ABSTRACT
Interleukin (IL)-9 is a pleiotropic cytokine, which can function as a positive or negative regulator of immune responses on multiple types of cells. The role of IL-9 was originally known in allergic disease and parasite infections. Interestingly, recent studies demonstrate its presence in the tumor tissues of mice and humans, and the association between IL-9 and tumor progression has been revisited following the discovery of T helper (Th) 9 cells. Tumor-specific Th9 cells are considered to be the main subset of CD4+ T cells that produce high level of IL-9 and exhibit an IL-9-dependent robust anti-cancer function in solid tumors. IL-9 exerts an unprecedented anti-tumor immunity not only by inducing innate and adaptive immune responses but also directly promoting apoptosis of tumor cells. The objective of this review is to summarize the latest advances regarding the anti-tumor mechanisms of IL-9 and Th9 cells.
KEYWORDS: Interleukin-9, T helper 9 cells, innate immune response, adaptive immune response, cancer immunotherapy
Introduction
The expression of cytokines influences the development of tumors in tumor micro-environment by at least two ways: cytokines affect tumor cells proliferation/apoptosis and drive anti-tumor immunity. Interleukin (IL)-9 is first identified for its function in autoimmune inflammations and allergic processes.1–3 Recently, its presence in the tumor bed of mice and humans has attracted much attention.4 IL-9 was originally identified as a T cell cytokine.5,6 As early as 1994, Renauld et al.7 found that IL-9 mediates proliferation of thymic lymphomas cell lines from in vitro study, and further indicated that dysregulated IL-9 is beneficial for the development of some T cell malignancies in vivo. Subsequently, IL-9 was certified to stimulate malignant T cell proliferation. Moreover, Fischer et al.8 observed increased expression level of IL-9 in the serum of Hodgkin’s lymphoma patients, suggesting that IL-9 might be essential for the growth of Hodgkin’s lymphoma. Although the aforesaid findings have shown that IL-9 is a cytokine with potential oncogenic activity in some hematological cancers, conversely, IL-9 is also shown to robustly kill tumor cells directly or exerts anti-tumor effects indirectly by inducing extraordinarily potent tumor-specific immune responses in recent studies.
It is known that IL-9 supports the growth of some T cell-derived hematological cancer cells. Interestingly, recent studies have observed that the effect of IL-9 on neoplasia may depend on different types of tumors. In most types of solid tumors, the blockage of IL-9/IL-9 receptor (IL-9R) resulted in a higher incidence or development of tumors, while exposed to exogenous recombinant IL-9 suppressed the growth of tumors.4 Notably, the association between IL-9 and tumor progression has been revisited following the discovery of T-helper (Th) 9 cells. Tumor-specific Th9 cells-mediated anti-tumor effects are highly dependent on IL-9 production by Th9 cells.9,10 Accordingly, IL-9 exerts a crucial anti-tumor effect not only acting on the growth of tumor cells directly, but also deriving both innate and robust adaptive immune responses, and further weakening the burden of tumor. In this review, we discuss the mechanism of IL-9 and Th9 cells in the anti-tumor function, which provides a powerful dynamic to explore the potential strategies for cytokine-based and cell-based immunotherapy in multiple types of tumors.
Overview of the IL-9
Description
IL-9 was first described as a T cell growth factor.5,6 The human and mouse Il9 gene is located on the long arm of chromosome 5 and chromosome 13, respectively.11,12 And human Il9 gene is located in the Th2 cytokine gene cluster.11 IL-9 is a 14kD glycoprotein, which has 144 amino acids and one signal peptide of 18 amino acids.12
IL-9 was originally categorized as a cytokine produced by Th2 cells.13 Recently, a novel IL-9-producing CD4+ Th9 cell subset is defined, which differentiated from naïve CD4+ T cells with transforming growth factor (TGF)-β and IL-4.14,15 Noticeably, Th9 cells are considered to be the main subset of CD4+ T cells that produce high level of IL-9 for an IL-9-dependent anti-cancer function in solid tumors. However, IL-9 is not specific to Th9 cells and also can be produced at a lower amount by innate immune cells such as mast cells and innate lymphoid cells type 2 as well as by Th2 cells, regulatory T (Treg) cells, and natural kill (NK) T cells.16–20 Notably, our study also suggested that naïve CD8+ T cells also can be differentiated into IL-9-producing CD8+ T (Tc9) cells under Th9 polarizing conditions.21
Function
IL-9 is a pleiotropic cytokine, which can function as a positive or negative regulator of immune responses on multiple types of cells.22 Previous studies have demonstrated that IL-9 plays an important role in various physiological processes, including allergic inflammatory processes, immunity against parasites and autoimmune diseases (Table 1).1–3 Recently, several studies have indicated that IL-9 also exerts immune regulation function in the development of tumors. Nevertheless, the effect of IL-9 on neoplasia may depend on different types of tumors. IL-9 has pro-tumor effects mainly in T cell-derived hematological cancer,23,24 which may due to IL-9 is a T cell growth factor. However, T9IL-33 cells, which is a population of IL-9-producing CD4+ and CD8+ T cells activated via the soluble stimulation 2 (ST2)-IL-33 pathway, have been shown to exert extraordinary anti-leukemic effects after adoptive cell transfer (ACT).25 Importantly, IL-9 exerts potent anti-tumor function in solid tumors, such as melanoma, lung cancer, colon cancer and breast cancers,4,9,10,26,27 suggesting its pleiotropic function in cancer settings (Table 1).
Table 1.
The functions of IL-9 in normal and tumor tissues
| Tissues | Functions | References | |
|---|---|---|---|
| Normal Tissues | Airway | Favors allergic inflammation, specifically in allergic asthma | Temann et al.1 |
| Intestinal | Immunity against Parasites | Licona-Limón et al.2 | |
| Tumor Tissues | Melanoma Lung cancer Colon cancer Breast cancer |
Anti-tumor | Lu et al.9 Lu et al.10 Purwar et al.4 Do Thi et al.26 You et al.27 |
| T-cell lymphomas | Pro-tumor | Qiu et al.23 Lavorgna et al.24 |
|
IL-9 modulation of anti-tumor immunity
IL-9 induces an innate-immune response
The recruitment of mast cells into solid tumor sites is associated with an improved prognosis in cancer. Several studies have highlighted that mast cells are essential for coordinating the anti-tumor events via targeting innate immunity following immunotherapies.28,29 In addition to T cells, IL-9 is also considered as a mast cell growth factor, which can promote mast cell activation and function.16 Purwar et al.4 showed that treatment with recombinant IL-9 could not inhibit the tumor growth in mast cells deficient mice, suggesting the presence of mast cells is essential for recombinant IL-9 exerted anti-tumor activity. Moreover, Abdul-Wahid et al.30 further found that IL-9 secreted by Th9 cells blocks the engraftment of cancer cells accompanied by activation of mast cells. The inhibition of mast cell degranulation could abolish the anti-tumor effect mediated by the vaccine-induced production of IL-9. Notably, IL-9 exerts an anti-tumor effect via targeting the activation and function of mast cells, which obtains direct cytotoxic activity and induces immune cells recruitment and activation against tumor cells (Figure 1). Therefore, activating mast cells is a potential mechanism for IL-9’s anti-tumor activity in vivo.
Figure 1.
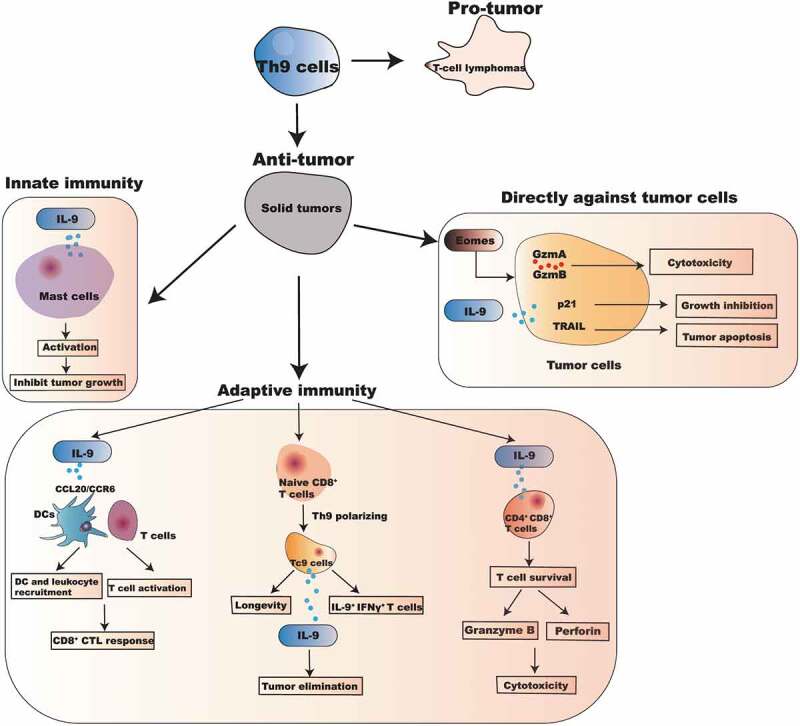
The pleiotropic mechanisms of IL-9 and Th9 cells in the anti-tumor function
IL-9 induces an adaptive anti-tumor immune response
While IL-9 triggers innate immune cells activation to prevent tumor growth, other studies definitely suggest that the anti-tumor activity of IL-9 is not only due to the activation of innate immune effectors. To further investigate the mechanisms of IL-9 developed anti-tumor immunity, Purwar et al.4 firstly injected melanoma cells into Rag1 deficient mice, which lack T and B cells. The results observed that the growth of melanoma is significantly inhibited in Rag1 deficient mice when exposed to recombinant IL-9, suggesting that T and B cells may not be required for the anti-tumor function of IL-9. However, our studies observed that host CD8+ T cells also could play a critical anti-tumor therapeutic role (Figure 1). IL-9 exerts anti-tumor effects, coinciding with a tremendously increased infiltration of tumor-reactive leukocyte subsets in tumor tissues, including CD4+ T cells and CD8+ T cells,9 suggesting adaptive immune response could also be derived by Th9/IL-9. The activation of CD8+ T cells is inhibited accompanied with reduced infiltration of leukocytes in IL-9 deficient mice.9 In line with our study, You et al.27 also demonstrated the expression of IL-9 is strikingly increased in CCR4− CCR6− CXCR3− CD4+ T cells of breast cancer patients and exerts anti-tumor effect by enhancing CD8+ T cell-mediated cytotoxicity.
Our study also demonstrates a potential mechanism on how Th9/IL-9 helped tumor-specific cytotoxic T lymphocyte (CTL) responses. While IL-9 may not directly act on CTL cells, it promotes the expression of co-stimulatory and major histocompatibility complex II molecules by enhancing DCs antigen presentation in vitro, which is crucial to the induction of anti-tumor immunity.31 We observed an IL-9-dependent induction of CCL20 and its receptor CCR6 production in lung tissue, which supports the recruitment of DCs and activated T cells into the tumor bed.9,32 This unique Th9 cell-induced expression of CCL20 bolsters the recruitment of CCR6+ DCs in an IL-9-dependent manner, ultimately derives robust CD8+ CTL-driven tumor destruction. Our study thus far highlights a new role of IL-9 in triggering of CD8+ CTL activation via provoking an inflammatory environment in tumor tissues (Figure 1). As a confirmation, Kim et al.33 also revealed that tumor necrosis factor receptor (TNFR)-related protein (GITR) agonistic antibody DTA-1-induced IL-9 expression strengthens functional maturation of tumor-specific CD8+ CTL responses through enhancing the function of DC in vivo.
In addition to CD8+ T cells, Parrot et al.34 found that CD4+CD8+ T cell is a new preferential target for IL-9 within the melanoma. IL-9 not only bolsters CD4+CD8+ double-positive T cells survival by enhancing apoptotic resistance and proliferation of these T cells, but also enhances cytotoxic properties of activated CD4+CD8+ T cells via secreting high levels of Granzyme B and Perforin (Figure 1). Finally, the production of tumor necrosis factor (TNF)-α and IL-13 were significantly increased in activated CD4+ CD8+ T cells in an IL-9-dependent manner.34
Finally, study from Do Thi et al.26 suggests that the ectopic expression of membrane-bound form of IL-9 (MB-IL-9) on CT26 colon cancer cells leads to a strong immune-stimulatory effect and further reduces tumorigenicity. In line with our observation, both CD4+ and CD8+ T cells are largely increased in splenocytes of MB-IL-9-CT26-bearing mice, suggesting the reduced tumorigenicity by MB-IL-9 is associated with tumor-specific T cell activation. Overall, the above results provided the rationale of using MB-IL-9 tumor clones as effective tumor vaccines.
IL-9’s direct effects on cancer cells
Fang et al.35 firstly described the direct anti-melanoma potential of IL-9 in human melanoma cell lines. Among several melanoma cell lines, IL-9 strongly inhibits HTB-72 and SK-Mel-5 melanoma cells growth and induces HTB-72 cells apoptosis in vitro. The growth and apoptosis of HTB-72 cells by IL-9 are associated with the upregulation of anti-proliferation molecule p21 and pro-apoptosis molecule TNF-related apoptosis-inducing ligand (TRAIL) in human melanoma (Figure 1). Furthermore, study from Miao et al.36 also highlights that specific Th9 cells induce the apoptosis of squamous cancer (SqC) cells possible because SqC cells express the IL-9R. Interestingly, adoptively transfer of SqC-specific Th9 cells in SqC-bearing mice shrinks tumors, which can be abolished with a neutralizing anti-IL-9 antibody. More recently, Chauhan et al.37 found that the number of PU.1+ Th9 cells and IL-9R were all significantly increased in human cervical cancer tissue. They indicated that IL-9 exerts anti-tumor effect through inhibiting proliferation and promoting apoptosis of cervical cancer cells (Figure 1). These results pinpoint that IL-9 may directly induce apoptosis of the IL-9R+ tumor cells in addition to their role of bolstering anti-tumor immunity.
Th9 as a new T cell paradigm to directly eliminate late-stage tumors
Recently, using mouse models of cancer, we9 and Zhao et al.38 have reported that IL-9-producing CD4+ Th9 cells promoted greater tumor clearance than the classic interferon (IFN)-γ-producing Th1 cells after adoptive transfer. However, the T cell features of Th9 cells beyond IL-9 production and whether these cells can be used to cure late-stage advanced tumors (a clinical-like scenario) have not been explored. Our recent studies showed that transfer of tumor-specific Th9 cells was able to exert greater anti-tumor responses against large established melanoma than Th1 and Th17 cells, and only Th9 cells could lead to a long-term tumor-free survival up to 300 days. The current advances in adoptive cell therapy suggest that the anti-tumor effector Th1 and Th17 cells represent two-T cell paradigms:39 “short-lived” cytolytic Th1 cells and “stem cell-like” memory Th17 cells. Unexpectedly, our results suggested that Th9 cells represent a novel third paradigm-they are less exhausted, fully cytolytic, and hyperproliferative. Indeed, Th9 cells exhibit cytolytic activity as strong as the classical cytolytic Th1 cells; but unlike polarized Th1 cells, they do not display an exhausted phenotype. Moreover, Th9 cells persisted as long as the so-called “stem cell-like” Th17 cells,10 but was driven by a unique Pu.1-Traf6-NF-κB signaling mediated hyperproliferative feature, which is not presented in other known CD4+ T cells subsets.
Utilizing tumor-specific Tc9 cells for adoptive cell therapy
Interestingly, IL-9 could not directly modulate CD8+ T cell cytotoxicity in vitro. They further found that blocking IL-9 resulted in a small but significant decrease in CD8+ T cell-mediated cytotoxicity, suggesting that IL-9 promotes CD8+ T cell response through at least in an indirect way.27 However, CD8+ T cells also can be differentiated into Tc9 cells under Th9 polarizing conditions.21 Our research also showed that Tc9 cells exert extraordinary anti-tumor responses depended on IL-9 production in vivo. Although type-I CD8+ cytotoxic T (Tc1) cells are essential for the established cancers treatment,40 Tc9 cells exhibit less cytolytic in vitro and persist longer in vivo than Tc1 cells. Strikingly, Tc9 cells produce higher IFN-γ and TNF-α, while Tc9-derived IL-9 is the key cytokine for Tc9 anti-tumor efficacy rather than IFN-γ. Therefore, these unique Tc9 cells are highly correlated with the improvement of CD8+ T cell-based adoptive immunotherapy.21
Transcription factors that control the anti-tumor activity of Th9 cells
Various studies have focused on the regulators of IL-9 transcription to identify the signaling pathways and transcriptional factors that dedicates Th9 cell differentiation and IL-9 production. The differentiation of Th9 cells is regulated by various transcription factors, such as interferon response factor (IRF), PU.1, sirtuin1 (SIRT1), inhibitor of DNA binding (Id) 3, NF-κB and retinoid-related orphan nuclear receptor γt (RORγt), which could promote the transcription of Il9 gene, thereby increasing Th9 cell differentiation.
IRF1/4/8 as positive regulators
Transcription factor IRF has a critical effect on immune response regulation and differentiation of Th9 cells. Among all the members of the IRF family, IRF1 promotes the effector function of Th9 cells through bolstering the expression of IL-9 from Th9 cells, and thus further enhances anti-tumor activity of Th9 cells.41 IRF4, another family member of IRF, is involved in the differentiation of Th9 cells. Study from Staudt et al.42 revealed that IRF4 controls IL-9 expression via binding to the promoter of Il9 to facilitate the development and function of IL-9-producing Th9 cells in asthma. Although IRF1 strengthens the activity of Il9 promoter with IRF4,41 the direct effect of IRF4 on the IL-9-producing Th9 cells remains unclear in tumors. IRF8, as an IRF4 homolog, is also essential for the Th9 lineage differentiation. Humblin et al.43 observed that mRNA and protein expression levels of IL-9 are significantly decreased in CD4+ T cells accompanied by enhanced tumor growth in Irf8-deficiency mice, suggesting that IRF8 is required for the anti-tumor effects of Th9 cells in vivo. Surprisingly, IRF8 does not activate the promoter of Il9, but function via interacting with IRF4, PU.1 and basic leucine zipper transcription factor, which significantly affects Il9 promoter activity and promotes the transcription of Il9.
NF-κB as a positive regulator
The transcription factor NF-κB has been reported to be crucial for IL-9 production.44 Kim et al.33 observed that DTA-1 induces IL-9 production by CD4+ T cells via NF-κB-tumor necrosis factor receptor-associated factor 6 (TRAF6) signaling pathway under Th9 polarizing conditions in various types of tumor models. Recently, our study demonstrated that RelA, which is a member of NF-κB family, could dramatically strengthen Il9 promoter transcriptional activity. IL-9 production can be suppressed by NF-κB pathway inhibitor or RelA small interfering RNA in Th9 cells.45 In addition, Fas signaling induces Th9 cells differentiation via activating NF-κB and further promote the anti-tumor activity of Th9 cells.46 Taken together, activation of NF-κB is essential for IL-9 production by Th9 cells in anti-tumor responses.
RORγt as a negative regulator
RORγt is known as a specific transcription factor for the development of Th17 cells.47 Interestingly, Purwar’s study suggests that RORγt promotes the growth of murine tumors in vivo.4 Unexpectedly, IL-9 production is significantly increased in Rorc-deficient CD4+ T cells. This result indicates that IL-9 contributes to the protective anti-tumor immunity in Rogc-deficient mice.
SIRT1 as a negative regulator
Histone deacetylase SIRT1 is a key inflammatory transcriptional regulator, which suppresses the inflammatory cytokines production by innate immune cells. Importantly, SIRT1 induces the differentiation of myeloid-derived suppressor cells with anti-tumor immunity.48,49 Recently, Wang et al.50 demonstrated that SIRT1 inhibits Th9 cell differentiation and the production of IL-9 in CD4+ T cells through negatively regulating mammalian target of rapamycin-hypoxia inducible factor-1α (mTOR-HIF1α) signaling to support the growth of tumor.
Id protein as a negative regulator
Id protein was originally identified as an inhibitor of E proteins, which is closely related to the development of various tumors.51 Among all members of Id family, Nakatsukasa et al.52 further showed that Id3 is a transcription factor to regulate Th9 cell differentiation from naïve CD4+ T cells. Importantly, Id3-deficiency enhanced anti-tumor immunity in melanoma-bearing mice, which is associated with the increased transcription and production of IL-9. These results revealed a new TAK1-Id3-E2A-GATA-3 signaling pathway in controlling the IL-9 production by Th9 cells for anti-tumor immunity.52
Th9/IL-9 signature favors response to immunotherapy in clinical studies
Treatment with Nivolumab is associated with a significant improvement in overall survival and progression-free survival, and 20% to 40% of patients obtain a long-term benefit.53,54 In a study conducted by Nonomura et al.,55 ~40% patients with advanced melanoma responded to Nivolumab (anti-PD-1 Abs). Of note, the increase in physiological Th9 cell counts during the treatment with Nivolumab has been reported to be associated with an improved clinical response for patients with metastatic melanoma. This observation highlights that the endogenous Th9 cell response might be a pharmacodynamic biomarker for Nivolumab-derived immunotherapy. Recently, Forget et al.56 also identified IL-9 as a potential pre-therapy predictive biomarker for response to adoptive TIL therapy in melanoma patients, and high IL-9 production is associated with the highest efficacy in adoptive TIL therapy. Therefore, utilizing IL-9 and related signaling have a broad prospect in clinical immunotherapy. The potential therapeutic strategies and product opportunities for the progress of Th9/IL-9 signature-based immunotherapies are shown in Table 2.
Table 2.
The potential therapeutic strategies and product opportunities for the progress of Th9/IL-9 signature-based immunotherapies
| Direction and Product | Application | Features | References |
|---|---|---|---|
| Cellular | Th9 cells for adoptive cell therapy | Exert anti-tumor effects by deriving robust CD8+ CTL response. | Lu et al.9 Kim et al.33 Zhao et al.38 |
| Exert greater anti-tumor responses against large established melanoma with exhibiting less exhausted, fully cytolytic and hyperproliferative. | Lu et al.10 | ||
| Directly effect on Sqc cells via inducing apoptosis of Sqc cells. | Miao et al.36 | ||
| Tc9 cells for adoptive cell therapy | Exert extraordinary anti-tumor through promoting CD8+ T cells response with exhibiting less cytolytic in vitro and persisting longer in vivo. | Lu et al.21 | |
| Allogeneic T9IL-33 cells for adoptive cell therapy | Increase IL-9 expression on T9 cells and further exhibit better anti-leukemic activity. | Ramadan et al.25 | |
| Protein | Exposed to exogenous recombinant IL-9 protein | Exerts anti-tumor activity through targeting the activation of mast cells. | Purwar et al.4 |
| Bolsters CD4+CD8+ double-positive T cells survival and enhances cytotoxic properties of activated CD4+CD8+ T cells. | Parrot et al.34 | ||
| Inhibits HTB-72 and SK-Mel-5 melanoma cells growth and induces HTB-72 cells apoptosis in vitro. | Fang et al.35 | ||
| Exerts anti-tumor effects through inhibiting proliferation and promoting apoptosis of cervical cancer cells. | Chauhan et al.37 | ||
| Treatment with Nivolumab (anti-PD-1 Abs) |
Favors melanoma-specific CD8+ T cell-mediated anti-tumor function. | Nonomura et al.,55 | |
| Transcription Factors | NF-κB | RelA (a member of NF-κB family) strengthens Il9 promoter transcriptional activity and further inhibits the growth of tumor. | Xue et al.45 |
| Fas signaling induces Th9 cells differentiation via activing NF-κB and thus enhances anti-tumor effect. | Shen et al.46 | ||
| RORγt | RORγt-deficiency leads to increased production of IL-9 for anti-tumor effects. | Purwar et al.4 | |
| SIRT1 | SIRT1 inhibitor enhances IL-9 production for anti-tumor effects. | Wang et al.50 | |
| Id3 | Id3-deficiency enhances anti-tumor immunity through inducing transcription and production of IL-9. | Nakatsukasa et al.52 |
The therapeutic implications for IL-9-based cancer immunotherapy in clinical
Although IL-9 may support the growth of some hematological cancers by promoting the proliferation of tumor cells, IL-9 has also been shown to support robust anti-tumor effects in tumor-bearing mice, based on the finding of these studies. In clinical, Purwar et al.4 showed that human memory Th9 cells could be detected in peripheral blood and skin of healthy human. Specially, the abundance of Th9 cells and IL-9 are significantly lower in the tumor-infiltrating lymphocytes (TIL) of melanoma lesions than the healthy blood and skin, reinforcing the relevance of IL-9 producing Th9 cells and melanoma in clinical immunotherapy. Nonomura et al.55 demonstrated that Th9 cells favor melanoma-specific CD8+ T cell-mediated anti-tumor function in patients treated with Nibolumab. Subsequently, You et al.27 further found that IL-9 and IL-9-expressing Th9 cells are strikingly increased in peripheral blood and circulating CD4+ T cells of breast cancer patients and exert anti-tumor effect by enhancing CD8+ T cell-mediated cytotoxicity. Recent study showed that Th9 cell counts significantly increased in human cervical cancer tissue, and IL-9 exerts anti-tumor immunity through effect on cervical cancer cells directly.37 Therefore, the above researches suggest that IL-9-based cancer immunotherapy has potential therapeutic implications in clinical (Table 3).
Table 3.
The therapeutic implications for IL-9-based cancer immunotherapy in clinical
| Years | Type of tumor | Tissues | Functions of Th9/IL-9 signature |
|---|---|---|---|
| 2012 | Melanoma | TIL | The decrease in tumor-infiltrating Th9 cells is associated with higher tumor development. |
| 2016 | CD8+ T cells | Th9 cells favor melanoma-specific CD8+ T cell-mediated anti-tumor function in patients treated with Nibolumab. | |
| 2017 | Breast cancer | Peripheral blood and circulating CD4+ T cells | Enhances CD8+ T cell-mediated cytotoxicity. |
| 2019 | Cervical cancer | Cervical | Inhibits proliferation and promote apoptosis of cervical cancer cells |
Prospects and challenges
As a pleiotropic cytokine, IL-9 exerts anti-tumor activity not only by bolstering tumor-specific immune responses, but also by directly inducing apoptosis of IL-9R+ cancer cells. It is well known that IL-9R can act through the IL-9/IL-9R axis. While Miao et al.36 reports that tumor-specific Th9 cells could induce the apoptosis of IL-9R+ SqC cells, our unpublished data suggests that majority of human and murine solid tumor cell lines and primary tumor cells do not express IL-9R, including breast cancer, lung cancer, pancreatic cancer, melanoma, ovarian cancer, head and neck cancer and colon cancer. Therefore, the downstream signals of IL-9 cannot be activated in these cancer cells which do not express IL-9R. However, the expression of IL-9R can be found in almost types of hematological malignancies especially in adult T-cell leukemia.57–59 Notably, only the proliferation of human T-cell acute lymphocytic leukemia and T-cell leukemia could be stimulated by IL-9.60 Comprehending the relation between IL-9/IL-9R and tumor cells may help develop novel strategies for the progress of Th9/IL-9-based immunotherapy.
Secondly, it is worth noting that various immune cells also produce IL-9. We have demonstrated that tumor-specific Th9 cells-mediated anti-tumor effects are highly dependent on IL-9 production by Th9 cells in murine tumor models of adoptive cell therapy.9,10 However, the unique maker(s) for Th9 cells in cancer patient settings remain to be identified. In human cancer tissues and peripheral organs, IL-9 may not be produced by Th9 cells, but instead, produced by immunosuppressive Th2 or Treg cells. The association of IL-9 to patient prognosis may be, in fact, a reflection of the role of Th2 or Treg cells in human cancers. Therefore, identification of more specific Th9 biomarkers in addition to IL-9 will facilitate our understanding of Th9 cells in human cancers.
As acclimating evidence that highlights the anti-tumor effects of Th9/IL-9 signature in various preclinical solid tumors, clinical studies have also confirmed the potential benefits of Th9-induction in the context of cancer immunotherapy strategies. We have recently characterized tumor-specific Th9 cells as a novel-third T cell paradigm for adoptive cell therapy-they are less exhausted, fully cytolytic, and hyperproliferative in the clinical-like scenario of ACT. We also believe that Th9 cells are endowed with the most critical T features required for long-lasting responses for cancer patients. The current advances in T cell receptor (TCR) and Chimeric antigen receptor (CAR) T technologies also enable us to target various tumors by TCR or CAR Th9 cells, and we are now working on the commercialization of human tumor-specific Th9 cells for adoptive cell therapy of cancer.
Funding Statement
This work was supported by grants from the National Cancer Institute (NCI, 4R00CA190910-03), the Elsa u. Pardee Foundation Award 2019, NCI P30 Administrative Supplement for Cell-Based Therapy (3P30CA012197-44S5), Daryl and Marguerite Errett Discovery Award 2020, ACS Research Scholar Grant (RSG-19-149-01-LIB), Wake Forest Baptist Comprehensive Cancer Center (WFBCCC) Push Pilot project and Wake Forest Start-up funds. Research reported in this publication was also supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR001420 (CTSI Pilot Grant Award 2018, CTSI Pilot Grant Award 2019, and CTSI Ignition Fund Pilot award).This study was also supported by the National Cancer Institute’s Cancer Center Support Grant award number P30CA012197 issued to the Wake Forest Baptist Comprehensive Cancer Center. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute.
Disclosure of potential conflicts of interest
There is no conflict of interest regarding the publication of this article.
References
- 1.Temann UA, Laouar Y, Eynon EE, Homer R, Flavell RA.. IL9 leads to airway inflammation by inducing IL13 expression in airway epithelial cells. Int Immunol. 2007;19:1–10. doi: 10.1093/intimm/dxl117. PMID: 17101709. [DOI] [PubMed] [Google Scholar]
- 2.Licona-Limón P, Arias-Rojas A, Olguín-Martínez E. IL-9 and Th9 in parasite immunity. Semin Immunopathol. 2017;39:29–38. doi: 10.1007/s00281-016-0606-9. PMID: 27900450. [DOI] [PubMed] [Google Scholar]
- 3.Yao W, Tepper RS, Kaplan MH. Predisposition to the development of IL-9-secreting T cells in atopic infants. J Allergy Clin Immunol. 2011;128:1357–60. doi: 10.1016/j.jaci.2011.06.019. PMID:21798577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Purwar R, Schlapbach C, Xiao S, Kang HS, Elyaman W, Jiang X, Jetten AM, Khoury SJ, Fuhlbrigge RC, Kuchroo VK, et al. Robust tumor immunity to melanoma mediated by interleukin-9-producing T cells. Nat Med. 2012;18:1248–53. PMID: 22772464. doi: 10.1038/nm.2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Uyttenhove C, Simpson RJ, Van Snick J. Functional and structural characterization of P40, a mouse glycoprotein with T-cell growth factor activity. Proc Natl Acad Sci U S A. 1988;85:6934–38. doi: 10.1073/pnas.85.18.6934. PMID: 3137580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Snick J, Goethals A, Renauld JC, Van Roost E, Uyttenhove C, Rubira MR, Moritz RL, Simpson RJ. Cloning and characterization of a cDNA for a new mouse T cell growth factor (P40). J Exp Med. 1989;169:363–68. doi: 10.1084/jem.169.1.363. PMID: 2521242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Renauld JC, van der Lugt N, Vink A, van Roon M, Godfraind C, Warnier G, Merz H, Feller A, Berns A, Van Snick J. Thymic lymphomas in interleukin 9 transgenic mice. Oncogene. 1994;9:1327–32. PMID: 8152793. [PubMed] [Google Scholar]
- 8.Fischer M, Bijman M, Molin D, Cormont F, Uyttenhove C, van Snick J, Sundström C, Enblad G, Nilsson G. Increased serum levels of interleukin-9 correlate to negative prognostic factors in Hodgkin’s lymphoma. Leukemia. 2003;17:2513–16. doi: 10.1038/sj.leu.2403123. PMID: 14562126. [DOI] [PubMed] [Google Scholar]
- 9.Lu Y, Hong S, Li H, Park J, Hong B, Wang L, Zheng Y, Liu Z, Xu J, He J, et al. Th9 cells promote antitumor immune responses in vivo. J Clin Invest. 2012;122:4160–71. PMID: 23064366. doi: 10.1172/JCI65459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu Y, Wang Q, Xue G, Bi E, Ma X, Wang A, Qian J, Dong C, Yi Q. Th9 cells represent a unique subset of CD4+ T cells endowed with the ability to eradicate advanced tumors. Cancer Cell. 2018;33:1048–60. doi: 10.1016/j.ccell.2018.05.004. PMID: 29894691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nicolaides NC, Holroyd KJ, Ewart SL, Eleff SM, Kiser MB, Dragwa CR, Sullivan CD, Grasso L, Zhang LY, Messler CJ, et al. Interleukin 9: a candidate gene for asthma. Proc Natl Acad Sci U S A. 1997;94:13175–80. PMID: 9371819. doi: 10.1073/pnas.94.24.13175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mock BA, Krall M, Kozak CA, Nesbitt MN, McBride OW, Renauld JC, Van Snick J. IL9 maps to mouse chromosome 13 and human chromosome 5. Immunogenetics. 1990;31:265–70. doi: 10.1007/bf00204898. PMID: 1970335. [DOI] [PubMed] [Google Scholar]
- 13.Veldhoen M, Uyttenhove C, van Snick J, Helmby H, Westendorf A, Buer J, Martin B, Wilhelm C, Stockinger B. Transforming growth factor-β ‘reprograms’ the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nat Immunol. 2008;9:1341–46. doi: 10.1038/ni.1659. PMID: 18931678. [DOI] [PubMed] [Google Scholar]
- 14.Schmitt E, Germann T, Goedert S, Hoehn P, Huels C, Koelsch S, Kühn R, Müller W, Palm N, Rüde E. IL-9 production of naive CD4+ T cells depends on IL-2, is synergistically enhanced by a combination of TGF-beta and IL-4, and is inhibited by IFN-gamma. J Immunol. 1994;153:3989–96. PMID: 7930607. [PubMed] [Google Scholar]
- 15.Dardalhon V, Awasthi A, Kwon H, Galileos G, Gao W, Sobel RA, Mitsdoerffer M, Strom TB, Elyaman W, Ho IC, et al. IL-4 inhibits TGF-β-induced Foxp3+ T cells and, together with TGF-β, generates IL-9+ IL-10+ Foxp3− effector T cells. Nat Immunol. 2008;9:1347–55. PMID: 18997793. doi: 10.1038/ni.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stassen M, Arnold M, Hültner L, Müller C, Neudörfl C, Reineke T, Schmitt E. Murine bone marrow-derived mast cells as potent producers of IL-9: costimulatory function of IL-10 and kit ligand in the presence of IL-1. J Immunol. 2000;164:5549–55. doi: 10.4049/jimmunol.164.11.5549. PMID: 10820228. [DOI] [PubMed] [Google Scholar]
- 17.Roediger B, Weninger W. Group 2 innate lymphoid cells in the regulation of immune responses. Adv Immunol. 2015;125:111–54. doi: 10.1016/bs.ai.2014.09.004. PMID: 25591466. [DOI] [PubMed] [Google Scholar]
- 18.Lu LF, Lind EF, Gondek DC, Bennett KA, Gleeson MW, Pino-Lagos K, Scott ZA, Coyle AJ, Reed JL, Van Snick J, et al. Mast cells are essential intermediaries in regulatory T-cell tolerance. Nature. 2006;442:997–1002. PMID: 16921386. doi: 10.1038/nature05010. [DOI] [PubMed] [Google Scholar]
- 19.Lauwerys BR, Garot N, Renauld JC, Houssiau FA. Cytokine production and killer activity of NK/T-NK cells derived with IL-2, IL-15, or the combination of IL-12 and IL-18. J Immunol. 2000;165:1847–53. doi: 10.4049/jimmunol.165.4.1847. PMID: 10925263. [DOI] [PubMed] [Google Scholar]
- 20.Noelle RJ, Nowak EC. Cellular sources and immune functions of interleukin-9. Nat Rev Immunol. 2010;10:683–87. doi: 10.1038/nri2848. PMID: 20847745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu Y, Hong B, Li H, Zheng Y, Zhang M, Wang S, Qian J, Yi Q. Tumor-specific IL-9-producing CD8+ Tc9 cells are superior effector than type-I cytotoxic Tc1 cells for adoptive immunotherapy of cancers. Proc Natl Acad Sci U S A. 2014;111:2265–70. doi: 10.1073/pnas.1317431111. PMID: 24469818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goswami R, Kaplan MH. A brief history of IL-9. J Immunol. 2011;186:3283–88. doi: 10.4049/jimmunol.1003049. PMID: 21368237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qiu L, Lai R, Lin Q, Lau E, Thomazy DM, Calame D, Ford RJ, Kwak LW, Kirken RA, Amin HM. Autocrine release of interleukin-9 promotes Jak3-dependent survival of ALK+ anaplastic large-cell lymphoma cells. Blood. 2006;108:2407–15. doi: 10.1182/blood-2006-04-020305. PMID: 16763206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lavorgna A, Matsuoka M, Harhaj EW. A critical role for IL-17RB signaling in HTLV-1 tax-induced NF-κB activation and T-cell transformation. PLoS Pathog. 2014;10:e1004418. doi: 10.1371/journal.ppat.1004418. PMID: 25340344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramadan A, Griesenauer B, Adom D, Kapur R, Hanenberg H, Liu C, Kaplan MH, Paczesny S. Specifically differentiated T cell subset promotes tumor immunity over fatal immunity. J Exp Med. 2017;214:3577–96. doi: 10.1084/jem.20170041. PMID: 29038366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Do Thi VA, Park SM, Lee H, Kim YS. Ectopically expressed membrane-bound form of IL-9 exerts immune-stimulatory effect on CT26 colon carcinoma cells. Immune Netw. 2018;18:e12. doi: 10.4110/in.2018.18.e12. PMID: 29503742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.You FP, Zhang J, Cui T, Zhu R, Lv CQ, Tang HT, Sun DW. Th9 cells promote antitumor immunity via IL-9 and IL-21 and demonstrate atypical cytokine expression in breast cancer. Int Immunopharmacol. 2017;52:163–67. doi: 10.1016/j.intimp.2017.08.031. PMID:28918288. [DOI] [PubMed] [Google Scholar]
- 28.Wang B, Li L, Liao Y, Li J, Yu X, Zhang Y, Xu J, Rao H, Chen S, Zhang L, et al. Mast cells expressing interleukin 17 in the muscularis propria predict a favorable prognosis in esophageal squamous cell carcinoma. Cancer Immunol Immunother. 2013;62:1575–85. PMID: 23912243. doi: 10.1007/s00262-013-1460-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oldford SA, Marshall JS. Mast cells as targets for immunotherapy of solid tumors. Mol Immunol. 2015;63:113–24. doi: 10.1016/j.molimm.2014.02.020. PMID: 24698842. [DOI] [PubMed] [Google Scholar]
- 30.Abdul-Wahid A, Cydzik M, Prodeus A, Alwash M, Stanojcic M, Thompson M, Huang EH, Shively JE, Gray-Owen SD, Gariépy J. Induction of antigen-specific TH9 immunity accompanied by mast cell activation blocks tumor cell engraftment. Int J Cancer. 2016;139:841–53. doi: 10.1002/ijc.30121. PMID: 27037842. [DOI] [PubMed] [Google Scholar]
- 31.Dabadghao S, Bergenbrant S, Anton D, He W, Holm G, Yi Q. Anti-idiotypic T-cell activation in multiple myeloma induced by M-component fragments presented by dendritic cells. Br J Haematol. 1998;100:647–54. doi: 10.1046/j.1365-2141.1998.00633.x. PMID: 9531329. [DOI] [PubMed] [Google Scholar]
- 32.Lu Y, Yi Q. Utilizing TH9 cells as a novel therapeutic strategy for malignancies. Oncoimmunology. 2013;2:e23084. doi: 10.4161/onci.23084. PMID: 23802062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim IK, Kim BS, Koh CH, Seok JW, Park JS, Shin KS, Bae EA, Lee GE, Jeon H, Cho J, et al. Glucocorticoid-induced tumor necrosis factor receptor-related protein co-stimulation facilitates tumor regression by inducing IL-9-producing helper T cells. Nat Med. 2015;21:1010–17. PMID: 26280119. doi: 10.1038/nm.3922. [DOI] [PubMed] [Google Scholar]
- 34.Parrot T, Allard M, Oger R, Benlalam H, Raingeard de la Blétière D, Coutolleau A, Preisser L, Desfrançois J, Khammari A, Dréno B, et al. IL-9 promotes the survival and function of human melanoma-infiltrating CD4+ CD8+ double-positive T cells. Eur J Immunol. 2016;46:1770–82. PMID: 27094152. doi: 10.1002/eji.201546061. [DOI] [PubMed] [Google Scholar]
- 35.Fang Y, Chen X, Bai Q, Qin C, Mohamud AO, Zhu Z, Ball TW, Ruth CM, Newcomer DR, Herrick EJ, et al. IL-9 inhibits HTB-72 melanoma cell growth through upregulation of p21 and TRAIL. J Surg Oncol. 2015;111:969–74. PMID: 25988864. doi: 10.1002/jso.23930. [DOI] [PubMed] [Google Scholar]
- 36.Miao BP, Zhang RS, Sun HJ, Yu YP, Chen T, Li LJ, Liu JQ, Liu J, Yu HQ, Zhang M, et al. Inhibition of squamous cancer growth in a mouse model by Staphylococcal enterotoxin B-triggered Th9 cell expansion. Cell Mol Immunol. 2017;14:371–79. PMID: 26388239. doi: 10.1038/cmi.2015.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chauhan SR, Singhal PG, Sharma U, Bandil K, Chakraborty K, Bharadwaj M. Th9 cytokines curb cervical cancer progression and immune evasion. Hum Immunol. 2019;pii:S0198-8859(18)31187-X. PMID: 31563404. doi: 10.1016/j.humimm.2019.09.009. [DOI] [PubMed] [Google Scholar]
- 38.Zhao Y, Chu X, J C, Wang Y, Gao S, Jiang Y, Zhu X, Tan G, W Z, Yi H, et al. Dectin-1-activated dendritic cells trigger potent antitumour immunity through the induction of Th9 cells. Nat Commun. 2016;7:12368. PMID: 27492902. doi: 10.1038/ncomms12368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muranski P, Borman ZA, Kerkar SP, Klebanoff CA, Ji Y, Sanchez-Perez L, Sukumar M, Reger RN, Yu Z, Kern SJ, et al. Th17 cells are long lived and retain a stem cell-like molecular signature. Immunity. 2011;35:972–85. PMID: 22177921. doi: 10.1016/j.immuni.2011.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat Rev Immunol. 2012;12:269–81. doi: 10.1038/nri3191. PMID: 22437939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Végran F, Berger H, Boidot R, Mignot G, Bruchard M, Dosset M, Chalmin F, Rébé C, Dérangère V, Ryffel B, et al. The transcription factor IRF1 dictates the IL-21-dependent anticancer functions of TH9 cells. Nat Immunol. 2014;15:758–66. PMID: 24973819. doi: 10.1038/ni.2925. [DOI] [PubMed] [Google Scholar]
- 42.Staudt V, Bothur E, Klein M, Lingnau K, Reuter S, Grebe N, Gerlitzki B, Hoffmann M, Ulges A, Taube C, et al. Interferon-regulatory factor 4 is essential for the developmental program of T helper 9 cells. Immunity. 2010;33:192–202. PMID: 20674401. doi: 10.1016/j.immuni.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 43.Humblin E, Thibaudin M, Chalmin F, Derangère V, Limagne E, Richard C, Flavell RA, Chevrier S, Ladoire S, Berger H, et al. IRF8-dependent molecular complexes control the Th9 transcriptional program. Nat Commun. 2017;8:2085. PMID: 29233972. doi: 10.1038/s41467-017-01070-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jash A, Sahoo A, Kim GC, Chae CS, Hwang JS, Kim JE, Im SH. Nuclear factor of activated T cells 1 (NFAT1)-induced permissive chromatin modification facilitates nuclear factor-κB (NF-κB)-mediated interleukin-9 (IL-9) transactivation. J Biol Chem. 2012;287:15445–57. doi: 10.1074/jbc.M112.340356. PMID: 22427656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xue G, Jin G, Fang J, Lu Y. IL-4 together with IL-1β induces antitumor Th9 cell differentiation in the absence of TGF-β signaling. Nat Commun. 2019;10:1376. doi: 10.1038/s41467-019-09401-9. PMID: 30914642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shen Y, Song Z, Lu X, Ma Z, Lu C, Zhang B, Chen Y, Duan M, Apetoh L, Li X, et al. Fas signaling-mediated TH9 cell differentiation favors bowel inflammation and antitumor functions. Nat Commun. 2019;10:2924. PMID: 31266950. doi: 10.1038/s41467-019-10889-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang XO, Pappu BP, Nurieva R, Akimzhanov A, Kang HS, Chung Y, Ma L, Shah B, Panopoulos AD, Schluns KS, et al. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR α and ROR γ. Immunity. 2008;28:29–39. PMID: 18164222. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang H, Bi Y, Xue L, Wang J, Lu Y, Zhang Z, Chen X, Chu Y, Yang R, Wang R, et al. Multifaceted modulation of SIRT1 in cancer and inflammation. Crit Rev Oncog. 2015;20:49–64. PMID: 25746104. doi: 10.1615/CritRevOncog.v20.i1-2. [DOI] [PubMed] [Google Scholar]
- 49.Liu G, Bi Y, Shen B, Yang H, Zhang Y, Wang X, Liu H, Lu Y, Liao J, Chen X, et al. SIRT1 limits the function and fate of myeloid-derived suppressor cells in tumors by orchestrating HIF-1α-dependent glycolysis. Cancer Res. 2014;74:727–37. PMID: 24351289. doi: 10.1158/0008-5472.CAN-13-2584. [DOI] [PubMed] [Google Scholar]
- 50.Wang Y, Bi Y, Chen X, Li C, Li Y, Zhang Z, Wang J, Lu Y, Yu Q, Su H, et al. Histone deacetylase SIRT1 negatively regulates the differentiation of interleukin-9-producing CD4+ T cells. Immunity. 2016;44:1337–49. PMID: 27317260. doi: 10.1016/j.immuni.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 51.Lasorella A, Benezra R, Iavarone A. The ID proteins: master regulators of cancer stem cells and tumour aggressiveness. Nat Rev Cancer. 2014;14:77–91. doi: 10.1038/nrc3638. PMID: 24442143. [DOI] [PubMed] [Google Scholar]
- 52.Nakatsukasa H, Zhang D, Maruyama T, Chen H, Cui K, Ishikawa M, Deng L, Zanvit P, Tu E, Jin W, et al. The DNA-binding inhibitor Id3 regulates IL-9 production in CD4+ T cells. Nat Immunol. 2015;16:1077–84. PMID: 26322481. doi: 10.1038/ni.3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, Hassel JC, Rutkowski P, McNeil C, Kalinka-Warzocha E, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320–30. PMID: 25399552. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 54.Topalian SL, Sznol M, McDermott DF, Kluger HM, Carvajal RD, Sharfman WH, Brahmer JR, Lawrence DP, Atkins MB, Powderly JD, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32:1020–30. PMID: 24590637. doi: 10.1200/JCO.2013.53.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nonomura Y, Otsuka A, Nakashima C, Seidel JA, Kitoh A, Dainichi T, Nakajima S, Sawada Y, Matsushita S, Aoki M, et al. Peripheral blood Th9 cells are a possible pharmacodynamics biomarker of nivolumab treatment efficacy in metastatic melanoma patients. Oncoimmunology. 2016;5:e1248327. doi: 10.1080/2162402X.2016.1248327. PMID: 28123885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Forget MA, Haymaker C, Hess KR, Meng YJ, Creasy C, Karpinets T, Fulbright OJ, Roszik J, Woodman SE, Kim YU, et al. Prospective analysis of adoptive TIL therapy in patients with metastatic melanoma: response, impact of anti-CTLA4, and biomarkers to predict clinical outcome. Clin Cancer Res. 2018;24:4416–28. PMID: 29848573. doi: 10.1158/1078-0432.CCR-17-3649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shang Y, Kakinuma S, Nishimura M, Kobayashi Y, Nagata K, Shimada Y. Interleukin-9 receptor gene is transcriptionally regulated by nucleolin in T-cell lymphoma cells. Mol Carcinog. 2012;51:619–27. doi: 10.1002/mc.20834. PMID: 21809393. [DOI] [PubMed] [Google Scholar]
- 58.Barata JT, Keenan TD, Silva A, Nadler LM, Boussiotis VA, Cardoso AA. Common gamma chain-signaling cytokines promote proliferation of T-cell acute lymphoblastic leukemia. Haematologica. 2004;89:1459–67. PMID: 15590396. [PubMed] [Google Scholar]
- 59.Baba H, Yamada Y, Mori N, Hayashibara T, Harasawa H, Tsuruda K, Sugahara K, Soda H, Takasaki Y, Tawara M. Multiple γc-receptor expression in adult T-cell leukemia. Eur J Haematol. 2002;68:362–69. doi: 10.1034/j.1600-0609.2002.00653.x. PMID: 12225394. [DOI] [PubMed] [Google Scholar]
- 60.Hoelzinger DB, Dominguez AL, Cohen PA, Gendler SJ. Inhibition of adaptive immunity by IL9 can be disrupted to achieve rapid T-cell sensitization and rejection of progressive tumor challenges. Cancer Res. 2014;74:6845–55. doi: 10.1158/0008-5472.CAN-14-0836. PMID: 25297635. [DOI] [PMC free article] [PubMed] [Google Scholar]


