2. Introduction
We experience the sleep-wake cycle as an alternation between a state of rest, during which consciousness is absent or altered, and a state of active interaction with the social and physical environment, during which the brain engages in many cognitive and other activities. Appropriate timing and adequate quality of the sleep-wake cycle are of paramount importance for successful functioning within our roles at home and at work. According to the conceptual framework of the circadian and homeostatic regulation of the sleep-wake cycle, timing of sleep and waking is achieved through the interaction of two endogenous oscillatory processes and external factors, such as the light-dark cycle and social constraints. Recent progress in the understanding of this interaction of the two endogenous oscillatory processes, the role of clock genes in regulating these oscillatory processes, as well as individual differences in the preferences for the timing of waking activities and sleep, are the topics of this chapter. We will list core and recent references and will also refer to three previous reviews 17;23;24.
3. Circadian and Homeostatic Regulation of Sleep and Cognition
a. Timing of Sleep and Waking Activities: Contribution of Environmental, Social and Internal Biological Factors
Bedtime and wake time are directly observable markers of the sleep-wake cycle, from which both the duration of sleep, as well as its phase relationship with clock time, social and geophysical cycles, can be computed. There is ample evidence demonstrating the impact of social factors on sleep timing. Most of us wake up to meet social requirements such as work schedules, and sleep timing and duration differ markedly between week-days and weekends. Evidence for the important role of light, i.e. the combined contribution of natural and artificial light, in the regulation of sleep and wakefulness comes from multiple sources, including blind individuals who often experience chronic sleep disturbances associated with non-synchronized circadian rhythms 59. The impact of the geophysical light-dark cycle (as opposed to the light-dark cycle we are exposed to) on the regulation of sleep in our industrialized societies is less profound. Sleep timing with respect to clock time and sleep duration are near constant across the seasons, even though in many highly populated areas on our planet the timing of dawn and dusk may change by several hours relative to local clock time, e.g. approximately 4 hours in London, UK. Furthermore, in most people, the sleep-wake cycle remains synchronized with clock time even when we change the phase relationship between clock time and the geo-physical light-dark cycle abruptly, such as occurs during the change to and from daylight savings time. The residual impact of the geophysical light-dark cycle on the timing of the sleep-wake cycle has, however, been observed in analyses of the timing of sleep relative to clock time in people living at similar latitude but different longitudes within one time zone 71. Evidence for the impact of endogenous biological factors on sleep-wake timing is plentiful. In fact, the magnitude of the effects of endogenous biological factors appears much larger than the impact of external environmental factors and is comparable to the impacts of social factors. This is not to underestimate the profound effect on sleep of local environmental factors, such as traffic noise and ambient temperature. Some characteristics of sleep including its timing and duration differ between men and women 22, and change throughout the life span 66;39, such that older people wake up earlier, while younger people sleep longer. Age-related changes in sleep duration persist when all social constraints are removed 50. When asked about the preferred timing of sleep and waking activities, including cognitive activities, individuals differ with respect to this diurnal preference. This trait-like characteristic, which differs between men and women and shifts towards morning preference with aging 44, has a heritability of around 50% 53. The magnitude of the differences in sleep timing across the life span and across the spectrum of diurnal preference, is considerable, i.e., as much as 2–3 hours between young adults and older people 70 and 2–3 hours between larks and owls 34;64. Despite these differences in timing of the sleep-wake cycle, most healthy people, with the noticeable exception of infants and young children, experience consolidated wake and sleep episodes, during which cognitive performance is upheld throughout the wake episode. We will first describe the endogenous oscillatory processes through which this consolidation of sleep and wakefulness is achieved, before we present the endogenous biological factors contributing to the individual differences in the timing of sleep and wakefulness.
b. Consolidation of Sleep-Wake Cycles Through Opposing Circadian and Homeostatic Sleep Propensity Rhythms
Research during the past 40–50 years has established that the consolidation and timing of the daily sleep-wake cycle is generated by the interaction of two oscillatory processes, the deep or stable circadian pacemaker, located in the suprachiasmatic nuclei (SCN) in the hypothalamus, and the labile oscillator or sleep homeostat, the locus of which is not known and may be diffuse. How the two processes interact has been elucidated through laboratory experiments in which the labile sleep-wake oscillator was scheduled to a periodicity well outside the circadian range 26;86;87. Because the deep circadian oscillator has a robust endogenous period near to 24-h, living on these non-circadian days leads to sleep and wake episodes occurring at all circadian phases, thereby allowing for an analysis of the contribution of these two oscillators to sleep-wake regulation (See Figure 1) Following initial reports, multiple experiments have now confirmed that the circadian process generates a sleep-wake propensity rhythm that has a phase relationship with the sleep-wake cycle which, although at first sight paradoxical, is highly functional: A circadian wake promoting signal becomes progressively stronger during the course of the biological day, reaching a maximum shortly before habitual bedtime, at a phase which has become known as the wake maintenance zone, or forbidden zone for sleep. This wake-promoting signal dissipates rapidly after the onset of nocturnal melatonin secretion. Circadian sleep propensity increases as the night progresses to reach a maximum at approximately 7–8 am in healthy young individuals and 6–7 am in older people, which is very close to habitual wake time 30. The circadian sleep-wake propensity rhythm oscillates in anti-phase with the homeostatic sleep-wake propensity rhythm: Homeostatic sleep propensity is at its minimum at the end of the sleep episode and then increases progressively throughout the waking episode, to reach a maximum just before the habitual sleep episode, which is initiated when the circadian drive for wakefulness subsides. Homeostatic sleep propensity can then dissipate throughout the sleep episode. The hypothesis that consolidation of the sleep-wake cycle is achieved through these opposing homeostatic and circadian sleep-wake propensity rhythms is supported by the observation that after lesions of the SCN, which is the hypothalamic locus of the deep circadian oscillator, the sleep-wake cycle becomes fragmented. Detailed analyses of sleep in intact and SCN-lesioned squirrel monkeys suggest that the SCN primarily generates a strong alerting signal 37. Other analyses suggest that the SCN also generates a sleep-inducing signal 61. The importance of the adequate phase relationship between circadian rhythmicity and sleep homeostasis is also underscored by the observation that disruption of the phase relationship between these two oscillators, such as occurs during shift work and jet-lag, leads to disruption of sleep and waking performance.
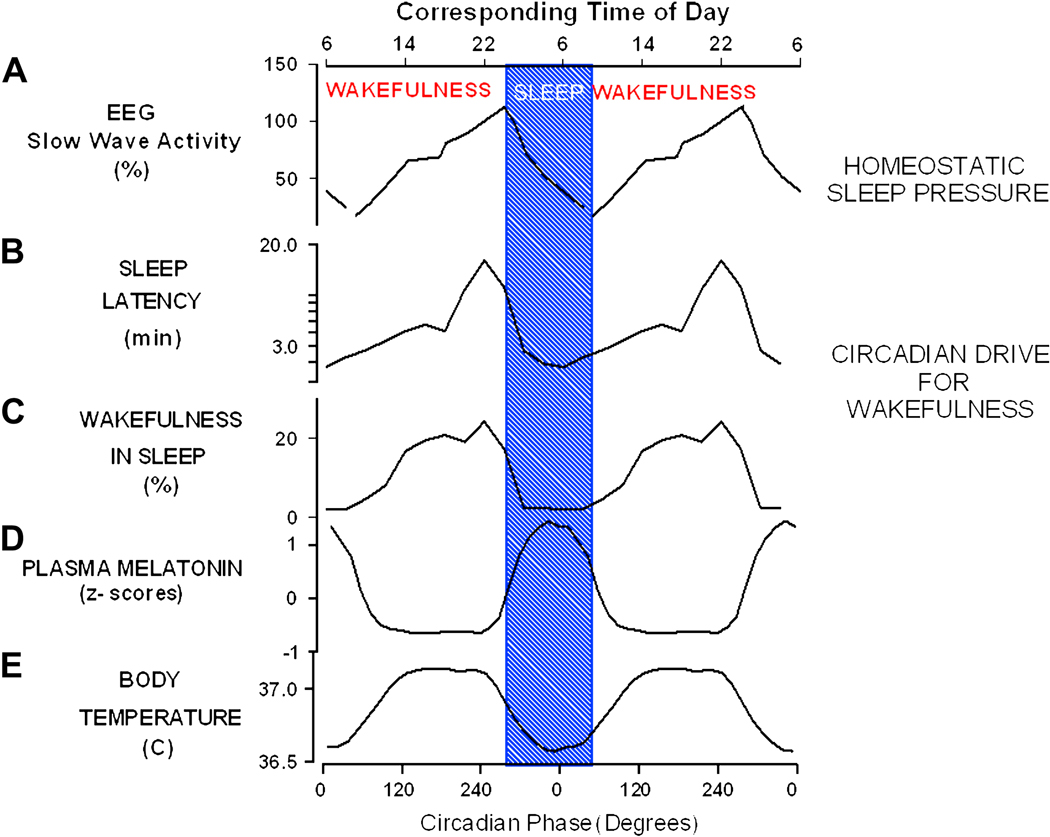
Fig 1.
Circadian and homeostatic regulation of sleep and wakefulness in humans. Panel A: Increase of homeostatic sleep pressure during wakefulness and its dissipation during sleep as reflected in EEG SWA during daytime naps and nocturnal sleep 25. Circadian variation in wake/sleep propensity as reflected in the latency to sleep onset (Panel B) after 18h:40 min of wakefulness and wakefulness (Panel C) in sleep opportunities, measured during forced desynchrony of the sleep-wake cycle and endogenous circadian rhythms of melatonin (Panel D) and core body temperature (Panel E) 30
Not only sleep-wake timing and sleep propensity, but also sleep physiology are profoundly influenced by these two oscillatory processes. This includes basic characteristics of sleep, such as the duration of Rapid Eye Movement Sleep (REMS), the density of rapid eye movements during REM sleep, alpha (8–12 Hz) EEG activity during REM sleep, the incidence, amplitude and frequency of sleep spindles, i.e. 12–14 Hz spindle-like EEG oscillations during nonREM sleep, high frequency EEG activity during nonREM sleep, as well as Slow Wave Activity (SWA; 0.75–4.5 Hz) in nonREM sleep 27;32;49;82. The latter variable is somewhat unique in that it is primarily determined by the homeostatic oscillator, and only minimally so by the circadian oscillator. In fact, SWA is considered a primary marker of the sleep-homeostat during sleep, also because sleep deprivation will lead to predictable increases in this variable.
The impact of the sleep-homeostat and circadian oscillator on the EEG is not limited to nonREM and REM sleep. The EEG recorded under controlled behavioral conditions during wakefulness is also profoundly influenced by these two oscillators.
For example, alpha activity during wakefulness exhibits a very distinct circadian rhythm with a maximum at approximately 4 pm and a minimum at 6 am, but is also influenced by time awake, such that it diminishes as time awake increases. By contrast, SWA and theta activity (4.5–8 Hz), while also displaying their circadian maxima during the biological day, increase as time awake increases 14. The latter two variables have been considered markers of the sleep-homeostat during wakefulness, also because sleep deprivation leads to a further increase in these EEG activities. Recent analyses of topographical differences with respect to the circadian and sleep-wake-dependent modulation of EEG activity have suggested that frontal areas are more affected by the sleep-homeostat than occipital areas, and particularly so for low frequency components of the EEG during sleep 12 and wakefulness14, as well as EEG spindle activity during sleep 51.
Thus, brain activity, as monitored through polysomnography and the analysis of the EEG during both wakefulness and sleep, is dynamically modulated by the circadian and homeostatic oscillator. The changes in low-frequency EEG activity associated with the duration of wakefulness have been hypothesized to represent use-dependent changes in neural networks 54;78. Such changes may be more prominent in areas that have been used more intensively, and evidence for local changes in SWA in response to local stimulation has been accumulating 43;47. Currently, it is thought that wakefulness and the intense sensory stimulation and experience associated with waking behaviors lead to local release of adenosine and growth factors, which in turn may lead to local increases in SWA 8;55.
c. Consolidation of Cognitive Performance Through Circadian Rhythmicity and Sleep Homeostasis
What we experience during wakefulness is our ability to perform. Waking performance includes both physical, e.g. athletic performance, and non-physical performance, e.g. sustained attention. Here we will only discuss aspects of the latter, which we will loosely refer to as cognitive performance (for a discussion on physical performance see 68). Cognitive performance comprises many different domains and elements including working memory, vigilance, attention, and executive function. A discussion of the brain basis and interrelationships between these domains and elements of cognitive performance is well beyond the scope of this chapter and the expertise of its authors. We merely summarize experiments in which the separate influences of the two oscillatory processes on cognitive performance have been assessed by using multiple performance tasks. Aschoff and Wever already reported that performance is influenced by both the circadian and sleep-wake oscillator 83. Subsequent experiments have shown that many aspects of performance deteriorate with time awake and improve with sleep. These observations are in accordance with the view that sleep serves to recover from the wear and tear of wakefulness, which interferes with our ability to perform. These experiments have also shown that the circadian influence on cognitive performance is such that performance is poor during the circadian night and better during the circadian day. This basic rhythm of performance has been observed for all performance tests analyzed to date, although minor differences in the timing of peak performance may exist between specific performance tasks 29;44;88. This suggests that both the circadian oscillator and the sleep-homeostat modulate general determinants of cognitive performance, e.g. arousal, rather than more specific aspects such as executive function only. This view is also consistent with the observation that variables such as core body temperature, which not only display circadian and sleep-wake dependent variation, are correlated with variation in performance on a number of tasks, as first reported by Kleitman and more recently re-investigated and confirmed 84. It should, however, be emphasized that very few experiments have been designed to investigate specifically how, within a cognitive psychology context, the circadian pacemaker and the sleep-homeostat interact to affect performance. Noticeable exceptions are the experiments conducted by Horowitz 42 and Santhi 72.These latter experiments indicate that the interaction of high sleep pressure and circadian misalignment leads to decrements in selective attention, and reduced accuracy (fast but sloppy search), as assessed in the visual search task. Harrison and Jones 40 used a sustained attention-to-response task thought to depend heavily on frontal lobe function, to investigate the contribution of time awake and the circadian system to errors related to failure of response (errors of omission) and errors in automatic aspects of responding, related to failure of inhibitory responses, (errors of commission). Interestingly, they reported that both errors of commission and omission were modulated by time awake but not by circadian phase, although the interaction between these two factors was highly significant for errors of commission.
In an analysis of the effects of sleep deprivation and circadian phase misalignment on performance, we found a statistically reliable deterioration on 11 out of 16 performance measures, with no clear differential sensitivity between executive and non-executive tasks 38.
The impact of circadian rhythmicity and sleep homeostasis on aspects of waking function is not limited to performance. The mood of healthy volunteers, as well as patients suffering from SAD, exhibits both sleep-wake-dependent and robust circadian modulation. Mood deteriorates with time awake, recovers during sleep and, from a circadian perspective, is worse in the early morning and best in the evening hours 9;52.
The impact of circadian and sleep-wake-dependent processes on performance extends beyond laboratory conditions. Thus, a contribution of the sleep-homeostat and the circadian oscillator to alertness and mood has been observed in abnormally-entrained and free-running blind individuals while living in their normal environment 58. These data from the blind also show that the wake-dependent deterioration of mood and performance is not solely dependent on light exposure, but also appears to be associated with wakefulness itself. All of the findings summarized thus far have emphasized the effects of the two oscillatory processes on acute aspects of waking performance. There is a rapidly growing interest in the role of sleep and circadian rhythms in the regulation of longer lasting aspects of performance, i.e. learning, memory and plasticity. Practice effects, i.e. the slow long-term improvements of performance that can be observed in throughput tasks such as the Digit Symbol Substitution and Addition task, are diminished when the phase relationship between the sleep-wake cycle and circadian rhythmicity is disrupted 85. Whether these differences are related to the sleep disruption or circadian misalignment, cannot be determined from the available data. Implementation of an implicit procedural memory task in experiments designed to elucidate the contribution of circadian rhythms and sleep to waking performance, has revealed that both circadian rhythmicity and sleep pressure modulate the improvement of the learned aspects of this task. Whether this improvement indeed reflects enhanced consolidation or just an improvement in observed performance cannot be deduced from the available data. In a separate line of research, evidence is accumulating that slow wave and sleep spindles are associated with consolidation of procedural and declarative tasks 43;74. In general, these experiments have emphasized the role of sleep rather than the role of circadian rhythmicity in learning, memory and plasticity, even though many aspects of sleep that are thought to be relevant to its memory consolidating effects, e.g. sleep spindles, are profoundly influenced by sleep homeostasis and circadian rhythmicity 27;32.
The data summarized in this section demonstrate that under conditions of normal entrainment, the phase relationship of the sleep-wake-dependent and circadian changes in performance are such that performance can be maintained throughout the waking episode, because the wake-dependent deterioration is countered by the circadian upswing. When wakefulness is extended into the biological night, performance deteriorates rapidly, because the wake-dependent decline is no longer opposed by the circadian arousal signal. The importance of sleep homeostasis and circadian rhythmicity is not limited to acute performance, but extends to mood as well as the long-term changes in performance associated with learning and memory. Next, we will discuss the nature of the interaction between the two processes, as well as their putative brain bases and neural correlates.
d. Interaction of Sleep Homeostasis and Circadian Rhythmicity: Observed Circadian Amplitude Depends on Homeostatic Sleep Pressure
The notion that the consolidation of sleep and waking performance are regulated by the sleep-homeostat and circadian rhythmicity is widely accepted. The two processes are, in general, thought to be independent: Conceptual and mathematical models have assumed that the two oscillatory processes shape sleep and waking performance in an additive manner 1. This implies that we can predict sleep propensity and waking performance at any given time by addition of the circadian and homeostatic process. This, in turn, leads to the prediction that the observed amplitude of the circadian modulation is independent of homeostatic sleep pressure, and suggests that the circadian and homeostatic process contribute independently to the variable of interest. There is now a wide range of observations that are at variance with such an additive contribution of the two processes. For example, the amplitude of the circadian rhythm of the propensity to wake up from sleep increases as sleep pressure dissipates. In the initial part of the sleep episode the circadian amplitude is small, i.e. we can sleep at all circadian phases. By contrast, at the end of the sleep episode, when homeostatic sleep pressure is low, the circadian amplitude of the propensity to wake up is very large: We can still maintain sleep consolidation at around the temperature nadir, but sleep becomes very much disrupted on its rising phase. This change in circadian amplitude has been observed for the duration of wakefulness 27 as well as both the frequency and duration of awakenings 28. The amplitude of the circadian rhythm of the propensity to initiate sleep also changes with sleep pressure 15;16.
One interpretation of this change in observed circadian amplitude is that increased homeostatic sleep pressure inhibits the circadian wake promoting signal and amplifies the circadian sleep promoting signal. Such an interaction between the circadian and homeostatic regulation will lead to more rapid transitions between the vigilance states. The amplitude of the circadian rhythm of EEG characteristics during sleep, such as sleep spindles and the density of Rapid Eye movements during sleep, also changes with homeostatic sleep pressure, such that its amplitude increases as homeostatic sleep pressure dissipates 49. Evidence for an interaction extends to the EEG during wakefulness, but now the observed amplitude increases as sleep pressure increases. This has been observed for delta and theta EEG activity but not alpha activity, the robust circadian amplitude of the latter variable is not affected by homeostatic sleep pressure 14.
The observed circadian amplitude for several aspects of waking performance also increases with homeostatic sleep pressure, such that the wake-dependent deterioration is minimal during the wake maintenance zone and most pronounced just after the core body temperature minimum 29 and a few hours after the melatonin maximum 88. This interaction has been observed for several measures on the psychomotor vigilance task, an addition task (See Figure 2), the digit symbol substitution task, the occurrence of slow eye movements and unintentional sleep onset during scheduled wake episodes. These interactions, which were not observed for the probed-recall memory task, were statistically reliable while living on a very long day (42.85 hour forced desynchrony with 28.57 hours of wakefulness) 86. The observed circadian amplitude of subjective sleepiness, as assessed by the Karolinska Sleepiness Scale, is very sensitive to changes in homeostatic sleep pressure and is already statistically reliable while living on a 20-h day 88.
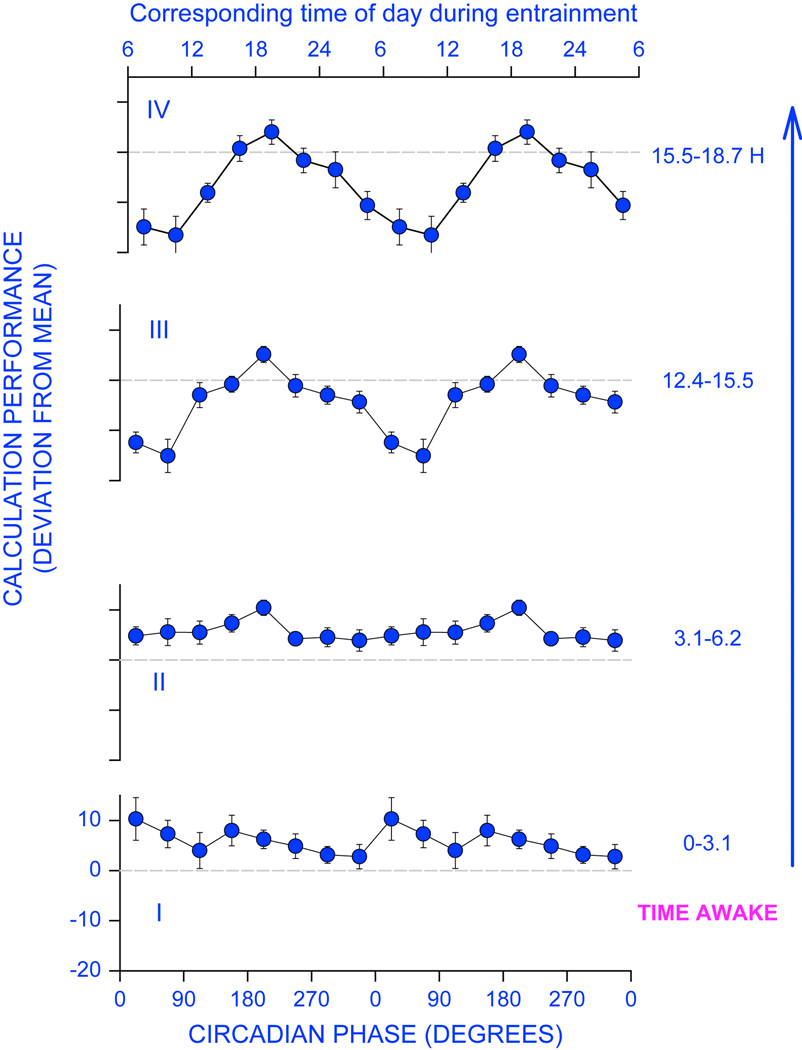
Fig 2.
Increase in the apparent circadian amplitude of calculation performance with increasing homeostatic sleep pressure. The circadian variation in performance was assessed while subjects were schedule to 28-h sleep-wake cycles in a forced desynchrony protocol, and data were segmented per quarter of the 18h:40 min scheduled wake episode. Whereas during the first quarter (0–3.1 hours awake), the circadian amplitude is very low, it increases progressively such that during the last quarter performance is very much impaired, in particular at and shortly after the nadir of the core body temperature rhythm (o degrees). Modified from 18;29.
Additional evidence for an interaction between the two oscillators stems from the observed modulation of the circadian period and amplitude of the melatonin rhythm by the sleep-wake cycle 13. Thus, even a hormone, the rhythm of which is driven through a multi-synaptic neural pathway from the SCN, is modulated by sleep-wake pressure, although these effects are relatively small 91. The implication of these observations is that sleep homeostasis and circadian rhythmicity are not independent. The amplitude of any observed circadian rhythm depends on the status of the sleep-homeostat.
The question then arises where in the central nervous system do the circadian and homeostatic oscillator meet and interact. Neuroanatomical and functional evidence suggests that output of the SCN reaches target areas such as the VLPO, TMN LH, thalamus and brain stem nuclei via the DMH 73. The diffuse activating systems serotonin, orexin, noradrenaline and histamine, which are all under circadian control, impinge on many areas including thalamic and cortical areas. This very much simplified neuroanatomical scheme of circadian modulation of the CNS already implies that the interaction with sleep homeostasis could take place at many different levels. In one scenario, for example, the circadian arousal signals a circadian rhythm of noradrenaline released from brain stem LC neurons 6, or serotonin from dorsal raphe nuclei, which impinge on cortical or thalamic networks to counter wake-dependent changes in these networks. The efficacy of these activating signals may be modified through adenosine, a putative mediator of homeostasis, or depend on the strength of local connectivity. Alternatively, homeostatic sleep pressure may modify the firing patterns of the LC, Dorsal Raphe, or other nuclei of the diffuse activating systems. Other areas in which interaction may occur are the VLPO and neurons synthesizing and releasing orexin. In fact, animal and human studies indicate that orexin is under both circadian and sleep-wake control 89;90 and SCN lesions indeed abolish the circadian rhythm of orexin 20;92.
Finally, evidence for feedback of the sleep-wake cycle and associated changes in SWA onto circadian rhythmicity has emerged at the level of multiple unit activity (MUA) of the SCN. This feedback concerns both acute changes in MUA in response to changes in SWA 21, as well as changes in the circadian amplitude of MUA in response to partial sleep deprivation 19. These observations show that the interaction of sleep homeostasis and circadian rhythmicity extends to an output that is very close to the core circadian oscillator. Whether sleep homeostasis indeed can modulate the amplitude of circadian rhythms at the level of clock gene expression and translation, which are thought to constitute the core of the circadian oscillations in humans, remains unknown, although animal studies have provided evidence for such interactions 60. Thus, there is now abundant evidence for an interaction of circadian rhythmicity at many different levels of description and many different areas in the brain. The discovery, that canonical clock genes are not only expressed in the loci of the circadian pacemaker but also in many other brain areas, adds another level of complexity with respect to the possible ways in which circadian rhythmicity and sleep homeostasis may interact.
Whatever the exact nature of locus of the interaction may be, one implication of the interaction is that differences in observed circadian amplitude may be related to differences in homeostatic sleep pressure.
4. Interindividual Differences in Circadian-Sleep Phenomenology and the Role of Clock Genes
Individuals differ with respect to the timing and duration of sleep, their preferred timing for waking activities including cognitive tasks, and their ability to maintain wakefulness and cognitive performance during sleep loss and circadian misalignment. Many of these characteristics have been shown to be trait-like and variations in circadian and sleep physiology and genetic makeup associated with these phenotypes are being investigated intensively.
a. Circadian correlates - physiology and clock gene expression
One line of investigation into circadian and sleep phenotypes is inspired by classical circadian entrainment theory, according to which the phase of a circadian pacemaker is determined by its intrinsic period (as well as light sensitivity and light exposure, which we will not discuss in this chapter). Variation in sleep-wake timing is predicted to be associated with variation in the phase of physiological markers of the circadian pacemaker, even when assessed in the absence of the sleep-wake cycle, i.e. constant routine conditions. These differences in phase are, in turn, predicted to be associated with differences in endogenous circadian period. The timing of the melatonin and core body temperature rhythms are earlier in morning types than evening types 35, and the endogenous period of these variables, as assessed during forced desynchrony, is shorter in the former group 33. The association between entrained phase and endogenous period is reliable in young individuals but not in older people. Associations between sleep-wake timing and circadian markers extend to the level of clock gene expression in vivo and in vitro. Many clock genes are rhythmically expressed in peripheral tissues and cells including peripheral blood cells in humans 5;10;56 (See Figure 3). In young adults, the timing of the rhythm of RNA expression of PER3 in leukocytes, as assessed under constant routine conditions, is associated with habitual sleep-wake timing, although this association is much weaker than the association with the melatonin rhythm 5. This association was not significant for the clock genes PER2 and BMAL1. The period of circadian gene expression, as monitored in vitro in fibroblast cell cultures through a luciferase reporter gene driven by the Bmal1 promoter, differs between morning and evening types 11. Morning types had an average period of 24.33 compared to 24.74 in evening types and entrained phase correlated with period length, although there was significant spread and overlap between the two sets of data. This implies that other factors could account for circadian behavioral variation. Differences in the amplitude of clock gene expression could affect phase of entrainment and the study showed that in individuals with normal circadian period, the morning subjects had low clock gene expression amplitude compared to high amplitude in the evening types, which also correlated with larger phase shifts in the morning types.
Fig 3.
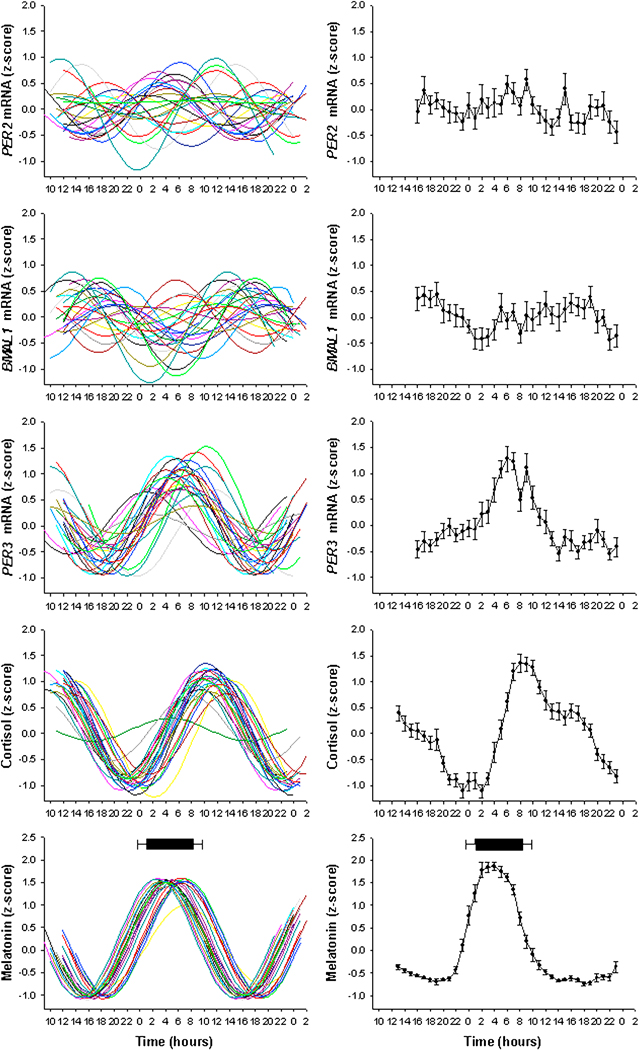
Individual and average oscillations in endocrine and mRNA markers of circadian rhythms assessed during constant routine conditions. Left Panel: z-scored normalized rhythms for PER2, BMAL1, PER3, melatonin and cortisol plotted relative to clock time. Right Panel: Average z-score curves (±SD). Mean sleep onset and wake times (±SD) are indicated by the black bar above the melatonin profile. With permission from 5.
The circadian oscillator has also been implicated in inter-individual differences in sleep duration. Short sleepers, have a shorter biological night, as indexed by the period of melatonin secretion, than long sleepers 3.
b. Homeostatic correlates
In another line of investigations, differences in sleep-wake timing and preference for the timing of waking activities are related to differences in the sleep-homeostat. It has long been known that morning types have more SWS and SWA, in particular in the beginning of the sleep episode, and a more rapid decline of SWA during sleep 48;57. This was recently confirmed in a series of analyses in which morning and evening types were subdivided into those who had an early or late circadian phase, and those who had a normal circadian phase, despite an extreme diurnal preference 62–64. These analyses have suggested that people may be morning types either because of an early circadian phase, or a more rapid build-up of homeostatic sleep pressure and associated changes in neural networks. In fact, it has been reported that during wakefulness, theta activity in the EEG increases more rapidly in morning types than evening types 76. Individual differences in SWS and SWA, primary markers of the sleep-homeostat, have been linked to polymorphisms in the Adenosine-2 receptor and adenosine deaminase 69, but whether these changes have functional consequences for waking performance remains to be established.
c. Amplitude correlates
Analyses of the association between diurnal preference, as assessed by the Horne-Östberg questionnaire 41, with patterns of clock gene expression in vitro have shown that part of the diurnal preference variation can be explained by differences in circadian period, but an approximately equal portion of the variance can be explained by differences in amplitude of clock gene expression 11. Young and older people differ with respect to their ability to perform when wakefulness is extended into the biological night. Whereas young people show a very strong deterioration of performance during the circadian night, the apparent circadian amplitude of this performance decrement is very much attenuated in older people 2. However, it should be noted that SWS and SWA are also very much reduced in older people. Because the observed circadian amplitude is a consequence of the interaction of homeostatic sleep pressure and circadian rhythmicity 27;29, it may very well be that differences in homeostasis underlie these age-related changes in circadian amplitude of performance deterioration.
d. Role of PER3 in the circadian and homeostatic regulation of sleep and cognition
The clock gene Per3 is thought to exert a statistically significant but minor impact on traditional circadian assays such as free-running period in animals 7;75. In humans, the role of this clock gene has been investigated by analyzing the association of polymorphisms in the gene with circadian and sleep phenotypes. To date polymorphisms in PER3 have been reported by a number of laboratories to be associated with diurnal preference and delayed sleep phase syndrome 4;36;67. We have reported that a primate-specific 81, variable number tandem repeat (VNTR) polymorphism in PER3, the allele frequency of which varies with ethnicity 65, exhibits a statistically significant but weak association with diurnal preference, as assessed with the Horne-Örtberg scales. Individuals homozygous for the longer, 5 repeat allele (PER35/5) are more likely to show morning preference than individual homozygous for the shorter, 4 repeat allele (PER34/4) 4 and the strength of this association declines with age 46. As we have seen above, diurnal preference is a complex phenotype and may be determined by differences in both circadian rhythmicity and sleep homeostasis, among other unidentified factors. To investigate the contribution of the VNTR PER3 polymorphism to circadian and sleep physiology, we conducted a prospective study in which individuals were selected only on the basis of their PER3 VNTR genotype. We next characterized sleep physiology, circadian physiology, and the effects of sleep loss and circadian misalignment on cognitive decline in subjects homozygous for the longer or shorter allele. No differences between the genotypes with respect to circadian physiological markers, such as the phase and amplitude of the melatonin and cortisol rhythm in plasma, or the phase and amplitude of clock gene expression in leukocytes, were observed. However, traditional markers of sleep homeostasis, i.e. SWA during sleep and the increase of theta activity during wakefulness and during sleep deprivation, as well as the autonomic control of heart rate during sleep 80 indicated a more rapid increase of homeostatic sleep pressure in PER35/579. This more rapid increase in homeostatic sleep pressure was associated with a more rapid decline in a composite measure of cognitive performance during the biological night, i.e. the apparent amplitude of the cognitive performance rhythm was much greater in PER35/5 than in PER34/4. (See Figure 4) Detailed analyses of the performance data showed that even though sleep loss and circadian phase misalignment affected many performance measures, the effects of genotype on deterioration of performance in the morning was particularly pronounced for measures of executive function, such as the verbal and spatial n-backs, paced visual serial addition tasks, and the delay in response on a serial addition task when a predictable sequence is replaced by a random sequence 38. This observation, when replicated, may suggest that clock genes could affect specific aspects of cognition, even though these specific aspects may also be mediated through effects on sleep homeostasis.
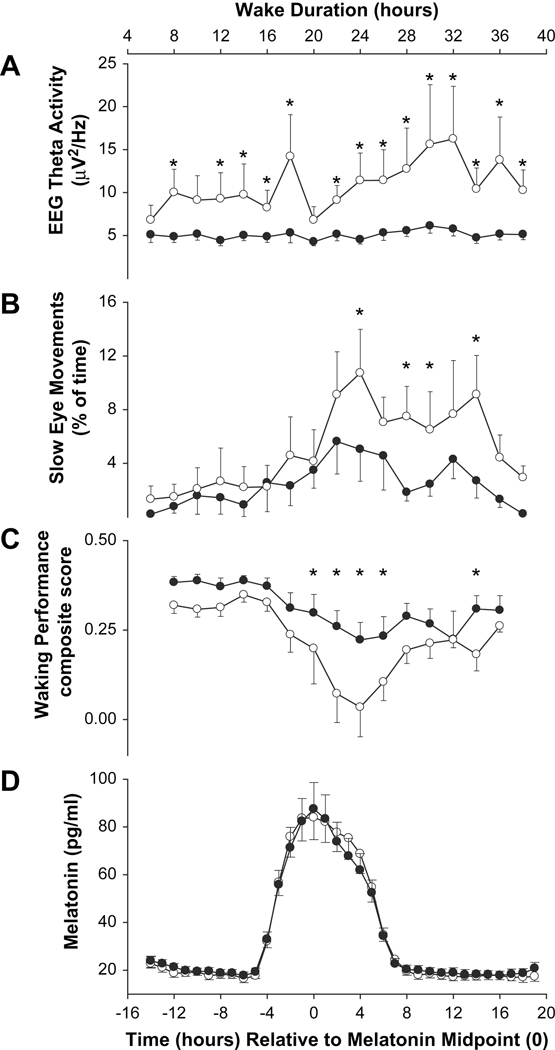
Fig 4.
Faster increase of homeostatic sleep pressure and more rapid deterioration of waking performance in PER35/5 (open symbols) than PER34/4 (filled symbols) during approximately 40 h of wakefulness. Homeostatic sleep pressure is assessed by increase of theta EEG activity and slow eye movements during wakefulness. Error bars represent SEMs. Data are plotted relative to the melatonin midpoint. With permission from 79.
e. Modeling the effects of PER3 on sleep homeostasis, circadian rhythmicity and the cognitive decline during sleep loss.
We developed a simple conceptual model to describe the effects of the VNTR polymorphism. In this model, it is assumed that the VNTR exerts its effect through effects on sleep homeostasis. The nature and mechanism of these effects are unknown, but could be related to effects on neural metabolisms that lead to more rapid changes in local connectivity in response to wakefulness in PER35/5, or alternatively reflect differences in activating influences on the EEG, which may appear as changes in sleep homeostasis. The time course and absolute values of SWA during baseline and recovery sleep are consistent with a more rapid buildup and decline of H in PER35/5 individuals 79, and this is reflected in the differential time course of process S (See Figure 5). Thus, during a normal sleep-wake cycle, the amplitude of the H oscillator is greater in PER35/5 than in PER34/4. The physiological and molecular circadian data are consistent with an identical amplitude and phase of a core circadian oscillator C, and we have, therefore, assumed Process C to be identical. Based on data from forced desynchrony protocols, we assume that this process C generates a wake-promoting signal and a sleep-promoting signal. The feedback of sleep homeostasis on the process implies that the amplitude of the wake and sleep promoting signals is modulated by H, such that the wake-promoting signal is inhibited by H and the sleep-promoting signal is amplified by H. As a consequence the amplitude of the C process modulated by H is greater in PER35/5 than in PER4/4. Performance is represented by the difference of this C*H process and H. Under these assumptions performance is near stable throughout a waking day in both PER35/5 and PER34/4, although minor differences between the two genotypes do emerge. Whereas in PER35/5 performance reaches its maximum just after awakening in the morning, in PER34/4 this maximum is reached in the evening hours, just prior to bed-time. When wakefulness is extended into the biological night, the differences between the two genotypes escalate. This is not because the difference in H becomes much greater between the two genotypes, but because of the interaction of H and C. The essence of the model is that a difference in the kinetics of a homeostatic process leads to differences in the negative effects of sleep loss in performance, in particular during the circadian morning. Thus, what is an apparent circadian phenotype derives from differences in sleep homeostasis.
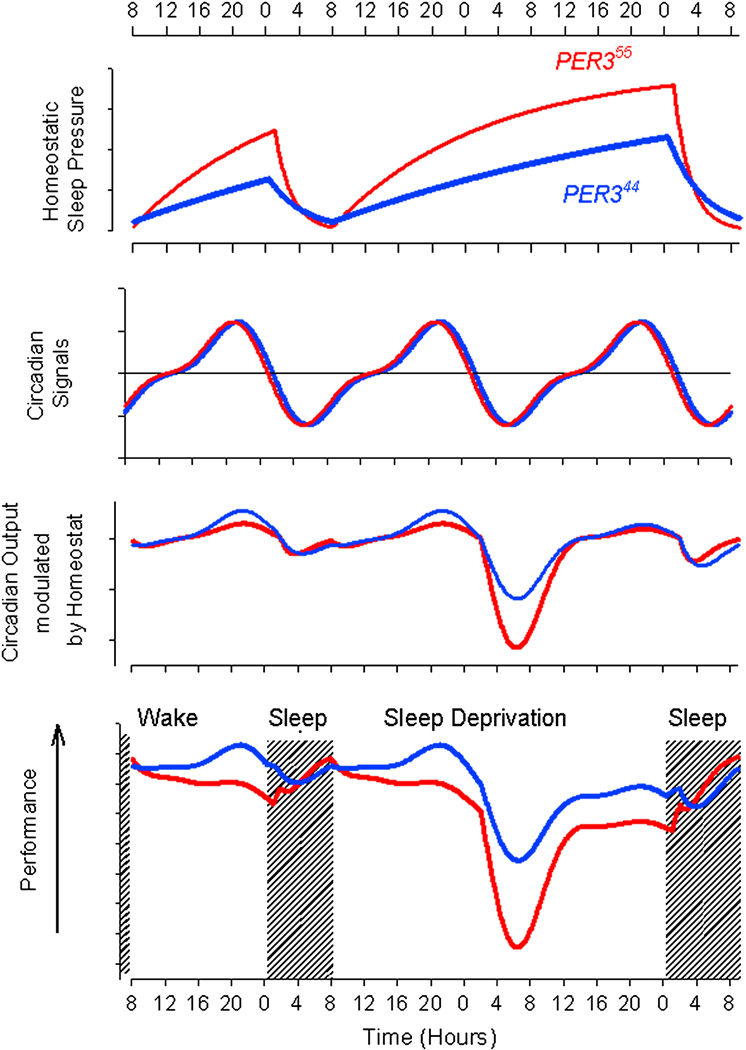
Fig 5.
Conceptual model for the regulation of sleep-wake and performance in PER35/5 and PER34/4. Top panel: A circadian signal promoting wakefulness (black) and sleep (red) does not differ in either phase or amplitude, between the genotypes. Second Panel: The homeostatic process S, increases during wakefulness and declines during S. The time constants of this process are shorter in PER35/5 than in PER34/4 and there the amplitude of the S oscillation during a normal sleep-wake cycle (left side of panel) is greater in PER35/5. During sleep deprivation (right side of panel) there is a prolonged increase in Process S followed by its return to baseline during recovery sleep.
Third Panel. The circadian process modulated by S. Please note that at the end of the waking day, the attenuation of the wake promoting signal by homeostatic sleep pressure is greater in PER35/5 than in PER34/4, during sleep deprivation, the sleep-promoting signal, which is maximal in the morning hours, is amplified by homeostatic sleep pressure and more so in PER35/5 than in PER34/4. Performance, which is a simple function of (C modulated by S) and S, is near stable during a normal waking day, although a small decline is observed in PER35/5 (typical for morning types) and in PER34/4 (typical for evening types) performance increases. During sleep deprivation performance is poorest in the early morning hours and in particular so in PER35/5. Please note the correspondence between this time course and the time course of performance in Fig 4.
A mutation in PER2 has been reported to affect the timing of sleep in members of a family afflicted with FASPS 77. To date, only effects on traditional circadian markers, i.e. phase and period 45, have been reported for this mutation and the available sleep physiology data are insufficient to warrant any conclusion with respect to effects on sleep homeostasis. However, animal experiments have shown that disruption to the clock genes Cry1, Cry2, Clock, Npas2, Bmal1 and Dbp all lead to effects on sleep physiology or EEG derived parameters of sleep homeostasis (reviewed in 31).
5. Conclusion
Maintenance of performance throughout consecutive waking episodes requires adequate alignment of a circadian arousal rhythm with the sleep-wake homeostat. The two systems interact dynamically and contribute to many aspects of sleep physiology and waking performance, including learning and memory. How aspects of sleep physiology and their circadian and homeostatic regulation relate to performance, remains largely unknown, although a role for slow-wave sleep is often implied. The close interaction between circadian rhythmicity and sleep homeostasis is underscored by the interdependence of circadian amplitude and homeostatic sleep pressure for many sleep and performance measures. Such a close interdependence is also consistent with the effects of clock genes on sleep homeostasis and the effects of the VNTR polymorphism in PER3 on markers of sleep homeostasis and cognitive decline in the early morning following sleep loss. Understanding the effects of these alterations in clock genes at the cellular level may provide insights into the nature of the sleep-homeostat and its interaction with circadian rhythmicity.
1. Synopsis.
Sleep physiology and waking performance are regulated through the interaction of an endogenous circadian process and a sleep-wake-dependent homeostatic process. The two processes are not independent: the observed circadian amplitude of waking performance depends on homeostatic sleep pressure, such that the negative effects of sleep loss are most pronounced in the early morning if homeostatic sleep pressure is high. Genes that are associated with circadian and/or sleep phenotypes in humans have been identified. The variable number tandem repeat polymorphism in PERIOD3, which is associated with morningness-eveningness, predicts inter-individual differences in cognitive decline in the early morning after sleep loss. This phenotype can be understood through the polymorphism’s effects on sleep homeostasis, which in turn leads to an apparent increase in the circadian amplitude of cognitive performance. These findings underscore the close interrelations between sleep, circadian rhythmicity and waking performance, and imply that some circadian phenotypes are related to changes in sleep regulatory processes. Understanding the effects of clock genes at the cellular and biochemical level may provide insights into the nature of the sleep-homeostat and its interaction with circadian rhythmicity in the regulation of waking performance.
Acknowledgements
Research of the authors and research summarized in this manuscript is supported by grants from the National Institutes of Health /NIA P01 AG09975; BBSRC (BB/F022883/1; BB/E003672/1, Wellcome Trust (069714/Z/02/Z & 0760667/Z04/Z) and AFOSR (FA9550-08-1-0080). Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the Office of Naval Research, The BBSRC or the Wellcome Trust.
We thank Wendy May for editorial assistance.
Reference List
- 1.Achermann P, Borbély AA. Simulation of daytime vigilance by the additive interaction of a homeostatic and a circadian process. Biol Cybern 1994;71115–121. [DOI] [PubMed] [Google Scholar]
- 2.Adam M, Retey JV, Khatami R, Landolt HP. Age-related changes in the time course of vigilant attention during 40 hours without sleep in men. Sleep 2006;29(1):55–57. [DOI] [PubMed] [Google Scholar]
- 3.Aeschbach D, Sher L, Postolache TT, Matthews JR, Jackson MA, Wehr TA. A longer biological night in long sleepers than in short sleepers. J Clin Endocrinol Metab 2003;88(1):26–30. [DOI] [PubMed] [Google Scholar]
- 4.Archer SN, Robilliard DL, Skene DJ, Smits M, Williams A, Arendt J et al. A length polymorphism in the circadian clock gene Per3 is linked to delayed sleep phase syndrome and extreme diurnal preference. Sleep 2003;26(4):413–415. [DOI] [PubMed] [Google Scholar]
- 5.Archer SN, Viola AU, Kyriakopoulou V, von SM, Dijk DJ. Inter-individual differences in habitual sleep timing and entrained phase of endogenous circadian rhythms of BMAL1, PER2 and PER3 mRNA in human leukocytes. Sleep 2008;31(5):608–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aston-Jones G, Chen S, Zhu Y, Oshinsky ML. A neural circuit for circadian regulation of arousal. Nature Neuroscience 2001;4(7):732–738. [DOI] [PubMed] [Google Scholar]
- 7.Bae K, Jin X, Maywood ES, Hastings MH, Reppert SM, Weaver DR. Differential functions of mPer1, mPer2, and mPer3 in the SCN circadian clock. Neuron 2001;30(2):525–536. [DOI] [PubMed] [Google Scholar]
- 8.Basheer R, Porkka-Heiskanen T, Strecker RE, Thakkar MM, McCarley RW. Adenosine as a biological signal mediating sleepiness following prolonged wakefulness. Biological Signals and Receptors 2000;9(6):319–327. [DOI] [PubMed] [Google Scholar]
- 9.Boivin DB, Czeisler CA, Dijk D-J, Duffy JF, Folkard S, Minors DS et al. Complex interaction of the sleep-wake cycle and circadian phase modulates mood in healthy subjects. Arch Gen Psychiatry 1997;54145–152. [DOI] [PubMed] [Google Scholar]
- 10.Boivin DB, James FO, Wu A, Cho-Park PF, Xiong H, Sun ZS. Circadian clock genes oscillate in human peripheral blood mononuclear cells. Blood 2003;102(12):4143–4145. [DOI] [PubMed] [Google Scholar]
- 11.Brown SA, Kunz D, Dumas A, Westermark PO, Vanselow K, Tilmann-Wahnschaffe A et al. Molecular insights into human daily behavior. Proc Natl Acad Sci U S A 2008;105(5):1602–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cajochen C, Foy R, Dijk D-J. Frontal predominance of relative increase in sleep delta and theta EEG activity after sleep loss in humans. Sleep Research Online 1999;2(3):65–69. [PubMed] [Google Scholar]
- 13.Cajochen C, Jewett ME, Dijk DJ. Human circadian melatonin rhythm phase delay during a fixed sleep-wake schedule interspersed with nights of sleep deprivation. J Pineal Res 2003;35(3):149–157. [DOI] [PubMed] [Google Scholar]
- 14.Cajochen C, Wyatt JK, Czeisler CA, Dijk D-J. Separation of circadian and wake duration-dependent modulation of EEG activation during wakefulness. Neuroscience 2002;114(4):1047–1060. [DOI] [PubMed] [Google Scholar]
- 15.Carskadon MA, Acebo C. Regulation of sleepiness in adolescents: update, insights, and speculation. Sleep 2002;25(6):606–614. [DOI] [PubMed] [Google Scholar]
- 16.Carskadon MA, Acebo C, Jenni OG. Regulation of adolescent sleep: implications for behavior. Ann N Y Acad Sci 2004;1021276–291. [DOI] [PubMed] [Google Scholar]
- 17.Czeisler CA, Dijk D-J. Human circadian physiology and sleep-wake regulation In: Takahashi JS, Turek FW, Moore RY editors. Handbook of Behavioral Neurobiology: Circadian Clocks. New York: Kluwer Academic/Plenum Publishing Co, 2001, 531–61 [Google Scholar]
- 18.Czeisler CA, Dijk D-J, Duffy JF. Entrained phase of the circadian pacemaker serves to stabilize alertness and performance throughout the habitual waking day In: Ogilvie RD, Harsh JR editors. Sleep Onset: Normal and Abnormal Processes. Washington, D.C.: American Psychological Association, 1994, 89–110 [Google Scholar]
- 19.Deboer T, Detari L, Meijer JH. Long term effects of sleep deprivation on the mammalian circadian pacemaker. Sleep 2007;30(3):257–262. [DOI] [PubMed] [Google Scholar]
- 20.Deboer T, Overeem S, Visser NA, Duindam H, Frolich M, Lammers GJ et al. Convergence of circadian and sleep regulatory mechanisms on hypocretin-1. Neuroscience 2004;129(3):727–732. [DOI] [PubMed] [Google Scholar]
- 21.Deboer T, VanSteensel MJ, Detari L, Meijer JH. Sleep states alter activity of suprachiasmatic nucleus neurons. Nat Neurosci 2003;6(10):1086–1090. [DOI] [PubMed] [Google Scholar]
- 22.Dijk DJ. Sleep of aging women and men: back to basics. Sleep 2006;29(1):12–13. [DOI] [PubMed] [Google Scholar]
- 23.Dijk DJ, Lockley SW. Invited Review: Integration of human sleep-wake regulation and circadian rhythmicity. J Appl Physiol 2002;92(2):852–862. [DOI] [PubMed] [Google Scholar]
- 24.Dijk DJ, von Schantz M. Timing and consolidation of human sleep, wakefulness, and performance by a symphony of oscillators. J Biol Rhythms 2005;20(4):279–290. [DOI] [PubMed] [Google Scholar]
- 25.Dijk D-J, Beersma DGM, Daan S. EEG power density during nap sleep: reflection of an hourglass measuring the duration of prior wakefulness. J Biol Rhythms 1987;2207–219. [DOI] [PubMed] [Google Scholar]
- 26.Dijk D-J, Czeisler CA. Paradoxical timing of the circadian rhythm of sleep propensity serves to consolidate sleep and wakefulness in humans. Neurosci Lett 1994;16663–68. [DOI] [PubMed] [Google Scholar]
- 27.Dijk D-J, Czeisler CA. Contribution of the circadian pacemaker and the sleep homeostat to sleep propensity, sleep structure, electroencephalographic slow waves and sleep spindle activity in humans. J Neurosci 1995;15(5):3526–3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dijk D-J, Duffy JF, Czeisler CA. Age-related increase in awakenings: impaired consolidation of nonREM sleep at all circadian phases. Sleep 2001;24(5):565–577. [DOI] [PubMed] [Google Scholar]
- 29.Dijk D-J, Duffy JF, Czeisler CA. Circadian and sleep/wake dependent aspects of subjective alertness and cognitive performance. J Sleep Res 1992;1112–117. [DOI] [PubMed] [Google Scholar]
- 30.Dijk D-J, Duffy JF, Riel E, Shanahan TL, Czeisler CA. Ageing and the circadian and homeostatic regulation of human sleep during forced desynchrony of rest, melatonin and temperature rhythms. J Physiol (Lond ) 1999;5162611–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dijk D-J, Franken P. Interaction of sleep homeostasis and circadian rhythmcity: dependent or independent systems In: Kryger MH, Roth T, Dement WC editors. Principles and Practice of Sleep Medicine, 4 edition Philadelphia: Elseviers Saunders, 2005, 418–34 [Google Scholar]
- 32.Dijk D-J, Shanahan TL, Duffy JF, Ronda JM, Czeisler CA. Variation of electroencephalographic activity during non-rapid eye movement and rapid eye movement sleep with phase of circadian melatonin rhythm in humans. J Physiol (Lond ) 1997;5053851–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duffy JF, Czeisler CA. Age-related change in the relationship between circadian period, circadian phase, and diurnal preference in humans. Neurosci Lett 2002;318(3):117–120. [DOI] [PubMed] [Google Scholar]
- 34.Duffy JF, Dijk D-J, Hall EF, Czeisler CA. Relationship of endogenous circadian melatonin and temperature rhythms to self-reported preference for morning or evening activity in young and older people. J Investig Med 1999;47141–150. [PMC free article] [PubMed] [Google Scholar]
- 35.Duffy JF, Rimmer DW, Czeisler CA. Association of intrinsic circadian period with morningness-eveningness, usual wake time, and circadian phase. Behav Neurosci 2001;115(4):895–899. [DOI] [PubMed] [Google Scholar]
- 36.Ebisawa T, Uchiyama M, Kajimura N, Mishima K, Kamei Y, Katoh M et al. Association of structural polymorphisms in the human period3 gene with delayed sleep phase syndrome. EMBO reports 2001;2(4):342–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Edgar DM, Dement WC, Fuller CA. Effect of SCN lesions on sleep in squirrel monkeys: Evidence for opponent processes in sleep-wake regulation. J Neurosci 1993;13(3)1065–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Groeger JA, Viola AU, Lo JC, von SM, Archer SN, Dijk DJ. Early morning executive functioning during sleep deprivation is compromised by a PERIOD3 polymorphism. Sleep 2008;31(8):1159–1167. [PMC free article] [PubMed] [Google Scholar]
- 39.Groeger JA, Zijlstra FR, Dijk DJ. Sleep quantity, sleep difficulties and their perceived consequences in a representative sample of some 2000 British adults. J Sleep Res 2004;13(4):359–371. [DOI] [PubMed] [Google Scholar]
- 40.Harrison Y, Jones K, Waterhouse J. The influence of time awake and circadian rhythm upon performance on a frontal lobe task. Neuropsychologia 2007;45(8):1966–1972. [DOI] [PubMed] [Google Scholar]
- 41.Horne JA, Östberg O. A self-assessment questionnaire to determine morningness- eveningness in human circadian rhythms. Int J Chronobiol 1976;497–110. [PubMed] [Google Scholar]
- 42.Horowitz TS, Cade BE, Wolfe JM, Czeisler CA. Searching night and day: a dissociation of effects of circadian phase and time awake on visual selective attention and vigilance. Psychol Sci 2003;14(6):549–557. [DOI] [PubMed] [Google Scholar]
- 43.Huber R, Ghilardi MF, Massimini M, Tononi G. Local sleep and learning. Nature 2004;430(6995):78–81. [DOI] [PubMed] [Google Scholar]
- 44.Johnson MP, Duffy JF, Dijk D-J, Ronda JM, Dyal CM, Czeisler CA. Short-term memory, alertness and performance: A reappraisal of their relationship to body temperature. J Sleep Res 1992;124–29. [DOI] [PubMed] [Google Scholar]
- 45.Jones CR, Campbell SS, Zone SE, Cooper F, DeSano A, Murphy PJ et al. Familial advanced sleep-phase syndrome: A short-period circadian rhythm variant in humans. Nature Medicine 1999;5(9):1062–1065. [DOI] [PubMed] [Google Scholar]
- 46.Jones KH, Ellis J, von SM, Skene DJ, Dijk DJ, Archer SN. Age-related change in the association between a polymorphism in the PER3 gene and preferred timing of sleep and waking activities. J Sleep Res 2007;16(1):12–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kattler H, Dijk D-J, Borbély AA. Effect of unilateral somatosensory stimulation prior to sleep on the sleep EEG in humans. J Sleep Res 1994;3159–164. [DOI] [PubMed] [Google Scholar]
- 48.Kerkhof GA, Lancel M. EEG slow wave activity, REM sleep, and rectal temperature during night and day sleep in morning-type and evening-type subjects. Psychophysiol 1991;28678–688. [DOI] [PubMed] [Google Scholar]
- 49.Khalsa SBS, Conroy DA, Duffy JF, Czeisler CA, Dijk DJ. Sleep- and circadian-dependent modulation of REM density. J Sleep Res 2002;11(1):53–59. [DOI] [PubMed] [Google Scholar]
- 50.Klerman EB, Dijk DJ. Age-related reduction in the maximal capacity for sleep--implications for insomnia. Curr Biol 2008;18(15):1118–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Knoblauch V, Martens WLJ, Wirz-Justice A, Krauchi K, Cajochen C. Regional differences in the circadian modulation of human sleep spindle characteristics. Eur J Neurosci 2003;18(1):155–163. [DOI] [PubMed] [Google Scholar]
- 52.Koorengevel KM, Beersma DG, Den Boer JA, van den Hoofdakker RH . Mood regulation in seasonal affective disorder patients and healthy controls studied in forced desynchrony. Psychiatry Res 2003;117(1):57–74. [DOI] [PubMed] [Google Scholar]
- 53.Koskenvuo M, Hublin C, Partinen M, Heikkila K, Kaprio J. Heritability of diurnal type: a nationwide study of 8753 adult twin pairs. J Sleep Res 2007;16(2):156–162. [DOI] [PubMed] [Google Scholar]
- 54.Krueger JM, Obal F. A neuronal group theory of sleep function. J Sleep Res 1993;2(2):63–69. [DOI] [PubMed] [Google Scholar]
- 55.Krueger JM, Obal F Jr. Sleep function. Front Biosci 2003;8d511-d519. [DOI] [PubMed] [Google Scholar]
- 56.Kusanagi H, Hida A, Satoh K, Echizenya M, Shimizu T, Pendergast JS et al. Expression profiles of 10 circadian clock genes in human peripheral blood mononuclear cells. Neurosci Res 2008;61(2):136–142. [DOI] [PubMed] [Google Scholar]
- 57.Lancel M, Kerkhof GA. Sleep structure and EEG power density in morning types and evening types during a simulated day and night shift. Physiol Behav 1991;491195–1201. [DOI] [PubMed] [Google Scholar]
- 58.Lockley SW, Dijk DJ, Kosti O, Skene DJ, Arendt J. Alertness, mood and performance rhythm disturbances associated with circadian sleep disorders in the blind. J Sleep Res 2008;17(2):207–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lockley SW, Skene DJ, Arendt J, Tabandeh H, Bird AC, Defrance R. Relationship between melatonin rhythms and visual loss in the blind. J Clin Endocrinol Metab 1997;82(11):3763–3770. [DOI] [PubMed] [Google Scholar]
- 60.Maret S, Dorsaz S, Gurcel L, Pradervand S, Petit B, Pfister C et al. Homer1a is a core brain molecular correlate of sleep loss. Proc Natl Acad Sci U S A 2007;104(50):20090–20095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mistlberger RE. Circadian regulation of sleep in mammals: role of the suprachiasmatic nucleus. Brain Res Brain Res Rev 2005;49(3):429–454. [DOI] [PubMed] [Google Scholar]
- 62.Mongrain V, Carrier J, Dumont M. Difference in sleep regulation between morning and evening circadian types as indexed by antero-posterior analyses of the sleep EEG. Eur J Neurosci 2006;23(2):497–504. [DOI] [PubMed] [Google Scholar]
- 63.Mongrain V, Carrier J, Dumont M. Circadian and homeostatic sleep regulation in morningness-eveningness. J Sleep Res 2006;15(2):162–166. [DOI] [PubMed] [Google Scholar]
- 64.Mongrain V, Lavoie S, Selmaoui B, Paquet J, Dumont M. Phase relationships between sleep-wake cycle and underlying circadian rhythms in Morningness-Eveningness. J Biol Rhythms 2004;19(3):248–257. [DOI] [PubMed] [Google Scholar]
- 65.Nadkarni NA, Weale ME, von Schantz M, Thomas MG. Evolution of a length polymorphism in the human PER3 gene, a component of the circadian system. J Biol Rhythms 2005;20(6):490–499. [DOI] [PubMed] [Google Scholar]
- 66.Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep 2004;27(7):1255–1273. [DOI] [PubMed] [Google Scholar]
- 67.Pereira DS, Tufik S, Louzada FM, edito-Silva AA, Lopez AR, Lemos NA et al. Association of the length polymorphism in the human Per3 gene with the delayed sleep-phase syndrome: does latitude have an influence upon it? Sleep 2005;28(1):29–32. [PubMed] [Google Scholar]
- 68.Reilly T, Atkinson G, Gregson W, Drust B, Forsyth J, Edwards B et al. Some chronobiological considerations related to physical exercise. Clin Ter 2006;157(3):249–264. [PubMed] [Google Scholar]
- 69.Retey JV, Adam M, Honegger E, Khatami R, Luhmann UF, Jung HH et al. A functional genetic variation of adenosine deaminase affects the duration and intensity of deep sleep in humans. Proc Natl Acad Sci U S A 2005;102(43):15676–15681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Roenneberg T, Kuehnle T, Pramstaller PP, Ricken J, Havel M, Guth A et al. A marker for the end of adolescence. Curr Biol 2004;14(24):R1038-R1039. [DOI] [PubMed] [Google Scholar]
- 71.Roenneberg T, Kumar CJ, Merrow M. The human circadian clock entrains to sun time. Curr Biol 2007;17(2):R44–R45. [DOI] [PubMed] [Google Scholar]
- 72.Santhi N, Horowitz TS, Duffy JF, Czeisler CA. Acute sleep deprivation and circadian misalignment associated with transition onto the first night of work impairs visual selective attention. PLoS ONE 2007;2(11):e1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature 2005;437(7063):1257–1263. [DOI] [PubMed] [Google Scholar]
- 74.Schmidt C, Peigneux P, Muto V, Schenkel M, Knoblauch V, Munch M et al. Encoding difficulty promotes postlearning changes in sleep spindle activity during napping. J Neurosci 2006;26(35):8976–8982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shearman LP, Jin X, Lee C, Reppert SM, Weaver DR. Targeted disruption of the mPer3 gene: subtle effects on circadian clock function. Mol Cell Biol 2000;20(17):6269–6275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Taillard J, Philip P, Coste O, Sagaspe P, Bioulac B. The circadian and homeostatic modulation of sleep pressure during wakefulness differs between morning and evening chronotypes. J Sleep Res 2003;12(4):275–282. [DOI] [PubMed] [Google Scholar]
- 77.Toh KL, Jones CR, He Y, Eide EJ, Hinz WA, Virshup DM et al. An hPer2 phosphorylation site mutation in familial advanced sleep phase syndrome. Science 2001;291(5506):1040–1043. [DOI] [PubMed] [Google Scholar]
- 78.Tononi G, Cirelli C. Sleep function and synaptic homeostasis. Sleep Medicine Reviews 2006;10(1):49–62. [DOI] [PubMed] [Google Scholar]
- 79.Viola AU, Archer SN, James LM, Groeger JA, Lo JC, Skene DJ et al. PER3 polymorphism predicts sleep structure and waking performance. Curr Biol 2007;17(7):613–618. [DOI] [PubMed] [Google Scholar]
- 80.Viola AU, James LM, Archer SN, Dijk DJ. PER3 polymorphism and cardiac autonomic control: effects of sleep debt and circadian phase. Am J Physiol Heart Circ Physiol 2008;295(5):H2156-H2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.von Schantz M, Jenkins A, Archer SN. Evolutionary history of the vertebrate period genes. J Mol Evol 2006;62(6):701–707. [DOI] [PubMed] [Google Scholar]
- 82.Wei HG, Riel E, Czeisler CA, Dijk D-J. Attenuated amplitude of circadian and sleep-dependent modulation of electroencephalographic sleep spindle characteristics in elderly human subjcts. Neurosci Lett 1999;26029–32. [DOI] [PubMed] [Google Scholar]
- 83.Wever RA.The circadian system of man: Results of experiments under temporal isolation. New York: Springer-Verlag, 1979, 1–276 [Google Scholar]
- 84.Wright KP Jr, Hull JT, Czeisler CA Relationship between alertness, performance, and body temperature in humans. Am J Physiol Regul Integr Comp Physiol 2002;283(6):R1370-R1377. [DOI] [PubMed] [Google Scholar]
- 85.Wright KP Jr, Hull JT, Hughes RJ, Ronda JM, Czeisler CA Sleep and wakefulness out of phase with internal biological time impairs learning in humans. J Cogn Neurosci 2006;18(4):508–521. [DOI] [PubMed] [Google Scholar]
- 86.Wyatt JK, Cajochen C, Ritz-De Cecco A, Czeisler CA, Dijk DJ. Low dose, repeated caffeine administration for circadian-phase dependent performance degradation during extended wakefulness. Sleep 2004;27(3):374–381. [DOI] [PubMed] [Google Scholar]
- 87.Wyatt JK, Dijk D-J, Czeisler CA. Sleep structure and neurobehavioral performance ina 20-HR forced desynchrony protocol: Sleep/ake homeostatic and circadian modualtion. Sleep Research. 1997. Ref Type: Abstract [Google Scholar]
- 88.Wyatt JK, Ritz-De Cecco A, Czeisler CA, Dijk D-J. Circadian temperature and melatonin rhythms, sleep, and neurobehavioral function in humans living on a 20-h day. Am J Physiol 1999;277R1152-R1163. [DOI] [PubMed] [Google Scholar]
- 89.Zeitzer JM, Buckmaster CL, Lyons DM, Mignot E. Locomotor-dependent and -independent components to hypocretin-1 (orexin A) regulation in sleep-wake consolidating monkeys. J Physiol 2004;557(Pt 3):1045–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zeitzer JM, Buckmaster CL, Parker KJ, Hauck CM, Lyons DM, Mignot E. Circadian and homeostatic regulation of hypocretin in a primate model: implications for the consolidation of wakefulness. J Neurosci 2003;23(8):3555–3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zeitzer JM, Duffy JF, Lockley SW, Dijk DJ, Czeisler CA. Plasma melatonin rhythms in young and older humans during sleep, sleep deprivation, and wake. Sleep 2007;30(11):1437–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang S, Zeitzer JM, Yoshida Y, Wisor JP, Nishino S, Edgar DM et al. Lesions of the suprachiasmatic nucleus eliminate the daily rhythm of hypocretin-1 release. Sleep 2004;27(4):619–627. [DOI] [PubMed] [Google Scholar]