Abstract
Over 20 years ago, our laboratory showed that growth hormone (GH) signals through the GH receptor-associated tyrosine kinase JAK2. We showed that GH binding to its membrane-bound receptor enhances binding of JAK2 to the GHR, activates JAK2, and stimulates tyrosyl phosphorylation of both JAK2 and GHR. The activated JAK2/GHR complex recruits a variety of signaling proteins, thereby initiating multiple signaling pathways and cellular responses. These proteins and pathways include: 1) Stat transcription factors implicated in the expression of multiple genes, including the gene encoding insulin-like growth factor 1; 2) Shc adapter proteins that lead to activation of the grb2-SOS-Ras-Raf-MEK-ERK1,2 pathway; 3) insulin receptor substrate proteins implicated in the phosphatidylinositol-3-kinase and Akt pathway; 4) signal regulatory protein α, a transmembrane scaffold protein that recruits proteins including the tyrosine phosphatase SHP2; and 5) SH2B1, a scaffold protein that can activate JAK2 and enhance GH regulation of the actin cytoskeleton. Our recent work has focused on the function of SH2B1. We have shown that SH2B1β is recruited to and phosphorylated by JAK2 in response to GH. SH2B1 localizes to the plasma membrane, cytoplasm and focal adhesions; it also cycles through the nucleus. SH2B1 regulates the actin cytoskeleton and promotes GH-dependent motility of RAW264.7 macrophages. Mutations in SH2B1 have been found in humans exhibiting severe early-onset childhood obesity and insulin resistance. These mutations impair SH2B1 enhancement of GH-induced macrophage motility. As SH2B1 is expressed ubiquitously and is also recruited to a variety of receptor tyrosine kinases, our results raise the possibility that effects of SH2B1 on the actin cytoskeleton in various cell types, including neurons, may play a role in regulating body weight.
Keywords: Growth hormone, JAK2, SH2B1, Signal transduction
1. Introduction
For many years, growth hormone (GH) has been known to be the primary hormone responsible for body growth. The tallest man on record (http://www.guinnessworldrecords.com/world-records/tallest-man-ever/), Robert Wadlow, had an untreated pituitary tumor that secreted abnormally high levels of GH [7]. He grew throughout life, achieving a height of 8 ft., 11 in. at the time of his death. Even today, the tallest people on record tend to achieve their great heights due to untreated pituitary tumors. The reverse is also true. Individuals who, for whatever reason, do not make normal levels of GH as children or have defective GH receptors, are short statured [14]. GH has also been recognized to regulate carbohydrate, protein and lipid metabolism. For example, GH decreases fat and increases lean body mass [16].
2. GH binding to its receptor activates the tyrosine kinase JAK2
In the mid 1980s, we asked the question of how GH acted at the level of the cell to bring about its diverse responses on body growth and metabolism. The GH receptor had been shown to be a membrane receptor that migrated as an ~ 110 kDa protein [11]. It was also known that defective GH receptors (patients with Laron Syndrome) resulted in short stature [20]. However, nothing was known about the signal transduction events that enabled GH binding to its plasma membrane-bound receptor to direct cellular responses. Around that time, a number of growth factors had been shown to bind to membrane receptors that had intrinsic tyrosine kinase activity. Since GH promoted growth, we hypothesized that GH might similarly bind to a membrane receptor with intrinsic tyrosine kinase activity or activate a tyrosine kinase. In support of this hypothesis, we showed that highly purified GH-GH receptor complexes co-purified with a tyrosine kinase [12]. Initial studies suggested that the GH receptor and the tyrosine kinase were the same protein since we saw essentially one band on sodium dodecyl sulfate polyacrylamide gels of highly purified and kinase-active GH receptor preparations. However, Leung et al. [33] cloned a GH receptor, which did not have tyrosine kinase activity. We solved the apparent discrepancy when we purified a truncated form of the GH receptor and saw that the truncated GH receptor co-purified with a tyrosine kinase the size of the full-length GH receptor [63]. This led us to search for a ~110 kDa tyrosine kinase, which in turn led us to the JAK family of tyrosine kinases. JAK1 and JAK2 were of the appropriate size, had been recently identified, and had no known function [67]. We showed that GH binding to its receptor increased the binding of JAK2 to GH receptor, activated JAK2, and increased phosphorylation of tyrosines within both JAK2 and GH receptor [3] (Fig. 1). This was an exciting finding because for the first time, it suggested a mechanism by which GH signal transduction was initiated – activation of JAK2. This finding was published back-to-back in Cell with an article by James Ihle’s group showing that erythropoietin similarly activated JAK2 [68]. These two publications were paradigm-shifting, since JAK family members have since been found to be activated in response to ligand binding to all members of the cytokine superfamily of receptors [6], a family numbering over 25 members. These ligands regulate such diverse and important physiological functions as satiety, immune function, milk production, hematopoiesis, and nerve function [23].
Fig. 1.
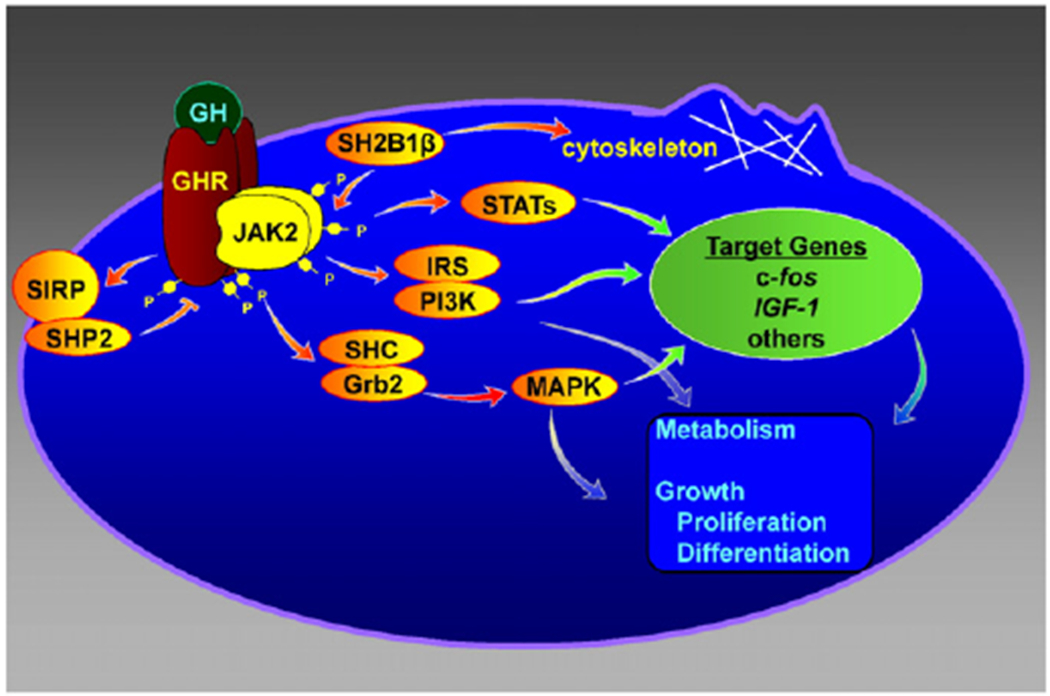
GH acts via a variety of signal transduction pathways. GH: growth hormone; GHR: growth hormone receptor; JAK2, Janus kinase 2; STAT: Signal Transducer and Activator of Transcription; MAPK: mitogen-activated protein kinase; IRS: insulin receptor substrate; PI3K: phosphatidyl inositol 3 kinase. Figure adapted from C Carter-Su, L Rui, J Herrington, M Stofega and M Diakonova, 2001, Targets for Growth Hormone and IGF-1 Action, pp31–43 © Bioscientifica Ltd. Adapted by permission.
3. JAK2 activation initiates signaling via multiple pathways
Having identified JAK2 as a critical and initiating cell signaling event for GH, we set out to determine the signaling events that are initiated as a consequence of JAK2 activation (Fig. 1). The first protein that caught our attention was p91, a transcription factor that had been identified in the context of the immune system. P91 was an intriguing candidate because of the finding that interferon (IFN)γ and IFNα, both of which were known to activate p91 [21,28,48,49] had recently been shown to activate members of the JAK family of tyrosine kinases (JAK2 and Tyk2 respectively) [62,64]. By investigating how GH regulates gene transcription, we were able to show that GH stimulated the tyrosyl phosphorylation of p91 [subsequently named Signal Transducer and Activator of Transcription 1 (Stat1)] and binding of p91 to the c-Sis-inducible element of the c-fos promoter [36]. Subsequently, we were among the first to show that GH also activates Stat3, Stat5a and Stat5b and promotes the accumulation of the activated form of Stats in the nucleus [9,25,50,51]. Stats 5a and 5b have been implicated in the synthesis of a variety of GH-sensitive genes, including insulin-like growth factor 1 (IGF-1), and acid labile subunit (ALS) which is a critical component of IGF binding protein complex (IGF1–IGFBP 3-ALS) [29]. They have been implicated in the transcription of a variety of GH regulated Cyp genes in the liver of mice [65]. Subsequent gene deletion studies [59] and human mutation identification [27] provide strong evidence that Stat5b is critical for GH’s effect on body height. These and other findings form the basis for the current canonical paradigm for GH signaling [31]: GH binding to its receptor activates JAK2, which in turns phosphorylates GH receptor on multiple tyrosines. These phosphorylated tyrosines, or tyrosines within JAK2, recruit various Stat proteins, which in turn are phosphorylated by JAK2 on a critical tyrosine. The phosphorylated Stat proteins are then released from the GH receptor/JAK2 complex, dimerize, move to the nucleus, and bind to Stat binding sites in GH-regulated genes. Stat proteins can also dimerize with other transcription factors, affecting the ability of those factors to bind to DNA and regulate gene transcription.
Although we recognized that Stat proteins are important for many actions of GH, we considered it likely that GH activation of JAK2 would initiate signaling pathways in addition to the Stat transcription factors. Over the years, we established that GH activates a number of additional signaling pathways. These include 1) the MAP kinase pathway; 2) insulin receptor substrate (IRS) proteins implicated in the activation of the phosphatidylinositol-3-kinase (PI3K) and Akt pathway; 3) signal regulatory protein α (SIRPα/SHPS1), a transmembrane scaffold protein that recruits proteins including the tyrosine phosphatase SHP2; and 4) SH2B1, a scaffold protein that can activate JAK2 and enhance GH regulation of the actin cytoskeleton.
Because JAK2 is a tyrosine kinase, we thought it likely that GH would be found to regulate the MAP kinases Erks 1 and 2. We showed that GH promotes the binding of the SH2 domain of Shc adapter protein to JAK2-GHR complexes, the tyrosyl phosphorylation of the 3 forms of Shc, and the binding of the adapter protein grb2 to Shc [60,61]. Further, we showed that GH stimulates association of the guanine nucleotide exchange factor SOS with Shc, and the activation of Ras, Raf, MEK and Erks 1 and 2 with a time course consistent with Erks 1 and 2 being activated via a Shc-grb2-SOS-Ras-Raf-MEK-Erk1/2 pathway. Erks have been shown to regulate a number of different types of molecules, including protein kinases, cytoskeletal proteins, phospholipases, and transcription factors [69]. Thus, GH activation of this pathway would be expected to regulate multiple responses in GH targeted cells.
The third pathway that we investigated was the IRS–PI3K pathway. Because under certain conditions, GH stimulates glucose transport in adipocytes [10], we hypothesized that GH might activate some of the pathways implicated in insulin regulation of glucose transport. Insulin and IGF-1 had been shown to activate IRS proteins, and activation of IRS proteins had been shown to recruit multiple PI3K proteins [56], which had been implicated in insulin stimulation of glucose transport [13]. We therefore hypothesized that GH activation of JAK2 would stimulate the tyrosyl phosphorylation of IRS proteins, which would recruit PI3K, leading to regulation of glucose transport and most likely other cellular responses. We demonstrated that indeed, GH stimulated that tyrosyl phosphorylation of both IRS1 and 2, as well as binding of the p85 regulatory subunit of PI3K to IRS1 and 2 and of the tyrosine phosphatase SHP2 to IRS2 [4,5]. These findings provide one possible mechanism by which GH causes the transient increase in glucose transport in adipocytes observed following a period of GH deprivation [10]. Longterm and in vivo, GH is considered diabetogenic and decreases insulin-sensitivity [1], which is likely to occur by a different mechanism. GH activation of IRS proteins also suggests a pathway by which GH could activate the transcription factor C/EBPβ. Activation of PI3K converts phosphatidylinositol (3,4)-bisphosphate (PIP2) lipids to phosphatidylinositol (3,4,5)-trisphosphate (PIP3). PIP3 recruits Akt to the plasma membrane, which enables the kinase PDK1 to access and phosphorylate T308 in Akt, leading to partial Akt activation [2]. Akt phosphorylation of glycogen synthase kinase 3 (GSK3) inhibits GSK3 activity. Decreased GSK3 activity results in decreased phosphorylation of a GSK3 phosphorylation site in C/EBPβ, and increased binding of a form of C/EBPβ, designated liver activating protein or LAP, to the c-fos promoter [44].
Because GH activates multiple pathways, we questioned whether multiple GH pathways might act together to regulate GH responses. One example of multiple pathways working together is GH regulation of expression of the c-fos gene (Fig. 2). We have shown that maximal expression of c-fos requires input from multiple GH signaling pathways. The promoter region of c-fos contains a binding site for Stat1 and Stat3 hetero or homodimers whose binding promotes c-fos gene expression [9,36,52]. The c-fos promoter also contains a serum response element that binds both serum response factor and ternary complex transcription factors, such as Elk1 [26,34,37]. GH stimulates the serine phosphorylation of Elk-1 via the MEK/Erk pathway, thereby enabling Elk-1 to mediate transcriptional activation. CREB and C/EBPβ, whose activity is also stimulated by phosphorylation by Erks 1 and/or 2 [17] but inhibited by phosphorylation by GSK3 [43,44], also bind to the promoter region of c-fos gene. Thus, c-fos gene expression in response to GH depends upon the balance of GH regulation of Stats, the MAPK pathway, the PI3K pathway and perhaps other pathways.
Fig. 2.
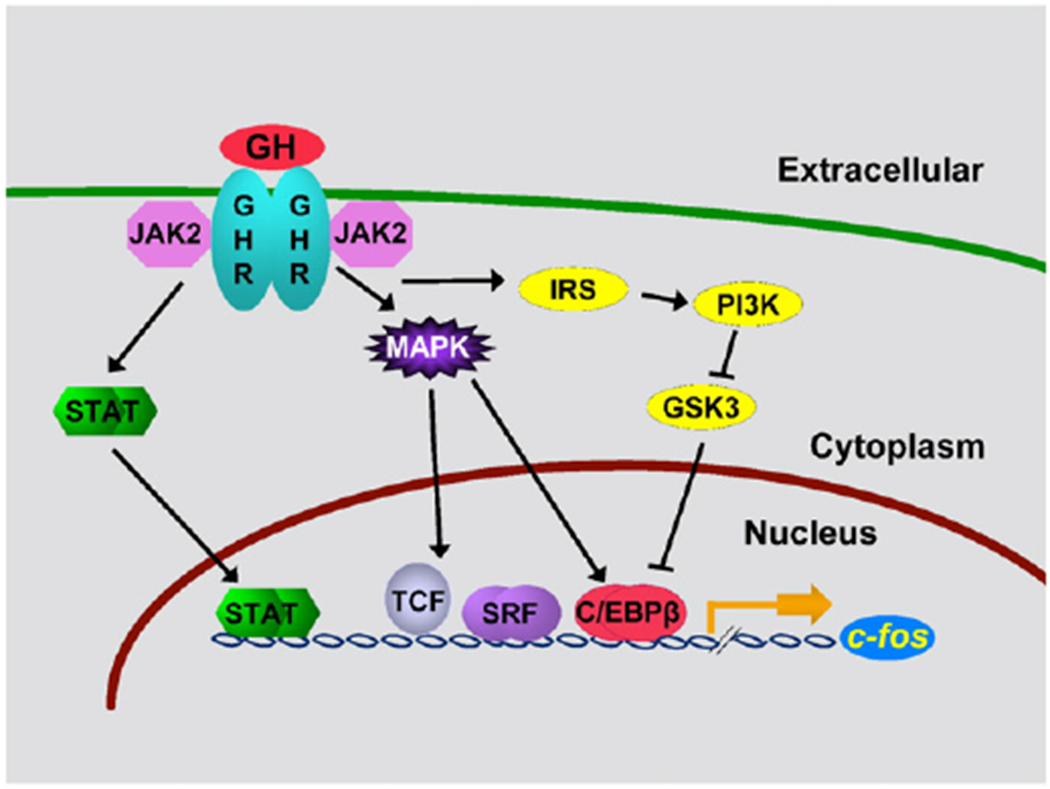
Multiple GH signaling pathways can contribute to specific GH responses. GH: growth hormone; GHR: growth hormone receptor; JAK2, Janus kinase 2; STAT: Signal Transducer and Activator of Transcription; MAPK: mitogen-activated protein kinase; IRS: insulin receptor substrate; PI3K: phosphatidyl inositol 3 kinase; GSK-3: glycogen synthase kinase-3; TCF: ternary complex factors; SRF: serum response factor; C/EBP: CCAAT enhancer binding protein. Figure adapted from Cesena et al., Molecular Genetics and Metabolism, 2007, 90, 126–133 © Bioscientifica Ltd (2007). Adapted by permission.
While trying to identify the tyrosine phosphatase(s) that dephosphorylate the GH receptor and/or JAK2, we identified signal regulatory protein α (SIRPα) as a JAK2 substrate [53,54]. SIRPα is a transmembrane glycoprotein that had been identified previously as a substrate of insulin receptor that recruits multiple SHP2 proteins. We found that in response to GH, JAK2 highly phosphorylates SIRPα1 and recruits SHP2 tyrosine phosphatases (Fig. 1). Recruitment of SHP2 to SIRPα1 appears to negatively regulate GH-JAK2 signaling.
4. JAK2 interacts with the scaffold protein SH2B1
To identify novel GH signaling proteins that are activated as a consequence of GH activation of JAK2, we performed a yeast 2-hybrid assay using the C-terminal amino acids of JAK2, which contains the kinase domain. When expressed in yeast, this portion of JAK2 is constitutively active. Of the potential JAK2 binding proteins identified in this assay, the adapter protein SH2B1 (SH2-B, PSM) was the most intriguing [47]. We pulled out the C-terminal 143 amino acids of a previously unidentified isoform of SH2B1. We designated this new isoform the β isoform. This isoform contains unique C-terminal 39 amino acids that lie just downstream of the SH2 domain. SH2B1 was originally cloned from mast cells because of its ability to bind in a yeast tribrid system to the tyrosyl-phosphorylated gamma subunit of the high-affinity immunoglobulin E (IgE) receptor [41]. Nothing else was known about the structure or function of SH2B1. This made it quite a challenge to identify the cellular function of SH2B1.
We established that SH2B1β is recruited to tyrosine (Tyr) 813 in activated JAK2 in response to GH and is phosphorylated on tyrosines 439 and 494 by JAK2 [30,39,47], suggesting that JAK2 phosphorylation of SH2B1β may recruit SH2 domain-containing signaling proteins to GH receptor/JAK2 complexes. We also showed that when overexpressed with JAK2, SH2B1β is a potent activator of JAK2 [46]. SH2B1β is also recruited to other members of the JAK family of tyrosine kinases, including JAK1 and JAK3 [40]. In the case of JAK1, SH2B1 is recruited to JAK1 and is phosphorylated by JAK1 but does not activate JAK1. In the case of JAK3, SH2B1 is recruited to Tyr785, the equivalent of Tyr813 in JAK2 [30]. However, we did not find that JAK3 phosphorylates SH2B1 nor did we find that SH2B1 activates JAK3. We hypothesized that SH2B1 was likely to bind additional proteins in a JAK3 independent manner, which would be recruited to JAK3 complexes when SH2B1 bound to JAK3.
5. SH2B1 regulates the actin cytoskeleton and cell motility
We have identified several other functions of SH2B1. When we observed the subcellular localization of GFP-tagged SH2B1, we found that SH2B1β localizes to membrane ruffles in cultured fibroblasts [24]. Membrane ruffles are formed at the leading edge of motile cells. This led us to hypothesize that SH2B1 interacts with and regulates the actin cytoskeleton. In support of this, we found that overexpressing SH2B1β increases membrane ruffling and cell motility in response to GH [18,24]. We next investigated whether GH acts as a chemoattractant to stimulate macrophage migration using a transwell migration assay. GH has been implicated in the migration of human monocytes [66] and both resting and activated human T cells [58]. We first showed that GH stimulates the migration of cultured RAW264.7 and bone marrow-derived mouse primary macrophages [55]. We then showed that overexpression of SH2B1β enhances GH-stimulated migration of RAW macrophages whereas reducing levels of SH2B1 using shRNA greatly impaired the GH-stimulated migration of RAW macrophages. As discussed above, we had previously shown that JAK2 phosphorylated SH2B1 on Tyr 439 and 494 [39]. We found that phosphorylation of these tyrosines appear to be required for SH2B1 to enhance GH-dependent macrophage motility since mutating them singly or together greatly impairs SH2B1 enhancement of GH-dependent macrophage motility [55]. Based on these and other migration studies using various truncated and mutated forms of SH2B1β, we speculate that SH2B1 regulates the actin cytoskeleton by recruiting proteins up to the plasma membrane where they are in close proximity to the actin cytoskeleton (Fig. 3). Some of these proteins are recruited by binding to tyrosines that are phosphorylated by JAK2 while others (e.g. Rac, [18]) bind constitutively to SH2B1β.
Fig. 3.
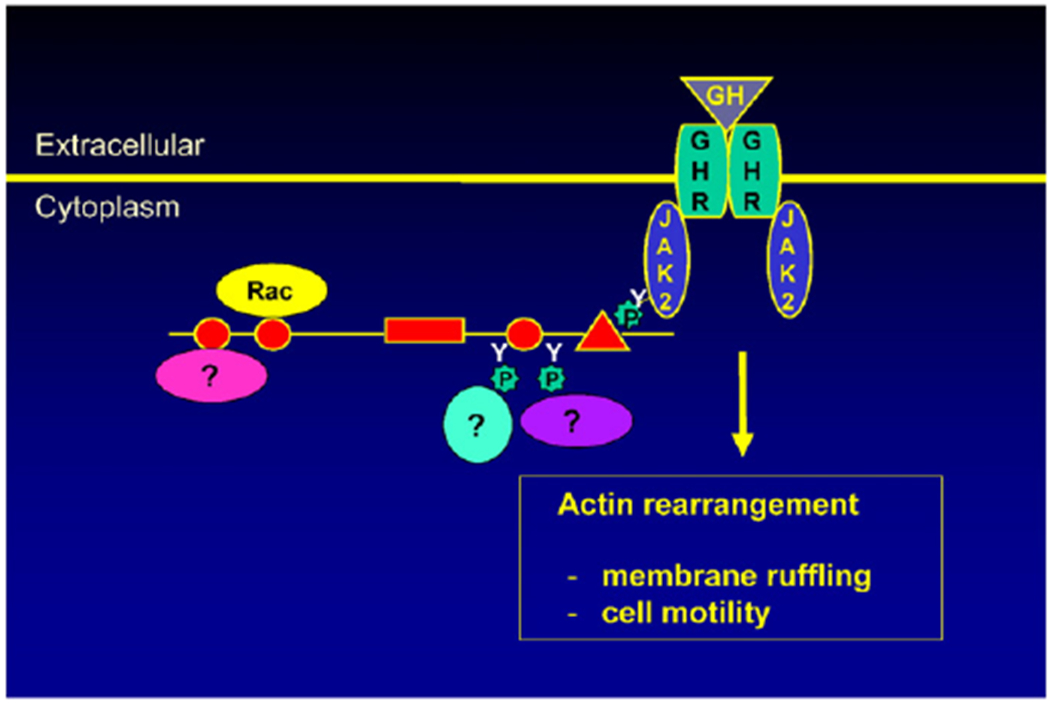
SH2B1β regulates the actin cytoskeleton and cell motility at least in part by serving as a scaffold protein for actin cytoskeleton-regulating proteins.
Cell migration relies, in part, on regulation of focal adhesion dynamics. Focal adhesions are large integrin-based macromolecular complexes that mediate cell-extracellular-matrix (ECM) attachment, facilitate direct signaling between the ECM and the cell and facilitate cell anchorage and motility (reviewed in [22]). We therefore looked to see if SH2B1β localizes to focal adhesions. Using confocal microscopy, we showed that SH2B1β colocalizes with vinculin, a focal adhesion protein [32]. Further, GH increases cycling of SH2B1β in and out of focal adhesions. Thus, SH2B1 may contribute to cell motility not only by interacting with and recruiting proteins to the actin cytoskeleton at the plasma membrane and in membrane ruffles, but also by serving as a scaffold protein for proteins in focal adhesions.
Because SH2B1 is recruited to JAK2 via its SH2 domain, we hypothesized that SH2B1 might be recruited to other cytokine receptors–JAK2 complexes or to activated receptor tyrosine kinases. In fact, we and others showed that SH2B1 is recruited to other receptors implicated in body growth, energy balance, and cell motility, including leptin receptor–JAK2 complexes and receptors for insulin, nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), and IGF-1 [35,42]. Variants in SH2B1 have been associated with obesity in genome wide association studies and gene deletion studies [8]. In addition, SH2B1−/− mice are obese [45]. Recently, individuals with point mutations in SH2B1 were identified among patients in the Genetics of Obesity Study (GOOS) [19]. These patients exhibit severe early onset childhood obesity, insulin resistance, hyperphagia and reduced height as adults. The SH2B1−/− mice similarly exhibit severe obesity, insulin resistance and hyperphagia. Some of the patients also exhibit maladaptive behavior, including social isolation, speech and language delay, and aggression.
We examined whether the human obesity mutations in SH2B1 impair SH2B1β-enhancement of GH-mediated macrophage migration. We found that overexpression of SH2B1β containing any of the three human obesity mutations tested, P90H, A175N and P322S completely blocked GH-stimulated macrophage migration [19]. These results raise the possibility that effects of SH2B1 on the actin cytoskeleton in other cell types, including neurons, and perhaps during development, may play a role in regulating body weight.
6. Growth hormone signaling pathways implicated in humans
Among the GH signaling proteins identified in these in vitro studies, only the GH receptor and Stat5b have been shown by human mutations to be associated with short stature in humans [20,27]. However, it is interesting to note that individuals with mutations in the IRS-1/PI3K pathway are short [15] as are individuals with RASopathies, which are diseases due to mutations in proteins in the MAP kinase pathway [57]. Some of the latter individuals are GH-deficient. Whether impaired GH signaling due to the mutations in these proteins contributes to the observed short stature or other altered phenotypes in these individuals is not known. However, GH has been shown to increase phosphorylation of Erk and PI3K in addition to Stat5 in cultured human fibroblasts [38], indicating that GH activates multiple pathways in cultured human cells, in addition to the cultured rodent cell lines used in our studies.
7. Summary
We have shown that GH binding to its receptor activates the GH receptor associated JAK2 tyrosine kinase. JAK2 in turn phosphorylates tyrosines within the GH receptor and within itself. These phosphorylated tyrosines can then recruit signaling proteins to GH receptor–JAK2 complexes in the plasma membrane. Proteins recruited to GH receptor-JAK2 complexes and phosphorylated by JAK2 include the transcription factors Stats 1, 3, 5a, and 5b that regulate GH sensitive genes including genes encoding c-Fos and IGF-1; IRS 1 and 2 which recruit PI3K and lead to activation of Akt and other proteins; Shc adapter proteins that initiate the Shc/grb2/SOS/Ras/Raf/MEK pathway leading to activation of Erks 1 and 2; SIRPα1 that recruits a tyrosine phosphatase that appears to be a negative regulator of JAK2 activity; and SH2B1, a scaffold protein that enhances GH-induced changes in the cytoskeleton leading to enhanced motility of cells, including macrophages. These pathways work together, presumably with other signaling proteins, to lead to a variety of responses to GH, including body growth, regulation of metabolism, and the ever-emerging actions of GH throughout the body.
Acknowledgements
This work was supported by grants from the National Institutes of Health (R01-DK54222 to CCS, R01-DK46072 to JS). We wish to acknowledge the help of Debbra Tackett and Joel Cline with manuscript preparation.
Abbreviations:
- GH
growth hormone
- IGF1
insulin-like growth factor 1
- IFN
interferon
- IRS
insulin receptor substrate
- PI3K
phosphatidylinositol-3-kinase
- SIRPα
signal regulatory protein α
- ALS
acid labile subunit
- GSK3
glycogen synthase kinase 3
- ECM
extracellular matrix
- Tyr
tyrosine
- Stat
Signal Transducer and Activator of Transcription
- TCF
ternary complex factors
- SRF
serum response factor
- C/EBP
CCAAT enhancer binding protein
Footnotes
Conflict of interest
There are no conflicts of interest.
References
- [1].Adamson U, On the diabetogenic effect of growth hormone in man: effects of growth hormone of glucagon and insulin secretion, Eur. J. Clin. Investig 11 (1981) 115–119. [DOI] [PubMed] [Google Scholar]
- [2].Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PR, Reese CB, Cohen P, Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Ba, Curr. Biol 7 (1997) 261–269. [DOI] [PubMed] [Google Scholar]
- [3].Argetsinger LS, Campbell GS, Yang X, Witthuhn BA, Silvennoinen O, Ihle JN, Carter-Su C, Identification of JAK2 as a growth hormone receptor-associated tyrosine kinase, Cell 74 (1993) 237–244. [DOI] [PubMed] [Google Scholar]
- [4].Argetsinger LS, Hsu GW, Myers MG Jr., Billestrup N, White MF, Carter-Su C, Growth hormone, interferon-γ, and leukemia inhibitory factor promoted tyrosyl phosphorylation of insulin receptor substrate-1, J. Biol. Chem 270 (1995) 14685–14692. [DOI] [PubMed] [Google Scholar]
- [5].Argetsinger LS, Norstedt G, Billestrup N, White MF, Carter-Su C, Growth hormone, interferon-γ, and leukemia inhibitory factor utilize insulin receptor substrate-2 in intracellular signaling, J. Biol. Chem 271 (1996) 29415–29421. [DOI] [PubMed] [Google Scholar]
- [6].Bazan JF, A novel family of growth factor receptors: a common binding domain in the growth hormone, prolactin, erythropoietin and IL-6 receptors, and the p75 IL-2 receptor B-chain, Biochem. Biophys. Res. Commun 164 (1989) 788–795. [DOI] [PubMed] [Google Scholar]
- [7].Behrens LH, Barr DP, Hyperpituitarism beginning in infancy: the alton giant, Endocrinology 16 (1932) 120–128. [Google Scholar]
- [8].Bochukova EG, Huang N,Keogh J, Henning E, Purmann C, Blaszczyk K, Saeed S,Hamilton-Shield J, Clayton-Smith J, O’Rahilly S, Hurles ME, Farooqi IS, Large, rare chromosomal deletions associated with severe early-onset obesity, Nature 463 (2010) 666–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Campbell GS, Meyer DJ, Raz R, Levy DE, Schwartz J, Carter-Su C, Activation of acute phase response factor (APRF)/Stat3 transcription factor by growth hormone, J. Biol. Chem 270 (1995) 3974–3979. [DOI] [PubMed] [Google Scholar]
- [10].Carter-Su C, Rozsa FA, Wang X, Stubbart JR, Rapid and transitory stimulation of 3-O-methylglucose transport by growth hormone, Am. J. Physiol 255 (1988) E723–E729. [DOI] [PubMed] [Google Scholar]
- [11].Carter-Su C, Schwartz J, Kikuchi G, Identification of a high affinity growth hormone receptor in rat adipocyte membranes, J. Biol. Chem 259 (1984) 1099–1104. [PubMed] [Google Scholar]
- [12].Carter-Su C, Stubbart JR, Wang X, Stred SE, Argetsinger LS, Shafer JA, Phosphorylation of highly purified growth hormone receptors by a growth hormone receptor-associated tyrosine kinase, J. Biol. Chem 264 (1989) 18654–18661. [PubMed] [Google Scholar]
- [13].Cheatham B, Vlahos CJ, Cheatham L, Wang L, Blenis J, Kahn CR, Phosphatidylinositol 3-kinase activation is required for insulin stimulation of pp70 S6 kinase, DNA synthesis, and glucose transporter translocation, Mol. Cell. Biol 14 (1994) 4902–4911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Cheek DB, Hill DE, Sawyer WH, Effect of growth hormone on cell and somatic growth, Handbook of Physiology, vol. 4, part 2, American Physiological Society, Washington, DC, 1974. sect. 7. [Google Scholar]
- [15].Chudasama KK, Winnay J, Johansson S, Claudi T, Konig R, Haldorsen I, Johansson B, Woo JR, Aarskog D, Sagen JV, Kahn CR, Molven A, Njolstad PR, SHORT syndrome with partial lipodystrophy due to impaired phosphatidylinositol 3 kinase signaling, Am. J. Hum. Genet 93 (2013) 150–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Corpas E, Harman SM, Blackman MR, Human growth hormone and human aging, Endocr. Rev 14(1993) 20–39. [DOI] [PubMed] [Google Scholar]
- [17].Cui TX, Kwok R, Schwartz J, Cooperative regulation of endogenous cAMP-response element binding protein and CCAAT/enhancer-binding protein beta in GH-stimulated c-fos expression, J. Endocrinol. Investig 196 (2008) 89–100. [DOI] [PubMed] [Google Scholar]
- [18].Diakonova M, Gunter DR,Herrington J, Carter-Su C, SH2-Bβ is a Rac-binding protein that regulates cell motility, J. Biol. Chem 277 (2002) 10669–10677. [DOI] [PubMed] [Google Scholar]
- [19].Doche MD, Bochukova EG, Su HW, Pearce L,Keogh JM, Henning E,Cline JM, Dale A, Cheetham T, Barroso I, Argetsinger LS, O’Rahilly SO, Rui L, Carter-Su C, Farooqi IS, SH2B1 mutations are associated with maladaptive behavior and obesity, J. Clin. Invest 122 (2012) 4732–4736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Eshet R, Laron Z, Pertzelan A, Amon R, Dintzman M, Defect of human growth hormone receptors in the liver of two patients with Laron-type dwarfism, Isr. J. Med. Sci 20 (1984) 8–11. [PubMed] [Google Scholar]
- [21].Fu X-Y, Schindler C, Improta T, Aebersold R, Darnell JE Jr., The proteins of ISGF-3, the interferon alpha-induced transcriptional activator, define a gene family involved in signal transduction, Proc. Natl. Acad. Sci. U. S. A 89 (1992) 7840–7843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Geiger B, Spatz JP, Bershadsky AD, Environmental sensing through focal adhesions, Nat. Rev. Mol. Cell Biol. 10 (2009) 21–33. [DOI] [PubMed] [Google Scholar]
- [23].Ghoreschi K, Laurence A, O’Shea JJ, Janus kinases in immune cell signaling, Immunol. Rev 228 (2009) 273–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Herrington J, Diakonova M, Rui L, Gunter DR, Carter-Su C, SH2-B is required for growth hormone-induced actin reorganization, J. Biol. Chem 275 (2000) 13126–13133. [DOI] [PubMed] [Google Scholar]
- [25].Herrington J, Rui L, Luo G, Yu-Lee L.-y., Carter-Su C, A functional DNA-binding domain is required for growth hormone-induced nuclear localization of Stat5B, J. Biol. Chem 274 (1999) 5138–5145. [DOI] [PubMed] [Google Scholar]
- [26].Hodge C, Liao J, Stofega M, Guan K, Carter-Su C, Schwartz J, Growth hormone stimulates phosphorylation and activation of Elk-1 and expression of c-fos, egr-1, and junB through activation of extracellular signal-regulated kinases 1 and 2, J. Biol. Chem 273 (1998) 31327–31336. [DOI] [PubMed] [Google Scholar]
- [27].Hwa V, Little B, Adiyaman P, Kofoed EM, Pratt KL, Ocal G, Berberoglu M, Rosenfeld RG, Severe growth hormone insensitivity resulting from total absence of signal transducer and activator of transcription 5b, J. Clin. Endocrinol. Metab 90 (2005) 4260–4266. [DOI] [PubMed] [Google Scholar]
- [28].Kessler DS, Veals SA, Fu X-Y, Levy DE, Interferon-alpha regulates nuclear translocation and DNA-binding affinity of ISGF3, a multimeric transcriptional activator, Genes Dev. 4 (1990) 1753–1765. [DOI] [PubMed] [Google Scholar]
- [29].Kurzer J, Carter-Su C, L. DE, Growth hormone induced activation and regulation of Jak2 and Stat proteins, in: Sehgal PB, Hirano T (Eds.), Signal Transducers and Activators of Transcription (STATs), Kluwer Academic Press, Dordrecht, Netherlands: 2003, pp. 177–190. [Google Scholar]
- [30].Kurzer JH, Argetsinger LS, Zhou Y-J, Kouadio J-L, O’Shea JJ, Carter-Su C, Tyrosine 813 is a site of JAK2 autophosphorylation critical for activation of JAK2 by SH2-Bβ, Mol. Cell. Biol 24 (2004) 4557–4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Lanning NJ, Carter-Su C, Recent advances in growth hormone signaling, Rev. Endocr. Metab. Disord 7 (2006) 225–235. [DOI] [PubMed] [Google Scholar]
- [32].Lanning NJ, Su HW, Argetsinger LS, Carter-Su C, Identification of SH2B1β as a focal adhesion protein that regulates focal adhesion size and number, J. Cell Sci 124 (2011) 3095–3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Leung DW, Spencer SA, Cachianes G, Hammonds RG, Collins C, Henzel WJ, Barnard R, Waters MJ, Wood WI, Growth hormone receptor and serum binding protein: purification, cloning and expression, Nature 330 (1987) 537–543. [DOI] [PubMed] [Google Scholar]
- [34].Liao J, Hodge C, Meyer D, Ho PS, Rosenspire K, Schwartz J, Growth hormone regulates ternary complex factors and serum response factor associated with the c-fos serum response element, J. Biol. Chem 272 (1997) 25951–25958. [DOI] [PubMed] [Google Scholar]
- [35].Maures TJ, Kurzer JH, Carter-Su C, SH2B1 (SH2-B) and JAK2: a multifunctional adaptor protein and kinase made for each other, Trends Endocrinol. Metab. 18 (2007) 38–45. [DOI] [PubMed] [Google Scholar]
- [36].Meyer DJ, Campbell GS, Cochran BH, Argetsinger LS, Larner AC, Finbloom DS, Carter-Su C, Schwartz J, Growth hormone induces a DNA binding factor related to the interferon-stimulated 91kD transcription factor, J. Biol. Chem 269 (1994) 4701–4704. [PubMed] [Google Scholar]
- [37].Meyer DJ, Stephenson EW, Johnson L, Cochran BH, Schwartz J, The serum response element can mediate the induction of c-fos by growth hormone, Proc. Natl. Acad. Sci. U. S. A 90 (1993) 6721–6725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Mul D, Wu S, de Paus RA, Oostdijk W, Lankester AC, Duyvenvoorde HA, Ruivenkamp CA, Losekoot M, Tol MJ, De Luca F, van de Vosse E, Wit JM, A mosaic de novo duplication of 17q21-25 is associated with GH insensitivity, disturbed in vitro CD28-mediated signaling, and decreased STAT5B, PI3K, and NF-kappaB activation, Eur. J. Endocrinol 166 (2012) 743–752. [DOI] [PubMed] [Google Scholar]
- [39].O’Brien KB, Argetsinger LS, Diakonova M, Carter-Su C, YXXL motifs in SH2-Bb are phosphorylated by JAK2, JAK1, and platelet-derived growth factor receptor and are required for membrane ruffling, J. Biol. Chem 278 (2003) 11970–11978. [DOI] [PubMed] [Google Scholar]
- [40].O’Brien KB, O’Shea JJ, Carter-Su C, SH2-B family members differentially regulate JAK family tyrosine kinases, J. Biol. Chem 277 (2002) 8673–8681. [DOI] [PubMed] [Google Scholar]
- [41].Osborne MA, Dalton S, Kochan JP, The yeast tribrid system – genetic detection of trans-phosphorylated ITAM–SH2-interactions, Biotechnology 13 (1995) 1474–1478. [DOI] [PubMed] [Google Scholar]
- [42].Pearce LR, Joe R, Doche MD, Su HW, Keogh JM, Henning E, Argetsinger LS, Bochukova EG, Cline JM, Garg S, Saeed S, Shoelson S, O’Rahilly S, Barroso I, Rui L, Farooqi IS, Carter-Su C, Functional characterisation of obesity-associated variants involving the alpha and beta isoforms of human SH2B1, Endocrinology 9 (2014) 3219–3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Piwien-Pilipuk G, Huo JS, Schwartz J, Growth hormone signal transduction, J. Pediatr. Endocrinol. Metab 15 (2002) 771–786. [DOI] [PubMed] [Google Scholar]
- [44].Piwien-Pilipuk G, Van Mater D, Ross SE, MacDougald OA, Schwartz J, Growth hormone regulates phosphorylation and function of CCAAT/enhancer-binding protein b by modulating Akt and glycogen synthase kinase-3, J. Biol. Chem 276 (2001) 19664–19671. [DOI] [PubMed] [Google Scholar]
- [45].Ren D, Li M, Duan C, Rui L, Identification of SH2-B as a key regulator of leptin sensitivity, energy balance and body weight in mice, Cell Metab. 2 (2005) 95–104. [DOI] [PubMed] [Google Scholar]
- [46].Rui L, Carter-Su C, Identification of SH2-Bβ as a potent cytoplasmic activator of the tyrosine kinase Janus kinase 2, Proc. Natl. Acad. Sci. U. S. A 96 (1999) 7172–7177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Rui L, Mathews LS, Hotta K, Gustafson TA, Carter-Su C, Identification of SH2-Bβ as a substrate of the tyrosine kinase JAK2 involved in growth hormone signaling, Mol. Cell. Biol 17 (1997) 6633–6644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Shuai K, Schindler C, Prezioso VR, DarnellJr JE., Activation of transcription by IFN-γ: tyrosine phosphorylation of a 91-kD DNA binding protein, Science 258 (1992) 1808–1812. [DOI] [PubMed] [Google Scholar]
- [49].Shuai K, Stark GR, Kerr IM, Darnell JE Jr., A single phosphotyrosine residue of Stat1 required for gene activation by interferon-γ, Science 261 (1993) 1744–1746. [DOI] [PubMed] [Google Scholar]
- [50].Smit LS, Meyer DJ, Billestrup N, Norstedt G, Schwartz J, Carter-Su C, The role of the growth hormone (GH) receptor and JAK1 and JAK2 kinases in the activation of Stats 1, 3, and 5 by GH, Mol. Endocrinol 10 (1996) 519–533. [DOI] [PubMed] [Google Scholar]
- [51].Smit LS, VanderKuur JA, Stimage A, Han Y, Luo G, Yu-lee L,Schwartz J, Carter-Su C, Growth hormone-induced tyrosyl phosphorylation and DNA binding activity of Stat5A and Stat5B, Endocrinology 138 (1997) 3426–3434. [DOI] [PubMed] [Google Scholar]
- [52].Sotiropoulos A, Moutoussamy S, Renaudie F, Clauss M, Kayser C, Gouilleux F, Kelly PA, Finidori J, Differential activation of Stat3 and Stat5 by distinct regions of the growth hormone receptor, Mol. Endocrinol 10 (1996) 998–1009. [DOI] [PubMed] [Google Scholar]
- [53].Stofega MR, Argetsinger LS, Wang H, Ullrich A, Carter-Su C, Negative regulation of growth hormone receptor/JAK2 signaling by signal regulatory protein a, J. Biol. Chem 275 (2000) 28222–28229. [DOI] [PubMed] [Google Scholar]
- [54].Stofega MR, Wang H, Ullrich A, Carter-Su C, Growth hormone regulation of SIRP and SHP-2 tyrosyl phosphorylation and association, J. Biol. Chem 273 (1998) 7112–7117. [DOI] [PubMed] [Google Scholar]
- [55].Su HW, Lanning NJ, Morris DL, Argetsinger LS, Lumeng CN, Carter-Su C, Phosphorylation of the adaptor protein SH2B1β regulates its ability to enhance growth hormone-dependent macrophage motility, J. Cell Sci 126 (2013) 1733–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Sun XJ, Rothenberg P, Kahn CR, Backer JM, Araki E, Wilden PA, Cahill DA, Goldstein BJ, White MF, Structure of the insulin receptor substrate IRS-1 defines a unique signal transduction protein, Nature 352 (1991) 73–77. [DOI] [PubMed] [Google Scholar]
- [57].Tamburrino F, Gibertoni D, Rossi C, Scarano E, Perri A, Montanari F, Fantini MP, Pession A, Tartaglia M, Mazzanti L, Response to long-term growth hormone therapy in patients affected by RAsopathies and growth hormone deficiency: patterns of growth, puberty and final height data, Am. J. Med. Genet. A (2015). [DOI] [PubMed] [Google Scholar]
- [58].Taub DD, Tsarfaty G, Lloyd AR, Durum SK, Longo DL, Murphy WJ, Growth hormone promotes human T cell adhesion and migration to both human and murine matrix proteins in vitro and directly promotes xenogeneic engraftment, J. Clin. Invest 94(1994) 293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Udy GB, Towers RP, Snell RG, Wilkins RJ, Park SH, Ram PA, Waxman DJ, Davey HW, Requirement of STAT5b for sexual dimorphism of body growth rates and liver gene expression, Proc. Natl. Acad. Sci. U. S.A 94 (1997) 7239–7244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].VanderKuur J, Allevato G, Billestrup N, Norstedt G, Carter-Su C, Growth hormone-promoted tyrosyl phosphorylation of Shc proteins and Shc association with Grb2, J. Biol. Chem 270 (1995) 7587–7593. [DOI] [PubMed] [Google Scholar]
- [61].VanderKuur JA, Butch ER, Waters SB, Pessin JE, Guan K-L, Carter-Su C, Signalling molecules involved in coupling growth hormone receptor to MAP kinase activation, Endocrinology 138 (1997) 4301–4307. [DOI] [PubMed] [Google Scholar]
- [62].Velazquez L, Fellous M, Stark GR, Pellegrini S, A protein tyrosine kinase in the interferon alpha/beta signaling pathway, Cell 70 (1992) 313–322. [DOI] [PubMed] [Google Scholar]
- [63].Wang X, Uhler MD, Billestrup N, Norstedt G, Talamantes F, Nielsen JH, Carter-Su C, Evidence for association of the cloned liver growth hormone receptor with a tyrosine kinase, J. Biol. Chem 267 (1992) 17390–17396. [PubMed] [Google Scholar]
- [64].Watling D, Guschin D, Muller M, Silvennoinen O, Witthuhn BA, Quelle FW, Rogers NC, Schindler C, Stark GR, Ihle JN, Kerr IM, Complementation by the protein tyrosine kinase JAK2 of a mutant cell line defective in the interferon-gamma signal transduction pathway, Nature 366 (1993) 166–170. [DOI] [PubMed] [Google Scholar]
- [65].Waxman DJ, O’Connor C, Growth hormone regulation of sex-dependent liver gene expression, Mol. Endocrinol 20 (2006) 2613–2629. [DOI] [PubMed] [Google Scholar]
- [66].Wiedermann CJ, Reinisch N, Braunsteiner H, Stimulation of monocyte chemotaxis by human growth hormone and its deactivation by somatostatin, Blood 82 (1993) 954–960. [PubMed] [Google Scholar]
- [67].Wilks AF, Harpur AG, Kurban RR, Ralph SJ, Zurcher G, Ziemiecki A, Two novel protein-tyrosine kinases, each with a second phosphotransferase-related catalytic domain, define a new class of protein kinase, Mol. Cell. Biol 11 (1991) 2057–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Witthuhn BA, Quelle FW, Silvennoinen O, Yi T, Tang B, Miura O, Ihle JN, JAK2 associates with the erythropoietin receptor and is tyrosine phosphorylated and activated following stimulation with erythropoietin, Cell 74 (1993) 227–236. [DOI] [PubMed] [Google Scholar]
- [69].Zehorai E, Yao Z, Plotnikov A, Seger R, The subcellular localization of MEK and ERK—a novel nuclear translocation signal (NTS) paves a way to the nucleus, Mol. Cell. Endocrinol 314 (2010) 213–220. [DOI] [PubMed] [Google Scholar]


