Abstract
Objectives
Cytokine release syndrome with elevated interleukin-6 (IL-6) levels is associated with multiorgan damage and death in severe coronavirus disease 2019 (COVID-19). Our objective was to perform a living systematic review of the literature concerning the efficacy and toxicity of the IL-6 receptor antagonist tocilizumab in COVID-19 patients.
Methods
Data sources were Ovid MEDLINE(R) and Epub Ahead of Print, In-Process & Other Non-Indexed Citations and Daily, Ovid Embase, Ovid Cochrane Central Register of Controlled Trials, Ovid Cochrane Database of Systematic Reviews, Web of Science, Scopus up, preprint servers and Google up to October 8, 2020. Study eligibility criteria were randomized controlled trials (RCTs) and observational studies at low or moderate risk of bias. Participants were hospitalized COVID-19 patients. Interventions included tocilizumab versus placebo or standard of care. We pooled crude risk ratios (RRs) of RCTs and adjusted RRs from cohorts, separately. We evaluated inconsistency between studies with I2. We assessed the certainty of evidence using the GRADE approach.
Results
Of 1156 citations, 24 studies were eligible (five RCTs and 19 cohorts). Five RCTs at low risk of bias, with 1325 patients, examined the effect of tocilizumab on short-term mortality; pooled RR was 1.09 (95%CI 0.80–1.49, I2 = 0%). Four RCTs with 771 patients examined the effect of tocilizumab on risk of mechanical ventilation; pooled RR was 0.71 (95%CI 0.52–0.96, I2 = 0%), with a corresponding number needed to treat of 17 (95%CI 9–100). Among 18 cohorts at moderate risk of bias with 9850 patients, the pooled adjusted RR for mortality was 0.58 (95%CI 0.51–0.66, I2 = 2.5%). This association was observed over all degrees of COVID-19 severity. Data from the RCTs did not show a higher risk of infections or adverse events with tocilizumab: pooled RR 0.63 (95%CI 0.38–1.06, five RCTs) and 0.83 (95%CI 0.55–1.24, five RCTs), respectively.
Conclusions
Cumulative moderate-certainty evidence shows that tocilizumab reduces the risk of mechanical ventilation in hospitalized COVID-19 patients. While RCTs showed that tocilizumab did not reduce short-term mortality, low-certainty evidence from cohort studies suggests an association between tocilizumab and lower mortality. We did not observe a higher risk of infections or adverse events with tocilizumab use. This review will continuously evaluate the role of tocilizumab in COVID-19 treatment.
Keywords: COVID-19, Meta-analysis, Mortality, Tocilizumab, Toxicity
Introduction
Since its emergence in December 2019, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has infected millions of people across the globe and claimed hundreds of thousands of human lives, as well as negatively impacting the economy of numerous countries. Although the majority of SAR-CoV-2-infected patients who develop coronavirus disease 2019 (COVID-19) manifest only mild symptoms, about 14% of patients develop severe symptoms and 5% develop critical disease defined by respiratory failure, shock and/or multiorgan failure [1].
The innate and adaptive immune responses react appropriately in the majority of infected people and lead to control of the infection with no significant damage to the host tissues. Macrophages are activated by damage-associated molecular patterns (DAMPs) from damaged cells (such as heat-shock proteins and hyaluronan fragments) and liberated pathogen-associated molecular patterns (PAMPS) such as viral RNA. These molecules activate toll-like receptors (TLRs) and NLRP3 inflammasome. Cytosolic DNA also triggers cGAS-STING and RIG-I-MAVS pathways. These responses lead to activation of antiviral immune responses (such as INF–I and –III response and production of other cytokines) that result in the amplification of the innate response and activation of adaptive immunity, leading to viral clearance and tissue repair [2,3].
Patients with severe COVID-19 disease manifest immune system dysregulation, which is believed to be triggered by a particular mode of programmed cell death called pyroptosis. This form of cell death induces several proinflammatory cytokines and chemokines—such as IL-1β, IL-2, IL-6, tumour necrosis factor α (TNF-α), and monocyte chemoattractant protein 1 (MCP1)—and lymphopenia with attrition of both CD4+ and CD8+ T cells and natural killer T cells [[3], [4], [5]]. IL-6 and IL-1® production promote neutrophil and cytotoxic T cell recruitment to the affected tissues, both of which contribute to tissue damage resulting in acute lung injury through production of oxygen free radicals and inflammatory mediators such as leukotrienes [2]. Indeed, elevated blood levels of IL-6 have been shown to correlate with COVID-19 disease severity and SARS-CoV-2 RNA blood levels in COVID-19 patients, and is also associated with a worse prognosis [6].
It is hypothesized that the elevation of cytokines in COVID-19 diseases is similar to other cytokine release syndromes (CRSs) observed with diseases such as haemophagocytic lymphohistiocytosis (HLH), macrophage activation syndrome (MAS) and chimeric antigen receptor T-cell therapy (CAR-T), and that this cytokine storm is responsible for the multiorgan damage observed in patients with severe COVID-19 [2]. A number of immunomodulatory therapies targeting these cytokines have recently garnered interest and have been tested in COVID-19 [[7], [8], [9], [10], [11], [12], [13], [14], [15]]. Among these, IL-6 receptor blockade with the humanized monoclonal antibody tocilizumab is used routinely as a disease-modifying agent in the treatment of rheumatoid arthritis [16], and has been shown to be effective in the treatment of CRS associated with CAR-T therapy [17]. These observations formed the basis for targeting IL-6 as a therapeutic approach for severe COVID-19 disease [18,19].
Several studies addressing the role of tocilizumab were subsequently published with variable results. Two recent systematic reviews [20,21] pooled crude unadjusted data from cohort studies and concluded that tocilizumab is associated with better outcomes in COVID-19. However, their conclusions are flawed by the confounding effect inherent in crude data from observational data. Moreover, the two reviews did not address the adverse events and did not evaluate the certainty of cumulative evidence. Therefore, we sought to perform a living systematic review of randomized trials and observational studies addressing the efficacy and safety of tocilizumab in the treatment of COVID-19 patients.
Methods
We followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines for reporting systematic reviews [22]. Our review is a living systematic review. A living systematic review is a cumulative synthesis that is updated regularly as new evidence becomes available [23].
Inclusion criteria
We included in separate analyses (a) RCTs or (b) cohort or case–control studies (at low or moderate risk of bias) reporting on the adjusted effect estimates (derived for example from conventional regression models or propensity score matching or analysis) of the association between tocilizumab use in COVID-19 patients and one of the following a-priori outcomes: in-hospital mortality, need for mechanical ventilation, need for ICU admission, composite outcomes if reported, adverse events (data from RCTs only), and infections (data from RCTs only).
Data sources and search strategies
A comprehensive search of several databases from 2019 to 8th October 2020, excluding animal studies, was conducted. The databases included Ovid MEDLINE(R) and Epub Ahead of Print, In-Process & Other Non-Indexed Citations and Daily, Ovid Embase, Ovid Cochrane Central Register of Controlled Trials, Ovid Cochrane Database of Systematic Reviews, Web of Science, and Scopus. The search strategy is available in the Appendix (Supplementary Material). We also searched for unpublished manuscripts using the medRxiv services and Researchsquare.com, Google, and the references of eligible studies and review articles.
Data extraction
Eight reviewers (IT, HT, MA, MA, MD, YA, TK and ZAK) in groups of two independently identified eligible studies and extracted the data into a prespecified data collection form in duplicate. Any discrepancies were resolved by two senior reviewers (IT and TK).
Living systematic review
In this living systematic review, two independent investigators (IT and ZK) receive an updated literature search file every 3 months and continuously include relevant newly published or unpublished studies as per above inclusion criteria. The relevant meta-analyses will be continuously updated, and if new evidence becomes available (judged by the author group in coordination with the Journal Editor in Chief), the results will be submitted for publication at regular intervals.
Quality assessment
Five reviewers (IT, HT, MA, TK and ZK) independently and in duplicate assessed the risk of bias for each study using the RoB 2 of the Cochrane risk-of-bias tool for randomized trials and the ROBINS-I (‘Risk Of Bias In Non-randomized Studies of Interventions’) for observational studies [24]. We also assessed all included cohort studies for risk of survivor bias (or immortal time bias). We considered the following analytical approaches as acceptable tools to account for survivor bias [[25], [26], [27]]: (a) tocilizumab use as a time-dependent variable in the regression analysis, (b) landmark analysis, (c) structural nested accelerated failure time model, and (d) marginal structure models. Reviewers judged each criterion for risk of bias and resolved any disagreements with a senior reviewer (IT). Finally, two reviewers (HT and IT) assessed the certainty of evidence for each of our outcomes using the GRADE (Grading of Recommendations Assessment, Development, and Evaluation) approach [28].
Statistical analysis
We evaluated heterogeneity between studies using the I2 statistic [29]. We pooled crude data from RCTs using the Mantel–Haenszel methods using fixed effect model [30]. Due to substantial heterogeneity in cohort studies, we pooled the adjusted effect estimates of the included studies using the DerSimonian–Laird random-effects model and constructed corresponding forest plots [31]. Prior to pooling, the ORs were converted to RRs using the method of Zhang and Yu [32]. For the main analyses, we included only the studies at low or moderate risk of bias.
We conducted a priori determined subgroup analyses to assess the impact of COVID-19 disease severity on response to tocilizumab therapy. We also conducted meta-regression using a priori chosen study level baseline characteristics of patients' populations to explore causes of heterogeneity of treatment effects across studies. These variables included characteristics of study population (age, sex, comorbidities), dosing regimen and number of tocilizumab doses, adjustment for survivor bias, and levels of ferritin, interleukin 6 (IL-6), C-reactive protein (CRP) and lactate dehydrogenase (LDH). Other considered variables were not included because of missing data. Finally, we constructed contour-enhanced funnel plots and performed an Egger precision-weighted linear regression test as a statistical test of funnel plot asymmetry and publication bias [33]. All analyses of cohort studies were conducted using Stata version 16 statistical software (StataCorp, College Station, Texas, USA) and those of RCTs using Review Manager, Version 5.4, The Cochrane Collaboration.
Results
Efficacy
Of 1156 citations, 24 studies (five RCTs and 19 cohorts) (Supplementary Material Tables S1 and S2) [7,[34], [35], [36], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58]] with 1325 patients (RCTs) and 10 021 patients (cohorts), including single-centre and multicentre studies from different countries, were included in our systematic review. Two studies [7,59] reported on the same cohort, and we included data from the report that focused on tocilizumab use [7]. To date, five RCTs (Supplementary Material Table S1) have been completed but only four have been published [47,54,56,57]. Preliminary data from another RCT (EMPACTA) [58] was identified on Google news as a press release and cross-identified on clinicaltrials.gov to identify its objectives and details (NCT04372186). Fig. 1 shows the result of our search strategy (PRISMA flow diagram). Supplementary Material Tables S1 and S2 (Table 1, Table 2) illustrate the general characteristics of the included studies. All studies reported on patients hospitalized with COVID-19 with varying degrees of disease severity.
Fig. 1.
Flow diagram of the assessment of studies identified in the systematic review.
Table 1.
Characteristics of completed randomized controlled trials of tocilizumab for patients with coronavirus disease 2019 (COVID-19)
| RCT | Design | Number of patients | Country, centres | Inclusion criteria | Tocilizumab | Primary outcome | Composite outcome used in meta-analysis | Completed versus stopped early |
|---|---|---|---|---|---|---|---|---|
| RCT-TCZ-COVID-19 NCT04346355 |
Open label | 60 TCZ versus 66 Controls | Italy, 24 centres |
COVID-19 pneumonia documented by radiological imaging, PaO2/FIO2 between 200 and 300 mmHg, and an inflammatory phenotype defined by fever and elevated CRP | 8 mg/kg up to a maximum of 800 mg, followed by a second dose after 12 hours | Composite outcome: entry into the ICU with MV, death from all causes, or clinical aggravation documented by the finding of a PaO2/FIO2 ratio <150 mmHg, whichever came first |
Death or continuous need for hospitalization at day 30 | Stopped early for futility |
| CORIMUNO-19 NCT04331808 |
Open label | 64 TCZ versus 67 controls | France, 9 centres |
COVID-19 and moderate or severe pneumonia requiring at least 3 L/min of oxygen but without ventilation or admission to ICU | 8 mg/kg on day 1 and on day 3 if clinically indicated | Scores >5 on the World Health Organization 10-point Clinical Progression Scale (WHO-CPS) on day 4 and survival without need of ventilation (including non-invasive ventilation) at day 14 | Death or continuous need for hospitalization at day 28 | Completed |
| BACC Bay Tocilizumab Trial NCT04356937 |
Double-blind, placebo-controlled trial | 161 TCZ versus 81 controls | USA, 7 centres |
(SARS-CoV-2) infection, hyperinflammatory states, and at least two of the following signs: fever (body temperature >38°C), pulmonary infiltrates, or the need for supplemental oxygen in order to maintain an oxygen saturation >92% | Single dose of tocilizumab 8 mg/kg |
Intubation or death | Mechanical ventilation or death at day 28 | Completed |
| COVACTA NCT04320615 |
Double-blind, placebo-controlled trial | 294 TCZ versus 144 controls | Nine countries (Canada, Denmark, France, Germany, Italy, The Netherlands, Spain, United Kingdom, United States), 67 centres |
Patients ≥18 years with severe COVID-19 pneumonia confirmed by PCR test in any body fluid and evidenced by bilateral chest infiltrates. Blood oxygen saturation ≤93% or partial pressure of oxygen/fraction of inspired oxygen <300 mm/Hg | 8 mg/kg infusion, maximum 800 mg second infusion could be administered 8–24 hours after the first | Clinical status on a 7-category ordinal scale at day 28 (1, discharged/ready for discharge; 7, death) | Death, withdrawal during hospitalization, transfer to ICU, or requirement for invasive mechanical ventilation within 28 days of baseline | Completed |
| EMPACTA NCT04372186 |
Double-blind, placebo-controlled trial | 194 TCZ versus 195 controls | Six countries (Brazil, Kenya, Mexico, Peru, South Africa, US), 69 centres |
COVID-19 pneumonia confirmed by PCR of any specimen and radiographic imaging SpO2 <94% while on ambient air |
8 mg/kg × 1, Possible second dose |
Death or MV by day 28 | Death or MV by day 28 |
NIV, non-invasive ventilation, PaO2/FIO2, ratio of partial pressure of arterial oxygen to fraction of inspired oxygen; HFNC, high-flow nasal cannula; MV, mechanical ventilation respiratory support; DNR, do not resuscitate; ARDS, acute respiratory distress syndrome; CRP, C-reactive protein; PaO2, arterial partial pressure of oxygen; FiO2, inspired fraction of oxygen; SpO2, oxygen pulse oximetry; ICU, intensive care unit; WHO, World Health Organization; PCR, polymerase chain reaction; TCZ, tocilizumab.
Table 2.
Characteristics of included cohort studies
| Study/Publication type | Country | No. of pts | Study type | Cohort selection | Treatment criteria | Exclusion criteria | Primary outcome | Analytical method | Variables adjusted for | Immortal time bias adjustment |
|---|---|---|---|---|---|---|---|---|---|---|
| Biran et al.a (same cohort as Ip et al.) | United States | 630 | Multicentre retrospective cohort | Patients admitted to ICU without prior exposure to tocilizumab | ARDS on MV, or worsening oxygenation with high oxygen requirements (80–100%) on high-flow nasal cannula or 15 L non-rebreather mask Symptoms had to be present for 7 days |
Pregnant, enrolled in an RCT, death within 24 h | 30-day mortality | Multivariate Cox regression with propensity score | Comorbidities, gender, age, race Steroids: no (equal in both groups) |
No |
| Colaneri et al.a | Italy | 42 | Single-center retrospective cohort | Hospitalized adult patients with a confirmed COVID-19 pneumonia | TCZ was administered if: CRP > 5 mg/dl, PCTI < 0.5 ng/mL, PF ratio <300 | ALT >500 U/L | ICU admission and 7-day in-hospital mortality rate. | Propensity matched logistic regression | Age, sex, LDH, neutrophils Steroids: (100%) received |
No |
| Eimerb et al. | Sweden | 44 | Single-center retrospective cohort | Patients admitted to ICU for severe ARDS | Rising O2 at least 5L/min, ≥ 7 days from symptom onset, CRP >100 mg/L or ferritin >500 ug/L and no contraindication to tocilizumab | Patients with COVID-19 admitted to ICU for primary diagnosis other than ARDS | 30-day all-cause mortality after ICU admission | Cox proportion-hazard model | Age, diabetes, hypertension, obesity, d-dimer, interlukin-6, troponin T and PaO2/FiO2 ratio | No |
| Garciaa et al. | Spain | 171 | Single-center Retrospective Cohort | COVID-19 patients with pneumonia who did not require ICU transfer during the first 24h of admission | Patients with pneumonia, progressive respiratory failure CRP ≥8 mg/dL or ferritin ≥800 ng/mL or lymphocyte count <800 | NR | In-hospital mortality or ICU admission | Multivariate logistic regression analysis with propensity scoring | Age, HTN, DM, heart disease, respiratory disease, lymphoma Steroids: No |
Inadequate: patients not requiring ICU admission within 24 hours |
| Gokhaleb et al. | India | 269 | Single-center Retrospective Cohort | Severe COVID-19 pneumonia with persistent hypoxia | Persistent hypoxia, bilateral pulmonary infiltrates and raised CRP, LDH and ferritin | Altered sensorium, terminal malignancy, EF < 20% | Survival | Multivariate Cox regression analysis | Age, oxygen saturation, creatinine, tocilizumab and invasive ventilation | No |
| Guaraldia et al. | Italy | 544 | Multi-center Retrospective Cohort | Patients with severe∗ COVID-19 infection | SaO2< 93% and a PaO2/FiO2 ratio <300 mm Hg in room air or a >30% decrease in their PaO2/FiO2 ratio in the past 24 h during hospitalization. | Coexisting infection, PaO2/FiO2 > 300, steroid use, neutrophils <500 platelet< 50,000 High risk for bowel perforation | Composite of mortality or invasive mechanical ventilation | Multivariate Cox regression analysis | Sex, age, recruiting center, duration of symptoms, and baseline SOFA score Steroids: yes |
Yes |
| Hillb el al. | United States | 88 | Multi-center retrospective cohort | Patients admitted to hospital and requiring supplemental oxygen | Persistent fever with impending or current respiratory failure, hemodynamic instability, IL-6 > 5 times normal. | Sepsis, transaminases >5 times normal, ANC <500 cells/mm3 or platelets <50 cells/mm3 | Clinical improvement i.e. two-point reduction in severity, discharge and 28 day mortality | Cox proportion-hazard model | Age, sex, ethnicity, BMI, diabetes, cardiovascular disease, hospital, code status, oxygen support category | No |
| Holtb et al. | United States | 62 | Single-center case-control study | Patients admitted to hospital meeting inclusion criteria | oxygen ≥ 4L and; IL-6 > 40 pg/mL, CRP >10 mg/dL, D- dimer >1 mcg/mL FEU, ferritin >1000 ng/mL, or LDH >350 units | NR | Effect of tocilizumab on mortality | Cox proportion-hazard model | Age, chronic hypoxia, nursing home, IL6 > 580, ferritin >1631, ICU admission, tocilizumab, hypoxia on admission, solid tumor, diabetes, Caucasian and altered mental status | No |
| Martinez-Sanzb et al. | Spain | 1229 | Multi-center Retrospective Cohort | COVID-19 patients. | Treatment criteria not reported | Death or transfer within 24 hours | In-hospital? Mortality | Multivariate Cox regression with Propensity score | Age, gender, comorbidities, lab values Steroids: NA |
Yes: Marginal structure model |
| Mikulskab et al. | Italy | 196 | Single-center Retrospective Cohort | Patients with severe∗ or critical∗∗ COVID-19 infection | Treatment criteria was not reported | Death intubation or discharge before day 3 of admission | Intubation mechanical ventilation or mortality | Overlap-weighted Cox proportional hazard regression model | Age, gender, presence of comorbidities, week treatment began, NIV, and labs Steroids: NA, Tcz compared with steroids |
Yes: landmark analysis |
| Naraina et al. | United States | 3098 | Multi-center Retrospective Cohort | Patients with cytokine storm defined as ferritin >700ng/mL or CRP >30mg/dL or LDH >300U/L | Treatment criteria not reported | Immunomodulatory drugs used prior to the diagnosis of cytokine storm | In-Hospital mortality | Multivariate Cox proportional hazard regression model | Age, gender, race, comorbidities, lab data, insurance status Steroids: NA, patients receiving steroids were in their own group |
No |
| Ramaswamyb et al. | United States | 86 | Multi-center Case-Control | Patients who died during hospitalization were cases, while patients discharged alive were controls. The exposure was treatment with tocilizumab | Treatment criteria was not reported | NR | In-hospital Mortality | IPSW Cox regression adjusted for competing risk of mortality | Age, race, gender, Elixhauser comorbidity score, MEWS score Steroids: included in IPW analysis |
No |
| Roomib et al. | United States | 176 | Single-center Retrospective Cohort | Hospitalized adult patients with a confirmed COVID-19 infection | Treatment criteria was not reported | NR | In-hospital mortality, ICU admission, mechanical ventilation | Multivariate logistic regression | Baseline comorbidities and medication use | No |
| Rossib et al. | France | 246 | Single-center Case Control | Patients with severe∗ COVID-19 pneumonia | Treatment criteria was not reported | Patients on IMV or admitted to the ICU | Composite of mortality and mechanical ventilation | IPSW Cox regression | Age, engagement status, systolic blood pressure SpO2/FiO2 ratio Steroids: Equal between groups after matching |
Inadequate: time between admission and inclusion was adjusted for in propensity-score matching |
| Rossottia et al. | Italy | 222 | Single-center Retrospective Cohort | Patients with severe cCOVID-19 infection | CT scan showing severe bilateral pneumonia; CRP >1 mg/dL, IL-6 >40 pg/mL, D-dimer >1.5 mcg/mL, or ferritin >500 ng/mL | ALT value > 5 x ULN; neutrophil cell count <500 cell/mmc; platelet count <50,000 cell/mmc | In-hospital Mortality | Cox regression models | NR Steroids: NA |
No |
| Roumierb et al. | France | 59 | Single-center Retrospective Cohort | NR | COVID-19 patients with requiring >6L O2, elevated CRP levels | NR | Mortality, Mechanical ventilation, and ICU admission | IPTW matched logistic regression | Age, gender and disease severity Steroids: No |
No |
| Somersa et al. | United States | 154 | Single-center retrospective Cohort | Critical dCOVID-19 patients requiring invasive mechanical ventilation | Abnormal chest imaging consistent with COVID-19, rapidly worsening respiratory status, suspicion of cytokine release storm, MV for <48 hours | Death within 48 hours of intubation, intubation for conditions unrelated to COVID-19 or enrolled into an RCT for sarilumab | In-hospital mortality | IPTW Cox regression model | Age, gender, race, ferritin, LDH, AST Steroids: No (equal between usage groups) |
Inadequate: patients who died within 48 hours of intubation were excluded |
| Tsaib et al. | United States | 132 | Single-center consecutive Cohort | Hospitalized patients who meet treatment criteria | Ferritin >300 ug/mL and SpO2 < 94% requiring supplemental oxygen or mechanical ventilation | NR | In-hospital mortality | Propensity matched logistic regression | Age, sex, body mass index, lactic acid, ferritin, LDH, procalcitonin, creatinine, hypertension and comorbidity score | No |
ALT: Alanine aminotransferase; ARDS: acute respiratory distress syndrome; CRP: C-reactive protein; DM: Diabetes mellitus; HTN: Hypertension; NR: Not reported; NA: Not applicable; IMV: Invasive mechanical ventilation; IPTW: Inverse probability treatment weighted; IPW: Inverse probability weighted; MV: Mechanical ventilation; UNL: upper normal limit; TCZ: Tocilizumab.
Peer-reviewed.
Pre-print.
Severe COVID-19 defined by one of the following 1) respiratory rate ≥30 breaths/min, 2) peripheral capillary oxygen saturation (SpO2) ≤ 93% while breathing room air, 3) PaO2/FiO2 ≤ 300 mmHg.
Critical patients requiring mechanical ventilation.
Four RCTs were at low risk of bias as per ROB 2 scale (Fig. 2 A). We could not assess the risk of bias in the EMPACTA trial even though it was a double-blind placebo-controlled trial. The quality of the observational studies was assessed using the ROBINS-I tool (Supplementary Material Fig. S1). Among included cohort studies, survivor bias was addressed in the analysis in four studies only [36,38,39,55] (Supplementary Material Table S2) .
Fig. 2.
A: Forest plot for the effect of tocilizumab on 28-30 days mortality in randomized controlled trials with corresponding risk of bias∗. B: Forest plot for the effect of tocilizumab on risk for mechanical ventilation in randomized controlled trials with corresponding risk of bias. C: Forest plot for the effect of tocilizumab on 28-30 days composite outcome in randomized controlled trials with corresponding risk of bias∗.
Randomized controlled trials
Five RCTs at low risk of bias, with 1325 patients, examined the effect of tocilizumab on short-term mortality; pooled risk ratio (RR) was 1.09 (95%CI 0.80–1.49, I2 = 0%) (Fig. 2A). Four RCTs with 771 patients examined the effect of tocilizumab on risk of mechanical ventilation; pooled RR was 0.71 (95%CI 0.52–0.96, I2 = 0%) (Fig. 2 B), with a corresponding number needed to treat of 17 (95%CI 9–100). Five RCTs at low risk of bias, with 1325 patients, examined the effect of tocilizumab on a composite of poor outcome; pooled RR was 0.71 (95%CI 0.56–0.89, I2 = 0%) (Fig. 2C). The definition of this composite outcome in each trial is summarized in Supplementary Material Table S1.
Data from the RCTs did not show higher risk of infections (Fig. 3 A) or adverse events (Fig. 3B) with tocilizumab; pooled RR 0.63 (95%CI 0.38–1.06, five RCTs) and 0.83 (95%CI 0.55–1.24, five RCTs), respectively.
Fig. 3.
A: Forest plot for relative risk of infections with tocilizumab vs. control in randomized controlled trials. B: Forest plot for relative risk of adverse events with tocilizumab vs. control in randomized controlled trials.
Cohort studies
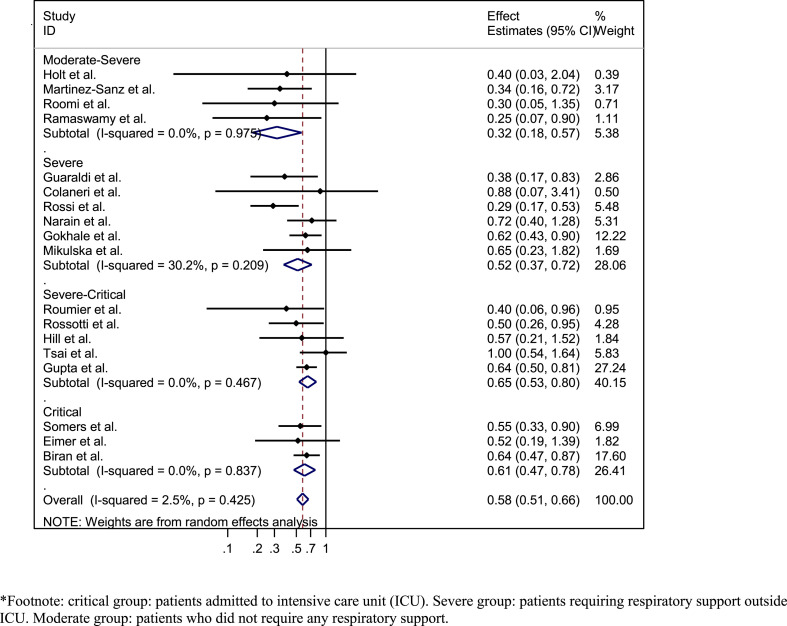
Data from 18 out of the 19 identified cohorts were used for this analysis. The study by Garcia et al. [60] did not report on mortality as an individual endpoint and therefore was not included in the quantitative analysis. Among 18 cohorts at moderate risk of bias, with 9850 patients, the pooled adjusted RR for mortality was 0.58 (95%CI 0.51–0.66, I2 = 2.5%) (Supplementary Material Fig. S2). This association was observed across all stages of disease severity (Fig. 4 ). Multivariate meta-regression analyses of the variables listed in the methods section did not identify any study level variable associated with RR of mortality. Contour-enhanced funnel plot and Egger's test for small-study effects (p 0.120) did not show evidence of publication bias (Supplementary Material Fig. S3). Only three studies reported on other outcomes (Supplementary Material Figs S4 and S5).
Fig. 4.
Forest plot of the association between tocilizumab use and short-term mortality in COVID-19 patients from cohorts at moderate risk of bias: stratified by disease severity∗.
All included cohort studies were at moderate risk of bias (Supplementary Material Fig. S1). Survivor bias was addressed in the analysis in four studies [36,38,39,55] only (Supplementary Material Table S2). To study the potential effect of survivor bias on the observed results, we performed a post-hoc analysis where we assumed that cohort studies which did not adjust for this bias as per above approaches reported over-estimated effect estimates. Based on data from a COVID-19 study [61] and another influenza study [62], relative risk for mortality increased by 40–60% when treatment (such as steroids or oseltamivir) was considered as a time-dependent variable in Cox regression analysis [61]. We therefore multiplied the RR or HR of studies that did not adjust for survivor bias and their corresponding 95%CIs by 1.6 and pooled the new adjusted effect estimates. The corrected pooled adjusted RR for mortality from the 18 cohorts was 0.77 (95%CI 0.63–0.95, I2 = 41%) (Supplementary Material Fig. S6).
GRADE of evidence
To facilitate interpretation and grading of the results, we calculated the absolute effect for mortality and other outcomes for both RCTs and cohort studies. Data from RCTs, at low risk of bias, showed that in hospitalized COVID-19 patients tocilizumab reduces the risk of mechanical ventilation with a corresponding number needed to treat of 17 (95%CI 9–100). Because of the imprecision of the 95%CI for the number needed to treat, this evidence was downgraded to moderate-certainty evidence. Although RCTs did not show that tocilizumab has an effect on mortality, the pooled RR of 1.09 (95%CI 0.80–1.49) was imprecise, with a wide 95%CI, suggesting that more studies may be needed for a definitive answer. In the five identified RCTs, the risk of mortality in the control group was 57/553 (10.3%). The sample size required for an RCT to detect an RR of 0.73 for mortality with tocilizumab (with 80% power and α 0.05) is 4506 patients (2253 in each arm). The total number of patients in the five RCTs is 772 patients in the tocilizumab group and 553 patients in the control group.
For the cohort studies, we used the baseline risk for mortality (27.3%) from the International Severe Acute Respiratory and Emerging Infection COVID-19 database [63]. The absolute risk difference in mortality was –11.5% (95%CI –13.4% to –9.3%) with a number needed to treat to prevent one death of 9 (95%CI 7–11). The overall quality of evidence (classified as low in observational studies) remained low given the moderate risk of study bias, low risk of publication bias, direct evidence, low inconsistency, and precise estimate.
Discussion
Main findings: efficacy
In this living systematic review and meta-analysis that included five RCTs and 18 cohorts as of October 2020, cumulative moderate certainty evidence shows that tocilizumab reduces the risk of mechanical ventilation in hospitalized COVID-19 patients. While RCTs showed that tocilizumab did not reduce short-term mortality, low-certainty evidence from cohort studies suggests an association between tocilizumab and lower mortality.
Although the effect of tocilizumab on mortality from RCTs are discordant with the results of observational studies, there are many possible explanations beyond study design and residual confounding [7,64]. These include different patient populations, inflammatory status, and timing of administration and dosing of tocilizumab. Moreover, the RCTs did not consider mortality as an individual primary endpoint, and therefore they were not powered enough to detect difference in mortality between tocilizumab and control groups as suggested by the wide 95%CI of their pooled RR 1.09 (95%CI 0.80–1.49). The sample size required for an RCT to detect an RR of 0.73 for mortality (similar RR to the cohort studies adjusted for survivor bias) is 4506 patients (2253 in each arm). The total number of patients in the five RCTs is 772 patients in the tocilizumab group and 553 patients in the control group. Finally, empirical studies have shown that pooled estimates from meta-analyses of observational studies yield similar estimates to those pooled from RCTs [[65], [66], [67], [68]].
Main findings: safety
Although IL-6 inhibition dampens the host immune response and may theoretically increase the risk of infections, we did not observe a higher risk of infections or adverse events with tocilizumab use in the RCTs. Moreover, many of the cohort studies included in our review assessed patients receiving tocilizumab in the ICU, of whom many were on mechanical ventilation. The high absolute incidence of secondary bacterial infections in the ICU [[69], [70], [71]] that was also observed in the cohort studies is probably related to critical illness and ICU admission as opposed to the use of tocilizumab. Tocilizumab use in CRS following CAR-T therapy was not associated with increased infections [72].
Mechanisms
IL-6 has pleotropic effects and plays important roles in host defence against invading organisms and tissue repair in acute environmental conditions such as trauma and burns [73,74]. It is produced by immune cells and several other types of cells in response to TLR activation and in response to other proinflammatory cytokines such as TNFα and IL-1®, and its production is also upregulated by coagulation factors like factor VIIa and thrombin [73]. IL-6 plays a pivotal role in acute-phase reaction and rapidly induces acute-phase proteins, including CRP, antitrypsin, serum amyloid protein A (SAA), fibrinogen, ferritin, hepcidin and others [73,74]. Some of these proteins are involved in host defence; CRP works as an opsonin through its binding to bacterial phosphorylcholine and activates the complement pathway, while antitrypsin inactivates proteases released by pathogens and damaged cells, and hepcidin possesses antimicrobial activities [73]. Moreover, IL-6 induces B-cell differentiation and antibody production, and induces cytotoxic-T-cell differentiation from CD8 T cells. It also promotes the induction of C3 complement and C5a receptor [73,74]. IL-6 also increases the production of intercellular adhesion molecule-1 (ICAM-1), MCP-1 and IL-8, which play roles in the migration of inflammatory cells to sites of inflammation and tissue damage [73].
All of these effects are very important in host defence against invading pathogens. However, persistent and dysregulated production of IL-6 may have detrimental effects, and IL-6 has been identified as an important mediator in different autoimmune and chronic inflammatory diseases such as rheumatoid arthritis, systemic lupus erythematosus (SLE), and others [73,74]. Additionally, the production of IL-6 has been shown to be significantly increased in conditions associated with CRS and cytokine storm, such as CAR-T therapy, MAS, HLH syndromes and viral infections associated with cytokine storm [73,74]. IL-6 plays a crucial role in the pathogenesis of CRS associated with these conditions. IL-6 increases vascular permeability leading to interstitial oedema, increased tissue pressure and damage through activation of the complement system, induction of vascular endothelial growth factor (VEGF) and histamine release from mast cells. It also activates the coagulation system through increased production of factor VIIa, thrombin and tissue factor, and induction of megakaryocyte maturation and platelet production. These procoagulation effects can lead to disseminated intravascular coagulation (DIC). Furthermore, IL-6 has direct myocardial depressing effects [73]. Therefore, these effects of IL-6 could explain the manifestations of COVID-19-associated cytokine storm which include hypoxaemia, hypotension, intravascular thrombosis, myocardial dysfunction and multiorgan failure. Several lines of evidence support this contention. For example, IL-6 injection in patients with breast and lung cancer have been shown to produce fever and influenza-like symptoms [75]. It has also been shown that injecting metastatic renal cancer patients with IL-6 induces coagulation activation as evidenced by increased thrombin–antithrombin III complexes and increased plasma levels of prothrombin activation fragment [76]. Additionally, daily subcutaneous IL-6 injections of 1–10 μg/kg/day for 7 days in patients receiving chemotherapy resulted in elevation of several acute-phase proteins, with the fastest response observed with CRP and serum amyloid protein A [77]. On the other hand, treatment of patient with CAR-T therapy induced cytokine storm with a single injection of the humanized anti-IL-6 monoclonal antibody, and tocilizumab led to a rapid resolution of the manifestations of this cytokine storm [17]. Further, in MAS, which is a well-known complication of systemic juvenile idiopathic arthritis (sJIA), it has been demonstrated that activation of haemophagocytic macrophages is linked to decreased functioning of natural killer cells with reduced expression of perforin and granzyme B, and it is believed that IL-6 plays a role in this process as treatment of patients with sJIA with tocilizumab results in normalization of reduced perforin and granzyme B [73]. The findings of our meta-analysis further support the role of IL-6 in CRS and establishes the potential therapeutic benefits of tocilizumab in CRS syndromes in general, and more specifically in severe COVID-19 disease.
Phenotypes/markers
Although IL-6 level determination is not performed routinely in most hospitals, acute-phase proteins can serve as surrogate markers for elevated IL-6 in COVID-19 disease, especially CRP and ferritin, as their assays are widely available. Therefore, CRP and ferritin can be used as markers of elevated IL-6 to help in selecting candidate COVID-19 patients for tocilizumab therapy. Indeed, in a multicentre study that included 1229 COVID-19 patients from Spain, it was found that tocilizumab reduced the risk of mortality only in patients with baseline CRP levels >150 mg/L (aHR 0.34, 95%CI 0.16–0.72, p 0.005) [38]. This indicates that patients with low CRP likely had low IL-6 levels and hence did not respond favourably to tocilizumab. Tocilizumab was also shown to significantly reduce CRP and ferritin among COVID-19 disease patients [78]. D-dimer—which is another biomarker that is usually elevated in patients with COVID-19 disease and which may indicate presence of intravascular thrombosis—was also found to predict outcomes to tocilizumab [78]. These studies indicate that not all COVID-19 patients respond equally to tocilizumab, which is likely reflecting the heterogeneous nature of the disease and the possibility that it encompasses different sub-phenotypes. Post-mortem studies lend support to this hypothesis as autopsy series from Italy and the USA showed that some COVID-19 patients exhibit extensive pulmonary inflammation and alveolar damage while others manifest severe vascular endothelial damage and intravascular thrombosis [79,80]. In addition, in the RECOVERY trial, only patients with underlying hypoxaemia had improved survival with dexamethasone therapy, reflecting the possible presence of different sub-phenotypes with differential responses to immunomodulatory therapies. A similar phenomenon has been observed in patients with sepsis. Re-analysis of the phase III trial of anti-IL-1® antibody (anakinra) therapy in sepsis patients revealed that only patients who had features of MAS (5.6% of patients) had markedly reduced mortality in response to anakinra [81]. Further studies are needed to identify COVID-19 patients who would respond favourably to immunomodulatory therapies.
Strengths and limitations
Our meta-analysis has several strengths. We included published and unpublished randomized trials, employed rigorous methodologies, and excluded unadjusted crude effect estimates from cohort studies. Our inclusion of real-world data from good-quality observational studies and randomized trials allowed us to explore sources of heterogeneity and to compare the results of the RCTs with those of cohort studies. Although observational studies are prone to different biases—including confounding by indication, survivor bias and residual confounding—empirical studies have shown that pooled estimates from meta-analyses of observational studies yield similar estimates to those pooled from RCTs [[65], [66], [67], [68]].
Conclusion
Cumulative moderate-certainty evidence shows that tocilizumab reduces the risk of mechanical ventilation in hospitalized COVID-19 patients. While RCTs showed that tocilizumab did not reduce short-term mortality, low-certainty evidence from cohort studies suggests an association between tocilizumab and lower mortality. We did not observe a higher risk of infections or adverse events with tocilizumab use. This review will continuously evaluate the role of tocilizumab in COVID-19 treatment.
Author contributions
ZK, MD, MR and HT contributed equally to this work. IT: conception and design of the study. IT, ZK, MD, HT, MA, YA, MA, RT, LH and TK: acquisition of the data. IT and MR: analysis of the data. IT, ZK, HT, MR and TK: interpretation of data. IT, ZK, LH and TK: drafting of the article. All authors: critical revision of the article for important intellectual content and final approval of the version to be submitted.
Transparency declaration
The authors declare no conflicts of interest. No funding was received for this work.
Editor: L. Leibovici
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2020.10.036.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Wiersinga W.J., Rhoades A., Cheng A., Peacock S., Prescott H. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324:782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 2.Vardhana S.A., Wolchok J.D. The many faces of the anti-COVID immune response. J Exp Med. 2020;217 doi: 10.1084/jem.20200678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tay M.Z., Poh C.M., Rénia L., MacAry P.A., Ng L.F. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20:363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen G., Wu D., Gou W., Cao Y., Huang D., Wang H. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130:2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y. Dysregulation of immune response in patients with Coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis. 2020;71:762–768. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen X., Zhao B., Qu Y., Chen Y., Xiong J., Feng Y. Detectable serum SARS-CoV-2 viral load (RNAaemia) is closely correlated with drastically elevated interleukin 6 (IL-6) level in critically ill COVID-19 patients. Clin Infect Dis. 2020;71:1937–1942. doi: 10.1093/cid/ciaa449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biran N., Ip A., Ahn J., Go R., Wang S., Mathura S. Tocilizumab among patients with COVID-19 in the intensive care unit: a multicentre observational study. Lancet Rheumatol. 2020;2:e603–e612. doi: 10.1016/S2665-9913(20)30277-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cavalli G., Luca G., Campochiaro C., Della-Torre E., Ripa M., Canetti D. Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study. Lancet Rheumatol. 2020;2:e325–e331. doi: 10.1016/S2665-9913(20)30127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Luca G., Cavalli G., Campochiaro C., Della-Torre E., Piera A., Tomelleri A. GM-CSF blockade with mavrilimumab in severe COVID-19 pneumonia and systemic hyperinflammation: a single-centre, prospective cohort study. Lancet Rheumatol. 2020;2:e465–e473. doi: 10.1016/S2665-9913(20)30170-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Favalli E.G., Caporali R. GM-CSF in the treatment of COVID-19: a new conductor in the pathogenesis of cytokine storm? Lancet Rheumatol. 2020;2:e448–e449. doi: 10.1016/S2665-9913(20)30185-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.King A., Vail A., O’Leary C., Hannan C., Brough D., Patel H. Anakinra in COVID-19: important considerations for clinical trials. Lancet Rheumatol. 2020;2:e379–e381. doi: 10.1016/S2665-9913(20)30160-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mehta P., Q Cron R., Hartwell J., Manson J., Tattersall R. Silencing the cytokine storm: the use of intravenous anakinra in haemophagocytic lymphohistiocytosis or macrophage activation syndrome. Lancet Rheumatol. 2020;2:e358–e367. doi: 10.1016/S2665-9913(20)30096-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Monteleone G., Sarzi-Puttini P.C., Ardizzone S. Preventing COVID-19-induced pneumonia with anticytokine therapy. Lancet Rheumatol. 2020;2:e255–e256. doi: 10.1016/S2665-9913(20)30092-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schulert G.S. Can tocilizumab calm the cytokine storm of COVID-19? Lancet Rheumatol. 2020;2:e449–e451. doi: 10.1016/S2665-9913(20)30210-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhong J., Tang J., Ye C., Dong L. The immunology of COVID-19: is immune modulation an option for treatment? Lancet Rheumatol. 2020;2:e428–e436. doi: 10.1016/S2665-9913(20)30120-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haraoui B., Casado G., Czirják L., Taylor A., Bernasconi C., Reiss W. Patterns of tocilizumab use, effectiveness and safety in patients with rheumatoid arthritis: core data results from a set of multinational observational studies. Clin Exp Rheumatol. 2017;35:899–906. [PubMed] [Google Scholar]
- 17.Maude S.L., Frey N., Shaw P., Aplenc R., Barrett D., Bunin N. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371:1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu X., Han M., Li T., Wang D., Fu B., Zhou Y. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci USA. 2020;117:10970–10975. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gritti G., Raimondi F., Ripamonti D., Riva I., Landi F., Alborghetti L. IL-6 signalling pathway inactivation with siltuximab in patients with COVID-19 respiratory failure: an observational cohort study. medRxiv. 2020 doi: 10.1101/2020.04.01.20048561. [DOI] [Google Scholar]
- 20.Aziz M. Efficacy of tocilizumab in COVID-19: a systematic review and meta-analysis. J Med Virol. 2020 doi: 10.1002/jmv.26509. [DOI] [PubMed] [Google Scholar]
- 21.Malgie J., Schoones J.W., Pijls B.G. Decreased mortality in COVID-19 patients treated with Tocilizumab: a rapid systematic review and meta-analysis of observational studies. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liberati A., Altman D., Tetzlaff J., Mulrow C., Gøtzsche P., Loannidis J. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elliott J.H., Turner T., Clavisi O., Thomas J., Higgins J., Mavergames C. Living systematic reviews: an emerging opportunity to narrow the evidence–practice gap. PLoS Med. 2014;11 doi: 10.1371/journal.pmed.1001603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sterne J.A., Hernán M., Reeves B., Savović J., Berkamn N., Viswanathan M. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tleyjeh I.M., Ghomrawi H., Steckelberg J., Montori V., Hoskin T., Enders F. Conclusion about the association between valve surgery and mortality in an infective endocarditis cohort changed after adjusting for survivor bias. J Clin Epidemiol. 2010;63:130–135. doi: 10.1016/j.jclinepi.2008.06.022. [DOI] [PubMed] [Google Scholar]
- 26.Arabi Y.M., Mandourah Y., Al-Hameed F., Sindi A., Almekhlafi G., Hussein M. Corticosteroid therapy for critically ill patients with Middle East Respiratory Syndrome. Am J Respir Crit Care Med. 2018;197:757–767. doi: 10.1164/rccm.201706-1172OC. [DOI] [PubMed] [Google Scholar]
- 27.Pazzagli L., Linder M., Zhang M., Vago E., Stang P., Myers D. Methods for time-varying exposure related problems in pharmacoepidemiology: an overview. Pharmacoepidemiol Drug Saf. 2018;27:148–160. doi: 10.1002/pds.4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guyatt G., Oxman A., Akl E., Kunz R., Vist G., Brozek J. GRADE guidelines: 1. Introduction—GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64:383–394. doi: 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 29.Higgins J.P., Thompson S., Deeks J., Altman G. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deeks J.J., Higgins J.P.T., Altman D.G. Chapter 10: analysing data and undertaking meta-analyses. In: Higgins J.P.T., Thomas J., Chandler J., Cumpston M., Li T., Page M.J., editors. Cochrane handbook for systematic reviews of Interventions version 6.1 (updated september 2020) Cochrane; 2020. [Google Scholar]
- 31.DerSimonian R., Laird N. Meta-analysis in clinical trials. Control Clin Trial. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 32.Zhang J., Yu K.F. What's the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998;280:1690–1691. doi: 10.1001/jama.280.19.1690. [DOI] [PubMed] [Google Scholar]
- 33.Peters J.L., Sutton A., Jones D., Abrams K., Rushton L. Contour-enhanced meta-analysis funnel plots help distinguish publication bias from other causes of asymmetry. J Clin Epidemiol. 2008;61:991–996. doi: 10.1016/j.jclinepi.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 34.Colaneri M., Bogliolo L., Valsecchi P., Sacchi P., Zuccaro V., Brandolino F. Tocilizumab for treatment of severe COVID-19 patients: preliminary results from SMAtteo COvid19 REgistry (SMACORE) Microorganisms. 2020;8:695. doi: 10.3390/microorganisms8050695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.A study to evaluate the safety and efficacy of tocilizumab in patients with severe COVID-19 pneumonia (COVACTA) 2020. https://clinicaltrials.gov/ct2/show/NCT04320615 Available from: [Google Scholar]
- 36.Guaraldi G., Meschiari M., Cozzi-Lepri A., Milic J., Tonelli R., Menozzi M. Tocilizumab in patients with severe COVID-19: a retrospective cohort study. Lancet Rheumatol. 2020;2:e474–e484. doi: 10.1016/S2665-9913(20)30173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martinez-Sanz J., Muriel A., Ron R., Herrera S., Perez-Molina J., Moreno S. Effects of tocilizumab on mortality in hospitalized patients with COVID-19: a multicenter cohort study. medRxiv. 2020 doi: 10.1101/2020.06.08.20125245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mikulska M., Nicolini L., Signori A., Di Biagio A., Cepulcri C., Russo C. Tocilizumab and steroid treatment in patients with severe COVID-19 pneumonia. medRxiv. 2020:2020. doi: 10.1101/2020.06.22.20133413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Narain S., Stefanov D., Chau A., Weber A., Marder G., Kaplan B. Comparative survival analysis of immunomodulatory therapy for COVID-19 ‘cytokine storm’: a retrospective observational cohort study. medRxiv. 2020 doi: 10.1101/2020.06.16.20126714. [DOI] [Google Scholar]
- 41.Ramaswamy M., Mannam P., Comer R., Sinclair E., McQuaid B., Schmidt M. Off-label real world experience using tocilizumab for patients hospitalized with COVID-19 disease in a regional community health system: a case–control study. medRxiv. 2020 doi: 10.1101/2020.05.14.20099234. [DOI] [Google Scholar]
- 42.Rossi B., Nguyen L., Zimmermann P., Boucenna F., Baucher L., Dubret L. Effect of tocilizumab in hospitalized patients with severe pneumonia COVID-19: a cohort study. medRxiv. 2020 doi: 10.1101/2020.06.06.20122341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rossotti R., Travi G., Ughi N., Matteo C., Biaguera C., Fumagalli R. Safety and efficacy of anti-Il6-receptor tocilizumab use in severe and critical patients affected by coronavirus disease 2019: a comparative analysis. J Infect. 2020;S0163–4453:30467–30469. doi: 10.1016/j.jinf.2020.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roumier M. Interleukin-6 blockade for severe COVID-19. medRxiv. 2020 doi: 10.1101/2020.04.20.20061861. [DOI] [Google Scholar]
- 45.Efficacy of early administration of tocilizumab in COVID-19 patients. 2020. https://clinicaltrials.gov/ct2/show/NCT04346355 Available from: [Google Scholar]
- 46.Somers E.C., Eschenauer G., Troost J., Golob J., Gandhi T., Wang L. Tocilizumab for treatment of mechanically ventilated patients with COVID-19. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rosas I., Bräu N., Water M., Go R., Hunter B., Bhagani S. Tocilizumab in hospitalized patients with COVID-19 pneumonia. medRxiv. 2020 doi: 10.1101/2020.08.27.20183442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eimer J., Vesterbacka J., Svensson A., Stojanovic B., Wagrell C., Sönnerborg A. Tocilizumab shortens time on mechanical ventilation and length of hospital stay in patients with severe COVID-19: a retrospective cohort study. J Intern Med. 2020 doi: 10.1111/joim.13162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tsai A., Diaware O., Nahass R., Brunetti L. Impact of tocilizumab administration on mortality in severe COVID-19. medRxiv. 2020 doi: 10.1101/2020.07.30.20114959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hill J.A., Menon M., Dhanireddy S., Wurfel M., Green M., Jain R. Tocilizumab in hospitalized patients with COVID-19: clinical outcomes, inflammatory marker kinetics, safety, and a review of the literature. medRxiv. 2020 doi: 10.1101/2020.08.05.20169060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gokhale Y., Mehta R., Karnik N., Kulkarni U., Gokhale S. Tocilizumab improves survival in severe COVID-19 pneumonia with persistent hypoxia: a retrospective cohort study with follow-up from Mumbai, India. BMC Infect Dis. 2020;24 doi: 10.1016/j.eclinm.2020.100467. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Holt G.E., Batra M., Murthi M., Kambali Shweta, Santos Kayo, Perez Bastidas Maria. Lack of Tocilizumab effect on mortality in COVID19 patients. Res Square. 2020 doi: 10.21203/rs.3.rs-39875/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roomi S., Ullah W., Ahmed F., Farooq S., Sadiq U., Chohan A. Efficacy of hydroxychloroquine and tocilizumab in patients with COVID-19: single-center retrospective chart review. J Med Internet Res. 2020;22 doi: 10.2196/21758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Salvarani C., Dolci G., Massari M., Merlo D., Cavuto S., Savoldi L. Effect of tocilizumab vs standard care on clinical worsening in patients hospitalized with COVID-19 pneumonia: a randomized clinical trial. JAMA Intern Med. 2020 doi: 10.1001/jamainternmed.2020.6615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gupta S., Wang W., Hayek S., Chan L., Mathews K., Melamed M. Association between early treatment with tocilizumab and mortality among critically ill patients with COVID-19. JAMA Intern Medic. 2020 doi: 10.1001/jamainternmed.2020.6252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hermine O., Xavier M., Tharaux P., Resche-Rigion M., Porcher R., Ravaud P., CORIMUNO-19 Collaborative Group Effect of tocilizumab vs usual care in adults hospitalized with COVID-19 and moderate or severe pneumonia: a randomized clinical trial. JAMA Intern Med. 2020 doi: 10.1001/jamainternmed.2020.6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stone J.H., Frigault M., Serling-Boyd N., Fernandes A., Harvey L., Foulkes A., BACC Bay Tocilizumab Trial Investigators Efficacy of tocilizumab in patients hospitalized with COVID-19. New Engl J Med. 2020 doi: 10.1056/NEJMoa2028836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Roche’s phase III EMPACTA study showed Actemra/RoActemra reduced the likelihood of needing mechanical ventilation in hospitalised patients with COVID-19 associated pneumonia. 2020. https://www.roche.com/media/releases/med-cor-2020-09-18.htm Available from: [Google Scholar]
- 59.Ip A., Berry D., Hansen E., Goy A., Pecora A., Sinclaire B. Hydroxychloroquine and tocilizumab therapy in COVID-19 patients—an observational study. medRxiv. 2020 doi: 10.1101/2020.05.21.20109207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moreno Garcia E., Caballero R., Albiach L., Aguero D., Ambrosioni J., Bodro M. Tocilizumab is associated with reduction of the risk of ICU admission and mortality in patients with SARS-CoV-2 infection. medRxiv. 2020 doi: 10.1101/2020.06.05.20113738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu J., Huang J., Zhu G., Liu Y., Xiao H., Zhou Q. Systemic corticosteroids show no benefit in severe and critical COVID-19 patients in Wuhan, China: a retrospective cohort study. medRxiv. 2020 doi: 10.1101/2020.05.11.20097709. [DOI] [Google Scholar]
- 62.Wolkewitz M., Schumacher M. Survival biases lead to flawed conclusions in observational treatment studies of influenza patients. J Clin Epidemiol. 2017;84:121–129. doi: 10.1016/j.jclinepi.2017.01.008. [DOI] [PubMed] [Google Scholar]
- 63.Hall M., Pritchard M., Dankwa E., Baillie J., Carson G., Docherty A. ISARIC COVID-19 clinical data report: 8 june 2020. medRxiv. 2020 doi: 10.1101/2020.07.17.20155218. [DOI] [Google Scholar]
- 64.Campochiaro C., Dagna L. The conundrum of interleukin-6 blockade in COVID-19. Lancet Rheumatol. 2020;2:E579–E580. doi: 10.1016/S2665-9913(20)30287-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Anglemyer A., Horvath H.T., Bero L. Healthcare outcomes assessed with observational study designs compared with those assessed in randomized trials. Cochrane Database Syst Rev. 2014 doi: 10.1002/14651858.MR000034.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shrier I., Boivin J., Steele R., Platt R., Furlan A., Kakuma R. Should meta-analyses of interventions include observational studies in addition to randomized controlled trials? A critical examination of underlying principles. Am J Epidemiol. 2007;166:1203–1209. doi: 10.1093/aje/kwm189. [DOI] [PubMed] [Google Scholar]
- 67.Concato J., Shah N., Horwitz R.I. Randomized, controlled trials, observational studies, and the hierarchy of research designs. N Engl J Med. 2000;342:1887–1892. doi: 10.1056/NEJM200006223422507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Benson K., Hartz A.J. A comparison of observational studies and randomized, controlled trials. N Engl J Med. 2000;342:1878–1886. doi: 10.1056/NEJM200006223422506. [DOI] [PubMed] [Google Scholar]
- 69.van Vught L.A., Klouwenberg P., Spitoni C., Scicluna B., Wiewel M., Horn J. Incidence, risk factors, and attributable mortality of secondary infections in the intensive care unit after admission for sepsis. JAMA. 2016;315:1469–1479. doi: 10.1001/jama.2016.2691. [DOI] [PubMed] [Google Scholar]
- 70.Beumer M.C., Koch R., Beuningen D., OudeLashof A., Veerdonk F., Kolwijck E. Influenza virus and factors that are associated with ICU admission, pulmonary co-infections and ICU mortality. J Crit Care. 2019;50:59–65. doi: 10.1016/j.jcrc.2018.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lim S.J., Choi J., Lee S., Cho Y., Jeong Y., Kim H. Intensive care unit-acquired blood stream infections: a 5-year retrospective analysis of a single tertiary care hospital in Korea. Infection. 2014;42:875–881. doi: 10.1007/s15010-014-0651-z. [DOI] [PubMed] [Google Scholar]
- 72.Frigault M.J., Nikiforow S., Mansour M., Hu Z., Horowitz M., Riches M. Tocilizumab not associated with increased infection risk after CAR T-cell therapy: implications for COVID-19? Blood. 2020;136:137–139. doi: 10.1182/blood.2020006216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tanaka T., Narazaki M., Kishimoto T. Immunotherapeutic implications of IL-6 blockade for cytokine storm. Immunotherapy. 2016;8:959–970. doi: 10.2217/imt-2016-0020. [DOI] [PubMed] [Google Scholar]
- 74.Mihara M., Hashizume M., Yoshida H., Suzuko M., Shiina M. IL-6/IL-6 receptor system and its role in physiological and pathological conditions. Clin Sci (Lond) 2012;122:143–159. doi: 10.1042/CS20110340. [DOI] [PubMed] [Google Scholar]
- 75.van Gameren M.M., Willemse P., Mulder N., Limburg P., Groen H., Vellenga E. Effects of recombinant human interleukin-6 in cancer patients: a phase I-II study. Blood. 1994;84:1434–1441. [PubMed] [Google Scholar]
- 76.Stouthard J.M., Levi M., Hack C., Veenhof C., Romijin H., Sauerwein H. Interleukin-6 stimulates coagulation, not fibrinolysis, in humans. Thromb Haemost. 1996;76:738–742. [PubMed] [Google Scholar]
- 77.Banks R.E., Forbes M., Storr M., Higginson J., Thompson D., Raynes J. The acute phase protein response in patients receiving subcutaneous IL-6. Clin Exp Immunol. 1995;102:217–223. doi: 10.1111/j.1365-2249.1995.tb06659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sciascia S., Aprà F., Baffa A., Baldovino S., Boaro D., Boero R. Pilot prospective open, single-arm multicentre study on off-label use of tocilizumab in patients with severe COVID-19. Clin Exp Rheumatol. 2020;38:529–532. [PubMed] [Google Scholar]
- 79.Carsana L., Sonzogni A., Nasr A., Rossi R., Pellegrinelli A., Zerbi P. Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: a two-centre descriptive study. Lancet Infect Dis. 2020;20:1135–1140. doi: 10.1016/S1473-3099(20)30434-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rapkiewicz A.V., Mai X., Carsons S., Pittaluga S., Kleiner D., Berger J. Megakaryocytes and platelet-fibrin thrombi characterize multi-organ thrombosis at autopsy in COVID-19: a case series. EClinicalMedicine. 2020;24:100434. doi: 10.1016/j.eclinm.2020.100434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shakoory B., Carcillo J., Chatham W., Amdur R., Zhao H., Dinarello C. Interleukin-1 receptor blockade is associated with reduced mortality in sepsis patients with features of macrophage activation syndrome: reanalysis of a prior phase III trial. Crit Care Med. 2016;44:275–281. doi: 10.1097/CCM.0000000000001402. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.