Abstract
Background:
Although pleural effusions are common among patients with acute heart failure, the relevance of pleural effusion size assessed on thoracic ultrasound has not been investigated systematically.
Methods:
In this prospective observational study, we included patients hospitalised for acute heart failure and performed a thoracic ultrasound early after admission (thoracic ultrasound 1) and at discharge (thoracic ultrasound 2) independently of routine clinical management. A semiquantitative score was applied offline blinded to clinical findings to categorise and monitor pleural effusion size.
Results:
Among 188 patients (median age 72 years, 62% men, 78% white, median left ventricular ejection fraction 38%), pleural effusions on thoracic ultrasound 1 were present in 66% of patients and decreased in size during the hospitalisation in 75% based on the pleural effusion score (P<0.0001). Higher values of the pleural effusion score were associated with higher pleural effusion volumes on computed tomography (P<0.001), higher NT-pro brain natriuretic peptide values (P=0.001) and a greater number of B-lines on lung ultrasound (P=0.004). Nevertheless, 47% of patients were discharged with persistent pleural effusions, 19% with large effusions. However, higher values of the pleural effusion score on thoracic ultrasound 2 did not identify patients at increased risk of 90-day heart failure rehospitalisations or death (adjusted hazard ratio (HR) 1.05, 95% confidence interval (CI) 0.92–1.19; P=0.46) whereas seven or more B-lines on lung ultrasound at discharge were independently associated with adverse events (adjusted HR 2.43, 95% CI 1.11–5.37; P=0.027).
Conclusion:
Among patients with acute heart failure, pleural effusions are associated with other clinical, imaging and laboratory markers of congestion and improve with heart failure therapy. The prognostic relevance of persistent pleural effusions at discharge should be investigated in larger studies.
Keywords: Ultrasound, pleural effusion, acute heart failure
Introduction
In patients hospitalised for acute heart failure (AHF) persistent signs and symptoms of congestion prior to discharge are important predictors of heart failure (HF) rehospitalisations and death.1 Pleural effusions (PEFs) represent a common finding in these patients.2 Depending on the imaging method, the reported prevalence of PEFs in AHF varies considerably, ranging from 47% and 89%.3–5 Similarly, the size of effusions (i.e. the amount of fluid) varies greatly among patients. The prognostic relevance of PEFs and the effect of treatment on PEFs (as well as the prognostic relevance of the response to treatment) have not been investigated systematically. Thoracic ultrasound (TUS) has been shown to detect PEFs with higher sensitivity and specificity than chest radiography (CXR) in several studies.6–9 TUS is increasingly used in the evaluation of patients with AHF and standardised approaches to quantifying lung B-lines as a dynamic surrogate for interstitial fluid have been described. By contrast, a standardised approach to the ultrasound quantification of pleural fluid is still lacking, and how pleural fluid detected by TUS changes following treatment in patients hospitalised with AHF has not been reported.
In this study of patients hospitalised for AHF, we quantified PEFs applying a semiquantitative TUS score. Based on this score, we examined the prevalence, distribution and change in PEF size in response to standard HF therapy during hospitalisation. Finally, we investigated the association between PEF size and clinical, laboratory and imaging markers of congestion, as well as the association between persistent PEFs and post-discharge outcomes.
Methods
Study population
This is a secondary analysis of a larger, prospective, observational study10 that enrolled 211 patients presenting with AHF at an academic hospital in the USA between April 2015 and August 2017.
Inclusion criteria for this study were: patients aged 18 years and older who were admitted to emergency observation or inpatient units with a diagnosis of AHF and requiring intravenous diuretics who underwent a clinically indicated, comprehensive transthoracic echocardiogram (TTE) early during admission. Patients with known or suspected HF with preserved ejection fraction were additionally required to have a NT-pro brain natriuretic peptide (BNP) level of 1400 pg/ml or greater during the current admission.
We excluded patients with important heart or lung disease known to impact lung ultrasound (LUS) findings, such as pneumonia or interstitial lung disease. Detailed inclusion and exclusion criteria for this study have previously been published.10 Eligible patients were identified using the daily echocardiographic laboratory schedule. All patients underwent treatment for HF with the treating clinicians blinded to the TUS findings. Clinical, laboratory and demographic data were extracted from patients’ hospital records by a trained investigator. This study complied with the Declaration of Helsinki and the local institutional review board approved the research protocol. Written informed consent was obtained from all study participants.
Thoracic and LUS imaging protocol
All patients underwent comprehensive TTEs by trained sonographers early during the admission. TTEs were performed using standard echocardiographic machines with phased array transducers. Immediately after the TTEs, an admission TUS (TUS1) for PEF assessment and a LUS (LUS1) for the investigation of B-lines were performed by a trained research assistant not involved in the patient’s clinical care using the same ultrasound equipment as for the TTEs.
To assess the presence of PEFs on TUS1, the transducer was positioned longitudinally (perpendicular to the ribs) in an intercostal space in the posterior axillary line with the patient in a semirecumbent position so that the liver or spleen, diaphragm and lung as well as the spine were clearly visualised in one image (Figure 1(a and b)). The presence of B-lines on LUS1 was examined in four standardised zones (two zones per hemithorax) and six second clips were recorded for each zone. The highest number of B-lines obtained in one intercostal space per zone was counted offline by two investigators blinded to clinical data as previously described.10 The sum of B-lines in all four zones was considered the total number of B-lines. A second TUS (TUS2) and LUS (LUS2) examination were performed close to hospital discharge.
Figure 1.
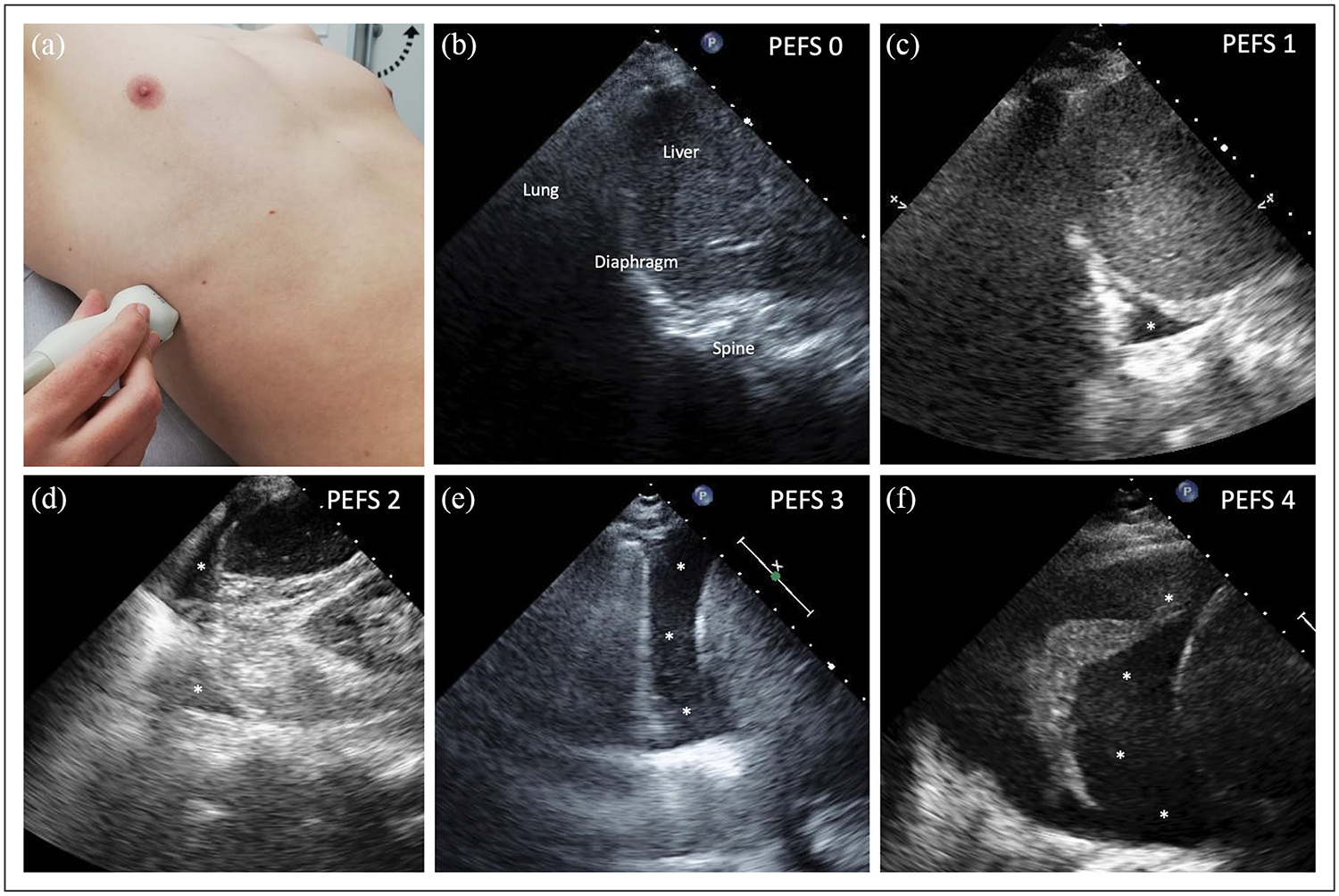
PEF score examples. Patient and probe positioning for PEF assessment (a). Example for increasing PEF scores assessed on thoracic ultrasound from 0 to 4 (b–f). Asterisk represents pleural fluid. PEF: pleural effusion score.
Semiquantitative PEF score
PEF size was categorised offline on de-identified six second clips by a single reader blinded to clinical, laboratory and outcome data as well as to the time point the clips were obtained using a semiquantitative score ranging from 0 to 4 points for each hemithorax. For the definition of the score see Table 1 and Figure 1. To estimate the total fluid burden for each patient the points of both hemi-thoraces were summed to a total PEF score ranging from 0 to 8.
Table 1.
Definition of the PEF score for each hemithorax.
| 0 | No PEF visible |
| 1 | PEF only visible in the CPA |
| 2 | PEF extends over the CPA without a clear separation of the lung base from the diaphragm |
| 3 | Clear separation between diaphragm and lung base at any point during the respiratory cycle |
| 4 | PEF occupies more than 50% of the basal pleural cavity visible in the standardised imaging plane |
PEF: pleural effusion; CPA: costophrenic angle.
CXR and CT image analyses
In patients who had undergone clinically indicated CXR and computed tomography (CT), these images were analysed by an experienced radiologist trained in cardiovascular imaging and blinded to the TUS measurements. On upright CXR images, PEFs were further quantified based on a modified, previously published CXR PEF score11,12 that is described in detail in the Supplementary files. PEF volumes on CT were measured for each hemithorax using the Vitrea Enterprise Suite (version 6.9.68.1) by Vital (Canon Medical Research, USA Inc.). On serial axial images the PEFs were manually traced, and the final volume was generated from the segmented three-dimensional image. Only CXR and CT studies that were obtained within 24 hours of the TUS examination were included in the analysis.
Outcome data collection
The time to first event was used for the primary endpoint (composite of HF readmission or all-cause death) for the 90 days after hospitalisation. HF hospitalisations were confirmed through patient follow-up phone calls, contacting the patients’ primary care physicians or cardiologists and review of electronic medical records and adjudicated by two experienced physicians. All-cause mortality was validated through review of the institution’s medical records, the social security index and obituaries.
Statistical analysis
Descriptive statistics for continuous variables are expressed as medians (interquartile range; IQR) and categorical variables as counts (percentages) unless otherwise noted. To analyse further the associations between increasing PEF size and other variables, the study population was divided into three groups based on the total PEF score: group 1: no PEFs (PEF score 0), group 2: total PEF score of 1–4, and group 3: total PEF score of 5–8. Between-group comparisons examining trends across the PEF study groups were assessed using Cuzick’s non-parametric trend test and changes of the PEF score using Wilcoxon signed rank tests. To investigate associations between the PEF score and volumetric measurements, we performed a subgroup analysis in all patients who underwent a clinically indicated CT examination within 24 hours of their TUS examination. A detailed presentation of this analysis as well as intra and inter-reader agreement of the PEF score can be found in the Supplementary files. For the analyses of time to death and/or hospitalisation unadjusted and adjusted Cox proportional hazard models were used to investigate both the independent effects of persistent PEFs prior to discharge on event-free survival and the prognostic value of PEFs in addition to persistent B-lines. For the B-line analysis patients were divided into tertiles (0–3 B-lines, 3–6 B-lines, ≥7 B-lines) based on the sum of pre-discharge B-lines (LUS2) as previously reported.10 To investigate the incremental prognostic value of PEFs, we added the PEF score on TUS2 as a continuous predictor to the B-line outcome model. All patients with complete data for both TUS2 and LUS2 were included in the analysis. Forward stepwise selection was applied to model building including age, sex, baseline systolic blood pressure, baseline log-transformed creatinine, baseline LVEF as well as baseline log-transformed NT-proBNP and variables with a P value less than 0.05 were added to the model. Statistical analyses were performed using Stata SE, version 14.2 (StataCorp., College Station, TX, USA; 2015) with P values less than 0.05 considered statistically significant.
Results
Study population
Of 211 patients enrolled in the study, 196 met the inclusion criteria and 188 had interpretable PEF images for TUS1 (Figure 2). In the overall cohort, the median age was 72 years (IQR 63–82), 62% were men, 72% presented with NYHA class III or IV and 51% had a reduced left ventricular ejection fraction (LVEF <40%) on admission.
Figure 2.

Study flow chart.
TUS: thoracic ultrasound; AHF: acute heart failure; HFpEF: heart failure with preserved ejection fraction; PEF: pleural effusion.
Demographic data
The baseline characteristics of the study population stratified by PEF study group on admission are presented in Table 2. Patients with higher PEF scores tended to be older, had a lower BMI and were less likely to be black, to have a prior diagnosis of chronic HF or obstructive sleep apnoea, and were less likely to take aldosterone inhibitors or diuretics as home medication, but were more likely to receive oxygen supplementation on admission. There was no significant difference in PEF size in men versus women (P=0.70).
Table 2.
Baseline clinical characteristics by PEF study groups on admission (n=188).
| n | PEF score 0 (n=63) | PEF score 1–4 (n=57) | PEF score 5–8 (n=68) | P (trend)* | |
|---|---|---|---|---|---|
| Demographics | |||||
| Age | 188 | 66 (53–78) | 72 (62–81) | 78 (70–84) | <0.001 |
| Male | 188 | 43 (68%) | 30 (53%) | 44 (65%) | 0.70 |
| Race | |||||
| White | 188 | 45 (72%) | 44 (77%) | 57 (84%) | 0.09 |
| Black | 188 | 14 (22%) | 12 (21%) | 6 (9%) | 0.04 |
| Admission exam | |||||
| BMI (kg/m2) | 188 | 33 (27–39) | 28 (24–32) | 24 (23–27) | <0.001 |
| Heart rate (bpm) | 188 | 77 (66–88) | 83 (71–97) | 79 (64–90) | 0.70 |
| Respiratory rate (breaths per min) | 188 | 19 (18–20) | 18 (18–20) | 18 (18–20) | 0.32 |
| Systolic blood pressure (mmHg) | 188 | 120 (106–136) | 120 (106–140) | 120 (103–137) | 0.71 |
| Diastolic blood pressure (mmHg) | 188 | 64 (60–76) | 69 (60–74) | 63 (58–71) | 0.18 |
| SpO2 (%) | 187 | 97 (95–98) | 97 (95–99) | 96 (95–98) | 0.10 |
| O2 supplementation | 187 | 14 (23%) | 18 (32%) | 32 (47%) | 0.003 |
| HF history | |||||
| NYHA class III or IV (n,%) | 188 | 45 (71%) | 42 (74%) | 49 (72%) | 0.94 |
| Prior HF diagnosis | 188 | 54 (86%) | 41 (72%) | 47 (69%) | 0.029 |
| HF admission within the past 6 months | 99 | 22 (51%) | 16 (70%) | 21 (64%) | 0.25 |
| HFrEF (EF <40%) | 188 | 35 (56%) | 28 (49%) | 32 (47%) | 0.34 |
| Medical history | |||||
| Hypertension | 188 | 50 (79%) | 49 (86%) | 57 (83%) | 0.58 |
| Atrial fibrillation | 188 | 33 (52%) | 31 (54%) | 37 (54%) | 0.81 |
| Myocardial infarction | 188 | 18 (29%) | 15 (26%) | 27 (40%) | 0.17 |
| CABG | 188 | 11 (17%) | 16 (28%) | 20 (29%) | 0.12 |
| ICD | 188 | 18 (29%) | 12 (21%) | 12 (18%) | 0.14 |
| Diabetes mellitus | 188 | 32 (51%) | 22 (37%) | 23 (34%) | 0.05 |
| COPD | 188 | 11 (17%) | 11 (19%) | 9 (13%) | 0.51 |
| OSA | 188 | 16 (25%) | 11 (19%) | 4 (6%) | 0.003 |
| Cancer | 188 | 17 (27%) | 16 (28%) | 22 (32%) | 0.50 |
| Home medication | |||||
| Beta-blocker | 188 | 54 (86%) | 45 (79%) | 55 (81%) | 0.48 |
| ACE inhibitor/ARB/ARNI | 188 | 33 (52%) | 26 (46%) | 28 (41%) | 0.20 |
| Diuretics | 188 | 50 (79%) | 34 (60%) | 42 (62%) | 0.035 |
| Spironolactone | 188 | 18 (29%) | 11 (19%) | 4 (6%) | 0.001 |
| Insulin | 188 | 18 (29%) | 13 (23%) | 13 (19%) | 0.20 |
| Admission laboratory results | |||||
| Sodium (mmol/l) | 188 | 140 (136–141) | 138 (135–140) | 139 (136–141) | 0.50 |
| Potassium (mmol/l) | 188 | 4.2 (3.9–4.8) | 4.3 (4–4.5) | 4.3 (4–4.6) | 0.82 |
| Haemoglobin (g/dl) | 188 | 11.5 (10.1–13.4) | 11.5 (10.2–12.8) | 11 (9.3–12.8) | 0.19 |
| Haematocrit (%) | 187 | 35 (31–40) | 36 (31–39) | 34 (29–38) | 0.20 |
| eGFR (ml/min/l.73m2) | 188 | 41 (24–61) | 46 (34–61) | 42 (21–65) | 0.79 |
| Hospitalisation data | |||||
| Hospital length of stay (days) | 188 | 7 (3–10) | 6 (4–9) | 6 (3–13) | 0.89 |
Data are presented as median (IQR) for continuous and as count (%) for categorical data.
P (trend) for PEF study groups 1–3.
PEF: pleural effusion; BMI: body mass index; SpO2: peripheral capillary oxygen saturation; O2: oxygen; NYHA: New York Heart Association; HF: heart failure; HFrEF: heart failure with reduced ejection fraction; CAD: coronary artery disease; CABG: coronary arterial bypass grafting; ICD: internal cardioverter-defibrillator; COPD: chronic obstructive pulmonary disease; OSA: obstructive sleep apnoea; ACE: angiotensin-converting enzyme; ARB: angiotensin receptor blocker; ARNI: angiotensin receptor neprilysin inhibitor, eGFR: estimated glomerular filtration rate.
Prevalence and size of PEFs on admission
Of the 188 patients included in the main analysis, 125 (66%) had PEFs on TUS1. PEFs were bilateral in 86 patients (69%). In the 39 patients (31%) with a unilateral effusion, 27 (69%) were right-sided. In patients presenting with PEFs, the median total PEF score was 5 (IQR 2–7). In patients with bilateral effusions, PEF scores on the right side tended to be higher than on the left side (4 (IQR 3–4) vs. 3 (IQR 1–4), P<0.001).
Prevalence and size of PEFs prior to discharge
Of the 111 patients with PEF data prior to discharge, 52 (47%) still had residual PEFs while 59 (53%) did not have any PEFs. The median PEF score of patients with PEFs at discharge was 4 (IQR 3–6).
Dynamic changes in PEF size during hospitalisation
Complete PEF data for both TUS1 and TUS2 were present in 108 patients. The median time between the two examinations was 6 days (IQR 3–8). In 25% of patients with PEFs on admission the total PEF score did not change while 50% improved by at least 2 points. The median change in the total PEF score was 1 (IQR 0–3). One-third of patients with PEFs on TUS1 showed no PEF on TUS2. For the change in PEF study groups see Figures 3 and 4.
Figure 3.

PEF study groups on admission (TUS1) (a) and discharge (TUS2) (b) (n=108).
PEF: pleural effusion; TUS: thoracic ultrasound.
Figure 4.

Change in PEF study group between TUS1 and TUS2 (n=108).
PEF: pleural effusion; TUS: thoracic ultrasound.
Clinical, laboratory and imaging markers of congestion
Only 34% of the patients with a PEF score between 5 and 8 on admission had diminished breath sounds on auscultation, although 16% of patients without PEFs on TUS1 also had this finding. There was an association between larger PEF size and both a higher number of B-lines on LUS (P=0.004) and signs of vascular congestion on the initial CXR (P=0.013), as well as a higher PEF score on CXR (P<0.001). Signs of pleural fluid on the admission CXR were detected in 97% of patients with a PEF score of 5–8 but only in 64% of patients with a PEF from 1 to 4 on the TUS examination. There were no significant differences in patient reported dyspnoea at rest or with exertion between the three study groups. However, patients with higher PEF scores had higher NT-proBNP levels (P=0.001) and a third heart sound was more frequently detected (P=0.024) (Table 3). Higher PEF scores by ultrasound were associated with larger PEF volumes measured on CT (P<0.001) (see Supplementary files).
Table 3.
Association of PEF study groups on admission to other markers of congestion (n=188).
| n | PEF score 0 (n=63) |
PEF score 1–4 (n=57) |
PEF score 5–8 (n=68) |
P (trend)* | |
|---|---|---|---|---|---|
| Physical exam1 | |||||
| JVD (≥10 cm) | 176 | 47 (80%) | 44 (85%) | 43 (66%) | 0.07 |
| Peripheral oedema (any) | 188 | 49 (78%) | 42 (74%) | 47 (69%) | 0.26 |
| Audible S3/4 | 186 | 3 (5%) | 4 (7%) | 11 (16%) | 0.024 |
| Rales/crackles (any) | 188 | 32 (51%) | 33 (58%) | 45 (66%) | 0.08 |
| Diminished breath sounds (any) | 188 | 10 (16%) | 12 (21%) | 23 (34%) | 0.016 |
| Thoracic and lung ultrasound | |||||
| PEF | 180 | 0 | 2 (1–3) | 7 (6–8) | N/A |
| B-lines | 178 | 4 (2–8) | 7 (4–9) | 7 (4–10) | 0.004 |
| Admission CXR1 | |||||
| Pleural effusions on upright CXR | 102 | 16 (54%) | 18 (64%) | 38 (97%) | 0.001 |
| PEF score on upright CXR (0–8) | 101 | 0 (0–2) | 2 (0–3) | 4 (3–5) | <0.001 |
| Cardiomegaly (CTR >0.5) | 135 | 34 (85%) | 41 (91%) | 48 (96%) | 0.07 |
| Vascular congestion | 134 | 24 (60%) | 29 (64%) | 41 (84%) | 0.013 |
| Interstitial oedema on CXR | 135 | 32 (80%) | 34 (76%) | 44 (88%) | 0.30 |
| Alveolar oedema on CXR | 135 | 2 (5%) | 6 (13%) | 8 (16%) | 0.12 |
| Dyspnoea | |||||
| At rest (NRS 1–10) | 186 | 5 (1–7) | 5 (1–7) | 4 (0–7) | 0.38 |
| On exertion (NRS 1–10) | 182 | 8 (7–9) | 8 (7–10) | 7 (5–9) | 0.38 |
| NT-proBNP (pg/ml)1 | 178 | 2861 (1683–5766) | 4327 (2712–7722) | 9012 (5091–15606) | 0.001 |
Data are presented as median (interquartile range) for continuous and as count (%) for categorical data.
P (trend) for PEF study groups 1–3.
Clinical, CXR and laboratory data were only included in the analysis if obtained within 24 hours of the admission TUS examination.
PEF: pleural effusion; JVD: jugular venous distension; TUS: thoracic ultrasound; CXR: chest radiography; NRS: numeric rating scale.
Outcomes
Among the 109 patients with data for both TUS2 and LUS2, there were 36 patients (33%) who experienced death or readmission for HF. In this cohort we did not observe a higher event rate in patients with higher PEF scores (adjusted HR 1.05 per point increase in score, 95% CI 0.92–1.19; P=0.46). Patients with seven or more B-lines at discharge were at higher risk of adverse events compared to patients with none to three B-lines (adjusted HR 2.43, 95% CI 1.11–5.37; P=0.027, c-statistic 0.734), after adjusting for baseline systolic blood pressure and baseline log-transformed creatinine. Further adjustment for the PEF score did not alter the relationship between B-lines and outcomes (adjusted HR 2.39, 95% CI 1.09–5.28; P=0.03, c-statistic 0.734), and in this model the PEF score was not significantly associated with the outcome (HR 1.04, 95% CI 0.91–1.18; P=0.61).
Discussion
In this study we investigated the prevalence of PEFs detected using TUS in patients with AHF and the characteristics of patients with and without PEFs. There were three main findings. First, the PEF score we propose might be a useful approach to semiquantitatively assess PEF size in patients with AHF and correlated well with CT quantification of PEF size. Second, PEFs are common in AHF and change in size during hospitalisation which can be quantified using the PEF score. Third, in this small cohort, persistent PEFs at discharge did not improve the identification of AHF patients at risk of adverse outcomes at 90 days.
Quantifying PEFs in patients with HF using TUS
CXR remains the most commonly used method to image the lungs and pleura of patients with AHF. Blackmore and colleagues compared PEF volumes on posterior–anterior and lateral CXR with those measured using CT scanning in 71 patients with a suspected PEF.12 The upright CXR was shown to miss PEFs with a volume of up to 200 ml and, conversely, patients with suspected PEFs on a CXR did not have a PEF confirmed on their CT. Moreover, PEFs on CXR in supine patients are even more challenging to detect and quantify.13 In a small cohort of intensive care unit patients with adult respiratory distress syndrome, supine CXR demonstrated a sensitivity as low as 39% and a specificity of 85% for detection of PEFs compared to CT.6 However, CT scanning involves more radiation exposure, is more costly and is not as widely available as CXR, and is unlikely to replace it as the first-line lung and pleural imaging modality in AHF.
TUS is another alternative and a variety of existing approaches to estimate the volumes of PEFs have been proposed.14–16 However, their value in patients with AHF may be limited. This is because some of these techniques require several measurements which are time consuming, and others are only applicable for at least moderate to large effusions, having been developed, primarily, to predict the safety of thoracentesis. Most importantly, an approach to estimate the fluid burden in both hemi-thoraces combined has not been developed. Kataoka17 proposed a semiquantitative strategy for unilateral PEFs that we have extended and transformed into a score for patients with AHF. Our novel score has important advantages for use in patients with AHF: it is easily obtainable at the bedside in semirecumbent patients using standard ultrasound imaging planes. Image acquisition requires little time (only one 6 second ultrasound clip per hemithorax) and the inter-reader agreement for the PEF score was very high compared to prior PEF studies using CXR.12 In this study, trained research assistants were able to obtain high quality images for the detection of PEFs which supports the feasibility of this imaging protocol in providers with little experience in point-of-care ultrasound imaging. A crucial benefit of this score over prior CXR scores is the opportunity for serial assessments without exposing the patient to radiation. This could facilitate frequent and cost-effective monitoring of change in PEF size in response to therapy over the course of hospitalisation in a range of clinical settings, not limited to patients with AHF.
Prevalence and size of PEFs in AHF
The prevalence of PEFs in this cohort was 66% and thus was higher than reported in previous studies using CXR (46–55%).3,4 This may in part be due to a higher sensitivity of TUS in detecting pleural fluid. Similarly, a prior small CT study reported a prevalence of 87% among patients with AHF.5 However, in this current analysis, the majority of patients underwent the TUS1 the day after their admission after receiving diuretic therapy which could have led to an underestimation of the prevalence of PEFs on admission.
The observed laterality of PEFs agrees with previous CXR and CT reports. In AHF, bilateral effusions are more common than unilateral effusions, accounting for between 58%3 and 87%5 of cases with PEFs. When unilateral, PEFs are more likely to be right-sided for reasons not completely understood.2,5,18 Furthermore, our findings also support and extend the previous observation that in patients with bilateral PEFs, the amount of fluid on the right side tends to be greater than on the left side, which has been described in both CXR and CT-based studies.5,18
Prior research10,19,20 has demonstrated that B-lines on LUS are a dynamic surrogate of pulmonary congestion and may be used to monitor the response to HF treatment, with a significant decrease in the number of B-lines during hospitalisation for AHF. Based on prior observations, both the development and complete resolution of PEFs have been thought to represent a slower aspect of pulmonary congestion (and de-congestion), possibly requiring days to weeks to accumulate (and resolve).5,21 In this analysis, only 25% of patients with PEFs on admission demonstrated no change in the PEF score, whereas in 33% with PEFs on admission we observed a complete regression of the effusions. This finding suggests that decongestive therapy has a measurable effect on PEF size within a median of 6 days and that PEF changes may be more dynamic than previously thought.
Prognostic implications of persistent PEFs at discharge
Prior studies have demonstrated that HF patients are frequently discharged despite having persistent clinical signs of congestion such as leg oedema or elevated jugular venous distention, and these patients are at increased risk of subsequent mortality and readmissions.1,22 Furthermore, studies investigating the prognostic implications of persistent B-lines on LUS demonstrated an association between an increased number of residual B-lines prior to discharge and adverse events.10,23 However, in most previously published LUS studies, larger PEFs were an exclusion criterion due to interference with certain zones of the LUS imaging protocol. The additional predictive value of PEFs on TUS has not been investigated systematically in larger studies. Although persistent PEFs were not an independent predictor of 90-day HF rehospitalisations or death in our cohort, only 50 patients were in this category at discharge and of these only 17 had an event. Consequently, we did not have the statistical power to examine this question. However, similar results were found in a small prospective study of ambulatory patients with chronic HF in which the presence of any PEFs combined with B-lines were associated with an increased risk of death or hospitalisation, while PEFs alone after adjusting for age, NT-proBNP and LVEF were not.24 Validation of these findings accounting for PEF size in larger HF cohorts is needed.
Limitations
This was a single centre analysis with a small but well characterised cohort. As the PEF score has its focus on the lung base it does not allow for further differentiation of very large effusions. This may result in a larger spectrum of volumes in hemithoraces with a PEF score of 4 and a change in size might not be captured by the score. However, based on prior CXR studies, the majority of the PEFs in patients with HF are reported to be small to moderate in size, occupying one third or less of the hemithorax which is in accordance with our findings.18,25 As the score is based on adding the score values of each hemithorax it might result in imprecise total estimates in the few patients with large unilateral effusions. However, the three PEF study groups showed a significant association of higher scores with larger volumes on CT. Comparison of the TUS to the CXR and CT findings should be viewed in the context that the imaging modalities were conducted up to 24 hours apart and that patients were receiving HF treatment in between. Also, patient positioning was different in the respective examinations, with the TUS performed in a semi-recumbent, the CXR in an upright and the CT scan in a supine position leading to a different pattern of fluid accumulation.
Conclusion
Our findings suggest that the proposed PEF score could be a useful method to semiquantitatively assess and monitor the size of PEFs in patients with AHF. PEFs on TUS were a common finding and a dynamic marker of pulmonary congestion in this cohort. While increasing values of the PEF score were associated with other clinical, imaging and laboratory markers of congestion, the question whether persistent PEFs at discharge represent a predictor of adverse events should be investigated in larger studies.
Supplementary Material
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This project was supported by a grant from the National Heart, Lung and Blood Institute (grant number K23HL123533) (EP). JJVM is supported by the BHF Centre of Research Excellence award number RE/18/6/34217
Footnotes
Conflict of interests
EP reports a grant from NIH/NHLBI, during the conduct of the study; JG reports grants from Amgen Pharmaceuticals, outside the submitted work; SDS reports grants and personal fees from Alnylam, grants and personal fees from Amgen, grants and personal fees from GSK, grants from Ionis, grants from Lone Star Heart, personal fees from BMS, grants from Celladon, grants and personal fees from Gilead, grants from Mesoblast, grants from MyoKardia, grants from NIH/NHLBI, grants and personal fees from Novartis, grants from Sanofi Pasteur, grants from Theracos, personal fees from Akros, personal fees from Bayer, personal fees from Corvia, personal fees from Ironwood, personal fees from Merck, personal fees from Pfizer, personal fees from Roche, personal fees from Takeda, personal fees from Theracos, personal fees from Quantum Genetics, personal fees from AoBiome, personal fees from Janssen, personal fees from Cardiac Dimensions, outside the submitted work; all other authors report no relevant conflicts of interest.
Supplementary material
Supplementary material for this article is available online.
References
- 1.Ambrosy AP, Pang PS, Khan S, et al. Clinical course and predictive value of congestion during hospitalization in patients admitted for worsening signs and symptoms of heart failure with reduced ejection fraction: findings from the EVEREST trial. Eur Heart J 2013; 34: 835–843. [DOI] [PubMed] [Google Scholar]
- 2.Natanzon A and Kronzon I. Pericardial and pleural effusions in congestive heart failure-anatomical, pathophysiologic, and clinical considerations. Am J Med Sci 2009; 338: 211–216. [DOI] [PubMed] [Google Scholar]
- 3.Morales-Rull JL, Bielsa S, Conde-Martel A, et al. Pleural effusions in acute decompensated heart failure: prevalence and prognostic implications. Eur J Intern Med 2018; 52:49–53. [DOI] [PubMed] [Google Scholar]
- 4.Davutoglu V, Yildirim C, Kucukaslan H, et al. Prognostic value of pleural effusion, CA-125 and NT-proBNP in patients with acute decompensated heart failure. Kardiol Pol 2010; 68: 771–778. [PubMed] [Google Scholar]
- 5.Kataoka H. Pericardial and pleural effusions in decompensated chronic heart failure. Am Heart J 2000; 139: 918–923. [DOI] [PubMed] [Google Scholar]
- 6.Lichtenstein D, Goldstein I, Mourgeon E, et al. Comparative diagnostic performances of auscultation, chest radiography, and lung ultrasonography in acute respiratory distress syndrome. Anesthesiology 2004; 100: 9–15. [DOI] [PubMed] [Google Scholar]
- 7.Kataoka H and Takada S. The role of thoracic ultrasonography for evaluation of patients with decompensated chronic heart failure. J Am Coll Cardiol 2000; 35: 1638–1646. [DOI] [PubMed] [Google Scholar]
- 8.Grimberg A, Shigueoka DC, Atallah AN, et al. Diagnostic accuracy of sonography for pleural effusion: systematic review. Sao Paulo Med J 2010; 128: 90–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zanobetti M, Poggioni C and Pini R. Can chest ultrasonography replace standard chest radiography for evaluation of acute dyspnea in the ED? Chest 2011; 139: 1140–1147. [DOI] [PubMed] [Google Scholar]
- 10.Platz E, Campbell RT, Claggett B, et al. Lung ultrasound in acute heart failure: prevalence of pulmonary congestion and short- and long-term outcomes. JACC Heart Fail 2019; 7: 849–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mammarappallil JG, Anderson SA, Danelson KA, et al. Estimation of pleural fluid volumes on chest radiography using computed tomography volumetric analysis: an update of the visual prediction rule. J Thorac Imaging 2015; 30: 336–339. [DOI] [PubMed] [Google Scholar]
- 12.Blackmore CC, Black WC, Dallas RV, et al. Pleural fluid volume estimation: a chest radiograph prediction rule. Acad Radiol 1996; 3: 103–109. [DOI] [PubMed] [Google Scholar]
- 13.Woodring JH. Recognition of pleural effusion on supine radiographs: how much fluid is required? AJR Am J Roentgenol 1984; 142: 59–64. [DOI] [PubMed] [Google Scholar]
- 14.Balik M, Plasil P, Waldauf P, et al. Ultrasound estimation of volume of pleural fluid in mechanically ventilated patients. Intensive Care Med 2006; 32: 318. [DOI] [PubMed] [Google Scholar]
- 15.Remerand F, Dellamonica J, Mao Z, et al. Multiplane ultrasound approach to quantify pleural effusion at the bedside. Intensive Care Med 2010; 36: 656–664. [DOI] [PubMed] [Google Scholar]
- 16.Dalen H, Gundersen GH, Skjetne K, et al. Feasibility and reliability of pocket-size ultrasound examinations of the pleural cavities and vena cava inferior performed by nurses in an outpatient heart failure clinic. Eur J Cardiovasc Nurs 2015; 14: 286–293. [DOI] [PubMed] [Google Scholar]
- 17.Kataoka H. Ultrasound pleural effusion sign as a useful marker for identifying heart failure worsening in established heart failure patients during follow-up. Congest Heart Fail 2012; 18: 272–277. [DOI] [PubMed] [Google Scholar]
- 18.Porcel JM and Vives M. Distribution of pleural effusion in congestive heart failure. South Med J 2006; 99: 98–99. [DOI] [PubMed] [Google Scholar]
- 19.Cortellaro F, Ceriani E, Spinelli M, et al. Lung ultrasound for monitoring cardiogenic pulmonary edema. Intern Emerg Med 2017; 12: 1011–1017. [DOI] [PubMed] [Google Scholar]
- 20.Volpicelli G, Caramello V, Cardinale L, et al. Bedside ultrasound of the lung for the monitoring of acute decompensated heart failure. Am J Emerg Med 2008; 26: 585–591. [DOI] [PubMed] [Google Scholar]
- 21.Stewart PB. The rate of formation and lymphatic removal of fluid in pleural effusions. J Clin Invest 1963; 42: 258–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Connor CM, Stough WG, Gallup DS, et al. Demographics, clinical characteristics, and outcomes of patients hospitalized for decompensated heart failure: observations from the IMPACT-HF registry. J Cardiac Fail 2005; 11: 200–205. [DOI] [PubMed] [Google Scholar]
- 23.Coiro S, Rossignol P, Ambrosio G, et al. Prognostic value of residual pulmonary congestion at discharge assessed by lung ultrasound imaging in heart failure. Eur J Heart Fail 2015; 17: 1172–1181. [DOI] [PubMed] [Google Scholar]
- 24.Gustafsson M, Alehagen U and Johansson P. Imaging congestion with a pocket ultrasound device: prognostic implications in patients with chronic heart failure. J Cardiac Fail 2015; 21: 548–554. [DOI] [PubMed] [Google Scholar]
- 25.Woodring JH. Distribution of pleural effusion in congestive heart failure: what is atypical? South Med J 2005; 98: 518–523. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


