Abstract
There is a growing recognition of sex differences in Alzheimer’s disease (AD). Females show an advantage over males on tests of verbal memory, which are used to diagnose AD and its precursor, amnestic mild cognitive impairment (aMCI). Women retain this advantage in aMCI despite reduced hippocampal volume and temporal lobe glucose metabolism. Here we examined whether this female advantage endures despite evidence of AD-specific pathology, cortical amyloid-β (Aβ) deposition measured with [18F]AV45 (florbetapir) positron emission tomography. Participants with normal cognition (N=304), aMCI (N=515), and AD dementia (N=175) were drawn from the Alzheimer’s Disease Neuroimaging Initiative (ADNI). Across and within diagnostic groups, we conducted linear regressions to examine the interaction of sex with cortical Aβ burden on immediate and delayed recall on the Rey Auditory Verbal Learning Test (RAVLT) adjusting for age, education, and APOE4. In the overall group, sex by cortical Aβ interaction was significant for delayed recall only. Overall, delayed recall performance was significantly better in women versus men among those with low to moderate Aβ burden, but women and men performed similarly among those with high Aβ burden. In diagnosis-stratified analyses, a significant sex by cortical Aβ interaction was observed for delayed recall in the aMCI group, but not in the normal or AD dementia groups. Thus, women maintain a verbal memory advantage over men in aMCI despite similar levels of AD pathology. Although this advantage may benefit women by delaying verbal memory impairment until more advanced pathology, it may also delay diagnosis of aMCI and treatment intervention.
Keywords: memory, cognitive reserve, amyloid, sex, positron-emission tomography
INTRODUCTION
The “cognitive reserve” theory is used to explain how individuals can vary in clinical measures of cognitive aging yet have a similar degree of Alzheimer’s disease (AD) brain pathology [1,2]. It proposes that high educational attainment, high IQ, and other cognitively advantageous characteristics confer a “reserve” capacity to engage compensatory brain networks or cognitive strategies so that cognitive function can be maintained despite brain pathology [1–3]. The theory predicts that, for a given level of clinical severity, persons with high reserve would have more brain pathology than persons with low cognitive reserve [1–3]. However, once brain pathology depletes brain resources past a threshold level, cognitive decline begins and is more accelerated in persons with high reserve because the pathology is more advanced at that point. In support of the cognitive reserve hypothesis, persons with high education show these predicted profiles at AD onset [4–6].
Throughout life, females perform better than males on verbal memory tests [7–8]. The cognitive reserve theory may explain the female advantage in verbal memory that persists in the precursor stage to AD dementia, amnestic mild cognitive impairment (aMCI) [9–11]. We previously showed that, in aMCI, women continued to outperform men in verbal memory despite moderate levels of hippocampal volume loss and temporal lobe hypometabolism, suggesting that women sustain their advantage despite advancing disease burden [9,10]. Others reported that the female advantage in verbal memory is eliminated or attenuated among patients with AD dementia [9–12]. Consistent with the cognitive reserve theory, the elimination of the female advantage in AD dementia suggests that women may experience more accelerated decline than men when transitioning from aMCI to AD dementia.
The female advantage in verbal memory is clinically relevant because verbal memory tests are used to diagnose aMCI and AD dementia, and the norms for these tests are not consistently sex-stratified or adjusted. Critically, the female advantage might mask underlying brain pathology particularly in earlier disease stages and, thereby, delay aMCI diagnosis.
Herein, we extended our previous findings of sex differences in how verbal memory performance relates to hippocampal volume and temporal metabolism to a hallmark pathologic characteristic specific to AD, amyloid-β (Aβ) plaque deposition. Aβ plaque deposition is an initial step in the AD pathologic cascade [13,14], and it is essential for AD diagnosis confirmation in autopsy [15]. [18F]AV45 (florbetapir) positron emission tomography (18F-AV45-PET) is a valid, in-vivo measure of brain Aβ deposition [16]. We examined whether the association between verbal memory performance and a summary measure of cortical [18F]AV45 Aβ burden differs by sex within and across diagnostic groups (Normal, aMCI, AD dementia). We hypothesized that the magnitude of the female advantage in verbal memory would vary by cortical Aβ burden. Specifically, women would perform significantly better on verbal memory tests at low and moderate levels of Aβ burden but not at high levels which indicate more advanced disease.
MATERIALS AND METHODS
Participants and Data Source
Cross-sectional data were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu) in July, 2014. Information about ADNI is provided at www.adni-info.org. ADNI is a longitudinal, multi-site, cohort study that began in 2003 as a public-private partnership, led by Principal Investigator Michael W. Weiner, MD. The broad aim of ADNI is to test whether neuroimaging markers, other biological markers, and clinical and neuropsychological assessment can be combined to measure the progression of mild cognitive impairment (MCI) and early Alzheimer’s disease (AD). ADNI recruitment targeted healthy older adults, individuals with early or late MCI and early AD patients [17]. See www.loni.ucla.edu/ADNI for recruitment procedures and www.adni-info.org/Scientists/ADNIGrant/ProtocolSummary.aspx for eligibility criteria. Our initial study sample consisted of the 1,006 participants who had concurrent diagnostic, [18F]AV45 PET, and verbal memory assessments from one visit cycle. We excluded seven participants that did not have apolipoprotein ε [ApoE] genotype, three participants who showed evidence of brain infection, infarction, or other focal lesions at their baseline MRI, and two participants with a MCI diagnosis that did not fulfill standard criteria for aMCI [18]. Thus, our final sample consisted of 994 participants (252 from ADNIGO and 742 from ADNI2). ADNI was approved by the institutional review board at each site and written consent was obtained from all participants.
Verbal Memory Outcomes
The Rey Auditory Verbal Learning Test (RAVLT) served as our verbal memory measure [19]. In each of five learning trials, the same list of 15 unrelated words is read aloud to the participant and the participant is asked to recall aloud as many words as possible. The number of correctly recalled words across the five learning trials comprises the “immediate recall score” (range: 0–75). After the learning trials, an interference list of 15 words that are unrelated to one another and the first list is read aloud to the participant, and the participant is asked to recall aloud as many of these words as possible. Then, the participant is asked to recall aloud as many words as possible from the first word list. After a 30-minute delay in which the participant completes only non-verbal tasks, the participant is again asked to recall as many words as possible from the first list as possible. The number of words correctly recalled after the delay comprises the “delayed recall score” (range: 0–15). The immediate and delayed recall scores served as our outcome measures because these outcomes demonstrated the strongest ability in differentiating between normal controls and AD among other RAVLT outcomes (e.g. hits and false positive errors on recognition) [20].
Diagnostic Criteria
ADNI criteria for an AD dementia diagnosis included a Mini Mental State Examination (MMSE) score between 20 and 26, a Clinical Dementia Rating (CDR) score of 0.5 or 1, and a diagnosis of probable AD dementia based on the NINCDS/ADRDA [21] criteria. Criteria for an aMCI diagnosis included a MMSE score between 24 and 30, a CDR of 0.5, a subjective memory complaint, and objective memory impairment as indicated by education-adjusted, but not age-adjusted, scores on the Wechsler Memory Scale Logical Memory II, but no significant impairment in non-memory cognitive domains or interference in everyday activities. An early aMCI diagnosis reflects modest impairment on delayed recall of Logical Memory II (score=9–11 for 16 or more years of education; score=5–9 for 8–15 years of education; score=3–6 for 0–7 years of education), whereas, a late aMCI diagnosis reflects more advanced impairment within the aMCI criteria (score≤8 for 16 or more years of education; score≤4 for 8–15 years of education; score≤2 for 0–7 years of education) [22]. Although late aMCI patients showed significantly worse performance than early aMCI patients on Logical Memory and RAVLT outcomes (p’s<0.05), early aMCI and late aMCI cases were combined into an aMCI group because statistical power was limited if the aMCI group was stratified by both early vs. late and sex. A “Normal” diagnosis required a MMSE score between 24 and 30, a CDR of 0, no subjective memory complaints, verified by a study partner, beyond what one would expect for age, and memory scores on the Logical Memory II Delayed Recall above the cutoffs for memory impairment.
AV45-PET
[18F]AV45 PET image acquisition is described in detail at http://www.adni-info.org. Participants were intravenously injected with an approximately 370 MBq bolus of the tracer, [18F]AV45. Following a 50-minute uptake period, a cranial PET scan was conducted and images were reconstructed using iterative algorithms immediately after the scan. Levels of tracer uptake from regions of interest (ROI) were measured and standardized by creating a standardized uptake value ratio (SUVR) of tracer uptake in the ROI to the whole cerebellum since the cerebellum stays relatively free of Aβ plaques in AD. T1-weighted, structural images from 1.5-T or 3-T MRI scanners were segmented and parcellated with Freesurfer (version 4.5.0) and used to define cortical ROIs and the cerebellar reference region. ROIs included lateral and medial frontal, anterior and posterior cingulate, lateral parietal, and lateral temporal regions. Mean [18F]AV45 SUVR were extracted from gray matter within ROIs. Scans were repeated if motion artifact was detected. Preprocessing of the scans was conducted by ADNI investigators at University of California, Berkeley as previously described [23]. Our primary analysis used a summary measure of Aβ deposition averaged across the four ROIs and divided by the whole cerebellum..
Statistical Analysis
In the overall sample and within diagnostic group, differences in sample characteristics and variables of interest (RAVLT scores and [18F]AV45 SUVR) between sexes were tested using independent t-tests for continuous variables and Chi-square tests for categorical variables. In the overall sample, multivariable linear regressions were conducted to test the independent and interactive associations of sex and temporal [18F]AV45 SUVR for the RAVLT immediate and delayed recall. Analyses were adjusted for age, education, apolipoprotein ɛ (ApoE) genotype, and diagnostic group (overall sample only). ApoE genotype was dichotomized as ApoE4 carriers versus non-carriers. In model one, we examined the independent effects of sex and cortical [18F]AV45 SUVR on RAVLT immediate and delayed recall. In model two, a sex × cortical [18F]AV45 SUVR interaction term was added to the model, but removed if not significant (p>0.05). Statistical procedures were repeated but were stratified by diagnostic group rather than covarying for diagnostic group. In light of our previous finding of a stronger association between RAVLT performance and hippocampal volume ratio (hippocampal volume/ intracranial volume) and temporal lobe glucose metabolic rate (TLGluMR) in women versus men, we repeated analyses adjusting for hippocampal volume ratio and TLGluMR to determine whether these imaging outcomes accounted for any cortical Aβ × sex interactions on RAVLT performance..
In exploratory analyses, we sought to determine the specificity of our findings to the sexually-dimorphic cognitive domain of verbal memory and not to sex neutral cognitive domains such as frontally-mediated, executive function. For this analysis, our executive function outcome was time to complete the Trail Making Test (TMT), Part B [24]. Our predictor variables were the independent and interactive associations of sex and cortical [18F]AV45 SUVR. All statistics were performed using SAS version 9.4 (SAS Institute Inc., Cary, N.C). Significance was defined as p<0.05 (two-sided).
RESULTS
Sample Characteristics
Our overall sample of 994 participants was comprised of 304 Normals, 515 aMCI (57% early aMCI), and 175 AD dementia patients. Sample characteristics are displayed by sex in the overall sample in Table 1 and by diagnostic group in Table 2. In the overall sample, women were younger, less educated, and had a lower mean severity rating on the CDR-SOB compared to men (p’s<0.05). As expected, women performed significantly better on the verbal memory measure, the Rey Auditory Verbal Learning Test (RAVLT) immediate and delayed recall compared to men (p≤0.001). Overall, cortical and [18F]AV45 standard uptake value ratios (SUVR) were similar between women and men. In diagnosis-stratified analyses, women were younger and less educated than men in each group (p’s<0.05). The female advantage on the RAVLT was evident in Normal and aMCI groups (p’s≤0.001) but not in the AD dementia group (p’s>0.05). Cortical [18F]AV45 SUVRs were higher in women versus men in the Normal and AD dementia groups (p’s<0.05), but not in the aMCI group (p>0.05). We categorized participants into Aβ positive and negative groups based on a previously-employed SUVR threshold of 1.11 which reflects an Aβ burden associated with a preclinical AD diagnosis [25,26]. The prevalence of Aβ positivity was significantly higher in Normal women versus Normal men (X2=4.6, p=0.03).
Table 1.
Overall sample characteristics by sex
| Parameters | Females N=455 Mean (SD) |
Males N=539 Mean (SD) |
p-value |
|---|---|---|---|
| Age | 71.8 (7.2) | 73.7 (7.1) | <0.001 |
| Education (years) | 15.6 (2.6) | 16.7 (2.6) | <0.001 |
| Race (% Caucasian) | 90.9 | 94.1 | 0.06 |
| APOE4 carrier (%) | 43.5 | 44.1 | 0.84 |
| Global cognition (MMSE) | 27.5 (2.9) | 27.3 (2.8) | 0.12 |
| CDR-SOB | 1.5 (1.9) | 1.7 (1.9) | 0.05 |
| RAVLT immediate recall | 40.4 (13.2) | 34.0 (12.3) | <0.001 |
| RAVLT delayed recall | 5.7 (4.7) | 4.2 (4.0) | <0.001 |
| Cortical [18F]AV45 SUVR | 1.21 (0.22) | 1.19 (0.23) | 0.37 |
Note. MMSE = Mini Mental State Exam. RAVLT = Rey Auditory Verbal Learning Test. CDR-SOB = Clinical Dementia Rating – Sum of Boxes. SUVR = Standardized uptake value ratio.
Table 2.
Sample characteristics by sex and diagnostic group
| Normals N=304 |
aMCI N=515 |
AD dementia N=175 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameters | Females N=158 (52%*) Mean (SD) |
Males N=146 (48%*) Mean (SD) |
p-value | Females N=226 (44%*) Mean (SD) |
Males N=289 (56%*) Mean (SD) |
p-value | Females N=71 (40%*) Mean (SD) |
Males N=104 (60%*) Mean (SD) |
p-value |
| Age | 73.0 (5.6) | 74.6 (6.1) | 0.02 | 70.8 (7.8) | 72.7 (7.3) | 0.005 | 72.3 (7.7) | 75.1 (7.3) | 0.02 |
| Education (years) | 15.9 (2.6) | 17.2 (2.5) | <0.001 | 15.6 (2.6) | 16.5 (2.7) | <0.001 | 15.0 (2.5) | 16.5 (2.5) | <0.001 |
| Race (% Caucasian) | 89.2 | 91.1 | 0.59 | 92.4 | 95.5 | 0.14 | 90.1 | 94.2 | 0.76 |
| APOE4 carrier (%) | 29.1 | 23.3 | 0.25 | 44.7 | 48.1 | 0.44 | 71.8 | 62.5 | 0.20 |
| MMSE score | 29.0 (1.3) | 28.9 (1.3) | 0.62 | 28.1 (1.8) | 28.0 (1.7) | 0.33 | 22.4 (3.1) | 22.9 (2.6) | 0.28 |
| CDR-Sum of Boxes | 0.05 (0.15) | 0.06 (0.24) | 0.46 | 1.4 (0.9) | 1.5 (1.0) | 0.21 | 5.0 (2.0) | 4.8 (2.0) | 0.51 |
| Early aMCI (%) | - | - | - | 57.6 | 58.1 | 0.64 | - | - | - |
| RAVLT immediate recall | 48.1 (9.1) | 43.0 (11.4) | <0.001 | 40.5 (11.7) | 34.1 (9.9) | <0.001 | 22.9 (8.3) | 21.1 (6.6) | 0.11 |
| RAVLT delayed recall | 8.4 (3.7) | 6.8 (4.2) | 0.001 | 5.5 (4.6) | 4.1 (3.6) | <0.001 | 0.4 (0.9) | 0.7 (1.2) | 0.17 |
| Cortical [18F]AV45 SUVR | 1.12 (0.18) | 1.08 (0.16) | 0.04 | 1.20 (0.22) | 1.21 (0.22) | 0.70 | 1.44 (0.16) | 1.33 (0.2) | 0.001 |
Note.
The proportion of females to males significantly varies across diagnostic groups, X2=7.83, p=0.02. aMCI = amnestic Mild Cognitive Impairment. AD = Alzheimer’s disease. MMSE = Mini Mental State Exam. RAVLT = Rey Auditory Verbal Learning Test. CDR = Clinical Dementia Rating. SUVR = standardized uptake value ratio.
Linear Regression Results – Overall Sample
Regression results are displayed in Table 2. Our hypothesis that the magnitude of the female advantage in verbal memory would depend on cortical Aβ burden was supported by a significant sex by cortical [18F]AV45 SUVR interaction for delayed recall (B=3.22, β=−0.12, SE=0.99, p=0.001) with a moderate and clinically-meaningful effect size [27]. On the other hand, the interaction was a non-significant trend for immediate recall (B=5.01, β=−0.12, SE=2.68, p=0.06 (see Figure 1A and 2A). Greater cortical [18F]AV45 SUVR was significantly associated with poorer RAVLT scores in both men and women; however, this association was stronger in women for delayed recall (B=−4.94, β=−0.25, SE=0.80, p<0.001for women versus B=−1.72, β=−0.09, SE=0.74, p=0.02 for men) and a similar pattern was seen on immediate recall (B=−11.47, β=−0.20, SE=2.16, p<0.0001 for women versus B=−6.46, β=−0.11, SE=1.99, p=0.001for men). This is reflected in the steeper slopes for delayed and immediate recall scores across cortical [18F]AV45 SUVR in women compared to men in Figures 1B and 1A. The female advantage on RAVLT outcomes was most evident in the low to moderate range of cortical [18F]AV45 SUVR (left side of the X axis), but, the female advantage disappeared among persons with higher cortical [18F]AV45 SUVR (right side of the X axis), with men and women performing similarly. When we added the hippocampal volume ratio (hippocampal volume/ intracranial volume) and temporal lobe glucose metabolic rate (TLGluMR) as covariates in analyses, the cortical [18F]AV45 SUVR × sex interaction remained significant for delayed recall (B=−3.38, SE=1.21, p=0.005).
Figure 1. RAVLT immediate recall scores as a function of cortical [18F]AV45 SUVR and sex in the (A) overall group, (B) Normals, (C) aMCI and (D) AD dementia.
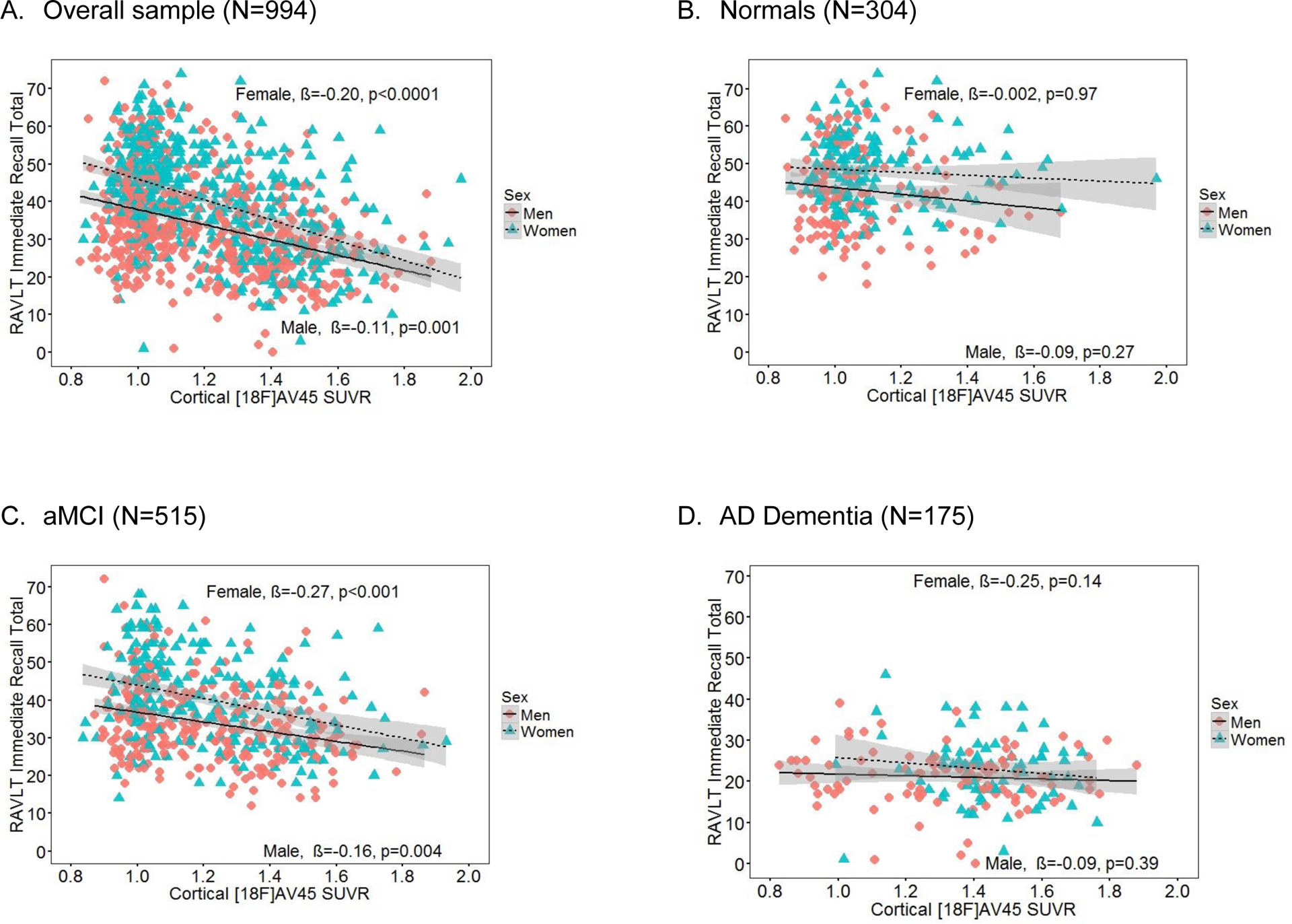
Note. Sex × cortical [18F]AV45 SUVR interaction just missed significance in the overall sample (A, p=0.06), and was not significant in diagnosis-specific groups of Normals (B), aMCI (C) and AD (D) (p’s>0.05). SUVR = standardized uptake value ratio, RAVLT = Rey Auditory Verbal Learning Test. β = sex-specific standardized regression coefficient of the relationship between RAVLT scores and cortical [18F]AV45 SUVR controlling for age, education and ApoE4 and diagnosis (overall sample only). aMCI = amnestic mild cognitive impairment. AD = Alzheimer’s disease.
Figure 2. RAVLT delayed recall scores as a function of cortical [18F]AV45 SUVR and sex in the (A) overall group, (B) Normals, (C) aMCI and (D) AD dementia.
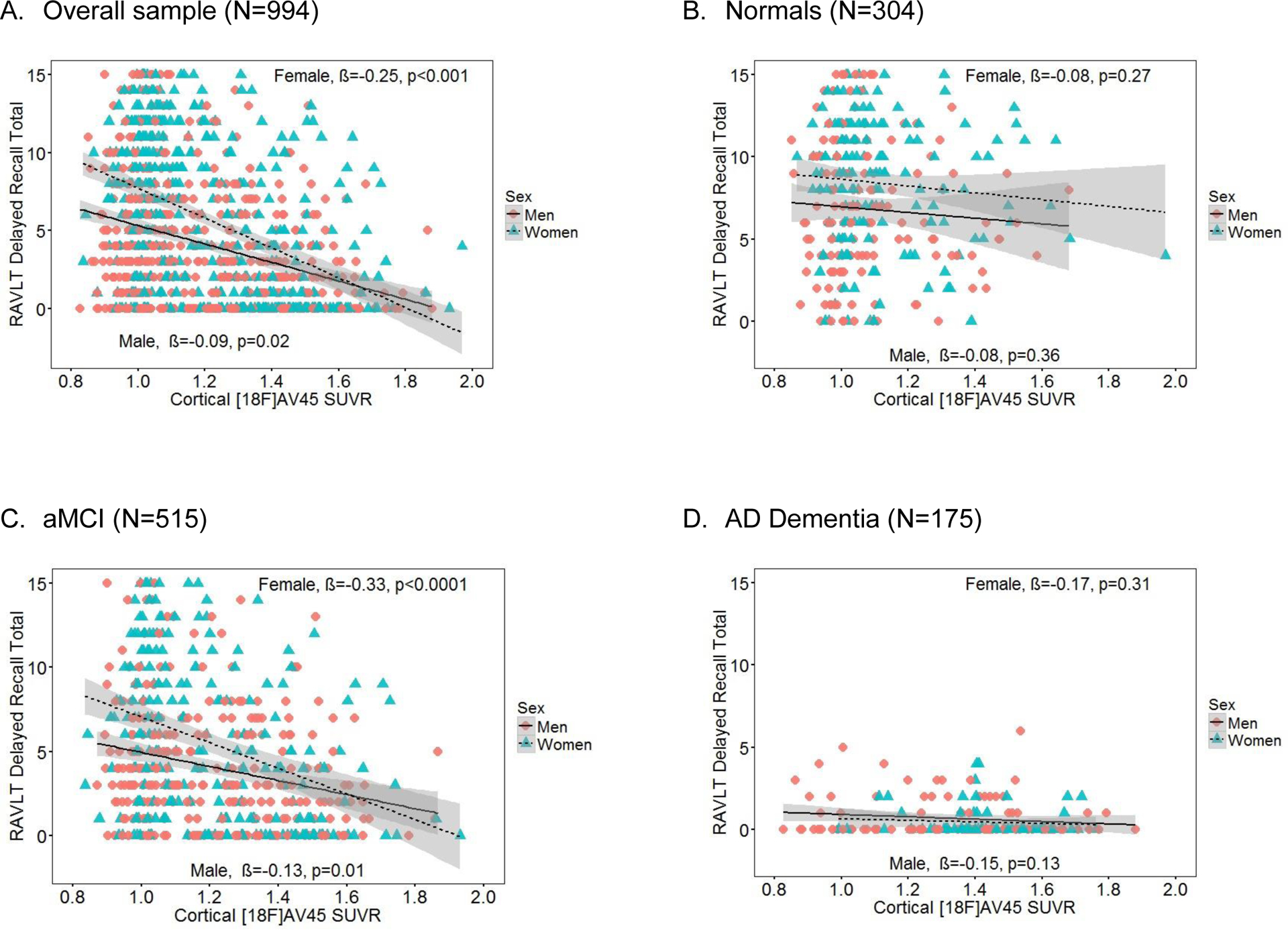
Note. Sex × cortical [18F]AV45 SUVR interaction was significant in the overall sample (A, p=0.0007) and in aMCI (C, p=0.009), but not in Normals (B) or AD (D) (p’s>0.05). SUVR= standardized uptake value ratio. RAVLT = Rey Auditory Verbal Learning Test. β = sex-specific standardized regression coefficient of the relationship between RAVLT scores and cortical [18F]AV45 controlling for age, education, ApoE4 and diagnosis (overall sample only). aMCI = amnestic mild cognitive impairment. AD = Alzheimer’s disease.
Linear Regression Analyses – Stratified by Diagnostic Group
In diagnosis-stratified analyses, the sex by cortical [18F]AV45 SUVR interaction was significant in the aMCI group for delayed recall (B=3.76, β=−0.14, SE=2.68, p=0.01) with a moderate and clinically-meaningful effect size [27], but it was not significant for immediate recall (B=5.87, β=−0.08, SE=3.96,p=0.14) (Figures 1C and 2C). The interaction was not significant for either RAVLT outcome in the AD dementia (Figures 1D and 2D) or Normal groups (Figures 1B and 2B). The significant sex by cortical [18F]AV45 SUVR interaction for delayed recall in the aMCI group had a similar pattern as the overall sample, whereby the association between RAVLT performance and cortical [18F]AV45 SUVR was stronger in women with aMCI (B=3.48, β=0.21, SE=0.57, p<0.001) compared to men with aMCI (B=1.92, β=0.12, SE=0.75, p=0.01). Figure 2C shows that the female advantage on delayed recall in aMCI is most evident in the low to moderate range of cortical [18F]AV45 SUVR (left side of the X axis), but, among persons with high cortical [18F]AV45 SUVR, the advantage disappears (right side of the X axis). Conversely, among Normals, women outperformed men on immediate (p<0.0001) and delayed recall (p<0.0003) regardless of cortical [18F]AV45 SUVR, and cortical [18F]AV45 SUVR was not associated with immediate (p=0.47) or delayed recall (p=0.46) (Figures 1B and 2B). Among AD dementia patients, women only outperformed men on immediate (p=0.02; Figure 1D) but not delayed recall (p=0.54; Figure 2D), and cortical [18F]AV45 SUVR was not associated with immediate (p=0.15) or delayed recall (p=0.08). As expected, in exploratory analysis examining the specificity of these sex differences, Trails B performance was significantly associated with cortical [18F]AV45 SUVR (B=0.33, SE=0.07, p<0.001) but not with sex (B=−0.002, SE=0.03, p=0.94) in the overall sample. The interactive association of sex × [18F]AV45 SUVR on Trails B performance was not significant in the overall group (B=0.02, SE=0.12, p=0.84). Results were the same in the diagnosis-specific groups.
DISCUSSION
We investigated whether the female advantage in verbal memory might represent a form of cognitive reserve by examining sex differences in the relationship between a clinical marker of AD (verbal memory performance) and an index of AD pathology (cortical Aβ burden). Consistent with the broader literature and prior findings from this sample, we found that women significantly outperformed men on immediate and delayed measures of verbal memory in the overall sample and in the Normal and aMCI groups [7–11]. As demonstrated previously [28,29], we confirmed that across the two sexes combined, greater cortical Aβ burden was related to poorer verbal memory performance in the overall sample and in the aMCI group. Our novel finding was a sex difference in the relationship between cortical Aβ burden and verbal memory performance in the overall sample. Specifically, females showed an advantage in memory performance when cortical Aβ burden was low to moderate, but not when Aβ burden was greater. This sex difference suggested an accelerated decline in verbal memory among women once the level of AD pathology reaches a critical threshold. Previously, we found a sex difference in how verbal memory performance relate to imaging markers of structural and functional decline (hippocampal volume and TLGluMR) [9,10]. The current results extend those findings more directly to neuropathology of AD dementia with an imaging marker that is more specific to AD pathology, Aβ plaque deposition.
In diagnosis-stratified analyses, as well as in previous studies [30,31], cortical Aβ burden was not related to RAVLT performance among Normals. This finding is expected given that Aβ accumulation in the brain can occur a decade or more before clinical deficits manifest [32,33]. The lack of relationship between Aβ burden and RAVLT performance in Normals may also reflect the more limited variability in Aβ deposition among Normals (SD=0.16) compared to the aMCI (SD=0.21) or AD (SD=0.30) groups. There was a significant effect of sex on Aβ burden among Normals in ADNI; women had a significantly higher cortical Aβ burden compared to men and, yet, showed significantly better verbal memory performance. Furthermore, we found that the prevalence of Aβ positivity was significantly higher in Normal women versus Normal men. Similarly, Jack et al. [34] reported a trend toward significantly greater cortical Aβ burden in Normal women versus Normal men after age 70 despite better verbal memory performance in women.
We found that in comparison with men, women in the Normal group had higher levels of Aβ burden and that a higher proportion met criteria for Aβ positivity. These findings support the cognitive reserve theory by showing that women are better able than men to maintain normal verbal memory performance despite accumulating Aβ pathology. In our prior reports, we found that hippocampal volume and metabolic imaging markers (FDG-PET) did not show a sex difference in Normals. This apparent discrepancy is explained by the well-established temporal evolution of AD biomarkers; Aβ deposition is thought to be the initial event followed by metabolic dysfunction and neurodegeneration and, ultimately, cognitive impairment [32,33].
Similar to our previous studies with imaging markers of hippocampal volume ratio or TLGluMR, results in the overall sample for the RAVLT delayed recall were driven by the aMCI group. As in the overall sample, women with aMCI outperformed men with aMCI on delayed recall when cortical Aβ burden was in the low to moderate range, but not at high levels of cortical Aβ burden. This suggests that women with aMCI sustain their verbal memory advantage over men despite having similarly moderate levels of cortical Aβ burden. Unlike our previous studies with hippocampal volume ratio and TLGluMR, the sex by cortical Aβ burden interaction was not significant for immediate recall in the overall sample or within the aMCI group. Within the broader framework of the temporal evolution of AD biomarkers, this inconsistency may reflect the looser coupling of cognitive impairment with Aβ deposition compared to neurodegenerative and metabolic markers. That is, Aβ deposition may be an early event which precedes cognitive decline by years. Biomarkers of neurodegeneration (volumetrics) and metabolism (FDG-PET) may reflect later processes more tightly linked with cognitive performance. The female advantage in verbal memory has been shown to be stronger for delayed versus immediate recall, and this could be why, with the looser coupling of amyloid to cognitive performance, a sex effect manifested with delayed recall only [8,35].
In AD dementia, men and women performed similarly on RAVLT delayed recall which supports our hypothesis that the female advantage in verbal memory would disappear among individuals with high Aβ burden; however, a floor effect with delayed recall scores limits interpretation. Inconsistent with hypotheses, women with AD dementia continued to significantly outperform men with AD dementia on immediate recall after adjusting for covariates; however, the female advantage was smaller (mean difference=1.8) compared to Normal (mean difference=5.1) and aMCI (mean difference=6.4) groups. Our results suggest a weakening of the female advantage in AD rather than an elimination or even a reversal as reported by others [11,12]. Cortical Aβ deposition was not associated with either RAVLT outcomes in AD dementia likely because Aβ aggregation plateaus prior to AD dementia diagnosis [36].
We suggest that the life-long, female advantage in verbal memory may represent a sex-specific form of cognitive reserve that enables women to maintain verbal memory performance for longer in the AD trajectory compared to men despite accumulating pathology. Importantly, when we applied a previously used cut-score for memory impairment on the RAVLT (<37 on immediate recall [37] and <8 on delayed recall [38]) to the current data, we found that women at this cut-score had a greater degree of cortical Aβ deposition compared to men for both immediate (~1.4 versus 1.1; see Figure 1A) and delayed recall (~1.0 versus 0.7; see Figure 2A). Therefore, in line with the cognitive reserve theory, verbal memory impairment clinically manifested in women at a more severe stage of AD pathology compared to men.
These findings might shed light on other reported sex differences in AD. Paradoxically, studies have reported higher incidence of AD dementia in women [39,40] whereas others have reported higher incidence of aMCI in men [41,42], although not consistently [43,44]. A higher risk of AD in women could reflect their overall higher Aβ burden in the Normal stage. However, if the female advantage in verbal memory allows women to delay verbal memory impairment until a later disease state, at which point they decline more rapidly as our study suggests, then the window of time for an aMCI diagnosis will be shorter in women versus men. In support of a more rapid decline in women after the onset of clinical impairment, an ADNI study reported that the rate of cognitive decline in women with MCI was two times faster compared to men with MCI [45]. Thus, among individuals that transition from Normal to AD dementia, women may transition more abruptly. Consequently, the aMCI stage would be less likely to be observed in women versus men in longitudinal studies with assessments occurring every 12 months or more. In accordance with this view, the Einstein Aging Study found that women were less likely to transition from normal to MCI but more prone to transition from normal to dementia than men [46].
Our study has limitations. Because our analysis was cross-sectional, we cannot determine the temporal relationship between verbal memory and cortical Aβ deposition. However, it is well-established that Aβ deposition can occur up to a decade before clinical memory impairment [32,33]. Our cross-sectional design precluded us from comparing rates of decline between men and women, which would provide a stronger test of the cognitive reserve theory. Longitudinal analyses are underway in ADNI in order to examine sex differences in rates of decline in verbal memory. Because men were significantly older than women in the overall sample and within all diagnostics groups, we adjusted for age in all analyses; however, this may not fully account for age effects. Lastly, ADNI is based on a convenience sample of volunteers, who are predominantly white and well-educated compared with the general US population, which limits generalizability of results.
To conclude, our results showed that the magnitude of the female advantage in verbal memory varies across levels of cortical Aβ burden. Specifically, women sustained the advantage despite cortical Aβ burden at minimal (i.e. mean difference of ~10 words in RAVLT immediate and ~3 words in RAVLT delayed) or moderate (i.e. mean difference of ~8 words in RAVLT Immediate and ~2 words in RAVLT delayed) levels; however, the female advantage was diminished when cortical Aβ burden was high. Our results have been consistent across three AD biomarkers which represent distinct pathologic hallmarks of the disease including: 1) hippocampal volume, a measure of neural loss; 2) TLGluMR, a measure of metabolic or neural dysfunction; and now 3) Aβ burden, a measure of AD-specific pathology. This set of findings provides compelling evidence that the female advantage in verbal memory may serve as a domain-specific form of cognitive reserve. Females may be able to sustain their advantage in verbal memory despite moderate levels of hippocampal atrophy, metabolic deficits and Aβ deposition. If replicated, our findings indicate the need to assess whether the female advantage paradoxically puts them at a disadvantage because a diagnosis of aMCI is made at a later disease stage compared to men. If so, then applying sex-based norms for all verbal memory tests may aid in identifying women at an earlier disease stage when currently available treatments are the most beneficial.
Table 3.
Results of multivariable linear regression analyses modeling the independent and interactive associations of sex and cortical Aβ burden with verbal memory performance.
| Multivariable Linear Regression Models | ||||||
|---|---|---|---|---|---|---|
| Model 1: No interactions in model | Model 2: Interaction included in model | |||||
| Sample/Outcome | Sex (male vs. female) | Cortical [18F]AV45 SUVR¥ | Sex × cortical [18F]AV45 SUVR¥ | |||
| B, β (SE) | p-value | B, β (SE) | p-value | B, β (SE) | p-value | |
| Overall Sample | ||||||
| Immediate recall | −5.43, −0.21 (0.62) | <0.0001 | −8.71, −0.15 (1.59) | <0.0001 | 5.01, −0.06 (2.68) | 0.06 |
| Delayed recall | −1.28, −0.14 (0.23) | <0.0001 | −3.16, −0.16 (0.59) | <0.0001 | 3.22, −0.12 (0.99) | 0.001 |
| Controls | ||||||
| Immediate recall | −5.45, −0.26 (1.16) | <0.0001 | −2.47, −0.04 (3.44) | 0.47 | −5.61, −0.07 (6.70) | 0.40 |
| Delayed recall | −1.69, −0.21 (0.47) | 0.0003 | −1.02, −0.04 (1.39) | 0.46 | 0.23, −0.01 (2.71) | 0.93 |
| aMCI | ||||||
| Immediate recall | −5.97, −0.26 (0.89) | <0.0001 | −10.74, −0.21 (2.22) | <0.0001 | 5.87, −0.08 (3.96) | 0.14 |
| Delayed recall | −1.33, −0.16 (0.34) | 0.0001 | −4.18, −0.22 (0.85) | <0.0001 | 3.76, −0.14 (1.51) | 0.01 |
| AD Dementia | ||||||
| Immediate recall | −2.82, −0.19 (1.22) | 0.02 | −4.16, −0.12 (2.88) | 0.15 | 5.40, −0.14 (6.19) | 0.38 |
| Delayed recall | 0.11, −0.05 (0.18) | 0.54 | −0.78, −0.15 (0.43) | 0.08 | 0.10, −0.02 (0.94) | 0.91 |
Note. All analyses were adjusted for age, education, ApoE status and diagnostic group (overall sample only). SUVR = standardized uptake value ratio. B = unstandardized regression coefficient. β = standardized regression coefficient. aMCI = amnestic Mild Cognitive Impairment. AD = Alzheimer’s disease.
ACKNOWLEDGMENTS
Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
Footnotes
CONFLICTS OF INTEREST
Dr. Sundermann reports no disclosures. Dr. Maki reports no disclosures. Dr. Rubin reports no disclosures. Dr. Lipton reports research support from the NIH: PO1 AG003949 (Program Director), PO1AG027734 (Project Leader), RO1AG025119 (Investigator), RO1AG022374–06A2 (Investigator), RO1AG034119 (Investigator), RO1AG12101 (Investigator) and has reviewed for the NIA and NINDS, Dr. Landau reports no disclosures. Dr. Biegon reports no disclosures.
Data used in preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. A complete listing of ADNI investigators can be found at: http://adni.loni.usc.edu/wp-content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf
References
- [1].Stern Y, Gurland B, Tatemichi TK, Tang MX, Wilder D, Mayeux R (1994) Influence of education and occupation on the incidence of Alzheimer’s disease. JAMA 271, 1004–1010. [PubMed] [Google Scholar]
- [2].Stern Y (2002) What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc 8, 448–460. [PubMed] [Google Scholar]
- [3].Stern Y, Zarahn E, Hilton HJ, Flynn J, DeLaPaz R, Rakitin B (2003) Exploring the neural basis of cognitive reserve. J Clin Exp Neuropsychol 25(5), 691–701. [DOI] [PubMed] [Google Scholar]
- [4].Stern Y, Albert S, Tang M-X, Tsai W-Y (1999) Rate of memory decline in AD is related to education and occupation: Cognitive reserve? Neurology 53, 1942–1947. [DOI] [PubMed] [Google Scholar]
- [5].Le Carret N, Auriacombe S, Letenneur L, Bergua V, Dartigues JF, Fabrigoule C (2005) Influence of education on the pattern of cognitive deterioration in AD patients: the cognitive reserve hypothesis. Brain Cogn 57, 120–126. [DOI] [PubMed] [Google Scholar]
- [6].Hall CB, Derby C, LeValley A, Katz MJ, Verghese J, Lipton RB (2007) Education delays accelerated decline on a memory test in persons who develop dementia. Neurology 69(17), 1657–1664. [DOI] [PubMed] [Google Scholar]
- [7].Kramer JH, Delis DC, Daniel MH (1988) Sex differences in verbal learning. J Clin Psychol 44, 907–915. [Google Scholar]
- [8].Aartsen MJ, Martin M, Zimprich D (2004) Gender differences in level and change in cognitive functioning. Results from the Longitudinal Aging Study Amsterdam. Gerontology 50, 35–38. [DOI] [PubMed] [Google Scholar]
- [9].Sundermann EE, Biegon A, Rubin LH, Lipton RB, Mowrey W, Landau S, Maki PM; Alzheimer’s Disease Neuroimaging Initiative (2016) Better verbal memory in women than men in MCI despite similar levels of hippocampal atrophy. Neurology; 86(15), 1368–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Sundermann EE, Maki PM, Rubin LH, Lipton RB, Mowrey W, Landau S, Biegon A; Alzheimer’s Disease Neuroimaging Initiative (2016) The female advantage in verbal memory: evidence of sex-specific cognitive reserve. Neurology, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Beinhoff U, Tumani H, Brettschneider J, Bittner D, Riepe MW (2008) Gender-specificities in Alzheimer’s disease and mild cognitive impairment. J Neurol 255(1), 117–122. [DOI] [PubMed] [Google Scholar]
- [12].Chapman RM, Mapstone M, Gardner MN, Sandoval TC, McCrary JW, Guillily MD, Reilly LA, DeGrush E (2011) Women have farther to fall: gender differences between normal elderly and Alzheimer’s disease in verbal memory engender better detection of Alzheimer’s disease in women. J Int Neuropsychol Soc 17(4), 654–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Schellenberg GD & Montine TJ (2012) The genetics and neuropathology of Alzheimer’s disease. Acta Neuropathol 124, 305–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Palop JJ & Mucke L (2010) Amyloid-beta-induced neuronal dysfunction in Alzheimer’s disease: from synapses toward neural networks. Nat. Neurosci 13, 812–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hyman BT & Trojanowski JQ (1997) Consensus recommendations for the postmortem diagnosis of Alzheimer disease from the National Institute on Aging and the Reagan Institute Working Group on diagnostic criteria for the neuropathological assessment of Alzheimer disease. J Neuropathol Exp Neurol 56, 1095–1097. [DOI] [PubMed] [Google Scholar]
- [16].Clark CM, Schneider JA, Bedell BJ, Beach TG, Bilker WB, Mintun MA, Pontecorvo MJ, Hefti F, Carpenter AP, Flitter ML, Krautkramer MJ, Kung HF, Coleman RE, Doraiswamy PM, Fleisher AS, Sabbagh MN, Sadowsky CH, Reiman EP, Zehntner SP, Skovronsky DM; AV45-A07 Study Group (2011) Use of florbetapir-PET for imaging beta-Aβ pathology. JAMA 305(3), 275–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Petersen RC, Aisen PS, Beckett LA, Donohue MC, Gamst AC, Harvey DJ, Jack CR Jr, Jagust WJ, Shaw LM, Toga AW, Trojanowski JQ, Weiner MW (2010) Alzheimer’s Disease Neuroimaging Initiative (ADNI): clinical characterization. Neurology 74(3), 201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E (1999) Mild cognitive impairment: clinical characterization and outcome. Archives of Neurology 56, 303–308. [DOI] [PubMed] [Google Scholar]
- [19].Schmidt M (1996) Rey auditory verbal learning test: A handbook, Western Psychological Services, Los Angeles, CA. [Google Scholar]
- [20].Ricci M, Graef S, Blundo C, Miller LA. Using the Rey Auditory Verbal Learning Test (RAVLT) to differentiate alzheimer’s dementia and behavioural variant fronto-temporal dementia. Clin Neuropsychol. 2012;26(6):926–41. [DOI] [PubMed] [Google Scholar]
- [21].McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM (1984) Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 34(7), 939–944. [DOI] [PubMed] [Google Scholar]
- [22].Aisen PS, Petersen RC, Donohue MC, Gamst A, Raman R, Thomas RG, Walter S, Trojanowski JQ, Shaw LM, Beckett LA, Jack CR Jr, Jagust W, Toga AW, Saykin AJ, Morris JC, Green RC, Weiner MW; Alzheimer’s Disease Neuroimaging Initiative (2010) Clinical Core of the Alzheimer’s Disease Neuroimaging Initiative: progress and plans. Alzheimers Dement 6, 239–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Jack CR Jr., Wiste HJ, Vemuri P, Weigand SD, Senjem ML, Zeng, Bernstein MA, Gunter JL, Pankratz VS, Aisen PS, Weiner MW, Petersen RC, Shaw LM, Trojanowski JQ, Knopman DS; Alzheimer’s Disease Neuroimaging Initiative (2010) Brain beta-amyloid measures and magnetic resonance imaging atrophy both predict time-to-progression from mild cognitive impairment to Alzheimer’s disease. Brain: a journal of neurology 133(11), 3336–3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Reitan RM, Wolfson D (1985) The Halstead-Reitan neuropsychological test battery, Neuropsychology Press, Tucson, AZ. [Google Scholar]
- [25].Johnson KA, Sperling RA, Gidicsin CM, Carmasin JS, Maye JE, Coleman RE, Reiman EM, Sabbagh MN, Sadowsky CH, Fleisher AS, Murali Doraiswamy P, Carpenter AP, Clark CM, Joshi AD, Lu M, Grundman M, Mintun MA, Pontecorvo MJ, Skovronsky DM; AV45-A11 study group (2013) Florbetapir (F18-AV-45) PET to assess amyloid burden in Alzheimer’s disease dementia, mild cognitive impairment, and normal aging. Alzheimers Dement 9(5 Suppl), S72–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Joshi AD, Pontecorvo MJ, Clark CM, Carpenter AP, Jennings DL, Sadowsky CH, Adler LP, Kovnat KD, Seibyl JP, Arora A, Saha K, Burns JD, Lowrey MJ, Mintun MA, Skovronsky DM; Florbetapir F 18 Study Investigators (2012) Performance Characteristics of amyloid PET with Florbetapir F 18 in Patients with Alzheimer’s Disease and Cognitively Normal Subjects. J Nucl Med 53, 378–384. [DOI] [PubMed] [Google Scholar]
- [27].Keith T (2015) Multiple regression and beyond: An introduction to multiple regression and structural equation modeling, Routledge, New York, NY. [Google Scholar]
- [28].Sperling RA, Johnson KA, Doraiswamy PM, Reiman EM, Fleisher AS, Sabbagh MN, Sadowsky CH, Carpenter A, Davis MD, Lu M, Flitter M, Joshi AD, Clark CM, Grundman M, Mintun MA, Skovronsky DM, Pontecorvo MJ; AV45-A05 Study Group (2013) Amyloid deposition detected with florbetapir F 18 ((18)F-AV-45) is related to lower episodic memory performance in clinically normal older individuals. Neurobiol Aging 34(3):822–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Pike KE, Savage G, Villemagne VL, Ng S, Moss SA, Maruff P, Mathis CA, Klunk WE, Masters CL, Rowe CC (2007) Beta-amyloid imaging and memory in non-demented individuals: evidence for preclinical Alzheimer’s disease. Brain 130(Pt 11):2837–44. [DOI] [PubMed] [Google Scholar]
- [30].Jack CR, Lowe VJ, Senjem ML, Weigand SD, Kemp BJ, Shiung MM, Knopman DS, Boeve BF, Klunk WE, Mathis CA, Petersen RC (2008) 11C PiB and structural MRI provide complementary information in imaging of Alzheimer’s disease and amnestic mild cognitive impairment. Brain 131, 665–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Rowe CC, Ellis KA, Rimajova M, Bourgeat P, Pike KE, Jones G, Fripp J, Tochon-Danguy H, Morandeau L, O’Keefe G, Price R, Raniga P, Robins P, Acosta O, Lenzo N, Szoeke C, Salvado O, Head R, Martins R, Masters CL, Ames D, Villemagne VL (2010) Amyloid imaging results from the Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging. Neurobiol Aging 31, 1275–83. [DOI] [PubMed] [Google Scholar]
- [32].Jack CR Jr, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, Petersen RC, Trojanowski JQ (2010) Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol 9(1), 119–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Jack CR JR, Vemuri P, Wiste HJ, Weigand SD, Aisen PS, Trojanowski JQ, Shaw LM, Bernstein MA, Petersen RC, Weiner MW, Knopman DS; Alzheimer’s Disease Neuroimaging Initiative (2011) Alzheimer’s Disease Neuroimaging Initiative. Evidence for ordering of Alzheimer disease biomarkers. Arch Neurol 68(12), 1526–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Jack CR Jr, Wiste HJ, Weigand SD, Knopman DS, Vemuri P, Mielke MM, Lowe V, Senjem ML, Gunter JL, Machulda MM, Gregg BE, Pankratz VS, Rocca WA, Petersen RC (2015) Age, Sex, and APOE ε4 Effects on Memory, Brain Structure, and β-amyloid Across the Adult Life Span. JAMA Neurol 72(5), 511–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Otero Dadín C, Rodríguez Salgado D, Andrade Fernández E. Natural sex hormone cycles and gender differences in memory. Actas Esp Psiquiatr. 2009;37(2):68–74. [PubMed] [Google Scholar]
- [36].Jack CR Jr, Wiste HJ, Lesnick TG, Weigand SD, Knopman DS, Vemuri P, Pankratz VS, Senjem ML, Gunter JL, Mielke MM, Lowe VJ, Boeve BF, Petersen RC (2013) Brain β-amyloid load approaches a plateau. Neurology 80(10), 890–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Stark SM, Yassa MA, Lacy JW, Stark CE (2013) A task to assess behavioral pattern separation (BPS) in humans: Data from healthy aging and mild cognitive impairment. Neuropsychologia 51(12), 2442–2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Lines CR, McCarroll KA, Lipton RB, Block GA. Prevention of Alzheimer’s In Society’s Elderly Study Group (2003). Telephone screening for amnestic mild cognitive impairment. Neurology 60(2), 261–266. [DOI] [PubMed] [Google Scholar]
- [39].Gao S, Hendrie HC, Hall KS, Hui S (1998) The relationships between age, sex, and the incidence of dementia and Alzheimer disease: a meta-analysis. Arch Gen Psychiatry 55, 809–815. [DOI] [PubMed] [Google Scholar]
- [40].Jorm AF, Korten AE, Henderson AS (1987) The prevalence of dementia: a quantitative integration of the literature. Acta Psychiatr Scand 76(5), 465–479. [DOI] [PubMed] [Google Scholar]
- [41].Roberts RO, Geda YE, Knopman DS, Cha RH, Pankratz VS, Boeve BF, Tangalos EG, Ivnik RJ, Rocca WA, Petersen RC (2012) The incidence of MCI differs by subtype and is higher in men: the Mayo Clinic Study of Aging. Neurology 78(5), 342–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Brodaty H, Heffernan M, Kochan NA, Draper B, Trollor JN, Reppermund S, Slavin MJ, Sachdev PS (2013) Mild cognitive impairment in a community sample: the Sydney Memory and Ageing Study. Alzheimers Dement 9(3), 310–317. [DOI] [PubMed] [Google Scholar]
- [43].Katz MJ, Lipton RB, Hall CB, Lipton RB, Hall CB, Zimmerman ME, Sanders AE, Verghese J, Dickson DW, Derby CA (2012) Age-specific and sex-specific prevalence and incidence of mild cognitive impairment, dementia, and Alzheimer dementia in blacks and whites: a report from the Einstein Aging Study. Alzheimer Dis Assoc Disord 26(4), 335–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Fitzpatrick AL, Kuller LH, Ives DG, Lopez OL, Jagust W, Breitner JC, Jones B, Lyketsos C, Dulberg C (2004) Incidence and prevalence of dementia in the Cardiovascular Health Study. J Am Geriatr Soc 52(2), 195–204. [DOI] [PubMed] [Google Scholar]
- [45].Lin KA, Choudhury KR, Rathakrishnan BG, Marks DM, Petrella JR, Doraiswamy PM; Alzheimer’s Disease Neuroimaging Initiative (2015) Marked gender differences in progression of mild cognitive impairment over 8 years. Alzheimers Dement (N Y) 1(2), 103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Song C, Kuo L, Derby CA, Lipton RB, Hall CB (2011) Multi-stage transitional models with random effects and their application to the Einstein aging study. Biom J 53(6), 938–955. [DOI] [PMC free article] [PubMed] [Google Scholar]


