Abstract
SARS-CoV-2 (COVID-19) is well known to have extrapulmonary manifestations, including acute renal failure. While radiologic findings of COVID-19 pulmonary-involvement have been described, renal findings associated with COVID-19 have not. We present a case of a 38-year-old Afro-Caribbean female diagnosed with COVID-19 whose renal ultrasound showed increased parenchymal echogenicity, decreased global color Doppler signal with elevated resistive indices, but no large vessel thrombi. Non-targeted renal biopsy demonstrated collapsing focal segmental glomerulosclerosis (FSGS), likely secondary to COVID-19 infection, which may be a specific manifestation of this disease that has been predominantly reported in Black patients. We report several findings on renal ultrasound with duplex Doppler not previously associated with COVID, specifically with FSGS, which in conjunction can be useful to both the radiologist and the clinician, potentially pointing them in the direction of this diagnosis and early treatment.
Keywords: COVID-19, Glomerulosclerosis, Focal segmental, Ultrasound
Highlights
-
•
Renal ultrasound provides benefit in cases of COVID-19 with acute kidney injury.
-
•
Collapsing focal segmental glomerulosclerosis can occur secondary to COVID-19.
-
•
This entity occurs nearly exclusively in Black patients.
-
•
Ultrasound may show echogenic kidneys, elevated resistive index, altered perfusion.
-
•
When these sonographic features are seen, clinicians should consider biopsy.
1. Introduction
While pulmonary manifestations of SARS-CoV-2 (COVID-19) are one of the most common presenting symptoms in affected patients and has been extensively described in the literature [[1], [2], [3]], COVID-19 also has significant extrapulmonary manifestations [4]. Renal failure is a relatively common extrapulmonary manifestation of COVID-19 with anywhere between 0.5 and 19% of patients expressing some degree of acute renal failure [5]. It is thought to be caused by the viral induced cytopathic effect within the proximal convoluted tubule cells of the kidney, resulting in nephrotic range proteinuria [6]. A case series of 26 patients in China demonstrated multiple post-mortem findings of intrinsic renal tubular disease, only two of which exhibited focal segmental glomerulosclerosis [7]. Recently, several case reports of surviving patients from outside of China showed that collapsing focal segmental glomerulosclerosis (FSGS) is a culprit of COVID-19 related renal failure, occurring predominantly in patients of African descent [[8], [9], [10], [11]].
This case contributes to the sparse existing literature regarding COVID-19 related collapsing FSGS in a living patient by adding radiologic findings related to the diagnosis, which has been limited in previous case reports. For patients with suspected acute kidney failure, ultrasound examination is a mainstay when imaging of acute or chronic renal failure is indicated, according to the ACR Appropriateness Criteria [12]. In our experience, imaging evaluation is often not needed in COVID-19 patients, as their renal function frequently improves with hydration. When indicated, ultrasound provides a quick, non-invasive assessment of renal cortical echogenicity, renal perfusion via Doppler imaging, and allows for obstructive uropathy to be quickly excluded, and this diagnostic examination should not be avoided due to COVID-19 diagnosis. Duplex Doppler imaging evaluates for large vessel clot in these patients who have contraindications to contrast enhanced CT, which is important given what is currently known about increased hypercoagulability in patients with COVID-19 [13,14]. As knowledge of complications from COVID-19 arise and become more prevalent, radiologists need to be aware of their associated imaging findings. These findings can lead to an appropriate use of non-targeted kidney biopsy versus conservative therapy while the patient remains infectious. The abnormal sonographic findings related to this case of FSGS are presented and correlated to the published pathologic, genetic, and epidemiologic findings.
2. Case report
A 38-year-old Afro-Caribbean female presented to the emergency department with a week of progressive dyspnea, cough, fatigue, nausea, and diarrhea. She denied any sick contacts or potential exposures to COVID-19. She had a past medical history significant for morbid obesity status post gastric bypass 15 years prior, well-controlled type two diabetes, and asthma. Her family history included a sister with end stage renal disease due to membranous nephritis diagnosed approximately 15 years prior, and a second sister with a recently diagnosed protein-losing enteropathy.
On presentation, she was afebrile with normal vital signs. Her exam was significant for bilateral respiratory crackles, 4–5 word conversational dyspnea, and the presence of oral thrush. Laboratory evaluation demonstrated an elevated creatinine level of 2.4 accompanied by a significantly low blood urea nitrogen level of 14 (baseline unknown, but had a serum creatinine of 1 approximately three years previously), suggestive of intrinsic renal abnormality, as opposed to the more commonly seen dehydration causes in COVID. In addition, she had microcytic anemia with a hemoglobin of 9.1 g/dL and mean corpuscular volume of 60.3 fL. She was lymphopenic with an absolute lymphocyte count of 0.59. Erythrocyte sedimentation rate (>130 mm/h), C-reactive protein(13.84 mg/dL), and lactate dehydrogenase (1170 unit/L) were all elevated. D-dimer was also high at 3.52 μg/mL, as was procalcitonin at 0.47 ng/mL. An initial chest radiograph was obtained which showed a patchy multifocal bilateral pneumonia, with atypical infections such as COVID-19 being within the differential (Fig. 1 ). An in-house performed COVID-19 PCR swab was obtained and subsequently returned as positive.
Fig. 1.
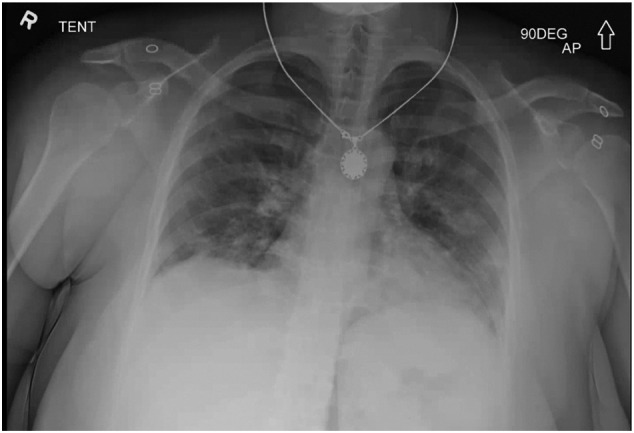
Portable AP chest radiograph demonstrating patchy bilateral parenchymal opacities, a pattern that can be seen in COVID-19 infection.
She was admitted to our hospitals' dedicated COVID-19 unit. The day after admission, her glomerular filtration rate continued to worsen significantly. A urinalysis was obtained and demonstrated >500 mg of protein; a follow-up 24-h urine protein demonstrated a nephrotic range proteinuria of 6.7 g. Her proteinuria was thought to be unrelated to her diabetes given her hemoglobin-A1-C of 6.0. Rheumatologic laboratory values were remarkable for only a weakly positive ANA (1:320 ratio) without any other abnormalities. Workup for HIV, Monospot, and hepatitis were also negative.
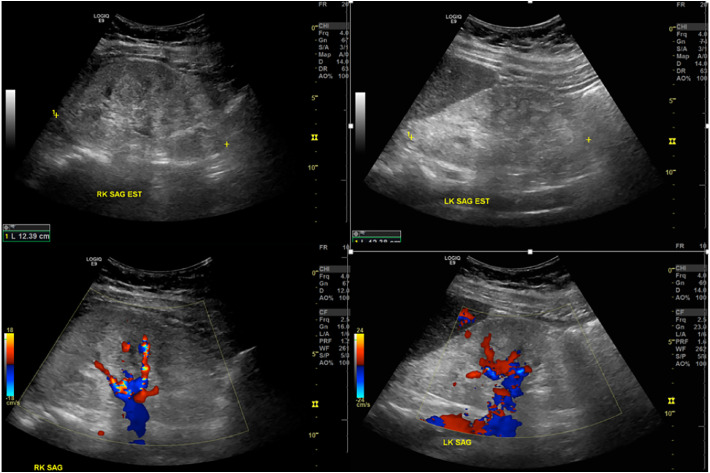
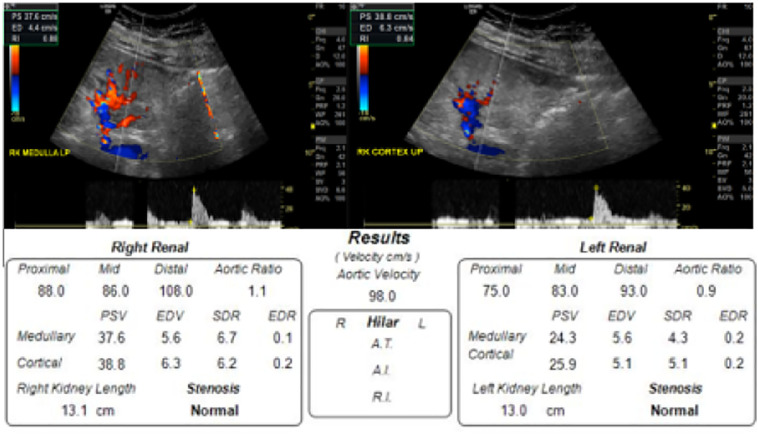
A renal ultrasound was performed to evaluate for intrinsic pathology, which demonstrated increased bilateral renal cortical echogenicity and loss of corticomedullary differentiation, along with globally diminished color Doppler flow to the parenchyma (Fig. 2 ). The kidneys were normal in size as was the cortical thickness. Duplex Doppler evaluation demonstrated patency of the major vessels with normal waveforms, however there were elevated resistive indices throughout the renal parenchyma, right side worse than left, and reduced diastolic velocities. The resistant flow was notable in both the renal arteries and parenchyma, accompanied by reduced diastolic flow velocities (Fig. 3 ).
Fig. 2.
Grayscale (above) and color Doppler (below) ultrasound images of both kidneys performed on a GE LogicQ E9. These show maintained size and cortical thickness, but increased echogenicity with loss of corticomedullary differentiation and globally decreased color Doppler flow to the parenchyma. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Fig. 3.
Example of duplex resistive indices obtained of the right kidney, with overall velocities obtained bilaterally.
Due to these sonographic and laboratory findings, the differential diagnosis remained intrinsic renal disease or renal microthrombi, as large vessel thrombus had been excluded. A non-targeted renal biopsy was requested as an inpatient and performed by an abdominal radiologist (images not shown) with pathology returning as collapsing FSGS. The pathologist noted that specifically, it was a collapsing glomerulopathy, which has been associated with both COVID-19 and HIV. The typical risk factors including HIV, autoimmune disease, and interferon therapy had been excluded, and given that the patient had several risk factors for development of this entity, the final diagnosis of FSGS was attributed to COVID-19 infection. No evidence of microthrombosis was seen. The patient was treated with supportive care both for her COVID-19 infection and for her FSGS-related renal failure, primarily with daily intravenous diuresis to maintain fluid balance, and ultimately did not require dialysis during her stay. The decision was made not to treat the patient's FSGS with glucocorticoids, because at the time, the infectious disease consult team recommended against patients with non-critical COVID receiving glucocorticoids. She was eventually discharged with improved dyspnea and cough, and improving but persistent laboratory signs of renal failure – her creatinine remained elevated at 3.16 (from a peak of 7.08) and blood urea nitrogen of 28, and she had increased urine output. In follow-up two weeks after discharge, her creatinine had improved to 2.4.
3. Discussion
To our knowledge, we have documented the sixth case in a living patient of collapsing focal segmental glomerulosclerosis that was likely related to or induced by COVID-19 infection, and the first to describe potentially associated imaging findings of this diagnosis in this population. Our patient has multiple sonographic findings that, while non-specific in isolation, were unusual in combination, and point towards an intrinsic renal tubular disease when seen in the setting of COVID-19 infection. While the findings are not specific enough to sonographically suggest FSGS, they can help guide management – renal function may not improve simply with hydration as standard treatment in pre-renal acute kidney injury, an obstructive process was excluded, and these findings may sway a clinician towards consideration of non-targeted renal biopsy earlier in the patients' hospital course. Radiologists who perform renal biopsies should be aware of these findings, and after discussion with the referring clinical team, the biopsy may be indicated to inform the patient's treatment plan. Glucocorticoids would be the first step in the treatment of biopsy-confirmed FSGS; however, currently the Infectious Disease Society of America (IDSA) only recommends glucocorticoids for hospitalized patients with severe or critical COVID-19. The IDSA has made the condition recommendation against using glucocorticoids in hospitalized patients with COVID-19 that is not severe or critical [15]. Patients who fall in that categorization, but have biopsy-proven FSGS, may benefit from glucocorticoids for renal function alone. Therefore, the management plan would be altered based on biopsy results and biopsy should be obtained while the patient is still infectious and not reflexively be delayed or avoided due to the patients COVID status.
Renal cortical echogenicity has long been used as a marker for intrinsic renal disease. The normal echogenicity of the renal cortex and medulla is equal to, or less than, that of normal hepatic or splenic tissue [16]. As demonstrated in Fig. 2, in our patient the kidneys were quite hyperechoic compared to the liver and splenic parenchyma. Fibrous tissue, which can be seen in renal injury, is echogenic as it reflects more echoes back to the ultrasound transducer than does normal parenchyma [17]. Both acute and chronic renal injury can have this pattern of increased cortical echogenicity related to fibrous/inflammatory reaction [16], however chronic renal failure is often accompanied by cortical thinning, which was absent in our patient. In addition, the rapidity with which her renal failure worsened and then improved, which mirrored the course of her pulmonary COVID symptoms, pointed to a more acute cause for her renal dysfunction. HIV-associated nephropathy can also demonstrate increased echogenicity, with decreased corticomedullary differentiation and may have a normal or enlarged kidney size [18]. The sonographic appearance of the kidneys in our patient exhibited many overlapping features with HIV nephropathy, and especially in the setting of COVID-19, HIV infection must be excluded, as was in our patient. Renal vein thrombus can also show the associated abnormality of increased echogenicity accompanied by enlargement, however color and duplex Doppler evaluation can exclude this, as in our patient [19].
Increased renal parenchymal resistive indices were the second finding demonstrated in this patient that point towards acute renal tubular disease. Vascular and color Doppler ultrasound has long been used to reveal both macroscopic and microscopic abnormalities in renal blood flow. In particular, evaluation of vascular impedance to flow at different areas of the renal parenchyma may directly suggesting microscopic functional or structural changes that provide valuable diagnostic and prognostic information [20]. A normal resistive index (RI) in the native kidney is between 0.5 and 0.7 [15], whereas in our patient RIs measured between 0.75 and 0.85, further supporting either a microvascular or tubular pathology. Elevated RI have been reported in diabetic patients [16], however our patient had a normal hemoglobin-A1-C, making this cause less likely. It is worth noting that a review article published by Tublin et al. in 2003 suggests that Doppler waveforms and resistive indices should be used cautiously when assessing for intrinsic renal disease, as the multifactorial process that produces the renal arterial waveform is not completely understood, and limits our ability to interpret the true meaning of these findings [21].
In addition to the above sonographic abnormalities, the kidneys in our patient showed decreased color Doppler flow globally to the parenchyma. Central vascularity was preserved (Fig. 2) and were grossly patent. Failure to detect color Doppler blood flow in general should prompt further evaluation with power Doppler, which is more sensitive [22]. This is commonly used in evaluation for areas of infarction in renal transplants, but given the potential for thrombotic complications associated with COVID-19, should have been considered in our case. Our institution has instituted limited ultrasound protocols so that our sonographers are in the patient room the smallest amount of time that is needed to answer the clinical question. While suboptimal, our lack of power Doppler ultimately did not affect the decision tree of proceeding to biopsy.
In addition to the above sonographic findings, it is worth noting that all six living patients who had biopsy proven collapsing FSGS during their COVID-19 infection, including the patient of our study, were of African descent. In addition, one case report noted that both of their patients were homozygous for apolipoprotein L1 (APOL1), which has been associated with FSGS in Black patients due to podocyte injury. It is hypothesized that the COVID-19 infection may act as a “second hit” to the podocytes that unmasked this underlying disease in this patient population [8]. With this in mind, our patient was tested for the gene and was founded to be heterozygous positive. It is possible that the timing of both FSGS and COVID-19 in our patient was coincidental, but given her risk factors, the unusual combination of ultrasound findings, pathology interpretation, and her clinical course, multidisciplinary review of this patient was undertaken by pathology, radiology and the clinical teams. Consensus was that coincidental presence of these two entities was less likely. Like many of COVID-19 manifestations, it is as of yet unclear the long-term sequela of this diagnosis and potential need for dialysis or transplant in the future.
4. Conclusions
Collapsing focal segmental glomerulosclerosis is an entity that is gaining more evidence as a culprit in COVID-19 related renal failure, particularly in patients of African descent. This case report details several sonographic findings including increased renal cortical echogenicity with loss of corticomedullary differentiation, increased resistive indices, and decreased color Doppler flow. These findings are nonspecific, but notable in combination. In the setting of COVID-19 infection, they can help the radiologist and clinical team refine the differential diagnosis of the patient's renal failure, and consider moving to biopsy when appropriate. Further case series and studies are required to further evaluate if the ultrasound findings in this report are generalizable to intrinsic renal failure in COVID-19 patients and to FSGS diagnosis in patients with COVID-19.
Funding
Not applicable.
Author contributions
All authors contributed significantly to the writing and editing of this manuscript.
Tyler Tancredi – Conceptualization, writing original draft.
Ami DeWaters – Conceptualization, review and editing.
Kathryn L. McGillen – Conceptualization, writing original draft, review and editing, supervision.
Declaration of competing interest
None.
Acknowledgements
None.
References
- 1.Chen D., Jiang X., Hong Y. Can chest CT features distinguish patients with negative from those with positive initial RT-PCR results for coronavirus disease (COVID-19)? [published online ahead of print, 2020 May 5] AJR Am J Roentgenol. 2020:1–5. doi: 10.2214/AJR.20.23012. [DOI] [PubMed] [Google Scholar]
- 2.Raptis C.A., Hammer M.M., Short R.G. Chest CT and coronavirus disease (COVID-19): a critical review of the literature to date [published online ahead of print, 2020 Apr 16] AJR Am J Roentgenol. 2020:1–4. doi: 10.2214/AJR.20.23202. [DOI] [PubMed] [Google Scholar]
- 3.Kanne J.P. Chest CT findings in 2019 novel coronavirus (2019-nCoV) infections from Wuhan, China: key points for the radiologist. Radiology. 2020;295(1):16–17. doi: 10.1148/radiol.2020200241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Behzad S., Aghaghazvini L., Radmard A.R., Gholamrezanezhad A. Extrapulmonary manifestations of COVID-19: radiologic and clinical overview [published online ahead of print, 2020 May 18] Clin Imaging. 2020;66:35–41. doi: 10.1016/j.clinimag.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zaim S., Chong J.H., Sankaranarayanan V., Harky A. COVID-19 and multiorgan response. Curr Probl Cardiol. 2020;45(8) doi: 10.1016/j.cpcardiol.2020.100618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu Z., Shi L., Wang Y. Pathological findings of COVID-19 associated with acute respiratory distress syndrome [published correction appears in Lancet Respir Med. 2020 Feb 25;:] Lancet Respir Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Su H., Yang M., Wan C. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020;98(1):219–227. doi: 10.1016/j.kint.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Magoon S., Bichu P., Malhotra V. COVID-19–related glomerulopathy: a report of 2 cases of collapsing focal segmental glomerulosclerosis [published online ahead of print, 2020 Jun 7] Kidney Med. 2020 doi: 10.1016/j.xkme.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kissling S., Rotman S., Gerber C. Collapsing glomerulopathy in a COVID-19 patient. Kidney Int. 2020;98(1):228–231. doi: 10.1016/j.kint.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peleg Y., Kudose S., D’Agati V. Acute kidney injury due to collapsing glomerulopathy following COVID-19 infection [published online ahead of print, 2020 Apr 28] Kidney Int Rep. 2020;5(6):940–945. doi: 10.1016/j.ekir.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Larsen C.P., Bourne T.D., Wilson J.D., Saqqa O., Sharshir M.A. Collapsing glomerulopathy in a patient with coronavirus disease 2019 (COVID-19) [published online ahead of print, 2020 Apr 9] Kidney Int Rep. 2020;5(6):935–939. doi: 10.1016/j.ekir.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Remer E.M., Papanicolaou N., Casalino D.D. ACR appropriateness criteria(®) on renal failure. Am J Med. 2014;127(11):1041–1048.e1. doi: 10.1016/j.amjmed.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 13.Tan C.W., Low J.G.H., Wong W.H., Chua Y.Y., Goh S.L., Ng H.J. Critically ill COVID-19 infected patients exhibit increased clot waveform analysis parameters consistent with hypercoagulability. Am J Hematol. 2020;95(7):E156–E158. doi: 10.1002/ajh.25822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spiezia L., Boscolo A., Poletto F. COVID-19-related severe hypercoagulability in patients admitted to intensive care unit for acute respiratory failure. Thromb Haemost. 2020;120(6):998–1000. doi: 10.1055/s-0040-1710018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhimraj A., Morgan R.L., Shumaker A.H. Infectious Diseases Society of America guidelines on the treatment and management of patients with COVID-19. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa478. https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/ Updated version 3.3.0, available online 9/25/2020 at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spatola L., Andrulli S. Doppler ultrasound in kidney diseases: a key parameter in clinical long-term follow-up. J Ultrasound. 2016;19(4):243–250. doi: 10.1007/s40477-016-0201-x. Published 2016 Apr 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Faubel S., Patel N.U., Lockhart M.E., Cadnapaphornchai M.A. Renal relevant radiology: use of ultrasonography in patients with AKI. Clin J Am Soc Nephrol. 2014;9(2):382–394. doi: 10.2215/CJN.04840513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Di Fiori J.L., Rodrigue D., Kaptein E.M., Ralls P.W. Diagnostic sonography of HIV-associated nephropathy: new observations and clinical correlation. AJR Am J Roentgenol. 1998;171(3):713–716. doi: 10.2214/ajr.171.3.9725302. [DOI] [PubMed] [Google Scholar]
- 19.Lockhart M.E., Robbin M.L. Renal vascular imaging: ultrasound and other modalities. Ultrasound Q. 2007;23(4):279–292. doi: 10.1097/ruq.0b013e31815adf4c. [DOI] [PubMed] [Google Scholar]
- 20.Viazzi F., Leoncini G., Derchi L.E., Pontremoli R. Ultrasound Doppler renal resistive index: a useful tool for the management of the hypertensive patient. J Hypertens. 2014;32(1):149–153. doi: 10.1097/HJH.0b013e328365b29c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tublin M.E., Bude R.O., Platt J.F. Review. The resistive index in renal Doppler sonography: where do we stand? AJR Am J Roentgenol. 2003;180(4):885–892. doi: 10.2214/ajr.180.4.1800885. [DOI] [PubMed] [Google Scholar]
- 22.Galgano S.J., Lockhart M.E., Fananapazir G., Sanyal R. Optimizing renal transplant Doppler ultrasound. Abdom Radiol (NY) 2018;43(10):2564–2573. doi: 10.1007/s00261-018-1731-9. [DOI] [PubMed] [Google Scholar]