composite cartilages: the trabecular, hypophyseal, acrochordal, and parachordal cartilages. The roof of the occipital region is comprised of the occipital arches and the tectum posterius (Figs 2 – 5). The parachordal cartilage and the occipital arches form first at the posterior aspect of the head as early as E12.5. The parachordal cartilage is ‘Y-shaped’, with the posterior aspects joining the occipital arches, and the foramen hypoglossum is positioned midway along this joint (Fig. 3). By the end of E12.5, the trabecular cartilage positioned anteriorly along the braincase floor, arises as the septum nasi, a narrow rod of cartilage at the midline of the cranium (Kawasaki & Richtsmeier, 2017).
↑ Fig. 5.

3D reconstruction of PTA-enhanced computed tomography images of the head of a mouse harvested at E15.5 showing lateral views (A, B, and C) and superior views (D, E, and F) of the developing lateral roof, lateral wall, and the braincase floor of the chondrocranium and their association with developing bones. Miniaturized views at far left show location of these structures within the developing head (lateral view at top; superior view at bottom). Only the left side is fully segmented and the otic and nasal capsules are not included. Using the eMOSS staging system (Musy et al., 2018), this specimen is staged at 372 ± 2 hours (15.5 days) post conception, a developmental age that closely matches its harvesting age. Lateral (A) and superior (D) views of the chondrocranium and lateral (C) and superior (F) views of early formation of the frontal, parietal, basioccipital, and exoccipital bones were segmented from the same mouse. Lateral (B) and superior (E) views of early development of the frontal, parietal, basioccipital, and lateral occipital bones manually superimposed on their associated aspects of the chondrocranium to show these associations. The exoccipital is visible in B but hidden from view in E due to the thickness of the cartilage. Abbreviations: AO, ala orbitalis; COP, orbitoparietal commissure; CSC, sphenocochlear commissure; H, hypophyseal cartilage; O, orbital cartilage; OA, occipital arch; P, parachordal cartilage; PP, parietal plate; T, trabecular cartilage; TP, tectum posterius; TTR, tectum transversum, Y, hypochiasmatic cartilage.
Fig. 3.

3D reconstruction of PTA-enhanced μCT images of the head of a mouse harvested at E13.5 showing the lateral roof, lateral wall, and braincase floor of the chondrocranium. Only the left side is fully segmented and the otic and nasal capsules are not included. Using the eMOSS staging system (Musy et al., 2018), this mouse is staged at 342.5 ± 2 hours or approximately 14.25 days post conception, revealing a developmental age that is older than its harvesting age. A) Lateral view of segmented regions within 3D volume rendering of transparent head to show relative placement of the chondrocranium; B) Lateral view of segmented regions of the chondrocranium and identification of chondrocranial features; C) Superior view of segmented regions within 3D volume rendering of transparent head to show relative placement of the chondrocranium within the head (the right side was not completely segmented); D) Superior view of segmented regions and identification of chondrocranial features. Scale bar on the left corresponds with A and C; scalebar on right corresponds with B and D. Abbreviations: AO, ala orbitalis; AR, acrochordal cartilage; fhg, foramen hypoglossum; H, hypophyseal cartilage; OA, occipital arch; P, parachordal cartilage; PP, parietal plate; T, trabecular cartilage; TP, tectum posterius; TTR, tectum transversum; Y, hypochiasmatic cartilage.
The hypophyseal and acrochordal cartilages, forming the medial portion of the braincase floor by E13.5. These two midline cartilages fuse to form the braincase floor with the parachordal cartilage as a continuous plate (Fig. 3). At this stage of development each occipital arch grows dorsally and forms a relatively thick plate (Kawasaki & Richtsmeier, 2017). By E13.5 the tectum posterius arises as a thin cartilage that joins ventrally with the occipital arch and rostrally with the parietal plate. Together, the tectum posterius, occipital arches, and parachordal cartilage join to form the boundary of the foramen magnum. By E14.5, the parachordal, acrochordal, hypophyseal, and trabecular cartilages fuse to form a continuous braincase floor (Fig. 4).
Fig. 4.

3D reconstruction of PTA-enhanced μCT images of the head of a mouse harvested at E14.5 showing the lateral roof and lateral wall and braincase floor of the chondrocranium. Only the left side is fully segmented and the otic and nasal capsules are not included. This mouse is developmentally staged (Musy et al., 2018) at 368 ± 2 hours, or E15.3 days post conception, revealing a developmental age that is older than its harvesting age. A) Lateral view of segmented regions within 3D volume rendering of transparent head to show relative placement of the chondrocranium; B) Lateral view of segmented regions of the chondrocranium and identification of chondrocranial features; C) Superior view of segmented regions within 3D volume rendering of transparent head to show relative placement of the chondrocranium within the head (the right side was not completely segmented); D) Superior view of segmented regions and identification of chondrocranial features. Scale bar on the left corresponds with A and C; scalebar on right corresponds with B and D. Abbreviations: AO, ala orbitalis; AR, acrochordal cartilage; COP, orbitoparietal commissure; CSC, sphenocochlear commissure; CSE, sphenethmoid commissure; fhy, hypophyseal fenestra, fhg, foramen hypoglossum; H, hypophyseal cartilage; O, orbital cartilage; OA, occipital arch; P, parachordal cartilage; PP, parietal plate; T, trabecular cartilage; TP, tectum posterius; TTR, tectum transversum; Y, hypochiasmatic cartilage.
During the period between E14.5 and E15.5, perichondral/endochondral ossification of the braincase floor begins with the basioccipital, and eventually replaces the parachordal cartilage as ossification progresses rostrally by E15.5 (Fig. 5). During this period, the exoccipital begins to mineralize through perichondral/endochondral ossification of the occipital arch (Table 1). This process begins at a middle portion of the occipital arch and spreads dorsoventrally (Fig. 5).
2. Lateral wall and the roof of the preoccipital region
The lateral wall and the roof and the preoccipital region are comprised of the sphenethmoid commissure, the ala orbitalis, the orbital cartilage, the hypochiasmatic cartilages, the tectum transversum, the orbitoparietal commissure, and the parietal plate (Figs 2 – 5). Between E12.5 and E13.5, the ala orbitalis arises medial to the globe of the eye narrowing anteriorly to form the sphenethmoid commissure, which joins to the nasal capsule. The caudal end of the ala orbitalis extends dorsally and this outgrowth becomes the base of the tectum transversum (Fig. 2). By E13.5 the tectum transversum arises as a relatively large apically expanding plate. The orbitoparietal commissure arises caudal to the globe of the eye by E13.5 and grows rostrally from the parietal plate, connecting the parietal plate to the base of the tectum transversum (Fig. 3). Between E12.5 and E13.5 the orbital cartilage and the hypochiasmatic cartilage arise as separate cartilages medial to the ala orbitalis (McBratney-Owen et al., 2008).
Between E13.5 and E14.5, the orbital cartilage merges with the ala orbitalis, as the orbital cartilage grows as a U-shaped rod anterolaterally (Eloff, 1948). At this point, the U-shaped rod of the orbital cartilage also grows medially and connects with the trabecular cartilage (Fig. 3). By E14.5, the hypochiasmatic cartilage extends medially to join with the trabecular cartilage (Fig. 4). During this period of development, the ala orbitalis expands apically as well as rostrocaudally and by E14.5 it is fan shaped. As the ala orbitalis expands, the sphenethmoid commissure becomes less distinct. Between E13.5 and E14.5 both the tectum transversum and the parietal plate appears to become thicker and continue to expand apically as well as rostrocaudally. The orbitoparietal commissure is formed when the rostral extension of the parietal plate reaches the caudal extension of the ala orbitalis. The tectum transversum grows apically from the posterior extension of the ala orbitalis (Fig. 4).
Prior to mineralization, the frontal bones are initially visible in optically cleared samples as a lattice-like matrix located superficial to the ala orbitalis by E14.5. At their initial stage of development, the unmineralized or lightly mineralized frontal bones are not dense enough to be detected by μCT. Mineralization occurs within this matrix; however, the exact location of initiation is variable (Kawasaki & Richtsmeier, 2017). By E15.5, this matrix mineralizes and spreads to cover the apical half of the ala orbitalis (Fig. 5). At this stage of development, the frontal bone does not extend beyond the rostrocaudal expanse of the ala orbitalis (Fig. 5).
Prior to mineralization, the osteoid of the parietal bones are initially visible as a lattice-like matrix by E14.5 that appears, in most instances, after the formation of the frontal bone matrix in most samples. This matrix lies superficial and overlaps slightly with the dorsal edge of the tectum transversum and grows dorsally (Kawasaki & Richtsmeier, 2017). Mineralization of the parietal bones begins between E14.5 and E15.5 superficial to the dorsal edge of the tectum transversum (Kawasaki & Richtsmeier, 2017). During this period, the orbitoparietal commissure becomes more distinctive and correspondingly there is more separation between the tectum transversum and the parietal plate (Fig. 4). By E15.5 the parietal bones expand apically and do not extend beyond the dorsoventral expanse of the tectum transversum (Fig. 5). Relative to the portion of the tectum transversum that is not covered by the parietal bone, the portion that is covered by the parietal bone appears to be weakly chondrified, the cartilage relatively thin and perforated (Fig. 5). As the parietal cartilage continues to expand apically, it remains thin. We propose that dermal bones develop superficial to thin cartilages, a phenomenon that is also observed in the parietal plate and ala orbitalis. Although not shown here, observations of E16.5 mice reveal that chondrocranial cartilages of the lateral wall continue to dissipate as dermal bones expand.
Discussion and Conclusions
Our observations reveal separate though related ontogenies of the chondrocranium and the dermatocranium in the laboratory mouse providing support for Patterson’s (1977) general conclusion that the vertebrate dermal skeleton and endoskeleton evolved as distinct structures and have remained distinct. Our observations also show that in the mouse, the chondrocranium initiates its formation at E12.5, with regions continuing to form and expand through E15.5. Data from our lab (not presented here) suggest that certain cartilages of the mouse chondrocranium (e.g., tectum transversum) begin to dissolve at E16.5 with the growth of associated dermal bones. Our description of two anatomical regions of the chondrocranium, the braincase floor cartilages and the cartilages of the pre-occipital lateral wall and roof, and their associations with the early development of the frontal, parietal, basioccipital, and exoccipital bones reveals distinctive patterns in developmental timing. The relative order of appearance of these cartilages is persistent. The relative order of the initial appearance of mineralizing bones – frontal, parietal, basioccipital, and exoccipital – is also fairly sTable, though the parietal may begin mineralization prior to the frontal in some individuals. In addition, the specific location of the initiation of mineralization of a dermal bone with reference to its associated cartilage is not uniform, revealing a degree of underlying variability in these spatial associations. For example, while the initial formation of the frontal bone is always superficial to the ala orbitalis, the position of the initial site of mineralization along the ala orbitalis is variable. The location of the initial mineralization of the parietal, basioccipital, and exoccipital bones appear to be less variable.
In addition to the recognized spatiotemporal patterning of cartilage bone that forms within some endocranial cartilages, our observations suggest a significance of the relationship between the lateral wall and roof of the chondrocranium and dermal bones that form via intramembranous ossification. Initial mineralization of the frontal and parietal bones invariably occurs after the appearance of their associated cartilages, and during their initial stage of growth, these two bones do not extend beyond the boundary of their associated cartilages (Table 1B). The frontal bone does not extend beyond the rostrocaudal expanse of the ala orbitalis and the parietal bone does not extend beyond the rostrocaudal expanse of the tectum transversum until after these cartilages begin to dissipate at E16.5. This suggests that the ala orbitalis and the tectum transversum act as boundaries for the developing frontal and parietal bones, respectively, and provides support for the hypothesis that the chondrocranium serves as a scaffold for developing intramembranous bones (Kawasaki & Richtsmeier, 2017). The nature of this scaffold; whether it is spatial, structural, or functional can be determined through experimentation.
If the chondrocranium serves as a scaffold, providing temporary structural support for the brain and other sense organs until the dermatocranium gains strength and acquires the essential size and shape, then exchange of information between the chondrocranium and dermatocranium would be required to signal for disassembly of portions of the chondrocranium when conditions are right. Boundaries between cell populations often provide the medium for communication, serving as tissue organizers or functioning as signaling interfaces during embryogenesis (Dahmann & Basler, 1999; Cheng et al., 2004; Merrill et al., 2006). Elements of the chondrocranium and dermatocranium form by migration, condensa-tion, and proliferation of separate cell populations (Hall & Miyake, 2000; Hall, 2015). Mesenchymal progenitors that give rise to osteoblasts and chondrocytes are initially marked by SOX9, and it is accepted that SOX9-positive cells are bi-potential, giving rise to both osteoblasts and chondrocytes (Long, 2012). Differentiation of SOX9-positive cells into osteoblasts is marked first by the expression of RUNX2 and then osterix (OSX), ultimately leading to mature osteoblasts. Cells expressing the Col2a1 gene encoding type-II collagen appear prior to cartilage formation. Our preliminary analyses of the boundary between the ala orbitalis and the frontal bone showed that while Col2a1-expressing chondroblast lineage cells distribute within the body of the ala orbitalis, within its perichondrium, and within its dorsal extension, the distribution of Runx2-expressing OLCs is limited principally to a narrow periosteal region of the frontal bone (Fig. 6). This suggests that chondroblast lineage cells forming the ala orbitalis and OLCs forming the frontal bone are separated by a boundary that is established early. Whether cells that occupy the boundary remain, or function in breaking down cartilage or building bone is not known. The function of cells that populate the chondrocranium/dermatocranium boundary over developmental time can be further investigated in embryonic laboratory mice by analysis of cellular processes, gene expression, and protein distribution using common laboratory protocols (e.g., detection of incorporated BrdU, TUNEL assay, immunohistochemistry, and in situ hybridization) and typically developing mice.
Fig. 6.

Distribution of chondroblast lineage cells and osteoblast lineage cells in the ala orbitalis (chondrocranium) and frontal bone (dermatocranium) in serial sections of a mouse at E14.5 detected by immunohistochemistry using an anti-RUNX2 antibody (A, C) and an anti-COL2A1 antibody (B, D). The distribution of RUNX2 is limited to the forming frontal bone (blue arrowhead in C). COL2A1 is detected in the cartilage matrix, in the perichondrium (red arrowhead in D), and in the dorsal extension of the ala orbitalis (green arrowhead in D) that extends apically to underlie the frontal.
Though we have presented our observations within an evolutionary context, and believe our results have implications across vertebrates, the mouse chondrocranium is unique. The presence of the tectum transversum has been reported only in the mouse (Kawasaki and Richtsmeier, 2017) and the Weddell seal (Fawcett, 1918) in mammals (de Beer, 1937). However, de Beer’s (1937) seminal work, the most comprehensive analysis of the chondrocranium of vertebrates to date, is limited by low numbers of specimens and developmental stages for each species. It is possible that de Beer, his forerunners, and more recent researchers missed the developmental window for the formation of the tectum transversum in other species – but until more data are available, we accept de Beer’s (1937) observation that the tectum transversum is confirmed only in the Weddell seal among the various mammals he studied. Analysis of the mouse chondrocranium indicates that the tectum transversum is tied to the development of the parietal bone, but if the majority of mammals do not develop a tectum transversum, this specific relationship may not extend to all mammals. This example underscores the importance of continued research into the temporal and spatial relationships of the chondrocranium and the development of cranial bones in additional taxa to illuminate the relationship between cartilaginous chondrocranial elements and intramembranous bones. Those studies coupled with experimental analysis of the laboratory mouse can reveal mechanisms of chondrocranial-dermatocranial integration in development and how these mechanisms contributed to the evolution of the skull.
Supplementary Material
Acknowledgements
The authors thank Drs. Tim Ryan, Brian Metscher, and Susan M. Motch Perrine and Mr. Tim Stecko at Penn State’s Center for Quantitative Imaging for obtaining high-quality μCT images and assisting in protocol review for PTA staining and cartilage visualization. Imaging studies were performed at Pennsylvania State University Center for Quantitative Imaging and mouse and wet lab studies were performed at Pennsylvania State University Department of Anthropology. Our segmentation procedures were aided immensely by the work of Dr. James Hanken, Professor of Zoology at Harvard University and Stephen Turney, Center for Brain Science, Harvard University who provided whole-slide imaging of our histological collection on a Huron TissueScope LE Slide Scanner (Huron Digital Pathology, Canada) that were examined using Girder software (Kitware). This work was supported by the National Institute of Dental and Craniofacial Research R01 DE027677 and the Eunice Kennedy Shriver National Institute of Child Health and Human Development P01HD078233.
References
- Behringer R, Gertsenstein M, Nagy KV & Nagy A (2014). Manipulating the Mouse Embryo: A Laboratory Manual. Cold Spring Harbor, Cold Spring Harbor Laboratory Press. [Google Scholar]
- Bellido T, Plotkin LI & Bruzzaniti A (2014). Bone cells, pp. 27–45 in: Burr DB & Allen MR (eds) Basic and Applied Bone Biology. San Diego, Academic Press. [Google Scholar]
- Boehm B, Rautschka M, Quintana L, Raspopovic J, Jan Ž & Sharpe J (2011). A landmark-free morphometric staging system for the mouse limb bud. Development, 138, 1227–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazeau M & Friedman M (2015). The origin and early phylogenetic history of jawed vertebrates. Nature, 520, 490–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervantes-Diaz F, Contreras P & Marcellini S (2017). Evolutionary origin of endochondral ossification: the transdifferentiation hypothesis. Development Genes and Evolution, 227, 121–127. [DOI] [PubMed] [Google Scholar]
- Chen JY, Huang DY & Li CW (1999). An early Cambrian craniate-like chordate. Nature, 402, 518–522. [Google Scholar]
- Cheng Y-C, Amoyel M, Qiu X, Jiang Y-J, Xu Q & Wilkinson DG (2004). Notch activation regulates the segregation and differentiation of rhombomere boundary cells in the zebrafish hindbrain. Developmental Cell, 6, 539–550. [DOI] [PubMed] [Google Scholar]
- Cionni M, Menke C & Stottmann RW (2014). The Mouse MC13 Mutant Is a Novel ENU Mutation in Collagen Type II, Alpha 1. PloS ONE, 9, e116104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahmann C & Basler K (1999). Compartment boundaries: at the edge of development. Trends in Genetics, 15, 320–326. [DOI] [PubMed] [Google Scholar]
- De Beer GR (1937). The Development of the Vertebrate Skull. Chicago, The University of Chicago Press. [Google Scholar]
- Depew MJ & Simpson CA (2006). 21st century neontology and the comparative development of the vertebrate skull. Developmental Dynamics, 235, 1256–1291. [DOI] [PubMed] [Google Scholar]
- Depew MJ, Tucker AS & Sharpe PT (2002). Craniofacial development, pp. 421–498 in: Rossant J & Tam PPL(eds) Mouse Development. San Diego, Academic Press. [Google Scholar]
- Donoghue PCJ, Sansom IJ & Downs JP (2006). Early evolution of vertebrate skeletal tissues and cellular interactions, and the canalization of skeletal development. Journal of Experimental Zoology (Molecular and Developmental Evolution), 306B, 278–294. [DOI] [PubMed] [Google Scholar]
- Eloff FC (1948). The early development of the skull of Otomys tropicalis. Annals of the Transvaal Museum, 21, 103–152. [Google Scholar]
- Eyre D (2001). Collagen of articular cartilage. Arthritis Research & Therapy, 4, 30–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett E (1917). The primordial cranium of Microtus amphibious (water-rat), as determined by sections and a model of the 25 mm stage. Journal of Anatomy, 51, 309–359. [PMC free article] [PubMed] [Google Scholar]
- Fawcett E (1918). The primordial cranium of Poecilophoca weddelli (Weddell’s seal), at the 27 mm. CR length. Journal of Anatomy, 52, 412–441. [PMC free article] [PubMed] [Google Scholar]
- Fawcett E (1923). The primordial cranium of Xerus (spiny squirrel) at the 17 and 19 millimeters stages. Journal of Anatomy, 57, 221–237. [PMC free article] [PubMed] [Google Scholar]
- Forey P (1998). History of the Coelacanth Fishes. London, Chapman & Hall. [Google Scholar]
- Franz-Odendaal TA, Hall BK & Witten PE (2006). Buried alive: How osteoblasts become osteocytes. Developmental Dynamics, 235, 176–190. [DOI] [PubMed] [Google Scholar]
- Gabner S, Böck P, Fink D, Glösmann M & Handschuh S (2020). The visible skeleton 2.0: phenotyping of cartilage and bone in fixed vertebrate embryos and foetuses based on X-ray microCT. Development, 147, 10.1242/dev.187633 [DOI] [PubMed] [Google Scholar]
- Gaiser KG, Maddox BK, Bann JG, Boswell BA, Keene DR, Garofalo S & Horton WA (2002). Y-position collagen II mutation disrupts cartilage formation and skeletal development in a transgenic mouse model of spondyloepiphyseal dysplasia. Journal of Bone and Mineral Research, 17, 39–47. [DOI] [PubMed] [Google Scholar]
- Gaupp E (1906). Die Entwickelung des Kopfskelettes, in: Hertwig O (ed) Entwickelungslehre der Wirbeltiere. Jena, Gustav Fischer. [Google Scholar]
- Goodrich ES (1930). Studies of the Structure and Development of Vertebrates. London, MacMillan. [Google Scholar]
- Gregory WK (1933). Fish skulls: a study of the evolution of natural mechanisms. Transactions of the American Philosphical Society 23, 457–481. [Google Scholar]
- Hall BK (2015). Bones and Cartilage: Developmental and Evolutionary Skeletal Biology. San Diego, Elsevier. [Google Scholar]
- Hall BK & Miyake T (1995). How do embryos measure time? pp. 3–20 in: McNamara KJ (ed.) Evolutionary Change and Heterochrony, Chichester, UK, John Wiley and Sons. [Google Scholar]
- Hall BK & Miyake T (2000). All for one and one for all: condensations and the initiation of skeletal development. Bioessays, 22, 138–147. [DOI] [PubMed] [Google Scholar]
- Hartmann C (2009). Transcriptional networks controlling skeletal development. Current Opinions in Genetic Development, 19, 437–443. [DOI] [PubMed] [Google Scholar]
- Hirasawa T & Kuratani S (2015). Evolution of the vertebrate skeleton: morphology, embryology, and development. Zoological Letters, 1, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hüppi E, Sánchez-Villagra MR, Tzika AC & Werneburg I (2018). Ontogeny and phylogeny of the mammalian chondrocranium: the cupula nasi anterior and associated structures of the anterior head region. Zoological Letters, 4, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janvier P & Arsenault M (2002). Calcification of early vertebrate cartilage. Nature, 417, 609. [DOI] [PubMed] [Google Scholar]
- Janvier P (2006). Early Vertebrates. New York, Oxford University Press. [Google Scholar]
- Janvier P (2015). Facts and fancies about early fossil chordates and vertebrates. Nature, 520, 483–489. [DOI] [PubMed] [Google Scholar]
- Kadam KM (1976). The development of the chondrocranium in the golden hamster, Mesocricetus auratus (waterhouse). Gegenbaurs Morphologisches Jahrbuch, 122, 796–814. [PubMed] [Google Scholar]
- Kamori T et al. (1997). Targeted disruption of Cbfa1 results in complete lack of bone formation owing to maturational arrest of osteoblasts. Cell, 89, 755–764. [DOI] [PubMed] [Google Scholar]
- Kardong KV (2018). Vertebrates: Comparative Anatomy, Function, Evolution. 8th edition. New York, McGraw-Hill Education. [Google Scholar]
- Kaucka M, Zikmund T, Tesarova M, Gyllborg D, Hellander A, Jaros J, Kaiser J, Petersen J, Szarowska B, Newton PT & Dyachuk V (2017). Oriented clonal cell dynamics enables accurate growth and shaping of vertebrate cartilage. Elife, 6, e25902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman MH (1992). Atlas of Mouse Development. London, Academic Press. [Google Scholar]
- Kawasaki K, Buchanan AB & Weiss KM (2009). Biomineralization in humans: making the hard choices in life. Annual Review of Genetics, 43, 119–142. [DOI] [PubMed] [Google Scholar]
- Kawasaki K & Richtsmeier JT (2017). Associations of the chondrocranium and dermatocranium, pp.52–78 in: Percival CJ & Richtsmeier JT (eds) Building Bones: bone development and formation in anthropology. Cambridge, Cambridge University Press. [Google Scholar]
- Keating JN, Marquart CL, Marone F & Donoghue PC (2018). The nature of aspidin and the evolutionary origin of bone. Nature Ecology & Evolution, 2, 1501–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyabu D, Maier W & Sánchez-Villagra MR (2012). Paleontological and developmental evidence resolve the homology and dual embryonic origin of a mammalian skull bone, the interparietal. Proceedings of the National Academy of Sciences United States of America, 109, 14075–14080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesciotto KM, Motch Perrine SM, Kawasaki M, Stecko T, Ryan TM, Kawasaki K & Richtsmeier JT (2020). Phosphotungstic acid-enhanced microCT: Optimized protocols for embryonic and early postnatal mice. Developmental Dynamics, 249, 573–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S-W, Prockop DJ, Helminen H, Fässler R, Lapveteläinen T, Kiraly K, Peltarri A, Arokoski J, Lui H & Arita M (1995). Transgenic mice with targeted inactivation of the Col2 alpha 1 gene for collagen II develop a skeleton with membranous and periosteal bone but no endochondral bone. Genes & Development, 9, 2821–2830. [DOI] [PubMed] [Google Scholar]
- Long F (2012). Building strong bones: molecular regulation of the osteoblast lineage. Nature Reviews Molecular Cell Biology, 13, 27–38. [DOI] [PubMed] [Google Scholar]
- Mahamid J, Sharir A, Gur D, Zelzer E, Addadi L & Weiner S (2011). Bone mineralization proceeds through intracellular calcium phosphate loaded vesicles: A cryoelectron microscopy study. Journal of Structural Biology, 174, 527–535. [DOI] [PubMed] [Google Scholar]
- Mallatt J & Chen JY (2003). Fossil sister group of craniates: predicted and found. Journal of Morphology, 258, 1–31. [DOI] [PubMed] [Google Scholar]
- Metscher BD (2009a) MicroCT for Developmental Biology: A Versatile Tool for High-Contrast 3D Imaging at Histological Resolutions. Developmental Dynamics, 238, 632–640. [DOI] [PubMed] [Google Scholar]
- Metscher BD (2009b) MicroCT for comparative morphology: simple staining methods allow high-contrast 3D imaging of diverse non-mineralized animal tissues. BMC Physiology, 9, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBratney-Owen B, Iseki S, Bamforth SD, Olsen BR & Morriss-Kay GM (2008). Development and tissue origins of the mammalian cranial base. Developmental Biology, 322, 121–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill AE, Bochukova EG, Brugger SM, Ishii M, Pilz DT, Wall SA, Lyons KM, Wilkie AO & Maxson RE Jr (2006). Cell mixing at a neural crest-mesoderm boundary and deficient ephrin-Eph signaling in the pathogenesis of craniosynostosis. Human Molecular Genetics, 15, 1319–1328. [DOI] [PubMed] [Google Scholar]
- Miles RS & Young GC (1977). Placoderm interrelationships reconsidered in the light of new ptyctodontids from Gogo, Western Australia, pp. 123–198 in: Andrews SM, Miles RS & Walker AD (eds) Problems in Vertebrate Evolution. New York, Academic Press. [Google Scholar]
- Miyake T, Cameron AM & Hall BK (1995). Detailed staging of inbred C57BL/6 mice between Theiler’s [1972] stages 18 and 21 (11 – 13 days of gestation) based on craniofacial development. Journal of Craniofacial Genetics and Developmental Biology, 6, 1–31. [PubMed] [Google Scholar]
- Moore WJ (1981). The Mammalian Skull. New York, Cambridge University Press. [Google Scholar]
- Musy M, Flaherty K, Raspopovic J, Robert-Moreno A, Richtsmeier JT & Sharpe J (2018). A quantitative method for staging mouse embryos based on limb morphometry. Development, 145, dev.154856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northcutt RG & Gans C (1983). The genesis of neural crest and epidermal placodes: a reinterpretation of vertebrate origins. The Quarterly Review of Biology, 58, 1–28. [DOI] [PubMed] [Google Scholar]
- Patterson C (1977). Cartilage bones, dermal bones and membrane bones, or the exoskeleton versus endoskeleton, pp. 77–121 in: Andrews SM, Miles RS & Walker AD (eds) Problems in Vertebrate Evolution. New York, Academic Press. [Google Scholar]
- Rintala M, Metsäranta M, Garofalo S, De Crombrugghe B, Vuorio E & Rönning O (1993). Abnormal craniofacial morphology and cartilage structure in transgenic mice harboring a Gly–> Cys mutation in the cartilage-specific type II collagen gene. Journal of Craniofacial Genetics and Developmental Biology, 13, 137–146. [PubMed] [Google Scholar]
- Rintala M, Metsäranta M, Säämänen A, Vuorio E & Rönning O (1997). Abnormal craniofacial growth and early mandibular osteoarthritis in mice harbouring a mutant type II collagen transgene. Journal of Anatomy, 190, 201–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romer AS (1959). The Vertebrate Story. Chicago, University of Chicago Press. [Google Scholar]
- Sánchez-Villagra MR & Forasiepi AM (2017). On the development of the chondrocranium and the histological anatomy of the head in perinatal stages of marsupial mammals. Zoological Letters, 3, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savontaus M, Metsranta M & Vuorio E (1996). Retarded skeletal development in transgenic mice with a type II collagen mutation. American Journal of Pathology, 149, 2169–2182. [PMC free article] [PubMed] [Google Scholar]
- Schoch RR (2006). Skull ontogeny: developmental patterns of fishes conserved across major tetrapod clades. Evolution & Development, 8, 524–536. [DOI] [PubMed] [Google Scholar]
- Schultze H-P (1993). Patterns of diversity in the skull of jawed fishes, pp. 189–254 in: Hanken J & HALL BK (eds) The Skull, Volume 2. Chicago, The University of Chicago Press. [Google Scholar]
- Sidor CA (2001). Simplification as a trend in synapsid cranial evolution. Evolution, 55, 1419–1442. [DOI] [PubMed] [Google Scholar]
- Smith MM & Hall BK (1990). Development and evolutionary origins of vertebrate skeletogenic and odontogenic tissues. Biological Review Cambridge Philosophical Society, 65, 277–373. [DOI] [PubMed] [Google Scholar]
- Tarazona OA, Slota LA, Lopez DH, Zhang G & Cohn MJ (2016). The genetic program for cartilage development has deep homology with Bilateria. Nature, 533, 86–89. [DOI] [PubMed] [Google Scholar]
- Theiler K (1989). The House Mouse: Atlas of Embryonic Development. New York: Springer-Verlag. [Google Scholar]
- Youssef EH (1966). The chondrocranium of the albino rat. Acta Anatomica, 64, 586–617. [DOI] [PubMed] [Google Scholar]
- Youssef EH (1969). Development of the membrane bones and ossification of the chondrocranium in the albino rat. Acta Anatomica, 72, 603–623. [DOI] [PubMed] [Google Scholar]
- Wanek N, Muneoka K, Holler-Dinsmore G, Burton R & Bryant SV(1989). A staging system for mouse limb development. Journal of Experimental Zoology, 249, 41–49. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Kimata K, Line S, Strong D & Gao Y-R (1994). Mouse cartilage matrix deficiency (cmd) caused by a 7 bp deletion in the aggrecan gene. Nature Genetics, 7, 154–157. [DOI] [PubMed] [Google Scholar]
- Werneburg I & Yaryhin O (2019). Character definition and tempus optimum in comparative chondrocranial research. Acta Zoologica, 100, 376–388. [Google Scholar]
- Werneburg I (2019). Morphofunctional categories and ontogenetic origin of temporal skull openings in amniotes. Frontiers in Earth Science, 7, 13. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


