Abstract
Background
While the relationship between long-term opioid therapy (LTOT) dose and overdose is well-established, LTOT’s association with all-cause mortality is less understood, especially among people living with HIV (PLWH). There is also limited information regarding the association of LTOT cessation or interruption with mortality.
Methods
Among PLWH and matched uninfected male veterans in care, we identified those who initiated LTOT. Using time-updated cox regression, we examined the association between all-cause mortality, unnatural death, and overdose, and opioid use categorized as 1–20 (reference group), 21–50, 51–90, and ≥ 91 mg morphine equivalent daily dose (MEDD).
Results
There were 22,996 patients on LTOT, 6,578 (29%) PLWH and 16,418 (71%) uninfected. Among 5,222 (23%) deaths, 12% were unnatural deaths and 6% overdoses. MEDD was associated with risk of all 3 outcomes; compared to patients on 1–20 mg MEDD, adjusted risk for all-cause mortality monotonically increased (Hazard Ratios (HR) [95% CI] for 21–50 mg MEDD = 1.36 [1.21, 1.52], 51–90 mg MEDD = 2.06 [1.82, 2.35], and ≥ 91 mg MEDD = 3.03 [2.71, 3.39]). Similar results were seen in models stratified by HIV. LTOT interruption was also associated with all-cause, unnatural, and overdose mortality (HR [95% CI] 2.30 [2.09, 2.53], 1.47 [1.13, 1.91] and 1.52 [1.04, 2.23], respectively).
Conclusions
Among PLWH and uninfected patients on LTOT we observed a strong dose-response relationship with all 3 mortality outcomes. Opioid risk mitigation approaches should be expanded to address the potential effects of higher dose on all-cause mortality in addition to unnatural and overdose fatalities.
Keywords: Long-term opioid therapy, mortality, overdose, HIV
1. Introduction
There is widespread concern over the increase in mortality associated with opioid use given the 200% increase in opioid-related deaths since 2000.1–3 Opioids have become one of the leading causes of injury-related mortality in the United States (US) over the past two decades.4 Within this context, it is now well recognized that long-term opioid therapy (LTOT) is associated with higher overdose mortality in a dose dependent manner. However, less is known about the impact of LTOT on all-cause mortality. Prior studies have reported that LTOT is associated with higher all-cause mortality in a dose-dependent fashion among general populations with chronic pain in Canada, Europe and Korea, and specifically among patients with Crohn’s disease and older patients with arthritis.5–9
While opioid therapy is still a part of pain management for people living with HIV (PLWH) and without HIV, there has been increasing attention to the potential harms associated with continued use in recent years.10,11 Of greatest concern is the risk of unintentional overdose death,12–15 the incidence of which has increased dramatically in recent years.16 Besides overdose death, other causes of death have been linked to opioid exposure, including trauma,17 violence, pulmonary disease,18 and cardiovascular disease;19 and others, such as invasive, potentially fatal infections such as pneumonia,20,21 have now also been linked to opioid exposure.
PLWH on opioid therapy may be more susceptible to complications compared to uninfected patients through mechanisms that may be ascribed to HIV-specific effects (e.g. greater vulnerability to the immunosuppressive effects of opioids)20 or HIV-related effects (e.g. higher prevalence among PLWH of conditions associated with overdose death, including psychiatric disorders and substance use disorders).22,23 Because of this broad spectrum of potential harmful effects, understanding risk requires consideration of specific opioid related causes of death as well as all-cause mortality. Appreciation of these risks may help PLWH and their providers make better-informed decisions about the use of opioid therapy.
Extending our previous work that considered one year of opioid receipt,24 and recognizing that opioid therapy changes over time, we sought to examine the association of opioids and mortality long-term. We were particularly interested in the effects of opioids among patients on LTOT and whether there is a discernable dose threshold above which risk increases disproportionately. Currently, opioid tapering to lower doses and cessation are being recommended as opioid risk reduction strategies. Available recent studies have suggested that opioid cessation may be associated with increased adverse outcomes including overdose and suicide mortality.25–28 There is no data regarding the effect of LTOT cessation or interruption on all-cause mortality risks.
In this study, we investigated the association between time-updated opioid therapy dose and 1) all-cause mortality, 2) unnatural death, and 3) fatal overdose among PLWH and uninfected patients on LTOT, and examined if there were differences by HIV status given PLWH’s susceptibility to opioid-related complications. We also examined whether LTOT prescription interruption was associated with mortality.
2. Methods
We used data from the Veterans Aging Cohort Study (VACS), described in detail elsewhere.29 In brief, VACS is a prospective study of all PLWH receiving care at Department of Veterans Affairs (VA) medical centers across the United States and their uninfected comparators. Each PLWH was matched with 2 uninfected patients on age, sex, race/ethnicity and site of care. VACS include data from the VA-based electronic health record, encompassing demographic data, medical diagnoses (based on International Classification of Diseases, Ninth Revision (ICD-9), Clinical Modification codes), laboratory results, pharmacy from the VA Pharmacy Benefits Management records system, and the National Death Index, which includes cause of death. This study was approved by the institutional review boards at VA Connecticut Healthcare System and Yale School of Medicine.
2.1. Study Population
We identified patients who initiated LTOT, defined as 90 consecutive days of opioid therapy allowing for up to 30-day gaps between medication fills30,31 with no more than 30 days’ receipt in the prior 180 days. The study period was between October 1, 1999 and September 30, 2014. Patients were excluded if they had greater than 30 days’ opioid receipt within the 180-day window prior to LTOT initiation (n=14,077), had a cancer diagnosis (except skin cancer, not including melanoma n=4,350), had evidence of palliative care (n=1,761) at the time of LTOT initiation, if HIV status was ambiguous (n=242), or if records had unclear pharmacy data (n=9). Patients with unusually high morphine equivalent daily dose (> 2000 mg MEDD) were excluded (n=602) under the rationale that their extreme outlier status could bias findings. We additionally excluded women (n=580), and race other/unknown (n=460) as these sub-sample sizes were too small to generate stable estimates or to examine separately.
2.2. Covariates
Demographic variables included age and race/ethnicity (white, black, and Hispanic). Clinical variables, based on ICD-9 codes, included diabetes; hypertension; congestive heart failure; coronary artery disease (CAD); chronic obstructive pulmonary disease (COPD); sleep apnea; pain-related diagnoses; psychiatric disorders (PTSD, major depression, bipolar disorder, and schizophrenia)32; opioid use disorder; substance use disorders (a composite of alcohol and drug use disorders); and smoking (classified as never, current or former). Hepatitis C virus (HCV) was based on ICD-9 codes and laboratory data. We categorized pain-related diagnoses as acute pain-related: abdominal pain, chest pain, fracture, or kidney stones; and chronic pain-related: back pain, extremity pain, headache, neck pain, neuropathy, osteoarthritis, other pain, rheumatoid arthritis, or temporomandibular joint pain. We also looked at the average pain score based on the numerical pain rating scale (0–10). HIV status was established based on ICD-9 codes (042. and V08.).
2.3. Time-updated covariates
Opioid profile (i.e. mg MEDD - our primary variable of interest and defined below), benzodiazepine receipt, and VACS index score 2.0—a validated measure of severity of illness (categorized as <75 (not ill), ≥75 (ill) and missing)33,34 —were time-updated in 30-day intervals from LTOT initiation date to event (i.e. death) or end of study. The VACS index score 2.0 is a physiologic score predicting risk of mortality that integrates data on age; HIV biomarkers (HIV-1 RNA viral load; CD4 cell count); and non-HIV biomarkers (hemoglobin, hepatitis C; FIB-4 to assess liver function, estimated glomerular filtration rate to assess renal function, albumin, body mass index, and white blood cell count). Benzodiazepine receipt was determined by pharmacy data indicating receipt of the following medications: alprazolam, chlordiazepoxide, clonazepam, clorazepam, diazepam, estazolam, flurazepam, lorazepam, midazolam, oxazepam, temazepam, and triazolam, and was dichotomized (yes/no). To determine opioid profile, we examined receipt of all outpatient oral and transdermal opioids, including codeine, hydrocodone, oxycodone, oxycodone sustained action (SA), morphine, morphine SA, fentanyl, hydromorphone, methadone and other (which includes the collapse category of the following low potency and/or low frequency opioids: dihydrocodeine, meperidine, pentazocine, propoxyphene, levorphanol, tramadol, and tapentadol). Non-formulary medications, including hydrocodone SA; hydromorphone SA; oxymorphone; oxymorphone SA; and codeine SA, were not dispensed.
Medications for the treatment of opioid use disorder (methadone via opioid treatment programs and buprenorphine) were excluded. Opioid dose was based on the average morphine-equivalent daily dosages (MEDD), estimated using standard conversion factors,30,31 and all outpatient fills of oral and transdermal opioids were converted into mg MEDD. Opioid dose was categorized into 1–20 (reference group), 21–50, 51–90, ≥ 91 mg MEDD, and suspected LTOT interruption, meaning patient did not receive or fill an opioid prescription at the VA for at least two continuous 30-day intervals (i.e. a period of ≥ 60 days). Consequently, because of how LTOT was defined, having a gap beyond what was allowed was deemed an interruption.
2.4. Outcomes
Our main outcome variables were all-cause mortality, unnatural death (i.e. injury [e.g. falls, motor vehicle accident, and homicide] or poisoning), and fatal overdose (defined as any intentional, unintentional, or indeterminate death from poisoning caused by any drug); and were defined using International Classification of Diseases (ICD) 9 and 10 code grouping from Centers for Disease Control and Prevention (CDC) drug overdose data and statistics.35 Cause of death was determined by matching decedent patient identifiers to the National Death Index file where causes of death are coded according to International Classification of Diseases, Tenth Revision. All-cause mortality included unnatural and overdose, and unnatural included overdose.
2.5. Statistical Analysis
We performed descriptive analysis of patients who initiated LTOT and evaluated their characteristics by HIV status using a chi-square test for categorical variables and T-test or Kruskal-Wallis for continuous variables at the time of LTOT initiation. Time-updated Cox proportional hazard models were used to examine the association between all-cause, unnatural, and overdose death, with opioid dose (as categorized above) adjusted for demographic and clinical factors (e.g. smoking). The strength of the Cox model is its ability to include covariates that change over time and to consider the relation of the survival outcome as a function of the change of the covariate.36 The proportional hazards assumption was evaluated by including interactions of the exposure variables with follow-up time. Models were then stratified by HIV status to evaluate if there were any substantive differences among PLWH versus uninfected. An interaction term including HIV, opioid exposure and each outcome was tested. We performed a sub-analysis examining all-cause mortality that did not include unnatural or overdose death, and the following sensitivity analyses: excluding patients who developed cancer during the observation period; retaining/using data from patients up until the date of a cancer diagnosis (i.e. censored); excluding patients with a gap in care of 18 months or more; and excluding patients with a VACS index score 2.0 > 100, which indicates a 20% risk of death in the next year and is a proxy for severe illness, to explore whether any of these factors significantly impacted our results.
All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, North Carolina, USA).
3. Results
We identified 22,996 patients who initiated LTOT, 6,578 (29%) PLWH and 16,418 (71%) uninfected. LTOT patients were predominately white, current smokers, had hypertension, and a chronic pain-related diagnosis (Table 1). PLWH on LTOT compared to uninfected patients on LTOT were more likely to be black, currently smoking, have HCV, acute pain diagnosis, substance use disorders, major depression, and higher VACS index score (Table 1).
Table 1.
Descriptive Statistics of Male Veterans on Long-term Opioids Therapy (LTOT)
| Variables, n (%) | Overall, n=22,996 | Uninfected, n=16,418 | PLWH, n=6,578 | p value |
|---|---|---|---|---|
| Age, mean (SD) | 51 (9) | 52 (9) | 51 (9) | 0.02 |
| Race: | <0.001 | |||
| White | 11,023 (48) | 7,970 (49) | 3,053 (46) | |
| Black | 10,258 (45) | 7,178 (44) | 3,080 (47) | |
| Hispanic | 1,715 (7) | 1,270 (8) | 445 (7) | |
| Smoking:m | <0.001 | |||
| Never | 4,544 (20) | 3,423 (21) | 1,121 (17) | |
| Current | 13,935 (61) | 9,819 (60) | 4,116 (63) | |
| Former | 3,641 (16) | 2,757 (17) | 884 (13) | |
| Diabetes | 7,738 (34) | 5,937 (36) | 1,801 (27) | <0.001 |
| Hepatitis C | 6,827 (30) | 3,758 (23) | 3,069 (47) | <0.001 |
| Sleep Apnea | 386 (2) | 339 (2) | 47 (1) | <0.001 |
| Hypertension | 10,721 (47) | 8,253 (50) | 2,468 (38) | <0.001 |
| Coronary artery disease | 2,443 (11) | 1,927 (12) | 516 (8) | <0.001 |
| Chronic obstructive pulmonary disease | 2,404 (10) | 1,729 (11) | 675 (10) | 0.55 |
| Congestive heart failure | 767 (3) | 573 (3) | 194 (3) | 0.04 |
| Opioid use disorder | 1,141 (5) | 641 (4) | 500 (8) | <0.001 |
| Pain related-diagnosis: | <0.001 | |||
| None | 4,027 (18) | 2,354 (14) | 1,673 (25) | |
| Acute | 1,075 (5) | 635 (4) | 440 (7) | |
| Chronic | 17,894 (78) | 13,429 (82) | 4,465 (68) | |
| Average pain score, IQR | 5.7 (3.0, 8.0) | 6.0 (3.0, 8.0) | 5.0 (2.5, 7.7) | <0.001 |
| Substance use disorders* | 5,879 (26) | 3,912 (24) | 1,967 (30) | <0.001 |
| Bipolar disorder | 1,796 (8) | 1,289 (8) | 507 (8) | 0.71 |
| Major depression | 2,824 (12) | 1,940 (12) | 884 (13) | 0.001 |
| PTSD | 3,593 (16) | 2,848 (17) | 745 (11) | <0.001 |
| Schizophrenia | 1,123 (5) | 849 (5) | 274 (4) | 0.001 |
| Psychiatric disorders** | 7,191 (31) | 5,340 (33) | 1,851 (28) | <0.001 |
| On ARTh | 4,068 (62) | n/a | n/a | n/a |
| MEDD, IQR | 20 (13, 32) | 20 (13, 30) | 21 (14, 37) | <0.001 |
| Benzodiazepine receipt | 4,549 (20) | 3,162 (19) | 1,387 (21) | 0.002 |
| Benzodiazepine dose, IQR | 440 (160, 990) | 450 (172, 1027) | 400 (150, 900) | 0.0006 |
| VACS index score 2.0, IQR | 36 (28, 49) | 32 (26, 39) | 55 (44, 68) | <0.001 |
| All-cause mortality | 5,222 (23) | 3,033 (18) | 2,189 (33) | <0.001 |
| Unnatural deaths | 619 (3) | 418 (2) | 201 (3) | 0.03 |
| Overdose | 337 (1) | 223 (1) | 114 (2) | 0.03 |
PLWH - people living with HIV; m 876 (4%) were missing smoking [7% in PLWH vs. 3% in uninfected].
Substance use disorders is a composite of alcohol and drug disorder
Psychiatric disorders is a composite of bipolar disorder, major depression, PTSD, and schizophrenia; h – among PLWH; ART – antiretroviral therapy; MEDD – Morphine equivalent daily dose.
There were 5,222 (23%) deaths (31/1000 person years [PY]) over the mean follow up time of 8 years. Twelve percent (619/5,222) of all-cause mortality was unnatural and 6% (337/5,222) was fatal overdose. The all-cause mortality rates were higher among higher opioid dose categories (1–20 mg MEDD 3/1000 PY, 21–50 mg MEDD 5/1000 PY, 51–90 mg MEDD 4/1000 PY, and ≥ 91 mg MEDD 12/1000 PY). PLWH had slightly higher mg MEDD and mortality compared to uninfected patients (table1).
In unadjusted Cox models, there was a clear and significant dose-response relationship between LTOT receipt and mortality. Compared to patients on 1–20 mg MEDD, those on 21–50, 51–90, and ≥ 91 had increasing risk of all-cause mortality, HR (95% CI) = 1.30 (1.16, 1.45), 1.92 (1.69, 2.19), and 3.00 (2.69, 3.35), respectively; unnatural death 1.38 (1.03, 1.85), 1.91 (1.36, 2.68), and 2.69 (1.99, 3.63), respectively; and overdose 1.55 (1.02, 2.36), 2.93 (1.87, 4.59), and 3.82 (2.53, 5.75), respectively. Patients who had LTOT interruption in their opioid receipt were also at increased risk for all three outcomes, HR (95 % CI) = 2.63 (2.39, 2.89), 1.80 (1.39, 2.33) and 1.88 (1.29, 2.74), respectively. A dose-response relationship was also evident in the unadjusted models stratified by HIV status (data not shown).
In models adjusted for age, race/ethnicity, smoking, substance use disorders, psychiatric disorders, benzodiazepine receipt, and severity of illness, the significant dose-response association between LTOT receipt and mortality remained (Table 2). For example, compared to patients on 1–20 mg MEDD there was a monotonic increasing risk of all-cause mortality by dose (i.e. 21–50 mg MEDD = 1.36 (1.21, 1.52), 51–90 mg MEDD = 2.06 (1.82, 2.35), and ≥ 91 mg MEDD = 3.03 (2.71, 3.39)). Other factors associated with all-cause mortality, unnatural death and overdose were current smoking, 1.63 (1.49, 1.78), 1.96 (1.47, 2.62), and 1.99 (1.32, 3.01), respectively; HCV, 1.56 (1.47, 1.65), 1.70 (1.44, 2.01), and 2.36 (1.88, 2.95), respectively; substance use disorders, 1.41 (1.32, 1.50), 1.85 (1.55, 2.20), and 2.18 (1.72, 2.76), respectively; and benzodiazepine receipt, 1.12 (1.04, 1.20), 1.73 (1.44, 2.07), and 2.00 (1.58, 2.54), respectively. Black race was inversely associated across all outcomes, 0.88 (0.83, 0.93), 0.68 (0.57, 0.81), and 0.66 (0.52, 0.83), respectively.
Table 2.
Adjusted Time-updated Cox Model Examining the Association between Opioid and Mortality (All-cause, Unnatural, and Overdose), among Male Veterans on Long-term Opioid Therapy (LTOT)
| All-cause | Unnatural | Overdose | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | |
| Opioid daily dose (MEDD) (ref 120 mg) | 1 | n/a | 1 | n/a | 1 | n/a |
| 21–50 | 1.36 (1.21, 1.52) | <0.001 | 1.27 (0.95, 1.69) | 0.11 | 1.38 (0.91, 2.10) | 0.13 |
| 51–90 | 2.06 (1.82, 2.35) | <0.001 | 1.68 (1.19, 2.36) | 0.003 | 2.47 (1.57, 3.87) | <0.001 |
| ≥ 91 | 3.03 (2.71, 3.39) | <0.001 | 2.01 (1.48, 2.72) | <0.001 | 2.65 (1.75, 4.02) | <0.001 |
| Suspected interruption in opioid receipt | 2.30 (2.09, 2.53) | <0.001 | 1.47 (1.13, 1.91) | 0.004 | 1.52 (1.04, 2.23) | 0.03 |
| Age per 10 years increment | 1.62 (1.57, 1.68) | <0.001 | 0.91 (0.82, 1.01) | 0.08 | 0.81 (0.70, 0.94) | 0.004 |
| Black | 0.88 (0.83, 0.93) | <0.001 | 0.68 (0.57, 0.81) | <0.001 | 0.66 (0.52, 0.83) | 0.001 |
| Smoking (ref never) | 1 | n/a | 1 | n/a | 1 | n/a |
| Current | 1.63 (1.49, 1.78) | <0.001 | 1.96 (1.47, 2.62) | <0.001 | 1.99 (1.32, 3.01) | 0.001 |
| Former | 1.10 (0.98, 1.23) | 0.10 | 1.25 (0.86, 1.82) | 0.24 | 1.22 (0.71, 2.09) | 0.47 |
| Hepatitis C | 1.56 (1.47, 1.65) | <0.001 | 1.70 (1.44, 2.01) | <0.001 | 2.36 (1.88, 2.95) | <0.001 |
| Substance use disorders* | 1.41 (1.32, 1.50) | <0.001 | 1.85 (1.55, 2.20) | <0.001 | 2.18 (1.72, 2.76) | <0.001 |
| Psychiatric disorders** | 0.89 (0.83, 0.95) | 0.0002 | 1.30 (1.10, 1.54) | 0.002 | 1.30 (1.03, 1.63) | 0.02 |
| Benzodiazepine receipt | 1.12 (1.04, 1.20) | 0.002 | 1.73 (1.44, 2.07) | <0.001 | 2.00 (1.58, 2.54) | <0.001 |
| VACS index score 2.0 (ref <75) | 1 | n/a | 1 | n/a | 1 | n/a |
| ≥75 | 5.68 (4.91, 6.58) | <0.001 | 1.24 (0.63, 2.47) | 0.53 | 1.27 (0.54, 2.95) | 0.58 |
| Missing | 3.51 (3.21, 3.82) | <0.001 | 2.42 (1.95, 3.00) | <0.001 | 2.28 (1.73, 3.02) | <0.001 |
Substance use disorders is a composite of alcohol and drug disorder
Psychiatric disorders is a composite of bipolar disorder, major depression, PTSD, and schizophrenia; and MEDD – Morphine equivalent daily dose. HR (95% CI) for smoking missing vs. never was 10.69 (9.53, 12.00), 15.26 (10.56, 22.04), and 14.02 (8.25, 23.82),respectively.
The proportional hazards assumption was evaluated by including an interaction term of the opioid exposure variable with follow-up time. The interaction was not significant for outcomes: unnatural (p=0.76), overdose (p=0.99), and all-cause mortality that excluded unnatural death (p=0.09). It was statistically significant for all-cause mortality overall, but the effect size was not substantive, 1.0003 (1.0000, 1.0007), p=0.05. The HR for opioid by time estimates the multiplicative change in the HR for opioid for each 30-day increase in follow-up time. For example, to estimate the HR at 6 months for mortality in 21–50 mg MEDD compared to 1–20, multiply the time 0 HR by the interaction HR raised to the 6th power (i.e. 1.32* 1.0003 * 1.0003 * 1.0003 * 1.0003 * 1.0003 * 1.0003 ≈ 1.32). The inclusion of the interaction term of LTOT with follow-up time suggests a very small increase in the hazards of all-cause mortality.
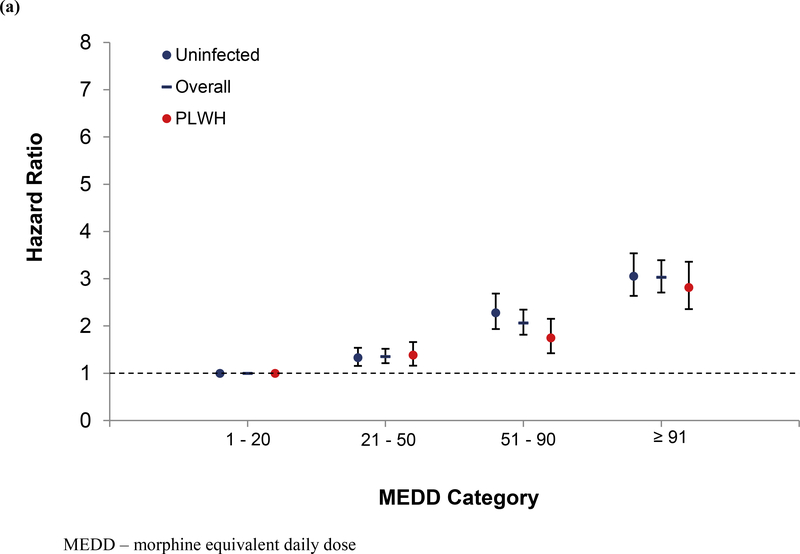
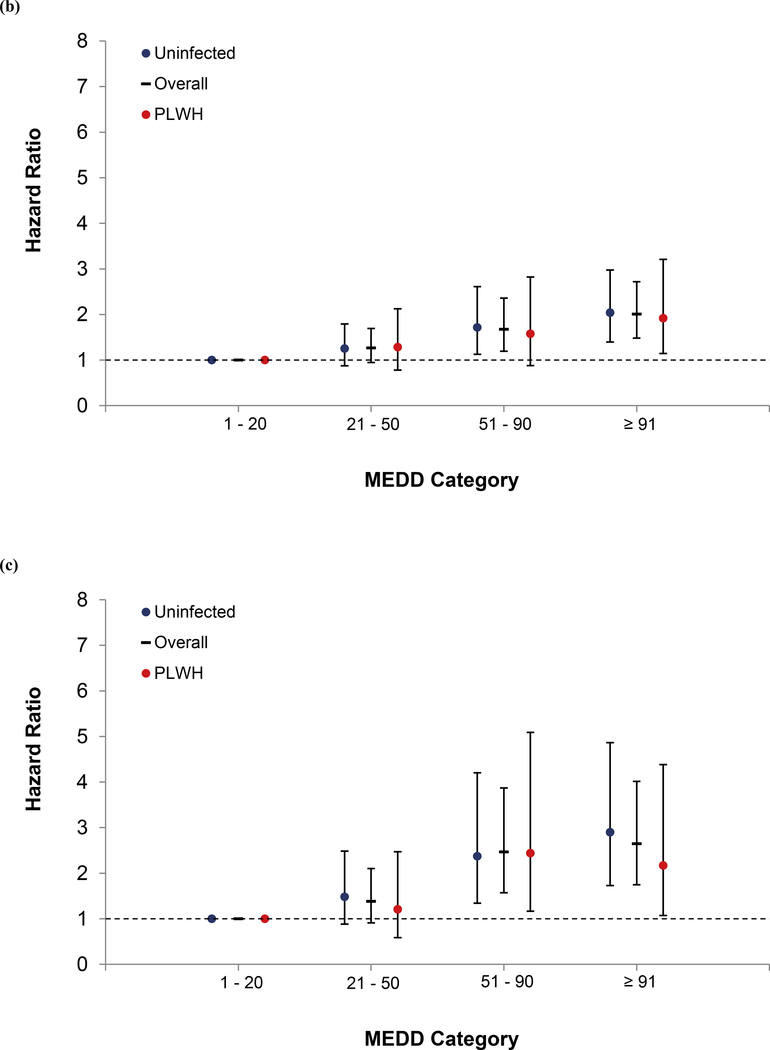
In models stratified by HIV, the dose-response relationship remained in uninfected, and largely in PLWH, except for overdose (Figure 1a, 1b, and 1c). LTOT interruption was also significant among uninfected patients for all outcomes, but only with all-cause mortality among PLWH. As in the unstratified model, current smoking, HCV, substance use disorders and benzodiazepine receipt were associated with mortality. An interaction term between opioid dose and HIV status was tested for each outcome, and it was not significant.
Figure 1.
Association of Opioid Dose with Mortality Overall and by HIV Status (a) All-cause, (b) Unnatural, and (c) Overdose
In a sub-analysis looking at all-cause mortality excluding unnatural death, compared to patients on 1–20 mg MEDD, those on 21–50, 51–90, and ≥ 91 had increasing risk; 1.37 (1.21, 1.54), 2.13 (1.85, 2.45), and 3.23 (2.86, 3.64), respectively. This remained true in models stratified by HIV. Among PLWH, those on 21–50, 51–90, and ≥ 91 showed increasing risk of all-cause mortality; 1.40 (1.15, 1.69), 1.77 (1.42, 2.21), and 2.95 (2.44, 3.57), respectively. Among uninfected the increase risk was 1.35 (1.15, 1.58), 2.40 (2.01, 2.87), and 3.29 (2.80, 3.85), respectively. In sensitivity analyses, the findings remained largely the same. A consistent dose-response relationship was observed in models excluding patients who were diagnosed with cancer during follow up, when we censored patients with an incident cancer diagnosis, when we excluded patients with an extended gap in care (i.e. 18 months or more), and when we excluded patients with a VACS index score 2.0 > 100 (Supplemental tables 1, 2, 3, and 4).
4. Discussion
In a large, diverse national sample of patients on LTOT with and without HIV, we observed a dose-response relationship between opioid use and mortality. Higher daily dose was associated not only with fatal overdose but also with all-cause mortality and unnatural death, independent of severity of illness. This association remained when all-cause mortality excluded unnatural death. LTOT interruption was also associated with increased all-cause, unnatural and overdose mortality. In addition, benzodiazepine receipt and substance use disorder (SUD) were important associated factors, more so with fatal overdose.
Similar to our findings, few prior studies have reported increased all-cause mortality associated with LTOT, but with limited information on association with dose levels.5–7;9 For example, a recent study from a nationally representative sample in South Korea between 2002 and 2015, a period of dramatic growth in prescription opioids similar to US, reported that LTOT with “strong opioids” (fentanyl, morphine, oxycodone, hydromorphone, and methadone) was associated with higher risk of all-cause mortality compared to “weak opioids” use and no opioid use.5 In a study among socio-economically disadvantaged patients in Ontario, Canada who received any opioid prescription between 2004 and 2008, both long and short term, Gomes et. al. reported increased risk of all-cause mortality and opioid related mortality (<10% of the deaths) similar to our study among those on high (201–400 MEDD) and very high doses (>400 MEDD) compared to moderate dose of ≤200 MEDD, a dose considered “high” in the current era.9 Our study adds a new level of knowledge by demonstrating that the risk increases monotonically even at lower, and currently commonly seen, levels of opioid doses.
Contrary to common notions, results of this and other studies are revealing that LTOT cessation or interruption may not be associated with a risk reduction as often expected37 but is associated with increased independent risk of overdose, unnatural and all-cause mortality and other adverse outcomes. A smaller single site study recently reported increased all-cause and overdose mortality associated with LTOT discontinuation.25 A study from the Vermont Medicaid population reported that opioid related hospitalizations increased following LTOT discontinuation.26 Another recent study from the US Veterans Health Administration database reported higher risk of overdose and suicide mortality during the 6 months immediately after cessation of both long and short term opioid prescriptions in a dose dependent manner and did not decrease below the risks associated with opioid continuation after the 6 months.27 They also reported that overdose and suicides deaths were also elevated during the first 6 months following opioid initiation. Our multi-site study extends these findings and adds to the emerging knowledge that LTOT initiation and continuation risks extends beyond overdose mortality to all unnatural and all-cause mortality, and these risks may further increase and not abate following LTOT discontinuation.
These findings together have significant clinical impact across the continuum of LTOT initiation, continuation, and discontinuation or interruption. The persistently elevated risks across the continuum of LTOT deployment should provide additional pause to providers and patients considering LTOT initiation, continuation, and discontinuation. It is particularly important for providers and patients engaging in opioid tapering to recognize that opioid discontinuation maybe a risky venture for many, and appropriate close monitoring, support, and risk mitigation strategies are essential. More studies investigating LTOT interruption are needed. The reasons for excess mortality with opioid discontinuation are not clear and should be investigated further.
The consistent and positive dose response association between opioids and all-cause mortality across all models, including the stratified, sub, and sensitivity models, indicate that patients and providers should be mindful of opioid dose irrespective of HIV status. The risk calculations associated with MEDD should also include the larger impact on all-cause mortality in addition to overdose and unnatural deaths. Furthermore, the excess overdose and suicide mortality risks related to co-prescription of benzodiazepines appears to persist beyond the one-year time reported earlier,24 and extend to all-cause mortality, albeit at a lower level than overdose risks. SUD, a known risk factor for adverse effects of LTOT was a strong contributing factor to all mortality outcomes in this study, suggesting caution when prescribing opioid dose, especially at higher doses among those with SUD. Interestingly, black patients had an inverse relationship in all models and all three outcomes. This is contrary to mortality rates in general and in most research arena, but similar to other research examining LTOT.19,24,38 We tested a posterior interaction term between black and opioid dose in relation to each outcome and it was not significant (data not shown). We are cautious in postulating the reasons for this association. One possible explanation is that given black patients are more likely to be monitored, more likely to be assigned a substance use disorder,39 and more likely to have opioids discontinued because of substance use, harm was mitigated. Further research is needed to understand this inverse association.
4.1. Limitations and Strengths
Our study has several limitations. From our observational study, we cannot make any causal inferences regarding the increased risk of mortality in association with LTOT. In addition, we did not have any data regarding the nature of LTOT interruption (slow vs abrupt, patient vs physician initiated, etc.), illicit use of opioids, nor other substances or supportive treatments received following LTOT discontinuation. Data on opioid prescriptions filled were restricted to those extracted from the VA Pharmacy Benefits Management outpatient records system; consequently, any opioid medications filled outside of the VA or in VA inpatient settings were not captured. However, this potential limitation may be negligible as most VA patients solely use VA pharmacies.40 Also, our sample was entirely of male veterans so findings may be less generalizable to women and non-veteran men. Finally, our cause of death ascertainment is only as complete as the National Death Index data. It is possible that a very small proportion of deaths may have been missed, but it is unlikely this would have substantially changed our results.
Our study also has several strengths. We were able to adjust for severity of illness using a validated risk index (VACS Index 2.0), ascertain validated mortality,41–43 and had complete cause of death from the National Death Index. Our sample was of patients receiving care within the national VA healthcare system, ensuring completeness of opioid profile captured and important confounders including demographics, HCV, and laboratory values were measured consistently across HIV status. Finally, we performed several sensitivity analyses to assess if results were driven by cancer or the severely ill, and our findings were unchanged.
5. Conclusions
Among patients receiving LTOT, we observed a strong dose-response relationship with all-cause mortality in addition to that with unnatural deaths and fatal overdoses among both PLWH and uninfected patients. LTOT interruption was also associated with increased mortality risk. Opioid risk mitigation approaches should be expanded to address the potential effects of higher dose on all-cause mortality among all receiving long term opioids and the potential risks following LTOT interruption.
Supplementary Material
Highlights.
Higher daily opioid dose was associated not only with fatal overdose, but also with all-cause mortality and unnatural death in patients with and without HIV.
LTOT cessation/interruption was also associated with increased risk of all-cause, unnatural and overdose mortality.
Benzodiazepine receipt and substance use disorders were strongly associated with all 3 outcomes, but more so with overdose fatality.
Acknowledgements
We would like to thank Dr. Adam Gordon for his insightful review of this manuscript. We would also like to thank the Veterans Healthcare Administration and the study coordinators, faculty, and staff on the Veterans Aging Cohort Study.
Role of funding source
COMpAAS Center and Observational study, and Veterans’ Aging Cohort Study were funded by NIAAA, Justice (PI) - U10-A013566–08, U24-AA02200, U01-AA020795, U01-AA020799, U24 AA022001, U24-AA020794, and U01-AA020790. The content of this manuscript is solely the responsibility of the authors and does not represent the official views of the Department of Veterans Affairs.
Footnotes
Conflict of interest
The authors report no conflict of interest.
Disclaimer: Any expressed views in this manuscript do not necessarily represent those of the Department of Veterans Affairs or the US Government.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hedegaard H, Warner M, Miniño AM. Drug overdose deaths in the United States, 1999–2016 NCHS Data Brief, no 294. Hyattsville, MD: National Center for Health Statistics; 2017. Available. from: https://www.cdc.gov/nchs/data/databriefs/db294.pdf [Google Scholar]
- 2.Hedegaard H, Miniño AM, Warner M. Drug overdose deaths in the United States, 1999–2017 NCHS Data Brief, no 329. Hyattsville, MD: National Center for Health Statistics; 2018 [Google Scholar]
- 3.Rudd RA, Aleshire N, Zibbell JE, Gladden RM. Increases in Drug and Opioid Overdose Deaths--United States, 2000–2014. MMWR Morb Mortal Wkly Rep 2016;64:1378–82 [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Web-based Injury Statistics Query and Reporting System (WISQARS) [online]. 2014. Retrieved from: http://www.cdc.gov/injury/wisqars/fatal.html.
- 5.Oh TK, Jeon Y-T, Choi JW. Trends in chronic opioid use and association with five-year survival in South Korea: a population-based cohort study. Br J Anaesth 2019;123(5):655–663. [DOI] [PubMed] [Google Scholar]
- 6.Ekholm O, Kurita GP, Hojsted J, Juel K, Sjogren P. Chronic pain, opioid prescriptions, and mortality in Denmark: A population-based cohort study. Pain 2014;155(12):2486–2490. [DOI] [PubMed] [Google Scholar]
- 7.Burr NE, Smith C, West R, Hull MA, Subramanian V. Increasing prescription of opiates and mortality in patients with inflammatory bowel diseases in England. Clin Gastroenterol Hepatol 2018;16(4):534–541. [DOI] [PubMed] [Google Scholar]
- 8.Solomon DH, Rassen JA, Glynn RJ, Lee J, Levin R, Schneeweiss S. The comparative safety of analgesics in older adults with arthritis. Arch Intern Med 2010;170(22):1968–1976. [DOI] [PubMed] [Google Scholar]
- 9.Gomes T, Juurlink DN, Dhalla IA, Mailis-Gagnon A, Paterson JM, Mamdani MM. Trends in opioid use and dosing among socio-economically disadvantaged patients. Open Med 2011;5(1):e13–e22. [PMC free article] [PubMed] [Google Scholar]
- 10.Chou R, Fanciullo GJ, Fine PG, et al. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain 2009;10(2):113–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chapman CR, Lipschitz DL, Angst MS, et al. Opioid pharmacotherapy for chronic noncancer pain in the United States: a research guideline for developing an evidence-base. J Pain 2010;11(9):807–829. [DOI] [PubMed] [Google Scholar]
- 12.Hall AJ, Logan JE, Toblin RL, et al. Patterns of abuse among unintentional pharmaceutical overdose fatalities. JAMA 2008;300(22):2613–2620. [DOI] [PubMed] [Google Scholar]
- 13.Dunn KM, Saunders KW, Rutter CM, et al. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med 2010;152(2):85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bohnert AS, Valenstein M, Bair MJ, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA 2011;305(13):1315–1321. [DOI] [PubMed] [Google Scholar]
- 15.Gomes T, Mamdani MM, Dhalla IA, Paterson JM, Juurlink DN. Opioid dose and drug-related mortality in patients with nonmalignant pain. Arch Intern Med 2011;171(7):686–691. [DOI] [PubMed] [Google Scholar]
- 16.Okie S A flood of opioids, a rising tide of deaths. [published correction appears in N Engl J Med. 2011 Jan 20;364(3):290]. N Engl J Med 2010;363(21):1981–1985.. [DOI] [PubMed] [Google Scholar]
- 17.Gomes T, Redelmeier DA, Juurlink DN, Dhalla IA, Camacho X, Mamdani MM. Opioid Dose and Risk of Road Trauma in Canada: A Population-Based Study. JAMA Intern Med 2013;173(3):196–201. [DOI] [PubMed] [Google Scholar]
- 18.Webster LR, Cochella S, Dasgupta N, et al. An Analysis of the Root Causes for Opioid-Related Overdose Deaths in the United States. Pain Med 2011;12 Suppl 2:S26–S35. [DOI] [PubMed] [Google Scholar]
- 19.Solomon DH, Rassen JA, Glynn RJ, Lee J, Levin R, Schneeweiss S. The comparative safety of analgesics in older adults with arthritis. [published correction appears in Arch Intern Med. 2011 Mar 14;171(5):403]. Arch Intern Med 2010;170(22):1968–1976. [DOI] [PubMed] [Google Scholar]
- 20.Edelman EJ, Gordon KS, Crothers K, et al. Association of prescribed opioids with increased risk of community-acquired pneumonia among patients with and without HIV. JAMA Intern Med 2019;179(3):297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roy S, Ninkovic J, Banerjee S, et al. Opioid drug abuse and modulation of immune function: consequences in the susceptibility to opportunistic infections. J Neuroimmune Pharmacol 2011;6(4):442–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malee KM, Mellins CA, Huo Y, et al. Prevalence, incidence and persistence of psychiatric and substance use disorders among mothers living with HIV. J Acquir Immune Defic Syndr 2014;65(5):526–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kraemer KL, McGinnis KA, Fiellin DA, et al. Low levels of initiation, engagement, and retention in substance use disorder treatment including pharmacotherapy among HIV-infected and uninfected veterans. J Subst Abuse Treat 2019;103:23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weisberg D, Gordon K, Barry D, et al. Long-term prescription opioids and/or benzodiazepines and mortality among HIV-infected and uninfected patients. J Acquir Immune Defic Syndr 2015;69(2):223–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.James JR, Scott JM, Klein JW, et al. Mortality after discontinuation of primary care-based chronic opioid therapy for pain: A retrospective cohort study. J Gen Intern Med 2019;34(12):2749–2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mark TL, Parish W. Opioid medication discontinuation and risk of adverse opioid-related health care events. Journal of substance abuse treatment. J Subst Abuse Treat 2019;103:58–63. [DOI] [PubMed] [Google Scholar]
- 27.Oliva EM, Bowe T, Manhapra A, et al. Associations between stopping prescriptions for opioids, length of opioid treatment, and overdose or suicide deaths in US veterans: observational evaluation. BMJ 2020;368:m283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Glanz JM, Binswanger IA, Shetterly SM, Narwaney KJ, Xu S. Association between opioid dose variability and opioid overdose among adults prescribed long-term opioid therapy. JAMA Netw Open. 2019;2(4):e192613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fultz SL, Skanderson M, Mole LA, et al. Development and verification of a” virtual” cohort using the National VA Health Information System. Med Care 2006;44(8 Suppl 2):S25–S30. [DOI] [PubMed] [Google Scholar]
- 30.Edelman EJ, Gordon K, Becker WC, et al. Receipt of opioid analgesics by HIV-infected and uninfected patients. J Gen Intern Med 2013;28(1):82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Von Korff M, Saunders K, Thomas Ray G, et al. De facto long-term opioid therapy for noncancer pain [published correction appears in Clin J Pain. 2014 Sep;30(9):830. Korff, Michael Von [corrected to Von Korff, Michael]]. Clin J Pain 2008;24(6):521–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goulet JL, Fultz SL, McGinnis KA, Justice AC. Relative prevalence of comorbidities and treatment contraindications in HIV-mono-infected and HIV/HCV-co-infected veterans. AIDS 2005;19 Suppl 3:S99–S105 [DOI] [PubMed] [Google Scholar]
- 33.Tate JP, Justice AC, Hughes MD, et al. An internationally generalizable risk index for mortality after one year of antiretroviral therapy. AIDS 2013;27(4):563–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tate JP, Sterne JAC, Justice AC; Veterans Aging Cohort Study (VACS) and the Antiretroviral Therapy Cohort Collaboration (ART-CC). Albumin, white blood cell count, and body mass index improve discrimination of mortality in HIV-positive individuals. AIDS 2019;33(5):903–912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.https://www.cdc.gov/drugoverdose/pdf/pdo_guide_to_icd-9-cm_and_icd-10_codes-a.pdf, accessed 9/8/17
- 36.Singer JD, Willett JB. Applied longitudinal data analysis: Modeling change and event occurrence. Oxford: Oxford University Press; 2003. [Google Scholar]
- 37.Dowell D, Haegerich TM, Chou R. CDC Guideline for prescribing opioids for chronic pain—United States, 2016. [published correction appears in MMWR Recomm Rep. 2016;65(11):295]. MMWR Recomm Rep 2016;65(1):1–49. [DOI] [PubMed] [Google Scholar]
- 38.Becker WC, Gordon K., Edelman EJ, et al. Trends in any and high-dose opioid analgesic receipt among aging patients with and without HIV. [published correction appears in AIDS Behav. 2017 Apr;21(4):1228]. AIDS Behav 2016;20(3):679–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hausmann LR, Gao S, Lee ES, Kwoh CK. Racial disparities in the monitoring of patients on chronic opioid therapy. Pain 2013;154(1):46–52. [DOI] [PubMed] [Google Scholar]
- 40.Smith MW, Joseph GJ. Pharmacy data in the VA health care system. Med Care Res Rev 2003;60(3 Suppl):92S–123S. [DOI] [PubMed] [Google Scholar]
- 41.Cowper DC, Kubal JD, Maynard C, Hynes DM. A primer and comparative review of major US mortality databases. Ann Epidemiol 2002;12(7):462–468. [DOI] [PubMed] [Google Scholar]
- 42.Fisher SG, Weber L, Goldberg J, Davis F. Mortality ascertainment in the veteran population: alternatives to the National Death Index. Am J Epidemiol 1995;141(3):242–250. [DOI] [PubMed] [Google Scholar]
- 43.Arnold N, Sohn MW, Maynard C, Hynes DM: VIReC Technical Report 2: VA-NDI Mortality Data Merge Project, Hines, IL, VA Information Resource Center, 2006 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.