Abstract
Background:
WHO does not recommend community-level health workers (CLHWs) using integrated community case management (iCCM) to treat 7–59 days old infants with fast breathing with oral amoxicillin, whereas World Health Organization (WHO) integrated management of childhood illness (IMCI) recommends it. We want to collect evidence to help harmonization of both protocols.
Methods:
A cluster, randomized, open-label trial will be conducted in Africa and Asia (Ethiopia, Malawi, Bangladesh and India) using a common protocol with the same study design, inclusion criteria, intervention, comparison, and outcomes to contribute to the overall sample size. This trial will also identify hypoxaemia in young infants with fast breathing. CLHWs will assess infants for fast breathing, which will be confirmed by a study supervisor. Enrolled infants in the intervention clusters will be treated with oral amoxicillin, whereas in the control clusters they will be managed as per existing iCCM protocol. An independent outcome assessor will assess all enrolled infants on days 6 and 14 of enrolment for the study outcomes in both intervention and control clusters. Primary outcome will be clinical treatment failure by day 6. This trial will obtain approval from the WHO and site institutional ethics committees.
Conclusions:
If the research shows that CLHWs can effectively and safely treat fast breathing pneumonia in 7–59 days old young infants, it will increase access to pneumonia treatment substantially for infants living in communities with poor access to health facilities. Additionally, this evidence will contribute towards the review of the current iCCM protocol and its harmonization with IMCI protocol.
Trial Registration:
The trial is registered at AZNCTR International Trial Registry as ACTRN12617000857303.
Keywords: Young infant, Enhanced case management, iCCM, Community level health worker, Pulse oximeter, Fast breathing pneumonia, Amoxicillin
INTRODUCTION
Pneumonia contributes to 15% of under-five mortality, with majority occurring among poor children in remote areas of sub-Saharan Africa and South Asia.1,2 The proportion of deaths among pneumonia cases varies from 0.1% to up to 30%, depending upon disease severity, timeliness, appropriateness and promptness of treatment.3–6 Most deaths occur outside hospitals.5 Despite significant reductions in global mortality, progress is sub-optimal in Sub-Saharan Africa and South Asia.2,6,7 Treatment is needed for an estimated 138 million pneumonia episodes annually.2
World Health Organization (WHO)/United Nations Children’s Fund (UNICEF) integrated community case management (iCCM) recommends community-level health workers (CLHW) to refer all sick young infants to a health facility (Table 1).8 A large proportion of families with sick young infants do not accept referral to a health facility due to various reasons.9–14 WHO or UNICEF integrated management of childhood illness (IMCI) protocol recommends oral amoxicillin for fast breathing pneumonia at health facilities.15–17 What can be done for children of families who cannot access health facilities?18,19
Table 1:
Key differences between standard iCCM and enhanced iCCM.8
| Age (7–59 days) | WHO/UNICEF iCCM | Enhanced iCCM tested in study |
|---|---|---|
| Fast breathing* | Refer to hospital | Treat with oral amoxicillin |
| Danger signs or severe chest indrawing | Refer to hospital | Refer to hospital |
| Hypoxaemia detected by pulse oximetry (SpO2<90%) | No | SpO2 measured by CLHW and refer hypoxaemia cases to hospital |
Respiratory rate equal to or more than 60 breaths per minute. CLHW: community-level health workers.
Increase access to pneumonia treatment is possible if evidence showed that 7–59 days old infants with fast breathing could be safely and effectively treated by CLHWs at community level. This would contribute to the evidence base for potential revision of iCCM protocol used at community level and its harmonization with the IMCI protocol used at health facility level.8,20
METHODS
Research question
Can 7–59 days old infants with fast breathing without general danger signs and/or hypoxaemia be treated effectively and safely with oral amoxicillin by CLHWs?
Primary objective
Determine and compare treatment failure rates of enhanced iCCM (Table 1) compared with standard iCCM (Table 1) for 7–59 days old infants with fast breathing without general danger signs and/or hypoxaemia, treated with oral amoxicillin by CLHWs.
Secondary objective
Evaluation of use of pulse oximetry and oxygen saturation (SpO2) as a component of community based pneumonia management algorithm.
Study design
The study was designed on cluster randomized controlled open-label trial.
Study setting and sites
The study will be conducted at four sites in Bangladesh, Ethiopia, India and Malawi (Table 2 and 3).
Table 2:
Study site characteristics.
| Country | Bangladesh | India | Ethiopia | Malawi |
|---|---|---|---|---|
| Geographical location | Six sub-districts in Barisal district under Barisal division: 176 community clinics | Palwal district, Haryana State:92 sub-centers | Four highland districts: 92 health posts attached to 20 health centers | Two districts: 44 health facilities in 248 village clinics |
| Population | ~1.1 million | ~1.2 million | ~0.5 million | ~0.5 million |
| Estimated number of under five year old children | 110,000 | 120,000 | 65,000 | 81,000 |
| Characteristics of clusters | ||||
| Name | Union | Health sub-centers | Health center | Health center |
| Median population | 25492 | 10318 | 25492 | 20926 |
| Characteristics of the CLHW | ||||
| Designation | CHCP | ASHA | HEW | HSA |
| Number of CLHWs | 176 | 876 | 198 | 289 |
| Basic education | 12th grade | Literate/ 8th grade | 10th grades | 12th grades |
| Training | 12 weeks training on primary health care | 42 weeks training of ASHA training modules | 12 months training in hygiene, sanitation, disease prevention and family health | 3 months training in health promotion and prevention |
| Place of work | Community clinic | Households | Health post | Village clinics |
| Population covered | 6000–7000 population | 1000 population | 1500–2500 population | 1600–1800 population |
CHCP: Community health care provider, ASHA: Accredited social health activist; HEW: Health extension worker; HSA: Health surveillance assistant.
Table 3:
Details of study site.
| In Bangladesh, the study will be conducted in six-sub-districts (Gaurnadi, Banaripara, Wazirpur, Babugonj, Barisal Sadar and Bakergonj) of Barisal district. The CLHW is known as CHCP, which is based at the ‘Community Clinic’ with a catchment population of around 6000–7000. Their referral health facility is ‘Upazila Health Complex’. About 54% receive pneumonia treatment from traditional doctors and pharmacies, and 50–60% of the families may refuse referral advice.28 |
| In Ethiopia the study will be conducted in four rural highland districts (woreda) Debark, Dabat, Janamora and Wogera of North Gondar zone in Amhara region. The CLHW is called HEW based at ‘health post’ covering a population of 1500–2500 people. Their referral health facility is a ‘health centre’. About 27% mothers or caretakers seek advice or treatment from health care provider or health facility for pneumonia.29 |
| In India the study will be conducted in the district of Palwal, Haryana state. The CLHW is called ASHA working in the community from their homes, covering 200–300 households, a population of up to 1500 people. Their referral level health facility is a ‘subcentre’ or a ‘primary health centre’. The private practitioners are the most common source of care seeking for children for all illnesses (~60%). A large proportion of these providers are unqualified. Around 60% of the families are unable to accept referral because mothers are unable to leave their homes or have other siblings to take care.30 |
| In Malawi the study will be carried out in Dedza and Ntchisi districts located in central Malawi. The CLHW is called ‘HSA’ working in the community either from home or a small health post covering a population of 1600–1800 people. Their referral level health facility is a ‘health centre’. Families seek care from HSAs for iCCM illnesses in 10–30% of cases.31,32 |
CHCP: Community health care provider, ASHA: Accredited social health activist; HEW: Health extension worker; HSA: Health surveillance assistant.
Participants
Infants 7–59 days old with fast breathing only, i.e., respiratory rate of 60 or more breaths per minute will be enrolled in the study. Infants who are unconscious, unable to feed at all or stopped feeding well, have movement with stimulation or no movement at all, convulsions, severe chest indrawing, high body temperature (≥38°C), low body temperature (<35.3°C), persistent vomiting, weight less than 2000 g on presentation, hypoxaemia (SpO2 <90%), previous inclusion in the study or do not consent to participate in the study, will be excluded.
Intervention
In intervention clusters all enrolled infants with fast breathing will be treated with 80 mg/kg/day oral amoxicillin in two divided doses for seven days. Dispersible amoxicillin 250 mg scored tablets will be used. CLHWs will administer the first dose at the place of enrolment and the remaining doses will be provided to caregivers for administering at home. Caretakers will be counselled to continue with the oral antibiotic twice daily for seven days, the importance of adherence, correct dose, timing and home care.
Control
In control clusters all sick young infants will be referred by CLHWs to a referral health facility for further management.
Identification of pregnancies and births, surveillance for illness
Existing pregnancy surveillance systems will be strengthened and an orientation session on identification of sick young infants will be organized for community level volunteers including CLHWs to identify newborns as soon as possible after birth and refer them to trained CLHWs. CLHW or community volunteers will visit every new birth at home on days 1, 3, and 7. During the home or facility visits, the CLHW will provide standard advice regarding newborn care.21
CLHWs will also be trained to recognize and record danger signs of illness as described in the WHO/UNICEF training package for CLHWs, and will refer or take any infant who has a danger sign to a referral health facility.22 Families will be encouraged to report to the CLHW or directly to the referral health facility if the infant has any danger sign at any point between the CLHW’s visits.
Demand generation activities
In the study communities, it is common practice that newborns are not taken out of their homes even if they are sick. In addition, the CLHWs have not previously provided any treatment to sick young infants in these communities. Information, education, and communication (IEC) activities in intervention clusters will be aimed to create awareness about the CLHWs’ new role as enhanced community based treatment providers for treating pneumonia in 7–59 days old infants. In the control clusters, the IEC activities will focus on sustaining the community’s demand for standard iCCM management provided by CLHWs. The materials will include posters with pictures and simple messages in the local languages and audio announcements. Posters or wall paintings will be placed at high visibility public places. Nameplates mentioning the CLHW’s name and phone number will be pasted outside each CLHW’s home in India and Malawi. Periodic audio announcements through loudspeakers will be made across the study areas. These methods are chosen as they are currently used in these areas to promote new activities. The IEC materials and audio announcements using loudspeakers will be separated for intervention and control clusters as per the standard and enhanced iCCM protocol. Where community support groups exist, their members will be sensitized about the signs of illness in infants and provided practical tips for dissemination of messages. Mothers/caregivers will be shown video clips on fast breathing at immunization sites and mothers’ gatherings. Pictorial leaflets will be distributed to caregivers. Information sessions will be held with school children on pneumonia signs and symptoms. Because CLHWs in Bangladesh and in Ethiopia cover a larger population compared to the CLHWs in India and Malawi, community volunteers in both countries will also be trained for home visits to identify sick young infants and link them to the CLHWs.
Screening and enrolment
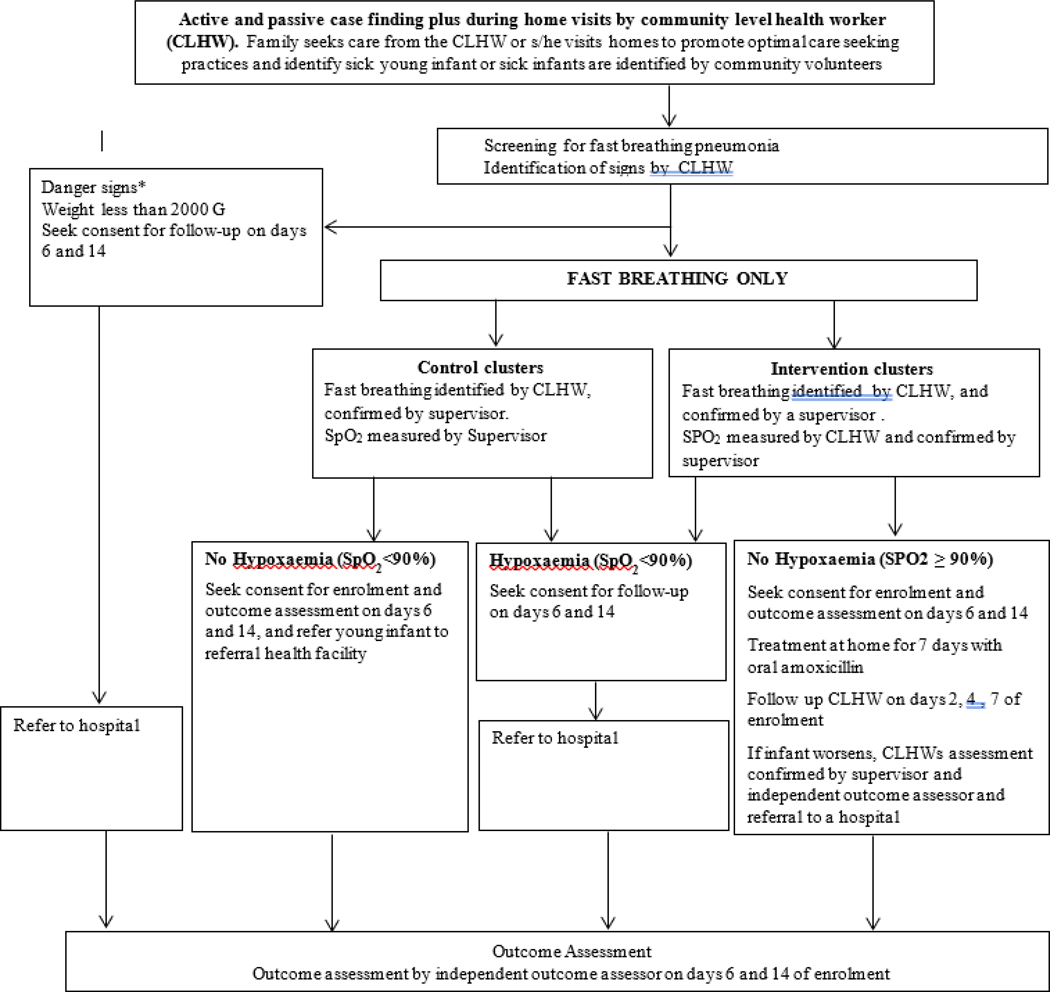
Young infants up to two months of age presenting to a CLHW with any illness will be evaluated for the eligibility criteria for enrolment (Figure 1). CLHW will use pulse oximetry on all fast breathing infants to identify hypoxaemia (SpO2 <90%) in intervention clusters. Infants with any danger sign or hypoxaemia, or unwilling to give consent will be referred by the CLHWs to a referral health facility. Those meeting the eligibility criteria will be examined by a study supervisor to validate the criteria before enrolment. A written informed consent will be obtained from a parent for eligible infants. A detailed history and clinical examination will be performed by the CLHW, and findings will be recorded on a standardized case recoding form (CRF). Screening, clinical assessment, enrolment and, provision of study medication will take place within one hour of identifying the case. Details of the implementation strategy are given in Table 4.
Figure 1: Overall study approach for screening, enrolment and management of young infants.
*Danger signs - Unconscious, movement only with stimulation or no movement at all, convulsions, stopped feeding well or unable to feed at all, severe chest indrawing, body temperature ≥38°C or <35.5°C, persistent vomiting (defined as vomiting following three attempts to feed the baby within ½ hour).
Table 4:
Implementation Strategy for intervention and control clusters.
| Activity | What | Who | When | Where | How |
|---|---|---|---|---|---|
| Intervention clusters | |||||
| Case identification | Detection of cases | Community-level health worker (CLHW) | Young infant is seen by the CLHW either at their place of work or during routine postnatal home visits | Community clinic in Bangladesh, Health Post in Ethiopia, Household in India and Malawi | Clinical assessment |
| Screening and enrolment | Detection of eligible cases | CLHW | Young infant is seen by the CLHW either at their place of work or during routine postnatal home visits | Community clinic in Bangladesh, Health Post in Ethiopia, Household in India and Malawi | Clinical assessment, pulse oximetry and consent |
| Confirmation of cases | Validation of eligible cases identified by CLHW | Study Supervisor | An eligible young infant is identified by a CLHW and supervisor is informed | Community clinic in Bangladesh, Health Post in Ethiopia, Household in India and Malawi | Clinical assessment, pulse oximetry and confirmation of CLHWs findings |
| Treatment provision | Oral amoxicillin | CLHW and parents/care givers | After enrolment and consent | First dose given at place of enrolment and remaining doses given at home | Practical demonstration of giving the first dose in front of mother/caregiver |
| Follow up | Follow up assessments of infants under intervention treatment | CLHW | On days 2,4 and 7 after enrolment | Community clinic in Bangladesh, Health Post in Ethiopia, Household in India and Malawi | Clinical assessment, pulse oximetry and checking treatment adherence |
| Supervision | Sub-sample on follow-up days for quality assurance | Study Supervisor | Follow-up visits on days 2, 4, and 7 | Community clinic in Bangladesh, Health Post in Ethiopia, Household in India and Malawi | Conformational assessment |
| Outcome assessment | Outcome assessment | Independent Outcome Assessor | Days 6, 14 and if patient deteriorated in between | Patients household or a hospital if patient was admitted | Clinical assessment and filling outcome assessment form |
| Control clusters | |||||
| Case identification | Detection of cases | CLHW | Young infant is seen by the CLHW either at their place of work or during routine postnatal home visits | Community clinic in Bangladesh, Health Post in Ethiopia, Household in India and Malawi | Clinical assessment |
| Screening and enrolment | Detection of eligible cases | CLHW | Young infant is seen by the CLHW either at their place of work or during routine postnatal home visits | Community clinic in Bangladesh, Health Post in Ethiopia, Household in India and Malawi | Clinical assessment, and consent |
| Confirmation of cases | Validation of eligible cases identified by CLHW | Study Supervisor | An eligible young infant is identified by a CLHW and supervisor is informed | Community clinic in Bangladesh, Health Post in Ethiopia, Household in India and Malawi | Clinical assessment, and confirmation of CLHWs findings, pulse oximetry |
| Treatment provision | Refer to a referral facility | CLHW | After enrolment and consent | First dose given at place of enrolment and refer to a referral facility | Counselling for referral |
| Follow up | NA | NA | NA | NA | NA |
| Supervision | NA | NA | NA | NA | NA |
| Outcome assessment | Outcome assessment | Independent Outcome Assessor | Days 6, 14 and if patient deteriorated in between | Patients household or a hospital if patient was admitted | Clinical assessment and filling outcome assessment form |
NA: not applicable.
Randomization
The cluster or unit of randomization will be a Union in Bangladesh, a Health Center in Ethiopia, a Health Subcenter in India, and a Health Center in Malawi. Stratified randomization will be carried out using the median or above population at the Union or health subcenters or health centers as first stratum and those below the median population as second stratum. WHO staff in Geneva not associated with site work will prepare the randomization lists. For Bangladesh, 52 clusters (26 intervention and 26 control) were selected out of 54; two were dropped due to odd numbers in both strata. For Ethiopia, 20 clusters (10 intervention and 10 control) were selected. For India site, 92 clusters (46 intervention and 46 control) were selected out of 95; three were dropped due to odd numbers in both strata. For Malawi, 44 clusters (22 intervention and 22 control) were selected out of 46 health centres, two were dropped due to odd number in both strata.
Sample size
If there is truly no difference between the standard iCCM and enhanced iCCM (assuming 10% treatment failure in both groups), with design effect of 1.2 and attrition of 10%, then 990 infants with fast breathing per group are required to be 90% sure that the upper limit of a two-sided 95% confidence interval will exclude a difference in favour of the standard iCCM of >5%. We are expecting 10 cases per cluster over the study period, so 198 clusters (99 per group) will be required for random allocation.
Use of pulse oximetry
Hypoxaemia is a major complication of pneumonia and is a major contributor to mortality.23–25 Hypoxaemia, defined as SpO2 <90% in low altitude, is difficult to identify by clinical signs alone.26 Non-invasive pulse oximetry technology can identify hypoxaemia early in infants who are at higher risk of poor outcome. They can be promptly referred and treated at a referral health facility with oxygen. Because the current iCCM tool does not identify hypoxaemic children, we will train CLHWs to use pulse oximetry to identify hypoxaemic infants for prompt referral to a hospital to increase the safety of study participants.
CLHWs, study supervisors and independent outcome assessors (IOAs) in intervention clusters and study supervisors and IOAs in control clusters will be provided with Masimo RAD-5v handheld portable pulse oximeters, and they will be trained on how to use and how to maintain them.
Follow-up
CLHW will follow all infants on days 2, 4 and 7 after enrolment in intervention clusters. The mother/caregiver will be advised to return to a Health Extension Worker (HEW) at the health post in Ethiopia, Community Health Care Provider (CHCP) at community clinic in Bangladesh, to accredited social health activist (ASHA) in India and health surveillance assistant (HSA) in Malawi for scheduled follow-up or any time if symptoms recur or the infant develops danger signs. CLHW will visit the infant at home if the infant does not present for follow-up. Each danger sign will be clearly explained to mother or caregiver. During follow-up visits, CLHWs will evaluate the infants and if any danger sign or sign of deterioration is observed, he/she will contact the study supervisor to physically confirm the signs and refer the young infant to the referral health facility as per iCCM protocol. Data will be recorded in the relevant CRF. Adverse drug reactions (anaphylactic reaction, severe diarrhoea, disseminated and severe rash) will be monitored actively by CLHW and if noticed will be confirmed by the supervisor and recorded in the relevant CRF.
Young infants who present with danger signs on screening or have hypoxaemia on screening in intervention and control clusters will be referred to a hospital after the first dose of amoxicillin. Parents/caregivers will be requested for consent for follow-up of these referred young infants on days 6 and 14 by an IOA.
Adherence to treatment
At each follow-up visit in intervention clusters, adherence will be evaluated by counting the remaining tablets. In instances when follow up visits scheduled after the completion of treatment or the parents/caregivers lose the remaining medicine, adherence will be evaluated by asking the parent/caregiver how much amoxicillin was administered and the number of tablets used. An enrolled young infant in the intervention clusters will be considered adherent to the antibiotic treatment if s/he has received either all oral antibiotic doses for 7 days or at least 80% (11/14 doses) during the entire treatment period. Adherence to treatment in the control clusters will also be attempted in the same manner.
Study outcomes
Primary outcome
Primary outcome will be treatment failure as defined in Table 5. Treatment failure will be only declared by an IOA when s/he will confirm it. We will also record withdrawal of informed consent at any time after enrolment, as well as hospitalization decided by the family, but those will not be included in the treatment failure criteria.
Table 5:
Definition of treatment failure.
| Death at any time within day 1 to 14 of enrolment. |
| Hospitalized for any reason or has any indication of hospitalization on day 6 of enrolment |
| Persistence of fast breathing on day 6 of enrolment. |
| Development of serious adverse effect of the study antibiotic/amoxicillin DT (anaphylactic reaction, severe diarrhoea, disseminated and severe rash) within day 1 to 6 of enrolment. |
Secondary outcomes
Evaluation of use of pulse oximetry to measure SpO2 will consist of the following three components: feasibility of using a pulse oximeter by CLHWs; accuracy of pulse oximetry when used by CLHWs against a standardized measurement by a trained supervisor; impact of pulse oximetry on referral and treatment outcomes when combined with increased scope for CLHWs to treat children i.e., does it increase safety of iCCM and improve referrals?
Separation between the outcome assessment and treatment teams
The proposed trial cannot be blinded due to the nature of the treatment by the CLHW. To reduce potential measurement bias, we will use IOA, who will be trained nurses, physicians or clinical field workers and will be independent of the enrolment and treatment to evaluate study outcomes. IOAs will be trained in standard iCCM, enhanced iCCM and study procedures. An IOA will visit all enrolled young infants on day 6 and 14 of enrolment to assess the outcome in both intervention and control clusters. Outcome assessment will be done at home or at the hospital for infants who are hospitalized on day of the visit.
Data collection instruments and procedures
Data will be collected by CLHWs, study supervisors and IOAs. Data on all pregnancy and births will be collected at the time of home visits by CLHWs, who will also screen infants for any illness. The supervisor will complete a confirmation form for each case before enrolment. A follow-up form (only in intervention clusters) and pulse oximeter use checklist will be completed for each case separately by the CLHWs and supervisors. An IOA will complete an outcome assessment form on days 6 and 14 for both intervention and control clusters. In case of serious adverse event (SAE), an IOA will fill a separate form describing this event. All completed forms will be reviewed by supervisors for completeness before data entry and any necessary corrections will be made. Supervisors and IOAs will submit CRFs to the study site office. A coordinator/co-investigator will check the CRFs to evaluate their completeness. Any queries will be sent back to the field before CRFs are transferred to the data entry teams.
Data management
Each individual site will have its own data management team, which will be responsible for their own day-to-day activities. A central data coordination centre (DCC), an independent institution will be responsible for overall data coordination for the trial. A common SQL-based database will be developed by the DCC that will be used by all sites, which will keep automated record of all changes. The database will have a conventional structure with two main components: a “back-end” and a “front-end” database. The back-end database will contain just the tables and data. The front-end database will contain the data-entry screens, queries, reports and programming and will be good clinical practices (GCP) compliant. The front-end will be linked into the tables contained at the back-end so that the two can operate together as a single unit. DCC data management team will help set up systems at all sites and will train the local data managers. DCC will establish a system of double data entry system as well as range and consistency checks at the country level. Data will be entered twice at each site and a clean dataset will be periodically uploaded at the central DCC site. The DCC will run periodic logical checks on the uploaded data and send the queries to the site-specific data managers for resolution.
Data analysis
Simple comparisons of means and proportions by intervention and control clusters will be carried out to evaluate baseline comparability of the two groups. The primary analyses will be per-protocol to compare treatment outcomes by comparing proportions of treatment failure (including deaths and deterioration) and between intervention and control clusters. Those who will be lost to follow-up and in whom consent would be withdrawn will not be included in the per-protocol analysis. An intention-to-treat analysis will also be conducted. The treatment outcomes between control and intervention clusters will be compared and the difference in the risk of treatment failure together with 95% confidence intervals will be calculated. Secondary analyses will be performed to investigate the effect on secondary outcomes. Additionally, univariate and multivariate regression analyses will be performed to identify predictors of treatment failure.
Quality control and assurance
Training of community level health workers and study staff
Training of CLHWs, study supervisors, IOAs, study coordinators and investigators for the study will be a two-step process. A six day training course for “master trainers” will be held for selected senior level participants facilitated by WHO expert facilitators at one of the sites using the study relevant standard operating procedures, and the WHO training modules for “caring for the sick child in the community” and “caring for the newborn in the community”.8,27 For intervention clusters we have adapted the iCCM young infants chart booklet and other training materials to include enhanced components of iCCM.8 These site-specific master trainers will subsequently train other study staff at their respective sites. For CLHWs the training modules/materials will be translated into the respective local languages. Additionally, all study staff will be trained in GCP. The study sites will also develop a system of periodic refresher training of CLHWs, supervisors and IOAs through video demonstration and or clinical practice including pulse oximetry.
Standardization
Periodic standardization exercises on a quarterly basis will be carried out for CLHWs, supervisors and IOAs for measurement of respiratory rate, temperature, mid upper arm circumference (MUAC), pulse oximetry and identification of danger signs to maintain their clinical skills.
Logistics and commodities
Trained CLHWs will be equipped with necessary medicines, equipment and logistics for pneumonia case management i.e., oral amoxicillin, pulse oximeter, digital thermometers, digital weighing scales, respiratory rate counting timers, MUAC tapes, CRFs and other necessary materials. The study teams will monitor the CLHW’s equipment for periodic standardisation and replace faulty devices if any. The consumables will be replenished as needed.
Supervision
All CLHWs will be supervised by a study supervisor. All infants who are assessed as potential enrolment will be validated by a supervisor in both intervention and control clusters to ensure quality. Pulse oximetry assessment by CLHWs will also be evaluated and confirmed by the supervisors through an on-spot independent assessment in intervention clusters. Supervised accompanied visits and independent unaccompanied visits will be carried out regularly by the supervisors. Principal investigators and co-investigators will also make random visits to study sites to check the project performance and quality.
Periodic meetings of the research implementation teams will be held to review enrolment, performance, field visit observations, address problems and identify solutions or required actions to overcome those. Action points will be followed up in the next meeting.
At each site the government district or sub-district level managers will also be involved in the supervision and monitoring activities.
Monitoring
Monthly progress reports will be submitted by all the sites to WHO for review and feedback. Regular conference calls will be held to discuss the progress and critical issues of the study. Data-based monitoring, as well as verification of SAE forms, will be carried out by the DCC with WHO study coordination team. Investigators or coordinators will randomly visit the field sites to observe CLHWs, supervisors and IOAs performing their activities. They will cross-check a small proportion of collected CRFs and share observations with supervisors and IOAs.
External monitoring
WHO monitors will visit each site twice a year to monitor the progress and observe the procedures using a checklist to monitor clinical trials. It will include assessment and enrolment procedures, clinical practices, other study procedures and data management. A proportion of completed CRFs will be checked, and all SAE reporting forms will be reviewed.
Ethical considerations
The protocol will be submitted for approval to all the local institutional ethical review committees at the study sites and WHO ethical review committee. Where required national and regional or state approvals will be obtained. The safety of enrolled infants in this trial will be ensured by close monitoring and follow-up. CLHWs will be trained to facilitate referral through counselling using iCCM guidance for assisting referral. The trial will follow CIOMS and GCP guidelines.
Written informed consent in local language will be obtained from the parent or guardian of the infant before inclusion in the study. Communities in both intervention and control clusters will be informed through community representatives.
Confidentiality and data handling
At enrolment, each participant will be given a unique participant ID number and the consent form, CRFs and any other forms linking participant personal information to study ID will be kept in locked filing cabinets. The key linking participant name and ID will be kept confidential in a secured location with limited access. Regular backup of the existing data will be done in appropriate intervals. All computers being used in the study will be password protected. Participants’ names or identifiers will not be used in any publications. The data/records will be kept until its use in any form including secondary analyses.
Patient safety
The study procedures and data collection will not pose any significant physical, psychological, social, legal or other kind of risks to the participants, as they are all routinely used in clinical practice. All enrolees will be followed up till completion of treatment and assessed on day 14 of the enrolment. Pulse oximetry will be used to identify higher risk hypoxaemic cases. Treatment failures will receive appropriate antibiotic therapy at referral health facility according to standard clinical practice. Those needing hospitalization will be referred to nearby sub-districtor districtor tertiary care hospitals immediately. All procedures will be explained in detail and any queries by parents will be answered. The mobile phone number of the CLHW and study supervisors will be provided to parentsor caregivers so that they could reach them if needed.
Data safety monitoring board
An independent Data Safety Monitoring Board (DSMB) will be constituted to monitor the trial at regular intervals.
Serious adverse events
In case of a SAE, the CLHW will contact the study supervisor and IOA. The IOA will document the SAE and convey the information to the study coordinator or investigator. SAEs such as death or either anaphylactic reaction, severe diarrhoea, disseminated or severe rash from antibiotic or oral amoxicillin will be reported to WHO within 48 hours of the occurrence. WHO will periodically report the SAE events to the DSMB.
Dissemination of results
Results will be disseminated in each study country and implications of results for implementation will be discussed. Results will also be disseminated at a regional and global level. Publication in national journals and presentation in national and international meetings will be encouraged.
Trial registration
The trial is registered at AZNCTR international Trial Registry as ACTRN12617000857303.
DISCUSSION
Equitable and increased access to diagnosis and standardised treatment are critical in reducing pneumonia mortality. We will compare treatment failure rates of fast breathing pneumonia in 7–59 days old infants with oral amoxicillin by CLHWs in community to current management at the health facility or hospital. Hypoxaemic young infants will be referred to a hospital. All enrolees will be followed regularly. Outcome assessment will be conducted by IOA to address bias. In control clusters, CLHWs will continue to refer all sick young infants including those with fast breathing pneumonia after providing first dose of amoxicillin.
The study has been carefully developed and pragmatically designed to allow collection of generalizable results without putting young infants at risk. While microbiological and/or radiological diagnosis may add improved specificity to the diagnosis, remote low-resource settings do not have access to these investigations. All personnel will be trained to follow the protocol and standard operating procedures. Standardized training, supervision, oversight, and monitoring will be undertaken to ensure quality, consistency, harmonized trial procedures and implementation. Study staff, investigators, and WHO coordinating team in Geneva will be engaged in close supervision and monitoring of the study, to assess compliance with human subjects, research regulations and guidelines, adherence to the study protocol and procedures, quality and accuracy of data collected, and quality of care and child safety.
Improving access to services and increasing awareness, and demand for care within communities are crucial for reducing pneumonia mortality. Without these, infants may never receive potentially lifesaving treatment. This study will help identify necessary actions to increase parents or caregivers recognition of sick young infants and improve health care seeking behaviours, decrease family mistrust in the public health system, and increase knowledge, skill and confidence of CLHWs for better management of infants with pneumonia.
Acknowledgments
Funding: This work is supported by Bill and Melinda Gates Foundation grant number OPP1109076
Footnotes
Conflict of interest: None declared
Ethical approval: This trial will obtain approval from the WHO and site institutional ethics committees
Contributor Information
Golam Mothabbir, Save the Children US, Bangladesh Office.
Shohel Rana, Save the Children US, Bangladesh Office.
Abdullah H. Baqui, Johns Hopkins University, USA
Salahuddin Ahmed, Johns Hopkins University-Bangladesh.
ASM Nawshad Ahmed, Child Health Research Foundation, Bangladesh.
Sunita Taneja, Center for Health Research and Development, Society for Applied Studies.
Sudarshan Mundra, Center for Health Research and Development, Society for Applied Studies.
Nita Bhandari, Center for Health Research and Development, Society for Applied Studies.
Suresh Dalpath, State Health System Resource Centre, Haryana, India.
Zemene Tigabu, University of Gondar, Gondar.
Gashaw Andargie, University of Gondar, Gondar.
Alemayehu Teklu, University of Gondar, Gondar.
Ashenafi Tazebew, University of Gondar, Gondar.
Kassahun Alemu, University of Gondar, Gondar.
Tadese Awoke, University of Gondar, Gondar.
Abebaw Gebeyehu, Amhara Regional Health Bureau.
Gomezgani Jenda, Save the Children, US, Malawi Office.
Humphreys Nsona, Ministry of Health, Malawi.
Don Mathanga, College of Medicine, Blantyre, Malawi.
Yasir Bin Nisar, Department of Maternal, Newborn, Child and Adolescent Health and Ageing, World Health Organization, Geneva, Switzerland.
Rajiv Bahl, Department of Maternal, Newborn, Child and Adolescent Health and Ageing.
Salim Sadruddin, Retired WHO staff.
Lulu Muhe, Retired WHO staff.
Peter Moschovis, Pediatric Pulmonary Medicine, Pediatric Global Health, Massachusetts General Hospital, Harvard Medical School, USA.
Samira Aboubaker, Retired WHO staff.
Shamim Qazi, Retired WHO staff.
REFERENCES
- 1.United Nations Inter-agency Group for Child Mortality Estimation (UN IGME). Levels and Trends in Child Mortality: Report 2019, Estimates developed by the United Nations Inter-agency Group for Child Mortality Estimation. New York, USA: United Nations Children’s Fund, 2019. Available at: https://data.unicef.org/resources/levels-and-trends-in-child-mortality/. Accessed on 3 October 2019. [Google Scholar]
- 2.McAllister DA, Liu L, Shi T, Chu Y, Reed C, Burrows J, et al. Global, regional, and national estimates of pneumonia morbidity and mortality in children younger than 5 years between 2000 and 2015: a systematic analysis. Lancet Glob Health. 2019;7(1):47–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kallander K, Hildenwall H, Waiswa P, Galiwango E, Peterson S, Pariyo G. Delayed care seeking for fatal pneumonia in children aged under five years in Uganda: a case-series study. Bull World Health Organ. 2008;86(5):332–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sutrisna B, Reingold A, Kresno S, Harrison G, Utomo B. Care-seeking for fatal illnesses in young children in Indramayu, west Java, Indonesia. Lancet. 1993;342(8874):787–9. [DOI] [PubMed] [Google Scholar]
- 5.Nair H, Simoes EA, Rudan I, Gessner BD, Azziz-Baumgartner E, Zhang JSF, et al. Global and regional burden of hospital admissions for severe acute lower respiratory infections in young children in 2010: a systematic analysis. Lancet. 2013;381(9875):1380–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.WHO Global Health Observatory. Proportions of child deaths by cause. 2019. Available at: https://apps.who.int/gho/data/node.main.ChildMortLevels?lang=en. Accessed on 24 January 2020.
- 7.Liu L, Oza S, Hogan D, Chu Y, Perin J, Zhu J, et al. Global, regional, and national causes of under-5 mortality in 2000–15: an updated systematic analysis with implications for the Sustainable Development Goals. Lancet. 2016;388(10063):3027–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization, UNICEF. Caring for the sick child in the community. Geneva, Switzerland: WHO Available at: https://www.who.int/maternal_child_adolescent/documents/caring-for-the-sick-child/en/. onAccessed on 3 October 2019.
- 9.Bhandari N, Bahl R, Bhatnagar V, Bhan MK. Treating sick young infants in urban slum setting. Lancet. 1996;347(9017):1774–5. [PubMed] [Google Scholar]
- 10.Baqui AH, El-Arifeen S, Darmstadt GL, Ahmed S, Williams EK, Seraji HR, et al. Effect of community-based newborn-care intervention package implemented through two service-delivery strategies in Sylhet district, Bangladesh: a cluster-randomised controlled trial. Lancet. 2008;371(9628):1936–44. [DOI] [PubMed] [Google Scholar]
- 11.Zaidi AK, Tikmani SS, Warraich HJ, Darmstadt GL, Bhutta ZA, Sultana S, et al. Community-based treatment of serious bacterial infections in newborns and young infants: a randomized controlled trial assessing three antibiotic regimens. Pediatr Infect Dis J. 2012;31(7):667–72. [DOI] [PubMed] [Google Scholar]
- 12.Bang AT, Bang RA, Baitule SB, Reddy MH, Deshmukh MD. Effect of home-based neonatal care and management of sepsis on neonatal mortality: field trial in rural India. Lancet. 1999;354(9194):1955–61. [DOI] [PubMed] [Google Scholar]
- 13.Tshefu A, Lokangaka A, Ngaima S, Engmann C, Esamai F, Gisore P, et al. Simplified antibiotic regimens compared with injectable procaine benzylpenicillin plus gentamicin for treatment of neonates and young infants with clinical signs of possible serious bacterial infection when referral is not possible: a randomised, open-label, equivalence trial. Lancet. 2015;385(9979):1767–76. [DOI] [PubMed] [Google Scholar]
- 14.Tshefu A, Lokangaka A, Ngaima S, Engmann C, Esamai F, Gisore P, et al. Oral amoxicillin compared with injectable procaine benzylpenicillin plus gentamicin for treatment of neonates and young infants with fast breathing when referral is not possible: a randomised, open-label, equivalence trial. Lancet. 2015;385(9979):1758–66. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization. Recommendations for Management of Common Childhood Conditions: Evidence for Technical Update of Pocket Book Recommendations: Newborn Conditions, Dysentery, Pneumonia, Oxygen Use and Delivery, Common Causes of Fever, Severe Acute Malnutrition and Supportive Care. Geneva, Switzerland: WHO; 2012. Available at: https://www.who.int/maternal_child_adolescent/documents/management_childhood_conditions/en/. Accessed on 3 October 2019. [PubMed]
- 16.World Health Organization. Revised WHO classification and treatment of childhood pneumonia at health facilities: Evidence summaries. Switzerland: WHO, 2014. Available at: https://www.who.int/maternal_child_adolescent/documents/child-pneumonia-treatment/en/. Accessed on 10 October 2019. [PubMed]
- 17.World Health Organization. Guideline: Managing possible serious bacterial infection in young infants when referral is not feasible. Switzerland: WHO, 2015. Available at: https://www.who.int/maternal_child_adolescent/documents/bacterial-infection-infants/en/. Accessed on 10 October 2019. [PubMed]
- 18.Lassi ZS, Middleton P, Bhutta ZA, Crowther C. Health care seeking for maternal and newborn illnesses in low- and middle-income countries: a systematic review of observational and qualitative studies. F1000Res. 2019;8:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amare Y, Paul S, Sibley LM. Illness recognition and appropriate care seeking for newborn complications in rural Oromia and Amhara regional states of Ethiopia. BMC Pediatr. 2018;18(1):265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization. Integrated Management of Childhood Illness: management of the sick young infant aged up to 2 months. IMCI chart booklet. Geneva, Switzerland: WHO, 2019. Available at: https://www.who.int/maternal_child_adolescent/documents/management-sick-young-infant-0-2-months/en/ Accessed on 10 October 2019.
- 21.World Health Organization, UNICEF. WHO/UNICEF Joint Statement: Home visits for the newborn child:a strategy to improve survival. Switzerland: WHO & UNICEF, 2009. Available at: https://www.who.int/maternal_child_adolescent/news_events/news/2009/09_07_08/en/. Accessed on 13 October 2019. [PubMed]
- 22.World Health Organization, UNICEF. Integrated Management of Childhood Illness: caring for newborns and children in the community. Switzerland: WHO & UNICEF, 2011. Available at: https://apps.who.int/iris/handle/10665/44398. Accessed on 13 October 2019.
- 23.Subhi R, Adamson M, Campbell H, Weber M, Smith K, Duke T. The prevalence of hypoxaemia among ill children in developing countries: a systematic review. Lancet Infect Dis. 2009;9(4):219–27. [DOI] [PubMed] [Google Scholar]
- 24.Lazzerini M, Sonego M, Pellegrin MC. Hypoxaemia as a Mortality Risk Factor in Acute Lower Respiratory Infections in Children in Low and Middle-Income Countries: Systematic Review and Meta-Analysis. PLoS One. 2015;10(9):e0136166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duke T, Mgone J, Frank D. Hypoxaemia in children with severe pneumonia in Papua New Guinea. Int J Tuberc Lung Dis. 2001;5(6):511–9. [PubMed] [Google Scholar]
- 26.World Health Organization. Oxygen therapy for children: a manual for health workers. Switzerland: World Health Organization, 2016. Available at: https://www.who.int/maternal_child_adolescent/documents/child-oxygen-therapy/en/. Accessed on 3 October 2019.
- 27.World Health Organization, UNICEF. Caring for newborns and children in the community: a training course for community health workers. Geneva, Switzerland: WHO, UNICEF. Available at: https://www.who.int/maternal_child_adolescent/documents/community-care-newborns-children/en/. Accessed on 3 October 2019.
- 28.National Institute of Population Research and Training (NIPORT), Mitra and Associates, Macro International. Bangladesh Demographic and Health Survey 2014. Dhaka, Bangladesh and Calverton, Maryland, USA: NIPORT, Mitra and Associates, and Macro International, 2016. Available at: https://dhsprogram.com/publications/index.cfm. Accessed on 10 October 2019.
- 29.Central Statistical Agency, ICF International. Ethiopia Demographic and Health Survey 2011. Addis Ababa, Ethiopia and Calverton, Maryland, USA: Central Statistical Agency and ICF International, 2012. Available at: https://dhsprogram.com/publications/index.cfm. Accessed on 13 October 2019.
- 30.Taneja S, Dalpath S, Bhandari N, Kaur J, Mazumder S, Chowdhury R, et al. Operationalising integrated community case management of childhood illnesses by community health workers in rural Haryana. Acta Paediatr. 2018;107:80–8. [DOI] [PubMed] [Google Scholar]
- 31.Amouzou A, Kanyuka M, Hazel E, Heidkamp R, Marsh A, Mleme T, et al. Independent Evaluation of the Integrated Community Case Management of Childhood Illness Strategy in Malawi Using a National Evaluation Platform Design. Am J Trop Med Hyg. 2016;94(6):1434–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zalisk K, Guenther T, Prosnitz D, Nsona H, Chimbalanga E, Sadruddin S. Achievements and challenges of implementation in a mature iCCM programme: Malawi case study. J Glob Health. 2019;9(1):010807. [DOI] [PMC free article] [PubMed] [Google Scholar]