ABSTRACT
The aim of the study was to investigate changes in the incidences of community-acquired pneumonia (CAP) and CAP-related hospitalizations following introduction of 13-valent pneumococcal conjugate vaccine (PCV13) in children ≤5 years of age into the national immunization programme (NIP) of Turkey. PCV7 was included in the NIP of Turkey in November 2008 and was replaced by PCV13 in late 2011. Changes in the incidences of CAP and CAP-related hospitalizations per 100,000 children admissions were investigated from 2011 to 2017. A total of 225,963 children visits were recorded; CAP was diagnosed in 4863 (2.15%) children and 1086 (22%) of them hospitalized between 2011 and 2017. The incidence of CAP declined from 5448 to 1144/100,000 from 2011 to 2017 (p = .001, r = −0.965). When the mean annual incidence of CAP between the transition period of PCV13 (2011/2012) was compared with a post-PCV13 period (2016/2017), CAP incidence was found to be 22% lower (p = .009). Also, the incidence of CAP-related hospitalization decreased significantly from 943 to 335/100,000 from 2011 to 2017 (p = .004 r = −0.91). Moreover, the mean incidence of CAP hospitalization declined 35% (p = .01) between the transition period of PCV13 and a post-PCV13 period. Thus, our study showed a significant reductions in the incidences of CAP and CAP-related hospitalization in children ≤5 years-old after the implementation of PCV13 into the NIP of Turkey.
KEYWORDS: Pneumococcal conjugate vaccine, community acquired pneumonia, pneumonia related hospitalization, children, national immunization programme, Turkey
Introduction
Community-acquired pneumonia (CAP) is a leading cause of childhood mortality and morbidity worldwide. Approximately 120 million of cases and 1.3 million deaths are reported annually worldwide in children under age 5 years.1 In developed countries, the annual incidence of pneumonia is estimated to be 33 per 10,000 in children younger than 5 years and 14.5 per 10,000 in children 0 to 16 years.2
CAP is a common cause of hospitalizations among children. An estimated 5,133,000 hospital admissions due to lower respiratory infections were recorded globally in 2016 among children younger than 5 years, and lower respiratory infections caused 13% of all deaths.3 Most deaths due to lower respiratory infection in children younger than 5 years occurred in the first year of life.3 A range of microorganisms have been implicated as etiologic agents of CAP, but the true prevalence of these agents in children is uncertain. Streptococcus pneumoniae is the most common bacterial cause of pneumonia in children, followed by Haemophilus influenzae, Staphylococcus aureus, Chlamydia pneumoniae, and Mycoplasma pneumoniae. Viruses alone including respiratory syncytial virus, rhinoviruses, human metapneumoviruses, adenoviruses, and influenza cause 14% to 40% of cases of CAP in children.4-6 On the other hand, determination of the cause of pediatric CAP is difficult due to the low sensitivity of conventional microbial tests, such as blood cultures and nasopharyngeal (NP) cultures, to accurately identify those bacteria involved in localized lung infection. Moreover, identification of viruses or bacteria in NP samples by molecular techniques do not accurately predict actual agents since children may be carriers of these microorganisms in their NP flora.
To date, 7-, 10- and 13-valent formulations of the pneumococcal conjugate vaccine (PCV) have been licensed (PCV7, PCV10, and PCV13).7 PCV7 was included in the National immunization programme (NIP) of Turkey in November 2008 and was used until late 2011. PCV13 replaced PCV7 and was introduced into the NIP in April 2011 with a 3-dose schedule at 2,4 and 6 months of age and booster dose at 12 months.8 No catch-up program was run for those children older than 12 months of age unvaccinated with PCV13. The World Health Organization (WHO) reported a national coverage for the PCV7 and PVC13 of 93-98% (mean 96%) during 2009–2017 for Turkish children younger than 12 months of age.9
Many clinical trials have demonstrated the effectiveness of the PCV vaccines in decreasing both invasive and noninvasive diseases among both children and adults.1 Many studies reported that PCV7, after introduction in NIP of countries such as Italy, Poland, US and Sweden, resulted in a 13–65% reduction of all-cause pneumonia.10–12 Although several post-PCV7 implementation studies reported an impact on pneumonia, since PCV13 was introduced later, only limited impact data are available for PCV13, especially for low- or middle-income countries.
The aim of this study was to investigate the impact of PCV13 vaccination on the incidence of over 7 years of CAP and hospitalization due to CAP in children ≤5 years of age after the implementation of PCV13 in the NIP of Turkey.
Materials and methods
We conducted a retrospective record review including all children ≤5 years of age presenting to study hospitals with suspected or clinical pneumonia between 2011 and 2017. This study was performed at Ataşehir Memorial Hospital and Şişli Memorial Hospital, located within 2 different districts (estimated population of the two districts: 690,000, nearly 4–5% of the whole city population) of Istanbul, Turkey. Both hospitals are large primary and tertiary care hospitals that work mainly with the private health insurance system. The patients were children aged 1 month to 5 years with a diagnosis of CAP given in our study hospitals’ pediatric outpatient or emergency room (ER) units from January 1, 2011 to December 31, 2017. They were identified according to the International Statistical Classification of Diseases and Related Health Problems-10th (ICD-10 codes): J12 (viral pneumonia), J13 (pneumonia due to Streptococcus pneumoniae), J14 (pneumonia due to Haemophilus influenzae), J15 (bacterial pneumonia), J16 (pneumonia due to other infectious organisms), J17 (pneumonia in diseases classified elsewhere), and J18 (pneumonia, unspecified organism). Patients diagnosed with the following codes were also included in the study: J85.1 (abscess of the lung with pneumonia), J86 (pyothorax, including empyema), J22 (acute lower tract infections other than acute bronchiolitis), and J90 (pleural effusion). During the whole study period there were no methodological changes for the diagnosis of CAP. The number of admitted children was detected by protocol numbers that also included the identification number of each child via hospital databases. The number of clinical visits was calculated by summing each child’s admissions to our study hospitals throughout the year by using the unique application form number that is linked to each child protocol number in our hospital database systems. Incidences were calculated as follows: incidence of CAP per 100,000 children admitted: number of CAP cases in a year/number of total admissions in a year X 100,000, incidence rate of CAP-related hospitalizations per 100,000 children admitted: number of CAP-related hospitalizations/number of total admissions in a year X 100,000; and incidence of adenovirus-positive acute gastroenteritis: number of adenovirus-positive acute gastroenteritis cases/number of total admissions in a year X 100,000. For comparison purposes as a control group, we considered the incidence of adenovirus-positive acute gastroenteritis, which is unlikely to be related to PCV vaccination, during the study period among children ≤5 years. The study was approved by the Şişli Memorial Hospital Ethical Committee.
Statistical analysis
Data were entered into Microsoft Office Excel 2007 (Microsoft, Redmond, WA, USA) and analyzed using Stata 10.0 Statistics/Data Analysis (StataCorp, Lakeway, Drive, TX, USA). The year 2011–2012 was considered a transition period of PCV13 immunization for children ≤5 years of age (PCV-13 was introduced in late 2011 in the Turkey NIP), and the year 2016–2017 was considered a full vaccination coverage period for children ≤5 years old. To measure the linear correlation between the study period (years) and the incidences of CAP and hospitalization due to CAP, we used the Pearson correlation coefficient (r). A t-test was performed to determine if there were differences in the mean incidences of CAP and CAP-related hospitalization between the PCV13 transition period and the post-PCV13 period. A p value <.05 was considered significant.
Results
Between 2011 and 2017, 454,777 children (mean; 64,968 children/year; range: 47,131–80,623 children/year) were admitted to ER and pediatric outpatient clinics (Table 1). The yearly admission number gradually increased with time, ranging from 47,131 in 2011 to 80,623 in 2017 (p < .05). Among them, 225,963 (49%) children (mean: 32,280 children/year; range: 12,719–56,093 children year) were ≤5 years old (Table 1). The PCV vaccine eligibility of the study population is shown in Table 2.
Table 1.
Incidence of community-acquired pneumonia, community-acquired pneumonia-related hospitalization and adenovirus positive acute gastroenteritis (control group) with respect to years
| Year | Number of total visits (n) | Number of admissions for children age ≤ 5 years (%) |
Number of cases of CAP for children age ≤ 5 years | Number of cases of CAP hospitalization for children age ≤ 5 years |
Number of cases of TT insertion for children age ≤ 5 years |
Incidence of CAP for children age ≤ 5 years* |
Incidence of CAP hospitalization children age ≤ 5 years* |
Incidence of adenovirus positive acute gastroenteritis children age ≤ 5 years* |
Incidence of total TT insertion for children age ≤ 5 years |
|---|---|---|---|---|---|---|---|---|---|
| 2011 | 47,131 | 12,719 (27) | 693 | 120 | 0 | 5448 | 943 | 2.40 | 0 |
| 2012 | 53,049 | 15,449 (29) | 674 | 121 | 2 | 4362 | 783 | 2.90 | 12.9 |
| 2013 | 61,795 | 25,398 (41) | 867 | 181 | 3 | 3413 | 712 | 1.60 | 11.8 |
| 2014 | 65,500 | 28,287 (43) | 971 | 216 | 4 | 3432 | 763 | 1.40 | 14.1 |
| 2015 | 67,832 | 40,473 (59) | 615 | 124 | 0 | 1519 | 306 | 1.80 | 0 |
| 2016 | 78,847 | 47,544 (60) | 501 | 136 | 0 | 1053 | 286 | 1.80 | 0 |
| 2017 | 80,623 | 56,093 (69) | 642 | 188 | 0 | 1144 | 335 | 1.80 | 0 |
CAP: community-aquired pneumonia, TT: thorax tube; *Incidence per 100,000 children admission: number of cases of in a year/number of admissions X 100,000.
Table 2.
Pneumococcal conjugate vaccines eligibility of study population with respect to years
| Children age ≤ 5 years |
||
|---|---|---|
| Year | Any PCV7a vaccination | Any PCV13b vaccination |
| 2011 | Yes for children age < 24 months | None |
| 2012 | None | Yes for children age < 12 months |
| 2013 | None | Yes for children age < 24 months |
| 2014 | None | Yes for children age < 36 months |
| 2015 | None | Yes for children age < 48 months |
| 2016 | None | Yes for children age < 60 months |
| 2017 | None | Yes for all |
aSeven valen pneumococcal conjugate vaccine bThirteen valen pneumococcal conjugate vaccine; World Health Organization (WHO) reported a national coverage for the PCV7 of 97% in 2009, 93% in 2010, 96% in 2011 and for the PVC13 of 97% in 2012, 97% in 2013, 96% in 2014, 97% in 2015, 98% in 2016 and 96% in 2017.
Incidences of community-acquired pneumonia and hospitalization
During the study period from 2011 to 2017, a total of 4863 children were diagnosed with CAP (mean 694 children/year; range: 501–971 children/year) (Table 1). In children ≤5 years of age, the annual incidences of CAP significantly decreased during the period 2011 to 2017 from 5448 to 1144 per 100,000 admissions (Figure 1) (p = .001, r = −0.96). On the other hand, in the control group, the incidence of adenovirus-positive acute gastroenteritis in children ≤ 5 years of age did not change significantly from 2011 to 2017 (p = .18 r = −0.57) (Figure 1). When the mean annual incidence of CAP was compared between the transition period of PCV13 (year 2011/2012) and the post-PCV13 period (year 2016/2017) in children ≤5 years, the incidence of CAP decreased significantly [4905 ± 765/100,000 vs 1098 ± 64/100,000; 95% CI: 14.65–61.48; p = .009].
Figure 1.
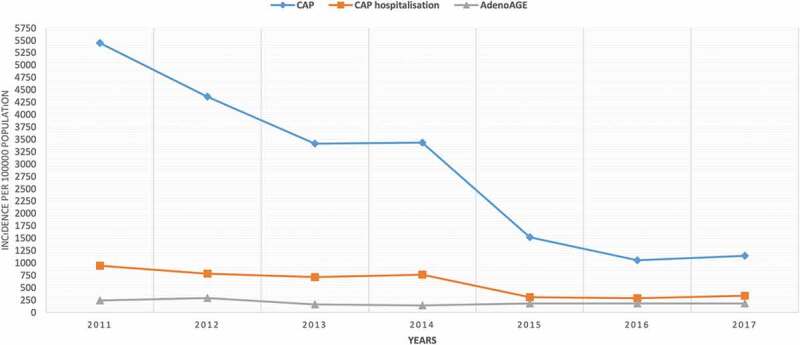
Trends in incidences of community-acquired pneumonia, community-acquired pneumonia-related hospitalization and adenovirus positive acute gastroenteritis (control group) with respect to years
A total of 1086 (22%) children ≤5 years were hospitalized due to CAP (mean: 155 children/year; range: 120–188 children/year) (Table 1) during the study period. The annual incidence of CAP hospitalization in children ≤5 years decreased significantly from the year 2011 to 2017 (p = .004, r = −0.91) (Figure 1). Moreover, when the mean annual incidence of hospitalization due to CAP was compared between the transition period of PCV13 (year 2011/2012) and the post- PCV13 period (year 2016/2017) among children ≤5 years, the incidence of hospitalization due to CAP decreased significantly [863 ± 113/100,000 vs 310 ± 34/100,000; 95% CI: 1.93–9.12; p = .009].
Discussion
To the best of our knowledge, this is the first study in Turkey to assess the impact of PCV13 of the incidences of CAP and hospitalization due to CAP in children ≤5 years of age following PCV13 introduction into the NIP. In this study, we showed a significant impact of PCV13 vaccination on the incidence of CAP and CAP-related hospitalization in children ≤5 years of age, 7 years after PCV13 introduction in Turkey. Our trend analysis revealed that after the implementation of PCV13 into the NIP of our country in late 2011, the incidence of CAP and CAP-related hospitalization decreased significantly over time in children ≤5 years of age.
Additionally, we observed a decrease in the mean incidence of CAP between the transition PCV13 period (years 2011/2012) and the post-PCV13 period (years 2016/2017) of 78%. In order to exclude secular trends following the example of previous studies we selected adenovirus-positive acute gastroenteritis as a control condition because its common and should be unaffected by PCV13.13 We saw that the incidence rate of adenovirus-positive AGE did not change significantly from 2011 to 2017.
Studies in different geographic regions and different countries of the world have shown a decrease in childhood pneumonia cases after the addition of conjugated pneumococcal vaccine to the national immunization schedules.14 These reduction rates may vary depending on the vaccine used, country and age. The PCV-related reduction in the incidence of pneumonia is more evident in children younger than 5 years of age, especially in children under 2 years of age, who have a high incidence of disease.14
One of the initial studies investigating the impact of PCV7, performed by the Kaiser Permanente Vaccine Study Group, reported that PCV7 reduced radiographically defined pneumonia by 30%.15 Studies in the United States since starting the PCV7 national immunization schedule in 2000 have shown a decrease in childhood pneumonia and associated hospitalizations.16 The decrement in the incidence of pneumonia and the pneumonia-related hospitalization in the postvaccination period may vary. This variability is due to the methodological differences of the studies (before and after assessments, interrupted time-series analyses, external and synthetic control analysis, and change-point analyses). PCV7 introduction into the US routine infant vaccination schedule was associated with reductions of 5% to 40% in all-cause pneumonia among different age groups of children.16
There are also epidemiological data showing a continued downward trend of community-acquired pneumonia incidence after changing the immunization schedule from PCV7 to PCV13. Additionally, substantial reductions in pneumonia hospitalization rates were observed among children in the decade after PCV7 introduction, and PCV13 implementation in 2010 was associated with further declines. PCV13 introduction was associated with a 21% and 17% reduction in all-cause pneumonia among children aged <2 years and 2–4 years, respectively, with no change observed for children 5–17 years in the US.16 As in the United States, in other developed countries, the inclusion of PCV in the NIP has led to a reduction in the incidence of pneumonia and pneumonia-related hospitalizations in children.
PCV7 was added to the UK immunization program in 2006, when compared with the prevaccination period, the observed reduction in the incidence of pneumoniae was 19% in children under 5 years of age and 30% in children under 2 years of age, and pneumonia-related hospitalization dropped by 31%.17 Thorrington et al. investigated the changes in the incidence of pneumococcal-related diseases between the 2 years before the introduction of PCV into the national immunization schedule in the UK and the post-PCV period, a period spanning 2004 to 2015. They found that the reduction in the incidence of pneumococcal pneumonia was 80%, pneumonia of unspecified causative organisms was 31% and empyema was 54% in children under 2 years of age.18
In continental Europe, PCV7 was gradually introduced from 2007–2009, followed by the 10- (PCV10) or 13-valent (PCV13) vaccines from 2009 onwards, into the Sweden NIP. Naucler et al. investigated the changing epidemiology of CAP in Sweden by using a nationwide registry system.19 They showed that the incidences of CAP decreased significantly by 36%, 20%, and 16%, respectively for ages <2, 2–4, and 5–17 years, when the pre-PCV period (years 2005–2006) was compared with the post-PCV period (years 2014–2015).19 Similar results have been observed in studies conducted in other European countries, including Italy, the Netherlands, Israel, and France.20-24
Our study is the second to evaluate long term (over at least 7 years) impact of PCV13 on CAP and CAP-related hospitalization. The most important finding of our study is a high impact (reduction) of PCV13 on CAP and CAP-related hospitalization incidences in children ≤5 years of age. We previously performed a prospective nasopharyngeal carriage surveillance study in Istanbul between September 2011 and September 2013 after PCV13 introduction in our country, and we showed that after the introduction of PCV13 into the NIP, overall (including vaccine type serotypes and nonvaccine type serotypes) nasopharyngeal pneumococcal carriage was decreased to 7%, and the greatest drop was in children ≤5 years of age.25
This study had several limitations. First, the study population did not include all of the country’s children, but Istanbul (nearly 20% of the country’s population) is the largest city of Turkey. Istanbul has more than 100 hospitals, including state and private hospitals. Ataşehir Memorial Hospital is located in the Asian region of Istanbul, and Şişli Memorial Hospital is located in the European region of Istanbul. Both hospitals are large hospitals that mainly work with the private health insurance system. We think that our study hospitals may be representative of the Istanbul population, as well as fairly representative the profile of our country as a whole, and may yield good data about the impact of PCV13 on CAP. Second, we could not calculate the true incidence of CAP and CAP-related hospitalizations in our study. We did not have data on the whole child population under 5 years in our hospital regions since our study hospitals are not the only medical units serving the populations in their regions. For this reason, we preferred to calculate the incidence of newly diagnosed CAP and CAP-related hospitalizations in our study population by using the incidence of cases per 100,000 child admissions and investigate the changes with respect to time. Third, we had no pre-PCV period cohort with which to compare our study population; therefore, we compared our results with those of the PCV transition period, but our data consisted of at least 7 years post-PCV13 follow-up. Our main findings were as follows: during the 7-year period following the introduction of PCV13 (2 years of the PCV7 period + 7 years of the PCV13 period), there was a significant reduction in the incidences of CAP and CAP-related hospitalization from PCV13 in children under 5 years of age.
This study supports the positive impact of PCV vaccination on the noninvasive pneumococcal disease in addition to bacteremia and meningitis in children in a developing country, Turkey. Eventually, 10 years after the implementation of PCV into the NIP of Turkey in a 3 + 1 (2, 4, 6, and 12 months), we have seen a dramatic decline in the pneumococcal disease burden in our country, and in 2019, the Turkish Ministry of Health reduced the PCV13 vaccination schedule from 3 + 1 to 2 + 1 doses (2 months, 4 months, and 12 months) for children.
Funding Statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Rudan I, O’Brien KL, Nair H, Liu L, Theodoratou E, Qazi S, Lukšić I, Fischer Walker CL, Black RE, Campbell H. Child Health Epidemiology Reference Group (CHERG). Epidemiology and etiology of childhood pneumonia in 2010: estimates of incidence, severe morbidity, mortality, underlying risk factors and causative pathogens for 192 countries. J Glob Health. 2013;3(1):010401. doi: 10.7189/jogh.03.010401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harris M, Clark J, Coote N, Fletcher P, Harnden A, McKean M, Thomson A.. British thoracic society standards of care committee British thoracic society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax. 2011;66(Suppl 2):ii1. doi: 10.1136/thoraxjnl-2011-200598. [DOI] [PubMed] [Google Scholar]
- 3.Troeger C, Blacker B, Khalil IA, Rao PC, Cao J, Zimsen SRM, Albertson SB, Deshpande A, Farag T, Abebe Z. GBD 2016 lower respiratory infections collaborators. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet Infect Dis. 2018;18(11):1191–210. doi: 10.1016/S1473-3099(18)30310-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jain S, Williams DJ, Arnold SR, Ampofo K, Bramley AM, Reed C, Stockmann C, Anderson EJ, Grijalva CG, Self WH, et al. CDC EPIC study team. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med. 2015;372(9):835–45. doi: 10.1056/NEJMoa1405870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Korppi M, Heiskanen-Kosma T, Kleemola M. Incidence of community-acquired pneumonia in children caused by Mycoplasma pneumoniae: serological results of a prospective, population-based study in primary health care. Respirology. 2004;9(1):109. doi: 10.1111/j.1440-1843.2003.00522.x. [DOI] [PubMed] [Google Scholar]
- 6.Kurz H, Göpfrich H, Wabnegger L, Apfalter P. Role of Chlamydophila pneumoniae in children hospitalized for community-acquired pneumonia in Vienna, Austria. Pediatr Pulmonol. 2009;44(9):873. doi: 10.1002/ppul.21059. [DOI] [PubMed] [Google Scholar]
- 7.Skinner JM, Indrawati L, Cannon J, Blue J, Winters M, Macnair J, Pujar N, Manger W, Zhang Y, Antonello J, et al. Pre-clinical evaluation of a 15-valent pneumococcal conjugate vaccine (PCV15- CRM197) in an infant-rhesus monkey immunogenicity model. Vaccine. 2011;29:8870–76. doi: 10.1016/j.vaccine.2011.09.078. [DOI] [PubMed] [Google Scholar]
- 8.Ceyhan M. Konjuge Pnömokok Aşılarında Son Gelişmeler: 13-Valanlı Konjuge Pnömokok Aşısı. J Pediatr Inf. 2011;5:68–73. doi: 10.5152/ced.2011.25. [DOI] [Google Scholar]
- 9.WHO World Health Organization . Immunization summary http://apps.who.int/gho/data/node.main.PCV3n?lang=en.
- 10.Berglund A, Ekelund M, Fletcher MA, Nyman L. All-cause pneumonia hospitalizations in children <2 years old in sweden, 1998 to 2012: impact of pneumococcal conjugate vaccine introduction. PLoS One. 2014;9(11):e112211. doi: 10.1371/journal.pone.0112211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lindstrand A, Bennet R, Galanis I, Blennow M, Ask LS, Dennison SH, Rinder MR, Eriksson M, Henriques-Normark B, Örtqvist Å, et al. Sinusitis and pneumonia hospitalization after introduction of pneumococcal conjugate vaccine. Pediatrics. 2014;134(6):e1528–e1536. doi: 10.1542/peds.2013-4177. [DOI] [PubMed] [Google Scholar]
- 12.Fitzwater SP, Chandran A, Santosham M, Johnson HL. The worldwide impact of the seven-valent pneumococcal conjugate vaccine. Pediatr Infect Dis J. 2012;31(5):501–08. doi: 10.1097/INF.0b013e31824de9f6. [DOI] [PubMed] [Google Scholar]
- 13.Grijalva CG, Nuorti JP, Arbogast PG, Martin SW, Edwards KM, Griffin MR. Decline in pneumonia admissions after routine childhood immunisation with pneumococcal conjugate vaccine in the USA: a time-series analysis. Lancet. 2007;369(9568):1179–86. doi: 10.1016/S0140-6736(07)60564-9. [DOI] [PubMed] [Google Scholar]
- 14.Cohen R, Cohen JF, Chalumeau M, Levy C. Impact of pneumococcal conjugate vaccines for children in high- and non-high-income countries. Expert Rev Vaccines. 2017;16(6):625–40. doi: 10.1080/14760584.2017.1320221. [DOI] [PubMed] [Google Scholar]
- 15.Hansen J, Black S, Shinefield H, Cherian T, Benson J, Fireman B, Lewis E, Ray P, Lee J. Effectiveness of heptavalent pneumococcal conjugate vaccine in children younger than 5 years of age for prevention of pneumonia: updated analysis using world health organization standardized interpretation of chest radiographs. Pediatr Infect Dis J. 2006;25:779–81. doi: 10.1097/01.inf.0000232706.35674.2f. [DOI] [PubMed] [Google Scholar]
- 16.Wiese AD, Griffin MR, Grijalva CG. Impact of pneumococcal conjugate vaccines on hospitalizations for pneumonia in the United States. Expert Rev Vaccines. 2019. April;18(4):327–41. doi: 10.1080/14760584.2019.1582337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elemraid MA, Rushton SP, Shirley MD, Thomas MF, Spencer DA, Eastham KM, Hampton F, Gorton R, Pollard K, Gennery AR, et al. North East of England paediatric respiratory infection study group. Impact of the 7-valent pneumococcal conjugate vaccine on the incidence of childhood pneumonia. Epidemiol Infect. 2013. August;141(8):1697–704. doi: 10.1017/S0950268812002257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thorrington D, Andrews N, Stowe J, Miller E, van Hoek AJ. Elucidating the impact of the pneumococcal conjugate vaccine programme on pneumonia, sepsis and otitis media hospital admissions in England using a composite control. BMC Med. 2018. 8;16(1):13. doi: 10.1186/s12916-018-1004-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Naucler P, Henriques-Normark B, Hedlund J, Galanis I, Granath F, Örtqvist Å. The changing epidemiology of community-acquired pneumonia: nationwide register-based study in Sweden. J Intern Med. 2019. July 6;286:689–701. doi: 10.1111/joim.12956. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 20.Durando P, Crovari P, Ansaldi F, Sticchi L, Sticchi C, Turello V, Marensi L, Giacchino R, Timitilli A, Carloni R, et al. Collaborative group for pneumococcal vaccination in Liguria. Universal childhood immunisation against Streptococcus pneumoniae: the five-year experience of Liguria Region, Italy. Vaccine. 2009;27(25–26):3459–62. doi: 10.1016/j.vaccine.2009.01.052. [DOI] [PubMed] [Google Scholar]
- 21.Azzari C, Serranti D, Nieddu F, Moriondo M, Casini A, Lodi L, de Benedictis FM, De Vitis E, Cavone F, Cortimiglia M, et al. Significant impact of pneumococcal conjugate vaccination on pediatric parapneumonic effusion: italy 2006–2018. Vaccine. 2019;37(20):2704–11. doi: 10.1016/j.vaccine.2019.04.012. [DOI] [PubMed] [Google Scholar]
- 22.van Deursen AMM, Schurink-Van’t Klooster TM, Man WH, van de Kassteele J, van Gageldonk-lafeber AB, Bruijning-Verhagen PCJL, de Melker HE, Sanders EAM, Knol MJ. Impact of infant pneumococcal conjugate vaccination on community acquired pneumonia hospitalization in all ages in the Netherlands. Vaccine. 2017;35(51):7107–13. doi: 10.1016/j.vaccine.2017.10.090. [DOI] [PubMed] [Google Scholar]
- 23.Greenberg D, Givon-Lavi N, Ben-Shimol S, Ziv JB, Dagan R. Impact of PCV7/PCV13 introduction on community-acquired alveolar pneumonia in children <5 years. vaccine. 2015;33(36):4623–29. doi: 10.1016/j.vaccine.2015.06.062. [DOI] [PubMed] [Google Scholar]
- 24.Ouldali N, Levy C, Minodier P, Morin L, Biscardi S, Aurel M, Dubos F, Dommergues MA, Mezgueldi E, Levieux K, et al. Long-term association of 13-valent pneumococcal conjugate vaccine implementation with rates of community-acquired pneumonia in children. JAMA Pediatr. 2019;173(4):362–70. doi: 10.1001/jamapediatrics.2018.5273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soysal A, Karabağ-Yılmaz E, Kepenekli E, Karaaslan A, Cagan E, Atıcı S, Atınkanat-Gelmez G, Boran P, Merdan S, Hasdemir U, et al. The impact of a pneumococcal conjugate vaccination program on the nasopharyngeal carriage, serotype distribution and antimicrobial resistance of Streptococcus pneumoniae among healthy children in Turkey. Vaccine. 2016;34:3894–900. doi: 10.1016/j.vaccine.2016.05.043. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- WHO World Health Organization . Immunization summary http://apps.who.int/gho/data/node.main.PCV3n?lang=en.


